Abstract
Individual differences in executive functioning and brain morphology are considerable. In this study, we investigated their interrelation in a large sample of healthy older individuals. Digit span, trail‐making, and Stroop tasks were used to assess different executive subfunctions in 367 nondemented community‐dwelling individuals (50–81 years). Task performance was analyzed relative to brain structure using voxel‐based morphometry, corrected for age and sex. Improved task performance was associated with increased local gray matter volume in task‐specific patterns that showed partial, but not complete overlap with known task‐specific functional imaging patterns. While all three tasks showed associations with prefrontal gray matter volume as expected for executive functioning, the strongest overlap between the three tasks was found in insular cortex, suggesting that it has a previously underestimated role for executive functions. The association between the insular cortex and executive functioning was corroborated using stereological region‐of‐interest measurement of insular volume in a subgroup of 93 subjects. Quantitatively, the volume of the single most strongly related region explained 2.4 ± 1.1% of the variance in executive performance over and above the variance explained by age, which amounted to 7.4 ± 4.1%. The age‐independent peak associations between executive performance and gray matter described here occurred in regions that were also strongly affected by age‐related gray matter atrophy, consistent with the hypothesis that age‐related regional brain volume loss and age‐related cognitive changes are linked. Hum Brain Mapp 34:3333–3346, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: age, human, insular cortex, neuropsychological testing, voxel‐based morphometry, stereology
INTRODUCTION
The human central executive is a multicomponent construct that modulates planning, cognitive flexibility, abstract thinking, rule acquisition, implementation, behavioral inhibition, and the selection of relevant sensory information [Baddeley and Hitch, 1974; Baddeley, 2002; Glisky, 2007], playing a key role in almost all aspects of cognition. These functions are critically dependent on the prefrontal cortex and its functional integration with other cortical and subcortical regions [Passingham, 1995; Roberts et al., 1998]. Normal aging has been shown to be associated with volume loss of the prefrontal cortex [Cowell et al., 2007, 1994; Tisserand et al., 2002; Salat et al., 2004; Tisserand et al., 2004], and given that there is a wealth of evidence to suggest that deterioration in executive function is a key contributor to specific domains of cognitive decline in normal aging [Glisky, 2007], a direct link between brain structure and executive cognition may exist, particularly in older adults. Indeed, previous studies in healthy adults suggested that executive performance is related to prefrontal volume [Elderkin‐Thompson et al., 2008; Newman et al., 2007; Tisserand et al., 2004]. However, given that executive functions and higher‐order fluid cognition in general are dependent on multiple cortical and subcortical networks [Knight, 2007], it is highly likely that the structural components underlying such functions are not limited to the prefrontal cortex. It is therefore important to consider how the entire brain structurally supports executive functions. The primary goal of the present study was to investigate the relationship between gray matter volume and executive function in a large sample of older individuals.
In conjunction with the prefrontal cortex, the insula exhibits accelerated age‐related decay of volume in both cerebral hemispheres [Bergfield et al., 2010; Good et al., 2001; Hutton et al., 2009; Raz et al., 2010]. Furthermore, executive functions may load on the insular cortices, as reflected in studies of task‐level control and focal attention [Nelson et al., 2010], directed attention using the Stroop task [Hart et al., 2010] and decision‐making [Thielscher and Pessoa, 2007]. The insula has an important role in many visceral, autonomic, and motor processes [Dupont et al., 2003; Flynn et al., 1999; Mesulam and Mufson, 1982a, 1982b] and in more cognitively orientated processes, such as the functional neuroanatomy of language [Dronkers, 1996; Keller et al., 2011; Ogar et al., 2006; Price, 2000; Wise et al., 1999]. On the basis of a database of published foci of imaging studies developed by Van Essen and coworkers [Van Essen, 2009; Van Essen and Dierker, 2007; Van Essen et al., 2001], Nelson et al. 2010 point out that the left and right anterior insular cortices are among the most commonly activated brain regions across all cognitive tasks, perhaps suggesting that this region may have a general role in attention and task‐level control. We were therefore particularly interested in the relationship between insular cortex volume and executive function in older adults.
Voxel‐based morphometry (VBM) is widely used on magnetic resonance images (MRI) to investigate brain structural alterations in patient samples and cognitive correlates of gray matter alterations in healthy individuals. The technique removes the need of an expert anatomist for time‐consuming volumetric estimation of predefined regions‐of‐interest (ROI), and is particularly useful to gain insight into regional brain alterations in an exploratory manner in large samples of subjects. However, there has been some discussion concerning validity of the VBM technique [Ashburner and Friston, 2001; Bookstein, 2001; Fein et al., 2006; Keller and Roberts, 2008; Pereira et al., 2008; Salmond et al., 2002]. To address this issue, and further corroborate our results, we supplemented VBM analyses with a mathematically unbiased and robust stereological approach to obtain volume estimates of the insular cortices, corresponding to our principal ROI. Stereology in conjunction with point counting has been recently shown to be a reliable and functionally significant method to estimate the volume of the insular cortices [Keller et al., 2011]. Therefore, we used both VBM and stereological approaches to examine the relationship between gray matter volume and executive function. We assessed executive functions using digit span, trail‐making, and Stroop paradigms given that these tasks of attentional and executive functions are among the first to decline with age [Hedden and Gabrieli, 2004]. In a first step, we used voxel‐level analyses, VBM‐based ROI analyses and stereological methods in a large cohort of nondemented older individuals to identify regions where gray matter volume was related to executive functions after correction for age and sex. In a second step, we then determined the extent of age‐related gray matter volume loss in these regions and the relative contributions of sex, age, and sex‐and‐age‐independent variations in regional gray matter volume to executive performance.
MATERIALS AND METHODS
Subjects
The Münster SEARCH study (Systematic evaluation and alteration of risk factors for cognitive health) examines the relationship between aging, cognitive functions and cardiovascular risk factors in community‐dwelling individuals. The research protocol has been approved by the local ethics committee and written informed consent was obtained from every participant. Participants from 40 to 85 years of age were randomly selected based on dates of birth from the population register of the city of Münster, Germany. They were invited to participate in the study by letter and recruited after giving informed consent. From a total of 3,500 invited citizens, 792 consented to participate. All participants received a structured clinical face‐to‐face interview, a physical examination by a trained study physician, blood sampling, structural MRI brain imaging and comprehensive neuropsychological assessment. Although the study was open to subjects between 40 and 85 years, the initial recruitment phase focused on participants above 50 years of age, with very few subjects below 50 included in the database at the time the present investigation was conducted. For the present investigation, we therefore selected subjects between 50 and 85 years of age whose structural MRI was free of artifacts or gross morphological abnormalities (brain tumors or mengingeomas, territorial stroke, arachnoideal cysts, massive subcortical atherosclerotic encephalopathy). To reduce confounding effects on neuropsychological performance, we excluded putatively depressed subjects (Beck's depression inventory score > 17 [Beck et al., 1961]), subjects with probable mild cognitive impairment (Mini‐Mental State Examination score < 27), subjects on psychotropic medication, subjects with atrial fibrillation [Knecht et al., 2008], and subjects with severe neurological or psychiatric conditions. In addition, we excluded subjects for whom gross segmentation errors occurred during the preprocessing of MRI scans for VBM. All segmented, normalized images were visually inspected and 25 subjects showing obvious segmentation errors (mostly classification of dura/scalp as gray matter) were excluded from analysis. These criteria left 367 subjects for the present study (172 male, 195 female). Characteristics of the study population and for the subsample for whom stereological analysis of the insula was performed are shown in Table 1.
