Abstract
Objectives: Recent anatomical–functional studies have transformed our understanding of cerebral motor control away from a hierarchical structure and toward parallel and interconnected specialized circuits. Subcortical electrical stimulation during awake surgery provides a unique opportunity to identify white matter tracts involved in motor control. For the first time, this study reports the findings on motor modulatory responses evoked by subcortical stimulation and investigates the cortico‐subcortical connectivity of cerebral motor control. Experimental design: Twenty‐one selected patients were operated while awake for frontal, insular, and parietal diffuse low‐grade gliomas. Subcortical electrostimulation mapping was used to search for interference with voluntary movements. The corresponding stimulation sites were localized on brain schemas using the anterior and posterior commissures method. Principal observations: Subcortical negative motor responses were evoked in 20/21 patients, whereas acceleration of voluntary movements and positive motor responses were observed in three and five patients, respectively. The majority of the stimulation sites were detected rostral of the corticospinal tract near the vertical anterior‐commissural line, and additional sites were seen in the frontal and parietal white matter. Conclusions: The diverse interferences with motor function resulting in inhibition and acceleration imply a modulatory influence of the detected fiber network. The subcortical stimulation sites were distributed veil‐like, anterior to the primary motor fibers, suggesting descending pathways originating from premotor areas known for negative motor response characteristics. Further stimulation sites in the parietal white matter as well as in the anterior arm of the internal capsule indicate a large‐scale fronto‐parietal motor control network. Hum Brain Mapp 34:3023–3030, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: awake surgery, motor mapping, motor control, negative motor network, subcortical electrostimulation
INTRODUCTION
Our understanding of cerebral motor control has changed over the last two decades. Brodmann differentiated area 4 (primary motor cortex) and area 6 [Brodmann, 1909] which later became known as the supplementary motor cortex [Penfield and Jasper, 1954]. Rizzolatti and his team split the supplementary motor area (SMA) into a SMA proper and a pre‐SMA according to differing function and cytoarchitecture [Matelli et al., 1991; Rizzolatti et al., 1996]. Furthermore, the premotor area was recognized as an additional secondary area of motor control [Fulton, 1935] involved in reaching movements [Geyer et al., 2000; Rizzolatti et al., 1988]. This knowledge led to a concept of hierarchical motor control, in which cortical motor areas possessed clearly defined functions while white matter received little attention. It was generally believed that cognitive, visual, and somatosensory inputs were integrated in the SMA. Initiation for movements would then be sent from the SMA and the premotor area to the primary motor area, which in turn would send movement commands to the spinal motoneurons [Campbell, 1905; Fulton, 1949; Rao et al., 1993; Wiesendanger, 1981; Wise, 1985]. This hierarchical concept, consisting of SMAs and premotor areas providing complex movement design to the primary motor cortex, and a primary motor cortex having a monopoly on motor control over the spinal cord, has recently been challenged by findings in the macaque monkey and in humans [Nachev et al., 2008; Rizzolatti et al., 1998].
Tracer injection into the cervical spinal cord of macaque monkeys showed that the SMA and the premotor area have projections to the spinal cord [Dum and Strick, 1991]. In fact, secondary motor areas of the frontal brain seem to contain nearly the same amount of corticospinal neurons as the primary motor cortex [Dum and Strick, 1991]. The secondary motor areas also appear to exert motor control directly through projections on spinal cord motoneurons in addition to their well‐known cortical influence on the primary motor cortex [Wise, 1996]. Projections from primary and secondary motor areas are markedly different in the monkey: stimulation on the primary motor cortex evoked larger and more frequent excitatory responses than stimulation in the SMA (88 versus 48%, respectively) [Maier et al., 2002].
