Abstract
The anterior insula and the dorsal anterior cingulate cortex (ACC) are regarded as key brain structures associated with the integration of perceived phobic characteristics of external stimuli and the perception of ones own body responses that leads to emotional feelings. To test to what extent the activity in these two brain structures anatomically and functionally overlap during phobic reactions and interoception, we submitted the same group of phobic participants (n = 29; either spider or blood‐injection‐injury (BII) phobics) and controls (n = 17) to both type of experimental paradigms. Results showed that there was a clear anatomical overlap in the Blood Oxygen Level‐Dependent (BOLD) responses within the anterior insula and ACC elicited during phobic symptom provocation and during interoceptive awareness. The activity within these two brain structures also showed to be correlated in the spider phobia group, but not in the BII phobic participants. Our results seem to support the idea that the activity within these two brain areas would be associated with the integration of perceived stimuli characteristics and bodily responses that lead to what we label as “fear.” However, that seems not to be the case in BII phobia, where more research is needed in order to clarify to what extent that could be associated with the idiosyncratic physiological response that these patients present in front of phobic stimuli (i.e., drop in heart rate and blood pressure). Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: phobias, specific phobia, fear, interoceptive, insula, cingulate gyrus, emotion
INTRODUCTION
It has been consistently shown that the amygdala, the insula, and the anterior cingulate cortex (ACC) play a prominent role in the brain network associated with anxiety and fear, both in healthy controls and anxiety disorder populations [see Etkin and Wager, 2007 for a recent meta‐analysis]. Moreover, the anterior insula and the dorsal ACC have also been implicated in interoceptive awareness and monitoring of bodily responses. In this respect, different studies have shown increased activity in these brain structures when participants were asked to attend to internal bodily responses, for example, to their heartbeats [Critchley et al., 2003, 2004; Pollatos et al., 2005, 2007]. Because of their involvement in both fear and interoception, it has been proposed that the anterior insula and the dorsal ACC will play a central role in integrating the perceived characteristics of stimuli and the perceived bodily responses to them, leading to emotional feelings [Critchley, 2004; Harrison et al., 2010; Paulus and Stein, 2010; Pollatos et al., 2007]. This is somehow supported by the fact that anxious participants do seem to show a superior performance than non‐anxious participants in tasks involving interoceptive awareness [Zoellner and Craske, 1999], and by the positive correlation shown of the activity in the right anterior insula during interoception with scores in subjective anxiety [Critchley et al., 2004]. However, we are still lacking evidence of the overlap in the activity of these brain areas during the perception of emotionally significant stimuli and during the perception of bodily responses.
Specific phobia offers an excellent ground to test this hypothesis. Presenting phobic participants with phobia‐related images invariably provokes a perceptible increase in the person's physiological arousal along with a subjective feeling of fear [e.g., Hamm et al., 1997]. According with our previous argument, we could assume that the anterior insula and the dorsal ACC will be involved in the integration of the perceived threatening value of the external stimuli and the perceived bodily changes—arousal—elicited by this threat, this process leading to the subjective experience of fear. In fact, presenting phobic participants with phobia‐related images results in increased activity in the anterior insula and the dorsal ACC—among other brain areas—[e.g., Dilger et al., 2003; Straube et al., 2006a, b]. However, no study has yet accounted for the potential overlap in the activity of these brain areas during a symptom provocation paradigm and during a non‐emotional interoceptive paradigm. Following our argument, we would expect the activity in the anterior insula and dorsal ACC while confronting phobic‐related stimuli and while attending bodily responses to overlap anatomically and functionally within the same phobic population.
To test this prediction, participants suffering from specific phobia and a group of healthy controls took part in both an interoceptive paradigm and a symptom provocation task. In addition, we were interested in testing our hypothesis in two different sub‐types of specific phobia: Animal phobia and Blood‐injection‐injury (BII) phobia. Most of what we know about the neurophysiology of specific phobia comes from research into animal phobics (more specifically, spider phobics [SP]). Very few studies have been run in any other subtypes of specific phobia (i.e., BII, natural environment, or situational) and the results so far available (only in the BII subtype) point toward the existence of potentially relevant differences [Caseras et al., 2010a, b; Hermann et al., 2007; Schienle et al., 2003]. Previous research has shown differences between BII and SP phobic participants in the activity of the anterior insula and the dorsal ACC [Caseras et al., 2010a] during symptom provocation; although these differences seem to be only present when phobic participants face long periods of phobia‐related stimulation (e.g., block designs), not when these stimuli are presented briefly and spaced by neutral stimulation (e.g., event‐related designs) [Caseras et al., 2010b]. Therefore, our secondary goal was to explore to what extent previously reported differences in the anterior insula and the dorsal ACC during symptom provocation between these two subtypes of specific phobia would extend into similar activity differences during a non‐emotional interoceptive paradigm. Considering both aims, we recruited non‐phobic healthy controls, participants suffering from SP but no BII, and participants with BII but not SP.
