Abstract
Parkinson's disease (PD) is surprisingly heterogeneous: some patients have a prominent resting tremor, while others never develop this symptom. Here we investigate whether the functional organization of the voluntary motor system differs between PD patients with and without resting tremor, and whether these differences relate to the cerebral circuit producing tremor. We compared 18 PD patients with marked tremor, 20 PD patients without tremor, and 19 healthy controls. Subjects performed a controlled motor imagery task during fMRI scanning. We quantified imagery‐related cerebral activity by contrasting imagery of biomechanically difficult and easy movements. Tremor‐related activity was identified by relating cerebral activity to fluctuations in tremor amplitude, using electromyography during scanning. PD patients with tremor had better behavioral performance than PD patients without tremor. Furthermore, tremulous PD patients showed increased imagery‐related activity in somatosensory area 3a, as compared with both healthy controls and to nontremor PD patients. This effect was independent from tremor‐related activity, which was localized to the motor cortex, cerebellum, and thalamic ventral intermediate nucleus (VIM). The VIM, with known projections to area 3a, was unique in showing both tremor‐ and imagery‐related responses. We conclude that parkinsonian tremor influences motor imagery by modulating central somatosensory processing through the VIM. This mechanism may explain clinical differences between PD patients with and without tremor. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc
Keywords: Parkinson's disease, motor subtypes, resting tremor, motor imagery, action planning, basal ganglia, thalamus, cerebellum, somatosensory cortex, premotor cortex
INTRODUCTION
Parkinson's disease (PD) is a surprisingly heterogeneous disorder. The classical triad of symptoms includes resting tremor, bradykinesia and rigidity, but the expression of these symptoms varies markedly between patients [Jankovic et al.,1990; Lewis et al.,2005]. For instance, while the phenotype of some PD patients is dictated by an early and prominent resting tremor (tremoulous PD), ∼25% of PD patients never develop this symptom [nontremor PD; Hoehn and Yahr,1967]. A large body of evidence suggests that the tremulous subtype is more benign than the nontremor subtype, as evidenced by slower progression of motor symptoms [Josephs et al.,2006; Louis et al.,1999], better motor planning abilities [Vakil and Herishanu‐Naaman,1998] and less cognitive dysfunction [Alves et al.,2006; Burn et al.,2006]. Thus, while PD patients with tremor have an additional symptom, they generally follow a more benign clinical course than PD patients without tremor.
These clinical findings suggest that different pathophysiological or compensatory mechanisms are involved in these PD subtypes, but clear evidence is lacking. Here we address this issue by focusing on the voluntary motor system, which harbors the core pathophysiological substrate of PD [Marsden,1982]. We consider two possible hypotheses. First, pathological differences between PD patients with and without tremor could lead to altered processing in planning‐related brain regions, independently from the pathological changes that produce tremor. Specifically, post‐mortem work has shown that nontremor PD patients have more severe loss of dopaminergic neurons in the ventrolateral midbrain that projects to the posterior putamen [Jellinger,2002]. This could lead to impaired processing in regions anatomically connected to this striatal subregion, for example the premotor cortex [Parent and Hazrati,1995; Takada et al.,1998]. Second, the presence of resting tremor could by itself change the functional organization of the cerebral motor system. Specifically, resting tremor has been linked to a cerebral circuit involving the anterior cerebellum [Deiber et al.,1993; Fukuda et al.,2004], the thalamic ventral intermediate nucleus [VIM; [Benabid et al.,1991; Lenz et al.,1994]], and the primary motor cortex [M1; [Fukuda et al.,2004]. M1 and the anterior cerebellum are also involved in motor execution [Hanakawa et al.,2003; Stoodley and Schmahmann,2009], while the VIM is involved in voluntary action planning [Paradiso et al.,2004] and it relays afferent input to somatosensory area 3a [Padberg et al.,2009]. Thus, tremor‐related responses in this circuit may influence voluntary action planning by interfering with central motor commands or by altering central somatosensory processing. This hypothesis predicts that PD patients with resting tremor show a spatial overlap between tremor‐ and planning‐related cerebral responses during voluntary action planning.
We investigate these hypotheses using an experimental design with three key features. First, we compared two carefully matched groups of PD patients that had either absent or prominent resting tremor, but who all displayed similar levels of akinesia and rigidity. Second, in order to reliably distinguish between the cerebral underpinnings of tremor and voluntary action planning, it is important to use a motor task that does not generate motor output itself. This separation is necessary because motor execution and tremor inevitably interact in the corticospinal tract. Furthermore, somatosensory afferences related to execution and tremor can compete in the peripheral nervous system [Jones et al.,1989]. These interdependencies make it difficult to attribute cerebral differences between PD subtypes to either central or peripheral mechanisms. Here we circumvented this problem by using a validated and well‐controlled motor imagery task that does not activate the corticospinal tract, and that produces no afferent feedback [Helmich et al.,2009; Parsons,1987]. This approach is justified by empirical evidence showing that motor imagery is sensitive to motor control variables [de Lange et al.,2006; Gentili et al.,2004], and that it uses neural operations involved in action planning [Cisek and Kalaska,2004]. Third, we wanted to quantify cerebral activity related to tremor itself. For this purpose, we localized tremor‐related brain activity by measuring fluctuations in tremor amplitude with surface electromyography (EMG) during scanning [Helmich et al.,2010]. This design led to the segregation of three different patterns of cerebral activity: (1) brain regions where PD patients with and without tremor have different responses during motor imagery; (2) brain regions involved in tremor‐related processing (this is indexed by cerebral activity cofluctuating with tremor amplitude); and (3) brain regions showing both tremor‐related responses and motor imagery‐related activity.
METHODS
Subjects
We included three carefully matched groups of 19 healthy controls (12 men, aged 58.6 ± 7.9 years; mean ± SD), 18 tremulous PD patients (10 men, 56.7 ± 10.0 years) and 20 nontremor PD patients (16 men, 59.1 ± 9.4 years). The distributions of age and gender were not significantly different between the three groups (P = 0.69 and P = 0.27, respectively). For further group characteristics and statistics, see Table I and Supporting Information Table I. All subjects gave written informed consent according to institutional guidelines of the local ethics committee (CMO region Arnhem‐Nijmegen, Netherlands). All subjects were right‐handed. Patients were included when they had idiopathic PD, diagnosed according to the UK Brain Bank criteria by an experienced movement disorders specialist (BR Bloem). The most important inclusion criterion was either clear presence or absence of resting tremor. Tremulous PD was defined as a Unified Parkinson's Disease Rating Scale (UPDRS) resting tremor score of ≥2 for at least one hand during physical examination, and an obvious history of resting tremor. Nontremor PD was defined as a UPDRS resting tremor score of 0 for each hand during physical examination and no history of resting tremor. The severity of action and postural tremor played no role in the definition of the two PD groups. Exclusion criteria were: clinical signs of dementia, other neurological diseases, and general exclusion criteria for MRI scanning (such as claustrophobia, pacemaker, and implanted metal parts). Before scanning, the patients' disease severity was assessed by one examiner (RC Helmich) using the Hoehn & Yahr stages and the UPDRS. Tremor severity was assessed using Part A of the Fahn‐Tolosa‐Marin Tremor Rating Scale (TRS), which involves a clinical score of 0–4 points for each extremity (hands and feet, left and right), separately for resting, postural and action tremor [Stacy et al.,2007]. Cognitive function was assessed with the Frontal Assessment Battery [FAB; [Dubois et al.,2000]]. We also collected the Dutch version of the Barratt Impulsiveness Scale (BIS‐11) [Patton et al.,1995] in a subset of PD patients (15 tremulous PD and 17 nontremor PD). The BIS‐11 is 30‐item questionnaire concerning control of thoughts and behavior. The scale is based on a tri‐factor model of impulsivity measuring: (a) “motor impulsiveness” measured by 11 items (e.g., I do things without thinking); (b) “attentional impulsiveness” measured by eight items (e.g., I do not pay attention); (c) “nonplanning impulsiveness” measured by 11 items (e.g., I plan tasks carefully). Twelve patients did not use any Parkinson medication; the others used dopaminergic medication (levodopa or dopamine‐agonists). The amount of dopaminergic medication [expressed as the levodopa equivalent daily dose; LEDD; Wenzelburger et al.,2002] was not significantly different between both PD groups (Table I). The experiments were performed in the morning, and the patients were asked not to take their medication the evening before the experiment. Thus, they were all off‐medication for at least 12 h during the experiment [i.e., in a practically defined off‐condition; Langston et al.,1992].
Table I.
