Abstract
Real‐time functional magnetic resonance imaging (rtfMRI) is a novel technique that has allowed subjects to achieve self‐regulation of circumscribed brain regions. Despite its anticipated therapeutic benefits, there is no report on successful application of this technique in psychiatric populations. The objectives of the present study were to train schizophrenia patients to achieve volitional control of bilateral anterior insula cortex on multiple days, and to explore the effect of learned self‐regulation on face emotion recognition (an extensively studied deficit in schizophrenia) and on brain network connectivity. Nine patients with schizophrenia were trained to regulate the hemodynamic response in bilateral anterior insula with contingent rtfMRI neurofeedback, through a 2‐weeks training. At the end of the training stage, patients performed a face emotion recognition task to explore behavioral effects of learned self‐regulation. A learning effect in self‐regulation was found for bilateral anterior insula, which persisted through the training. Following successful self‐regulation, patients recognized disgust faces more accurately and happy faces less accurately. Improvements in disgust recognition were correlated with levels of self‐activation of right insula. RtfMRI training led to an increase in the number of the incoming and outgoing effective connections of the anterior insula. This study shows for the first time that patients with schizophrenia can learn volitional brain regulation by rtfMRI feedback training leading to changes in the perception of emotions and modulations of the brain network connectivity. These findings open the door for further studies of rtfMRI in severely ill psychiatric populations, and possible therapeutic applications. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: real‐time fMRI, schizophrenia, insula, face emotion recognition, self‐regulation, neurofeedback
INTRODUCTION
Since the 1960s, a variety of studies has demonstrated that subjects can be trained by neurofeedback to gain voluntary control of different components of the electroencephalographic spectrum [Birbaumer, 2006; Birbaumer and Cohen, 2007; Strehl et al., 2006]. In comparison, the use of metabolic activity as a method to achieve brain self‐regulation has been much more recent, becoming feasible due to progress in real‐time functional magnetic resonance imagining (rtfMRI), a noninvasive method that can be used for online feedback of the blood oxygenation‐level dependent (BOLD) signal [Sitaram et al., 2008]. Because of its high spatial and temporal resolution, rtfMRI has enabled subjects to achieve volitional control of the activity of circumscribed brain areas [deCharms et al., 2005; Hamilton et al., 2011; Rota et al., 2009; Yoo et al., 2006]. Hence, rtfMRI has arisen as a complete novel approach for cognitive neuroscience, in which the manipulation of the brain activity can be used as an independent variable that allows the study of subsequent behavioral modifications. However, despite its promising achievements, and the repeated statement of the potential therapeutic benefits of its use on psychiatric disorders [Caria et al., 2007, 2010; deCharms, 2008; Posse et al., 2003; Weiskopf et al., 2004, 2007], no study has yet demonstrated the application of rtfMRI in these populations.
In a recent study of our group, Caria et al. demonstrated for the first time that healthy individuals can acquire control of the BOLD activation in left anterior insula by rtfMRI training, and that learned self‐regulation modulates the response to aversive stimuli [Caria et al., 2010]. The successful self‐regulation of the anterior insula is of exceptional importance due to its potential application to mental disorders caused by dysfunction of brain networks with critical involvement of this region [Nagai et al., 2007; Naqvi and Bechara, 2009; Shapira et al., 2003].
Schizophrenia is a chronic, often devastating disorder in which the hypothesis that insular dysfunction plays a central role in its psychopathology has been supported by recent reports of insula volume reduction [Makris et al., 2006; Meisenzahl et al., 2008; Paillere‐Martinot et al., 2001; Price et al., 2010; Saze et al., 2007; Sigmundsson et al., 2001; Takahashi et al., 2004], perfusion abnormalities linked to general symptomatology and cognitive dysfunctions [Crespo‐Facorro et al., 2001b; Curtis et al., 1998; Farrer et al., 2004; Surguladze et al., 2001; Yoo et al., 2005], and deficits in emotional processing [Crespo‐Facorro et al., 2001a; Paradiso et al., 2003; Sanjuan et al., 2007].
A specific role of the insula in the recognition of emotional expressions has been recognized, particularly for the recognition of disgust faces (a primitive function related to the appraisal of distasteful or dangerous stimuli), based on functional imaging [Phillips et al., 1997, 2004; Sambataro et al., 2006; Wicker et al., 2003; Williams et al., 2005], lesion studies [Adolphs et al., 2003; Calder et al., 2000], and findings in clinical populations [Hennenlotter et al., 2004; Kipps et al., 2007]. Abnormal perception of emotional faces is central to the psychopathology of schizophrenia [Morrison et al., 1988], is associated with poor social functioning [Couture et al., 2006] and is known to persist over the course of the illness [Addington and Addington, 1998; Hooker and Park, 2002]. Several reports have stated that this deficit is more noticeable for negative emotions, and might be specially pronounced for disgust faces [Chambon et al., 2006; Kohler et al., 2000, 2003; Schneider et al., 2006]. Besides the fronto‐limbic dysfunction suggested as the neural basis for the deficit in face emotion recognition [Das et al., 2007; Fakra et al., 2008], a diminished activation of anterior insula could be associated with the particular deficit to recognize expressions of disgust in schizophrenia [Phillips et al., 1999; Williams et al., 2007].
In the current study, we applied for the first time rtfMRI in a psychiatric population, i.e., schizophrenia patients, and attempted to answer crucial questions for therapeutic applications of this technique. Our first specific aim was to evaluate if patients with schizophrenia can learn self‐control of the BOLD signal of the anterior insula cortex by rtfMRI neurofeedback, and to investigate if self‐regulation capability is correlated with clinical measures and symptom severity. Most rtfMRI studies have used a short period of training of 1 or 2 days, and few neurofeedback sessions. In the present study, we used an extended protocol spanning more than 2 weeks, as we expected that schizophrenia patients might need more sessions of training to achieve self‐regulation.
Our second aim was to examine whether the enhancement of insula activity by self‐regulation is accompanied by a behavioral change related to a particular impairment in schizophrenia. Considering the role of insula in the recognition of disgust, we hypothesized that schizophrenia patients perform better in the recognition of disgust emotional faces following learned self‐regulation of insula cortex.
The final aim of the study was to explore changes in the connectivity of the brain network associated with the neurofeedback training. The majority of previous studies have only examined single region of interest (ROI) modulations by rtfMRI. However, the modulation of a brain network by voluntary control is of special interest for cognitive neuroscience in general and schizophrenia in particularly, in light of the growing evidence linking cognitive and emotional processing deficits and an abnormal neural connectivity in this disease [Boksman et al., 2005; Das et al., 2007; Fakra et al., 2008; Fletcher et al., 1999; Honey et al., 2005; Meyer‐Lindenberg, 2010; Meyer‐Lindenberg et al., 2005; Witthaus et al., 2008]. We investigated whether the rtfMRI training modifies the connectivity of insula with other areas of the emotional network involved in self‐regulation, particularly amygdala and medial prefrontal cortex [MPFC; Ochsner et al., 2004; Phan et al., 2002], regions whose dysfunction and abnormal connectivity are considered to be central to the schizophrenia psychopathology [Das et al., 2007; Fakra et al., 2008; Pomarol‐Clotet et al., 2010].