METHOD
Participants
Participants were recruited among undergraduate students from the Autonomous University of Barcelona, who completed an online version of the Fear of Spiders Questionnaire [FSQ; Szymanski and O'Donohue, 1995] and the Mutilation Questionnaire [MQ; Kleinknecht and Thorndike, 1990]. Both questionnaires have reported excellent psychometric properties and the ability to discriminate between phobics and non‐phobics. Participants scoring above the highest quartile on one of the questionnaires and below the lowest quartile on the other were provisionally pre‐selected as SP or BII phobics. Participants scoring below the lowest quartile on both questionnaires were pre‐selected as controls. Candidates were then interviewed over the phone and administered a brief screening questionnaire based on DSM‐IV‐TR criteria for specific phobia, and the Mini International Neuropsychiatric Interview [MINI, Sheehan et al., 1998]. Candidates were invited to participate if they met criteria for specific phobia (except controls) and did not meet criteria for depression, bipolar disorder, panic disorder, substance abuse or dependence and psychosis, were right handed, and suitable for a Magnetic Resonance Imaging ((MRI) scan. None of the participants included in the present study qualified for any other DSM‐IV psychiatric diagnosis than specific phobia (none in the case of controls). To ensure sufficient severity in the BII group, we included the additional criterion of history of fainting in situations involving medical interventions or the sight of blood.
A total of 52 participants were scanned—34 phobics and 18 controls. From that total, 6 participants had to be excluded from the symptom provocation paradigm due to problems with data acquisition or excessive head movement, resulting in 46 participants—29 phobics (14 SP and 15 BII) and 17 controls—with data from both paradigms (i.e., symptom provocation and interoceptive).
Experimental Procedure
The symptom provocation task and the interoceptive paradigm were presented in counterbalanced order across participants. The former consisted of a 7‐min event‐related task involving the presentation of single visual stimuli—90 neutral and 15 phobia‐related images. Each image was presented for 4 sec and phobic‐related images were always separated by a gap of 20 sec (i.e., five neutral images). More details about this task can be found in the work by Caseras et al. [ 2010b].
The interoceptive paradigm consisted of two conditions (i.e., exteroceptive and interoceptive) that alternated five times. For each condition, participants were instructed to attend to an exteroceptive (i.e., sound) or an interoceptive (i.e., heartbeats) stimulus depending on a visual cue presented on the screen (colored circle). A green circle indicated “attend to the sound,” and a red circle “attend to your heartbeat.” Participants were asked to concentrate as much as possible on the targeted stimulus so that when the green or red circle changed color into gray they could count as many sounds or heartbeats as possible. Participants responded during the gray circles by pressing a key with their right index finger every time that a sound or a heartbeat was perceived. The attending phase (i.e., green or red circle) always lasted for 20 sec and the counting phase (i.e., gray circle) for 10 sec, although participants were unaware of these timings. At the beginning and the end of the task a 30‐sec fixation cross block was presented as a low level baseline. Participants were trained in the task prior to the scan. Once in the scanner, the exteroceptive cue (i.e., sound) was played at a volume that made it difficult to be heard over the noise of the scanner. This volume was set in an individual basis by asking the participants to report when the sound could be just heard over the noise of the scanner while the operator was progressively increasing the volume. The noise was presented through headphones all along the task and the visual cues through goggles.
Heartbeats were monitored using an MRI‐compatible pulse oximeter (Model 4500MRI, Invivo corp. Orlando, FL) with the fiber optic SpO2 probe placed on the participants' left index finger; and were recorded using a personal computer running a data logger developed in‐house with Labview 8.0 software (National instruments corp. Austin, TX).
Before the scanning session, all participants completed the Spielberger Trait and State Anxiety Inventory [STAI; Spielberger et al., 1983] and the Anxiety Sensitivity Index [ASI, Peterson and Reiss, 1992]. After the scanning, participants completed a short questionnaire about the perceived difficulty counting the sounds and their heartbeats and the aversive value of the stimuli presented during the symptom provocation paradigm.
Written consent to participate was obtained from all the participants and the study protocol was reviewed and approved by the Ethics Committee at the Autonomous University of Barcelona.
Image Acquisition
Gradient‐echo echo planar images were acquired on a 1.5‐T MRI GE scanner. For each volume obtained (210 for the symptom provocation paradigm, 180 for the interoceptive paradigm) 22 non‐contiguous axial planes parallel to the intercommisural plane were collected with the following parameters: repetition time (TR) 2,000 msec, echo time (TE) 50 msec, slice thickness 4 mm, 1.5mm gap, a 24‐cm field of view (FOV) and an image acquisition matrix 64 × 64. Four dummy acquisitions were also made at the beginning of each run to set longitudinal magnetization into steady state.