Table 1.
Characterization of participants
| Complete group (n = 367) | Stereology subgroup (n = 93) | |
|---|---|---|
| Age [years] | 63.7 ± 6.7 (50–81) | 64.7 ± 6.9 (50–79) |
| Gender | 53.1% female | 51.0% female |
| BDI score | 5.2 ± 4.0 (0–17) | 5.5 ± 4.3 (1–17) |
| Performance on neuropsychological tests | ||
| Digit span forward [digits] | 7.6 ± 1.6 (3–12) | 7.7 ± 1.6 (4–12) |
| Digit span backward [digits] | 6.7 ± 1.7 (2–12) | 6.7 ± 1.8 (2–11) |
| TMT part A [s] | 33 ± 10 (14–76) | 33 ± 10 (14–68) |
| TMT part B [s] | 83 ± 30 (30–260) | 82 ± 27 (30–165) |
| Stroop test part II [s] | 20 ± 4 (10–35) | 20 ± 3 (15–29) |
| Stroop test part III [s] | 40 ± 10 (20–76) | 39 ± 8 (23–65) |
Data are given as mean ± SD (range) of the raw values. Mean values in the subgroup were not significantly different from the total group.
Neuropsychological Assessment
Trained technicians supervised by a clinical neuropsychologist conducted the neuropsychological assessment, consisting of digit span forward and backward, trail‐making tests (TMT) parts A and B and Stroop tests parts II and III. Table 2 provides details on the procedure and the functions assessed by the different paradigms. Difference scores were calculated to isolate underlying executive subfunction as detailed in Table 2.
Table 2.
Neuropsychological tests of attention and executive functions used in this study
| Task group | Test | Task description | Performance index | Measured functions | Origin |
|---|---|---|---|---|---|
| Digit spans | Digit span forward | Digit sequences of 3 to 8 digits are read out and have to be reproduced in the presented order | Number of correctly remembered digits | Short‐term memory [Kaufman et al, 1991] | Wechsler Memory Scale Revised, German version [Härting et al., 2000] |
| Digit span backward | Digit sequences of 3 to 8 digits are read out and have to be reproduced in inverse order | Number of correctly remembered digits | Short‐term memory, data manipulation [Lezak, 1995] | ||
| Digits difference score | – | Calculated: Digit span forward ‐ digit span backward | Data manipulation [Wager and Smith, 2003] | ||
| Trail‐making tests (TMT) | TMT part A | 25 numbers on a sheet of paper have to be connected by pencil in ascending order | Time to completion | Attention, visual searching, psychomotor speed [Shum et al., 1990] | Compendium of neuropsychological tests [Spreen and Strauss 1998] |
| TMT part B | Same as TMT A, but numbers and letters have to be alternated in ascending order | Time to completion | Attention, visual searching, psychomotor speed, set shifting [Shum et al., 1990] | ||
| TMT difference score | – | Calculated: TMT part B ‐ part A | Set shifting [Spreen and Strauss, 1998] | ||
| Stroop tests | Stroop test part II | Colored rectangles are presented. Subjects have to name the colors. | Time to completion | Color perception, color naming, speed of processing [MacLeod, 1991] | [Stroop, 1935]; German version: Nürnberger Altersinventar (Nuremberg Gerontopsychological Inventory) [Oswald and Fleischmann, 1997] |
| Stroop test part III | Colored rectangles with conflicting color words printed on them are presented. Subjects have to name the colors of the rectangles. | Time to completion | Color perception, color naming, speed of processing, response inhibition [MacLeod, 1991] | ||
| Stroop difference score | – | Calculated: Stroop test part III ‐ part II | Response inhibition [Zajano and Gorman, 1986] |
MRI Data Acquisition
Magnetic resonance imaging (MRI) was performed on a 3 T MRI system (Gyroscan Intera T30, Philips Medical System, Best, The Netherlands) using a structural T1‐weighted 3D turbo‐field‐echo sequence (matrix 256 × 256 × 160 over a field of view of 25.6 × 25.6 × 16 cm3, reconstructed after zero filling to 512 × 512 × 320 cubic voxels with an edge length of 0.5 mm). The system was a whole body scanner equipped with master gradients (slew rate 150 mT/m/ms; maximal gradient strength 33 mT/m). A circularly polarized transmit‐receive quadrature head coil with HF reflecting screen at the cranial end was employed for spin excitation and resonance signal acquisition. All MR images were resampled to a voxel size of 1 mm × 1 mm × 1 mm and corrected for image inhomogeneity using in‐house software prior to VBM and stereological analyses.
Voxel‐Based Morphometry
MRI data preprocessing for VBM
The principles of voxel‐based morphometry were employed as described by Ashburner and Friston (2000). Data preprocessing and analysis was performed with SPM5 (Wellcome Department of Imaging Neuroscience, University College London, UK) running under Matlab 7.1 (Mathworks, Sherborn, MA), using the VBM5 toolbox (Structural Brain Mapping Group, University of Jena, Germany). Default settings were used unless otherwise indicated. Data were resliced to a voxel size of 2 × 2 × 2 mm3. The unified segmentation algorithm of SPM5 [Ashburner and Friston, 2005] was used to segment and spatially normalize the structural MR images. Voxel values of the resulting normalized gray matter segments indicate the probability (between 0 and 1) that a specific voxel belongs to gray matter. Images were corrected for volume changes during normalization by modulating each voxel by the Jacobian determinant derived from the normalization, allowing analysis of local gray matter volumes [Good et al., 2001]. Analyses of gray matter concentration from unmodulated gray matter image were not performed in this study. Finally, images were smoothed using an isotropic Gaussian kernel of 10 mm full‐width at half‐maximum. In a preliminary explorative analysis we did not find any impact of the intracranial volume to factors investigated in this study. Therefore, we did not adjust for differences in head size.
Whole‐brain VBM analysis at the voxel‐level
Voxel‐wise multiple linear regression analyses were performed using the general linear model as implemented in SPM5, determining the association between regional gray matter volume and performance in the specified neuropsychological test, after controlling for sex and age. Voxels with a gray matter probability < 0.2 in the mean unmodulated gray matter segment (averaged over all included subjects) were excluded from statistical analysis. Given that (i) we had a priori hypotheses about the relationships between regional atrophy, aging, and executive function, (ii) that the associations between cognition and macroscopic morphology of gray matter are likely to be subtle, and (iii) that we followed up VBM analyses with stereological analysis, we applied a liberal threshold of P < 0.001 (uncorrected) across the whole brain for exploratory purposes.