METHODS AND MATERIALS
Participants
Nine right handed schizophrenia patients (paranoid subtype, five female, mean age: 26.3 years) were recruited, diagnosed, and subtyped using the Structured Clinical Interview for DSM‐IV disorders [Wittchen et al., 1997]. All patients were receiving atypical antipsychotic medication without modification for at least 4 weeks (more information regarding the participants can be found in Supporting Information). The general psychopathological assessment included the Positive and Negative Syndrome Scale [PANSS; Kay et al., 1987] and the Calgary Depression Scale for Schizophrenia [Addington et al., 1990; Table I]. All participants of the study were naive to neurofeedback and fMRI experiments. After complete description of the study to the participants, written informed consent was obtained. The study was approved by the Ethics Committee of the Medical Faculty of the University of Tübingen.
Table I.
Demographic and clinical characteristics of schizophrenia patients (n = 9)
| Mean | SD | |
|---|---|---|
| Age, years | 26.3 | 4.5 |
| Duration of illnessa | 4 | 2.1 |
| Chlorpromazine equivalents | 951 mg | 385 |
| PANSS – P | 14 | 3.7 |
| PANSS – N | 16.2 | 4.7 |
| PANSS – G | 32.1 | 5.6 |
| Calgary depression scale | 4.3 | 3.6 |
Abbreviations: PANSS‐N: PANSS negative symptoms sub‐score; PANSS‐P: PANSS positive symptoms sub‐score; PANSS‐G: PANSS general psychopathology sub‐score.
Duration of illness was calculated from the first psychotic episode.
Experimental Protocol
The general design of the study can be seen in Figure 1.
Figure 1.
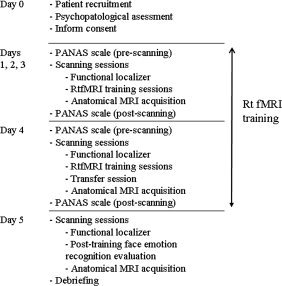
Experimental protocol.
RtfMRI self‐regulation training
Schizophrenia patients participated in 4 days of training, conducted in 2 weeks (2 days per week). Every day, they performed three scanning sessions of self‐regulation training (two patients could only complete 10 training sessions instead of 12). Each session consisted of alternating upregulation (self‐regulation; n = 6), and baseline blocks (n = 7; 30 s each block). During upregulation blocks, patients were asked to increase the BOLD signal in the target ROI (left and right anterior insula), guided by contingent rtfMRI visual feedback.
The rtfMRI‐system was implemented at the Institute of Medical Psychology and Behavioral Neurobiology, Tübingen [for a complete description of the system, see Caria et al., 2007; Sitaram et al., 2008]. A functional localizer session was performed on each patient and each day in order to delineate ROI1 in a block‐based paradigm consisting of four blocks in which patients had to use imagery to recall emotionally relevant experiences, alternating with five resting blocks (22.5 s each). The ROI1 comprised two rectangular areas (left and right anterior insula cortex) encompassing 4 × 5 voxels on a single slice of 5 mm thickness. The reference ROI, namely ROI2, was selected as a background area encompassing a slice at the location z = 60 (passing through upper brain structures, e.g., top part of motor cortex and parietal areas), to cancel global effects and to average out any unspecific activation.
For the feedback presentation during the training, the difference between ROI1 and ROI2 time‐courses was calculated and normalized to the baseline. The feedback signal was computed as (BOLDreg − BOLDrest)ROI1 − (BOLDreg − BOLDrest)ROI2. BOLDreg and BOLDrest constituted the BOLD signal during the current scan of upregulation, and the average BOLD signal from the previous baseline (rest) block, respectively. Feedback was presented to the patients during up‐regulation blocks in the form of a graphical thermometer (using a projector in the scanner), which displayed red bars that increased or decreased in number according to the increase or decrease of the feedback signal, as computed above. For the feedback, bars were continually updated at intervals of 1.5 s with new fMRI information. Patients were instructed that the recall of emotionally relevant past experiences combined with the feedback could enable them to control the thermometer bars. No specific emotional cues or recall strategies were given. Baseline and upregulation blocks were cued by blue and red colored backgrounds of the thermometer window. During baseline blocks, thermometer bars remained static (no feedback), and patients were instructed to relax in order to bring back the BOLD signal to the baseline level.
On the last day of training, every patient performed a single “transfer” session, during which they were instructed to perform self‐regulation without any feedback information, intended to test whether training effects persist in the absence of feedback.
On each day of training, at the beginning and end of the fMRI sessions, patients answered the Positive and Negative Affect Schedule [PANAS; Watson et al., 1988], a psychometric scale developed to measure the independent constructs of positive and negative affect. PANAS contains a list of 10 descriptors for positive scale: attentive, interested, alert, excited, enthusiastic, inspired, proud, determined, strong, and active; and 10 descriptors for negative scale: distressed, upset‐distressed; hostile, irritable‐angry; scared, afraid‐fearful; ashamed, guilty; nervous, and jittery. In our study, the scale was used to investigate if current state of affect correlates with the capability to self‐regulate, and to explore if any unpleasant affect could be present after the sessions of insula self‐regulation. To further explore any possible negative effect of the training, patients were instructed to report any discomfort during the study, and were actively asked about it after each scanning session.
MR acquisition
Functional images were obtained on a 3.0 Tesla body scanner, with standard 12‐channels head coil (Siemens Magnetom Tim TRIO, Siemens, Erlangen, Germany). A standard echo‐planar imaging sequence was used (EPI; TR = 1.5 s, matrix size = 64 × 64, TE = 30 ms, flip angle α = 70°). Sixteen oblique slices (voxel size = 3.3 × 3.3 × 5.0 mm3, slice gap = 1 mm), AC/PC aligned in axial orientation were acquired. For superposition of functional maps upon brain anatomy a high‐resolution T1‐weighted structural scan of the whole brain was collected each day of scanning, from each participant (MPRAGE, matrix size = 256 × 256, 160 partitions, 1 mm3 isotropic voxels, TR = 2,300 ms, TE = 3.93 ms, TI = 1100 ms, α = 8°).
