Abstract
Background. Patients with major depressive disorder (MDD) display impairments in recollection, which have been explained by both hippocampal and prefrontal dysfunction. Here, we used an event‐related fMRI design, to dissociate hippocampal and prefrontal contributions to the neural processes involved in recollection success and recollection attempt early in the course of MDD. Methods. To disentangle state‐ and trait‐effects of depression, we included 20 medication‐naive patients with a first depressive episode, 20 medication‐free patients recovered from a first episode, and 20 matched, healthy controls in an event‐related fMRI study using a source recollection paradigm. Results. Group comparisons revealed that during the acute state of depression there is an increase in left prefrontal activity related to recollection attempt, while there were no differences in neural correlates of successful recollection. Conclusions. Our results indicate that in the early course of depression, depressive state is associated with increased left prefrontal activation during the attempt to recollect source information suggesting an increased need for executive control during recollection in MDD. In this sample of first‐episode MDD patients we found no evidence for hippocampal dysfunction. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: MDD, fMRI, hippocampus, prefrontal cortex, memory, retrieval
INTRODUCTION
Patients with major depressive disorder (MDD) display impairments in declarative memory retrieval [Burt et al.,1995; Clark et al.,2009; Zakzanis et al.,1998]. Studies of human memory have revealed a fundamental distinction between two ways of gaining access to past experiences: recollection and familiarity [Mandler,1980; Yonelinas,2001]. Recollection involves the recognition of a stimulus including the retrieval of contextual information that accompanied the original encounter, whereas familiarity refers to a feeling that a stimulus has been encountered before without contextual retrieval [Mandler,1980]. MDD is characterized by a selective deficit in recollection, with preserved familiarity [Degl'Innocenti and Backman,1999; Drakeford et al.,2010; MacQueen et al.,2002; Ramponi et al.,2004] whereby in particular patients with several past episodes are more likely to display deficits in recollection [Basso and Bornstein,1999; Fossati et al.,2004; Gorwood et al.,2008; MacQueen et al.,2002].
Recollection is the result of an interplay of multiple, interdependent processes within the brain [Buckner and Wheeler,2001; Cabeza et al.,2008; Dobbins et al.,2002; Eichenbaum et al.,2007]. The medial temporal lobe, and the hippocampus in particular, is critical for successful recollection, as the hippocampus binds together an item and its context [Ranganath et al.,2004]. The prefrontal cortex (PFC) on the other hand is required for “control” processes that guide recollection attempt [Buckner and Wheeler,2001; Buckner et al.,1999; Dobbins et al.,2002] and these control processes are in general insensitive to the outcome of the recollection process [Buckner et al.,1998; Dobbins et al.,2003; Kahn et al.,2004].
Two different hypotheses have been put forward in the literature to explain recollection deficits in MDD. The first hypothesis states that impairment in recollection in MDD is the result of hippocampal dysfunction [Bearden et al.,2006; Gorwood et al.,2008; MacQueen et al.,2002,2003; Sheline et al.,1999]. Several arguments support this hypothesis. Recollection is a typical form of hippocampally dependent memory, whereas preserved types of memory in MDD such as familiarity or implicit memory are not hippocampally dependent. Second, volume reduction of the hippocampus is the most robust neuropathological finding in MDD [Campbell et al.,2004; Clark et al.,2009; Videbech and Ravnkilde,2004] and is likely to cause hippocampal dysfunction. Structural changes of the hippocampus correlate with the number of past depressive episodes and other indices of illness chronicity [Bell‐McGinty et al.,2002; Frodl et al.,2008; Gorwood et al.,2008; MacQueen et al.,2003; Sheline et al.,2003], which is consistent with the pattern of more pronounced memory problems in advanced stages of the disease, independent of current mood state [Fossati et al.,2004; Gorwood et al.,2008; MacQueen et al.,2002]. However, it is still unclear which role the hippocampus plays in the early course of MDD. Most studies that investigated patients with a first episode of MDD neither found prominent memory deficits [Albus et al.,1996; Basso and Bornstein,1999; Fossati et al.,2004] nor volume reductions in the hippocampus [MacQueen et al.,2003; van Eijndhoven et al.,2009]. This does not rule out “subclinical” functional changes, however, that may precede structural deficits of the hippocampus [MacQueen et al.,2003].
The second hypothesis states that impairments in recollection result from an inefficiency of declarative memory retrieval, caused by prefrontal dysfunction [Fossati et al.,2004; Ramponi et al.,2004]. The left PFC mediates the executive control during a recollection attempt [Buckner and Wheeler,2001]. The pattern of memory deficits in MDD patients, which typically shows more impairment on free recall than cued recall and recognition, suggests that these deficits reflect executive problems of depressed patients to implement retrieval strategies and perform effortful tasks [Fossati et al.,2004]. Moreover, reduced specificity of autobiographical memory in MDD, which reflects an impairment in recollection of personally experienced past events [Williams and Scott,1988], is related to poor executive control [Dalgleish et al.,2007; Raes et al.,2006; Ramponi et al.,2004]. PFC deficits in executive control are thought to be especially related to the depressive state, although some impairment may persist in remitted cases [Clark et al.,2009].
Functional neuroimaging studies are needed to disentangle the contribution of hippocampal and prefrontal dysfunction to recollection deficits in MDD. Here, we investigated the neural correlates of recollection in MDD with an event‐related fMRI design [Tendolkar et al.,2007; Weis et al.,2004b]. By using a paradigm that allows to investigate both neural activity related to successful recollection and recollection attempt, we were able to disentangle hippocampal and prefrontal function in MDD (see [Kahn et al.,2004] for a similar paradigm). Our study sample included a group of medication‐naïve patients with a first major depressive episode (MDE), a group of medication‐free patients who had recovered from a first MDE, and a group of matched healthy individuals. This approach allowed us to (1) examine MDD without the effects of prolonged depression on brain structure and (2) to disentangle state and trait effects of depression on the neural correlates of recollection [van Wingen et al.,2010].
