Abstract
Previous studies have shown that white matter in the deaf brain changes due to hearing loss. However, how white‐matter development is influenced by early hearing experience of deaf people is still unknown. Using diffusion tensor imaging and tract‐based spatial statistics, we compared white‐matter structures among three groups of subjects including 60 congenitally deaf individuals, 36 acquired deaf (AD) individuals, and 38 sex‐ and age‐matched hearing controls (HC). The result showed that the deaf individuals had significantly reduced fractional anisotropy (FA) values in bilateral superior temporal cortex and the splenium of corpus callosum compared to HC. The reduction of FA values in acquired deafness correlated with onset age of deafness, but not the duration of deafness. To explore the underlying mechanism of FA changes in the deaf groups, we further analyzed radial and axial diffusivities and found that (1) the reduced FA values in deaf individuals compared to HC is primarily driven by higher radial diffusivity values and (2) in the AD, higher radial diffusivity was correlated with earlier onset age of deafness, but not the duration of deafness. These findings imply that early sensory experience is critical for the growth of fiber myelination, and anatomical reorganization following auditory deprivation is sensitive to early plasticity in the brain. Hum Brain Mapp, 2012. © 2010 Wiley Periodicals, Inc.
Keywords: congenitally deaf; acquired deaf; white matter; diffusion tensor imaging; fractional anisotropy, tractography
INTRODUCTION
Sensory experience from birth to old age exerts an important influence on brain development [Nithianantharajah and Hannan,2006]. Deaf individuals provide us with a unique opportunity to investigate how the human brain changes as a result of sensory deprivation. Numerous functional neuroimaging studies have shown that deaf individuals show activation of auditory cortex in response to various tasks, such as sign language [Emmorey et al.,2002,2005; MacSweeney et al.,2002,2006; Neville et al.,1998; Petitto et al.,2000; Sakai et al.,2005], nonlinguistic visual stimuli [Finney et al.,2001,2003; MacSweeney et al.,2004], and tactile stimuli [Auer et al.,2007; Levanen et al.,1998]. These results suggest that the auditory cortex in the deaf has been functionally reorganized and colonized by other sensory modalities.
Despite these advances, the exact neurobiological mechanisms of cross‐modal reorganization in deaf individuals remain largely unknown [Bavelier and Neville,2002; Merabet and Pascual‐Leone,2010]. Previous magnetic resonance imaging (MRI) studies have found decreased white‐matter volume in auditory cortex and surrounding areas in congenitally or early deaf individuals when compared with normal hearing individuals [Allen et al.,2008; Emmorey et al.,2003; Shibata,2007]. The abnormal white matter in the auditory cortex may be related to the cross‐modal plasticity in this region.
Diffusion tensor imaging (DTI) provides measures of white‐matter microstructure in the human brain by using the diffusion properties of water molecules [Basser et al.,1994]. DTI is sensitive to the hindrance of water diffusion resulting from local tissue boundaries. Water molecules diffuse more rapidly along the length of an axon and more slowly perpendicular to it owing to myelin and membranes, causing diffusion to be anisotropic. Fractional anisotropy (FA), a DTI‐derived quantitative measure of the directional dependence of water diffusion, reflects anatomical features of white matter, such as axon caliber and myelination. Water diffusion can be quantitatively measured with axial diffusivity (λ∥) and radial diffusivity (λ⟂), DTI‐derived indices, which have been demonstrated to be selectively affected by certain pathological processes in animal studies [Song et al.,2002]. Axial diffusivity (λ∥) indicates diffusion of water parallel to an axon tract, and radial diffusivity (λ⟂) indicates diffusion of water perpendicular to an axon tract. Recently, it has been suggested that increased λ∥ is indicative of pathology of the axon itself, and increased λ⟂ is indicative of myelin abnormalities [Kraus et al.,2007; Song et al.,2003,2005]. Moreover, probabilistic tractography techniques [Behrens et al.,2003b; Parker and Alexander,2003; Parker et al.,2002] can also be used to delineate the white‐matter tracts and the relative strengths of different routes between regions by determining the dominant direction of water diffusion at the microstructural level. This method has been used effectively in both healthy and diseased individuals [Kloppel et al.,2008; Parker and Alexander,2005). To date, only one study has used DTI to investigate the alteration of white matter in the deaf brain [Kim et al.,2009], in which FA was examined in 13 early deaf individuals, who became deaf before 3 years old, and 29 hearing controls (HC). The early deaf individuals showed decreased FA when compared with HC in right superior temporal gyrus (STG), internal capsule, superior longitudinal fasciculus, and inferior frontal regions [Kim et al.,2009]. This study provides evidence for the structural alteration of the white matter in the deaf brain.
However, two important issues remain open for investigation. First, how white‐matter development is influenced by early hearing experience in deaf people is still unknown. Previous studies have indicated that brain plasticity is greatest in the early period of postnatal life, which has been referred to as the sensitive period [Hensch,2005]. Experimental evidence for sensitive periods has been found in primary sensory cortex in several species [Hensch,2004]. Kim and colleagues [2009] did not differentiate between congenital and acquired deafness, though they may impact white‐matter maturation differently. In addition, previous studies have not investigated the effect of onset of deafness and the duration of deafness on the alteration of brain structure. Such an investigation is critical to determine whether white‐matter alteration in deaf people is sensitive to the period when auditory deprivation occurs or is just simply determined by the lack of hearing experience. If the onset but not duration of deafness in humans is the main factor influencing white‐matter organization, this would suggest a sensitive period of white‐matter development. Second, reduction of FA in deaf people could be due to either axial diffusivity (λ∥) or radial diffusivity (λ⟂), or both. Analysis of diffusivity may provide important information [Pierpaoli et al.,2001; Song et al.,2002], which could shed insight into whether the alteration of white‐matter results from differences in degree of myelination or pathology of the axon. It is not known whether axial and/or radial diffusivity is affected by deafness.