Posttraining face emotion recognition evaluation
After the completion of the self‐regulation training, a face emotion recognition task was performed to evaluate the effect of the acquired insula self‐regulation on the perception of emotional faces. The evaluation consisted of eight scanning sessions, each session comprising alternating upregulation and baseline tests. Each upregulation test consisted of an upregulation block (30 s), followed by a picture presentation block (5 s) and a subjective rating (evaluation) block (10 s) (see Fig. 2). During the upregulation blocks patients had to self‐regulate anterior insula cortex, aided by the contingent rtfMRI visual feedback, just as they had learned during the training. During the picture presentation block, one happy (out of 20) or one disgust face (out of 20) were displayed for 5 s, and patients were instructed to select the type of emotion (among seven options: fear, anger, disgust, sad, happy, surprise, or neutral), and its emotional intensity in a scale varying from 1 (without emotion) to 9 (extremely intense). During rating blocks participants indicated their choices: type of emotion and emotion intensity (5 s for each choice), using MR‐compatible buttons, via an adapted version of the Self Assessment Manikin [Bradley and Lang, 1994]. Each baseline test consisted of first a baseline block (as in the training sessions), followed by a picture presentation block and a rating block, as above. Although the main goal of the task was to evaluate the effect of self‐regulation on recognition of disgust, we included happy faces in the paradigm to explore results in an emotion of opposite valence. No information about the fact that only two emotions would be displayed was given to the patients. To enable comparison of the ratings of the pictures, the same happy and disgust faces were presented during upregulation and baseline tests. To avoid repetition effects, the order of presentation of faces were pseudo‐randomized across upregulation and baseline tests.
Figure 2.
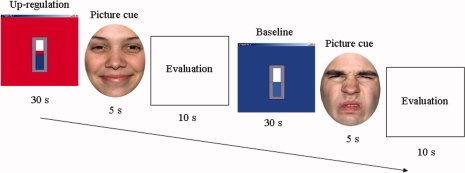
Posttraining face emotion recognition evaluation (Day 5). Experimental design for the evaluation of emotional faces displaying an upregulation and a baseline (nonregulation) test. Upregulation and baseline blocks were cued with red and blue backgrounds, respectively.
Off‐Line Data Analysis
Insula cortex self‐regulation
ROI analysis of the BOLD signal increase in left and right anterior insula with respect to baseline in each session of self‐regulation training was compared by using the Fisher score (FS), which measures the discriminability between BOLD signals of two conditions, in this case “baseline” and “upregulation” blocks. While conventional measures of the BOLD signal change in a pre‐selected ROI are calculated only by the mean difference between two conditions, FS considers both the variance and the mean [Bishop, 1995; Lal et al., 2004]. With two time series (defined as X1 = BOLD time series during upregulation blocks and X2 = BOLD time series during baseline), the FS is then defined as ratio of the square of the difference between the mean BOLD values in each time‐series to the sum of the variance in the time‐series.
In our study, for the calculation of the FS, BOLD values were extracted from two rectangles, each of size 4 × 5 voxels (∼15 × 20 mm2), encompassing the MNI coordinates: x: −27, −42; y: 10, 35; z: −5, 5; for left insula, and x: 27, 42; y: 10, 35; z: −5, 5; for right insula.
The capability to volitionally regulate anterior insula was quantified by the average FS of the sessions of training, and the slope of the regression line (learning curve) of the FS values computed across the training sessions. To confirm that patients successfully regulated insula during the emotion recognition evaluation, we applied the same procedure, by extracting the information from baseline and upregulation blocks.
All distributions of the data set resulting from the behavioral tests, psychopathological scales, and FS were checked for normality. Non‐parametric test were used for non‐normally distributed data. All analyses were two‐tailed.
Connectivity analysis
Effective connectivity analysis was conducted using Granger causality modeling (GCM), methodology that uses temporal information in one or more time‐series of signals from a certain brain region to predict signal time courses in another, so that temporally directed influences rather than only correlations in activity between different brain areas can be generated [Abler et al., 2006; Seth, 2005, 2010].
To evaluate changes in effective connectivity during feedback training, causal density (CD) of the emotional brain network involved in self‐regulation was computed. Causal Density is a measure of connectivity, defined by the fraction of interactions among ROIs in a network that are causally significant, and is represented by the equation: CD = GC/N(N − 1), where GC is the total number of significant causal connections observed, and N is the number of ROIs [Seth, 2005, 2010]. A set of unconnected ROIs will have low CD. To determine ROIs to be included in the analysis, we used a mixed approach. First, we included brain areas that have been consistently reported in the literature as important in the cognitive regulation of emotions [Ochsner et al., 2004]. Second, a group GLM analysis of functional images was performed to determine the top‐most clusters of activations associated with successful self‐regulation in the patients. The analysis was performed using random effect analysis, by a paired t test that compared the last day of training (three sessions) and the first day of training (three sessions), (for further details please see Supporting Information). Eight ROIs were chosen to be include in the GCM (center MNI coordinates x,y,z): MidFG/middle frontal gyrus (−50, 2, 42), MPFC/medial prefrontal cortex (−6, 48, 40), ACC/anterior cingulate cortex (−3,7,25), left Insula (−46,10,0), right insula (46,0,0), amygdala (−18,−10,−14), SMG/supra marginal gyrus (−66,−26,25), and precuneus (−10,−49, 40). Averaged time‐series of BOLD activations from the self‐regulation blocks of the experiments for each of these ROIs was extracted from the preprocessed (i.e., realigned, time resliced, normalized, and spatially smoothed) fMRI data. For each ROI, the time series were obtained from a box containing a central voxel and the immediately adjacent 26 voxels. To correct for intersubject differences in BOLD magnitudes, mean BOLD value for each subject was subtracted from his/her time‐series data, in each ROI.
The connectivity of the network involved in self‐regulation was analyzed by comparing the CD of the weakest and the strongest session of regulation per subject (according to the FS), of the first and last day of training, respectively.
RESULTS
Insula self‐regulation
The BOLD increase during upregulation compared to baseline, improved with the number of training sessions, showing that patients successfully learned to control the BOLD‐magnitude in the anterior insula, with rtfMRI training (see Fig. 3). A group linear regression analysis performed across all sessions of training showed a significant increase in BOLD signal (measured by the FS) for both left [y = 0.015x + 0.07, P < 0.001, R 2 = 0.1 ] and right anterior insula [y = 0.023x + 0.03, P < 0.001, R 2 = 0.25]. In a post hoc test, involving a comparison of later sessions with the first session of training, a significant higher FS was found in session number 8 (z = 2.02, P = 0.04), 9 (z = 2.07, P = 0.03), 10 (z = 1.96, P = 0.05), and 12 (z = 2.2, P = 0.02) for left anterior insula. For right anterior insula, sessions with significant higher FS compared with the first session were Session 3 (z = 1.96, P = 0.05), 7 (z = 2.19, P = 0.02), 8 (z = 2.55, P < 0.01), 9 (z = 2.56, P = 0.01), 10 (z = 2.67, P < 0.01), 11 (z = 2.03, P = 0.04), and 12 (z = 2.37, P = 0.01; Wilcoxon signed‐rank test). Bold‐times series of a representative patient can be seen in Figure 1 of Supporting Information. Patients reported the use of emotional imagery and recall of positive and negative emotional autobiographic episodes as strategies for gaining volitional control of the BOLD response.
Figure 3.