Electrical stimulation of the human cortex performed either to localize an epileptic focus or for functional mapping during surgery has provided us with valuable information on cortical motor control [Duffau, 2001; Ikeda et al., 2009]. A positive motor response may be readily evoked through electrostimulation of the primary motor cortex [Penfield and Bouldrey, 1937]. The secondary motor areas such as the SMA and the premotor area, however, remain unresponsive after standard stimulation and lead to the typical complex movements of the extremities only with a longer duration of stimulation [Graziano et al., 2002].
Penfield and Jasper were the first in 1954 to observe cessation of voluntary muscle contraction without alteration of consciousness when selected areas rostral of the primary motor cortex were electrically stimulated [Penfield and Jasper, 1954]. Lüders et al. confirmed these findings, localized the areas within the posterior part of the inferior frontal gyrus and the preSMA and termed the identified locations “negative motor areas” (NMA) [Lüders et al., 1987; Lüders et al., 1995].
Additional NAMs were detected anterior to the primary motor cortex [Mikuni et al., 2006], corresponding to the premotor area and the caudal SMA. Electrical stimulation in this area during awake surgery elicited cessation of movements of the upper extremities with additional cessation of movements of the leg and the tongue in areas rostral to the reciprocal representation areas within the precentral gyrus. Furthermore, a negative motor response (NMR) was detected in the superior parietal lobule after electrical stimulation [Mikuni et al., 2006], implying that cortical NMAs are not restricted to the frontal lobe. However, the subcortical connectivity of these areas remains unknown.
In awake surgeries for diffuse low‐grade gliomas (LGG), we use both cortical and subcortical electrostimulation mapping to identify and preserve essential pathways [Duffau et al., 2002]. Subcortical stimulation provides an equally unique possibility to investigate axonal connectivity [de Benedictis and Duffau, 2011]. To better understand the negative motor phenomenon, we moved on from superficial cortical areas to investigate the underlying white matter connections and extend our knowledge on the hodology of motor control. We report here on a series of patients in whom surgery was performed for frontal, insular, and parietal LGG. In addition, subcortical stimulation sites that led to cessation or acceleration of movement, and the characteristics of the evoked (negative) motor responses were documented. We also identify the origin and course of the newly detected fiber tracts. To our knowledge, this is the first description of subcortical NMRs.
PATIENTS AND METHODS
Patient Population
Twenty‐one patients with diffuse LGGs in the frontal, insular, or parietal lobe were selected from those admitted to the Department of Neurosurgery, Gui de Chauliac Hospital, Montpellier, France, from 2008 to 2011. Informed consent was obtained from all patients prior to surgery. Intraoperative subcortical electrostimulation mapping and motor monitoring through continuous movements of the extremities were performed in the 21 selected patients, who underwent awake surgery.
All patients received a neurological examination prior to surgery. Patients were also evaluated using Karnofsky Performance Status. Language was assessed by a speech therapist using the Boston Diagnostic Aphasia Examination.
Intraoperative Electrostimulation Mapping
All patients underwent surgery under local anesthesia so that functional cortical and subcortical mapping could be carried out using direct brain stimulation. This method, including the electrical parameter, was previously described by the authors [Duffau et al., 2002; Duffau et al., 2005]. A bipolar electrode with 5‐mm spaced tips delivering a biphasic current (pulse frequency of 60 Hz, single‐pulse phase duration of 1 ms, amplitude from 1 to 4 mA—Nimbus*, Hemodia) was applied to the brain of the awake patients. The current intensity used for the individual patients was determined by progressively increasing the amplitude in 1‐mA increments (from a baseline of 1 mA) until a functional response was elicited. Cortical electrical stimulation was conducted for the identification of eloquent cortical areas in every case (bipolar electrode, 5‐mm spaced tips, 60 Hz, single‐pulse phase duration of 1 ms, 1.5–2 mA). In the first stage, tumor boundaries were also obtained using ultrasonography. Then, before starting with the resection, cortical mapping was performed. In all cases, sensory‐motor mapping was conducted first (to elicit transient movement and/or paresthesia). All positive stimulation sites were marked with a tag number. This enabled to define the optimal threshold of stimulation. However, our cortical mapping protocol did not include continuous movements of the extremities, and therefore NMAs were not identified.