Analysis of VBM‐based ROI volumes
To determine if gray matter associations with cognitive functions were highly localized to peaks (as detected by voxel‐based analysis) or extended to larger functional regions, we complemented the analysis by correlations of mean gray matter within each of 10 ROIs that were defined according to previous knowledge on locations of the investigated cognitive functions. Regional masks were defined using the WFU pickatlas tool (Functional MRI Laboratory, Wake Forest University School of Medicine, Winston‐Salem, CA) as follows: orbitofrontal cortex (OFC, defined as the conjunction of Brodmann areas [Babcock and Salthouse, 1990]10 and 11), ventrolateral prefrontal cortex (VLPFC, BA 44/45/47), dorsolateral prefrontal cortex (DLPFC, BA46/9), anterior cingulate cortex (ACC, BA24/32), insular cortex, posterior parietal cortex (BA 5/7/40/39), medial temporal lobe (hippocampus and parahippocampal gyrus), primary visual cortex (BA17), thalamus, and cerebellum. Individual gray matter volume within each of these regions was calculated by summing voxel values of the smoothed modulated normalized images within the corresponding region. The association between gray matter volume within ROIs and cognitive performance was assessed by partial correlations, correcting for age and sex. This type of analysis is called ROI‐VBM analysis throughout the article to differentiate it from stereological ROI analysis (see below).
Stereological Measurement of Insular Volume
Because of the labor‐intensive nature of stereological point counting analysis, a subset of 93 participants was randomly selected for this part of the study. This subgroup was large enough to be representative of the overall cohort in terms of age, sex, and cognitive performance (Table 1). Volumes of the left and right insula were estimated using the Cavalieri method of modern design stereology in conjunction with point counting [Roberts et al., 2000] using Easymeasure software [Keller et al., 2007], based on the principles of our previous study [Keller et al., 2011] (Fig. 4A–C). Prior to point counting, the original sagittal MR images were converted to coronal images, and contrast and brightness was optimized for all subjects using MRIcro (http://www.cabiatl.com/mricro/). For point counting, an array of test probes (i.e., points) was projected over coronal sections of the T1‐weighted MR images, and points intersecting the insula were counted. The insular ROI was the entire insular cortex, including all segments of the long and short insular gyri, which were demarcated from adjacent brain regions by the circular insular sulcus. Separation between test points was five points and slice interval was every fifth section (5 × 5 approach, 2.5 mm × 2.5 mm). This differed from our previous study [Keller et al., 2011], which used a 4 × 4 approach. This was due to the vastly increased sample size in the present study (93 vs. 25), and that the reduced sampling density permitted a more rapid analysis (one brain analyzed in 30 min, as opposed to the 40 min per brain using the 4 × 4 approach). Importantly, in reducing the density of the stereological parameters to 5 × 5, it was crucial to maintain a coefficient of error (CE) lower than the recommended 5% [Roberts et al., 2000]. We found that a 5 × 5 approach on MR images of voxel size 1 mm × 1 mm × 1 mm resulted in CE's of 4.23% and 4.76% for the left and right insula, respectively. An estimate of insular volume was obtained as the sum of the estimated areas of the structure transects on consecutive systematic sections multiplied by the distance between the sections. Further information for insular volume estimation can be found in [Keller et al., 2011]. Inter‐rater reliability studies were performed between two raters (SB and SSK) and indicated high levels of reproducibility of measures (intraclass correlation coefficient = 0.89 left insula, 0.86 right insula). Total insular volumes were obtained by adding left and right insular volumes. The association between insular gray matter volume and cognitive performance was assessed by partial correlations, correcting for age and sex.
Figure 4.
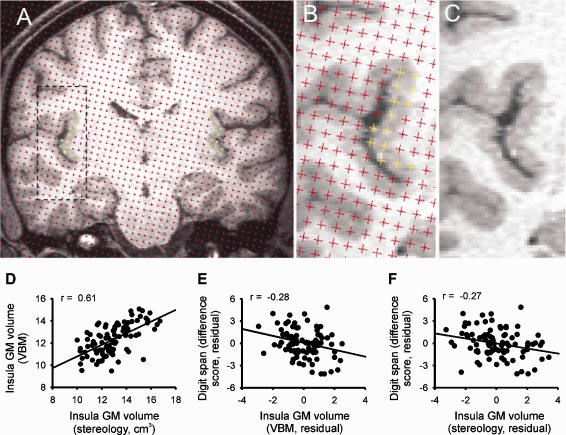
Comparison of stereological methods and voxel‐based morphometry for measurement of insular cortex volume. A–C: Stereology in conjunction with point counting used to estimate the volume of the insular cortices in a randomly selected individual. A: Point counting within the left and right insula is highlighted in yellow. B: Magnification of point counting within the left insula. C: Magnification of the left insula without the projected points for anatomical clarity. D: Correlation between insular volumes as quantified by stereology and by ROI‐VBM analysis (P < 0.001). E, F: Partial correlation plots (after correction for age and gender) of both insular measures with the digit span difference score (P < 0.05). Note that smaller difference scores indicate better executive performance. GM, gray matter; VBM, voxel‐based morphometry. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Choice of Statistical Model
When assessing the full possible range of regional gray matter volumes, one might expect a sigmoid relationship with cognition, exhibiting a threshold (below that further decrease of volume has no effect on cognition), an approximately linear middle part and a zone of saturation (above that the region contributes maximally to cognition). In this study, we assumed that the lower tail of the sigmoid curve was cut by excluding demented subjects. Using linear regression analysis, we then sensitized the analysis to regions that in cognitively healthy subjects are critically related to the function under investigation, i.e., where subjects in the normal age‐adjusted range of cognitive performance fall in the approximately linear middle region of the sigmoid curve. Not finding an association between regional gray matter volume and a certain neuropsychological test may thus mean that (1) gray matter volume in the region is not related to performance in this test or (2) regional gray matter is related to performance in the test, but healthy subjects have regional gray matter volumes in the saturation part of the sigmoid curve, such that small variations in volume do not have an effect on performance.
Assessment of Regional Power Differences
The power to detect associations in voxel‐ or region‐based morphometry as used in this study depends critically on the regional signal‐to‐noise ratio. Regional differences in the amount of “noise” (i.e., interindividual variation not caused by the investigated parameter) in gray matter volume may thus produce regional differences in the power to detect a signal, leading to preferential detection of signal in regions of low noise. As signal is small in comparison to noise in the present setting, regional variability of gray matter volume may serve as an estimation of noise. Coefficients of variation of gray matter volume were similar among gray matter regions (Supporting Information Fig. S1). The power to detect a signal should therefore be similar for all regions.