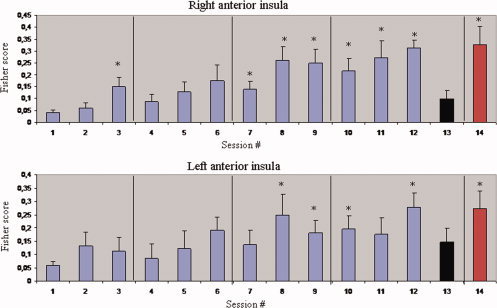
Group analysis of BOLD signal change in left and right anterior insula, across the 4 days of rtfMRI training and the emotion recognition evaluation. Blue bars represent the Fisher Score (and standard error) during training sessions. The black bars represent the transfer sessions, and the red bars represent the mean of the FS (and standard error) of the sessions of the emotion recognition evaluation. *P < 0.05, for the Wilcoxon signed‐rank test comparing the values of each session with the first session of training.
For the transfer session, although the FS values were higher compared with the first session of training, this difference was not significant for left insula (z = 1.481, P = 0.14) and was close to significant for the right insula (z = 1.84, P = 0.07; Wilcoxon signed‐rank test). The FS of the transfer sessions were positively correlated with the mean FS of the sessions of training, for both left (Pearson r = 0.75, P = 0.01) and right insula (r = 0.77, P = 0.01).
The mean FS calculated from the sessions of the emotion recognition evaluation, was also significantly higher than the first session of training both for left insula (z = 2.66, P < 0.01), and right insula (z = 2.66, P < 0.01; Wilcoxon signed‐rank test).
No significant differences were found for the PANAS scores before and after the scanning sessions, neither in the negative affects scale (median prescan = 16, median postscan = 15; z = −1.25, ns, r = 0.17), nor in the positive affects scale (median prescan = 25, median postscan = 25; z = −0.38, ns, r = 0.05, Wilcoxon signed‐rank test). Patients did not report any discomfort during the training sessions, apart for occasional mild “tiredness.”
Self‐regulation and Psychopathology
The enhancement of insular activation, as measured by the mean FS across the sessions of training, was negatively correlated with the negative symptoms (measured by the PANSS‐N scale), (left insula: Pearson r = −0.68, P = 0.04; right insula: r = −0.72, P = 0.03), and the duration of the illness for both left and right insula (left insula: r = −0.72, P = 0.03; right insula: r = −0.79, P = 0.01; tested for normality, Shapiro‐Wilks test).
No significant correlations were found between the mean FS across training or the learning curve and other clinical measures such us, the positive symptoms (measured by the PANSS‐P scale), PANSS‐G, age of onset of the disease, chlorpromazine equivalents, the calgary depression scale, or the positive and negative affect scores of the PANAS.
Posttraining Face Emotion Recognition Evaluation
Following upregulation, patients correctly identified significantly more disgust faces (48.3% ± 23.97%, mean ± SD) than following baseline (37.7% ± 17.69%, mean ± SD; paired t test, t(8) = −2.8, P = 0.02; tested for normality, Shapiro‐Wilks test), indicating a significantly higher sensitivity toward disgust faces following up‐regulation than baseline.
Patients correctly identified significantly fewer happy faces following upregulation (60.5% ± 18.78%, mean ± SD) than after baseline blocks (73.3% ± 15.41%, mean ± SD; paired t test, t(8) = 2.7, P = 0.02; tested for normality, Shapiro‐Wilks test) (see Fig. 4), showing lower sensitivity for happy faces following upregulation. There were no significant differences in the specificity of recognition when compared between upregulation and baseline conditions, either for happy faces (specificity baseline = 98%; specificity upregulation = 99%), or disgust faces (specificity baseline = 98%; specificity upregulation = 98%), (specificity measures the proportion of true negatives which are correctly identified, and was defined by the equation = number of true negatives/(number of true negatives + number of false positives)).
Figure 4.
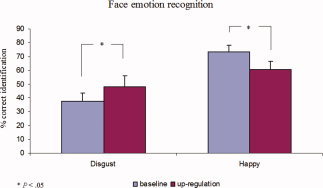
Percentage of correct identification (mean and standard error) for disgust and happy faces during the emotion recognition evaluation.
The analysis of the labeling errors showed that most disgust faces that were wrongly identified, were mislabeled as angry faces, following both baseline and upregulation (Table II). A significant reduction of mislabeling disgust faces as angry faces was observed following upregulation compared with baseline (z = −2.44, P = 0.01, r = −0.57; Wilcoxon signed‐rank test). No difference was found for the intensity ratings for disgust faces following upregulation (5.53 ± 0.98, mean ± SD) and baseline blocks (5.71 ± 0.87, mean ± SD), (t(8) = −1.9, P = 0.08; tested for normality, Shapiro‐Wilks test). Similar lack of significant difference was observed for intensity ratings for happy faces, between baseline (4.58 ± 0.78, mean ± SD) and upregulation (4.48 ± 0.62, mean ± SD; t(8) = 0.79, ns.).
Table II.
Face emotion recognition evaluation (group analysis)
| Disgust faces | Happy faces | ||||||
|---|---|---|---|---|---|---|---|
| Answers | Baseline | UpRegulation | P = | Answers | Baseline | UpRegulation | P = |
| Disgust | 68 | 87 | 0.02 | Happiness | 132 | 109 | 0.02 |
| Sadness | 30 | 34 | n.s. | Sadness | 2 | 5 | n.s. |
| Fear | 13 | 16 | n.s. | Fear | 4 | 1 | n.s. |
| Anger | 57 | 35 | 0.015 | Anger | 0 | 3 | n.s. |
| Neutral | 4 | 7 | n.s. | Neutral | 20 | 29 | n.s. |
| Surprise | 6 | 0 | n.s. | Surprise | 19 | 31 | n.s. |
| Happiness | 2 | 1 | n.s. | Disgust | 3 | 2 | n.s. |
| Total | 180 | 180 | Total | 180 | 180 | ||
The table displays results of the emotion recognition test in all patients following conditions “baseline” and “upregulation.” Numbers represent the total number of selections of the emotions indicated in the first left columns, under the headings “disgust faces” and “happy faces.” As 20 numbers of happy and 20 numbers of disgust faces were presented per condition (up‐regulation and baseline) to each subject (n = 9), the total number of possible answers for each of these two emotions was 180. Right columns under the headings “disgust faces” and “happy faces” show the P‐values for the statistics comparing the number of selections for each emotion between baseline and upregulation conditions.
Correlation Between Self‐regulation and Emotion Recognition
A positive correlation (Spearman r = 0.76, P = 0.018) was found between the capability to self‐regulate right anterior insula (measured by the average FS of the sessions of the emotion recognition evaluation), and the increase in the sensitivity for recognizing disgust faces (measured as the difference between the sensitivity for recognizing disgust following upregulation minus the sensitivity for recognizing disgust following baseline).