METHODS
Subjects
Twenty medication‐naive MDD patients with a current first episode (mean age ± SD 34.1 ± 11.6 years; range 18–56 years), 20 medication‐free MDD patients recovered from a first episode (mean age ± SD 35.8 ± 11.6 years; range 18–53 years), and 20 healthy controls (mean age ± SD 37.3 ± 12.7 years; range 18–53 years) participated in the study. MDD was diagnosed using the Structured Clinical Interview for DSM‐IV (SCID) [First et al.,1996] by a trained psychiatrist (P.vE.). Inclusion criterion for the depressed group was a moderate to severe depression defined as a Hamilton Rating Scale for Depression [Hamilton,1960] (HDRS) 17‐item score ≥18. Inclusion criteria for the recovered group were the absence of clinically relevant symptoms over the preceding 6 to 24 months defined as an HDRS 17‐item score ≤7 [Frank et al.,1991] and discontinuation of antidepressant therapy for at least 2 months. Patients with other current or lifetime DSM‐IV Axis‐I disorders as assessed with the Mini International Neuropsychiatric Interview (MINI) [Sheehan et al.,1998] were excluded. Exclusion criteria for healthy controls consisted of lifetime DSM‐IV Axis‐I disorder as assessed with the MINI, and a history of psychiatric disorders in first degree relatives, because heritable trait effects in the control group could confound the comparisons with the recovered MDD group. All participants were physically healthy, and did not use any medication other than hormonal contraceptives. Other exclusion criteria were a history of substance abuse or dependence, a history of traumatic brain injury, claustrophobia, mental implants, and for women, postpartum depression, pregnancy, lactation, or menopause. The study was approved by the local ethics committee (CMO region Arnhem‐Nijmegen, The Netherlands). Participants were recruited via the outpatients' clinic of the Department of Psychiatry and by local newspaper advertisements. All participants gave written informed consent prior to participation. All participants underwent a neuropsychological assessment to address possible differences in neuropsychological characteristics between the groups.
Experimental Design
After a brief training session, participants viewed in total 270 photographs of either buildings or natural landscapes (135 for each category), selected to be similar in complexity, brightness, and contrast [Takashima et al.,2006; Tendolkar et al.,2007,2008; Weis et al.,2004a]. The experiment was divided into six study‐test cycles with a short break in between. During each of the study phases, subjects viewed 30 pictures, sequentially presented for 1,400 ms with a randomized interstimulus interval (ISI) of 2,500 to 4,100 ms (mean 3,300 ms). These stimuli were dyed in red, blue, or green shades and subjects were required to make a color‐decision by a button press. After every study phase, memory was tested during a subsequent test phase, whereby 45 stimuli (30 old and 15 new) were presented as plain gray‐scale photographs. Participants were required to make a combined recognition/source recollection judgment. Specifically, the subject made one of four responses by a button press: (1) “Old‐Red”, (2) “Old‐Green” or (3) “Old‐Blue” indicated that the subject recognized the item as being old and at the same time recollected in which color the item was initially presented. (4) “New” indicated that the subject did not recognize the item as studied (see Fig. 1) [Kahn et al.,2004]. Stimuli were counterbalanced across participants so that six subjects saw the same setup of pictures. For each of these six subjects, pictures each were dyed in a different order in red, blue, and green shades and presented in a pseudorandom order, so that no two subjects saw the pictures in the same color or order. During the test phase, stimuli were presented sequentially for 1400 ms with a randomized interstimulus interval (ISI) of 2,500 to 4,100 ms (mean 3,300 ms). Additionally, 90 trials of baseline stimulation (i.e. black screen), each lasting 2,000 ms, were randomly intermixed as so‐called null events. Both the ISI variation and the inclusion of null events have been shown to increase the statistical efficiency of event‐related designs [Friston et al.,1999].
Figure 1.
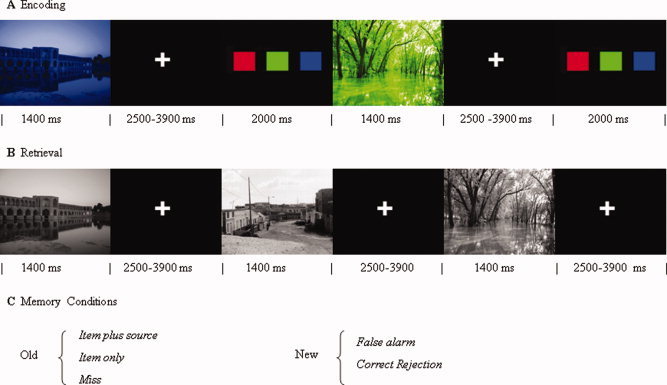
Experimental design. A. Encoding conditions with stimulus presentation in red, green, or blue, randomized interstimulus interval and a color‐decision task. B. Retrieval conditions with studied and unstudied items intermixed and presented in grey color, followed by a one‐step old‐new recognition and source memory task. C. Possible memory outcomes for the studied (old) and unstudied (new) items. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The memory conditions consisted of (1) old items, that were recognized and accompanied by correct source recollection (Item plus Source), (2) old items that were recognized without correct source recollection (Item only), (3) old items that were forgotten (Misses), (4) new items misclassified as old (False Alarms), and (5) new items that were correctly rejected as novel lures (Correct Rejections). Thus, measures of source recollection success (Item plus Source vs. Item only) and source recollection attempt (Item only vs. Misses) were obtained for all old pictures. Differences in memory performance between groups were analyzed with mixed model ANOVAs in SPSS 15.0. Accuracy of item recognition was assessed by the difference in probabilities of a correct old judgment and an old judgment for a new item (Pr = probability hit − probability false alarm). Response bias (Br) was calculated with the formula derived from Snodgrass and Corwin: probability false alarm divided by 1 − Pr [Snodgrass and Corwin,1988]. Accuracy of source memory was assessed by the percentage of “Item plus Source” trials of old trials.
fMRI Data Acquisition
MRI data were acquired during encoding and retrieval with a 1.5T Siemens (Erlangen, Germany) Sonata MR scanner, equipped with a standard head coil. T2*‐weighted BOLD images were acquired using EPI, with each image volume consisting of 33 axial slices (3 mm, 0.5 mm slice‐gap, TR = 2,290 s, TE = 30 ms, 64 × 64 matrix, FOV = 224 mm, FA = 90°). In addition, a high resolution T1‐weighted structural MR image was acquired for spatial normalization procedures (3D MP‐RAGE, 1 × 1 × 1 mm3 voxels).
fMRI Data Analysis
Image analysis was performed with SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). The first five EPI‐volumes were discarded to allow for T1 equilibration, and the remaining images were realigned to the first volume. Images were then corrected for differences in slice acquisition time, spatially normalized to the Montreal Neurological Institute (MNI) T1 template, resampled into 2 × 2 × 2 mm3 voxels, and spatially smoothed with a Gaussian kernel of 8 mm FWHM.