Clinical characteristics
| Group | PD with tremor (n = 18) | PD without tremor (n = 20) | P‐value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age | 56.7 | 10 | 59.1 | 9.4 | 0.44 | |
| Gender | 10 M/8 F | 16 M/4 F | 0.11 | |||
| Duration | 4.3 | 2.1 | 4.5 | 2.6 | 0.83 | |
| H&Y | 2 | 0.3 | 2.1 | 0.2 | 0.57 | |
| LEDD | 220.6 | 419 | 348.6 | 247.7 | 0.25 | |
| FABa | Conceptualization | 2.94 | 0.25 | 3 | 0 | 0.27 |
| Mental Flexibility | 2.5 | 0.63 | 2.8 | 0.55 | 0.21 | |
| Motor Programming | 2.7 | 0.48 | 2.7 | 0.67 | 0.85 | |
| Sensitivity to Interference | 2.9 | 0.25 | 2.8 | 0.41 | 0.25 | |
| Inhibitory Control | 2.8 | 0.44 | 2.9 | 0.31 | 0.24 | |
| Environmental Autonomy | 3.0 | 0 | 3.0 | 0 | 1.0 | |
| Attention Span | 6.6 | 0.63 | 6.7 | 0.88 | 0.74 | |
| Total | 16.8 | 1.1 | 17.1 | 1.2 | 0.47 | |
| BIS‐11b | Motor | 17.1 | 2.9 | 16.2 | 2.8 | 0.38 |
| Attention | 18.6 | 3.5 | 19.1 | 4.7 | 0.76 | |
| No Planning | 24.9 | 5.2 | 25.1 | 3.4 | 0.87 | |
| Total | 60.6 | 5.5 | 60.4 | 8.6 | 0.94 | |
| UPDRS part III | Total | 27.2 | 8.1 | 27.9 | 9 | 0.79 |
| Hand | 16.2 | 4.8 | 14.6 | 5.1 | 0.34 | |
| Foot | 4.2 | 2.2 | 3.9 | 2 | 0.64 | |
| Bradykinesia | 10.8 | 4.2 | 12.5 | 4.3 | 0.25 | |
| Rigidity | 5.5 | 2.3 | 6.4 | 2.9 | 0.3 | |
| “Face” | 1.9 | 1.2 | 2.9 | 1.6 | 0.034 | |
| “Body” | 3.4 | 1.8 | 4.8 | 2.4 | 0.05 | |
| Tremor (TRS part A) | Rest | 4.2 | 2 | 0.2 | 0.52 | <0.001 |
| Posture | 2.9 | 1.9 | 2.4 | 1.1 | 0.053 | |
| Action | 1.1 | 1.3 | 0.9 | 1.4 | 0.63 | |
Three groups of subjects were measured: 19 healthy controls, 18 PD patients with marked tremor and 20 PD patients without tremor. All subjects were consistent right‐handers. All PD patients were tested in a practically defined off‐state [more than 12 h after having taken their last medication; (Langston et al.,1992)]. Disease duration is shown in years, and defined as the time since patients noticed their first symptoms. Tremor scores are shown as points on the Tremor Rating Scale [maximum score per item ranges from 0 to 4 points per effector and per side, i.e., maximum score is 8 points per side; (Stacy et al.,2007)]. Three nontremor patients had a very subtle resting tremor while off‐medication at the day of testing, explaining the nonzero resting tremor score. Bradykinesia refers to the sum of UPDRS items 23–26, rigidity to UPDRS item 22, “Face” to the sum of UPDRS items 18–19 and “Body” to UPDRS items 27–31 [Stochl et al.,2008]. The right row shows the P‐value resulting from the statistical comparison of the three groups (ANOVA) and of the two PD groups (two‐sample t‐test). All groups were matched in terms of age and gender; the difference between the PD groups was confined to resting tremor and to facial and gait/axial symptoms. UPDRS, Unified Parkinson's Disease Rating Scale part III (highest possible score is 108 points); H&Y, Hoehn and Yahr rating scale (highest stage is 5). M, male; F, female; FAB, frontal assessment battery [Dubois et al.,2000]; BIS‐11 = Dutch version of the Barratt Impulsiveness Scale [Patton et al.,1995]; PD, Parkinson's disease; LEDD, levodopa equivalent daily dose. See also Supporting Information Table 1 for the asymmetry of bradykinesia, rigidity and tremor.
FAB scores were collected in 16 PD patients with tremor and in all 20 nontremor PD patients.
BIS‐11 scores were collected in 15 PD patients with tremor and in 17 nontremor PD patients.
Age was compared across the three groups using a one‐way analysis of variance (ANOVA). Gender was compared using a chi‐square test. Disease characteristics were compared across the two PD groups using two‐sample t‐tests. First, we compared the severity of tremor symptoms across groups by using the resting, action and postural tremor scores from the TRS part A, separately for each type of tremor. Second, we compared nontremor symptoms across groups by using different items from the UPDRS‐III. We divided the total UPDRS score into four different subscores [Stochl et al.,2008]: bradykinesia of the extremities (sum of items 23–26), rigidity (item 22), speech/hypomimia (sum of items 18–19) and axial/gait bradykinesia (sum of items 27–31).
Motor Imagery
Experimental design
We evoked and quantified motor imagery on a trial‐by‐trial basis by using the laterality judgment task [Parsons,1987]. During this task, subjects were presented with pictures of hands and feet (Fig. 1a), and they were required to judge whether the presented stimulus depicted a left or a right body part. Importantly, stimuli could be shown in a biomechanically easy or difficult orientation. Biomechanically easy stimuli consisted of hand stimuli where the fingers pointed medially and of foot stimuli where the toes pointed upwards (both with respect to the vertical body axis). Biomechanically difficult stimuli consisted of hand stimuli where the fingers pointed laterally and of foot stimuli where the toes pointed downwards. By comparing these conditions (biomechanically difficult vs. easy), we were able to identify the network that is specifically involved in imagined movements, while avoiding the interpretational and methodological problems that arise when using a secondary task to control for visuospatial processes only loosely related to movement simulation [de Lange et al.,2006; Helmich et al.,2007; Parsons,1987]. Because both sets of stimuli are matched for visual complexity and rotation (with respect to the canonical stimulus position), this procedure inherently controls for effects related to stimulus presentation, mental rotation/visual imagery, and response delivery. Thus, we used this experimental manipulation to assess the behavioral and cerebral correlates of action planning. For each orientation, the hand‐ and foot‐stimuli could be shown in four different rotations (45°, 75°, 105°, and 135°) and in two different views (hands: palmar or radial view; feet: lateral or medial view). Stimulus rotation and stimulus view were not of primary interest for this study, but their inclusion in the experimental design was important to ensure that subjects used motor imagery to solve the laterality judgment task, rather than alternative processes like spatial mappings between position of a stimulus feature and laterality. This yielded a total of 64 different stimuli (total of 640 trials during the whole fMRI experiment).
Figure 1.
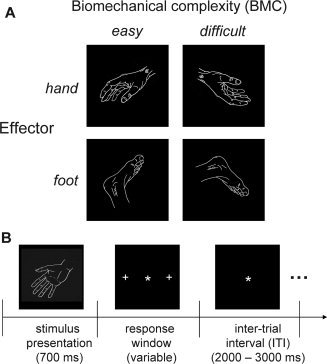
Experimental design. A: Experimental conditions. Subjects were presented with a picture of a hand or a foot, and they were asked to judge whether it represented a left or a right body part. There were two levels of motor planning difficulty, depending on the biomechanical complexity of the imagined movement towards the position indicated by the stimulus. B: Time course of one trial. The star indicates the fixation point; the crosses on the left and on the right indicate the targets for the saccade used by the subjects to respond during the imagery task. The drawings in Panels A and B illustrate representative stimuli configurations sampled from the set of 64 pictures used in this study.
Experimental procedures
Subjects started with a training session outside the scanner until they could perform the task (see Supporting Information). The task in the scanner was divided in two consecutive sessions of ∼30 min each, with a break of ∼15 min in between. Subjects were lying supine, facing the bore of the magnet, unable to see their hands. The stimuli were presented via a mirror onto a screen, using Presentation software (Neurobehavioral systems, Albany, USA). The stimuli subtended a visual angle of <5°. Each trial started with the fixed presentation of the stimulus for 700 ms, followed by a variable response window (tailored to the individual's reaction times), and it ended with a variable inter‐trial interval that was randomly jittered between 2,000 and 3,000 ms (Fig. 1b). Subjects responded to left and right body parts by making a saccade to a target depicted on the left or on the right side of the screen (eccentricity of ∼9°). Eye movements were monitored using a video‐based infrared eyetracker (Sensomotoric Instruments, Berlin, Germany). By using saccades as response modality, we minimized interferences between resting tremor, motor imagery, and button presses. The subjects' trial‐to‐trial reaction times (RT; defined as the time between stimulus onset and onset of the saccade) were calculated off‐line using custom‐made Matlab software (MathWorks, Natick, MA). Following visual inspection of each trial, saccade onset was defined as the first time point (since trial onset) at which the change in pupil position (first temporal derivative) was larger than three standard deviations (SD) above the mean.
