Abstract
Understanding how the developing brain processes auditory information is a critical step toward the clarification of infants' perception of speech and music. We have reported that the infant brain perceives pitch information in speech sounds. Here, we used multichannel near‐infrared spectroscopy to examine whether the infant brain is sensitive to information of pitch changes in auditory sequences. Three types of auditory sequences with distinct temporal structures of pitch changes were presented to 3‐ and 6‐month‐old infants: a long condition of 12 successive tones constructing a chromatic scale (600 ms), a short condition of four successive tones constructing a chromatic scale (200 ms), and a random condition of random tone sequences (50 ms per tone). The difference among the conditions was only in the sequential order of the tones, which causes pitch changes between the successive tones. We found that the bilateral temporal regions of both ages of infants showed significant activation under the three conditions. The stimulus‐dependent activation was observed in the right temporoparietal region of the both infant groups; the 3‐ and 6‐month‐old infants showed the most prominent activation under the random and short conditions, respectively. Our findings indicate that the infant brain, which shows functional differentiation and lateralization in auditory‐related areas, is capable of responding to more than single tones of pitch information. These results suggest that the right temporoparietal region of the infants increases sensitivity to auditory sequences, which have temporal structures similar to those of syllables in speech sounds, in the course of development. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: music, neuroimaging, NIRS, speech perception, temporal cortex
INTRODUCTION
Speech and musical sounds comprise a continuous time series of phonemes and tones. Listeners are able to perceive temporal orders of these components, which form auditory information. For example, when we listen to an auditory sequence of ‘C (do) – D (re) – E (mi),’ we detect each tone individually and then collate the order of tones to identify the sequence. If we only detect tones singly, or if we only detect multiple tones holistically and intermixedly, the sequence would not emerge from the tones. Unraveling these translations naturally provoke the fundamental question: how do infants perceive speech and musical sounds? Previous studies on infants have successfully applied behavioral paradigms to determine how the perception of speech and musical sounds develops [for reviews, see Aslin et al.,1998; Jusczyk,1999; Trehub and Hannon,2006].
Recent neuroimaging studies on neonates and infants reported inconsistent results as to cortical activation in response to musical stimuli. While two studies showed weak bilateral responses to musical stimuli [Dehaene‐Lambertz et al.,2010; Kotilahti et al.,2010], a functional magnetic resonance imaging (fMRI) study reported the main effect of a music condition in contrast to a silence condition in the right temporal and parietal regions of neonates [Perani et al.,2010]. Because musical sounds contain multiple information of melody, rhythm, harmony, and so on, activation patterns would be affected by changes in all these variables. A useful strategy to reveal functional roles of cortical regions is to focus on a single information of auditory sequences. Telkemeyer et al. (2009) used temporally structured sounds and reported that neonates showed bilateral and right‐lateralized responses to rapid and slow acoustic modulations, respectively. Their results suggested the hemispheric difference in the processing of auditory sequences.
Infants are also capable of discerning unique speech melodies, i.e., speech pitch information. We conducted studies to clarify how infants perceive pitch contours in speech sounds [Homae et al.,2006,2007]. Using multichannel near‐infrared spectroscopy (NIRS), we demonstrated that the right temporoparietal region of 3‐ and 10‐month‐old infants is responsive to speech pitch information (red and blue circles in Fig. 1) and that comparisons between the activation patterns of the two ages show developmental changes. The sensitivity of infants to discern pitch contour changes in speech sounds cannot be observed if infants only process segmental information or the pitch height of each syllable individually, suggesting that infants perceive pitch changes over multiple syllables in speech sounds. Thus, the right temporoparietal region is a strong candidate for the neural substrates of the processing of pitch changes over multiple tones in auditory sequences. In this study, we examined this possibility by using three types of auditory stimuli with distinct temporal structures of pitch changes (50, 200, and 600 ms). The continuity of pitch changes in several hundred milliseconds corresponds to syllabic transition rates in speech sounds. There would be sensitive time windows of the infant brain for processing pitch changes in auditory sequences. To measure the cortical activation of infants, we used multichannel NIRS. We predefined a region of interest (ROI) in the right temporoparietal region (a green trapezoid in Fig. 1) according to our previous studies [Homae et al.,2006,2007] and studies on adults [Griffiths et al.,1997; Patterson et al.,2002; Zatorre et al.,1994]. Furthermore, we tested whether the patterns of cortical activation show developmental changes between 3 months old and 6 months old, at which age infants can memorize musical melodies by using pitch information [Plantinga and Trainor,2005]. Prior behavioral studies have reported development of auditory and speech perception at these ages [Aslin et al.,1998; Kuhl,2004].
Figure 1.
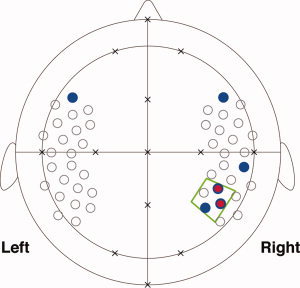
The ROI in the right hemisphere. We have reported cortical activation in the right temporoparietal region of 3‐month‐old infants (red circles) [Homae et al.,2006] and 10‐month‐old infants (blue circles) [Homae et al.,2007], which are related to the processing of pitch information in speech sounds (P < 0.01). The two red circles overlaps blue circles. In this study, we set a ROI (a green trapezoid) to examine whether this region of the infant brain is sensitive to information of pitch changes in auditory sequences. The arrangement of measurement channels (open circles) in our previous studies was used in the measurement of 3‐month‐old infants (see Fig. 3). Several landmarks from the international 10‐20 system are shown (crosses).
We prepared three types of tone sequences as the auditory stimuli, all of which were made from a single sequence by changing the temporal order of the 96 tones, and thus the number of appearances of each tone was equal among the three conditions. The difference among the conditions was only in the sequential order of the tones, which causes pitch changes between the successive tones. By investigating whether the infant brain showed different activation patterns for tone sequences of the three conditions, we tested the functional role of the right temporoparietal region in the pitch processing.
MATERIALS AND METHODS
Participants
Forty‐six full‐term healthy Japanese infants participated in this study. Twenty‐two infants were 3 months old (7 girls and 15 boys; mean age: 115.5 days; range: 95–128 days), and 24 infants were 6 months old (15 girls and 9 boys; mean age: 193.8 days; range: 182–209 days). All infants were sleeping quietly while they were studied. An additional 38 (3 months old: N = 19 and 6 months old: N = 19) infants were studied, but they were excluded from the analysis due to either producing large head movements resulting in motion artifacts in the signals (3 months old: N = 12 and 6 months old: N = 8) or probe obstruction by hair (3 months old: N = 7 and 6 months old: N = 11). A success rate of 3‐ and 6‐month‐old infants was 53.7% and 55.8%, respectively. The measurement was stopped when infants awoke from their sleep during the experiments (3 months old: N = 19 and 6 months old: N = 41). Informed consent was obtained from the parents of the infants prior to the initiation of the experiments. The study was approved by the ethics committee of the Graduate School of Education, University of Tokyo.