Statistical analysis was performed within the framework of the general linear model [Friston et al.,1995]. Together with null events, explanatory variables were temporally convolved with the canonical hemodynamic response function of SPM5. The following explanatory variables were entered in the model: Item plus Source, Item only, Misses False Alarms, and Correct Rejections. The onsets of these events were time locked to the onset of the visual presentation of the stimuli and all lasted 1,400 ms. In addition, the realignment parameters were included to model potential movement artifacts, as well as a high‐pass filter (cut‐off at 1/128 Hz). The relevant parameter images contrasting each condition to null events were entered in a mixed‐model ANOVA with nonsphericity correction for dependent measures.
Based on our research question we were interested in state and trait effects of depression on recollection success and recollection attempt. State effects were assessed by comparing the group of acutely depressed patients with the combined group of recovered patients and healthy controls, while trait effects were assessed by comparing the combined patient group (acutely depressed and recovered patients) with healthy controls. Statistical tests were family‐wise error rate corrected for multiple comparisons across the entire brain (P < 0.05), using random field theory‐based cluster correction with an initial height‐threshold of P < 0.001 [Friston et al.,1993]. Based on our a priori hypothesis about involvement in memory and depression, data for the hippocampus were corrected for a reduced search region based on the anatomically defined hippocampus [Maldjian et al.,2003]. In addition, mean activity of significant clusters in the hippocampus related recollection success and mean activity of significant clusters in the prefrontal cortex related to recollection attempt were extracted for subsequent analyses with state (depressed vs. recovered and control) and trait (depressed and recovered vs. control) as factors and age and sex as covariates. Peak activations of significant clusters are reported in MNI coordinates.
RESULTS
Subject Characteristics
The HRSD scores confirmed that the currently depressed patients had a moderate to severe depression, and that the currently asymptomatic patients had recovered from depression. As expected, the depressed patients scored higher on negative mood (BDI) and state and trait anxiety (STAI) on the self‐report questionnaires than the recovered patients, and the recovered patients scored higher than the healthy individuals (all P < 0.05; see Table I). However, no significant differences between the groups were observed on any of the neuropsychological tests, including measures of IQ, visual and declarative memory, attention, psychomotor speed, and executive function (see Table I). These results show that this medication free population of first‐episode depressed patients had no apparent impairment in neuropsychological functioning.
Table I.
Demographical, clinical, and neuropsychological characteristics of the MDD patients and the healthy controls
| Depressed (N = 20) | Recovered (N = 20) | Healthy (N = 20) | Group effecta (P) | |
|---|---|---|---|---|
| Age (yr) | 34.1 (11.6) | 35.8 (11.7) | 37.3 (12.7) | 0.71 |
| sex (male/female) | 7/13 | 6/14 | 7/13 | 0.92b |
| Handedness (left/right) | 0/20 | 1/19 | 1/19 | 0.64b |
| Educational level (1–5)c | 3.75 (0.85) | 3.60 (0.60) | 3.95 (0.69) | 0.40b |
| HDRS 17‐item score | 21.08 (4.03) | 3.40 (2.04) | — | <0.001 d |
| STAI | ||||
| State | 43.6 (9.0) | 36.0 (7.2) | 33.8 (8.2) | 0.001 |
| Trait | 53.3 (12.4) | 43.7 (9.1) | 31.7 (7.3) | <0.001 |
| Age at onset (yr) | 33.7 (11.4) | 33.4 (11.5) | — | 0.85d |
| Duration episode (mo) | 7.1 (5.5) | 21.6 (14.3) | — | <0.001 d |
| Duration since onset (mo) | 7.1 (5.5) | 33.7 (17.3) | — | <0.001 d |
| Medication use (number/all)e | — | 18/20 | — | |
| Duration medication use (mo) | — | 16.7 (11.7) | — | |
| DART‐IQ | 93.45 (13.70) | 100.05 (8.08) | 100.40 (17.38) | 0.20 |
| Episodic memory | ||||
| AVLT | ||||
| Immediate recall (no) | 50.95 (10.15) | 52.75 (8.77) | 49.45 (7.78) | 0.51 |
| Delayed recall (no) | 10.9 (3.1) | 11.1 (2.25) | 10.9 (2.2) | 0.96 |
| Complex figure task | 22.73 (5.5) | 21.78 (6.12) | 23.05 (4.77) | 0.75 |
| Attention and psychomotor speed | ||||
| TMT, test A (s) | 30.75 (11.07) | 26.20 (6.65) | 28.95 (6.47) | 0.23 |
| DSST, completed (no) | 62.00 (9.62) | 64.20 (8.49) | 59.00 (8.90) | 0.20 |
| Executive function | ||||
| WCST | ||||
| Categories completed, no | 5.0 (1.9) | 5.1 (1.7) | 5.8 (0.9) | 0.23 |
| Perseverative errors, no | 14.4 (15.5) | 16.3 (13.2) | 9.4 (9.6) | 0.26 |
| TMT, interference (%) | 52.6 (43.4) | 54.1 (56.5) | 50.5 (33.0) | 0.97 |
Data are expressed as mean (SD).
Bold values imply significant group differences.
AVLT, Dutch modified version of the Rey Auditory Verbal Learning Test; CFT, Rey‐Osterrieth Complex; Figure Test; DART, Dutch version of the National Adult Reading Test; DSST, Digit Symbol Substitution Test; HDRS, Hamilton Rating Scale for Depression; STAI, State Trait Anxiety Inventory; TMT, Trail Making Test; WCST, Wisconsin Card Sorting Test.
One‐way ANOVA.
P value of likelihood ratio (categorical variables).
Educational level is coded level 1 to 5 (5=academic), according to the Dutch education system [Loozen and Post, 1991]
One‐way ANOVA with two groups only (=two‐sample t‐test).
Seventeen patients used SSRIs or SNRIs (sertraline, paroxetine, citalopram, venlafaxine), 1 patient used SSRI (paroxetine) followed by TCA (amitryptiline).
Behavioural Results
An overview of the mean memory performance and reaction times is listed in Table II.
Table II.