To investigate these issues, the current study examined the structural connectivity of white matter using DTI in three groups, including 60 congenitally deaf (CD) individuals who were deaf at birth, 36 acquired deaf (AD) individuals who became deaf between 6 months and 6 years, and 38 sex‐ and age‐matched HC. We used tract‐based statistical analysis (TBSS) [Smith et al.,2006] and probabilistic tractography [Behrens et al.,2003a] to examine structural connectivity alterations. Our specific objectives were (1) to explore FA changes in CD and AD compared to HC; (2) to further explore the basis of FA differences with measuring axial diffusivity (λ∥) and radial diffusivity (λ⟂); (3) to investigate how such changes are related to the age of onset of deafness (AOD) and the duration of deafness.
METHODS
Participants
A large sample of 134 participants with no history of neurological (other than deafness) or psychiatric illness was recruited for this study. All participants were right handed, with scores on the Edinburgh Handedness Inventory above +90. There were 60 CD individuals (38 females, mean age, 21.1 ± 2.26 years; range, 17–28), 36 AD individuals (16 females, mean age, 21.5 ± 1.54 years; range, 19–26), and 38 HC (21 females, mean age, 21.8 ± 2.25 years; range, 17–28). Each deaf individual achieved a normal intelligence quotient (IQ) score on Raven's standard progressive matrices (SPM) (all deaf individuals had higher scores than 50% on the appropriate norms. Twenty‐eight of 96 deaf individuals fell into 50–75%, 43 of 96 deaf individuals fell into 75–90%, and 25 of 96 deaf individuals had greater than 90%). All deaf individuals exhibited profound hearing loss (CD: better ear: mean 98.3 ± 7.00 dB; range, 91–120; left ear: mean 100.1 ± 7.73 dB; range, 91–120; right ear: mean 101.3 ± 7.68 dB; range, 91–120; AD: better ear: mean 99.7 ± 8.87 dB; range, 91–120; left ear: mean 100.4 ± 7.75 dB; range, 92–120; right ear: mean 102.0 ± 9.34 dB; range, 91–125). There were no significant differences in level of hearing in better ear (t = 0.881, P = 0.381), left ear (t = 0.214, P = 0.831), or right ear (t = 0.417, P = 0.678) between the two deaf groups. All deaf participants did not wear hearing aids before 6 years old, and no individuals used a hearing aid consistently in the past 3 years. Chinese Sign Language was the primary language of all deaf participants. All CD participants were deaf due to genetic, pregnancy‐related virus (cytomegalovirus or maternal rubella), or unknown causes. The causes of AD were meningitis, otitis media, ototoxic medications. Age ranges of deafness onset in AD individuals are from 6 months to 6 years (mean age, 2.59 years).1 Among these three groups, no significant difference in either age (F = 1.392, P = 0.252) or sex (χ2 = 3.268, P = 0.195) was observed. The protocol was approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neuroscience and Learning at Beijing Normal University. Informed written consent was obtained from all participants before the scanning. Characteristics of CD individuals and AD individuals are listed in Supporting Information Tables I and II online, respectively.
Data Acquisition
The study was carried out in the State Key Laboratory of Cognitive Neuroscience and Learning in Beijing Normal University. DTI was performed on a Siemens 3T Tim Trio scanner with a 12‐channel phased array head coil. The head was immobilized using foam pads, and ear protection was used for each individual. The DTI dataset was acquired in the transverse orientation using a twice‐refocused, spin‐echo, diffusion‐weighted EPI sequence. The sequence parameters are as follows: 64 nonlinear directions, b‐value = 1,000 s/mm2, repetition time = 8,000 ms, echo time = 104 ms, slice thickness = 2.5 mm without gap, data matrix = 128 × 128, field of view = 230 mm × 230 mm, and voxel size = 1.8 × 1.8 × 2.5 mm3. Forty‐seven slices were acquired covering the whole cerebrum.
Imaging Processing
DTI dataset was analyzed using the software package FSL version 4.1 (http://www.fmrib.ox.ac.uk/fsl) [Smith et al.,2004]. Briefly, nonbrain voxels were first extracted using the brain extraction tool [Smith,2002]. Next, the images were corrected for head motion and residual eddy current distortion using the FMRIB's diffusion toolbox (FDT). The FA maps were then reconstructed using the toolbox FDT. Finally, the TBSS registration and tract skeletonization process were performed as given in the description of Smith et al. [2006]. A lower threshold of FA of 0.2 was used to prevent including nonskeleton voxels. The skeletonized FA data obtained using the TBSS process were then used for voxel‐wise cross‐subject statistical analysis.
Statistical Analyses
The statistical analysis is based on a nonparametric approach using permutation test theory with a standard general linear model design matrix. Monte Carlo permutation testing was performed to generate the random permutations. Group differences among CD, AD, and HC were found with a one‐way ANOVA. The threshold‐free cluster enhancement approach was used to correct for multiple comparisons [Smith and Nichols,2009]. Then, we conducted post hoc t tests to compare mean FA of these regions among three groups, using Bonferroni correction for dealing with the multiple comparison issue. To further examine the basis of any FA differences, we also conducted TBSS on diffusion parameters λ∥ and λ⟂ in regions that exhibited differences in FA among the three groups.
To examine whether such anatomical reorganization was related to AOD and/or duration of deafness, we conducted a correlation analysis. Regions that showed differences between the AD and HC were submitted to correlation analysis to determine the effect of auditory experience on FA, λ∥, and λ⟂. P < 0.05 was regarded as significant in the correlation analysis.