To facilitate coregistration of the functional MRI (fMRI) data into standard space, a fast SPGR‐Inversion Recovery image was also obtained from each participant using the following parameters: TR 11.85 msec, TE 4.2 msec, inversion time 400 msec, number of averages 1, 130 slices of 1.2 mm thickness (no gap), flip angle 15°, acquisition matrix 256 × 256.
Statistical Analyses
Demographic data and behavioral response
Demographic and behavioral data were compared across the three groups using Chi‐square or ANOVA tests followed by pair‐wise comparisons, as required.
Behavioral responses during the interoceptive paradigm were lost for 9 participants; consequently, analyses on accuracy only included 16 control, 14 SP, and 13 BII participants. A correct response was awarded every time that a key press followed between 100 and 800 msec after a sound or a heartbeat; responses falling out this time range were considered non‐valid. To control for non‐specific performance effects during scanning an interoceptive accuracy index was calculated as the percentage change relative to performance in the exteroceptive condition. Three participants showed outlier scores (i.e., values 3 standard deviations from the mean) in this index and were excluded from all analyses that included this behavioral measure. This accuracy index was compared across the three groups using ANOVA.
fMRI data
fMRI data processing was carried out using FSL (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl). The following pre‐processing steps were applied to both paradigms: motion correction using MCFLIRT [Jenkinson et al., 2002]; non‐brain removal using BET [Smith, 2002]; spatial smoothing using a Gaussian kernel of full width at half maximum 5 mm; grand‐mean intensity normalization of the entire 4D dataset; highpass temporal filtering; and registration to individual T1 anatomical images.
For the symptom provocation paradigm, in the general lineal model (GLM) model an event was specified every time a phobia‐related image was presented, convolved with a gamma variate and the temporal derivative was included. The resulting functional images were converted to standard Montreal Neurological Institute (MNI) space using FLIRT. Higher‐level analysis was carried out using FLAME (FMRIB's Local Analysis of Mixed Effects) [Beckmann et al., 2003; Woolrich et al., 2004]. A more detailed explanation of the analysis of this paradigm can be found in the work by Caseras et al. [ 2010b]. Regarding the interoceptive paradigm, in the GLM model, a separate event was specified for each of the attending phases (i.e., to the exteroceptive or interoceptive target) and each of the counting periods (i.e., count of sounds or heartbeats), and convolved with a gamma variate. The temporal derivatives were also included. The fixation cross periods at the beginning and end of the paradigm were used as a baseline and the contrast exteroceptive > interoceptive and interoceptive > exteroceptive computed for each participant. The resulting functional images were converted to standard MNI space using FLIRT. Higher‐level analysis was carried out using FLAME (FMRIB's Local Analysis of Mixed Effects) [Beckmann et al., 2003; Woolrich et al., 2004], which included a three level between‐participants factor (i.e., healthy controls, SP, and BII). Z‐statistic images were thresholded using clusters determined by Z > 2 and P = 0.05 that corrected for multiple comparisons cluster significance [Worsley, 2001].
Regions of interest (ROI) were drawn in the insula and ACC by projecting the activation maps resulting from the contrast interoception > exteroception using the whole sample onto the anatomical maps for these ROIs taken from the Harvard‐Oxford cortical and subcortical structural atlases. Each resulting ROI mask represented the sections within the right or left insula or the anterior cingulate that showed enhanced activity during heartbeat detection.
To perform a conjunction analysis, the previous ROI masks were projected onto the masks for the same brain regions obtained using a similar procedure on the symptom provocation activation maps. The final ROI included the overlapping areas of the right insula and the ACC activated during both paradigms. BOLD signal change from each task was then extracted using these ROIs for the 46 participants that completed both tasks, and the correlation was calculated by means of Pearson's r.
RESULTS
Demographic, Psychological, and Behavioral Measures
There were no group differences regarding gender distribution or age. In all the anxiety measures obtained, controls showed lower scores than BII participants; with SP phobics lying midway between the other two groups (see Table I).
Table I.
Mean and standard deviations (sd) for demographics, psychological measures, and accuracy indexes across groups
| Controls (n = 18), Mean (sd) | SP (n = 16), Mean (sd) | BII (n = 18), Mean (sd) | |
|---|---|---|---|
| Gender (% male) | 11.1% | 12.5% | 11.1% |
| Age | 21.72 (2.80) | 21.62 (2.57) | 22.78 (2.62) |
| STAI‐S | 9.88 (6.61)a | 14.12 (8.53) | 15.38 (5.50) |
| STAI‐T | 12.88 (9.02)a | 17.56 (7.80) | 22.61 (8.72) |
| ASI | 6.27 (3.33)a, b | 14.43 (8.07)c | 19.38 (8.19) |
| Accuracyd | (n = 16) | (n = 14) | (n = 13) |
| Correct Intero. | 26.89 (14.82) | 34.85 (18.36) | 38.05 (27.41) |
| Correct Extero. | 78.85 (14.15) | 62.85 (23.93) | 65.25 (25.93) |
| IAe | −64.90 (24.11)b | −41.38 (26.08) | −51.21 (30.95) |
STAI‐S: State scale of the Spielberger State and Trait Anxiety Index.