Overview memory performance and reaction times (mean and standard deviations) for acutely depressed patients (n = 20), recovered patients (n = 20), and healthy controls (n = 20)
| Depressed | Recovered | Healthy | P a | |
|---|---|---|---|---|
| Memory PERFORMANCE | ||||
| Old items | ||||
| Source hits (%) | 43.6 (±16.6) | 41.7 (±15.2) | 43.9 (±15.0) | 0.88 |
| Item hits (%) | 36.2 (±11.1) | 33.1 (±9.3) | 34.0 (±9.3) | 0.61 |
| Overall hits (%) | 79.8 (±12.3) | 74.8 (±15.3) | 77.9 (±15.0) | 0.56 |
| Misses (%) | 19.3 (±12.1) | 23.7 (±15.3) | 19.7 (±12.7) | 0.51 |
| New items | ||||
| Correctly rejected (%) | 69.3 (±17.8) | 67.8 (±15.9) | 69.8 (±13.7) | 0.91 |
| False alarms (%) | 29.6 (±17.6) | 31.3 (±15.3) | 28.0 (±14.3) | 0.77 |
| Reaction times | ||||
| Old items | ||||
| Source hits (ms) | 1,838 (±537) | 1,700 (±483) | 1,935 (±557) | 0.39 |
| Item hits (ms) | 2,000 (±564) | 1,849 (±582) | 2,140 (±657) | 0.34 |
| Misses (ms) | 1,782 (±546) | 1,614 (±461) | 1,723 (±452) | 0.56 |
| New items | ||||
| Correctly rejected (ms) | 1,599 (±460) | 1,449 (±395) | 1,609 (±445) | 0.45 |
| False alarms (ms) | 2,071 (±584) | 1,826 (±610) | 2,142 (±702) | 0.28 |
Data are expressed as mean (SD). Memory performance is expressed as percentage of old or new items. Reaction times are reported in milliseconds.
One‐way ANOVA.
Recognition accuracy did not differ between groups (mean (± s.d.): Prcurrent = 0.50 (±0.20), Prrecovered = 0.44 (±0.14), and Prcontrol = 0.50 (±0.17), F (2,57) = 0.93, P = 0.40, and it was well above chance level (t (59) = 21.7; P < 0.001). In addition, response bias did not differ between groups: Brcurrent = 0.58 (±0.22), Brrecovered = 0.56 (±0.22), and Brcontrol = 0.56 (±0.20); F (2,57) = 0.05, P = 0.96. The accuracy of source memory did not differ between groups (mean correct ± s.d %): current depression = 43.6 ± 17%, recovered = 41.7 ± 15%, control = 43.9 ± 15%; F (2,57) = 0.12, P = 0.88) and was well above chance level (t (59) = 11.4, P < 0.001).
Across groups, reaction times for memory decisions were fastest for correct rejections and slowest for false alarms (correct rejections (1,548 ± 431 ms) < misses (1,703 ± 484 ms) < item plus source (1,819 ± 525 ms) < item only (1,991 ± 603 ms) < false alarms(2,006 ± 637 ms); F (4,57) = 44.8, P < 0.001), but no significant effects or interactions with the factor group were observed (F (2,57) = 1,0, P = 0.37). Thus, the pattern of reaction times parallels the absence of behavioural differences in memory performance.
Neural Correlates of Recollection Success and Recollection Attempt
First we identified brain responses that were associated with recollection success by investigating which brain regions showed larger activity for Item plus Source trials as opposed to Item only trials. This analysis gave rise to significant activation in the left and right anterior hippocampus, as expected. In addition, more brain activity for Item plus Source trials as opposed to Item only trials was found in left anterior cingulate cortex, inferior frontal gyrus, precuneus, and left insula as well as left and right middle temporal gyrus and right angular gyrus (Table III and Fig. 2a).
Table III.
Main effect of recollection success and recollection attempta
| MNI coordinates | Cluster size | Z | |||
|---|---|---|---|---|---|
| x | y | z | |||
| Recollection success (item plus source hits > item only) | |||||
| L anterior cingulate cortex | −4 | 46 | 6 | 2,242 | 5.48 |
| L middle temporal gyrus | −56 | −36 | −10 | 1,805 | 5.17 |
| R angular gyrus | 54 | −52 | 30 | 835 | 5.00 |
| L insula | −30 | 10 | −16 | 187 | 4.94 |
| L precuneus | 0 | −52 | 36 | 1,205 | 4.70 |
| R middle temporal gyrus | 68 | −38 | 0 | 415 | 4.68 |
| L hippocampus | −18 | −12 | −16 | 108 | 4.30 |
| R hippocampus | 18 | −4 | −12 | 46 | 3.92 |
| Recollection attempt (item only > misses) | |||||
| L inferior frontal gyrus | −46 | 8 | 32 | 2,703 | >10 |
| L inferior parietal lobule | −36 | −50 | 40 | 1,909 | 7.29 |
| L caudate nucleus | −10 | 6 | 6 | 1,078 | 6.46 |
| L superior frontal gyrus | −2 | 16 | 48 | 446 | 5.88 |
| R caudate nucleus | 10 | 14 | −6 | 512 | 5.60 |
| L insula | −32 | 26 | 0 | 138 | 5.33 |
The local maxima of the significant clusters (P cluster < 0.05) are reported in MNI coordinates with cluster size and Z‐value.
All peak p fdr−corr values are ≤0.001.
Figure 2.
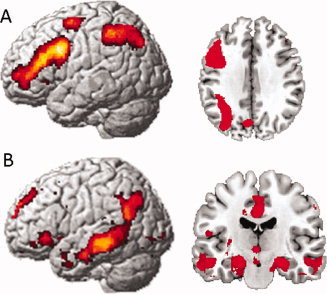
Task effects across depressed, recovered, and healthy individuals (P cluster < 0.05, FWE corrected). (A) Recollection attempt: item only > misses (rendered brain and transverse slice, z = 36). (B) Recollection success: source hits > item only hits (rendered brain and coronal slice, y = −5). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Second, we investigated brain responses that were associated with recollection attempt in the absence of recollection success by investigating which brain regions showed a larger activity for Item only hits as opposed to Misses (Table III and Fig. 2b). Across the groups, recollection attempt elicited activation within the left inferior frontal gyrus, the left and right inferior parietal lobules, the left and right caudate nucleus, the left superior frontal gyrus, and the left insula.
