Abstract
A fear of being rejected can cause perceptions of more insecurity and stress in close relationships. Healthy individuals activate the dorsal anterior cingulate cortex (dACC) when experiencing social rejection, while those who are vulnerable to depression deactivate the dACC presumably to downregulate salience of rejection cues and minimize distress. Schizotypal individuals, characterized by unusual perceptual experiences and/or odd beliefs, are more rejection sensitive than normal. We tested the hypothesis, for the first time, that individuals with high schizotypy also have an altered dACC response to rejection stimuli. Twenty‐six healthy individuals, 14 with low schizotypy (LS) and 12 with high schizotypy (HS), viewed depictions of rejection and acceptance and neutral scenes while undergoing functional MRI. Activation maps in LS and HS groups during each image type were compared using SPM5, and their relation to participant mood and subjective ratings of the images was examined. During rejection relative to neutral scenes, LS activated and HS deactivated the bilateral dACC, right superior frontal gyrus, and left ventral prefrontal cortex. Across both groups, a temporo‐occipito‐parieto‐cerebellar network was active during rejection, and a left fronto‐parietal network during acceptance, relative to neutral scenes, and the bilateral lingual gyrus during rejection relative to acceptance scenes. Our finding of dACC‐dorso‐ventral PFC activation in LS, but deactivation in HS individuals when perceiving social rejection scenes suggests that HS individuals attach less salience to and distance themselves from such stimuli. This may enable them to cope with their higher‐than‐normal sensitivity to rejection. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: schizotypal personality, rejection, acceptance, dorsal anterior cingulate cortex, lingual gyrus, fMRI
INTRODUCTION
Rejection sensitivity (RS) is the tendency to expect rejection by significant people in a person's life [Downey and Feldman,1996]. Rejection‐sensitive individuals perceive more insecurity and stressors in close relationships, express more vulnerability towards people around them and feel more anxious in social situations (Langens and Schuler,2005; Mehrabian and Ksionzky,1974; Sokolowski et al.,2000; Vorauer et al.,2003]. Increased RS in the form of reflected appraisals of vulnerability in turn create “authenticity doubts”, that is where the person feels that the significant other expresses more positive regard than he/she truly feels [Lemay and Clark,2008].
Across various psychiatric disorders, greater RS is associated with greater perceived social stress and fewer perceived coping resources [Rusch et al.,2009]. Patients with a diagnosis of schizotypal personality disorder show greater RS than healthy individuals [Torgersen et al.,2002]. In addition, a high level of criticism by a relative towards the patient aggravates communication disorder in patients with schizophrenia [Rosenfarb et al.,1995,2000], and such patients are more likely to endorse stronger beliefs about the consequences of being rejected [Grant and Beck,2009]. A high level of expressed emotion, the negative emotion expressed by a family member towards a patient in the form of criticism, hostility, rejection, emotional over‐involvement or decreased warmth [Leff and Vaughn,1985], is associated with a greater likelihood of relapse to psychosis (Bailer et al.,1994; Kreisman et al.,1988; Kuipers et al.,2010; Rutter and Brown,1966] and a greater number of psychotic exacerbations [Heresco‐Levy et al.,1992].
The neural basis of RS has been examined in healthy populations. In healthy individuals, greater RS activates the dorsal anterior cingulate cortex (dACC) (Burklund et al.,2007; Eisenberger et al.,2003; Kross et al.,2007; Masten et al.,2009; Somerville et al.,2006]. For instance, a higher level of RS was associated with greater activity in the dACC when watching video clips of people expressing disapproval compared to no emotion, anger, or disgust [Burklund et al.,2007]. A frontal lobe network that included the dACC was activated when healthy individuals viewed images of individuals experiencing social rejection than acceptance [Kross et al.,2007]. The dACC is also known to be involved in conflict detection [Carter et al.,2001; Somerville et al.,2006] and emotional decision‐making in healthy individuals [Walton et al.,2004,2007]; these additional cognitive‐emotional processes may contribute towards perception of rejection. High RS individuals show greater activation in the dACC, VLPFC, and SFG when viewing social rejection scenes compared to low RS individuals [Kross et al.,2007]. The VLPFC and SFG are involved in empathizing with others [Hooker et al.,2010b; Kramer et al.,2010; Sommer et al.,2010] and regulating emotion (Hooker et al.,2010a; Mak et al.,2009].