STAI‐T: Trait scale of the Spielberger State and Trait Anxiety Index.
ASI: Anxiety Sensitivity Index.
Control ≠ BII.
Control ≠ SP.
SP ≠ BII.
Percentage of stimuli correctly identified (by pressing the key) during the response‐required phase of each condition.
(Correct Intero − Correct Extero)/Correct Extero; after outliers exclusion (SP n = 13, BII n = 11).
In general, participants made more responses during the exteroceptive condition than during interoception, t(42) = 6.648, P < 0.001; suggesting that they were more successful at perceiving the sound than their own heartbeats. In fact, participants correctly detected on average 69.5% of the sounds presented versus only 32.8% of heartbeats. When comparing across groups, no significant differences were found in performance (i.e., number of correctly detected trials) during either the interoceptive or exteroceptive conditions (Table I). Nevertheless, the ANOVA for the interoceptive accuracy index showed a tendency toward significance, F(2, 39) = 2.83, P = 0.07. Pair‐wise comparisons revealed a significant better performance for SP patients compared with controls (P < 0.05) (Table I).
Regarding their heart rate responses, during the symptom provocation paradigm there were no overall differences in heart rate between phobic groups in response to phobic stimuli, while both groups showed a rise in heart rate compared to controls, the later showing a decrease after phobic images. However, SP phobic participants showed increased HR acceleration relative to BII phobics between 2 and 4 sec after the presentation of phobic images [see Caseras et al., 2010b for a more comprehensive description). In the interoceptive paradigm during the heartbeat detection phase, there was a trend toward significance (F(2,42) = 2.87, P = 0.068) for controls to show a slower heart rate than the two patient groups, who were not different from each other (mean [sd] heart rate for controls, SP, and BII, respectively: 57.13 [9.08], 66.46 [11.81], and 65.53 [13.89]); considering the whole of the interoceptive task, there were no group differences in HR.
fMRI Results
Paradigms' validity
The symptom provocation paradigm activated the expected brain network, including the insula and ACC bilaterally. Both phobic groups showed increased activity in these brain areas compared to controls; but were not different from each other [a thorough analysis of this paradigm has been published elsewhere—Caseras et al., 2010b].
With regard to the non‐emotional interoceptive paradigm and considering all the participants in the study, the contrast interoceptive > exteroceptive yielded significant activity within the left and right insula, the dorsal anterior cingulate gyrus (BA 32), the supplementary motor area (BA 6), the thalamus, the right superior temporal lobe (BA 22), and the right somatosensory cortex (BA 3) (Fig. 1 and Table II). The opposite contrast yielded significant activation in the bilateral orbitofrontal and ventromedial prefrontal cortex (BA 10, 11) extending into the amygdala, the rostral parts of the ACC (BA 32), caudate and putamen, the temporal lobe (BA 21, 22), left postcentral and precentral gyry (BA 1, 2, 3, 4, 43), and occipitoparietal cortex (BA 7, 18, 19) extending into the posterior cingulate (BA 30) (Fig. 1 and Table II).
Figure 1.