Behavioral analyses
We analyzed the influence of factors GROUP (controls, tremulous PD, nontremor PD), EFFECTOR (hand, foot), and ORIENTATION (biomechanically easy, difficult) on median reaction times (RT) and on normalized error rates [ER; arcsine transformation; Sheskin,2003], using three‐way repeated measures ANOVA on the behavioral data collected during scanning. Alpha‐level was set at P = 0.05.
fMRI Image Acquisition and Preprocessing
Functional images were acquired on a Siemens TRIO 3 T MRI system (Siemens, Erlangen, Germany) equipped with echo planar imaging (EPI) capabilities, using an eight‐channel head coil for radio frequency transmission and signal reception. Blood oxygenation level‐dependent (BOLD) sensitive functional images were acquired using a single shot gradient EPI‐sequence [TE/TR = 30/2,380 ms; 35 axial slices, voxel size = 3.5 × 3.5 × 3.0 mm3; inter‐slice gap of 0.5 mm; field of view (FOV) = 224 mm. High‐resolution anatomical images were acquired using an MP‐RAGE sequence (TE/TR = 2.92/2,300 ms; voxel size = 1.0 × 1.0 × 1.0 mm3, 192 sagittal slices; FOV = 256 mm; scanning time ∼5 min).
All data were preprocessed and analyzed with SPM5 (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm). First, functional EPI images were spatially realigned using a least squares approach and a six parameter (rigid body) spatial transformation [Friston et al.,1995]. Subsequently, the time‐series of each voxel was realigned temporally to acquisition of the first slice (slice time correction). Anatomical images were spatially coregistered to the mean of the functional images [Ashburner and Friston,1997] and segmented using a unified segmentation approach. The resulting transformation matrix was then used to normalize the anatomical and functional images. The normalized functional images were resampled at an isotropic voxel size of 2 mm and smoothed with an isotropic 8 mm full‐width‐at‐half‐maximum (FWHM) Gaussian kernel.
Analysis of Task‐Related Cerebral Effects
General linear model
The preprocessed fMRI time series was analyzed at the first level using an event‐related approach in the context of the General Linear Model (GLM). The GLM considered the factors LATERALITY (left, right), EFFECTOR (hand, foot) and ORIENTATION (biomechanically easy, difficult), leading to eight different conditions. Trials were modeled as square‐wave functions time‐locked to stimulus onset, and durations corresponding to the mean reaction time across all imagery trials of the subject. The effect of stimulus ROTATION on cerebral activity was separately modeled for each condition using a linear basis function (parametric modulation with four levels corresponding to 45°, 75°, 105°, and 135°). In addition, our first‐level model included separate regressors of no interest: two regressors modeling incorrect and missed trials, two regressors describing the signal intensity averaged on each scan over the segmented white matter and over a blank portion of the MR images (Out of Brain signal, OOB), and 36 regressors describing head motion [linear, quadratic and cubic effects of the six movement parameters belonging to each volume and also the first derivative of each of those regressors, to control for spin‐history effects; Lund et al.,2005]. In PD patients with tremor, we also included a regressor modeling the changes in tremor amplitude during scanning (see below). Parameter estimates for all regressors were obtained by maximum‐likelihood estimation, while using a temporal high‐pass filter (cut‐off 128 s), and modeling temporal autocorrelation as a first‐order auto‐regressive ‐AR(1)‐ process. At the first level, we defined a contrast that combined left and right stimuli for each effector and orientation, and the four resulting contrast images (hand‐easy; hand‐difficult; foot‐easy; foot‐difficult) were taken to the second level and entered into a full factorial repeated measures ANOVA with factors Group, Effector, and Orientation. Although the gender distribution was not significantly different between groups, we added this information as a covariate to the second level analysis, to correct for possible gender‐related cerebral differences during motor imagery [Seurinck et al.,2004].
Region of interest analysis
Besides a whole brain search, we also performed an region of interest (ROI) analysis on the bilateral dorsal premotor cortex (PMd). This was done because we expected subtype‐specific differences in the PMd, as outlined in the introduction, and because the PMd plays a dominant role in motor imagery, as previsouly shown [Cisek and Kalaska,2004; de Lange et al.,2006; Johnson et al.,2002]. Specifically, we took the PMd coordinates from a previous study that performed the same motor imagery task in healthy subjects [MNI coordinates, left PMd: [‐24 −8 54]; right PMd: [30−8 56]; [de Lange et al.,2006]], and we searched for altered imagery‐related activity in two spheres of 10 mm radius around these coordinates.
Correlation with disease duration
On the basis of the finding that motor symptoms progress more slowly in tremor‐dominant than in nontremor patients [Jankovic and Kapadia,2001], we quantified the effect of disease duration on cerebral activity. This was done for brain regions with differential group effects (i.e., left PMd, left/right BA3a, left OP4). Thus, for each group we calculated a Spearman's rho coefficient (two‐tailed) between disease duration and imagery‐related activity (difference between beta value for biomechanically difficult vs. easy trials).
Analysis of Tremor‐Related Cerebral Effects
During MR scanning, we measured activity in the extensor digitorum communis (EDC) or flexor carpi radialis (FCR) muscle (depending on the tremor characteristics) of the most‐affected forearm of each PD patient. The same was done for the right FCR in 17 out of 19 healthy controls. We used these data for the following purposes. First, by testing for brain responses temporally correlated to fluctuations in tremor power, we could investigate the cerebral network involved in tremor genesis. Specifically, for each tremulous PD patient we determined the individual “tremor frequency” (Fig. 4a) and extracted the EMG power at this frequency. This produced a regressor describing the scan‐by‐scan fluctuations in tremor‐related EMG power. After normalization and convolution with the hemodynamic response function (hrf; Fig. 4b), we added this regressor to the first‐level model described above. For each patient, this produced one contrast image representing tremor amplitude‐related effects. To calculate effects over the whole group, we reoriented these contrast images such that the left side was always the most‐affected side. This was done because all patients had an asymmetric tremor (Supporting Information Table I), but they were not all affected on the same side. Then we tested for significant tremor‐related effects over the whole brain, by performing a one‐sample t‐test. In addition, we used a more sensitive within‐patients comparison to detect tremor‐related effects, i.e., we compared tremor‐related responses between homotopic regions in the most‐ and least‐affected hemisphere. We applied this procedure to: (1) the tremor‐related regions localized above; (2) regions where we expected tremor‐related responses [primary somatosensory cortex, using anatomical ROI's of BA1, 2, 3a, and 3b from the Anatomy Toolbox; [Eickhoff et al.,2005]]; (3) two control regions where we expected no tremor‐related effects [visual cortex: BA17 and 18; [Eickhoff et al.,2005]]. Finally, by comparing averaged EMG power between groups and task conditions (using the same statistical model as for RT), we could rule out that subjects made actual movements during the motor imagery task, and we could test whether tremor amplitude of tremulous PD patients increased as a function of the task conditions. For further details, see Supporting Information.
Figure 4.
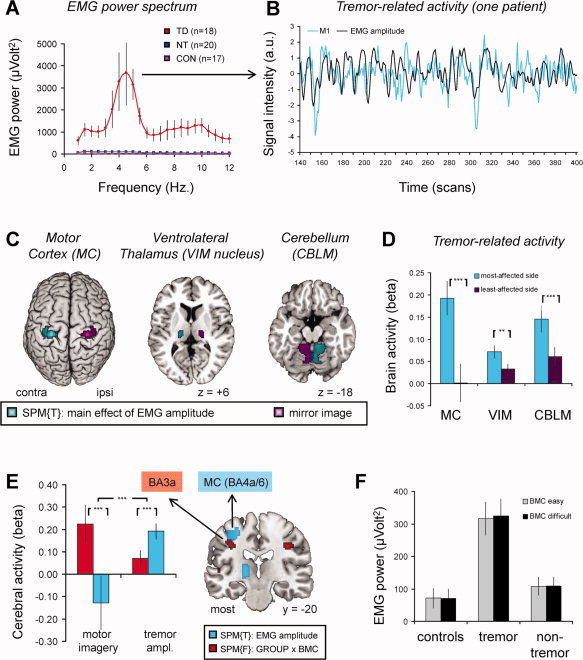
Tremor‐related brain activity. Panel A shows rectified EMG power (y‐axis) as a function of frequency (x‐axis), averaged across 18 PD patients with tremor (in red), 17 controls (in violet) and 20 nontremor PD patients (in blue). For each patient, a regressor describing scan‐by‐scan variations in EMG power at tremor frequency (∼4 to 5 Hz) was used to localize brain regions with tremor‐related responses (i.e., regions where cerebral activity cofluctuated with tremor amplitude). Panel B illustrates this procedure for one patient, over a period of 260 scans (∼10 min, on the x‐axis). In this patient, cerebral activity in the contralateral motor cortex (M1, blue line, time course filtered at f > 0.008 and z‐normalized) was correlated with tremor amplitude (black line, z‐normalized). Panel C shows the anatomical distribution of tremor amplitude‐related brain activity (in cyan, SPM of a t‐contrast across 18 PD patients with tremor, shown at an uncorrected threshold of P < 0.001). The left side represents the side contralateral to the tremulous hand. In purple, the homotopic regions in the other (least‐affected) hemisphere are shown. Panel D shows the tremor‐related responses (mean beta values ± SEM, on the y‐axis) for the motor cortex (MC, BA4a/6), ventrolateral thalamus (VIM nucleus) and cerebellum (CBLM), separately for the most‐affected hemisphere (blue bars) and for the least‐affected hemisphere (purple bars). Panel E shows that the imagery‐related effects in BA3a (in red, same contrast as in Fig. 3D) are independent from the tremor‐related effects in neighboring BA4a/6 (in blue, same contrast as in panel C). That is, imagery‐related brain activity was significantly larger in BA3a than in BA4a/6 (left two bars, the y‐axis shows the difference between biomechanically difficult and easy conditions; average ± SEM). Conversely, tremor‐related brain activity was significantly larger in BA4a/6 than BA3a (right two bars, the y‐axis shows the average beta value ± SEM). Panel F shows rectified EMG power (average ± SEM, on the y‐axis) during imagery of biomechanically easy and difficult movements (colored bars), across the three groups (x‐axis). The results show increased EMG activity in PD patients with tremor compared to controls and nontremor PD patients, but EMG power was not modulated by the biomechanical complexity (BMC) of the imagined movement. Other conventions as in Figures 2 and 3. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Analysis of Overlapping Task‐ and Tremor‐Related Cerebral Effects
In tremulous PD patients, we searched for regions showing both task‐related effects (as indexed by larger activity for biomechanically difficult vs. easy trials) and tremor‐related effects (as indexed by cofluctuations between cerebral activity and tremor amplitude). Thus, we entered these two sets of contrast images into a second level analysis (full factorial model). All images were reoriented such that the left side was always the most‐affected side. We then performed a formal conjunction analysis on these two conditions [Nichols et al.,2005], and searched for cerebral effects in the bilateral motor cortex, cerebellum, and VIM (i.e., ROI analysis in areas with significant tremor‐related effects, shown in Fig. 4c).