State and Trait Depression Effects on Neural Correlates of Recollection Success and Attempt
State‐effects of depression were assessed by comparing currently depressed individuals with the combined group of recovered and healthy individuals [van Wingen et al.,2010]. A state effect on recollection attempt, i.e. independent of recollection success, was found within the left inferior frontal gyrus ((−48, 8, 28), T = 4.27, P = 0.025 cluster corrected) (Fig. 3). Mean activity within the corresponding significant memory cluster on a group level was extracted to address differences between individual groups. Subsequent two‐group ANCOVAs with age and sex as covariates showed that the currently depressed group differed significantly from both the recovered group (F (1,36) = 5.65, P = 0.005) and the control group (F (1,36) = 10.78, P < 0.001). The recovered group did not differ significantly from the control group (F (1,36) = 1.69, P = 0.19) confirming that the observed effect in the left inferior frontal gyrus is indeed a genuine state‐effect. In addition, Figure 3 shows the pattern of activity within all memory conditions in the three groups within the left inferior frontal gyrus. This pattern of activity is based on the mean value of β‐weights that were extracted from the significant memory cluster in the left inferior frontal gyrus on group level. To test possible differences in limbic deactivation between the groups, the reverse contrast (misses vs. item only hits) was investigated. This contrast did not reveal any significant differences between the three groups within limbic or other brain regions. We did not find a state effect on successful contextual retrieval either.
Figure 3.
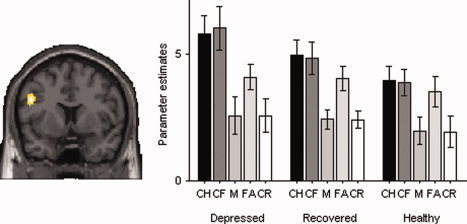
State effect during recollection attempt within the left inferior frontal gyrus. The figures show a coronal slice (y = 11; left = left) at the peak of the significant cluster (P cluster = 0.025 FWE corrected) for the contrast between item only trials versus misses (recollection attempt). The right panel shows the corresponding parameter estimates, extracted from the functional ROI defined at whole group level for depressed, recovered and healthy individuals in the different conditions (CH = source hits, CF = item hits, M = misses, FA = false alarms, CR = correct rejections). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To address trait‐effects, the combined group of currently depressed and recovered patients was compared to the group of healthy individuals. This analysis did not reveal any trait effects on either recollection success or recollection attempt. A direct comparison between recovered patients and healthy controls did not reveal differences in neural activity related to recollection retrieval either.
As the whole brain analyses of successful recollection revealed no specific state or trait‐effects of depression within the hippocampus, we extended our analyses with a region of interest (ROI) analysis of the left and right hippocampus to investigate possible differences in hippocampal activation between the three groups with greater sensitivity. To this end, mean activity within the significant clusters in the left and right hippocampus across the three groups was extracted to address state and trait effects. Again, this analysis revealed neither state (left hippocampus: F (1,55) = 0.022, P = 0.88; right hippocampus: F (1,55) = 0.44, P = 0.51) nor trait‐effects (left hippocampus: F (1,55) = 0.50, P = 0.48; right hippocampus: F (1,55) = 0.84, P = 0.36) of depression on recollection success in the hippocampus.
DISCUSSION
The main goal of this study was to investigate state‐ and trait‐related effects of first episode depression on brain regions that were associated with successful recollection and recollection attempt [Kahn et al.,2004]. In particular, we sought to address two distinct hypotheses with regard to the underlying cause of recollection deficits in depression, i.e. hippocampal and prefrontal dysfunction. Our results indicate that the depressed state specifically affects the neural correlates of a recollection attempt. This neural effect consisted of increased activity within the left ventrolateral prefrontal cortex (VLPFC), which is consistent with executive control involvement during an recollection attempt [Buckner et al.,1998; Dobbins et al.,2002,2003; Kahn et al.,2004]. Our data further suggest that the neural correlates of recollection success remain unaffected during depressive state and trait in first episode MDD.
Group comparisons of behavioural data of memory performance revealed no differences in memory performance in this sample of first episode patients. This could be explained by the good neuropsychological functioning of the participants as neither the medication‐naive first‐episode depressed patients nor the medication‐free patients who had recovered from a first depressive episode showed neuropsychological impairments. The absence of recollection deficits is in line with most other studies that investigated memory performance in the early course of MDD [Albus et al.,1996; Basso and Bornstein,1999; Fossati et al.,2004] and indicates that recollection deficits in MDD are probably more associated with longer duration of depressive illness or more severe forms of depression. The absence of performance differences is advantageous for interpretation of the imaging results, because differences in performance may change the observed neural effects [Morcom et al.,2007].
Exploratory brain analyses of the group as a whole were in line with the existing literature on distinct neural correlates that underlie recollection success and recollection attempt [Buckner et al.,1998; Dobbins et al.,2003; Kahn et al.,2004]. Left lateralized prefrontal and parietal activations were seen during recollection attempts, whereas medial temporal lobe structures were differentially more active during successful recollection. The state effect of depression on recollection attempt was found within the left inferior frontal gyrus, primarily within BA 44 and 45, corresponding to the pars opercularis and triangularis, which are part of the mid to posterior VLPFC [Amunts et al.,1999; Petrides,2005]. This region is generally acknowledged for its role in the cognitive control of memory (for a review see [Badre and Wagner,2007]).
The left VLPFC plays an important role in the cognitive control of episodic memory retrieval, and source memory retrieval in particular [Henson et al.,1999; Nolde et al.,1998; Rugg et al.,1999]. This role is independent from recollection success [Buckner and Wheeler,2001; Buckner et al.,1998; Dobbins et al.,2003; Kahn et al.,2004; Konishi et al.,2000; Lundstrom et al.,2005; Wheeler and Buckner,2003], reflecting the mere attempt to retrieve contextual information. It serves two different aspects in the control of episodic retrieval that are relevant in the current context: (1) top‐down control of memory retrieval and (2) selection operating on active representations that emerge through bottom‐up and top‐down retrieval that is relevant for the task at hand [Badre and Wagner,2007; Badre et al.,2005].
Top‐Down Control of Memory Retrieval
Activity in the left VLPFC increases in response to the demand for a controlled search process, for example in situations when bottom‐up processes are insufficient for automatic retrieval. Wheeler and Buckner have nicely demonstrated this function and also shown that these control processes are not primarily modulated by the mnemonic status, as also new trials that were incorrectly attributed to a source condition (i.e. false alarms) showed increased activity within the left ventrolateral prefrontal cortex, BA 44 [Wheeler and Buckner,2003]; for similar results see [Kahn et al.,2004; Lundstrom et al.,2005]. These results are in accordance with the increased activity during false alarms within the left VLPFC that we found in our experiment in contrast with misses and correct rejections (Fig. 3). It likely reflects the high demand for a controlled search strategy, when an attempt is made to retrieve the color of the picture that is falsely recognized as old. It is important to note that Figure 3 suggests that this controlled search process for false alarms is equally evident in patients and controls.