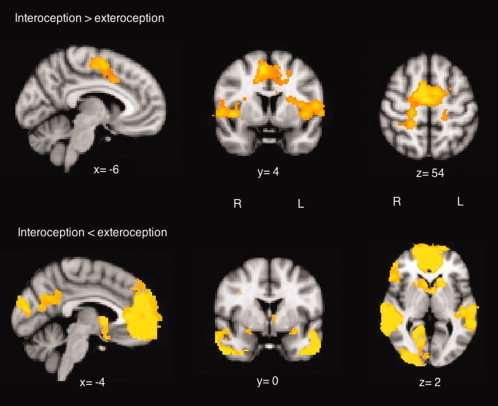
Significant BOLD responses (cluster P < 0.05, corrected) from the contrast interoceptive > exteroceptive (top row) and interoceptive < exteroceptive (bottom row) for all participants in the study (n = 52). This image is shown in radiological convention (i.e., right cerebrum in the left side of the image). The functional data are superimposed on a high‐resolution anatomical MNI template using fslview (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Local maximas obtained when contrasting the interoceptive and exteroceptive conditions for the whole sample (n = 52)
| Location | Side | Coordinates | Z value |
|---|---|---|---|
| Enhanced activity during heartbeat detection (interoceptive > exteroceptive) | |||
| Medial prefrontal cortex | R | 14, −8, 58 | 4.67 |
| L | −22, −8, 44 | 4 | |
| Insula | R | 42, 8, 0 | 4.38 |
| Precentral gyrus | R | 18, −16, 60 | 4.13 |
| Postcetral gyrus | R | 22, −32, 62 | 4.06 |
| Superior temporal gyrus | R | 52, 0, 2 | 3.92 |
| Thalamus | R | 20, −22, 16 | 3.52 |
| Reduced activity during heartbeat detection (interoceptive < exteroceptive) | |||
| Poscentral gyrus | L | −42, −30, 60 | 5.85 |
| Medial frontal gyrus | R | 10, 40, −8 | 5.62 |
| Ventromedial prefrontal cortex | L | −18, 44, −10 | 5.14 |
| Rostral anterior cingulate | L | −12, 46, −8 | 5.07 |
| Middle temporal gyrus | R | 60, −24, −4 | 5.01 |
| Superior frontal gyrus | R | 10, 56, −6 | 4.96 |
| Superior temporal gyrus | R | 48, −26, −8 | 4.64 |
| Precentral gyrus | L | −58, −18, 44 | 4.19 |
Enhanced activity during heartbeat detection (i.e., interoception > exteroception) in the right insula correlated with interoceptive accuracy, r = 0.31, P < 0.05. The activity in the left insula correlated with anxiety trait and state (STAI‐state r = 0.28, P < 0.05; STAI‐trait r = 0.26, P = 0.07). No other significant correlations between the activity in the insula or the ACC and psychological/behavioral measures were found.
Examining group differences in enhanced activity during heartbeat detection within the pre‐defined ROIs (i.e., insula and ACC) by means of an ANOVA including the three groups, the only significant effect (P < 0.05 corrected for multiple comparisons) resulted in controls showing a greater activity than SP participants in the right insula (Fig. 2).
Figure 2.

The figure in the left depicts the significant difference (corrected cluster P value < 0.05) in activity between controls and SP participants within the right insula for the contrast interoceptive > exteroceptive. The boxplot on the right presents with the mean %BOLD signal change within this cluster for the three groups. This image is shown in radiological convention (i.e., right cerebrum in the left side of the image). The functional data are superimposed on a high‐resolution anatomical MNI template using fslview (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Conjunction analysis
As predicted, BOLD responses during interoception and symptom provocation showed a clear anatomical overlap within the anterior insula and the dorsal ACC (Fig. 3). Moreover, the %BOLD signal change within the right insula during each of the paradigms used here showed a negative correlation that approached significance, r = −0.489, P = 0.09 for SP participants. However, this correlation was clearly non‐significant for BII phobics, r = 0.212, P > 0.1 (Fig. 3a). The same effect was shown in the ACC, with BOLD signal change correlating r = −0.499, P = 0.083 in SP participants, but r = −0.024, P > 0.1 in BII phobics (Fig. 3b). The correlation within the left insula was non‐significant for either SP or BII participants, r = 0.020 and r = 0.128, respectively, both P > 0.1. However, the comparison between SP and BII participants' correlation coefficients only resulted in a tendency towards a significant difference for the right insula (Fisher's Z = 1.79, P = 0.07).
Figure 3.

Anatomical overlap between the activity in the right insula and the ACC during the symptom provocation paradigm and the interoceptive paradigms. The functional data are superimposed on a high‐resolution anatomical MNI template using fslview (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl). The scatter plots reflect the correlation between the %BOLD signal change during both paradigms within each ROI. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
The aim of the present study was to investigate the extent to which the activity in the anterior insula and dorsal ACC during interoception overlaps with the activity in the same brain regions during phobic symptom provocation, and to compare these across specific phobia subtypes (i.e., animal and BII phobics).
Both behavioral paradigms—the symptom provocation and the non‐emotional interoceptive paradigm—produced the expected activity within the brain, including significant activity within the ROI for this study, that is, the anterior insula and the ACC. In the symptom provocation task, phobic patients showed significant increases in activity within the insula and ACC compared to healthy controls. Moreover, fear induced changes in HR correlated with BOLD signal change in the right and left anterior insula in SP participants and with BOLD in right and left ACC in BII (these results alongside a thorough analysis of this task have been published elsewhere, see Caseras el al. 2010b]. Regarding the interoceptive paradigm reported here, this task appeared to be rather difficult, with participants able to correctly identify only approximately one‐third of the heartbeats recorded. However, it was effective in activating the brain network previously associated with interoception [Critchley, 2004; Critchley et al., 2004; Pollatos et al., 2007]. The paradigm produced enhanced activity during heartbeat detection in bilateral insula, the ACC, right somatosensory cortex, and supplementary motor area. It should be noted that for the insula, the peak of activation reported here laid slightly posterior to the peaks previously published by Critchley and Critchley et al. [ 2004] and Pollatos et al. [ 2005, 2007]. However, our activity peak can still be considered anatomically in the anterior insula (y = 8), at about only 6 mm away from Critchley and Pollato's reported peaks (y = 14). Moreover, our activity cluster extended slightly anterior from the peak. Therefore, we are confident that we are reporting activity in a functionally equivalent brain area to those previously reported by other authors. The insula, ACC, right somatosensory cortex, and the supplementary motor area showed positive significant BOLD responses during both conditions—interoceptive and exteroceptive—but resulted significantly greater during the heartbeat detection (i.e., interoceptive condition). Importantly, the activity within the right anterior insula correlated positively with the interoceptive accuracy index, validating the role of this brain structure in the awareness of interoceptive information. The BOLD response in the left insula, though, only correlated with the anxiety scores (STAI trait and state), suggests that the left insula might be more associated with a pure emotional response rather than the integration of emotional value of stimuli and interoceptive perception that lead to emotional feelings.