Statistical Inference
Statistical inference was performed at the cluster‐level, using a threshold of P < 0.05 corrected for multiple comparisons over the whole brain, on the basis of an intensity threshold of t > 3.1 [Friston et al.,1996]. We applied the Non‐Stationary Cluster Extent Correction for SPM toolbox (http://fmri.wfubmc.edu/cms/NS-General) to calculate cluster‐level statistics for F‐contrasts. Following significant interactions between groups and conditions (whole‐brain corrected), we calculated the average beta values across these clusters using MarsBar [http://marsbar.sourceforge.net], and performed further statistical testing on these values in SPSS 15.0. Finally, when performing analyses in regions of interest (e.g., in the PMd), we applied a voxel‐level family‐wise error (FWE) correction within these search volumes.
RESULTS
Clinical Characteristics
PD patient with and without tremor were matched for general disease severity (duration, total UPDRS, H&Y scale, FAB, BIS‐11; see Table I). Bradykinesia and rigidity scores were also similar across groups. PD patients with tremor had significantly more resting and postural tremor than the nontremor group, while action tremor severity did not differ between groups. In contrast, nontremor PD patients had significantly more axial and gait symptoms, as well as symptoms related to speech and hypomimia. This is consistent with the clinical characteristics of these subtypes [Jankovic et al.,1990].
Behavioral Results
Reaction times
Overall, patients and controls were equally fast in responding (no main effect of GROUP: F(1,54) = 0.14; P = 0.87). All groups were significantly slower for motor imagery of biomechanically difficult than easy trials (main effect of ORIENTATION: F(1,54) = 109.0; P < 0.001; Fig. 2a,b). This effect was stronger for feet than for hands (Effector × Orientation interaction: F(1,54) = 58.0; P < 0.001), but clearly present for both effectors (main effect of Orientation; hand stimuli: F(1,54) = 36.2; P < 0.001; foot stimuli: F(1,54) = 112.8; P < 0.001). The effects of biomechanical complexity were similar across groups (F < 1 for all interactions with GROUP). These results indicate that all groups were sensitive to the biomechanical constraints associated with imaging hand and foot movements, i.e., the subjects used motor imagery to solve the task. All groups were equally fast for imagery of hand and foot movements (no main effect of Effector: F(1,54) = 1.2; P = 0.28; no interaction with Group). Having used a task that patients can solve as well as controls, it becomes meaningful to compare cerebral responses across groups during performance of correct trials [Price and Friston,1999].
Figure 2.
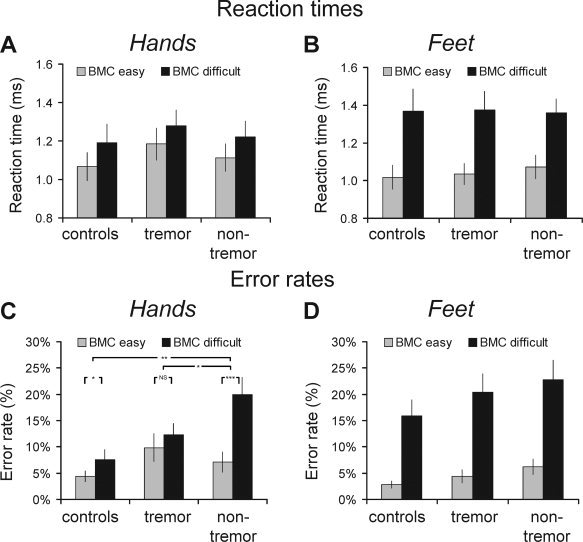
Behavioral results: effects of biomechanical complexity. Reaction times (RT, in seconds; Panels A, B) and error rates (ER, in % correct; Panels C, D) for biomechanically easy (gray bars) and difficult (black bars) conditions are shown separately for hand (panels A‐C) and foot (panels B‐D) trials, across the three different groups (x‐axis). Subjects were consistently slower for biomechanically difficult (as compared with easy) trials; this effect was larger for foot than for hand stimuli, but it was equal across groups. Subjects made more errors for biomechanically difficult (as compared with easy) trials, and this effect was larger for foot than for hand stimuli. Nontremor PD patients had relatively high error rates specifically for imagery of biomechanically complex hand movements. Controls = 19 healthy controls; tremor = 18 PD patients with tremor; nontremor = 20 nontremor PD patients. In panel C, * indicates P < 0.05, ** indicates P < 0.01 and *** indicates P < 0.001. BMC = biomechanical.
Error rates
All groups adequately performed the task with low error rates of 7.7 ± 6.1% (controls), 11.7 ± 7.9% (tremulous PD) and 14.0 ± 9.6% (nontremor PD; mean ± SD; no significant difference across groups). All groups made more errors for motor imagery of biomechanically difficult than easy trials (main effect of Orientation: F(1,54) = 109.6; P < 0.001; Fig. 2c,d); this effect was stronger for feet than for hands (Effector × Orientation interaction: F(1,54) = 32.5; P < 0.001), but clearly present for both effectors (main effect of Orientation; hand stimuli: F(1,54) = 26.6; P < 0.001; foot stimuli: F(1,54) = 126.6; P < 0.001). Contrary to the matched performance in terms of reaction times, we found that nontremor PD patients had relatively high error rates specifically for imagery of biomechanically difficult hand movements (Group × Effector × Orientation interaction: F(2,54) = 3.3; P = 0.045; hands: Group × Orientation interaction: F(2,54) = 6.1; P = 0.004; feet: no Group × Orientation interaction: F(2,54) = 0.1; P = 0.89). This pattern set the nontremor PD group aside from both controls (hands; Group × Orientation interaction: F(1,37) = 9.7; P = 0.004) and from PD patients with tremor (hand; Group × Orientation interaction: F(1,36) = 7.0; P = 0.012).
fMRI Results: Motor Imagery‐Related Effects
Shared cerebral activity across groups
In all three groups, we observed motor imagery‐related activity in the bilateral dorsal premotor cortex (PMd), posterior parietal cortex, ventral premotor cortex, insula, pallidum, thalamus, cerebellum, occipito‐temporal cortex, pre‐SMA, and the left dorsolateral prefrontal cortex (effect of Orientation: biomechanically difficult > easy, conjunction analysis across groups [Nichols et al.,2005]; Fig. 3a,b; Supporting Information Table II). This finding is consistent with previous studies that showed the involvement of this network in motor imagery, both in healthy subjects [de Lange et al.,2006] and in PD patients [Helmich et al.,2007]. Furthermore, all groups showed effector‐specific cerebral effects (hand vs. foot imagery; see Supporting Information Material for details). Specifically, imagery of hands was associated with specific activity in lateral portions of Brodmann Area (BA) 3b and in BA17, while imagery of feet was associated with specific responses in dorso‐medial BA6, the superior parietal lobule (SPL) and the inferior parietal cortex (IPC; Supporting Information Fig. 1a,b). Furthermore, responses in BA3b, BA6, and IPC were sensitive to the laterality of the stimulus, such that activity in the right hemisphere was larger for imagery of left‐ than right‐lateralized movements and vice versa (Supporting Information Fig. 1c–f). This somatotopy strongly suggests that all subjects used first‐person kinesthetic motor imagery to solve the task, rather than visual imagery.
Figure 3.