Probabilistic Tractography
We were also interested in whether there were notable differences among the three groups in the main directions or pathways of white‐matter tracts at the locations where there were significant differences in FA. Probablistic tractography was performed using FDT [Behrens et al.,2003b]. We first ran Markov Chain Monte Carlo sampling to build up distributions of diffusion parameters at each voxel in an individual's diffusion space. We modeled 2 fibers per voxel as done in a previous study [Behrens et al.,2007]. We then transformed the clusters having altered FA values between groups to the diffusion space of each individual. Next, we sampled the distribution of voxel‐wise principal diffusion directions from the seed masks of the three FA clusters showing group differences to generate probabilistic tractography (per cluster per participant). By performing this tractography, it was possible to build up the a posteriori connectivity distribution from the seed.
To compare the resulting tracts among CD, AD, and HC, we generated a group “averaged” distribution map [Rouw and Scholte,2007]. For each participant, we set a fixed threshold to determine the presence (1) or absence (0) of a tract at each voxel. Then, we added the 1 s and 0 s at each voxel across all CD, AD, and HC. The sum was then divided by the total number of CD, AD, and HC. For the resulting maps, a value of 70% indicated a voxel in which 70% participants from the group had positive results from fiber tracking for that location.
RESULTS
Regions of Significant Difference in FA Among the Three Groups
Significant differences in fractional anisotropy (FA) within the whole‐brain TBSS skeleton were identified among CD, AD, and HC, including three white‐matter clusters (cluster size > 10 mm3) (Table I). The anatomic locations are in right STG extending to Heschl's gyrus (HG) and planum temporale, in left HG extending to planum temporale, and in splenium of corpus callosum (SCC) (Fig. 1). These areas with FA differences are consistent with previous DTI studies of early deaf individuals [Kim et al.,2009] and with conventional MRI [Allen et al.,2008; Emmorey et al.,2003; Shibata,2007]. Post hoc tests showed that CD exhibited lower FA values, when compared with HC, in right STG (P = 8.56 × 10−12), in left HG (P = 7.96 × 10−6), and in SCC (P = 1.91 × 10−5). Moreover, CD exhibited significantly lower FA values compared to AD in the right STG (P = 4.45 × 10−4) and SCC (P = 3.66 × 10−2). In addition, AD had a reduced FA values compared to HC in the right STG (3.43 × 10−3) (see Fig. 2). No other significant differences were found.
Table I.
Location of white‐matter areas where CD, AD, and HC groups had significant differences
| Location | Cluster size (mm3) | P value | MNI coordinates (mm) | Mean FA in cluster | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | CD | AD | HC | |||
| Right superior temporal gyrus | 612 | 0.005 | 53 | −19 | 2 | 0.374 | 0.393 | 0.412 |
| Left Heschl's gyrus | 110 | 0.034 | −40 | −27 | 7 | 0.401 | 0.417 | 0.437 |
| Splenium of corpus callosum | 11 | 0.046 | 17 | −36 | 28 | 0.675 | 0.697 | 0.713 |
P value is the maximum p value of the permutation test; MNI coordinates are those of the corresponding maximum P value point of each cluster. FA, fractional anisotropy; CD, congenital deaf individuals; AD, acquired deaf individuals; HC, hearing controls. Minimum cluster size 10 mm3.
Figure 1.
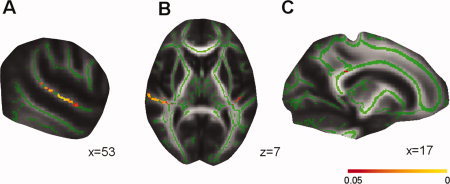
Differences in fractional anisotropy (FA) between congentially deaf (CD), acquired deaf (AD), and hearing controls (HC). The white‐matter skeleton (green) is projected onto the MNI brain (gray). The location of differences in FA value between CD, AD, and HC is indicated (Red–Yellow). (a) Areas in right superior temporal gyrus (STG) with difference in FA between CD, AD, and HC. (b) Areas in right STG (left side) and left Heschl's gyrus (right side) with differences in FA between CD, AD, and HC. The left side of the image corresponds to the participant's right hemisphere. (c) Areas in splenium of corpus callosum with differences in FA between CD, AD, and HC. A threshold‐free cluster enhancement corrected threshold of P < 0.05 is used. The color bar represents the P value.
Figure 2.
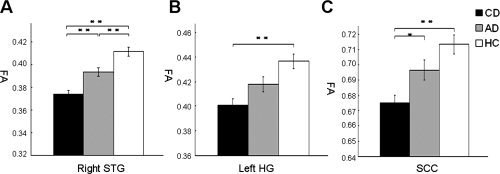
Reduced fractional anisotropy (FA) in deaf individuals compared to hearing controls (HC). (a) Lower FA values in congentially deaf (CD) compared to acquired deaf (AD) and in both deaf groups compared to HC in right superior temporal gyrus (STG). (b) Lower FA values in CD compared to HC in left Heschl's gyrus (HG). (c) Lower FA values in CD compared to HC in splenium of corpus callosum (SCC). Values are presented in mean millimeters (standard error). *P < 0.05; **P < 0.01.