Stimuli
The stimuli were digitally recorded tone sequences, which were made by the Musical Instrument Digital Interface sequencer and converted to WAV‐format files (16 bit, 22,050 Hz). We prepared a chromatic scale containing 25 semitones from C3 to C5 of piano sounds (Fig. 2a), each of which exhibits a harmonic structure. The duration of each tone was 50 ms. Each tone sequence consisted of 96 tones (duration: 50 ms × 96 tones = 4800 ms per tone sequence). We made three types of tone sequences. First, the ascending or descending scales, which consisted of the 24 semitones shown in Fig. 2a, were divided into two scales of 12 semitones (600 ms). The ascending or descending series of 12 semitones were pseudorandomly presented as the long condition (Fig. 2b). These scales started from C3, C4, or C5. In a single sequence of the long condition, we presented eight chromatic scales covering an octave in pseudorandomized orders (Fig. 2b). As all chromatic scales begin with C and end with B (ti) or C sharp, a tone sequence consisted of 96 tones under the long condition, which can be perceived as a continuous sequence if the infants were sensitive to pitch chroma [Demany and Armand,1984]. Second, the ascending or descending scales, which consisted of the 12 semitones in Fig. 2b, were divided into three scales of four semitones (200 ms). These ascending or descending series of four semitones were pseudorandomly presented as the short condition (Fig. 2c). The four tones constituting a chromatic scale were placed at regular pitch intervals (i.e., semitone), and the fourth tone was followed by a larger gap of the pitch interval. Third, the ascending or descending scales, which consisted of the four semitones in Fig. 2c, were divided into individual semitones (50 ms). Semitones were pseudorandomly presented as the random condition (Fig. 2d). These processes allowed us to make tone sequences containing all 96 tones in each trial under each condition. The only difference among the conditions was the order of tones. The mean pitch changes between two successive tones under the long, short, and random conditions were 1.6, 3.3, and 9.0 semitones, respectively.
Figure 2.
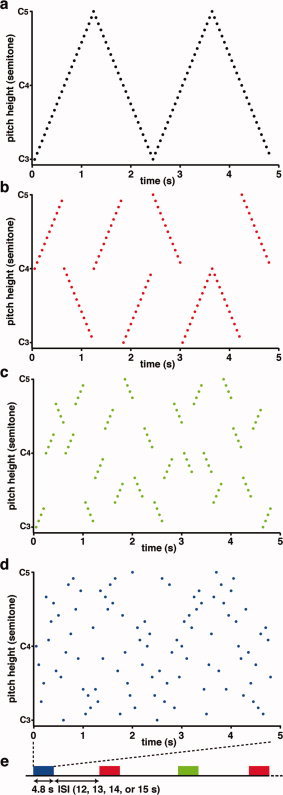
The tone sequences. (a) The original tone sequence consists of 96 tones from C3 to C5. The abscissa indicates time (s), and the ordinate indicates pitch height (semitone). (b–d) Examples of the tone sequences under the three conditions. The red, green, and blue‐filled circles indicate tones presented in the long (b), short (c), and random (d) sequences, respectively. Under each of the three conditions, an identical set of tones made from the original tone sequence (a) was presented. The only difference among the conditions was the order of tones alone. (e) Stimulus presentation. The red, green, and blue‐filled squares indicate the single trials for the long, short, and random sequences, respectively. The tone sequences (duration: 4.8 s) were presented in a pseudorandomized order. During the interstimulus interval (ISI: 12, 13, 14, or 15 s), no sound was presented.
Stimulus Presentation
All experiments were conducted in a sound‐attenuated room (the background noise: less than 30 dB SPL). The infant was held in an experimenter's arms during the measurement of cortical activation, and the stimuli were presented while the infant was in daytime sleep. The infants were almost motionless and slept soundly throughout the experimental sessions. We previously reported that NIRS recordings from infants in daytime sleep provide long‐duration and motion‐free data with a sufficiently high signal‐to‐noise ratio, and the obtained data can be used to evaluate cortical responses to speech sounds [Homae et al.,2006,2007; Nakano et al.,2008,2009; Taga et al.,2007]. Stimulus‐dependent cortical responses to speech sounds have been reported in both sleeping adults [Portas et al.,2000] and infants [Dehaene‐Lambertz et al.,2002; Peña et al.,2003].
Tone sequences were presented at a maximum amplitude of 45 dB SPL using a BOSE MMS‐1 speaker system placed in front of the infant. We presented 12 sequences per condition to each infant. The presentation of tone sequences was counterbalanced among the infants. No sound was presented during the interstimulus intervals (duration: 12, 13, 14, or 15 s; Fig. 2e).
NIRS Recordings
We used multichannel NIRS instruments (ETG‐100 and ETG‐7000 for 3‐ and 6‐month‐old infants, respectively; Hitachi Medical Corporation, Tokyo, Japan). The NIRS instruments exploit the optical properties of hemoglobin, which has oxygenated (oxy‐Hb) and deoxygenated (deoxy‐Hb) forms with different absorption spectra in the NIR wavelength region. By using two NIR wavelengths (780 and 830 nm in ETG‐100 and 785 and 830 nm in ETG‐7000) and applying the data analyses based on the modified Lambert–Beer law, these instruments measure the relative changes in the concentrations of oxy‐Hb and deoxy‐Hb in the cerebral cortex at preset measurement points. Detailed descriptions of the principles underlying NIRS have been previously described [Jöbsis,1977; Maki et al.,1995; Obrig and Villringer,2003; Reynolds et al.,1988; Villringer and Chance,1997]. NIRS has been successfully used to investigate cortical activation in infants in response to auditory stimuli [Homae et al.,2006,2007; Kotilahti et al.,2005,2010; Minagawa‐Kawai et al.,2007; Nakano et al.,2008,2009; Peña et al.,2003; Taga and Asakawa,2007; Taga et al.,2007; Telkemeyer et al.,2009]. NIR light was emitted from laser diodes through incident optical fibers. The maximum intensity of NIR light was set at 0.6 mW for 3‐month‐old infants and 1.2 mW for 6‐month‐old infants [Homae et al.,2010]. The received light was detected by avalanche photodiodes through detection via optical fibers and separated into individual light sources, depending on each wavelength. For the 3‐month‐old infants, four sets of 3 × 3 arrays were used composed of five incident and four detection fibers mounted on a flexible cap over the frontal, temporal, and temporoparietal areas of each hemisphere. For the 6‐month‐old infants two sets of 3 × 10 arrays composed of 15 incident and 15 detection fibers were mounted on a flexible cap over the frontal, temporal, temporoparietal, and occipital areas of each hemisphere. Each pair of adjacent incident and detection fibers defined a single measurement channel, which enabled us to simultaneously measure the time course of oxy‐Hb and deoxy‐Hb signals with a 0.1‐s time resolution. The distance between incident and detection fibers was set at approximately 2 cm [Taga et al.,2007]. The measurement channels were correctly positioned by reference to the international 10‐20 system of electrode placement using landmarks of external auditory pores, vertex, and inion from each infant. Because few available atlases exist for the infant brain, we used previous studies on adults [Herwig et al.,2003; Homan et al.,1987; Okamoto et al.,2004; Steinmetz et al.,1989] to estimate the craniocerebral correlation for each measurement channel. A recent MRI study suggested that the cortical structure in infants is similar to adults in many aspects [Hill et al.,2010]. We have reported functional mapping for audiovisual stimuli in infants, which is consistent with an estimated map from the craniocerebral correlation [Watanabe et al.,2008,2010].