During a second surgical stage, the tumor was removed by alternating resection and subcortical stimulation, with the same electrical parameters than at the cortical level, without notification of the patient [Duffau et al., 2005]. Language was analyzed by a speech therapist present during the awake phase [Duffau et al., 2002]. Furthermore, our protocol of functional monitoring during tumor resection required patients to perform continuous movements of the contralateral upper extremity: this test consisted of repetitive and alternating flexion and extension of the arm, hand, and fingers at a frequency at approximately 0.5 Hz. Analogical continuous movements of the contralateral lower extremity were analyzed during subcortical resection of the mesial SMA. A NMR was defined as cessation of the described continuous movements without loss of consciousness [Lüders et al., 1987; Lüders et al., 1995]. The corresponding subcortical sites were marked with a number in case of reproducible motor interference, that is, when symptoms were induced by at least three stimulations. An intraoperative photography was taken, featuring the positive cortical stimulation sites (motor, sensory, and speech) as assessed by preresection cortical mapping and marked by a number, as well as the subcortical stimulation sites that led to motor interference. The characteristics of the motor interference were noted together with eventual positive motor responses and speech anomalies. To lessen the intraoperative burden of the patient, the lower contralateral extremity was tested only in selected cases of mesial SMA, and motor interferences on the ipsilateral extremities were not assessed.
Surgical Resection
Resection was extended up to functional boundaries assessed by stimulation mapping that respected both eloquent cortical areas and essential subcortical pathways. This approach limits neurologic deficits while maximizing the extent of resection which is thought to have a positive impact on the further course of LGG [Smith et al., 2008; Soffietti et al., 2010]. The neurologic status was assessed immediately after surgery and again after 3 months analogical to the preoperative assessment.
Localization of Stimulation Sites
The exact localization of intraoperative subcortical stimulation sites was determined by its spatial relation to various anatomical (gyri, sulci, midline, and lateral ventricle) and functional (motor, sensory, and speech) landmarks as documented on intraoperative photography. The determined location of the stimulation site was plotted into the postoperative magnetic resonance imaging (MRI). Indeed, beyond the assessment of the extent of resection, this imaging enabled the analysis of the anatomical location of the eloquent areas, i.e. in essence, at the periphery of the cavity, where the resection was stopped according to functional boundaries—a methodology that we previously reported [Duffau et al., 2002]. The anterior commissure–posterior commissure, vertical lines tangential to the posterior margin of the anterior and posterior commissure (VAC and VPC, respectively) were used as a reference frame for stimulation sites (Fig. 1). The NMR locations were copied onto a mid‐sagittal and a coronal (at the level of the anterior leg of the internal capsule) schema of the human brain (Fig. 1).
Figure 1.
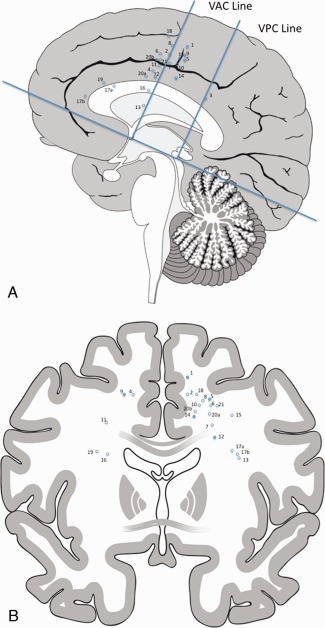
Sagittal (A) and coronal (B) planes. Sites with movement inhibition upon subcortical stimulation are marked with (○). Sites with movement inhibition and positive motor response are marked with a (•). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
RESULTS
Patient Population
The mean patient age was 35 years (range, 24–50 years). Of the 9 men and 12 women, 17 patients were right‐handed and 4 were left‐handed according to the Edinburgh inventory. All 21 patients had a Karnofsky Performance Status of 90 or 100%. Seizures were the presenting symptoms in all patients. Four patients had a slight speech deficit and none had a motor or sensory deficit prior to surgery. The preoperative MRI localized the tumor within the SMA in 19 patients, the insula in one patient and subjacent to the retrocentral gyrus in another patient (Table 1).