To further determine the basis of FA differences among CD, AD, and HC, we also conducted comparisons on other diffusion parameters, λ∥ and λ⟂, in regions that showed differences in FA between the three groups. One‐way ANOVA analyses of λ∥ and λ⟂ (Table II) showed significant differences in axial diffusivity (λ∥) among CD, AD, and HC at the SCC only (F 2,131 = 3.377, P = 0.037). Post hoc tests showed increased λ∥ values in AD when compared with CD (P = 0.047). Radial diffusion (λ⟂) showed significant differences among CD, AD, and HC in all three clusters (right STG: F 2,131 = 16.478, P = 4.14 × 10−7; left HG: F 2,131 = 16.564, P = 3.86 × 10−7; SCC: F 2,131 = 11.356, P = 2.83 × 10−5). Post hoc tests showed that CD showed increased λ⟂ compared to HC in all three clusters (right STG: P = 1.88 × 10−7; left HG: P = 1.75 × 10−7; SCC: P = 1.67 × 10−5). Increased λ⟂ values were also found in AD when compared with HC in all three clusters (right STG: P = 0.0049; left HG: P = 0.0074; SCC: P = 0.0091) (see Fig. 3). No other significant differences were found. These analyses showed that auditory deprivation results in reduced FA values in auditory cortex and corpus callosum, which is primarily driven by a higher λ⟂ values.
Table II.
Comparisons of axial diffusivity (λ∥) and radial diffusivity (λ⟂) in regions that showed group FA differences between CD, AD, and HC
| Location | Mean λ∥ in cluster (×10−3 mm2/s) | Mean λ⟂ in cluster (×10−3 mm2/s) | ||||||
|---|---|---|---|---|---|---|---|---|
| CD | AD | HC | P value | CD | AD | HC | P value | |
| Right STG | 1.07 | 1.08 | 1.08 | 0.470 | 0.61 | 0.59 | 0.57 | 4.14 × 10−7 ** |
| Left HG | 1.12 | 1.12 | 1.09 | 0.057 | 0.59 | 0.57 | 0.54 | 3.86 × 10−7 ** |
| SCC | 1.49 | 1.54 | 1.49 | 0.037* | 0.42 | 0.41 | 0.38 | 2.83 × 10−5 ** |
Location is the anatomical areas in regions that showed group FA differences between CD, AD, and HC; CD, congenital deaf individuals; AD, acquired deaf individuals; HC, hearing controls.
P < 0.05;
P < 0.01.
Figure 3.
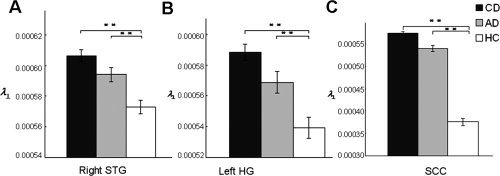
Increased radial diffusivity (λ⟂) in deaf individuals compared to hearing controls. (a) Higher λ⟂ values in congenitally deaf (CD) compared to hearing controls (HC) as well as higher λ⟂ values in acquired deaf (AD) compared to HC in right superior temporal gyrus (STG). (b) Higher λ⟂ values in CD compared to HC as well as higher λ⟂ values in AD when compared with HC in left Heschl's gyrus (HG). (c) Higher λ⟂ values in CD and AD compared to HC in splenium of corpus callosum (SCC). Values are presented in mean millimeters (standard error). *p < 0.05; **p < 0.01.
Relationships Between Age of Onset of Deafness and FA in AD Individuals
To determine the role of the age of onset of auditory deprivation in regions that showed group differences in fractional anisotropy (FA), Pearson's correlations (one‐tailed) were calculated between FA values and deafness characteristics. The analyses showed that the mean FA values in right STG and SCC had significant correlations with AOD (right STG: r = 0.286, P = 0.045; SCC: r = 0.306, P = 0.035), but not with duration of deafness (right STG: r = −0.061, P = 0.361; SCC: r = −0.198, P = 0.124). Mean FA values in left HG marginally correlated with AOD (r = 0.233, P = 0.086), but not with duration of deafness (r = 0.031, P = 0.428). To eliminate the influence of age and gender, we re‐ran the correlations between mean FA values and AOD after partialing both variables and found very similar results. The correlation is significant in SCC (r = 0.312, P = 0.036); marginally significant in right STG (r = 0.275, P = 0.057) but not in left HG (r = 0.208, P = 0.119). After partialing for duration of deafness, correlations between mean FA values and AOD were statistically significant in right STG (r = 0.318, P = 0.032) and left HG (r = 0.324, P = 0.029), marginally correlated in SCC (r = 0.238, P = 0.084) (see Fig. 4). These results show that early auditory experience (AOD) plays an important role in white‐matter tract integrity.
Figure 4.
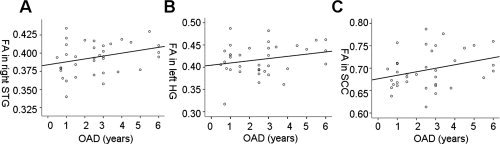
Correlations between age of onset of deafness (AOD) in acquired deaf individuals and the mean fractional anisotropy (FA) in regions showing group FA differences. (a) Later AOD is correlated with higher FA values in right superior temporal gyrus (STG) (r = 0.286, P = 0.045). (b) Later AOD is correlated (not significantly) with higher FA values in left Heschl's gyrus (HG) (r = 0.233, P = 0.086). (c) Later, AOD is correlated with higher FA values in splenium of corpus callosum (SCC) (r = 0.306, P = 0.035). The correlations for right STG and left HG were still significant after partialing for duration of deafness (right STG: r = 0.318, P = 0.032; left HG: r = 0.324, P = 0.029).
To further determine the basis of lower FA values in acquired deaf (AD) individuals, we conducted Pearson correlation analyses (one‐tailed) on the other diffusion parameters, λ∥ and λ⟂, in regions that showed group FA differences. The correlations between mean λ∥ values and AOD were significant in left HG (r = −0.398, P = 0.008), but not in right STG (r = −0.053, P = 0.380) or SCC (r = −0.093, P = 0.295). No significant correlations were found between duration of deafness and mean λ∥ values (right STG: r = −0.175, P = 0.154; left HG: r = 0.249, P = 0.07; SCC: r = 0.007, P = 0.484). After partialing for the duration of deafness, the correlations between mean λ∥ values and AOD were significant in left HG (r = −0.320, P = 0.030), but not in right STG (r = −0.210, P = 0.112) or SCC (r = 0.113, P = 0.259).