Data Analysis
We applied the same analysis methods that we have previously reported [Homae et al.,2006,2007; Taga et al.,2007]. We examined the variation in the oxy‐Hb signals, which estimated changes in the regional cerebral blood oxygenation during brain activation. In addition, we also analyzed the deoxy‐Hb signals. We evaluated relative changes in oxy‐Hb and deoxy‐Hb signals contingent on an arbitrarily assigned zero baseline from the start of the measurement period, which was based on the modified Lambert–Beer law. Because the precise optical path length of the light traveling through brain tissue cannot be completely evaluated by continuous‐wave NIRS, the units of oxy‐Hb and deoxy‐Hb signals were determined by multiplying the molar concentration by length (mM·mm). To evaluate the cortical activation of similar regions in the two age groups, we analyzed the signal changes of 64 measurement channels, which covered the frontal, temporal, and temporoparietal regions, in the data obtained from the 6‐month‐old infants. We examined the possible effect of the difference in head sizes, by randomly picking up 100 head‐size data values of 3‐ and 6‐month‐old infants from our database and calculating their mean values: 41.1 cm for 3‐month‐old infants and 43.1 cm for 6‐month‐old infants. The possible difference in head size was estimated to be less than 5%. Because we set the distance between incident and detection fibers at approximately 2 cm in both infant groups, the head‐size difference, if any, between age groups and our channel arrangements affected the sensitivity of NIRS measurement only slightly, if at all.
In each individual dataset, we initially extracted data blocks from the time course data. Each data block ranged from 0.5 s prior to stimulus onset to 16.5 s after stimulus onset. By detecting rapid changes in the summation of oxy‐Hb and deoxy‐Hb signals, we eliminated data blocks with a low signal‐to‐noise ratio due to obstruction by hair and those with movement artifacts. Following these screening processes, we only used data that contained a minimum of six data blocks under each condition (range: 6–12 blocks; mean: 11.7 and 11.6 for 3‐ and 6‐month‐old infants, respectively). All 3‐month‐old infants analyzed surpassed this criterion in all measurement channels. In 6‐month‐old infants, at least 18 infants surpassed this criterion in all measurement channels (range: 18–24 infants; mean: 23.7). We then calculated the mean signal of the first 11 time points (i.e., from 0.5 s prior to stimulus onset, during which no sounds was presented, to 0.5 s after stimulus onset) in each data block and used this value as the baseline for each block. We corrected for a linear trend of signal changes using a linear fit to the baseline and the mean signal of the last 11 time points in each data block. This method was used in our previous studies [Homae et al.,2006,2007; Nakano et al.,2008; Taga et al.,2007; Watanabe et al.,2008], and baseline correction using first 11 time points was validated by our recent study [Watanabe et al.,2010]. By averaging the signal changes over data blocks for each subject under each condition, we obtained the hemodynamic responses at each measurement channel.
To identify the activated regions under each condition, we evaluated the individual data as random effects and performed t‐tests and analyses of variance (ANOVAs) for each channel. First, a t‐test of the signal changes under each condition was performed against the zero baseline (one‐sample, two‐tailed t‐test). We applied a conjunction analysis to reveal the cortical regions activated under all three conditions [Nichols et al.,2005]. Then, we compared the cortical activation among the three conditions (one‐way repeated measures ANOVA and Ryan's method). Multiple comparisons among the measurement channels were considered by adopting an all‐measurement‐channels false discovery rate correction at Q < 0.05 (3‐month‐old infants: 48 channels, 6‐month‐old infants: 64 channels) [Benjamini and Hochberg,1995; Genovese et al.,2002; Singh and Dan,2006]. Based on our previous studies [Homae et al.,2006,2007], we predefined the ROI in the right temporoparietal region. We would report significant signal changes in the ROI even if the changes were observed in a single channel in the ROI. The NIRS instrument can reliably map localized absorbers of NIR light inside biological tissue and scattering medium [Maki et al.,1995; Yamashita et al.,1996]. We reported that 2‐ to 4‐month‐olds and 10‐month‐old infants showed localized and reproducible activation in a single channel [Homae et al.,2007; Taga et al.,2003]. Thus, we considered that all activations that persist beyond the conservative threshold warrant reporting, and we did not use a cluster filter. Because we determined in a previous study on infants that oxy‐Hb signals displayed a better signal‐to‐noise ratio than deoxy‐Hb signals [Homae et al.,2007], we focused on oxy‐Hb signal changes to examine the responsiveness of cortical regions to tone sequences.
RESULTS
Through the use of multichannel NIRS, we found that 3‐ and 6‐month‐old infants showed hemodynamic responses to tone sequences in both the left and right hemispheres (Figs. 3 and 4). The temporal and temporoparietal regions under the three conditions demonstrated remarkable signal changes. These regions showed both increases in oxy‐Hb signals, as well as decreases in deoxy‐Hb signals. Moreover, the oxy‐Hb signal changes were larger than the deoxy‐Hb signal changes. To quantify the hemodynamic responses to tone sequences, we created an average time course of all the measurement channels for the three conditions in both infant groups (Fig. 5). The maximal change in the oxy‐Hb signals occurred at 6.6 s. In the statistical analyses, we used the mean changes of the oxy‐Hb signals in the time window from 5.0 to 9.3 s after the onset of the stimulus, in which the oxy‐Hb signal change was greater than two standard deviations for all time points of the averaged time course.