The contrast exteroceptive > interoceptive resulted in a more complex picture than the former. In fact, examining the activity maps for each condition separately, it was clear that the significant BOLD responses within the prefrontal cortex (orbital and medial parts), the posterior cingulated, and the inferior parietal/temporal cortices resulted from a greater deactivation during the interoceptive than the exteroceptive condition. Interestingly, these brain structures correspond largely to the default network [Damoiseaux et al., 2006; Raiche et al., 2001]. The default network has been shown to consistently deactivate when subjects engage in cognitively demanding tasks compared with baseline states [Harrison et al., 2008] and therefore our results might be simply reflecting an increased cognitive demand in performing the interoceptive relative to the exteroceptive condition. As for the increased activity in bilateral auditory cortices and thalamus extending into the striatum, the likely explanation is the attention to an external auditory cue during the exteroceptive condition. More difficult to explain is the increased activity in the amygdala/hippocampus complex since this paradigm did not include any emotional factor. However, the activity in the amygdala has also been associated with non‐emotional cognitive processing, such as working memory [Schaefer et al., 2006], and it could well be the case that the increased counting of/responding to the external stimuli in our paradigm explains the increased BOLD response in this brain structure. Regardless, the focus of this report was the interoceptive > exteroceptive contrast and we will only refer to this aspect further here.
When comparing the enhanced brain activity within the anterior insula and ACC during interoception across experimental groups, control participants showed a significantly greater BOLD response in the right anterior insula compared to SP participants. Interestingly, when comparing performance using the interoceptive accuracy index across groups, control participants showed a significantly lower accuracy than SP participants. This might be suggesting a lower efficiency of the interoceptive network (more specifically in the right anterior insula) in control participants, who required greater activity to perform the task whilst still performing at a lower level than SP participants. Previous research has suggested greater interoceptive awareness in high anxious compared to low anxious participants [Dunn et al., 2010; Zoellner and Craske, 1999] and the results reported here would support this hypothesis regarding SP participants. In the case of BII participants, their results lay in between the other two groups both in accuracy and BOLD responses within the right anterior insula, not being significantly different to either of them. More research using larger samples and a potentially less difficult interoceptive task could shed light on the potential differences between BII phobia and controls and/or other subtypes of specific phobia such as SP. Moreover, it would also allow a better control for experimental confounders such as the fact that the interoceptive condition demands more cognitive resources than the exteroceptive due to increased difficulty.
Regarding the main aim of our study, there was a clear anatomical overlap between the BOLD responses in the anterior insula and in the dorsal ACC obtained during the phobic symptom provocation and the non‐emotional interoceptive paradigms in phobic participants. This result suggests, as previously proposed by others [Craig, 2004; Critchley, 2004; Critchley et al., 2004; Pollatos et al., 2007], that these two brain areas are involved in both the perception of emotional stimuli and the perception of bodily responses dissociated from any emotional content. In fact, Harrison et al. [ 2010] have recently shown an overlap in the activity of the anterior insula to the perception of disgusting scenes associated with scenes of surgical procedures and the personal experience of disgust measured through gastric and cardiac responses. Following with this argument, in our case the %BOLD signal change within the right anterior insula and the dorsal ACC during heartbeat detection and symptom provocation was negatively correlated in SP participants. This can be interpreted as the more severe the phobia and, therefore, the more intense the brain response to fearful stimuli, the more efficient the interoception brain network, with more fearful participants requiring less activity within this network to perform at a similar behavioral level. In fact, when dividing SP participants according to a median‐split of the activity in the right insula or ACC during symptom provocation, no significant differences were detected in their performance during the interoceptive task, with the interoceptive index showing very similar values for both groups. This result again validates the hypothesis that the right anterior insula and the dorsal ACC are associated with the integration of emotional and physiological perceptions to generate emotional feelings. As the James–Lange theory proposed more than a century ago, emotional feeling will originate in the perception of bodily changes induced by the encounter with external stimuli [James, 1890], according to our results and others', these processes will take place within the right anterior insula and ACC.