Table 1.
Patient characteristics and motor responses with stimulation
| Patient | Age | Sex | Side | Handedness | Affected lobe | Characteristics of Stimulation Results | |||
|---|---|---|---|---|---|---|---|---|---|
| Negative motor response | Acceleration | Positive motor response | Speech arrest | ||||||
| 1 | 26 | M | L | R | Frontal | Inhibition of arm and leg movements | Arm and leg movements | No | |
| 2 | 28 | M | L | R | Frontal | Inhibition of arm and leg movements | No | No | |
| 3 | 36 | F | L | R | Parietal | Inhibition of arm movements | No | No | |
| 4 | 25 | F | R | L | Frontal | Inhibition of arm movements | No | Yes | |
| 5 | 25 | M | L | R | Frontal | Inhibition of arm movements | Arm movements | No | |
| 6 | 44 | F | L | R | Frontal | Inhibition of arm movements | No | No | |
| 7 | 43 | M | L | R | Frontal | Inhibition of arm movements | No | No | |
| 8 | 35 | F | L | R | Frontal | Inhibition of arm movements | No | No | |
| 9 | 42 | F | R | L | Frontal | Inhibition of arm movements | Arm movements | No | |
| 10 | 39 | F | L | R | Frontal | Inhibition of arm movements | No | Yes | |
| 11 | 38 | M | R | L | Frontal | Inhibition of arm movements | No | No | |
| 12 | 40 | M | L | R | Frontal | Inhibition of arm movements | Arm movements | No | |
| 13 | 31 | F | L | R | Insula | None | Acceleration of arm movements | No | No |
| 14 | 41 | F | L | R | Frontal | Inhibition of arm movements | Arm movements | No | |
| 15 | 41 | M | L | R | Frontal | Inhibition of arm movements | No | Yes | |
| 16 | 34 | F | R | L | Frontal | Inhibition of arm movements | No | No | |
| 17 | 28 | F | L | R | Frontal | Site A: Inhibition of arm movements | No | Yes | |
| Site B: Inhibition of arm movements | No | Yes | |||||||
| 18 | 32 | M | L | R | Frontal | Inhibition of arm movements | No | Yes | |
| 19 | 25 | F | R | R | Frontal | None | Acceleration of arm movements | No | No |
| 20 | 50 | M | L | R | Frontal | Site A: Inhibition of arm movements | Acceleration of arm movements | No | No |
| Site B: Inhibition of arm movements | No | No | |||||||
| 21 | 27 | F | L | R | Frontal | Inhibition of arm movements | No | No | |
Abbreviations: M, male; F, female; R, right; and L, left.
Characteristics of Subcortical Stimulation Sites
Cortical electrical stimulation was performed to localize the precentral gyrus and speech areas according to our mapping protocol. Subcortical electrical stimulation identified a total of 23 sites with motor interference in all 21 patients (Fig. 1). The sites identified during subcortical electrical stimulation were located veil‐like in a coronal plane in 19 patients, ranging 2 cm subcortical down to the anterior arm of the internal capsule rostral to the primary motor fibers (Fig. 1). Three additional stimulation sites were localized further anterior to the VAC line within the frontal white matter and one site was found subjacent to the retrocentral gyrus. In five patients, electrical stimulation evoked positive motor response of the arm (and of the leg in one patient) when at rest, in addition to inhibition of movement during voluntary continuous movements at the same stimulation site (Fig. 1). Inhibition of voluntary movement was evoked in 20 patients, and acceleration of movement in three patients. Continuous movement in the contralateral lower extremity was monitored only in two cases of mesial SMA resection, and both patients showed additional movement inhibition of the lower extremity during subcortical stimulation. The motor arrest observed following electrical stimulation always affected the continuous movement of the entire extremity and lasted for a few seconds followed by normal continuation of the transitory interrupted movement. Consciousness was never impaired during the NMR. In three patients, cessation of movements of the superior extremity alternated with acceleration of the continuous movements at the same stimulation site.