Figure 3.
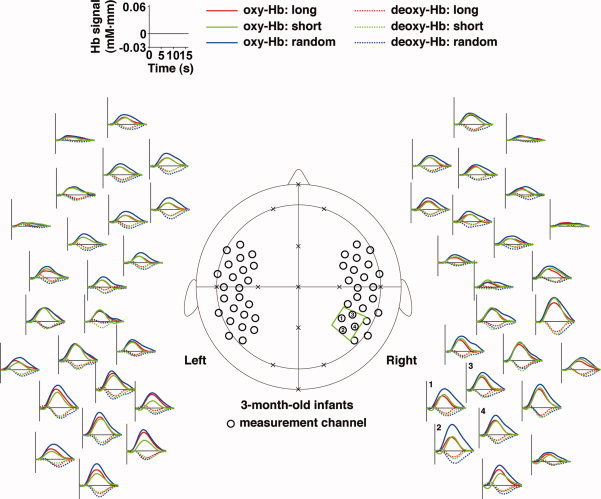
The signal changes in the 3‐month‐old infants obtained by measurements using multichannel near‐infrared spectroscopy (NIRS). Measurement channels (black open circles in the central panel) were placed over the bilateral frontal, temporal, and temporoparietal regions of infants. These channels were correctly positioned using the 10‐20 system (crosses). The green trapezoid indicates the ROI (four channels). The numbers indicate the channels in the predefined ROI. Mean time courses of the oxygenated hemoglobin (oxy‐Hb) and deoxygenated hemoglobin (deoxy‐Hb) signals of all the infants are arranged around the central panel. The red, green, and blue lines indicate the responses to the long, short, and random sequences, respectively. The abscissa indicates time (s), and the ordinate indicates the Hb signal (mM·mm). The zero time‐point represents the onset of the stimulus. For the purpose of display, the time course data were low‐pass filtered.
Figure 4.
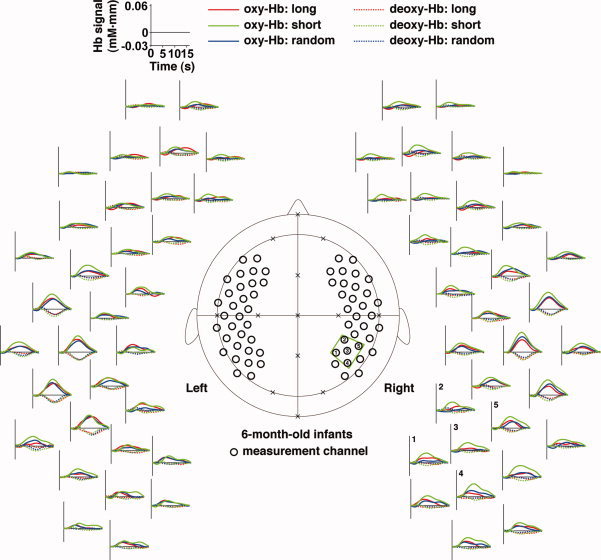
The signal changes in the brains of the 6‐month‐old infants. Measurement channels (black open circles in the central panel) were placed over bilateral frontal, temporal, and temporoparietal regions of infants. These channels were correctly positioned using the 10‐20 system (crosses). The green trapezoid indicates the ROI (five channels). The numbers indicate the channels in the predefined ROI. Mean time courses of the oxy‐Hb and deoxy‐Hb signals among all infants are arranged around the central panel. The red, green, and blue lines indicate responses for the long, short, and random sequences, respectively. For display, the time course data were low‐pass filtered.
Figure 5.
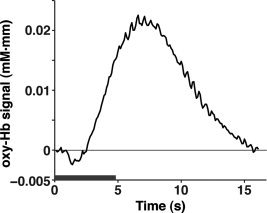
The averaged time course of oxy‐Hb signal changes in all the measurement channels for the three conditions. The time courses of the 3‐ and 6‐month‐old infants are averaged. The gray bar indicates the period during which the auditory sequences were presented (4.8 s).
By conducting statistical analyses on the mean changes in oxy‐Hb signals, we examined the cortical activation in response to the tone sequences. We found that the bilateral prefrontal, temporal, temporoparietal regions of the 3‐month‐old infants showed significant activation for all three sequences (gray‐filled circles in Fig. 6a). The activation patterns in the bilateral regions of infants were consistent with our previous results using speech sounds [Homae et al.,2006]. A representative activation pattern was observed in the left and right temporal channels that was located about 1 cm above T3 and T4, respectively, of the international 10‐20 system. Based on the craniocerebral correlation derived from adult studies, these channels were placed near the sylvian fissure, and the activation in the early auditory areas resulted in the signal changes in the temporal channels. Figure 6b shows the signal changes in the right temporal channel (the auditory area of 3‐month‐old infants, AA_3M) under each condition. This measurement channel indicated equivalent signal changes under each of the three conditions.
Figure 6.
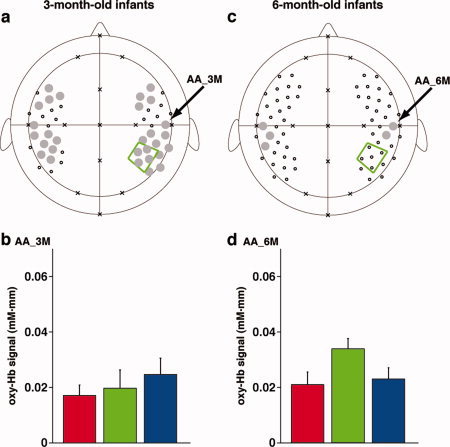
The cortical activation under the three conditions in the 3‐ and 6‐month‐old infants. (a) The regions revealed by the conjunction analyses of activation for the long, short, and random sequences in the 3‐month‐old infants. We used the time from 5.0 to 9.3 s after the onset of the stimulus for analyses. The gray‐filled circles indicate the channels that surpassed the statistical thresholds under all three conditions. Small open circles indicate measurement channels that did not show significant activation. An arrow indicates the right auditory area (AA_3M) in the temporal region. (b) Oxy‐Hb signal changes in the AA_3M. The red, green, and blue bars indicate the mean signal changes for the long, short, and random sequences, respectively. Error bars show the standard error for each test condition. (c) The regions revealed by the conjunction analyses of activation in the 6‐month‐old infants. The right auditory area (AA_6M) in the temporal region, which is the same channel as AA_3M, is indicated by an arrow. (d) Oxy‐Hb signal changes in AA_6M. The red, green, and blue bars represent the mean signal changes for the long, short, and random sequences, respectively. Error bars show the standard error among all subjects.