Negative correlations between AOD and mean λ⟂ values in regions that showed group FA differences were significant in left HG (r = −0.382, P = 0.011) and SCC (r = −0.351, P = 0.018), marginally correlated in right STG (r = −0.235, P = 0.084). No significant correlations were found between duration of deafness and mean λ⟂ values (right STG: r = −0.064, P = 0.356; left HG: r = 0.072, P = 0.339; SCC: r = 0.202, P = 0.120). The same results were found between mean λ⟂ values and AOD after partialing for age and gender. The correlations were significant in left HG (r = −0.381, P = 0.013) and SCC (r = −0.359, P = 0.019), but not in right STG (r = −0.216, P = 0.110). After partialing for the duration of deafness, negative correlations between mean λ⟂ values and AOD were significant in all three clusters (right STG: r = −0.352, P = 0.019; left HG: r = −0.432, P = 0.005; SCC: r = −0.295, P = 0.043) (Fig. 5). These results indicate that earlier auditory deprivation is associated with higher λ⟂ values, which drives a reduction in FA value in deaf individuals.
Figure 5.
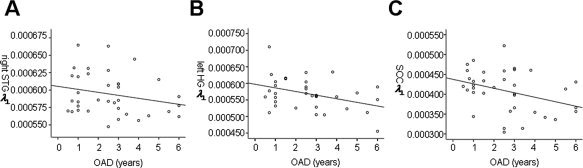
Correlations between age of onset of deafness (AOD) in acquired deaf individuals and mean radial diffusivity (λ⟂) values in regions showing group FA differences. (a) Later, AOD is correlated (not significantly) with lower λ⟂ values in right superior temporal gyrus (STG) (r = −0.235, P = 0.084). (b) Later, AOD is correlated with lower λ⟂ values in left Heschl's gyrus (HG) (r = 0.382, P = 0.011). (c) Later, AOD is correlated with lower λ⟂ values in splenium of corpus callosum (SCC) (r = 0.351, P = 0.018). The correlations for all three clusters were significant after partialing for duration of deafness (right STG: r = −0.352, P = 0.019; left HG: r = −0.432, P = 0.005; SCC: r = −0.295, P = 0.043).
Probabilistic Tractography
We calculated probabilistic diffusion tractography for the three regions that showed group FA differences to investigate whether the main direction of the macrostructural white‐matter tracts differ among three groups. Tractography showed that the area of lower FA value in the right STG and left STG were parts of local association fibers in STG and the inferior longitudinal fasciculus/inferior fronto‐occipital fasciculus that connect distant brain regions. But we did not find any qualitative group differences in the main direction of the white‐matter tracts (see Fig. 6).
Figure 6.
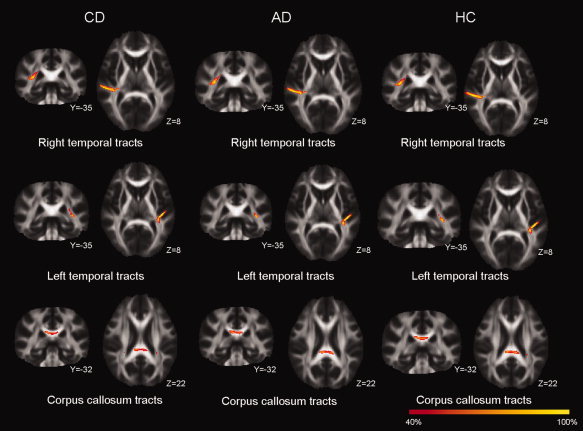
Tractography distributions in congentially deaf (CD), acquired deaf (AD), and hearing controls (HC). The results were very similar for these three groups for right superior temporal gyrus (STG, top row), left Heschl's gyrus (HG, middle row), and splenium of corpus callosum (SCC, bottom row). The color bar represents the percent of participants having positive results from fiber tracking for that voxel.
DISCUSSION
Using DTI, we showed that auditory deprivation induces decreased FA in right STG for both congentially deaf (CD) and AD compared to HC and decreased FA in left HG for CD compared to HC. Decreased FA values were also found in right STG and SCC for CD compared to AD. These results are compatible with the previous findings of either less white‐matter volume or decreased FA within the temporal cortex in congenital or early deafness [Kim et al.,2009]. We extend these findings in two important ways. First, the reduction in FA values in the deaf brain depends on the age of onset of deafness of auditory deprivation. We found that CD individuals who were deaf at birth showed lower FA values than AD individuals becoming deaf after birth. Moreover, the reduction of FA values in acquired deaf is correlated with earlier age of onset of deafness (AOD), but not duration of deafness. Second, the reduction of FA values in deaf individuals is largely due to an increased radial diffusivity (λ⟂), which is also correlated with AOD rather than duration of deafness.
The changes of white matter in the temporal cortex of the deaf brain may be caused by neural atrophy, because hearing loss may leave this region unused for auditory processing. If this is true, we should observe a correlation between the reduction of FA values and the duration of deafness. However, our results did not support this possibility. Instead, we found that the reduction of FA values are correlated with earlier AOD, indicating the changes of white matter are more likely related to functional re‐organization of this region [Bavelier and Neville,2002; Merabet and Pascual‐Leone,2010]. Our results are also compatible with one previous functional study, which revealed that people with early deafness (AOD < 2 years) and people with late deafness (AOD > 5 years) showed different activation in STG during sign language comprehension [Sadato et al.,2004]. These findings suggest that the alteration of structural connectivity of the auditory cortex in the deaf brain depends on the age of onset of auditory deprivation. The earlier the hearing loss occurs, the greater the white‐matter changes. This is consistent with the findings that the brain is most receptive to change in response to sensory deprivation during an early period of postnatal life [Merabet and Pascual‐Leone,2010).