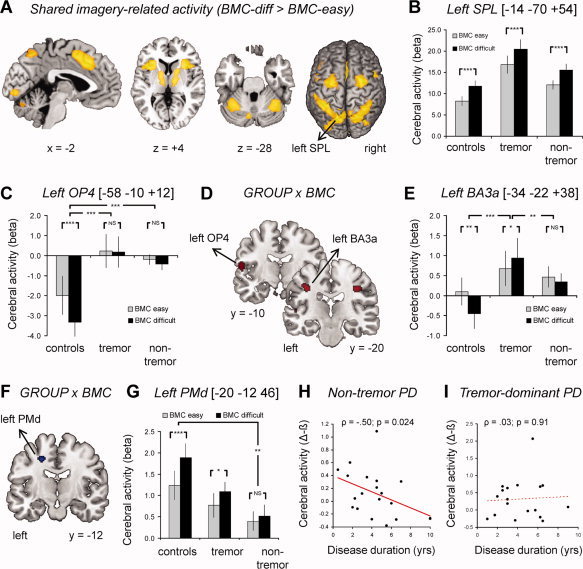
Motor imagery‐related brain activity. Panels A and B show shared motor imagery‐related activity between the three groups. In panel A, the statistical parametric map (SPM, in yellow) of the t‐contrast: “biomechanically difficult > easy” [conjunction analysis over all groups; (Nichols et al.,2005)] is shown at an uncorrected threshold of P < 0.001 (for graphical purposes). In panel B, imagery‐related cerebral activity (mean beta values ± SEM, on the y‐axis) is plotted for the superior parietal lobule (SPL), shown separately for biomechanically easy and difficult conditions (colored bars) and across the three groups (x‐axis). Panels C–G show differential motor imagery‐related activity between the three groups. In panels D and F, the SPM of the F‐contrast: “GROUP × ORIENTATION interaction” is shown at an uncorrected threshold of P < 0.001 (for graphical purposes), superimposed on three coronal sections of a representative brain of the MNI series. Panels C, E, and G show the effects size (mean beta values ± SEM, on the y‐axis) of imagery‐related cerebral activity in the left BA3a (panel C), left OP4 (panel E) and left PMd (panel G), plotted separately for biomechanically easy and difficult conditions (colored bars) and across the three groups (x‐axis). Panels H and I show that imagery‐related activity in the left PMd (difference between beta values for biomechanically difficult and easy trials, on the y‐axis) decreased as a function of disease duration (in years, on the x‐axis) for nontremor PD patients (panel H), but not for PD patients with tremor (panel I). In panels C, E, and G, * indicates P < 0.05, ** indicates P < 0.01 and *** indicates P < 0.001. NS indicates no significant difference. BA, Brodmann area. Other conventions as in Figure 2. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Between‐groups differential cerebral activity
There was a significant interaction between Group (controls, tremulous PD, nontremor PD) and Orientation (biomechanically easy, difficult) in the somatosensory cortex both in the left hemisphere (local maximum at MNI [−34 −22 + 38], 80 voxels; F = 12.8, z = 4.39; P = 0.019 corrected) and in the right hemisphere (local maximum at MNI [+36 −20 + 36], 69 voxels; F = 13.7, z = 4.57; P = 0.047 corrected; Fig. 3c,d). Anatomically, both regions could be assigned to BA3a with high probabilities of 70% (left hemisphere) and 90% [right hemisphere; [Eickhoff et al.,2005]]. In both regions, PD patients with tremor showed increased somatosensory activity during biomechanically difficult (as compared with easy) trials (left BA3a: t(17) = −2.8, P = 0.013; right BA3a: t(17) = −3.3, P = 0.005), while controls showed decreased somatosensory activity during biomechanically difficult (as compared with easy) trials (left BA3a: t(18) = 3.6, P = 0.002; right BA3a: t(18) = 3.4, P = 0.003). This was visible in a significant Group (controls, tremulous PD) by Orientation (biomechanically difficult, easy) interaction in both the left (F(1,35) = 20.3, P < 0.001) and the right (F(1,35) = 22.2, P < 0.001) BA3a. Conversely, nontremor PD patients showed an absent modulation of somatosensory activity across biomechanically difficult or easy trials (left BA3a: t(19) = 1.4, P = 0.18; right BA3a: t(19) = −0.3, P = 0.77). This was visible in a significant Group by Orientation interaction with respect to the controls (left BA3a: F(1,37) = 5.9, P = 0.020; right BA3a: F(1,37) = 8.8; P = 0.005) and the tremulous PD patients (left BA3a: F(1,36) = 8.9, P = 0.005; right BA3a: F(1,36) = 6.1; P = 0.018). In contrast to the effects seen in BA3b, responses in the left and right BA3a were not sensitive to the laterality of the stimulus (left or right; see Supporting Information Fig. 2). Since nontremor PD patients had enhanced error rates during hand imagery (see Fig. 2), it might be argued that their absent modulation of BA3a activity by biomechanical complexity was driven by task disengagement. This possibility is unlikely, however, because the somatosensory effects were present for both hand and foot imagery, and because these effects were present for nontremor subgroups with high and low error rates (Supporting Information Fig. 3).
In addition to these effects in primary somatosensory cortex, there was a strong trend towards a Group × Orientation interaction in the left secondary somatosensory cortex (local maximum at MNI [−58 −10 + 12], 133 voxels; F = 10.6, z = 3.83; P = 0.070 corrected; Fig. 3a,e). Anatomically, this region could be assigned to the parietal operculum (OP4) with a probability of 60% [Eickhoff et al.,2005]. Within this region, controls showed decreased cerebral activity during biomechanically difficult (as compared to easy) trials (t(18) = −8.0, P < 0.001), while both PD groups showed an absent modulation of OP4 activity (tremulous PD: t(17) = 0.31, P = 0.77; nontremor PD: t(1,19) = 1.4, P = 0.18). There were no brain regions where orientation‐related activity changed as a function of both effector and group, indicating that the differences between patients and controls generalized to imagery of both hand and foot movements.
Finally, we searched for group differences in the bilateral premotor cortex [based on ROIs from [de Lange et al.,2006]]. Our results demonstrate a strong trend towards a GROUP (controls, tremulous PD, nontremor PD) by ORIENTATION (biomechanically easy, difficult) interaction in the left PMd (MNI coordinate: [−20 −12 46]; F = 7.6; z = 3.2; P = 0.069, FWE‐corrected; Fig. 3f,g). Direct t‐tests between groups revealed significantly larger effects of biomechanical complexity in controls than in nontremor PD patients (T = 3.9; P = 0.009, FWE‐corrected), while PD patients with tremor showed an intermediate pattern that was not significantly different from the other two groups (P > 0.2). Imagery‐related activity in the left PMd decreased with disease duration in the nontremor group (ρ = −0.50; P = 0.024; Fig. 3h), but not in the tremulous PD group (ρ = 0.03; P = 0.91; Fig. 3i). There were no significant group‐differences in the right PMd.
fMRI Results: Tremor‐Related Cerebral Activity
In tremulous PD patients, EMG bursts were observed at a typical tremor frequency of 4–5 Hz (Fig. 4a). We used scan‐by‐scan fluctuations of EMG power at the patient‐specific tremor frequency to localize tremor‐related cerebral responses, operationalized as those regions where activity cofluctuated with variations in tremor amplitude (Fig. 4b). We performed four different analyses.
First, we conducted a whole‐brain search for tremor‐related cerebral regions. This revealed three significant areas Fig. 4c): the precentral gyrus contralateral to the tremulous limb (MNI [±28 −26 + 60], 401 voxels, T = 8.39, P < 0.001 corrected), the contralateral thalamus (MNI [±16 −18 0], 138 voxels, T = 5.53, P = 0.029 corrected) and the ipsilateral cerebellum (MNI [±6 −56 −24], 930 voxels, T = 6.09, P < 0.001 corrected). The local maximum of the precentral cluster was located at the border of BA 4a and 6, with a probability of 40% for either BA [Eickhoff et al.,2005]. The local maximum of the thalamic cluster was located in a region that is anatomically connected to the primary motor, somatosensory, and premotor cortices [Behrens et al.,2003]; [http://www.fmrib.ox.ac.uk/connect]. Since the VIM is situated in this region of the thalamus [Fukuda et al.,2004], and generally thought to play a dominant role in the generation of resting tremor [Benabid et al.,1991; Lenz et al.,1994], we labeled this cluster accordingly. The cerebellar cluster was located in Lobules IV and V [Diedrichsen et al.,2009], in a region that is consistently activated during various sensorimotor tasks [Stoodley and Schmahmann,2009]. The involvement of these three regions in resting tremor is consistent with previous PET studies [Fukuda et al.,2004; Kassubek et al.,2001].
Second, since all tremulous PD patients had a more pronounced tremor on one hand than the other, we directly compared tremor‐related responses between homotopic regions in the most‐ and least‐affected hemisphere. This revealed significantly larger tremor‐related responses in the most‐ than least‐affected motor cortex [t(17) = 3.8, P < 0.001], VIM [t(17) = 3.1; P = 0.003] and cerebellum [t(17) = −4.3; P < 0.001; Fig. 4c,d]. Furthermore, using this sensitive within‐patients comparison, we found significantly larger tremor‐related responses in the most‐ than least‐affected somatosensory cortex (BA1: t(17) = 2.6, P = 0.017; BA3a: t(17) = 2.7, P = 0.016; BA3b: t(17) = 2.9, P = 0.011; Supporting Information Fig. 3b), but no asymmetry in the visual cortex (BA17: P = 0.26; BA18: P = 0.46; Supporting Information Fig. 3c).