In the 6‐month‐old infants, we found that the bilateral temporal regions showed significant activation under all three conditions (gray‐filled circles in Fig. 6c). While the activation pattern in the bilateral temporal regions was consistent with the results for the 3‐month‐old infants (Fig. 6a), the activated regions of the 6‐month‐old infants were localized to the temporal regions. Figure 6d shows the signal changes in the right temporal channel (the auditory area of the 6‐month‐old infants, AA_6M) under the three conditions. This region was located very close to AA_3M of the 3‐month‐old infants, as shown in Figure 6a. The signal changes in the auditory area did not show significant difference between the infant groups (Table I).
Table I.
Direct comparison of signal changes in the right auditory area
| The right auditory area (AA_3M, AA_6M) | ||
|---|---|---|
| Age (3‐ and 6‐month‐old infants) | F(1,44) = 1.58 | P > 0.1 |
| Condition (long, short, and random) | F(2,88) = 3.41 | P < 0.05 |
| Age × condition | F(2,88) = 0.83 | P > 0.1 |
We investigated the differences in cortical activation in response to the three types of tone sequences. We found that the right temporoparietal region of the 3‐month‐old infants (TP_3M; the black‐filled circle in Fig. 7a), which was located in the predefined ROI (the green trapezoid in Fig. 7a), showed differences among the three conditions [F(2,42) = 8.89, P < 0.001; the results in the predefined ROI were shown in Table II]. This region showed significant signal changes under all three conditions (Fig. 6a), and the post hoc tests confirmed that the signal changes of TP_3M for the random sequence were greater than changes under the long and short conditions (Fig. 7b). TP_3M of the random condition showed the highest signal changes among the three conditions of all the channels, when the signal changes of each condition were averaged among the subjects (Fig. 7c), while the same channel of the long and short conditions showed lower signal changes. Given the bilateral activation in the temporal regions, our findings demonstrate symmetric (in the auditory area) and asymmetric (in the temporoparietal region) responses to tone sequences in the 3‐month‐old infant brain.
Figure 7.
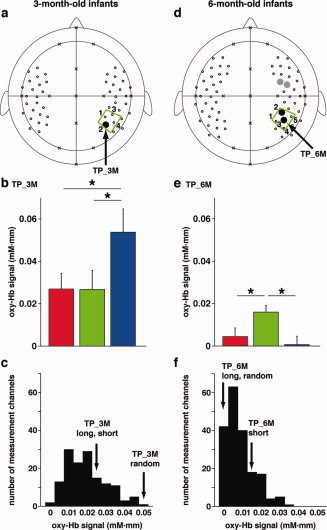
The stimulus‐dependent activation patterns in the 3‐ and 6‐month‐old infants. (a) The activated region revealed by the ANOVA analysis for the long, short, and random sequences in the 3‐month‐old infants. TP_3M in the right temporoparietal ROI (the green trapezoid) showed significant differences among the three test conditions. (b) Oxy‐Hb signal changes in TP_3M. The red, green, and blue bars indicate the mean signal changes for the long, short, and random sequences, respectively. Error bars show the standard error among all subjects. Asterisks (*) denote P < 0.05 in the post hoc tests. (c) The histogram of mean signal changes of the 3‐month‐old infants. We calculated subject‐averaged signal changes in each channel for each condition and counted the number of channels in each bin (bin size = 0.005 mM·mm). The total number of the counts was 144 (48 channels under the three conditions). TP_3M of the random condition is sorted in the highest bin (0.05–0.055 mM·mm) as shown by an arrow, whereas TP_3M of the long and short conditions are sorted in the middle bin (0.025–0.030 mM·mm). (d) ANOVA revealed the regions activated on stimulation by the long, short, and random sequences in the 6‐month‐old infants. Two channels in the right temporoparietal ROI showed significant differences among the three test conditions (the black‐filled circles). The representative channels in this region (TP_6M) is indicated by an arrow. The prefrontal region also showed the significant differences (the gray‐filled circles). (e) Oxy‐Hb signal changes in TP_6M. Asterisks (*) denote P < 0.05 in the post hoc tests. (f) The histogram of mean signal changes of the 6‐month‐old infants. The total number of the counts was 192 (64 channels under the three conditions). The signal changes of TP_6M under the short condition was greater than the signal changes of this channel under the long and short conditions.
Table II.
The right temporoparietal ROI of the 3‐ and 6‐month‐old infants
| The 3‐month‐old infants | The 6‐month‐old infants | ||||
|---|---|---|---|---|---|
| 1 | F(2,42) = 3.13 | P = 0.05 | 1 | F(2,46) = 2.68 | P = 0.08 |
| 2 (TP_3M) | F(2,42) = 8.89 | P = 0.0006 | 2 | F(2,44) = 8.33 | P = 0.0009 |
| 3 | F(2,42) = 1.50 | P = 0.23 | 3 (TP_6M) | F(2,46) = 6.90 | P = 0.0024 |
| 4 | F(2,42) = 1.72 | P = 0.19 | 4 | F(2,46) = 3.81 | P = 0.029 |
| 5 | F(2,44) = 1.70 | P = 0.14 | |||
The ANOVAs applied to the data of the 6‐month‐old infants revealed that the right temporoparietal region of this infant group also showed differences among the three conditions (the black‐filled circles in Fig. 7d). One of the channels (TP_6M, the temporoparietal region of the 6‐month‐old infants; F(2,46) = 6.62, P < 0.005; the results in the predefined ROI were shown in Table II), which was in the predefined ROI and the adjacent channel to TP_3M in the 3‐month‐old infants (Fig. 7a), showed significant signal changes only in response to the short sequence, and furthermore, the post hoc tests confirmed that the signal changes for the short sequence were greater than changes for the long and random sequences (Fig. 7e,f). This pattern of signal changes was observed in the prefrontal region of the 6‐month‐old infants (the gray‐filled circles in Fig. 7d; P < 0.005). Both the 3‐ and 6‐month‐old infants showed stimulus‐dependent activation in the right temporoparietal ROI, and the stimulus‐dependency of the activation in the 6‐month‐old infants was different from that observed in the 3‐month‐old infants. The interaction between the infant groups and the three conditions was significant (Table III).
Table III.