All patients recovered well from surgery and were discharged home within 1 week following surgery. Fifteen patients experienced postoperative worsening of speech. Akinesia was noted in the contralateral arm in one patient and in both the contralateral arm and the leg in another patient. All patients with neurological worsening underwent rehabilitation at home. On re‐examination at 3 months, all patients had regained their respective preoperative level.
DISCUSSION
Our understanding of cortical motor control has changed radically over the last decades. The modulatory and hierarchical concept of a SMA‐generating motor sequence commands and a primary motor cortex with a monopoly on the transmission of motor commands to the spinal cord motor neurons has been challenged by the findings of additional motor areas within the frontal and parietal lobe [Matelli et al., 1985; Filimon, 2010] and by the detection of corticospinal fibers originating from secondary motor areas [Dum and Strick, 1991]. Furthermore, the lack of clear anatomical and functional borders between various motor areas [Graziano et al., 2002] has questioned the strictly localizationist view. Surgical resection of the SMA leads to variable, but mostly transient, motor, and speech deficits called the SMA syndrome [Krainik et al., 2001; Krainik et al., 2004]. The transient nature of the SMA syndrome and the lack of permanent motor deficits in patients with damage to the SMA have been attributed to cortical plasticity in conjunction with a vast subcortical network [Krainik et al., 2004]. To comprehend the complex functioning of motor control, an investigation of the cortico‐subcortical network is mandatory [de Benedictis and Duffau, 2011].
Cortical origin of the Identified Fibers
As previously mentioned, our protocol of cortical mapping did not include continuous movements of the extremities, and therefore cortical NMAs were not recorded. Motor interference (inhibition, acceleration, or positive response) was nevertheless reliably recorded in all patients throughout the resection and subcortical mapping. However, several cortical areas are known to present identical responses upon electrical stimulation, such as the here‐described subcortical fibers. These so‐called “NMAs” are located within the pre‐SMA and the inferior frontal gyrus in front of the primary motor face presentation Brodmann area 44 [Lüders et al., 1987; Lüders et al., 1995; Penfield and Jasper, 1954], as well as in front of the primary motor cortex over the entire convexity [Mikuni et al., 2006] and, in a single reported case, in the postcentral gyrus [Mikuni et al., 2006]. The pre‐SMA is known to have vast connections to prefrontal regions, but not to the central region or parietal lobe or spinal tract [Dum and Strick, 1991; Hutchins et al., 1988; Rizzolatti et al., 1996]. Furthermore, the pre‐SMA and the anterior SMA‐proper were invaded and destroyed by the tumor in most of our patients, making them unlikely to be the origin of the here‐described subcortical fibers located just rostral of the corticospinal tract (CST). The veil‐like distribution makes the NMA in Brodmann area 44 and the parietal NMA equally unlikely to be the corresponding cortical area for all stimulated fibers although a subset of the fibers might be part of a parieto‐frontal loop. However, origin from the cortex within the depth of the precentral sulcus cannot be excluded. In addition, five patients showed positive motor response when at rest in addition to the described NMR at the same stimulation site near the VAC line. These stimulated fibers were too far anterior to be part of the primary motor fibers originating from the precentral gyrus. We therefore believe these fiber bundles, which show both inhibitory and excitatory characteristics, to be of premotor origin, including the posterior part of the SMA‐proper.