Postretrieval Selection
Activity within the left VLPFC also increases when there is a need to select task‐relevant contextual details from competing alternatives. This selection mechanism is not restricted to episodic remembering, but is also present in the context of semantic retrieval and working memory, whenever tasks involve selection or interference resolution en route to generating a response [Badre and Wagner,2005; Bunge et al.,2001; D'Esposito et al.,1999; Jonides and Nee,2006; Postle et al.,2004]. It is a domain‐general selection process that may bias active representations maintained in working memory to overcome conflict, thereby permitting selection of relevant representations from noise because of other active competitors [Thompson‐Schill et al.,1997]. In contrast to controlled retrieval, selection is required under conditions of competition from among retrieved representations and task‐irrelevant information. The need for selection is dependent on the amount of retrieved contextual information, and as such is sensitive to the item history during retrieval attempt, because the amount of contextual details differs as a function of item history [Badre and Wagner,2007].
In the light of no behavioral differences between the groups, the state related increase in left PFC activity could be estimated as being compensatory in nature [Price and Friston,2002], as more brain activation is needed to get normal levels of memory performance.
It is unlikely, however, that this compensatory activity reflects overcoming of weaker memory signals as a result of poorer encoding or deficits in retrieval process within the medial temporal lobe. If the increased activity would compensate for weaker memory signals from the MTL one would expect either more activity during successful recollection in MDD patients (in the item plus source condition) or behavioral differences in recollection between the groups, which are both not the case. Instead, the increase in left prefrontal activity likely reflects an increase in activity that is needed to maintain the right level of retrieval orientation to search memory strategically and focus attention on task‐relevant retrieved information [Bunge et al.,2001; Dobbins et al.,2003].
As is evident from Figure 3, activation within the VLPFC is modulated by MDD for the item plus source hits (CH) and the item only hits (CF), while the level of activity of false alarms (FA) does not differ between depressed patients and the comparison groups. This indicates that MDD specifically affects postretrieval selection. It is unlikely that this increased demand for selection (to maintain similar levels of performance) in MDD is the result of an excess of contextual information that is retrieved during recollection, as it would have affected levels of performance (higher in MDD) and activity within the medial temporal lobe (increased activity in MDD). Rather, our findings indicate that the increased demand for selection is the result of interference from competing processes such as depressive ruminations.
Our results are in line with a number of studies that have found an increase in left prefrontal regions in MDD patients during working memory tasks. In particular, these studies show that depressed patients require greater recruitment of left prefrontal regions to maintain similar levels of task performance during conditions of increased cognitive load [Harvey et al.,2005; Matsuo et al.,2007; Walsh et al.,2007; Walter et al.,2007]. Depressed patients may use their attentional resources primarily to focus on task‐irrelevant or specifically depression‐relevant thoughts [Hartlage et al.,1993], caused by maladaptive functional interactions in limbic‐cortical regions [Harvey et al.,2005; Mayberg,1997]. Increased left frontal activation serves to resolve this interference during cognitive demanding tasks and may compensate for a lack of deactivation of limbic structures such as the medial prefrontal cortex and the amygdala [Harvey et al.,2005]. However, we did not find evidence for a lack of deactivation within limbic or other brain structures in our study. It remains possible that increased activation of the left prefrontal cortex may effectively prevent limbic overactivity during recollection attempts. Dichter et al. found amygdala overreactivity in MDD patients in a passive viewing condition, but not in the cognitive control task which was characterized by increased left prefrontal activation [Dichter et al.,2009]. An alternative explanation would be that the increase of activity within VLPFC reflects compensation for an inherent inefficiency of this task‐related network [Berman et al.,2011; Segrave et al.,2010]
A reduction on autobiographical memory specificity (AMS) in depressed subjects [Williams and Scott,1988], which is thought to be part of the broader memory deficit in retrieving the specific details of the context in which a memory was acquired [Raes et al.,2006; Ramponi et al.,2004], has also been related to an impaired cognitive control associated with depressed mood [Dalgleish et al.,2007] and in particular rumination [Ramponi et al.,2004; Watkins et al.,2000]. Interestingly, McGuire et al. recently found that activity within the left VLPFC (−51, 12, 27) correlated with self‐reports of experienced decision costs and individual differences in effort‐based choice. Their results imply that decision costs may be registered based on the degree to which control mechanisms are recruited during decision‐making. Together with our results, this may explain the high level of experienced effort of MDD patients during cognitive demanding tasks such as recollection.
Increased resources for cognitive control during recollection were apparent in the acute phase of depression, and differed significantly from both recovered patients and healthy controls. Visual inspection of Figure 3 shows, however, that the pattern of left prefrontal activation is not completely resolved in the remitted patients, which may suggest a persisting vulnerability. This is in line with several studies that have shown that executive control, which is compromised across a range of paradigms in MDD [Elliott et al.,1996; Rogers et al.,2004], improves substantially as depressive episodes remit, although some impairments persist in remitted cases [Clark et al.,2009].
The data from our study did not reveal differences in hippocampal function between the groups, which is in line with several structural imaging studies who did not detect volumetric differences in the hippocampus early in the course of MDD either [Frodl et al.,2002; MacQueen et al.,2003; van Eijndhoven et al.,2009]. We previously reported state and trait effects on amygdala structure and function in the early course of depression without evidence of hippocampal deficits [van Eijndhoven et al.,2009; van Wingen et al.,2010]. Our results suggest that dysfunction of the hippocampus does not seem to play a major role in the early course of depression. It should be noted, however, that the absence of differences between the groups could also reflect Type II errors, related to limitations of the current design in its sensitivity to detect differences. The context that is introduced by the color in the current study is more intrinsic than for example context that is introduced by different encoding tasks, such as the study by Kahn et al. [2004]. Although both our study and previous studies using a similar experimental set‐up found clear evidence of hippocampal involvement during color‐retrieval ([Tendolkar et al.,2008; Weis et al.,2004b], it remains possible that more complex binding of item and context would be more sensitive to detect hippocampal dysfunction in depression. Moreover, the one‐step yes‐no recognition, plus source recollection paradigm that was adopted in our study leads to a number of chance source hits, which may have resulted in a dilution of the recollection success effect that may have masked differences between the groups in this contrast. While we previously reported on increased amygdala reponsivity during encoding of emotionally neutral stimuli as a trait factor in depression, we did not find evidence of increased amygdala activity during retrieval of neutral information [van Eijndhoven et al.,2009; van Wingen et al.,2010].