Direct comparison of signal changes in the right temporoparietal region
| The right temporoparietal region (TP_3M, TP_6M) | ||
|---|---|---|
| Age (3‐ and 6‐month‐old infants) | F(1,44) = 11.15 | P < 0.005 |
| Condition (long, short, and random) | F(2,88) = 3.77 | P < 0.05 |
| Age × condition | F(2,88) = 13.70 | P < 0.0001 |
| Simple main effect of condition | ||
| 3‐month‐old infants (TP_3M) | F(2,88) = 13.80 | P < 0.0001 |
| 6‐month‐old infants (TP_6M) | F(2,88) = 3.67 | P < 0.05 |
The right temporoparietal region of the 3‐ and 6‐month‐old infants showed significant difference among the conditions, whereas the difference was not observed in the left hemisphere. To confirm the hemispheric differences in the temporoparietal regions of the 3‐ and 6‐month‐old infants, we applied direct comparisons between the signal changes in the right temporoparietal regions (TP_3M or TP_6M) and their left homologues (Table IV). These comparisons were based on the assumption that the optical path lengths in homologous regions were equivalent. In both infant groups, we found the significant interaction between the hemispheres (right and left) and the conditions (long, short, and random). While the right temporoparietal region showed the significant simple main effect in both infant groups, the left homolog did not show the significant effect. These findings suggest the lateralization of the auditory pitch processing in the right hemisphere of infants.
Table IV.
Direct comparison of signal changes in the right and left temporoparietal regions
| The temporoparietal region of the 3‐month‐old infants (TP_3M, the left homolog) | ||
|---|---|---|
| Hemisphere (right and left) | F(1,21) = 0.59 | P > 0.1 |
| Condition (long, short, and random) | F(2,42) = 6.33 | P < 0.005 |
| Hemisphere × condition | F(2,42) = 9.66 | P < 0.0005 |
| Simple main effect of condition | ||
| Right temporoparietal region | F(2,84) = 8.90 | P < 0.0005* |
| Left temporoparietal region | F(2,84) = 4.54 | P < 0.05 |
| The temporoparietal region of the 6‐month‐old infants (TP_6M, the left homolog) | ||
| Hemisphere (right and left) | F(1,23) = 0.94 | P > 0.1 |
| Condition (long, short, and random) | F(2,46) = 3.35 | P < 0.05 |
| Hemisphere × condition | F(2,46) = 6.11 | P < 0.005 |
| Simple main effect of condition | ||
| Right temporoparietal region | F(2,92) = 6.84 | P < 0.005* |
| Left temporoparietal region | F(2,92) = 0.75 | P > 0.1 |
The P values with asterisks (*) in the simple main effect satisfied the statistical threshold with the Bonferroni correction (3‐month‐old infants, four channels in the ROI; 6‐month‐old infants, five channels in the ROI).
DISCUSSION
This study examined whether cortical regions show stimulus‐dependent differential activation under the long, short, and random sequence conditions; such activation differences were observed in the right temporoparietal region of both infant age groups, as we have expected. If infants detected individual tones singly, or detected all tones in a sequence holistically, the three conditions we tested would have elicited similar amounts of activation in all cortical regions. The present results dismissed these possibilities, and instead support the possibility that infants detect more than single tones of auditory information, namely pitch changes in successive tones. Our findings confirmed the expectation that the right temporoparietal region is involved in processing pitch changes in not only speech sounds [Homae et al.,2006,2007], but also changes in auditory sequences. While the bilateral auditory areas showed significant activation in the three conditions, the hemispheric difference of the temporoparietal region was observed both in the 3‐ and 6‐month‐old infants (Table IV and Fig. 7). These results showed that both bilateral and right‐lateralized activation observed in previous studies [Dehaene‐Lambertz et al.,2010; Kotilahti et al.,2010; Perani et al.,2010] simultaneously occurred in the infant brain and further demonstrated functional differentiation for pitch processing in the auditory‐related regions of the infant brain.
In the 3‐month‐old infant group, the most prominent activation in the right temporoparietal region was observed when stimulated with the random sequence (Fig. 7a,b), which corresponded to the largest pitch changes between successive tones. This result suggests that the 3‐month‐old infants are sensitive to pitch changes in a short span of time. Otherwise, the 3‐month‐old infants can detect discontinuity in global pattern of pitch changes, which carries the lowest expectation what appears in the following sequences, in a 4.8‐s random sequence. Given that the pitch changes and discontinuities in pitch contours exist also in speech sounds, the right temporoparietal region of the 3‐month‐old infants would process these features when the infants perceive speech sounds.
The stimulus‐dependency of the right temporoparietal activation showed developmental changes from 3 to 6 months of age (Table III and Fig. 7). In the 6‐month‐old infants, the most prominent activation in the right temporoparietal region was observed when stimulated by the short sequence composed of four tones placed at regular pitch intervals, with the fourth tone followed by a larger pitch interval (Fig. 2c). This finding suggests that information extracted from auditory sequences changes with infant development. Although the exact reason why the 3‐ and 6‐month‐old infants exhibited different patterns of stimulus dependency is unexplained, we can raise two possibilities as follows. The first possibility is that a time window for pitch processing in the 6‐month‐old infants is being tuned to continuity of pitch changes in several hundred milliseconds, which corresponds to syllabic transition rates in speech sounds. Around the 6 months old, infants become sensitive to language‐specific information of vowels [for review, Kuhl,2004]. The increased sensitivity to frequency changes in several hundred milliseconds would help infants to detect vowels in the speech sounds. This possibility suggests a hypothesis that the activation pattern of 6‐month‐old infants to speech sounds would be different from the pattern of the 3‐month‐old infants.
The second possibility is that the 6‐month‐old infants could perceive pitch information of not just two successive tones but also multiple tones, including auditory ‘edges’ caused by sudden changes in pitch intervals and chroma that occur only in the short condition. Behavioral studies reported that infants during the second half of the first year of life can perceive the differences between two melodic contours, one of which changes the temporal order of tones presented in an original sequence [Trehub et al.,1984,1987]. Infants may also use changes in pitch to remember musical melodies [Plantinga and Trainor,2005], suggesting that infants can successfully use both pitch information and the temporal order of multiple tones to assess differences between musical melodies. The cortical mechanisms involved in the processing of sequential auditory events and edges were examined in fMRI studies on adults, and the right temporoparietal region was a candidate for these mechanisms [Herdener et al.,2007; Mustovic et al.,2003]. When auditory sequences enter auditory memory, sequential groups of auditory information may be formed [Koelsch and Siebel,2005]. Therefore, this region may be related to the formation of groups of auditory sequences, such as the most representative properties of music. Future studies are required to clarify how the developing brain processes the continuity and discontinuity of auditory information.