RESULTS
Association of Cognitive Performance With Local/Regional Gray Matter Volume
Associations between cognitive performance and local/regional gray matter volume were assessed after correction for age and sex. For all tasks (digit span forward and backward, TMT parts A and B and Stroop test parts II and III), improved performance was associated with increased local gray matter volume in various cortical clusters as determined using whole‐brain VBM statistics. These clusters included several prefrontal and posterior parietal regions, the insular cortex, cerebellum, and, in the tests relying on visual processing (TMT, Stroop), parts of the occipital cortex (Supporting Information Fig. S2, Table S1). No clusters were found where improved performance was associated with decreased gray matter volume. Correlations between task performance and gray matter were not limited to highly localized peaks (as determined by voxel‐based statistics), but were also detected when analyzing mean gray matter volume within the predefined larger regions (ROI‐VBM analysis), again involving various prefrontal regions, the insula, cerebellum and some regions related to specific tasks (Fig. 1). Both with whole‐brain VBM and with ROI‐VBM statistics, it was evident that while there was an association between gray matter and performance in the basic tasks (digit span forward, TMT part A, Stroop test part II), the association was considerably greater in the tasks that put additional demand on executive functioning (digit span backward, TMT part B, Stroop test part III). To isolate regions that were selectively associated with executive functions, we used the difference scores (digit span forward‐backward; TMT part B‐A; Stroop test part III‐II). Better executive performance (as quantified by lower difference scores) was associated with larger gray matter volume both in whole‐brain VBM and ROI‐VBM statistics, showing task‐specific patterns overlapping in several prefrontal regions, the insula and the cerebellum (Figs. 2 and 3A, Table 3).
Figure 1.
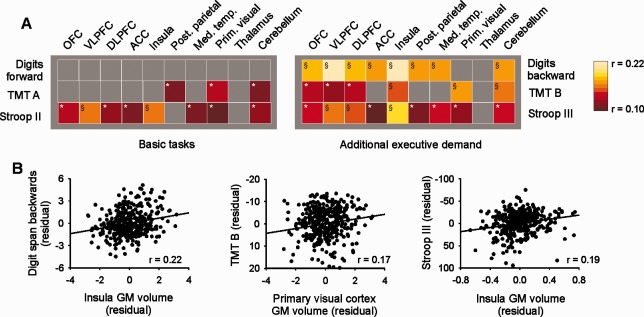
Association of cognitive performance with regional gray matter volume. A: The association between mean gray matter volume within prespecified ROIs (ROI‐VBM analysis) and test scores was assessed by partial correlation (corrected for age and sex). Correlations are illustrated using color coding as indicated. *Significant at P < 0.05 (uncorrected); §significant at P < 0.05 after Bonferroni correction for 10 comparisons (corresponding to the number of ROIs tested). All correlations were positive, i.e., better performance was associated with larger gray matter volume. B: Partial correlation plots (after correction for age and gender) of digit span backward, TMT part B and Stroop test part III with the most strongly associated ROIs. GM, gray matter. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
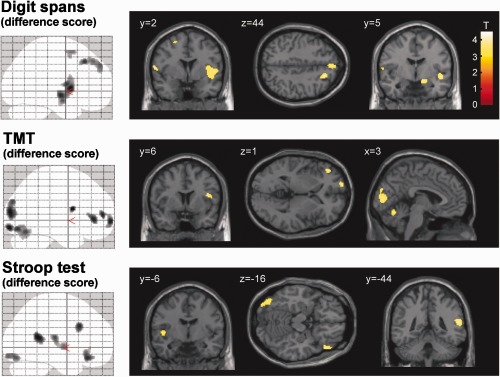
Association of executive performance with local gray matter volume. Significant associations (after correction for age and sex) with the respective difference score (at P < 0.001, uncorrected and a cluster extent threshold of 100 voxels) are illustrated superimposed on the Montreal Neurological Institute (MNI) high‐resolution single subject T1 image. Note that smaller difference scores indicate better executive performance. Blobs show significant negative associations between difference scores and gray matter volume, illustrating regions where better performance was associated with larger gray matter volume. No significant positive correlations were found between difference scores and gray matter volume throughout the brain. Coordinates are according to the MNI atlas. Right side in the figure corresponds to right side of the brain. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
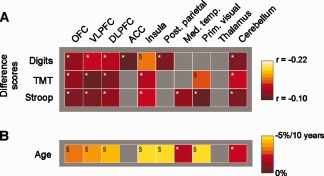
Association of regional gray matter with executive performance and age. A: The association between mean gray matter volume within prespecified ROIs (ROI‐VBM analysis) and the respective difference score was assessed by partial correlation (corrected for age and sex). Correlations are illustrated using color coding as indicated. Note that smaller difference scores indicate better executive performance. All correlations were negative, i.e. better performance, indicated by smaller difference scores, was associated with larger gray matter volume within the respective ROIs. B: Gray matter decrease within prespecified ROIs per 10 years of age was assessed using linear regression (corrected for sex), converted to percentage using the gray matter volume of a 50‐year‐old male as extrapolated from the linear regression as reference. *Significant at P < 0.05 (uncorrected); §significant at P < 0.05 after Bonferroni correction. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Associations between executive functioning and local gray matter volume as determined by whole‐brain VBM statistics
| Digits (difference score) | TMT (difference score) | Stroop (difference score) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anatomical region | Side | Brodmann areas | Peak coordinate (x y z) | Peak T value | Brodmann areas | Peak coordinate (x y z) | Peak T value | Brodmann areas | Peak coordinate (x y z) | Peak T value |
| Insula | R | 50 8 4 | 4.45 | 40 8 16 | 3.91 | |||||
| Insula | L | −46 −18 16 | 3.58 | |||||||
| Frontal | ||||||||||
| Frontopolar cortex | L | 10 | −18 66 −2 | 3.83 | ||||||
| Medial prefrontal cortex | R | 8 | 18 30 44 | 4.08 | ||||||
| Medial prefrontal cortex | R + L | 9/8 | 2 54 38 | 3.74 | ||||||
| Lateral prefrontal cortex | L | 45/47/46 | −46 40 2 | 3.78 | 46/9 | −52 20 32 | 3.76 | |||
| Lateral prefrontal cortex | R | 47/45 | 48 32 −16 | 3.75 | ||||||
| Premotor cortex | L | 6 | −62 2 10 | 3.99 | ||||||
| Premotor cortex | L | 6 | −24 10 58 | 3.60 | ||||||
| Temporal and parietal | ||||||||||
| Medial temporal lobe | R | 28, Amygdala | 14 −4 −14 | 3.82 | 28/34 | 14 −10 −20 | 3.59 | |||
| Superior temporal gyrus/ inferior parietal lobe | R | 22/40 | 58 −46 16 | 3.93 | ||||||
| Occipital | ||||||||||
| Occipital cortex | R | 18 | 22 −78 −12 | 3.66 | ||||||
| Occipital cortex | L | 18/17 | −12 −90 −12 | 3.91 | 18/19 | −42 −78 −16 | 3.55 | |||
| Occipital cortex | L | 18/19 | −4 90 12 | 3.90 | ||||||
| Cerebellum | ||||||||||
| Cerebellum | L | −2 −66 −18 | 3.57 | −18 −60 −62 | 3.94 | |||||
| Cerebellum | R | 8 −68 −18 | 3.54 | |||||||
Clusters of ≥100 contiguous voxels above a threshold of P < 0.001 (uncorrected) are tabulated in terms of the brain region and the corresponding Brodmann's area. Note that smaller difference scores indicate better executive performance. All associations shown in the table were negative, i.e., better performance was related to larger local gray matter. Coordinates are according to the Montreal Neurological Institute (MNI) atlas.