Interestingly, the relationship between the elicited activity within the insula and ACC during each experimental paradigm does not seem to apply to BII participants in our study. For this subtype of specific phobia, an anatomical overlap between the BOLD responses during each task was found, but the intensity of the BOLD response to each condition appears unrelated. It should be noted though, that the between‐groups comparison of these correlation coefficients did not show significant differences, and only a trend to significance was observed for the right insula. In any case, these results clearly suggest that in the case of BII patients it is more difficult to conclude that these brain areas are playing a similar role when activated in response to phobic stimuli or to an interoceptive paradigm, and it might simply indicate that these brain structures are involved in the perception of fear and the perception of body responses, but being part of different brain networks that operate separately. It can also be the case that this lack of relationship is idiosyncratic for BII phobia and somehow related to the particular physiological response to phobic stimuli in this group; this is, the vasovagal syncope that causes the heart rate and the blood pressure to drop rather than keep raising as is the case for all other subtypes of specific phobia [Thyer et al., 1985]. Unfortunately, we lack a golden standard to conclude that this pattern from BII participants deviates from normality, since our controls did not respond intensely to either type of phobic stimuli (they were selected for showing a low level of fear to either blood‐injection‐injuries or spiders) and therefore we cannot perform the same analysis in this group. Furthermore, differences between phobic groups cannot be interpreted as an artifact driven by differences in cardiac responses that could have affected BOLD; our results show no differences in the pattern of heart rate responses between SP and BII participants on either paradigm. We could have expected our BII participants to show the characteristic vasovagal syncope (drop in heart rate) in response to BII images, but due to the nature of our paradigm in which phobic stimuli were presented briefly (4 sec), only the initial component of this syncope (heart rate acceleration) was visible. In this respect, our BII phobics did not show different heart rate patterns than the SP phobics included here or any other prototypical phobic patient presented with phobia‐related stimulation. Further research using a less fearless sample of controls or more intensely salient negative stimuli should shed light into this issue, allowing to resolve whether this lack of association between the activity in these two brain structures during the perception of fear and of ones own heartbeat in BII is idiosyncratic of this subtype of specific phobia or common in the general population; making the association found in SP participants distinctive.
Study Limitation
There are several limitations from the present report that need to be acknowledged. Firstly, despite thoroughly training our participants on heart beat detection prior to scanning, this condition was rather difficult to perform. It produced less responses (i.e., detections) than the exteroceptive condition (sound detection), meaning that both conditions were not equal in difficulty. The most obvious problem caused by this is that some of the brain activity that we attribute to heart detection from the interoceptive > exteroceptive contrast, could be better explained simply by an increased cognitive demand (load effect). However, the fact that previous research using other interoceptive paradigms [Critchley et al., 2004; Pollatos et al., 2007] has reported activity in the same brain structures validates the use of the present paradigm. Secondly, another important caveat to note is the small sample size. The fact that we wanted to explore the potential differences between SP and BII and that both groups showed critical differences did not allow us to combine both groups into a “Phobic vs Healthy participants” contrast thus boosting statistical power. As a consequence of this, some of the effects reported here are marginally significant and therefore require replication. Nevertheless, this report represents a novel approach to the role of the anterior insula and ACC in integrating perceived emotional value of stimuli and somatic information, this added to pre‐existing literature increase our knowledge on the involvement of these brain structures in the production of emotional feelings.
CONCLUSIONS
Summarizing, the results from the present study support the previously described involvement of the right anterior insula and dorsal ACC during interoception and fear perception. We also showed that the activity in the anterior insula and the dorsal ACC clearly overlaps in terms of anatomy when participants are engaged in a symptom provocation paradigm and in a non‐emotional interoceptive paradigm. Moreover, the activity in the right anterior insula and dorsal ACC during both paradigms appeared to be correlated, indicating also an overlap in the function of these two brain structures when reacting to the perception of external phobic‐related stimuli and to the perception of ones own heartbeat. Interestingly, there seems to be a lateralization effect in the anterior insula, with the right anterior insula involved in both emotional value and interoceptive perception and the left insula only reacting to the emotional value of the external stimuli. However, this result was only detected in SP phobic participants, but not in the BII phobia group, although the correlation coefficients were not statistically different between groups, suggesting that the function of these brain structures might be at the core of the distinction between subtypes of specific phobia.