There are a variety of white‐matter properties that can be changed resulting in FA reduction in auditory deprivation, both microstructural properties such as degree of myelination as well as axonal diameter and density [Beaulieu,2002; Mori and Zhang,2006; Rouw and Scholte,2007; Tamnes et al.,2009; Wozniak and Lim,2006] and macrostructural properties of intravoxel and intervoxel fiber‐tract coherence [Basser and Pierpaoli,1996]. With probabilistic tractography, however, we did not find notable differences among CD, AD, and HC in the main direction of macrostructural white‐matter tracts that connected distant brain regions, for example, temporal and occipital areas. So, alteration of microstructural properties of the white matter rather than the main direction of fiber tract coherence may be the principle factor accounting for the structural differences between deaf and hearing individuals.
We further showed that the reduction of FA in the deaf individuals was mainly due to higher radial diffusivity (λ⟂) rather than a change in axial diffusivity (λ∥). The increase in λ⟂ for deaf individuals is compatible with findings seen in early blind individuals [Shu et al.,2009]. Developmental studies have also shown age‐related FA increases [Barnea‐Goraly et al.,2005; Bonekamp et al.,2007; Eluvathingal et al.,2007; Giorgio et al.,2008; Klingberg et al.,1999; Morriss et al.,1999; Mukherjee et al.,2001; Qiu et al.,2008; Snook et al.,2005; Tamnes et al.,2009], which appear to be primarily driven by a λ⟂ decrease [Snook et al.,2005]. Therefore, the decreased FA values for the deaf people in our study suggest that maturity of their auditory cortex lags as a consequence of early deprivation. In another words, auditory deprivation primarily prevents λ⟂ values from declining, which drives a reduction in FA value in deaf individuals. More importantly, we also showed that the increase of λ⟂ values was significantly correlated with earlier AOD. This further suggests that early sensory experience plays an important role in white‐matter development.
Analysis of axial diffusivity (λ∥) and radial diffusivity (λ⟂) may provide important insights into the potential physical mechanisms that underlie changes in FA values [Pierpaoli et al.,2001; Song et al.,2002]. It has been suggested that higher λ∥ may reflect pathology of the axon itself, whereas λ⟂ increases appear to be related to lower axon myelination [Kraus et al.,2007; Song et al.,2003,2005]. Our results imply that sensory deprivation results in lower axon myelination rather than pathology of the axon, suggesting early sensory experience is an important factor for the growth of fiber myelination. In addition to the growth of fiber myelination, neuronal connections are also dynamically pruned and strengthened over development [Huttenlocher,1979; Huttenlocher and Dabholkar,1997; Huttenlocher and de Courten,1987; Huttenlocher et al.,1982]. This may result in more coherent axonal connections [Barnea‐Goraly et al.,2005], accounting for the progressively increased FA values in childhood and adolescence [Barnea‐Goraly et al.,2005]. In our study, the changes in white‐matter structure in deaf individuals could be due to decreased use‐dependent pruning and/or immature myelination. The pruning and/or myelination of neuronal connections in sensory cortex depends on, at least in part, activity‐based competition among different sensory inputs [Innocenti and Price,2005]. However, lack of auditory input may suspend the normal pruning of connections in auditory cortex in deaf people [Emmorey et al.,2003; Penhune et al.,2003]. So, we speculate that the redundant neuronal connections maintained in the auditory cortex of deaf people may provide the structural foundation of functional reorganization, allowing neural rewiring that allows auditory cortex to process visual and tactile stimuli [Auer et al.,2007; Finney et al.,2001,2003; Levanen et al.,1998]. Specifically, earlier deafness may result in greater remaining redundant connections, allowing for more potential of functional cross‐modal plasticity. However, it is still not clear whether and how greater redundant connections relate to lower FA values. If they do, this may account for the finding that the CD group has lowest FA value.
The locations of alterations of FA in bilateral temporal cortex were not symmetrical. Reduced FA values were mainly located in right STG (extending to right HG). In contrast to STG, we found significant differences in FA in deaf people compared to HC in bilateral HG. Numerous functional studies have reported plasticity of secondary and association auditory areas in deaf people, showing these regions respond to sign language [Emmorey et al.,2002,2005; MacSweeney et al.,2002,2006; Neville et al.,1998; Petitto et al.,2000; Sakai et al.,2005], nonlinguistic visual stimuli [Finney et al.,2001,2003] and tactile stimuli [Auer et al.,2007; Levanen et al.,1998], but few have found plasticity within HG [Finney et al.,2001]. Similar results have also been shown in animal studies [Merabet and Pascual‐Leone,2010]. It may be that primary areas are more resistant to cross‐modal reorganization [Sadato et al.,2004]. Then, the reduced FA in HG may be due to lower axon myelination rather than remaining redundant connections and is less likely associated with cross‐modal plasticity. Different from most previous studies [Emmorey et al.,2003; Penhune et al.,2003], we also found reduced FA in the SCC from which fibers project to bilateral temporal cortex (Fig. 6). This is consistent with recent research that used tensor‐based morphometric analysis of voxel‐wise volumetric differences in congenitally and prelingually deaf people compared to HC [Lepore et al.,2009]. Reduced FA in SCC may indicate decreased connection between bilateral auditory cortices. Similar reduced white‐matter integrity between contra‐hemispheric visual cortex has been found in the blind [Park et al.,2007; Yu et al.,2007].