Subcortical Trajectory of the Descending “modulatory motor pathways”
The pre‐Rolandic cortical NMAs located mainly within the premotor area and the caudal SMA [Mikuni et al., 2006] lie in the same coronal plane as the subcortical stimulation sites in this study. These pre‐Rolandic NMAs therefore seem to be the most likely origin of the fibers detected in our patients. The veil‐like distribution of subcortical stimulation sites in our study, ranging down to the anterior arm of the internal capsule in a coronal plane, indicates a possible course toward the spinal cord. The premotor area is known to comprise motor representations of the arm [Gentilucci et al., 1988; Gerbella et al., 2010; Matelli et al., 1985; Rizzolatti et al., 1988] and to have projections to the spinal cord motor neurons [Dum and Strick, 1991; Maier et al., 2002]. In fact, an average of 29% of all fibers at the cervical medullar level originate from the SMA, and 31% from the premotor area, implying a direct access of the SMA and the premotor area to the spinal cord [Dum and Strick, 1991]. Thus, control of movement by the SMA and premotor areas seems to be exerted directly on a spinal level in parallel to the corticospinal input from the primary motor cortex [Dum and Strick, 1991]. It is of interest that all patients exhibited motor interference of the upper extremity. This is in line with anatomical–functional findings in the macaque monkey, which showed that the premotor area rostral of the primary motor cortex contains vast motor representation areas of the arm, but not of the leg [Gentilucci et al., 1988], whereas the SMA also comprises leg motor representation [Mitz and Wise, 1987; Rizzolatti et al., 1996]. In accordance with these studies, we observed motor interference in two patients during stimulation of the mesial frontal white matter (Fig. 1).
Possible Involvement of the Parietal Lobe and Fronto‐Parietal Connections
Furthermore, we evoked movement inhibition in the white matter subjacent to the retrocentral gyrus and further anterior within the frontal lobe (Fig. 1). Connections of the detected fibers to known cortical NMAs in the pre‐SMA, the pre‐motor area, and Brodmann area 44 are suggested by the similarity of the motor response [Lüders et al., 1987; Mikuni et al., 2006; Penfield and Bouldrey, 1937]. A single case of a parietal cortical NMA within the retrocentral gyrus has been described [Mikuni et al., 2006]. The parietal lobe, however, is known to have strong connections to premotor areas of the frontal lobe [Filimon et al., 2010; Matelli and Luppino, 2001] and to contain motor functions as demonstrated through cortical stimulation in macaques and lesion studies in humans [Fogassi and Luppino, 2005; Jackson et al., 2009]. Our findings of subcortical fibers with inhibitory motor characteristics give further evidence that the motor control network extends beyond the frontal lobe into parietal areas.
Limitations of This Study
Maximizing tumor resection while avoiding permanent neurologic deficits was the principal goal of surgery in these 21 patients. Electrical stimulation was performed to detect functional boundaries and was therefore restricted to certain areas. As a result, further stimulation sites leading to various motor responses might have been missed.
In addition, only 2 of the 19 patients (11%) harboring tumors within the SMA proper showed transient motor deficits (motor SMA syndrome). This stands in contrast to historical cases, which showed motor SMA syndromes in 48% of cases after resection of tumors within the SMA proper [Krainik et al., 2001]. One could suspect that this low rate of motor SMA syndrome might be indicative of a selection of very anteriorly located frontal tumors. However, Figure 1 shows the localization of our stimulation points on the posterior border of the resection cavity. One can thus see that the resections encompassed the SMA proper in all frontal cases, ruling out a selection of anteriorly located tumors as the reason for the low rate of SMA syndromes.
On the other hand, it is worth noting that the functional recovery following SMA syndrome was not always complete. In the same article by Krainik et al. 2001, at 1 year after surgery, three patients kept fine movement disorders (underutilization of the upper limb) and two patients had bimanual coordination impairment. This is the reason why in the present series, our surgical strategy was to remove only noneloquent tissue and to stop resection after any interference with motor function. This led to a termination of surgery after the identification of inhibition and acceleration of movement in anatomical sites just anteriorly to the presumed corticospinal tract, and not because of positive motor responses owing to stimulation of primary motor neurons themselves. In other words, we decided to stop the resection according to functional boundaries, including the network subserving motor control. Therefore, it is likely that sparing fibers with motor modulatory functions had influenced the occurrence rate of motor SMA syndrome, as well as the quality of long‐term recovery. Indeed, on reexamination at 3 months, all patients had regained their respective preoperative level. However, our patient numbers is too small for a definite assessment of the importance of those spared fibers for functional outcome. Further studies are required to investigate the influence of subcortical motor mapping on the occurrence of postoperative SMA syndrome.