Third, given the close proximity between tremor‐related responses in BA4a/6 (Fig. 4c) and task‐related responses in BA3a (Fig. 3c), we wanted to rule out that these effects were part of one underlying pattern. Accordingly, we directly compared tremor‐ and task‐related activity (factor PROCESS) across the two clusters (factor REGION, i.e. BA3a and BA4a/6, both calculated for the most‐affected hemisphere) and found a double dissociation in their activity patterns (Process × Region interaction: F(1,17) = 31.1, P < 0.001; Fig. 4e). Thus, while tremor‐related responses were significantly larger in BA4a/6 than in BA3a (t(17) = −7.3, P < 0.001), the opposite was true for task‐related processing (t(17) = 3.9, P = 0.001). It should be noted, however, that these differences in BA3a occurred in the context of significant tremor‐related effects (t(17) = 2.0; P = 0.031, one‐tailed) and task‐related effects (t(17) = 2.7; P = 0.008, one‐tailed), as expected on the basis of our previous findings (Fig. 3d and Supporting Information Fig. 3b).
Fourth, we calculated the average EMG power for each trial to test whether resting tremor amplitude was modulated by the task conditions. Tremulous PD patients showed significantly higher EMG activity than the other two groups (main effect of Group: F(2,49) = 11.7, P < 0.001, controls vs. tremulous PD: P = 0.001; nontremor PD vs. tremulous PD: P = 0.001; two‐sample t‐tests; Fig. 4f), while controls and nontremor PD patients showed similar EMG activity (P = 0.38). Crucially, EMG activity did not differ across conditions (F < 2) and these effects were similar across groups (all interactions with Group: F < 2). This indicates that altered imagery‐related brain activity across groups cannot be explained by actual movements or by task‐related changes in tremor amplitude.
fMRI Results: Overlap Between Tremor‐ and Motor Imagery‐Related Cerebral Activity
In tremulous PD patients, we tested for cerebral regions showing both tremor‐related responses (indexed by the EMG amplitude regressor) and imagery‐related activity (indexed by a contrast between biomechanically difficult and easy trials), using a conjunction analysis of both contrasts [Nichols et al.,2005]. This revealed significant effects in the most‐affected VIM (MNI coordinates: [±14 −22 + 6], T = 4.1; P = 0.005 FWE corrected; [±16 −22 + 2], T = 3.9; P = 0.011 FWE corrected; Fig. 5c) and in the least‐affected VIM (MNI coordinates: [±18 −16 + 4], T = 4.0; P = 0.005 FWE corrected; Fig. 5c). These three thalamic regions were reported to have maximal anatomical connectivity with the posterior parietal cortex, the somatosensory cortex, and the premotor cortex, respectively [http://www.fmrib.ox.ac.uk/connect; Behrens et al.,2003]. Conversely, tremor‐ and imagery‐related cerebral effects were strictly segregated in the (pre)motor cortex (Fig. 5a), with task‐related activity being located more rostrally (in BA 6/8) than tremor‐related activity (in BA4a/6). Likewise, we found a clear spatial segregation in the cerebellum (Fig. 5b), with task‐related activity in lateral (Lobule VI and Crus I) and tremor‐related activity in more medial areas [Lobules IV and V; [Diedrichsen et al.,2009]].
Figure 5.
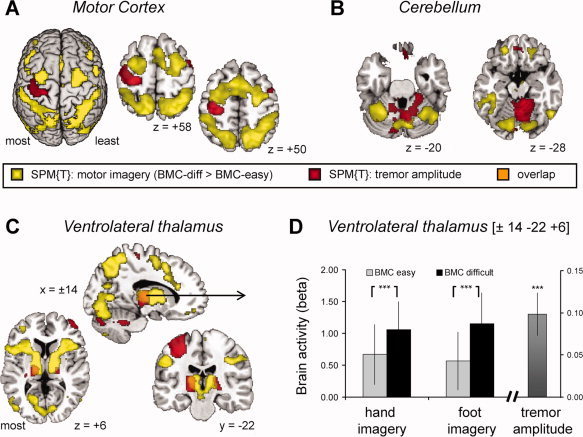
Overlap between imagery‐ and tremor‐related brain activity. Panels A–C show the anatomical distribution of tremor‐related brain activity (in yellow, SPM of the t‐contrast: “biomechanically difficult > easy”) and tremor‐related brain activity (in red, SPM of the t‐contrast: “tremor amplitude”) across 18 PD patients with tremor. Both contrasts are shown at a threshold of P < 0.01 uncorrected, to best visualize the pattern of overlap and segregation. Panel A focuses on the motor cortex, Panel B on the cerebellum and Panel C on the thalamus. The results demonstrate that imagery‐ and tremor‐related effects are clearly separated in the motor cortex and cerebellum, but that they overlap in the ventrolateral thalamus (orange voxels in Panel C). Panel D shows both imagery‐related activity (indexed by larger activity for biomechanically difficult than easy trials, colored bars on the left side) and tremor‐related responses (indexed by cofluctuation of cerebral activity with tremor amplitude, bar on the right side) in a thalamic voxel where these two effects converged [tested with a conjunction analysis; (Nichols et al.,2005)]. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
We investigated whether the functional organization of the cerebral motor system differs between PD patients with and without tremor. Planning‐related activity was investigated using a controlled motor imagery task, and quantified by comparing imagery of biomechanically difficult and easy movements. In addition, we measured tremor‐related brain activity using EMG recordings during scanning. There are three main findings. First, we found that PD patients with tremor had increased imagery‐related activity in the somatosensory cortex (BA3a). Second, we observed tremor‐related brain activity in the primary motor cortex, cerebellum and ventral intermediate nucleus (VIM) of the thalamus. Third, PD patients with tremor showed an overlap between tremor‐ and imagery‐related activities in the VIM, which has known anatomical projections to BA3a. These findings suggest that tremor‐related activity in the VIM may influence motor imagery by altering central processing of somatosensory input. In addition to these main findings, we observed a tendency towards differential effects in the dorsal premotor cortex (PMd). Specifically, controls showed robust imagery‐related responses in PMd, while tremulous PD patients showed reduced responses and nontremor PD patients showed absent responses. Furthermore, imagery‐related activity in PMd decreased with disease duration in nontremor PD, but not in tremulous PD. In the following sections, we discuss how these cerebral differences may account for some of the clinical and behavioral differences observed between PD patients with and without tremor.
Altered Somatosensory Activity During Motor Imagery in Parkinson's Disease
The enhanced imagery‐related BA3a activity in PD patients with tremor, defined as the disparity between biomechanically difficult and easy movements, was functionally dissociated from tremor‐related responses. That is, imagery‐related BA3a activity was modulated by the biomechanical complexity of the imagined movement, but tremor amplitude was not. This indicates that the increased BA3a activity was not primarily driven by increased tremor‐related input from the periphery, but rather by altered central gating of somatosensory signals. Altered central gating of afferent signals in PD patients with tremor may be mediated by the VIM, which was unique in showing both imagery‐ and tremor‐related activities. That is, electrophysiological recordings in PD patients have shown that many VIM neurons with tremor characteristics also respond to somatosensory input and are activated during voluntary movements [Lenz et al.,1994]. Furthermore, anatomical studies in nonhuman primates have shown that the homologue of the human VIM [ventroposterior lateral nucleus, pars oralis; VPLo; Percheron et al.,1996] receives somatosensory input from the spinal cord [Stepniewska et al.,2003], and sends projections specifically to BA3a within the somatosensory cortex [Padberg et al.,2009]. The VPLo is also densely connected with regions of the motor system, i.e. M1 [Holsapple et al.,1991; Hoover and Strick,1999] and the cerebellum [Evrard and Craig,2008]. This pattern of connectivity allows the VIM to provide a neural interface where somatosensory input is modulated by cerebellar output before it reaches the cortex [Stepniewska et al.,2003]. Accordingly, the thalamic region where we observed overlapping responses was previously found to have high anatomical connectivity with both the somatosensory cortex and the premotor cortex, as measured with diffusion tensor imaging [Behrens et al.,2003; Johansen‐Berg et al.,2005]. Interestingly, the overlap between tremor‐ and task‐related responses in the VIM was significant for both hemispheres. This was caused by the spatial distribution of tremor‐related responses, which were strictly lateralized in M1 (i.e., only seen in the hemisphere contralateral to the tremulous limb), but more bilateral (although asymmetric) in the VIM and cerebellum. This finding may explain why the increased imagery‐related BA3a activity of PD patients with tremor also occurred in both hemispheres.