To further corroborate the association between insular volume and executive performance, we obtained stereological measures of insular volume from a randomly chosen subgroup (n = 93, Fig. 4A–C). The subgroup did not significantly differ from the complete group in age, gender, BDI scores, and neuropsychological performance (Table 1). Insular volumes as quantified by VBM (sum of voxel values within the insular ROI) and by stereology were significantly correlated (Fig. 4D). Correlations of both measures with the digit span difference score were similar (Fig. 4E,F). Correlations with the TMT and Stroop difference scores did not reach significance for either insular volume parameter within this smaller group.
The Effect of Age in Cortical Regions Related to Cognitive Functions
To assess if the regions showing an age‐independent association between cognitive performance and gray matter volume (as described above) are also affected by age‐related gray matter atrophy, we determined regional age‐related gray matter decrease in the same regions (ROI‐VBM analysis) investigated for cognitive functions (Fig. 3B). Age‐related gray matter loss in these regions (ROI‐VBM analysis) ranged from nonsignificant (<1.5% per 10 years) to highly significant (5% per 10 years) and was largest in the insula and posterior parietal cortex.
Variance in Cognitive Functions Explained by Sex, Age, and Sex‐and‐Age‐Independent Regional Gray Matter
We used linear regression analysis to determine the cumulative association of sex, age, and sex‐and‐age‐independent gray matter variability (within the regions most strongly related to the respective function according to ROI‐VBM analysis) with cognitive functions. Regression coefficients for the difference scores are shown in Table 4. While sex made only a minor contribution to the variance of each of the three difference scores (<0.5%), age explained a significant portion of the variance of the TMT and Stroop difference scores (7.0% and 7.7%, respectively) but not of the digit span difference score. The percentage of variance explained by the gray matter volume of the most strongly related region according to ROI‐VBM analysis (primary visual cortex for the TMT difference score and insular cortex for the two remaining difference scores) ranged between 1.9 and 2.4%. On average, the additional variance explained by ROI gray matter volume amounted to 41% of the variance already explained by age. For the original (not difference) test scores, results were quantitatively similar. The variance explained by age ranged from 2.6 % (Stroop test part II) to 12.8 % (TMT part A) and the variance explained by sex‐and‐age‐independent regional gray matter within the most strongly associated ROI ranged from 0.8% (digit span forward) to 4.6% (digit span backward).
Table 4.
Association of sex, age, and sex‐and‐age‐independent regional gray matter variability with cognitive functions
| Independent variable | % of variance explained | Standardized beta (in complete model) | Significance (P) |
|---|---|---|---|
| Regression 1: Digit span difference score | |||
| Sex | 0.5% | 0.072 | 0.17 |
| Age | 0.2% | 0.046 | 0.38 |
| Insular cortex gray matter volume | 2.4% | −0.154 | 0.003 |
| Total: | 3.1% | 0.01 | |
| Regression 2: TMT difference score | |||
| Sex | 0.3% | 0.071 | 0.16 |
| Age | 7.0% | 0.266 | <0.001 |
| Primary visual cortex gray matter volume | 2.2% | −0.149 | 0.003 |
| Total: | 9.5% | <0.001 | |
| Regression 3: Stroop difference score | |||
| Sex | 0.4% | −0.04 | 0.42 |
| Age | 7.7% | 0.279 | <0.001 |
| Insular cortex gray matter volume | 1.9% | −0.136 | 0.07 |
| Total | 10.0% | <0.001 | |
Hierarchical linear regression was performed with cognitive test difference scores as dependent variable and sex, age and regional gray matter volume (as determined by ROI‐VBM analysis, corrected for age and sex) successively entered as independent variables. For each cognitive test, the region exhibiting the strongest association with the respective difference score was used. Lower difference scores indicate better executive performance (see Table II).
DISCUSSION
This study shows that task‐specific regional gray matter volume patterns for different cognitive tasks can be identified by VBM in analogy to the task‐specific regional activation patterns identified by functional neuroimaging. Basic tasks were less closely related to local gray matter volume than the corresponding tasks with additional executive demand, consistent with the notion that executive functioning places special demand on cortical resources.
Gray Matter Volume Representations of Cognitive Functions and Their Relation to Previous Knowledge on Functional Representations
Performance on the digit span backwards task was related to gray matter volume in various cortical regions. Some of these regions were consistent with cortical regions activated during working memory tasks in functional imaging, e.g., VLPFC [storage of information in phonological loop (Wager and Smith, 2003)], DLPFC and posterior parietal cortex [data manipulation (D'Esposito et al., 1998; Fletcher and Henson, 2001; Wager and Smith, 2003)] and ACC [attention and monitoring (Gazzaniga et al., 2002)]. However, the strongest correlation was detected with lateral temporal lobe gray matter, a region not typically associated with working memory but rather with visual processing [ventral visual pathway, (Ungerleider and Haxby, 1994)]. A possible interpretation might be that visualization of the digit sequence is a strategy used when performing the digit span backwards [Cabeza and Nyberg, 2000; Wager and Smith, 2003]. The digit span difference score, thought to isolate the data manipulation component of the task, was mainly related to the insula and dorsal medial prefrontal cortex. The latter is in accordance with the “ventral storage, dorsal executive” hypothesis of working memory, although dorsolateral rather than dorsomedial regions have been associated with data manipulation in functional imaging studies [Owen, 2000; Wager and Smith, 2003].
Performance in the TMT (both parts and difference score) was related to occipital and cerebellar gray matter, consistent with its visuomotor demands. The difference score, focusing on the set‐shifting component of the task, was additionally associated with left VLPFC, DLPFC and frontopolar cortex, consistent with the functional representation of set‐shifting [Collette and Van der Linden, 2002; Loose et al., 2006]. In agreement with our results, functional imaging during TMT has identified activations in left DLPFC and insula [Moll et al., 2002; Zakzanis et al., 2005]. Additional activations have been reported in premotor, mid‐cingulate and posterior parietal and temporal regions [Moll et al., 2002; Zakzanis et al., 2005]. Similar to the present results, associations between TMT performance and gray matter volume have been reported in left ventrolateral cortex and occipital cortex [Newman et al., 2007]. Thus, it can be concluded that left VLPFC and DLPFC are associated with set‐shifting both in functional and structural imaging studies.