Effective Connectivity Changes
Time‐series of BOLD activations in a number of ROIs (n = 8) were extracted and submitted to the GCM analysis (Fig. 5a,b). Network connections among insula cortex, amygdala and MPFC increased with feedback training, as found by comparing between the weakest regulation session (CD: 0.16) and the strongest regulation session (CD: 0.58). In addition, the CD of the network as a whole was enhanced with training. This change is reflected in the larger causal density in the strongest session of regulation compared with the weakest session considering the entire network (0.61 vs. 0.46, respectively), wherein new connections appeared among anterior cingulate cortex, precuneus, supramarginal gyrus, and the rest of the ROIs.
Figure 5.
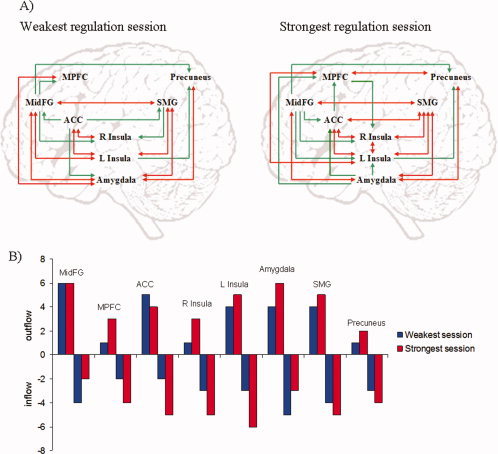
Group analysis of effective connectivity changes due to rtfMRI training using Granger causality modeling (GCM). MidFG: middle frontal gyrus (−50, 2, 42; MNI center coordinates x,y,z), MPFC: medial prefrontal cortex (−6, 48, 40), ACC: anterior cingulate cortex (−3,7,25), L Insula (−46,10,0), R Insula (46,0,0), Amygdala (−18,−10,−14), SMG: supra marginal gyrus (−66,−26,25), precuneus (−10,−49, 40). (A) Directed influences of the emotional network during upregulation, in the weakest and the strongest regulation sessions. Red arrows indicate bidirectional influences between ROIs. (B) Number of outgoing and incoming connections of each ROI of the emotional network during upregulation. Outflow: number of outgoing connections from each of the ROIs. Inflow: number of incoming connections to each of the ROIs.
DISCUSSION
The results show that patients with schizophrenia are able to learn volitional control of the activity of anterior insular cortex, with neurofeedback based on rtfMRI. Interestingly, two factors, namely negative symptoms and the duration of the illness were negatively correlated with the success of self‐regulation. Reductions in the size of insula in schizophrenia patients with predominantly negative symptomatology (affective flattening, alogia, avolition) have been reported [Sigmundsson et al., 2001; Takahashi et al., 2004]. Therefore, if insula abnormalities are part of the neural substrates of negative symptoms, the difficulty to learn self‐regulation in patients with more severe negative symptomatology could reflect the abnormality of the insular structure. Considering the use of emotional imagery as a strategy to achieve self‐regulation, an alternative hypothesis could be that the difficulty to self‐regulate in patients with more negative symptoms could reflect a more general emotional disturbance expressed in that regulatory deficit. In fact, patients with prominent negative symptomatology have several deficits in emotional processing and expression [Addington et al., 1998; Henry et al., 2007; Schneider et al., 1995]. In view of the relatively short duration of the disease in our patients, it is noteworthy that a negative correlation was found between the capability to self‐regulate and illness duration. This finding suggests that factors interfering with self‐regulation may start in early stages of the disease and develop rapidly thereafter. On the other hand, these results also suggest that in schizophrenia patients, some particular aspects of the disease (i.e., negative symptoms) could predict the chances of successful learned brain self‐regulation. In this sense, it is also arguable that other factors, like a general capability to perform imagery, a cognitive deficit, or attention impairment, might play also a role in learned self‐regulation. This possibility has not yet been studied in any previous rtfMRI study, and certainly should be addressed in future works. Similarly, as the patients in our sample were all of paranoid subtype (a subgroup of schizophrenia patients that might have less cognitive impartment than other subtypes), future studies can also compare the self‐regulation performance in different schizophrenia subtypes, or among patients with different cognitive capabilities.
This is the first rtfMRI study to have included such a long training, fact of importance for future therapeutics in severely ill psychiatric and/or neurological patients, in which long period of trainings can be expected. The transfer session showed a reduction in the FS compared to the later sessions of training, indicating that self‐regulation could be best achieved in the presence of feedback. However, as patients performed a transfer session only once at the end of the training, it might be possible that the novelty of the transfer session could have interfered with their performance. Interestingly, a positive correlation was found between the general capability to self‐regulate during the training sessions and the capability to self‐regulate during the transfer session, suggesting that the patients who can better regulate in the presence of feedback, can also have better achievement of self‐regulation in its absence, after the training. In future studies, feedback and transfer sessions could be interspersed in a systematic fashion to improve regulation performance during transfer. This improved protocol could also be used to explore the factors that mediate improvement in performance.
Regarding the behavioral results, patients detected significantly more disgust faces following upregulation of anterior insula, than after the baseline condition, in line with our hypothesis. Furthermore, the increased sensitivity following up‐regulation was correlated with the enhancement in right anterior insula activation. Although previous findings regarding laterality are not consistent, this result supports the reports of right insula activation in the recognition of disgust faces [Anderson et al., 2003; Phillips et al., 1997, 2004; Schroeder et al., 2004]. Also, these findings provide further support, albeit indirectly, to the hypothesis that a dysfunction in insula cortex plays a crucial role in patients' deficit to recognize expressions of disgust. The over‐representation of anger emotion in the labeling errors is concordant with the literature showing that these two emotions tend to be confused, being proximally located in two‐dimensional models of affect [Bullock and Russell, 1986; Gerber et al., 2008]. It is not surprising therefore that the increased sensitivity for recognizing disgust faces following insula upregulation was accompanied by a significant decrease in the erroneous labeling of disgust as anger. Although it is not possible with the current design to totally disentangle the role of some particular form of emotional imagery priming disgust emotion recognition, some arguments make us strongly believe that the increase in anterior insula activity is the crucial and necessary event that leads to the enhanced capability to recognize disgust faces. First, the already mentioned significant correlation found between right insula BOLD levels and the enhanced sensibility toward disgust shows a relationship between brain activation of this region and recognition of disgust. Second, the fact that patients in the study used a variety of positive and negative emotional imagery strategies for self‐regulation of insula, makes it difficult to think that the increased sensitivity toward disgust was due merely to the priming effect caused by one specific form of emotional imagery preceding the recognition test. The diminished capability to recognize happy faces following upregulation of anterior insula was an unexpected finding. Insula activity has been associated with the report of negative valance of emotional pictures [Anders et al., 2004; Viinikainen et al., 2010], and in our previous rtfMRI study of insula regulation, learned self‐regulation led to an increased negative valance on the perceived aversion of emotionally negative pictures in healthy subjects [Caria et al., 2010]. These findings may lead one to speculate that heightened activations of anterior insula might tilt the balance from positive valance stimuli (in this case happy emotional faces), towards a more negative valence. However, the error pattern for the identification of happy faces (displayed in Table II) does not provide clear support for this explanation, as happy faces were mislabelled following upregulation as some negative emotions, but also as neutral and surprise. An alternative explanation could be that learned single ROI self‐regulation leads to modifications in the connectivity of brain networks, in addition to changes in the region itself. Face emotion recognition may involve the functional interaction among certain brain areas considered to be specialized for recognition of specific emotions [for a review see Adolphs, 2002], for example, amygdala for fear [Adolphs et al., 1994; Morris et al., 1996] and insula for disgust [Phillips et al., 1997, 2004; Sambataro et al., 2006; Wicker et al., 2003; Williams et al., 2005]. Therefore, it is conceivable that the volitional control of one of these particular regions could in turn modify the functional connectivity with other regions involved in face emotion recognition, leading to modulations of the detection of other emotions, e.g., happy emotions as shown in our results. Although it is not possible to test this hypothesis with our current design, it nevertheless stresses the importance of examining brain connectivity changes associated with rtfMRI training.