We found that the right prefrontal region of the 6‐month‐old infants showed the prominent activation in response to the short sequence (Fig. 7d). The measurement channel of the prefrontal region was positioned approximately at the midpoint between C4 and F8 of the international 10‐20 system, which may have corresponded to the ventral part of the prefrontal sulcus [Okamoto et al.,2004]. This channel may detect the activation in the inferior frontal gyrus and the ventral subregion of the premotor cortex. The functional roles of the right prefrontal region for auditory processing have been introduced in adult studies, such as the detection of auditory deviance, the temporal maintenance of auditory information, and the processing of musical structures [Doeller et al.,2003; Koelsch et al.,2005; Schubotz and von Cramon,2002; Zatorre et al.,1994]. The anatomical connection between the prefrontal region and the temporoparietal region has been reported in primate studies [Petrides and Pandya,1988; Romanski et al.,1999] and in human studies using diffusion tensor imaging (DTI) [Makris et al.,2005; Tomassini et al.,2007]. A small tract of the superior longitudinal fasciculus, which connects these regions, was identified in 3‐ to 12‐month‐old infants [Zhang et al.,2007], although myelination of the fiber progresses slowly in neonates, infants, and toddlers. Furthermore, the arcuate fasciculus can be reconstructed by tractography of DTI in 1‐ to 4‐month‐old infants [Dubois et al.,2009], suggesting that the connection at least partly exists in early infancy. These reports of anatomical connections in infants and our observation of stimulus‐dependent coactivation in these regions provide a hypothesis that the prefrontal region works in cooperation with the temporoparietal region for processing pitch information, and these regions may form a functional network even in infancy. The interaction among multiple regions would play an important role in constructing a biological foundation for the processing of auditory information, including information from musical notes and speech sounds.
The activated cortical regions in response to auditory stimuli of all the three conditions become localized to the bilateral temporal cortex during the course of development (Fig. 6a,c), whereas cortical regions that are related to pitch processing in the auditory sequences may evolve into a broader network including the right temporoparietal and prefrontal regions (Fig. 7a,d). The latter tendency is consistent with our previous report that while the right temporoparietal region of 3‐month‐old infants is related to the processing of speech pitch information, both the prefrontal and temporoparietal regions of 10‐month‐old infants are involved in the pitch processing (Fig. 1) [Homae et al.,2006,2007]. The developmental changes in activation patterns may be affected by both cognitive development and maturation of cortico‐cortical connections, as well as thalamo‐cortical connections, in the developing brain [Berl et al.,2006; Casey et al.,2005; Homae et al.,2010]. The present results suggest that the neural development proceeds in two directions, localization and globalization, to organize functional networks for perception, cognition, and behavior.
Acknowledgements
The authors thank Kayo Asakawa for her technical and administrative assistance.
REFERENCES
- Aslin RN, Jusczyk PW, Pisoni DB ( 1998): Speech and auditory processing during infancy: Constraints on and precoursors to language In: Kuhn D, Siegler RS, editors. Handbook of Child Psychology, Vol. 2, Cognition, Perception, and Language, 5th ed. New York: Wiley; pp 147–198. [Google Scholar]
- Benjamini Y, Hochberg Y ( 1995): Controlling the false discovery rate—A practical and powerful approach to multiple testing. J R Stat Soc Ser B: Methodol 57: 289–300. [Google Scholar]
- Berl MM, Vaidya CJ, Gaillard WD ( 2006): Functional imaging of developmental and adaptive changes in neurocognition. Neuroimage 30: 679–691. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S ( 2005): Imaging the developing brain: What have we learned about cognitive development? Trends Cogn Sci 9: 104–110. [DOI] [PubMed] [Google Scholar]
- Dehaene‐Lambertz G, Dehaene S, Hertz‐Pannier L ( 2002): Functional neuroimaging of speech perception in infants. Science 298: 2013–2015. [DOI] [PubMed] [Google Scholar]
- Dehaene‐Lambertz G, Montavont A, Jobert A, Allirol L, Dubois J, Hertz‐Pannier L, Dehaene S ( 2010): Language or music, mother or Mozart? Structural and environmental influences on infants' language networks. Brain Lang 114: 53–65. [DOI] [PubMed] [Google Scholar]
- Demany L, Armand F ( 1984): The perceptual reality of tone chroma in early infancy. J Acoust Soc Am 76: 57–66. [DOI] [PubMed] [Google Scholar]
- Doeller CF, Opitz B, Mecklinger A, Krick C, Reith W, Schroger E ( 2003): Prefrontal cortex involvement in preattentive auditory deviance detection: neuroimaging and electrophysiological evidence. Neuroimage 20: 1270–1282. [DOI] [PubMed] [Google Scholar]
- Dubois J, Hertz‐Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene‐Lambertz G ( 2009): Structural asymmetries in the infant language and sensori‐motor networks. Cereb Cortex 19: 414–23. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Rees A, Witton C, Cross PM, Shakir RA, Green GGR ( 1997): Spatial and temporal auditory processing deficits following right hemisphere infarction—A psychophysical study. Brain 120: 785–794. [DOI] [PubMed] [Google Scholar]
- Herdener M, Esposito F, Di Salle F, Lehmann C, Bach DR, Scheffler K, Seifritz E ( 2007): BOLD correlates of edge detection in human auditory cortex. Neuroimage 36: 194–201. [DOI] [PubMed] [Google Scholar]
- Herwig U, Satrapi P, Schonfeldt‐Lecuona C ( 2003): Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr 16: 95–99. [DOI] [PubMed] [Google Scholar]
- Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, Coalson T, Van Essen D ( 2010): A surface‐based analysis of hemispheric asymmetries and folding of cerebral cortex in term‐born human infants. J Neurosci 30: 2268–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Asakawa K, Taga G ( 2006): The right hemisphere of sleeping infant perceives sentential prosody. Neurosci Res 54: 276–280. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Taga G ( 2007): Prosodic processing in the developing brain. Neurosci Res 59: 29–39. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Otobe T, Nakano T, Go T, Konishi Y, Taga G ( 2010): Development of global cortical networks in early infancy. J Neurosci 30: 4877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan RW, Herman J, Purdy P ( 1987): Cerebral location of international 10–20 system electrode placement. Electroencephalogr Clin Neurophysiol 66: 376–382. [DOI] [PubMed] [Google Scholar]
- Jöbsis FF ( 1977): Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198: 1264–1267. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW ( 1999): Narrowing the distance to language: one step at a time. J Commun Disord 32: 207–222. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Siebel WA ( 2005): Towards a neural basis of music perception. Trends Cogn Sci 9: 578–584. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Fritz T, Schulze K, Alsop D, Schlaug G ( 2005): Adults and children processing music: An fMRI study. Neuroimage 25: 1068–1076. [DOI] [PubMed] [Google Scholar]