Response inhibition in the Stroop task has been mainly associated with activation of the DLPFC and the dorsal ACC [Bush et al., 2000; Leung et al., 2000; Liu et al., 2004; Peterson et al., 1999]. We found an association of the Stroop difference score with gray matter volume in the left DLPFC, but association with the ACC was relatively weak (Stroop test part III) or absent (difference score).
Although functional and gray matter volume representations of the cognitive functions tested in the present study overlap in many respects, there are also major differences, e.g. the lack of association between Stroop task and gray matter volume in the ACC and the association of the digit span difference score with medial rather than lateral dorsal prefrontal cortex. These results suggest that some regions, although being strongly activated by a specific function, nonetheless have a structural and/or functional reserve for that function, so that gray matter volume decrease (within the range encountered in the presently investigated non‐demented population) does not lead to reduced function. This reserve may depend on the same region and/or other regions (partially) taking over the function of the original region.
Regions Associated With Cognitive Functions Across Different Tasks: DLPFC and Insular Cortex
Executive functioning has been divided into different subfunctions (neurocognitive processes) such as directed attention, working memory, set‐shifting and response inhibition [Suchy, 2009]. Specific activation patterns relevant for each subfunction have been identified in functional imaging studies that overlap in executive “core” regions such as the DLPFC and possibly the posterior parietal cortex [Collette and Van der Linden M., 2002; Collette et al., 2005; Suchy, 2009; Wager and Smith, 2003]. The executive subfunctions investigated here (data manipulation, set‐shifting and response inhibition) were related to gray matter volume in several prefrontal regions, with a partial overlap in left DLPFC. However, both the voxel‐level analysis (Table 3) and the regional analysis (Fig. 3A) show that the largest overlap between the different subfunctions was present in the insular cortex. Given that there may be methodological issues associated with VBM that may affect results [Ashburner and Friston, 2001; Bookstein, 2001; Fein et al., 2006; Keller and Roberts, 2008; Pereira et al., 2008; Salmond et al., 2002], we complemented the analysis by obtaining stereological measures of insular volume in a subgroup of participants. As the results obtained by both methods were similar, we suggest that insular cortex volume has a relationship with executive functioning. Indeed, some previous functional imaging studies have reported prominent, mostly bilateral insular activity during different neuropsychological tasks, including Stroop test [Leung et al., 2000; Liu et al., 2004], TMT [Zakzanis et al., 2005], Go/NoGo task [Laurens et al., 2005; Menon et al., 2001], infrequent target detection [Huettel et al., 2004], selective attention [Corbetta et al., 1991] and working memory [Grasby et al., 1994; Paulesu et al., 1993]. In addition, insular lesions have been associated with speech and language disorders [Cereda et al., 2002; Dronkers, 1996; Nestor et al., 2003] and insular atrophy is related to impaired executive functioning in Huntington's disease and alcohol dependence [Chanraud et al., 2007; Peinemann et al., 2005]. Our results show association with insular volume not only in the difference scores but also in two of the three basic tasks (digit span forward and Stroop test part II), albeit at a lower level. Thus, results from both structural and functional imaging suggest that the insular cortex, in addition to other functions, may be a “core” cognitive region, most prominently involved in but not limited to executive processes.
Functional Implications of Association Between Regional Gray Matter Volume and Cognitive Function
In the present study, cognitive performance was positively associated with gray matter volume in several cortical regions. It has been emphasized that gray matter volume differences are not necessarily due to differences in neuronal numbers or density of synaptic contacts, but may also reflect different amount of glial cells or even extracellular fluid [Draganski et al., 2006; May and Gaser, 2006]. Nonetheless, the most straightforward interpretation of our results is that the cognitive task under study is critically dependent on gray matter volume in the associated brain regions, meaning that pre‐existing or acquired variations in gray matter volume have a direct impact on performance in the respective task. However, gray matter volume may also change according to demand, a process that has been termed use‐dependent plasticity [Draganski and May, 2008]. Thus, decreased use of specific cognitive functions during certain stages of life (e.g., retirement) might lead to both reduced cognitive performance and gray matter volume reduction in regions associated with these functions. Higher levels of education may have the contrary effect. Longitudinal studies will be needed to address direction of causality. Furthermore, it will be interesting to investigate the relationship between executive performance and white matter architecture using diffusion tensor imaging techniques. In particular, it may be hypothesized that the gray matter correlations with cognition observed in the present study relate to the integrity and connectivity of white matter between these regions [Bendlin et al., 2010; Voineskos et al., 2010].
Both executive performance and gray matter volume decrease with age [Good et al., 2001; Hedden and Gabrieli, 2004]. In a cross‐sectional study, it is not possible to determine if the two relationships form a causal sequence (i.e., aging leads to gray matter atrophy, gray matter atrophy leads to cognitive decline) or are independent but parallel manifestations of age. Again, longitudinal studies will be needed to address this question. However, the age‐independent peak associations between executive performance and gray matter volume described here occurred in regions that were also strongly affected by age‐related gray matter atrophy. Supposing that age‐dependent gray matter atrophy has the same effects as age‐independent gray matter atrophy, the results are consistent with the causal sequence proposed above.
LIMITATIONS
This study used a cross‐sectional approach, precluding investigation of the impact of gray matter volume changes on cognitive function over time within the same individual. In addition, the study was limited to nondemented, cognitively healthy subjects. Because variations in both local gray matter volume and cognitive performance within this population were small compared with that of clinical populations with evident neuropsychological impairments, only subtle associations between gray matter volume and cognitive performance could be expected and were found. It remains to be determined if and how the associations reported here can be extrapolated into the pathological range, i.e., for populations with clearly impaired cognitive performance. Finally, this study did not use a specific control task. This is due to the fact that nonexecutive control tasks are not feasible because the performance of all cognitive tasks relies to some extent on executive functioning [Fuster, 2001]. However, this study assessed pairs of tasks with different executive demand, and indeed association of the basic tasks (digit span forward, TMT A, Stroop test part II) with the prefrontal cortex and the insula were absent or much smaller than for the corresponding tasks with high executive demand (digit span backward, TMT B, Stroop test part III), providing some degree of control for the association of these regions with executive demand.
CONCLUSIONS
The present study showed that task‐specific regional gray matter volume patterns exist and partly overlap with activation patterns seen in functional imaging. Cortical regions that did not overlap may be those that have a structural and/or functional reserve for a specific function in spite of the strong activation during performance of the task. The largest overlap between different cognitive functions was found in the insular cortex, a finding observed using more than one quantitative MRI approach. Together with the results of previous functional imaging studies, this suggests that the insular cortex, in addition to its other functions, may be a “core” cognitive region. Performance in cognitive tests is determined by multiple factors, with, as determined in the present study, on average 2‐3 % of the variance explained by the gray matter volume of the single most strongly predictive region. While this is small in absolute numbers, it adds on average about one third to the variance explained by age. The interplay of multiple exogenic and endogenous factors like genetic predisposition, education, experience, use‐dependent trophic effects, age, gender, nutrition, metabolism, and disease may account for the interindividual differences in regional gray matter volume.