REFERENCES
- Beckmann C, Jenkinson M, Smith SM ( 2003): General multi‐level linear modelling for group analysis in fMRI. NeuroImage 20: 1052–1063. [DOI] [PubMed] [Google Scholar]
- Caseras X, Giampietro V, Lamas A, Brammer M, Vilarroya O, Carmona S, Rovira M, Torrubia R, Mataix‐Cols D ( 2010a): The functional neuroanatomy of blood‐injection‐injury phobia: A comparison with spider phobics and healthy controls. Psychol Med 40: 125–134. [DOI] [PubMed] [Google Scholar]
- Caseras X, Mataix‐Cols R, Trasovares MV, Lopez‐Sola M, Ortiz H, Pujol J, Soriano‐Mas C, Giampetro V, Brammer MJ, Torrubia R ( 2010b): Dynamics of brain responses to phobic‐related stimulation in specific phobia subtypes. Eur J Neurosci, 32: 1414–1422. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 10: 59–70. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2004): Human feelings: Why are some more aware than others? Trends Cogn Sci 8: 239–241. [DOI] [PubMed] [Google Scholar]
- Critchley HD ( 2004): The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 101: 6333–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ ( 2003): Human cingulated cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 126: 2139–2152. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ ( 2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF ( 2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger S, Straube T, Mentzel HJ, Fitzek C, Reichenbach JR, Hecht H, Krieschel S, Gutberlet I, Miltner WHR ( 2003): Brain activation to phobia‐related pictures in spider phobic humans: An event‐related functional magnetic resonance imaging study. Neurosci Lett 348: 29–32. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Stefanovitch I, Evans D, Oliver C, Hawkins A, Dalgleish T ( 2010). Can you feel the beat? Interoceptive awareness is an interactive function of anxiety‐ and depression‐specific dimensions. Behav Res Ther 48: 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD ( 2007): Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Cuthbert BN, Goblisch J, Vaitl D ( 1997): Fear and the startle reflex: Blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology 34: 97–107. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez‐Sola M, Hernandez‐Ribas R, Deus J, Ortiz H, Soriano‐Mas C, Yucel M, Pantelis Ch, Cardoner N ( 2008): Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci 105: 9781–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Gianaros PJ, Critchley HD ( 2010): The embodiment of emotional feelings in the brain. J Neurosci 22: 12878–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A ( 2007): Diminished medial prefrontal cortex activity in blood‐injection‐injury phobia. Biol Psychol 75: 124–130. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S ( 2002): Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Kleinknecht RA, Thorndike RM ( 1990): The Mutilation Questionnaire as a predictor of blood/injury fear and fainting. Behav Res Ther 28: 429–437. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB ( 2010): Interoception in anxiety and depression. Brain Struct Funct 214: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RA, Reiss S ( 1992): Anxiety Sensitivity Index Manual, 2nd ed Worthington, OH: International Diagnostic Systems. [Google Scholar]
- Pollatos O, Kirsch W, Schandry R ( 2005): Brain structures involved in interoceptive awareness and cardioafferent signal processing: A dipole source localization study. Hum Brain Mapp 26: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Dorothee PA, Kaufmann Ch ( 2007): Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res 1141: 178–187. [DOI] [PubMed] [Google Scholar]
- Raiche ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Braver TS, Reynolds JR, Burgess GC, Yarkoni T, Gray JR ( 2006): Individual differences in amygdala activity predict response speed during working memory. J Neurosci 26: 10120–10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Stark R, Walter B, Kirsch P, Vailt D ( 2003): Disgust processing in phobia of blood‐injection‐injury. An fMRI study. J Psychophysiol 17: 87–93. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC ( 1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59 ( Suppl 20): 22–33. [PubMed] [Google Scholar]
- Smith S ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. 1983. Manual for the State‐Trait Anxiety Inventory (Form Y self‐evaluation questionnaire). Palo Alto: Consulting Psychologist Press. [Google Scholar]
- Straube T, Mentzel HJ, Miltner WHR ( 2006a): Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry 59: 162–170. [DOI] [PubMed] [Google Scholar]
- Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WHR ( 2006b): Effects of cognitive‐behavioral therapy on brain activation in specific phobia. Neuroimage 29: 125–135. [DOI] [PubMed] [Google Scholar]
- Szymanski J, O'Donohue W ( 1995): Fear of Spiders Questionnaire. J Behav Ther Exp Psychiatry 26: 31–34. [DOI] [PubMed] [Google Scholar]
- Thyer BA, Himle J, Curtis GC ( 1985): Blood‐injury‐illness phobia: A review. J Clin Psychol 41: 451–459. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM ( 2004): Multi‐level linear modelling for FMRI group analysis using Bayesian inference. NeuroImage 21: 1732–1747. [DOI] [PubMed] [Google Scholar]
- Worsley KJ ( 2001): Statistical analysis of activation images In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. New York: Oxford University Press; pp 251–271. [Google Scholar]
- Zoellner LA, Craske MG ( 1999): Interoceptive accuracy and panic. Behav Res Ther 37: 1141–58. [DOI] [PubMed] [Google Scholar]