It is possible that some of the group differences between the congentially and AD result from different etiologies of deafness. To examine whether there were obvious differences in the central nervous system between groups, the anatomic MR images of all participants in this study were screened by an experienced neuroradiologist, and no participant showed significant brain abnormalities. We also tested the IQ of all deaf participants with Raven's SPM, and all achieved a normal score. This suggests that the anatomy and function of the central nervous system of the deaf participants were not abnormal. However, this evidence cannot exclude the possibility that there might be subtle effects on central nervous. In addition, most cases of deafness caused by otitis media are associated with transient hearing loss. However, some people who have chronic suppurative otitis media usually suffer severe deafness. In our study, seven of our AD individuals with profound hearing loss suffered from chronic suppurative otitis media. To examine whether the results were driven by these seven participants, we re‐analyzed the FA data among three groups eliminating these participants. The results were very similar to that showed in Figure 1, suggesting that the deaf participants with otitis media did not influence the results (Supporting Information Fig. 1).
In conclusion, we found alterations in white‐matter integrity in auditory processing areas in deaf individuals affecting microstructral aspects of white matter. Further analyses showed that that the magnitude of this alteration in the AD was related to the age of onset of deafness, but not the duration of deafness. These findings revealed that anatomical reorganization of auditory cortex in deaf individuals is determined primarily by early plasticity in the brain, suggesting an early sensitive period of auditory cortex development.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information
Acknowledgements
We thank Hui Wu and Litao Zhu for technical assistance, and all the participants for participating the experiment.
Footnotes
The age that deaf people report they acquired deafness is usually the age they were found to be deaf. When this age is very young, for example, less than 6 months, it is difficult to verify whether they were hearing before they were found to be deaf. We excluded those deaf people who report acquiring deafness less than 6 months to reduce the risk of misclassifying a congenitally deaf individual as an acquired deaf one. In addition, if one acquired deafness very young (e.g., less than 6 months), his or her neural development may not differ very much from one who is deaf at birth. Of course, using 6 months as a cut‐off is somewhat arbitrary.
Contributor Information
Guosheng Ding, Email: Dinggsh@bnu.edu.cn.
Danling Peng, Email: pdl3507@bnu.edu.cn.
REFERENCES
- Allen JS, Emmorey K, Bruss J, Damasio H ( 2008): Morphology of the insula in relation to hearing status and sign language experience. J Neurosci 28: 11900–11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer ET Jr, Bernstein LE, Sungkarat W, Singh M ( 2007): Vibrotactile activation of the auditory cortices in deaf versus hearing adults. Neuroreport 18: 645–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea‐Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL ( 2005): White matter development during childhood and adolescence: A cross‐sectional diffusion tensor imaging study. Cereb Cortex 15: 1848–1854. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C ( 1996): Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson B 111: 209–219. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D ( 1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson B 103: 247–254. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Neville HJ ( 2002): Cross‐modal plasticity: Where and how? Nat Rev Neurosci 3: 443–452. [DOI] [PubMed] [Google Scholar]
- Beaulieu C ( 2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15: 435–455. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen‐Berg H, Woolrich MW, Smith SM, Wheeler‐Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. ( 2003a): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6: 750–757. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM ( 2003b) Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50: 1077–1088. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW ( 2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Mori S, Horska A ( 2007): Diffusion tensor imaging in children and adolescents: Reproducibility, hemispheric, and age‐related differences. Neuroimage 34: 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing‐Cobbs L ( 2007): Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17: 2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Damasio H, McCullough S, Grabowski T, Ponto LL, Hichwa RD, Bellugi U ( 2002): Neural systems underlying spatial language in American Sign Language. Neuroimage 17: 812–824. [PubMed] [Google Scholar]
- Emmorey K, Allen JS, Bruss J, Schenker N, Damasio H ( 2003): A morphometric analysis of auditory brain regions in congenitally deaf adults. Proc Natl Acad Sci USA 100: 10049–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Grabowski T, McCullough S, Ponto LL, Hichwa RD, Damasio H ( 2005): The neural correlates of spatial language in English and American Sign Language: A PET study with hearing bilinguals. Neuroimage 24: 832–840. [DOI] [PubMed] [Google Scholar]
- Finney EM, Fine I, Dobkins KR ( 2001): Visual stimuli activate auditory cortex in the deaf. Nat Neurosci 4: 1171–1173. [DOI] [PubMed] [Google Scholar]
- Finney EM, Clementz BA, Hickok G, Dobkins KR ( 2003): Visual stimuli activate auditory cortex in deaf subjects: Evidence from MEG. Neuroreport 14: 1425–1427. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen‐Berg H ( 2008): Changes in white matter microstructure during adolescence. Neuroimage 39: 52–61. [DOI] [PubMed] [Google Scholar]
- Hensch TK ( 2004): Critical period regulation. Annu Rev Neurosci 27: 549–579. [DOI] [PubMed] [Google Scholar]
- Hensch TK ( 2005): Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6: 877–888. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR ( 1979): Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Res 163: 195–205. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS ( 1997): Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387: 167–178. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C ( 1987): The development of synapses in striate cortex of man. Hum Neurobiol 6: 1–9. [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H ( 1982): Synaptogenesis in human visual cortex—Evidence for synapse elimination during normal development. Neurosci Lett 33: 247–252. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ ( 2005): Exuberance in the development of cortical networks. Nat Rev Neurosci 6: 955–965. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Park SY, Kim J, Lee DH, Park HJ ( 2009): Alterations of white matter diffusion anisotropy in early deafness. Neuroreport 20: 1032–1036. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M ( 1999): Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport 10: 2817–2821. [DOI] [PubMed] [Google Scholar]