Supporting information
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Table S1. Associations between cognitive functions and local grey matter volume as determined by whole‐brain VBM statistics. Clusters above a threshold of p<0.001 (uncorrected) are tabulated in terms of the brain region and the corresponding Brodmann' s area. * p<0.05, FWE‐corrected. Extent thresholds: digit span forward: 25 voxels; TMT part A, Stroop test part II: 50 voxels, remaining tests: 100 voxels. All associations were positive, i.e. better performance was related to larger local grey matter. Coordinates are according to the Montreal Neurological Institute (MNI) atlas. Common superscripts (1) to (7) indicate regions that were part of the same cluster, respectively.
REFERENCES
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry—The methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2001): Why voxel‐based morphometry should be used. Neuroimage 14:1238–1243. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Babcock RL, Salthouse TA (1990): Effects of increased processing demands on age differences in working memory. Psychol Aging 5:421–428. [DOI] [PubMed] [Google Scholar]
- Baddeley A (2002): Fractionating the central executive In: Stuss DT, Knight RT, editors.Principles of Frontal Lobe Function.Oxford:Oxford University Press; p246. [Google Scholar]
- Baddeley A, Hitch GJ (1974): Working memory In: Bower GA, editor.The psychology of learning and motivation.New York:Academic Press; p47. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961): An inventory for measuring depression. Arch Gen Psychiatry 4:561–571. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Kastman EK, Thiel BW, Rowley HA, Lazar M, Alexander AL, Johnson SC (2010): White matter in aging and cognition: A cross‐sectional study of microstructure in adults aged eighteen to eighty‐three. Dev Neuropsychol 35:257–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, Moeller JR, Alexander GE (2010): Age‐related networks of regional covariance in MRI gray matter: Reproducible multivariate patterns in healthy aging. Neuroimage 49:1750–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein FL (2001): “Voxel‐based morphometry” should not be used with imperfectly registered images. Neuroimage 14:1454–1462. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition. II. An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12:1–47. [DOI] [PubMed] [Google Scholar]
- Cereda C, Ghika J, Maeder P, Bogousslavsky J (2002): Strokes restricted to the insular cortex. Neurology 59:1950–1955. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL (2007): Brain morphometry and cognitive performance in detoxified alcohol‐dependents with preserved psychosocial functioning. Neuropsychopharmacology 32:429–438. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M (2002): Brain imaging of the central executive component of working memory. Neurosci Biobehav Rev 26:105–125. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der LM, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E (2005): Exploring the unity and diversity of the neural substrates of executive functioning. Hum Brain Mapp 25:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE (1991): Selective and divided attention during visual discriminations of shape, color, and speed: Functional anatomy by positron emission tomography. J Neurosci 11:2383–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell PE, Sluming VA, Wilkinson ID, Cezayirli E, Romanowski CA, Webb JA, Keller SS, Mayes A, Roberts N (2007): Effects of sex and age on regional prefrontal brain volume in two human cohorts. Eur J Neurosci 25:307–318. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE (1994): Sex differences in aging of the human frontal and temporal lobes. J Neurosci 14:4748–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J (1998): Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res 7:1–13. [DOI] [PubMed] [Google Scholar]
- Draganski B, May A (2008): Training‐induced structural changes in the adult human brain. Behav Brain Res 192:137–142. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A (2006): Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 26:6314–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF (1996): A new brain region for coordinating speech articulation. Nature 384:159–161. [DOI] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M (2003): Functional anatomy of the insula: New insights from imaging. Surg Radiol Anat 25:113–119. [DOI] [PubMed] [Google Scholar]
- Elderkin‐Thompson V, Ballmaier M, Hellemann G, Pham D, Kumar A (2008): Executive function and MRI prefrontal volumes among healthy older adults. Neuropsychology 22:626–637. [DOI] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, Barakos J, Moon K, Di SV, Shumway R (2006): Statistical parametric mapping of brain morphology: sensitivity is dramatically increased by using brain‐extracted images as inputs. Neuroimage 30:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN (2001): Frontal lobes and human memory: insights from functional neuroimaging. Brain 124:849–881. [DOI] [PubMed] [Google Scholar]
- Flynn FG, Benson DF, Ardila A (1999): Anatomy of the insula—Functional and clinical correlates. Aphasiology 13:55–78. [Google Scholar]
- Fuster JM (2001): The prefrontal cortex—An update: Time is of the essence. Neuron 30:319–333. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Ivry RB, Mangun GR (2002):Cognitive Neuroscience.New York:Norton. [Google Scholar]
- Glisky EL (2007): Changes in cognitive function in human aging In: Riddle DR, editor.Brain Aging: Models, Methods and Mechanisms.Boca Raton (FL):CRC Press. [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14:21–36. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston KJ, Simpson J, Fletcher PC, Frackowiak RS, Dolan RJ (1994): A graded task approach to the functional mapping of brain areas implicated in auditory‐verbal memory. Brain 117:1271–1282. [DOI] [PubMed] [Google Scholar]
- Hart SJ, Green SR, Casp M, Belger A (2010): Emotional priming effects during Stroop task performance. Neuroimage 49:2662–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härting C, Markovitsch HJ, Neufeld H, Calabrese P, Dejerine J, Deisinger K (2000):Wechsler‐Gedächtnistest—revidierte Fassung.Bern:Huber. [Google Scholar]
- Hedden T, Gabrieli JD (2004): Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci 5:87–96. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Misiurek J, Jurkowski AJ, McCarthy G (2004): Dynamic and strategic aspects of executive processing. Brain Res 1000:78–84. [DOI] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N (2009): A comparison between voxel‐based cortical thickness and voxel‐based morphometry in normal aging. Neuroimage 48:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, McLean JE, Reynolds CR (1991): Analysis of WAIS‐R factor patterns by sex and race. J Clin Psychol 47:548–557. [PubMed] [Google Scholar]
- Keller SS, Roberts N (2008): Voxel‐based morphometry of temporal lobe epilepsy: An introduction and review of the literature. Epilepsia 49:741–757. [DOI] [PubMed] [Google Scholar]
- Keller SS, Highley JR, Garcia‐Finana M, Sluming V, Rezaie R, Roberts N (2007): Sulcal variability, stereological measurement and asymmetry of Broca's area on MR images. J Anat 211:534–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Roberts N, Garcia‐Finana M, Mohammadi S, Ringelstein EB, Knecht S, Deppe M (2011): Can the language‐dominant hemisphere be predicted by brain anatomy? J Cogn Neurosci 23:2013–2029. [DOI] [PubMed] [Google Scholar]
- Knecht S, Oelschlager C, Duning T, Lohmann H, Albers J, Stehling C, Heindel W, Breithardt G, Berger K, Ringelstein EB, Kirchhof P, Wersching H (2008): Atrial fibrillation in stroke‐free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J 29:2125–2132. [DOI] [PubMed] [Google Scholar]