The results of our study have to be interpreted in the context of a number of strengths and limitations. To our knowledge, this is the first study that used an event‐related fMRI design to investigate neural correlates of recollection in MDD. The study sample consisted of a group of first episode MDD patients who were medication naïve and a sample of recovered first episode MDD patients who were free of medication use. By studying MDD patients in the early course of their disease we avoided effects of on‐going depression on brain structures [van Eijndhoven et al.,2009], which makes it more easy to interpret functional changes. The cross‐sectional design of our study is a clear limitation and the observed effects should be confirmed in a longitudinal design.
In sum, in this sample of first episode MDD patients, we found evidence for a state‐related increase of left prefrontal activity during recollection, while recollection success and related hippocampal activity remained unaffected. A possible explanation that has to be taken up in future studies is that depressed patients require more resources for cognitive control during recollection to resolve interference of maladaptive affective processes.
REFERENCES
- Albus M, Hubmann W, Wahlheim C, Sobizack N, Franz U, Mohr F ( 1996): Contrasts in neuropsychological test profile between patients with first‐episode schizophrenia and first‐episode affective disorders. Acta Psychiatr Scand 94: 87–93. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K ( 1999): Broca's region revisited: Cytoarchitecture and intersubject variability. J Comp Neurol 412: 319–341. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare‐Blagoev EJ, Insler RZ, Wagner AD ( 2005): Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 47: 907–918. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD ( 2005): Frontal lobe mechanisms that resolve proactive interference. Cereb Cortex 15: 2003–2012. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD ( 2007): Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45: 2883–2901. [DOI] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA ( 1999): Relative memory deficits in recurrent versus first‐episode major depression on a word‐list learning task. Neuropsychology 13: 557–563. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Glahn DC, Monkul ES, Barrett J, Najt P, Villarreal V, Soares JC ( 2006): Patterns of memory impairment in bipolar disorder and unipolar major depression. Psychiatry Res 142: 139–150. [DOI] [PubMed] [Google Scholar]
- Bell‐McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF III, Becker JT ( 2002): Brain morphometric abnormalities in geriatric depression: Long‐term neurobiological effects of illness duration. Am J Psychiatry 159: 1424–1427. [DOI] [PubMed] [Google Scholar]
- Berman MG, Nee DE, Casement M, Kim HS, Deldin P, Kross E, Gonzalez R, Demiralp E, Gotlib IH, Hamilton P, Joormann J, Waugh C, Jonides J ( 2011): Neural and behavioral effects of interference resolution in depression and rumination. Cogn Affect Behav Neurosci 11: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE ( 1999): Frontal cortex contributes to human memory formation. Nat Neurosci 2: 311–314. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Wagner AD, Rosen BR ( 1998): Functional‐anatomic study of episodic retrieval using fMRI. I. Retrieval effort versus retrieval success. Neuroimage 7: 151–162. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME ( 2001): The cognitive neuroscience of remembering. Nat Rev Neurosci 2: 624–634. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD ( 2001): Prefrontal regions involved in keeping information in and out of mind. Brain 124: 2074–2086. [DOI] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G ( 1995): Depression and memory impairment: A meta‐analysis of the association, its pattern, and specificity. Psychol Bull 117: 285–305. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M ( 2008): The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci 9: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM ( 2004): Lower hippocampal volume in patients suffering from depression: a meta‐analysis. Am J Psychiatry 161: 598–607. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ ( 2009): Neurocognitive mechanisms in depression: Implications for treatment. Annu Rev Neurosci 32: 57–74. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Jonides J, Smith EE ( 1999): The neural substrate and temporal dynamics of interference effects in working memory as revealed by event‐related functional MRI. Proc Natl Acad Sci USA 96: 7514–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T, Williams JM, Golden AM, Perkins N, Barrett LF, Barnard PJ, Yeung CA, Murphy V, Elward R, Tchanturia K, Watkins E ( 2007): Reduced specificity of autobiographical memory and depression: The role of executive control. J Exp Psychol Gen 136: 23–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degl'Innocenti A, Backman L ( 1999): Source memory in major depression. J Affect Disord 54: 205–209. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ ( 2009): Affective context interferes with cognitive control in unipolar depression: An fMRI investigation. J Affect Disord 114: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD ( 2002): Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron 35: 989–996. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL ( 2003): Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuropsychologia 41: 318–333. [DOI] [PubMed] [Google Scholar]
- Drakeford JL, Edelstyn NM, Oyebode F, Srivastava S, Calthorpe WR, Mukherjee T ( 2010): Recollection deficiencies in patients with major depressive disorder. Psychiatry Res 175: 205–210. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C ( 2007): The medial temporal lobe and recognition memory. Annu Rev Neurosci 30: 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, Paykel ES ( 1996): Neuropsychological impairments in unipolar depression: The influence of perceived failure on subsequent performance. Psychol Med 26: 975–989. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW ( 1996): Structured Clinical Interview for DSM‐IV Axis‐I Disorders, Clinical Version (SCID‐CV). Washington: American Psychiatric Press. [Google Scholar]
- Fossati P, Harvey PO, Le Bastard G, Ergis AM, Jouvent R, Allilaire JF ( 2004): Verbal memory performance of patients with a first depressive episode and patients with unipolar and bipolar recurrent depression. J Psychiatr Res 38: 137–144. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM ( 1991): Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry 48: 851–855. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC ( 1993): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1: 210–220. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM ( 1999): Stochastic designs in event‐related fMRI. Neuroimage 10: 607–619. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ ( 2002): Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 159: 1112–1118. [DOI] [PubMed] [Google Scholar]
- Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, Scupin I, Reiser M, Moller HJ, Meisenzahl EM ( 2008): Depression‐related variation in brain morphology over 3 years: Effects of stress? Arch Gen Psychiatry 65: 1156–1165. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Corruble E, Falissard B, Goodwin GM ( 2008): Toxic effects of depression on brain function: Impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am J Psychiatry 165: 731–739. [DOI] [PubMed] [Google Scholar]