Nontremor PD patients also showed altered imagery‐related activity in BA3a (as compared to controls), but these changes were significantly smaller than in PD patients with tremor. Specifically, in nontremor PD patients BA3a activity was not modulated by the biomechanical complexity of imagined movements, with BOLD values intermediate between healthy controls and tremulous PD. This suggests that the somatosensory alterations observed in tremulous PD occur in the presence of a more general, PD‐wide impairment in central somatosensory processing, which fits with a large body of evidence showing clear proprioceptive deficits in PD [Boecker et al.,1999; Konczak et al.,2009; Seiss et al.,2003]. Here we extend these previous findings by showing abnormal activity in the somatosensory cortex during imagined movements in PD. The primary cause of PD‐wide changes in somatosensory processing is likely located within the dopamine‐depleted cortico‐striatal circuit. Accordingly, we have recently shown that PD patients have altered connectivity between the putamen and somatosensory cortex [Helmich et al.,2010], and parkinsonian primates have nonspecific pallidal responses to proprioceptive input [Filion et al.,1988]. Taken together, our findings suggest that PD‐related changes in the cortico‐striatal circuit and tremor‐related changes in the cerebello‐thalamic circuit both contribute to altered processing in the somatosensory system. These somatosensory alterations may influence voluntary action planning in PD, as outlined below.
Functional Implications
Healthy controls showed reduced activity in the somatosensory cortex during imagery of biomechanically difficult movements, which fits with previous studies showing diminished (or gated) central processing of somatosensory afferents during motor imagery [Cheron and Borenstein,1992; Jahn et al.,2004; Rossini et al.,1996]. The inhibition of somatosensory afferents during motor imagery has been linked to the ability of the healthy brain to generate sensory predictions about planned actions [known as forward models Wolpert,2007]. Specifically, forward models use the efference copy of an action plan [Sperry,1950] to predict the somatosensory consequences of that action. When the action plan is executed, the predicted somatosensory input is compared with the actual input, and the resultant mismatch is used to adjust the ongoing action plan. When no mismatch occurs, this mechanism allows the brain to ignore the sensory input generated by a movement [Blakemore et al.,1998]. During motor imagery, the gain of the actual somatosensory input is minimized, and the imagined action unfolds exclusively under the influence of internal predictions [Grush,2004; Shadmehr and Krakauer,2008].
The absence of somatosensory gating in both PD groups suggests that these patients were impaired in generating somatosensory predictions derived from their imagined movements (forward models). This interpretation fits with empirical evidence that PD patients have an impaired dynamic representation of arm position during movements [Contreras‐Vidal and Gold,2004; Klockgether et al.,1995]. However, a link between altered somatosensory gating and impaired forward models does not explain why PD patients with tremor showed larger somatosensory alterations and better behavioral performance. One possibility is that tremor‐related increases in somatosensory processing may have beneficial effects on voluntary action planning. For instance, the robust afferent input generated by a tremulous limb may improve somatosensory uncertainty, and dynamic (tremor‐related) somatosensory input may be better integrated into upcoming motor plans than static somatosensory input [Gelissen and Cools,1987]. In a similar vein, it has been suggested that the physiological tremor observed in healthy subjects is an exploitable phenomenon that may reduce limb inertia [Goodman and Kelso,1983]. Future studies may further focus on possible beneficial aspects of parkinsonian resting tremor, for example by testing whether limb estimation before movement onset is different between PD patients with and without tremor. An alternative possibility is that the clinical benefits of tremor‐dominant PD patients [Alves et al.,2006; Louis et al.,1999], and the better behavioral performance observed here, are related to other pathophysiological differences between both subtypes.
Interpretational Issues
The differential effects of biomechanical complexity across groups were localized in the same BA3a region for hand and foot imagery. This result is not caused by limited functional resolution, since we could show lateralized and effector‐specific cerebral effects in adjacent parts of the somatosensory cortex (i.e. BA3b), as well as in the premotor cortex (see Supporting Information Fig. 1). Rather, it could be related to the peculiar organization and properties of BA3a, namely large receptive [Krubitzer et al.,2004] and highly fractured topographic representations [Huffman and Krubitzer,2001]. The increased BA3a responses in PD patients with tremor were present for both effectors, although half of these patients did not have a clinically noticeable foot tremor. However, more sophisticated behavioral measurements could reveal a classical resting tremor in apparently nontremulous limbs [Beuter et al.,2005], provided that the PD patient had a visible tremor in another limb. Furthermore, basal ganglia recordings in PD patients with tremor could reveal tremor‐related activity even when there was no noticeable tremor [Levy et al.,2001]. This suggests that in those PD patients developing resting tremor, this feature is distributed throughout the whole motor system, which is consistent with our findings.
Finally, although nontremor PD patients were carefully selected on the basis of absent tremor in clinical history and at prior clinical examination, three patients nevertheless showed a slight resting tremor at the day of testing (Table I). Two of these patients did not display any tremor in the fMRI scanner (no EMG peak at 4–5 Hz), and one patient displayed a very low‐amplitude tremor in the scanner (i.e. 10 times lower than in the tremor group). All three PD patients had marked bradykinesia and rigidity, and exclusion of these subjects did not change our findings in the bilateral BA3a (Supporting Information). Therefore, we are confident that these three patients belong to a relatively nontremulous PD subtype, and that inclusion of these subjects did not confound our comparison with tremulous PD.
CONCLUSION
Our results demonstrate functional differences in the voluntary motor system of PD patients with and without resting tremor. PD patients with tremor showed increased imagery‐related activity in the somatosensory cortex (BA3a), and tremor‐ and imagery‐related responses overlapped in a thalamic region connected to the somatosensory cortex. We conclude that tremor‐related responses in the cerebello‐thalamic circuit may influence voluntary action planning by modulating the transmission of somatosensory input to the cortex.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
REFERENCES
- Alves G, Larsen JP, Emre M, Wentzel‐Larsen T, Aarsland D ( 2006): Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord 21: 1123–1130. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K ( 1997): Multimodal image coregistration and partitioning—A unified framework. Neuroimage 6: 209–217. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen‐Berg H, Woolrich MW, Smith SM, Wheeler‐Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM ( 2003): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6: 750–757. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J ( 1991): Long‐term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337: 403–406. [DOI] [PubMed] [Google Scholar]
- Beuter A, Barbo E, Rigal R, Blanchet PJ ( 2005): Characterization of subclinical tremor in Parkinson's disease. Mov Disord 20: 945–950. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD ( 1998): Central cancellation of self‐produced tickle sensation. Nat Neurosci 1: 635–640. [DOI] [PubMed] [Google Scholar]
- Boecker H, Ceballos‐Baumann A, Bartenstein P, Weindl A, Siebner HR, Fassbender T, Munz F, Schwaiger M, Conrad B ( 1999): Sensory processing in Parkinson's and Huntington's disease: Investigations with 3D H(2)(15)O‐PET. Brain 122 ( Part 9): 1651–1665. [DOI] [PubMed] [Google Scholar]
- Burn DJ, Rowan EN, Allan LM, Molloy S, O'Brien JT, McKeith IG ( 2006): Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 77: 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Borenstein S ( 1992): Mental movement simulation affects the N30 frontal component of the somatosensory evoked potential. Electroencephalogr Clin Neurophysiol 84: 288–292. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF ( 2004): Neural correlates of mental rehearsal in dorsal premotor cortex. Nature 431: 993–996. [DOI] [PubMed] [Google Scholar]
- Contreras‐Vidal JL, Gold DR ( 2004): Dynamic estimation of hand position is abnormal in Parkinson's disease. Parkinsonism Relat Disord 10: 501–506. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Helmich RC, Toni I ( 2006): Posture influences motor imagery: An fMRI study. Neuroimage 33: 609–617. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Pollak P, Passingham R, Landais P, Gervason C, Cinotti L, Friston K, Frackowiak R, Mauguiere F, Benabid AL ( 1993): Thalamic stimulation and suppression of parkinsonian tremor. Evidence of a cerebellar deactivation using positron emission tomography. Brain 116 ( Part 1): 267–279. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N ( 2009): A probabilistic MR atlas of the human cerebellum. Neuroimage 46: 39–46. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B ( 2000): The FAB: A frontal assessment battery at bedside. Neurology 55: 1621–1626. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Craig AD ( 2008): Retrograde analysis of the cerebellar projections to the posteroventral part of the ventral lateral thalamic nucleus in the macaque monkey. J Comp Neurol 508: 286–314. [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L, Bedard PJ ( 1988): Abnormal influences of passive limb movement on the activity of globus pallidus neurons in parkinsonian monkeys. Brain Res 444: 165–176. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ ( 1995): Spatial registration and normalization of images. Hum Brain Mapp 3: 165–189. [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD ( 1996): Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 4: 223–235. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Barnes A, Simon ES, Holmes A, Dhawan V, Giladi N, Fodstad H, Ma Y, Eidelberg D ( 2004): Thalamic stimulation for parkinsonian tremor: Correlation between regional cerebral blood flow and physiological tremor characteristics. Neuroimage 21: 608–615. [DOI] [PubMed] [Google Scholar]