- Knight RT (2007): Neuroscience. Neural networks debunk phrenology. Science 316:1578–1579. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Kiehl KA, Liddle PF (2005): A supramodal limbic‐paralimbic‐neocortical network supports goal‐directed stimulus processing. Hum Brain Mapp 24:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC (2000): An event‐related functional MRI study of the stroop color word interference task. Cereb Cortex 10:552–560. [DOI] [PubMed] [Google Scholar]
- Lezak MD (1995):Neuropsychological Assessment.New York (NY):Oxford University Press. [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL (2004): Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event‐related fMRI. Neuroimage 22:1097–1106. [DOI] [PubMed] [Google Scholar]
- Loose R, Kaufmann C, Tucha O, Auer DP, Lange KW (2006): Neural networks of response shifting: Influence of task speed and stimulus material. Brain Res 1090:146–155. [DOI] [PubMed] [Google Scholar]
- MacLeod CM (1991): Half a century of research on the Stroop effect: an integrative review. Psychol Bull 109:163–203. [DOI] [PubMed] [Google Scholar]
- May A, Gaser C (2006): Magnetic resonance‐based morphometry: A window into structural plasticity of the brain. Curr Opin Neurol 19:407–411. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL (2001): Error‐related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ (1982a): Insula of the old world monkey. I. Architectonics in the insulo‐orbito‐temporal component of the paralimbic brain. J Comp Neurol 212:1–22. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ (1982b): Insula of the old world monkey. III. Efferent cortical output and comments on function. J Comp Neurol 212:38–52. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira‐Souza R, Moll FT, Bramati IE, Andreiuolo PA (2002): The cerebral correlates of set‐shifting: An fMRI study of the trail making test. Arq Neuropsiquiatr 60:900–905. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE (2010): Role of the anterior insula in task‐level control and focal attention. Brain Struct Funct 214:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR (2003): Progressive non‐fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain 126:2406–2418. [DOI] [PubMed] [Google Scholar]
- Newman LM, Trivedi MA, Bendlin BB, Ries ML, Johnson SC (2007): The relationship between gray matter morphometry and neuropsychological performance in a large sample of cognitively healthy adults. Brain Imaging Behav 1:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N (2006): Clinical and anatomical correlates of apraxia of speech. Brain Lang 97:343–350. [DOI] [PubMed] [Google Scholar]
- Oswald DW, Fleischmann UM (1997):Neuropsychological Aging Inventory (NAI).Göttingen, Germany:Hogrefe Verlag. [Google Scholar]
- Owen AM (2000): The role of the lateral frontal cortex in mnemonic processing: The contribution of functional neuroimaging. Exp Brain Res 133:33–43. [DOI] [PubMed] [Google Scholar]
- Passingham R (1995):The Frontal Lobes and Voluntary Action.Oxford:Oxford University Press. [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS (1993): The neural correlates of the verbal component of working memory. Nature 362:342–345. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Schuller S, Pohl C, Jahn T, Weindl A, Kassubek J (2005): Executive dysfunction in early stages of Huntington's disease is associated with striatal and insular atrophy: A neuropsychological and voxel‐based morphometric study. J Neurol Sci 239:11–19. [DOI] [PubMed] [Google Scholar]
- Pereira JM, Nestor PJ, Williams GB (2008): Impact of inconsistent resolution on VBM studies. Neuroimage 40:1711–1717. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC (1999): An fMRI study of Stroop word‐color interference: Evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry 45:1237–1258. [DOI] [PubMed] [Google Scholar]
- Price CJ (2000): The anatomy of language: Contributions from functional neuroimaging. J Anat 197( Part 3):335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U (2010): Trajectories of brain aging in middle‐aged and older adults: Regional and individual differences. Neuroimage 51:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Weiskrantz L (1998):The Prefrontal Cortex—Executive and Cognitive Functions.Oxford:Oxford University Press. [Google Scholar]
- Roberts N, Puddephat MJ, McNulty V (2000): The benefit of stereology for quantitative radiology. Br J Radiol 73:679–697. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B (2004): Thinning of the cerebral cortex in aging. Cereb Cortex 14:721–730. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha‐Khadem F, Connelly A, Gadian DG, Friston KJ (2002): Distributional assumptions in voxel‐based morphometry. Neuroimage 17:1027–1030. [PubMed] [Google Scholar]
- Shum DH, McFarland KA, Bain JD (1990): Construct validity of eight tests of attention: Comparison of normal and closed head injured samples. Clin Neuropsychol 4:151–162. [Google Scholar]
- Spreen O, Strauss E (1998):A Compendium of Neuropsychological Tests.Oxford:Oxford University Press. [Google Scholar]
- Stroop JR (1935): Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662. [Google Scholar]
- Suchy Y (2009): Executive functioning: Overview, assessment, and research issues for non‐neuropsychologists. Ann Behav Med 37:106–116. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Pessoa L (2007): Neural correlates of perceptual choice and decision making during fear‐disgust discrimination. J Neurosci 27:2908–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB (2002): Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel‐based morphometry. Neuroimage 17:657–669. [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J (2004): A voxel‐based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex 14:966–973. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV (1994): ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol 4:157–165. [DOI] [PubMed] [Google Scholar]
- Van Essen DC (2009): Lost in localization‐‐but found with foci?! Neuroimage 48:14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Dierker DL (2007): Surface‐based and probabilistic atlases of primate cerebral cortex. Neuron 56:209–225. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH (2001): An integrated software suite for surface‐based analyses of cerebral cortex. J Am Med Inform Assoc 8:443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, Pollock BG, Mulsant BH (2010): Age‐related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiol Aging doi:10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE (2003): Neuroimaging studies of working memory: A meta‐analysis. Cogn Affect Behav Neurosci 3:255–274. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK (1999): Brain regions involved in articulation. Lancet 353:1057–1061. [DOI] [PubMed] [Google Scholar]
- Zajano MJ, Gorman A (1986): Stroop interference as a function of percentage of congruent items. Percept Mot Skills 63:1087–1096. [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ (2005): An fMRI study of the Trail Making Test. Neuropsychologia 43:1878–1886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Table S1. Associations between cognitive functions and local grey matter volume as determined by whole‐brain VBM statistics. Clusters above a threshold of p<0.001 (uncorrected) are tabulated in terms of the brain region and the corresponding Brodmann' s area. * p<0.05, FWE‐corrected. Extent thresholds: digit span forward: 25 voxels; TMT part A, Stroop test part II: 50 voxels, remaining tests: 100 voxels. All associations were positive, i.e. better performance was related to larger local grey matter. Coordinates are according to the Montreal Neurological Institute (MNI) atlas. Common superscripts (1) to (7) indicate regions that were part of the same cluster, respectively.