- Kotilahti K, Nissilä I, Huotilainen M, Mäkelä R, Gavrielides N, Noponen T, Björkman P, Fellman V, Katila T ( 2005): Bilateral hemodynamic responses to auditory stimulation in newborn infants. Neuroreport 16: 1373–1377. [DOI] [PubMed] [Google Scholar]
- Kotilahti K, Nissila I, Nasi T, Lipiainen L, Noponen T, Merilainen P, Huotilainen M, Fellman V ( 2010): Hemodynamic responses to speech and music in newborn infants. Hum Brain Mapp 31: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK ( 2004): Early language acquisition: Cracking the speech code. Nat Rev Neurosci 5: 831–843. [DOI] [PubMed] [Google Scholar]
- Maki A, Yamashita Y, Ito Y, Watanabe E, Mayanagi Y, Koizumi H ( 1995): Spatial and temporal analysis of human motor activity using noninvasive NIR topography. Med Phys 22: 1997–2005. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Pandya DN ( 2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15: 854–869. [DOI] [PubMed] [Google Scholar]
- Minagawa‐Kawai Y, Mori K, Naoi N, Kojima S ( 2007): Neural attunement processes in infants during the acquisition of a language‐specific phonemic contrast. J Neurosci 27: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustovic H, Scheffler K, Di Salle F, Esposito F, Neuhoff JG, Hennig J, Seifritz E ( 2003): Temporal integration of sequential auditory events: silent period in sound pattern activates human planum temporale. Neuroimage 20: 429–434. [DOI] [PubMed] [Google Scholar]
- Nakano T, Homae F, Watanabe H, Taga G ( 2008): Anticipatory cortical activation precedes auditory events in sleeping infants. PLoS One 3: e3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Watanabe H, Homae F, Taga G ( 2009): Prefrontal cortical involvement in young infants' analysis of novelty. Cereb Cortex 19: 455–463. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB ( 2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Obrig H, Villringer A ( 2003): Beyond the visible—Imaging the human brain with light. J Cereb Blood Flow Metab 23: 1–18. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Oda I, Isobe S, Suzuki T, Kohyama K, Dan I ( 2004): Three‐dimensional probabilistic anatomical cranio‐cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 21: 99–111. [DOI] [PubMed] [Google Scholar]
- Patterson RD, Uppenkamp S, Johnsrude IS, Griffiths TD ( 2002): The processing of temporal pitch and melody information in auditory cortex. Neuron 36: 767–776. [DOI] [PubMed] [Google Scholar]
- Peña M, Maki A, Kovacic D, Dehaene‐Lambertz G, Koizumi H, Bouquet F, Mehler J ( 2003): Sounds and silence: An optical topography study of language recognition at birth. Proc Natl Acad Sci USA 100: 11702–11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Spada D, Andreolli G, Rovelli R, Baldoli C, Koelsch S ( 2010): Functional specializations for music processing in the human newborn brain. Proc Natl Acad Sci USA 107: 4758–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN ( 1988): Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol 273: 52–66. [DOI] [PubMed] [Google Scholar]
- Plantinga J, Trainor LJ ( 2005): Memory for melody: Infants use a relative pitch code. Cognition 98: 1–11. [DOI] [PubMed] [Google Scholar]
- Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD ( 2000): Auditory processing across the sleep–wake cycle: Simultaneous EEG and fMRI monitoring in humans. Neuron 28: 991–999. [DOI] [PubMed] [Google Scholar]
- Reynolds EOR, Wyatt JS, Azzopardi D, Delpy DT, Cady EB, Cope M, Wray S ( 1988): New non‐invasive methods for assessing brain oxygenation and hemodynamics. Br Med Bull 44: 1052–1075. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman‐Rakic PS, Rauschecker JP ( 1999): Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci 2: 1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY ( 2002): Predicting perceptual events activates corresponding motor schemes in lateral premotor cortex: An fMRI study. Neuroimage 15: 787–796. [DOI] [PubMed] [Google Scholar]
- Singh AK, Dan I ( 2006): Exploring the false discovery rate in multichannel NIRS. Neuroimage 33: 542–549. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Fürst G, Meyer BU ( 1989): Craniocerebral topography within the international 10–20 system. Electroencephalogr Clin Neurophysiol 72: 499–506. [DOI] [PubMed] [Google Scholar]
- Taga G, Asakawa K ( 2007): Selectivity and localization of cortical response to auditory and visual stimulation in awake infants aged 2 to 4 months. Neuroimage 36: 1246–1252. [DOI] [PubMed] [Google Scholar]
- Taga G, Asakawa K, Maki A, Konishi Y, Koizumi H ( 2003): Brain imaging in awake infants by near‐infrared optical topography. Proc Natl Acad Sci USA 100: 10722–10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga G, Homae F, Watanabe H ( 2007): Effects of source‐detector distance of near infrared spectroscopy on the measurement of the cortical hemodynamic response in infants. Neuroimage 38: 452–460. [DOI] [PubMed] [Google Scholar]
- Telkemeyer S, Rossi S, Koch SP, Nierhaus T, Steinbrink J, Poeppel D, Obrig H, Wartenburger I ( 2009): Sensitivity of newborn auditory cortex to the temporal structure of sounds. J Neurosci 29: 14726–14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Klein JC, Behrens TEJ, Pozzilli C, Matthews PM, Rushworth MFS, Johansen‐Berg H ( 2007): Diffusion‐weighted imaging tractography‐based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. J Neurosci 27: 10259–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehub SE, Hannon EE ( 2006): Infant music perception: Domain‐general or domain‐specific mechanisms? Cognition 100: 73–99. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Bull D, Thorpe LA ( 1984): Infants' perception of melodies: The role of melodic contour. Child Dev 55: 821–830. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Thorpe LA, Morrongiello BA ( 1987): Organizational processes in infants' perception of auditory patterns. Child Dev 58: 741–749. [PubMed] [Google Scholar]
- Villringer A, Chance B ( 1997): Non‐invasive optical spectroscopy and imaging of human brain function. Trends Neurosci 20: 435–442. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Homae F, Nakano T, Taga G ( 2008): Functional activation in diverse regions of the developing brain of human infants. Neuroimage 43: 346–357. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Homae F, Taga G ( 2010): General to specific development of functional activation in the cerebral cortexes of 2‐ to 3‐month‐old infants. Neuroimage 50: 1536–1544. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Maki A, Koizumi H ( 1996): Near‐infrared topographic measurement system: Imaging of absorbers localized in a scattering medium. Rev Sci Instrum 67: 730–732. [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E ( 1994): Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci 14: 1908–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Evans A, Hermoye L, Lee SK, Wakana S, Zhang WH, Donohue P, Miller MI, Huang H, Wang XQ, van Zijl PCM, Mori S ( 2007): Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage 38: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]