- Hamilton M ( 1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlage S, Alloy LB, Vazquez C, Dykman B ( 1993): Automatic and effortful processing in depression. Psychol Bull 113: 247–278. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Pochon JB, Levy R, Lebastard G, Lehericy S, Allilaire JF, Dubois B ( 2005): Cognitive control and brain resources in major depression: An fMRI study using the n‐back task. Neuroimage 26: 860–869. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ ( 1999): Recollection and familiarity in recognition memory: An event‐related functional magnetic resonance imaging study. J Neurosci 19: 3962–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Nee DE ( 2006): Brain mechanisms of proactive interference in working memory. Neuroscience 139: 181–193. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD ( 2004): Functional‐neuroanatomic correlates of recollection: Implications for models of recognition memory. J Neurosci 24: 4172–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL ( 2000): Neural correlates of episodic retrieval success. Neuroimage 12: 276–286. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM ( 2005): The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage 27: 824–834. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT ( 2003): Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 100: 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Galway TM, Hay J, Young LT, Joffe RT ( 2002): Recollection memory deficits in patients with major depressive disorder predicted by past depressions but not current mood state or treatment status. Psychol Med 32: 251–258. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mandler G ( 1980): Recognizing: The judgment of previous occurrence. Psychol Rev 87: 252–271. [Google Scholar]
- Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, Najt P, Sanches M, Zamarripa F, Li J, Lancaster JL, Fox PT, Gao JH, Soares JC ( 2007): Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry 12: 158–166. [DOI] [PubMed] [Google Scholar]
- Mayberg HS ( 1997): Limbic‐cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci 9: 471–481. [DOI] [PubMed] [Google Scholar]
- McGuire JT, Botvinick MM ( 2010): Prefrontal cortex, cognitive control, and the registration of decision costs. Proc Natl Acad Sci U S A 107: 7922–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD ( 2007): Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cereb Cortex 17: 2491–2506. [DOI] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, D'Esposito M ( 1998): Left prefrontal activation during episodic remembering: An event‐related fMRI study. Neuroreport 9: 3509–3514. [DOI] [PubMed] [Google Scholar]
- Petrides M ( 2005): Lateral prefrontal cortex: Architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci 360: 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Brush LN, Nick AM ( 2004): Prefrontal cortex and the mediation of proactive interference in working memory. Cogn Affect Behav Neurosci 4: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ ( 2002): Functional imaging studies of neuropsychological patients: Applications and limitations. Neurocase 8: 345–354. [DOI] [PubMed] [Google Scholar]
- Raes F, Hermans D, Williams JM, Demyttenaere K, Sabbe B, Pieters G, Eelen P ( 2006): Is overgeneral autobiographical memory an isolated memory phenomenon in major depression? Memory 14: 584–594. [DOI] [PubMed] [Google Scholar]
- Ramponi C, Barnard PJ, Nimmo‐Smith I ( 2004): Recollection deficits in dysphoric mood: An effect of schematic models and executive mode? Memory 12: 655–670. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M ( 2004): Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 42: 2–13. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N ( 2004): Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neurosci Res 50: 1–11. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Chua PM, Dolan RJ ( 1999): The role of the prefrontal cortex in recognition memory and memory for source: An fMRI study. Neuroimage 10: 520–529. [DOI] [PubMed] [Google Scholar]
- Segrave RA, Thomson RH, Cooper NR, Croft RJ, Sheppard DM, Fitzgerald PB ( 2010): Upper alpha activity during working memory processing reflects abnormal inhibition in major depression. J Affect Disord 127: 191–198. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC ( 1998): The mini‐international neuropsychiatric interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59( Suppl 20): 22–33; quiz 34–57. [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC ( 2003): Untreated depression and hippocampal volume loss. Am J Psychiatry 160: 1516–1518. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH ( 1999): Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 19: 5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J ( 1988): Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen 117: 34–50. [DOI] [PubMed] [Google Scholar]
- Takashima A, Jensen O, Oostenveld R, Maris E, van de Coevering M, Fernandez G ( 2006): Successful declarative memory formation is associated with ongoing activity during encoding in a distributed neocortical network related to working memory: A magnetoencephalography study. Neuroscience 139: 291–297. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Arnold J, Petersson KM, Weis S, Anke B‐D, van Eijndhoven P, Buitelaar J, Fernandez G ( 2007): Probing the neural correlates of associative memory formation: A parametrically analyzed event‐related functional MRI study. Brain Res 1142: 159–168. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Arnold J, Petersson KM, Weis S, Brockhaus‐Dumke A, van Eijndhoven P, Buitelaar J, Fernandez G ( 2008): Contributions of the medial temporal lobe to declarative memory retrieval: manipulating the amount of contextual retrieval. Learn Mem 15: 611–617. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ ( 1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA 94: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijndhoven P, van Wingen G, van Oijen K, Rijpkema M, Goraj B, Jan Verkes R, Oude Voshaar R, Fernandez G, Buitelaar J, Tendolkar I ( 2009): Amygdala volume marks the acute state in the early course of depression. Biol Psychiatry 65: 812–818. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Eijndhoven P, Cremers HR, Tendolkar I, Verkes RJ, Buitelaar JK, Fernandez G ( 2010): Neural state and trait bases of mood‐incongruent memory formation and retrieval in first‐episode major depression. J Psychiatr Res 44: 527–534. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B ( 2004): Hippocampal volume and depression: A meta‐analysis of MRI studies. Am J Psychiatry 161: 1957–1966. [DOI] [PubMed] [Google Scholar]
- Walsh ND, Williams SC, Brammer MJ, Bullmore ET, Kim J, Suckling J, Mitterschiffthaler MT, Cleare AJ, Pich EM, Mehta MA, Fu CH ( 2007): A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry 62: 1236–1243. [DOI] [PubMed] [Google Scholar]
- Walter H, Wolf RC, Spitzer M, Vasic N ( 2007): Increased left prefrontal activation in patients with unipolar depression: An event‐related, parametric, performance‐controlled fMRI study. J Affect Disord 101: 175–185. [DOI] [PubMed] [Google Scholar]
- Watkins E, Teasdale JD, Williams RM ( 2000): Decentring and distraction reduce overgeneral autobiographical memory in depression. Psychol Med 30: 911–920. [DOI] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G ( 2004a): Neural correlates of successful declarative memory formation and retrieval: The anatomical overlap. Cortex 40: 200–202. [DOI] [PubMed] [Google Scholar]
- Weis S, Specht K, Klaver P, Tendolkar I, Willmes K, Ruhlmann J, Elger CE, Fernandez G ( 2004b): Process dissociation between contextual retrieval and item recognition. Neuroreport 15: 2729–2733. [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL ( 2003): Functional dissociation among components of remembering: Control, perceived oldness, and content. J Neurosci 23: 3869–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Scott J ( 1988): Autobiographical memory in depression. Psychol Med 18: 689–695. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP ( 2001): Components of episodic memory: The contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci 356: 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E ( 1998): On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol 11: 111–119. [PubMed] [Google Scholar]