- Gelissen M, Cools A ( 1987): Movements of cats on a rotating cylinder: Role of the substantia nigra pars reticulata and the deeper layers of the superior colliculus. Behav Brain Res 25: 83–96. [DOI] [PubMed] [Google Scholar]
- Gentili R, Cahouet V, Ballay Y, Papaxanthis C ( 2004): Inertial properties of the arm are accurately predicted during motor imagery. Behav Brain Res 155: 231–239. [DOI] [PubMed] [Google Scholar]
- Goodman D, Kelso JA ( 1983): Exploring the functional significance of physiological tremor: A biospectroscopic approach. Exp Brain Res 49: 419–431. [DOI] [PubMed] [Google Scholar]
- Grush R ( 2004): The emulation theory of representation: Motor control, imagery, and perception. Behav Brain Sci 27: 377–396. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Immisch I, Toma K, Dimyan MA, Van Gelderen P, Hallett M ( 2003): Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol 89: 989–1002. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Aarts E, de Lange FP, Bloem BR, Toni I ( 2009): Increased dependence of action selection on recent motor history in Parkinson's disease. J Neurosci 29: 6105–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, De Lange FP, Bloem BR, Toni I ( 2007): Cerebral compensation during motor imagery in Parkinson's disease. Neuropsychologia 45: 2201–2215. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I ( 2010): Spatial remapping of cortico‐striatal connectivity in Parkinson's disease. Cereb Cortex 20: 1175–1186. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD ( 1967): Parkinsonism: Onset, progression and mortality. Neurology 17: 427–442. [DOI] [PubMed] [Google Scholar]
- Holsapple JW, Preston JB, Strick PL ( 1991): The origin of thalamic inputs to the “hand” representation in the primary motor cortex. J Neurosci 11: 2644–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Strick PL ( 1999): The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci 19: 1446–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L ( 2001): Area 3a: Topographic organization and cortical connections in marmoset monkeys. Cereb Cortex 11: 849–867. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T ( 2004): Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage 22: 1722–1731. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Kapadia AS ( 2001): Functional decline in Parkinson disease. Arch Neurol 58: 1611–1615. [DOI] [PubMed] [Google Scholar]
- Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I ( 1990): Variable expression of Parkinson's disease: A base‐line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 40: 1529–1534. [DOI] [PubMed] [Google Scholar]
- Jellinger KA ( 2002): Recent developments in the pathology of Parkinson's disease. J Neural Transm Suppl 62: 347–376. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, Matthews PM ( 2005): Functional‐anatomical validation and individual variation of diffusion tractography‐based segmentation of the human thalamus. Cereb Cortex 15: 31–39. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Rotte M, Grafton ST, Hinrichs H, Gazzaniga MS, Heinze HJ ( 2002): Selective activation of a parietofrontal circuit during implicitly imagined prehension. Neuroimage 17: 1693–1704. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Halonen JP, Shawkat F ( 1989): Centrifugal and centripetal mechanisms involved in the ‘gating’ of cortical SEPs during movement. Electroencephalogr Clin Neurophysiol 74: 36–45. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Matsumoto JY, Ahlskog JE ( 2006): Benign tremulous parkinsonism. Arch Neurol 63: 354–357. [DOI] [PubMed] [Google Scholar]
- Kassubek J, Juengling FD, Hellwig B, Knauff M, Spreer J, Lucking CH ( 2001): Hypermetabolism in the ventrolateral thalamus in unilateral Parkinsonian resting tremor: A positron emission tomography study. Neurosci Lett 304: 17–20. [DOI] [PubMed] [Google Scholar]
- Klockgether T, Borutta M, Rapp H, Spieker S, Dichgans J ( 1995): A defect of kinesthesia in Parkinson's disease. Mov Disord 10: 460–465. [DOI] [PubMed] [Google Scholar]
- Konczak J, Corcos DM, Horak F, Poizner H, Shapiro M, Tuite P, Volkmann J, Maschke M ( 2009): Proprioception and motor control in Parkinson's disease. J Mot Behav 41: 543–552. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Huffman KJ, Disbrow E, Recanzone G ( 2004): Organization of area 3a in macaque monkeys: Contributions to the cortical phenotype. J Comp Neurol 471: 97–111. [DOI] [PubMed] [Google Scholar]
- Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R ( 1992): Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 7: 2–13. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Martin RL, Tasker RR, Dostrovsky JO, Lenz YE ( 1994): Single unit analysis of the human ventral thalamic nuclear group. Tremor‐related activity in functionally identified cells. Brain 117 ( Part 3): 531–543. [DOI] [PubMed] [Google Scholar]
- Levy R, Dostrovsky JO, Lang AE, Sime E, Hutchison WD, Lozano AM ( 2001): Effects of apomorphine on subthalamic nucleus and globus pallidus internus neurons in patients with Parkinson's disease. J Neurophysiol 86: 249–260. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA ( 2005): Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry 76: 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K ( 1999): Progression of parkinsonian signs in Parkinson disease. Arch Neurol 56: 334–337. [DOI] [PubMed] [Google Scholar]
- Lund TE, Norgaard MD, Rostrup E, Rowe JB, Paulson OB ( 2005): Motion or activity: Their role in intra‐ and inter‐subject variation in fMRI. Neuroimage 26: 960–964. [DOI] [PubMed] [Google Scholar]
- Marsden CD ( 1982): The mysterious motor function of the basal ganglia: The Robert Wartenberg Lecture. Neurology 32: 514–539. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB ( 2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Padberg J, Cerkevich C, Engle J, Rajan AT, Recanzone G, Kaas J, Krubitzer L ( 2009): Thalamocortical connections of parietal somatosensory cortical fields in macaque monkeys are highly divergent and convergent. Cereb Cortex 19: 2038–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso G, Cunic D, Saint‐Cyr JA, Hoque T, Lozano AM, Lang AE, Chen R ( 2004): Involvement of human thalamus in the preparation of self‐paced movement. Brain 127: 2717–2731. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN ( 1995): Functional anatomy of the basal ganglia. I. The cortico‐basal ganglia‐thalamo‐cortical loop. Brain Res Brain Res Rev 20: 91–127. [DOI] [PubMed] [Google Scholar]
- Parsons LM ( 1987): Imagined spatial transformations of one's hands and feet. Cognit Psychol 19: 178–241. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES ( 1995): Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51: 768–774. [DOI] [PubMed] [Google Scholar]
- Percheron G, Francois C, Talbi B, Yelnik J, Fenelon G ( 1996): The primate motor thalamus. Brain Res Brain Res Rev 22: 93–181. [PubMed] [Google Scholar]
- Price CJ, Friston KJ ( 1999): Scanning patients with tasks they can perform. Hum Brain Mapp 8: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Caramia D, Bassetti MA, Pasqualetti P, Tecchio F, Bernardi G ( 1996): Somatosensory evoked potentials during the ideation and execution of individual finger movements. Muscle Nerve 19: 191–202. [DOI] [PubMed] [Google Scholar]
- Seiss E, Praamstra P, Hesse CW, Rickards H ( 2003): Proprioceptive sensory function in Parkinson's disease and Huntington's disease: Evidence from proprioception‐related EEG potentials. Exp Brain Res 148: 308–319. [DOI] [PubMed] [Google Scholar]
- Seurinck R, Vingerhoets G, de Lange FP, Achten E ( 2004): Does egocentric mental rotation elicit sex differences? Neuroimage 23: 1440–1449. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW ( 2008): A computational neuroanatomy for motor control. Exp Brain Res 185: 359–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheskin DJ ( 2003): The Handbook of Parametric and Nonparametric Statistical Procedures. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- Sperry RW ( 1950): Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43: 482–489. [DOI] [PubMed] [Google Scholar]
- Stacy MA, Elble RJ, Ondo WG, Wu SC, Hulihan J ( 2007): Assessment of interrater and intrarater reliability of the Fahn‐Tolosa‐Marin Tremor Rating Scale in essential tremor. Mov Disord 22: 833–838. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Sakai ST, Qi HX, Kaas JH ( 2003): Somatosensory input to the ventrolateral thalamic region in the macaque monkey: Potential substrate for parkinsonian tremor. J Comp Neurol 455: 378–395. [DOI] [PubMed] [Google Scholar]
- Stochl J, Boomsma A, Ruzicka E, Brozova H, Blahus P ( 2008): On the structure of motor symptoms of Parkinson's disease. Mov Disord 23: 1307–1312. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD ( 2009): Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. Neuroimage 44: 489–501. [DOI] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Nambu A, Inase M ( 1998): Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: Segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp Brain Res 120: 114–128. [DOI] [PubMed] [Google Scholar]
- Vakil E, Herishanu‐Naaman S ( 1998): Declarative and procedural learning in Parkinson's disease patients having tremor or bradykinesia as the predominant symptom. Cortex 34: 611–620. [DOI] [PubMed] [Google Scholar]
- Wenzelburger R, Zhang BR, Pohle S, Klebe S, Lorenz D, Herzog J, Wilms H, Deuschl G, Krack P ( 2002): Force overflow and levodopa‐induced dyskinesias in Parkinson's disease. Brain 125: 871–879. [DOI] [PubMed] [Google Scholar]
- Wolpert DM ( 2007): Probabilistic models in human sensorimotor control. Hum Mov Sci 26: 511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