- Kloppel S, Draganski B, Golding CV, Chu C, Nagy Z, Cook PA, Hicks SL, Kennard C, Alexander DC, Parker GJ, Tabrizi SJ, Frackowiak RS. ( 2008): White matter connections reflect changes in voluntary‐guided saccades in pre‐symptomatic Huntington's disease. Brain 131( Pt 1): 196–204. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM ( 2007): White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 130( Pt 10): 2508–2519. [DOI] [PubMed] [Google Scholar]
- Lepore N, Vachon P, Lepore F, Chou YY, Voss P, Brun CC, Lee AD, Toga AW, Thompson PM ( 2009): 3D Mapping of brain differences in native signing congenitally and prelingually deaf subjects. Hum Brain Mapp 31: 970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanen S, Jousmaki V, Hari R ( 1998): Vibration‐induced auditory‐cortex activation in a congenitally deaf adult. Curr Biol 8: 869–872. [DOI] [PubMed] [Google Scholar]
- MacSweeney M, Woll B, Campbell R, McGuire PK, David AS, Williams SC, Suckling J, Calvert GA, Brammer MJ ( 2002): Neural systems underlying British Sign Language and audio‐visual English processing in native users. Brain 125( Pt 7): 1583–1593. [DOI] [PubMed] [Google Scholar]
- MacSweeney M, Campbell R, Woll B, Giampietro V, David AS, McGuire PK, Calvert GA, Brammer MJ ( 2004): Dissociating linguistic and nonlinguistic gestural communication in the brain. Neuroimage 22: 1605–1618. [DOI] [PubMed] [Google Scholar]
- MacSweeney M, Campbell R, Woll B, Brammer MJ, Giampietro V, David AS, Calvert GA, McGuire PK ( 2006): Lexical and sentential processing in British Sign Language. Hum Brain Mapp 27: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Pascual‐Leone A ( 2010): Neural reorganization following sensory loss: The opportunity of change. Nat Rev Neurosci 11: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Zhang J ( 2006): Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51: 527–539. [DOI] [PubMed] [Google Scholar]
- Morriss MC, Zimmerman RA, Bilaniuk LT, Hunter JV, Haselgrove JC ( 1999): Changes in brain water diffusion during childhood. Neuroradiology 41: 929–934. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, McKinstry RC ( 2001): Normal brain maturation during childhood: Developmental trends characterized with diffusion‐tensor MR imaging. Radiology 221: 349–358. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Bavelier D, Corina D, Rauschecker J, Karni A, Lalwani A, Braun A, Clark V, Jezzard P, Turner R ( 1998): Cerebral organization for language in deaf and hearing subjects: Biological constraints and effects of experience. Proc Natl Acad Sci USA 95: 922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ ( 2006): Enriched environments, experience‐dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7: 697–709. [DOI] [PubMed] [Google Scholar]
- Park HJ, Jeong SO, Kim EY, Kim JI, Park H, Oh MK, Kim DJ, Kim SY, Lee SC, Lee JD ( 2007): Reorganization of neural circuits in the blind on diffusion direction analysis. Neuroreport 18: 1757–1760. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Alexander DC ( 2003): Probabilistic Monte Carlo based mapping of cerebral connections utilising whole‐brain crossing fibre information. Inf Process Med Imaging 18: 684–695. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Alexander DC ( 2005): Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci 360: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GJ, Wheeler‐Kingshott CA, Barker GJ ( 2002): Estimating distributed anatomical connectivity using fast marching methods and diffusion tensor imaging. IEEE Trans Med Imaging 21: 505–512. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Cismaru R, Dorsaint‐Pierre R, Petitto LA, Zatorre RJ ( 2003): The morphometry of auditory cortex in the congenitally deaf measured using MRI. Neuroimage 20: 1215–1225. [DOI] [PubMed] [Google Scholar]
- Petitto LA, Zatorre RJ, Gauna K, Nikelski EJ, Dostie D, Evans AC ( 2000): Speech‐like cerebral activity in profoundly deaf people processing signed languages: Implications for the neural basis of human language. Proc Natl Acad Sci USA 97: 13961–13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P ( 2001): Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13( 6, Pt 1): 1174–1185. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL ( 2008): Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: Voxel‐wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41: 223–232. [DOI] [PubMed] [Google Scholar]
- Rouw R, Scholte HS ( 2007): Increased structural connectivity in grapheme‐color synesthesia. Nat Neurosci 10: 792–797. [DOI] [PubMed] [Google Scholar]
- Sadato N, Yamada H, Okada T, Yoshida M, Hasegawa T, Matsuki K, Yonekura Y, Itoh H ( 2004): Age‐dependent plasticity in the superior temporal sulcus in deaf humans: A functional MRI study. BMC Neurosci 5: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai KL, Tatsuno Y, Suzuki K, Kimura H, Ichida Y ( 2005): Sign and speech: Amodal commonality in left hemisphere dominance for comprehension of sentences. Brain 128( Pt 6): 1407–1417. [DOI] [PubMed] [Google Scholar]
- Shibata DK ( 2007): Differences in brain structure in deaf persons on MR imaging studied with voxel‐based morphometry. AJNR Am J Neuroradiol 28: 243–249. [PMC free article] [PubMed] [Google Scholar]
- Shu N, Li J, Li K, Yu C, Jiang T ( 2009): Abnormal diffusion of cerebral white matter in early blindness. Hum Brain Mapp 30: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE ( 2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44: 83–98. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 ( Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. ( 2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C ( 2005): Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage 26: 1164–1173. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH ( 2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH ( 2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20: 1714–1722. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC ( 2005): Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26: 132–140. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due‐Tonnessen P, Walhovd KB ( 2009): Brain maturation in adolescence and young adulthood: Regional age‐related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex 20: 534–548. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Lim KO ( 2006): Advances in white matter imaging: A review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci Biobehav Rev 30: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Shu N, Li J, Qin W, Jiang T, Li K ( 2007): Plasticity of the corticospinal tract in early blindness revealed by quantitative analysis of fractional anisotropy based on diffusion tensor tractography. Neuroimage 36: 411–417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information