CONCLUSIONS
The evidence of motor modulatory responses upon subcortical electrical stimulation allows us to integrate recent findings of cortical stimulation and anatomical studies, and to postulate a veritable cortico‐subcortical network of motor control. This motor control network comprises frontal and parietal fibers as well as a descending pathway with inhibitory and excitatory characteristics, likely representing a direct projection from the premotor areas to the spinal cord. The knowledge of this network will help to guide surgery close to these structures in an attempt to diminish postoperative neurologic deficits. Further investigation of this network will shed light on functional deficits following lesions of the involved structures. Furthermore, a renewed classification of spinal cord projections originating from frontal premotor areas seems needed to distinguish these motor control fibers from the classical CST originating from the precentral gyrus.
ACKNOWLEDGMENTS
The authors received editing support for the final manuscript by Susan Wieting, Bern University Hospital, Department of Neurosurgery, Bern, Switzerland. The authors thank Daniela Miescher for graphic support, Department of Neurosurgery, Bern, Switzerland.
REFERENCES
- Brodmann K (1909): Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig:Barth. [Google Scholar]
- Campbell AW (1905): Histological Studies on the Localisation of Cerebral Function. Cambridge:Cambridge University Press. [Google Scholar]
- de Benedictis A, Duffau H (2011): Brain hodotopy: From esoteric concept to practical surgical applications. Neurosurgery 68:1709–1723. [DOI] [PubMed] [Google Scholar]
- Duffau H (2001): Acute functional reorganisation of the human motor cortex during resection of central lesions: A study using intraoperative brain mapping. J Neurol Neurosurg Psychiatry 70:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP, Bitar A, Fohanno D (2002): Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo‐functional study. Brain 125:199–214. [DOI] [PubMed] [Google Scholar]
- Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, van Effenterre R, Capelle L (2005): Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985‐96) and with (1996‐2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry 76:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL (1991): The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11:667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filimon F (2010): Human cortical control of hand movements: Parietofrontal networks for reaching, grasping, and pointing. Neuroscientist 16:388–407. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G (2005): Motor functions of the parietal lobe. Curr Opin Neurobiol 15:626–631. [DOI] [PubMed] [Google Scholar]
- Fulton JF (1935): Definitions of the motor and pre‐motor areas. Brain 58:311–316. [Google Scholar]
- Fulton JF (1949): Physiology of the Nervous System. New York:Oxford University Press. [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G (1988): Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp Brain Res 71:475–490. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G (2010): Cortical connections of the anterior (F5a) subdivision of the macaque ventral premotor area F5. Brain Struct Funct 216:43–65. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K (2000): Functional neuroanatomy of the primate isocortical motor system. Anat Embryol (Berl) 202:443–474. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T, Cooke DF (2002): The cortical control of movement revisited. Neuron 36:349–362. [DOI] [PubMed] [Google Scholar]
- Hutchins KD, Martino AM, Strick PL (1988): Corticospinal projections from the medial wall of the hemisphere. Exp Brain Res 71:667–672. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Hirasawa K, Kinoshita M, Hitomi T, Matsumoto R, Mitsueda T, Taki JY, Inouch M, Mikuni N, Hori T, Fukuyama H, Hashimoto N, Shibasaki H, Takahashi R (2009): Negative motor seizure arising from the negative motor area: Is it ictal apraxia? Epilepsia 50:2072–2084. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Newport R, Husain M, Fowlie GE, O'Donoghue M, Bajaj N (2009): There may be more to reaching than meets the eye: Re‐thinking optic ataxia. Neuropsychologia 47:1397–1408. [DOI] [PubMed] [Google Scholar]