Effective connections among insula cortex, amygdala, and MPFC were enhanced at the end of the training. Furthermore, the CD of the network involved in emotion processing was enhanced as a whole: increased number of connections was observed in the regions: ACC (attention and performance monitoring [Botvinick et al., 2004]), precuneus (episodic memory retrieval and self‐processing operations [Cavanna et al., 2006]), and supramarginal gyrus (first‐person perspective [Vogeley et al., 2004]). Abnormal brain connectivity during emotional processing has been reported in schizophrenia [Das et al., 2007; Fakra et al., 2008]. Therefore, if rtfMRI can be used to “repair” neural disconnection, this could have important therapeutic implications for schizophrenia, and also for other brain diseases in which the coupling of different brain areas is disrupted [Just et al., 2007; Noonan et al., 2009; Wang et al., 2007; Zhang et al., 2010].
The study has some limitations. The sample size of patients was relatively small. However, the longer training resulting in such a consistent effect compensates for the sample size. The rtfMRI training and behavioral post‐training evaluation, spanned over several days, was a demanding setting that included more than 180 fMRI scanning sessions (and more than 50,000 brain volumes), making this study exceptionally large in terms of the amount of data acquired. The high consistency and the large number of data points increase the statistical reliability of our data. Because of the lack of a control group of patients trained with non‐contingent (sham) feedback, it could be argued that the increase of insula control may constitute a session repetition or time effect. However, we did not include a group of patients trained with sham feedback for ethical reasons, and also considering the consistent evidence coming from all previous rtfMRI studies [Caria et al., 2007, 2010; deCharms et al., 2004, 2005; Rota et al., 2009; Yoo et al., 2006], that have demonstrated that volitional control can only be achieved by contingent feedback. Furthermore, our group tested this effect in a previous study using a similar rtfMRI‐training protocol, in healthy participants. Again, only the group trained with contingent feedback resulted in a learning effect. Emotional imagery without feedback and noncontingent feedback had no behavioral effects and no BOLD control was observed [Caria et al., 2007, 2010]. On the other hand, practice or time effect is accounted for in our design, as behavioral comparisons were made between upregulation and baseline conditions that were interspersed in the same sessions. If repetition did indeed influence learning, then it should have occurred for both baseline and upregulation conditions, cancelling the effect in our analysis. Furthermore, the feedback was computed online by subtracting global signal changes from the signal in the anterior insula, thereby removing unspecific effects and retaining task specific activations.
CONCLUSIONS AND FUTURE APPLICATIONS
This study shows for the first time that rtfMRI can be applied to psychiatric populations, leading to behavioral modifications and neural connectivity enhancement. Particularly for schizophrenia, it has not been previously shown that patients would be able to self‐induce neural activation modifications, leading to behavioral effects. Here we could demonstrate for the first time a completely novel approach, involving functional brain changes due to self‐regulation of cerebral activation patterns. We believe that these results open the door for future therapeutic applications of rtfMRI feedback, and can serve as a model for psychiatric populations for stimulating self‐efficacy and the possibility to remodel their own brain.
Some questions, out of the scope of this study, are still to be answered. At a behavioral level, further research should evaluate the translation of this approach to clinical treatment of mental disorders. At the neural level, enhancement of brain connections in the present study was achieved as a positive by‐product of single ROI self‐regulation training. However, further progress in rtfMRI methodology is expected to enable the real‐time computation and direct feedback of the coupling between several brain areas, making it possible to train patients to directly modulate the connection strengths of a network, creating exciting possibilities for the direct modulation of brain networks by self‐regulation.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
REFERENCES
- Abler B, Roebroeck A, Goebel R, Hose A, Schonfeldt‐Lecuona C, Hole G, Walter H ( 2006): Investigating directed influences between activated brain areas in a motor‐response task using fMRI. Magn Reson Imaging 24: 181–185. [DOI] [PubMed] [Google Scholar]
- Addington D, Addington J, Schissel B ( 1990): A depression rating scale for schizophrenics. Schizophr Res 3: 247–251. [DOI] [PubMed] [Google Scholar]
- Addington J, Addington D ( 1998): Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr Res 32: 171–181. [DOI] [PubMed] [Google Scholar]
- Adolphs R ( 2002): Neural systems for recognizing emotion. Current Opin Neurobiol 12: 169–177. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR ( 2003): Dissociable neural systems for recognizing emotions. Brain Cogn 52: 61–69. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A ( 1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669–672. [DOI] [PubMed] [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N ( 2004): Brain activity underlying emotional valence and arousal: A response‐related fMRI study. Hum Brain Mapp 23: 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD ( 2003): Neural correlates of the automatic processing of threat facial signals. J Neurosci 23: 5627–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N ( 2006): Breaking the silence: Brain‐computer interfaces (BCI) for communication and motor control. Psychophysiology 43: 517–532. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Cohen LG ( 2007): Brain‐computer interfaces: Communication and restoration of movement in paralysis. J Physiol 579: 621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C ( 1995): Neural Networks for Pattern Recognition, 2nd ed. UK: Oxford University Press. [Google Scholar]
- Boksman K, Theberge J, Williamson P, Drost DJ, Malla A, Densmore M, Takhar J, Pavlosky W, Menon RS, Neufeld RW ( 2005): A 4.0‐T fMRI study of brain connectivity during word fluency in first‐episode schizophrenia. Schizophr Res 75: 247–263. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS ( 2004): Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci 8: 539–546. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ ( 1994): Measuring emotion: The self‐assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry 25: 49–59. [DOI] [PubMed] [Google Scholar]
- Bullock M, Russell JA, editor ( 1986): Concepts of Emotion in Developmental Psychology. Cambridge: Cambridge University Press. [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW ( 2000): Impaired recognition and experience of disgust following brain injury. Nat Neurosci 3: 1077–1078. [DOI] [PubMed] [Google Scholar]
- Caria A, Veit R, Sitaram R, Lotze M, Weiskopf N, Grodd W, Birbaumer N ( 2007): Regulation of anterior insular cortex activity using real‐time fMRI. Neuroimage 35: 1238–1246. [DOI] [PubMed] [Google Scholar]
- Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N ( 2010): Volitional control of anterior insula activity modulates the response to aversive stimuli. A real‐time functional magnetic resonance imaging study. Biol Psychiatry 68: 425–432. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Chambon V, Baudouin JY, Franck N ( 2006): The role of configural information in facial emotion recognition in schizophrenia. Neuropsychologia 44: 2437–2444. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL ( 2006): The functional significance of social cognition in schizophrenia: A review. Schizophr Bull 32 ( Suppl 1): S44–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo‐Facorro B, Paradiso S, Andreasen NC, O'Leary DS, Watkins GL, Ponto LL, Hichwa RD ( 2001a): Neural mechanisms of anhedonia in schizophrenia: A PET study of response to unpleasant and pleasant odors. JAMA 286: 427–435. [DOI] [PubMed] [Google Scholar]
- Crespo‐Facorro B, Wiser AK, Andreasen NC, O'Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD ( 2001b): Neural basis of novel and well‐learned recognition memory in schizophrenia: A positron emission tomography study. Hum Brain Mapp 12: 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis VA, Bullmore ET, Brammer MJ, Wright IC, Williams SC, Morris RG, Sharma TS, Murray RM, McGuire PK ( 1998): Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 155: 1056–1063. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Flynn G, Harris AW, Liddell BJ, Whitford TJ, Peduto A, Gordon E, Williams LM ( 2007): Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res 90: 284–294. [DOI] [PubMed] [Google Scholar]
- deCharms RC ( 2008): Applications of real‐time fMRI. Nat Rev Neurosci 9: 720–729. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JD ( 2004): Learned regulation of spatially localized brain activation using real‐time fMRI. Neuroimage 21: 436–443. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC ( 2005): Control over brain activation and pain learned by using real‐time functional MRI. Proc Natl Acad Sci USA 102: 18626–18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakra E, Salgado‐Pineda P, Delaveau P, Hariri AR, Blin O ( 2008): Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res 100: 191–205. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Frith CD, Decety J, Georgieff N, d'Amato T, Jeannerod M ( 2004): Neural correlates of action attribution in schizophrenia. Psychiatry Res 131: 31–44. [DOI] [PubMed] [Google Scholar]
- Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ ( 1999): Abnormal cingulate modulation of fronto‐temporal connectivity in schizophrenia. Neuroimage 9: 337–342. [DOI] [PubMed] [Google Scholar]
- Gerber AJ, Posner J, Gorman D, Colibazzi T, Yu S, Wang Z, Kangarlu A, Zhu H, Russell J, Peterson BS ( 2008): An affective circumplex model of neural systems subserving valence, arousal, and cognitive overlay during the appraisal of emotional faces. Neuropsychologia 46: 2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton P, Glover GH, Hsu J, Johnson R ( 2011): Modulation of subgenual anterior cingulate cortex activity with real‐time neurofeedback. Hum Brain Mapp 32: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Haslinger B, Stahl R, Weindl A, von Einsiedel HG, Lange KW, Ceballos‐Baumann AO ( 2004): Neural correlates associated with impaired disgust processing in pre‐symptomatic Huntington's disease. Brain 127: 1446–1453. [DOI] [PubMed] [Google Scholar]
- Henry JD, Green MJ, de Lucia A, Restuccia C, McDonald S, O'Donnell M ( 2007): Emotion dysregulation in schizophrenia: Reduced amplification of emotional expression is associated with emotional blunting. Schizophr Res 95: 197–204. [DOI] [PubMed] [Google Scholar]
- Honey GD, Pomarol‐Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC ( 2005): Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain 128: 2597–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C, Park S ( 2002): Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res 112: 41–50. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ ( 2007): Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex 17: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA ( 1987): The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Duggins AJ, McCusker EA, Calder AJ ( 2007): Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington's disease. J Cogn Neurosci 19: 1206–1217. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Bilker W, Hagendoorn M, Gur RE, Gur RC ( 2000): Emotion recognition deficit in schizophrenia: Association with symptomatology and cognition. Biol Psychiatry 48: 127–136. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, Gur RE, Gur RC ( 2003): Facial emotion recognition in schizophrenia: Intensity effects and error pattern. Am J Psychiatry 160: 1768–1774. [DOI] [PubMed] [Google Scholar]
- Lal TN, Schroder M, Hinterberger T, Weston J, Bogdan M, Birbaumer N, Scholkopf B ( 2004): Support vector channel selection in BCI. IEEE Trans Biomed Eng 51: 1003–1010. [DOI] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ ( 2006): Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res 83: 155–171. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Koutsouleris N, Bottlender R, Scheuerecker J, Jager M, Teipel SJ, Holzinger S, Frodl T, Preuss U, Schmitt G, Burgermeister B, Reiser M, Born C, Moller HJ ( 2008): Structural brain alterations at different stages of schizophrenia: A voxel‐based morphometric study. Schizophr Res 104: 44–60. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A ( 2010): From maps to mechanisms through neuroimaging of schizophrenia. Nature 468: 194–202. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF ( 2005): Regionally specific disturbance of dorsolateral prefrontal‐hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62: 379–386. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ ( 1996): A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383: 812–815. [DOI] [PubMed] [Google Scholar]
- Morrison RL, Bellack AS, Mueser KT ( 1988): Deficits in facial‐affect recognition and schizophrenia. Schizophr Bull 14: 67–83. [DOI] [PubMed] [Google Scholar]
- Nagai M, Kishi K, Kato S ( 2007): Insular cortex and neuropsychiatric disorders: A review of recent literature. Eur Psychiatry 22: 387–394. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A ( 2009): The hidden island of addiction: The insula. Trends Neurosci 32: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Muller RA ( 2009): Aberrant functional connectivity in autism: Evidence from low‐frequency BOLD signal fluctuations. Brain Res 1262: 48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ ( 2004): For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23: 483–499. [DOI] [PubMed] [Google Scholar]
- Paillere‐Martinot M, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, Recasens C, Attar‐Levy D, Martinot JL ( 2001): Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res 50: 19–26. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Andreasen NC, Crespo‐Facorro B, O'Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD ( 2003): Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry 160: 1775–1783. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I ( 2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS ( 1997): A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–498. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, Williams SC, David AS ( 1999): A differential neural response to threatening and non‐threatening negative facial expressions in paranoid and non‐paranoid schizophrenics. Psychiatry Res 92: 11–31. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SC, Morgan M, Young AW, Gray JA ( 2004): Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage 21: 1484–1496. [DOI] [PubMed] [Google Scholar]