- Krainik A, Lehéricy S, Duffau H, Vlaicu M, Poupon F, Capelle L, Cornu P, Clemenceau S, Sahel M, Valery CA, Boch AL, Mangin JF, Bihan DL, Marsault C (2001): Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology 57:871–878. [DOI] [PubMed] [Google Scholar]
- Krainik A, Duffau H, Capelle L, Cornu P, Boch AL, Mangin JF, Le Bihan D, Marsault C, Chiras J, Lehéricy S (2004): Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology 62:1323–1332. [DOI] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Morris HH (1987): Negative Motor Responses Elicited by Stimulation of the Human Cortex. Advances in Epileptology. New York:Raven Press; pp229–231. [Google Scholar]
- Lüders HO, Dudley S, Dinner S (1995): The negative motor areas In: Fahn S, Hallett M, Lüders HO, Marsden CD, editors.Negative Motor Phenomenon. Philadelphia:Lippinscott‐Raven Publishers. [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN (2002): Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex 12:281–296. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G (2001): Parietofrontal circuits for action and space perception in the macaque monkey. NeuroImage 14:S27–S32. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G (1985): Patterns of cytochrome oxidase activity in the frontal agranular cortex of the macaque monkey. Behav Brain Res 18:125–136. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G (1991): Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol 311:445–462. [DOI] [PubMed] [Google Scholar]
- Mikuni N, Ohara S, Ikeda A, Hayashi N, Nishida N, Taki J, Enatsu R, Matsumoto R, Shibasaki H, Hashimoto N (2006): Evidence for a wide distribution of negative motor areas in the perirolandic cortex. Clin Neurophysiol 117:33–40. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Wise SP (1987): The somatotopic organization of the supplementary motor area: Intracortical microstimulation mapping. J Neurosci 7:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M (2008): Functional role of the supplementary and pre‐supplementary motor areas. Nat Rev Neurosci 9:856–869. [DOI] [PubMed] [Google Scholar]
- Penfield W, Bouldrey E (1937): Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60:389–443. [Google Scholar]
- Penfield W, Jasper J (1954): Mechanisms of voluntary movement. Brain 77:1–17. [DOI] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LG (1993): Functional magnetic resonance imaging of complex human movements. Neurology 43:2311. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M (1988): Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res 71:491–507. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M (1996): The classic supplementary motor area is formed by two independent areas. Adv Neurol 70:45–56. [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M (1998): The organization of the cortical motor system: New concepts. Electroencephalogr Clin Neurophysiol 106:283–296. [DOI] [PubMed] [Google Scholar]
- Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS (2008): Role of extent of resection in the long‐term outcome of low‐grade hemispheric gliomas. J Clin Oncol 26:1338–1345. [DOI] [PubMed] [Google Scholar]
- Soffietti R, Baumert BG, Bello L, von Deimling A, Duffau H, Frénay M, Grisold W, Grant R, Graus F, Hoang‐Xuan K, Klein M, Melin B, Rees J, Siegal T, Smits A, Stupp R, Wick W (2010): Guidelines on management of low‐grade gliomas: Report of an EFNS–EANO* Task Force. Eur J Neurol 17:1124–1133. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M (1981): Organization of the secondary motor areas of the cerebral cortex. Handbook of Physiology. Bethesda:American Physiological Society; pp1121–1147. [Google Scholar]
- Wise SP (1985): The primate premotor cortex: past, present, and preparatory. Ann Rev Neurosci 8:1–19. [DOI] [PubMed] [Google Scholar]
- Wise SP (1996): Corticospinal efferents of the supplementary sensorimotor area in relation to the primary motor area. Adv Neurol 70:57–69. [PubMed] [Google Scholar]