- Pomarol‐Clotet E, Canales‐Rodriguez EJ, Salvador R, Sarro S, Gomar JJ, Vila F, Ortiz‐Gil J, Iturria‐Medina Y, Capdevila A, McKenna PJ ( 2010): Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry 15: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Moore GJ, Schneider F ( 2003): Real‐time fMRI of temporolimbic regions detects amygdala activation during single‐trial self‐induced sadness. Neuroimage 18: 760–768. [DOI] [PubMed] [Google Scholar]
- Price G, Cercignani M, Chu EM, Barnes TR, Barker GJ, Joyce EM, Ron MA ( 2010): Brain pathology in first‐episode psychosis: magnetization transfer imaging provides additional information to MRI measurements of volume loss. Neuroimage 49: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota G, Sitaram R, Veit R, Erb M, Weiskopf N, Dogil G, Birbaumer N ( 2009): Self‐regulation of regional cortical activity using real‐time fMRI: the right inferior frontal gyrus and linguistic processing. Hum Brain Mapp 30: 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Dimalta S, Di Giorgio A, Taurisano P, Blasi G, Scarabino T, Giannatempo G, Nardini M, Bertolino A ( 2006): Preferential responses in amygdala and insula during presentation of facial contempt and disgust. Eur J Neurosci 24: 2355–2362. [DOI] [PubMed] [Google Scholar]
- Sanjuan J, Lull JJ, Aguilar EJ, Marti‐Bonmati L, Moratal D, Gonzalez JC, Robles M, Keshavan MS ( 2007): Emotional words induce enhanced brain activity in schizophrenic patients with auditory hallucinations. Psychiatry Res 154: 21–29. [DOI] [PubMed] [Google Scholar]
- Saze T, Hirao K, Namiki C, Fukuyama H, Hayashi T, Murai T ( 2007): Insular volume reduction in schizophrenia. Eur Arch Psychiatry Clin Neurosci 257: 473–479. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Gur RE, Shtasel DL ( 1995): Emotional processing in schizophrenia: Neurobehavioral probes in relation to psychopathology. Schizophr Res 17: 67–75. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Koch K, Backes V, Amunts K, Shah NJ, Bilker W, Gur RE, Habel U ( 2006): Impairment in the specificity of emotion processing in schizophrenia. Am J Psychiatry 163: 442–447. [DOI] [PubMed] [Google Scholar]
- Schroeder U, Hennenlotter A, Erhard P, Haslinger B, Stahl R, Lange KW, Ceballos‐Baumann AO ( 2004): Functional neuroanatomy of perceiving surprised faces. Hum Brain Mapp 23: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK ( 2005): Causal connectivity of evolved neural networks during behavior. Network 16: 35–54. [DOI] [PubMed] [Google Scholar]
- Seth AK ( 2010): A MATLAB toolbox for Granger causal connectivity analysis. J Neurosci Methods 186: 262–273. [DOI] [PubMed] [Google Scholar]
- Shapira NA, Liu Y, He AG, Bradley MM, Lessig MC, James GA, Stein DJ, Lang PJ, Goodman WK ( 2003): Brain activation by disgust‐inducing pictures in obsessive‐compulsive disorder. Biol Psychiatry 54: 751–756. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B ( 2001): Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry 158: 234–243. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Wiskopf N, Caria A, Veit R, Erb M, Birbaumer N ( 2008): fMRI brain‐computer interfaces: A tutorial on methods and applications. IEEE Signal Process Mag Special Issue BCI 25: 99–106. [Google Scholar]
- Strehl U, Leins U, Goth G, Klinger C, Hinterberger T, Birbaumer N ( 2006): Self‐regulation of slow cortical potentials: A new treatment for children with attention‐deficit/hyperactivity disorder. Pediatrics 118: e1530–e1540. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Calvert GA, Brammer MJ, Campbell R, Bullmore ET, Giampietro V, David AS ( 2001): Audio‐visual speech perception in schizophrenia: An fMRI study. Psychiatry Res 106: 1–14. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Hagino H, Zhou SY, Kawasaki Y, Nohara S, Nakamura K, Yamashita I, Seto H, Kurachi M ( 2004): Bilateral volume reduction of the insular cortex in patients with schizophrenia: A volumetric MRI study. Psychiatry Res 131: 185–194. [DOI] [PubMed] [Google Scholar]
- Viinikainen M, Jaaskelainen IP, Alexandrov Y, Balk MH, Autti T, Sams M ( 2010): Nonlinear relationship between emotional valence and brain activity: Evidence of separate negative and positive valence dimensions. Hum Brain Mapp 31: 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR ( 2004): Neural correlates of first‐person perspective as one constituent of human self‐consciousness. J Cogn Neurosci 16: 817–827. [DOI] [PubMed] [Google Scholar]
- Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T ( 2007): Altered functional connectivity in early Alzheimer's disease: A resting‐state fMRI study. Human Brain Mapp 28: 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A ( 1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Scharnowski F, Veit R, Goebel R, Birbaumer N, Mathiak K ( 2004): Self‐regulation of local brain activity using real‐time functional magnetic resonance imaging (fMRI). J Physiol Paris 98: 357–373. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Sitaram R, Josephs O, Veit R, Scharnowski F, Goebel R, Birbaumer N, Deichmann R, Mathiak K ( 2007): Real‐time functional magnetic resonance imaging: Methods and applications. Magn Reson Imaging 25: 989–1003. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G ( 2003): Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron 40: 655–664. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell B, Olivieri G, Peduto A, Brammer MJ, Gordon E ( 2005): BOLD, sweat and fears: fMRI and skin conductance distinguish facial fear signals. Neuroreport 16: 49–52. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Olivieri G, Peduto AS, David AS, Gordon E, Harris AW ( 2007): Fronto‐limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res 155: 29–44. [DOI] [PubMed] [Google Scholar]
- Wittchen H‐U, Wunderlich U, Gruschwitz S, Zaudig M, editors ( 1997): Strukturiertes Klinisches Interview für DSM‐IV,Achse‐I (SKID) [Structured Clinical Interview for DSM IV ]. Göttingen: Hogrefe. [Google Scholar]
- Witthaus H, Brune M, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, Heinz A, Klingebiel R, Juckel G ( 2008): White matter abnormalities in subjects at ultra high‐risk for schizophrenia and first‐episode schizophrenic patients. Schizophrenia Res 102: 141–149. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Choi BG, Juh RH, Park JM, Pae CU, Kim JJ, Lee SJ, Lee C, Paik IH, Lee CU ( 2005): Working memory processing of facial images in schizophrenia: fMRI investigation. Int J Neurosci 115: 351–366. [DOI] [PubMed] [Google Scholar]
- Yoo SS, O'Leary HM, Fairneny T, Chen NK, Panych LP, Park H, Jolesz FA ( 2006): Increasing cortical activity in auditory areas through neurofeedback functional magnetic resonance imaging. Neuroreport 17: 1273–1278. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Liu B, Ma ZL, Yang M, Zhang ZJ, Teng GJ ( 2010): Resting brain connectivity: Changes during the progress of Alzheimer disease. Radiology 256: 598–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