The neural basis of elevated RS has been explored in depression, but not in schizophrenia. Patients in remission from depression, but not healthy people, deactivate the dACC on hearing maternal criticisms [Hooley et al.,2005,2009]. An explanation for this finding was that people who are vulnerable to depression down‐regulate salience to criticism by deactivating the dACC [Hooley et al.,2005,2009].
This study examined the neural basis of RS in individuals with a high level of schizotypal personality traits, precursory to a study of patients with a schizophrenia or schizoaffective diagnosis. The advantage of examining the response to social rejection in a group of healthy individuals with schizotypal traits over patients with a schizophrenia or schizoaffective diagnosis is that observed effects would not be confounded by illness chronicity and medication. Schizotypy is a personality trait within the normal range of the schizophrenia spectrum, but related to schizophrenia at the clinical [Mason et al.,2005; Cochrane et al.,2010], genetic [Fanous et al.,2001,2007], neuropsychological [Gooding et al.,2006], and neurophysiological levels (Bollini et al.,2007; Ettinger et al.,2005]. Schizotypal traits include magical thinking, unusual perceptual experience, odd behaviour and speech [Mason et al.,1995] that are thought to correspond to positive symptoms of psychosis (Cochrane et al.,2010; Mason et al.,1995,2005] and anhedonia that is thought to correspond to negative symptoms [Vollema and van den Bosch,1995]. Studying the neural basis of RS in schizotypal individuals may help to understand how RS interacts with other stress‐provoking situations and interpretation of one's emotions at the neural level. Individuals with high levels of schizotypal personality traits may have altered ways of perceiving rejection cues [Torgersen et al.,2002] to minimize distress and may downregulate salience to rejection cues, similar to what was observed in recovered depressed patients [Hooley et al.,2009].
Given the conflicting evidence for the level of neural response to rejection between high RS healthy individuals and formerly depressed clinical individuals, and following earlier reports of greater RS in schizotypal personality disorder patients [Torgersen et al.,2002], it was hypothesized that normal individuals with a high level of schizotypal traits (high schizotypy, HS group) compared with individuals with a low level of schizotypal traits (low schizotypy, LS group) would show deactivation in the dACC in the neural response to social rejection scenes.
METHODS AND MATERIALS
Subjects
Participants were drawn from the general population with no known psychiatric diagnosis and selected only if they had very low or high levels of unusual experiences related to schizotypal personality [Mason et al.,2005]. Of 26 selected participants, 12 had a high level of unusual experiences [HS group, i.e. scored ≥7 on the Unusual Experiences (UE) subscale of the short form of the Oxford and Liverpool Inventory of Feelings and Experiences, O‐LIFE] [Mason et al.,2005] and 14 had a very low level of UE (LS group, i.e. scored ≤2 on the O‐LIFE UE subscale). The UE subscale of the O‐LIFE was chosen to identify HS and LS participants, as a high score on this subscale is associated with greater positive symptom severity in patients with schizophrenia [Cochrane et al.,2010]. A score of ≥7 of a maximum score of 12 on the UE subscale on the O‐LIFE short form was based on a score of >1 standard deviation above the UE scores found in the normal population [Mason et al.,2005].
The O‐LIFE was also used to measure other facets of schizotypy, namely cognitive disorganization that measures aspects of poor attention and concentration and is thought to relate to thought disorder and other disorganized aspects of psychosis, introvertive anhedonia that measures lack of enjoyment from social and physical sources of pleasure and is thought to relate to weakened forms of negative symptoms, and impulsive nonconformity that measures forms of behavior suggesting a lack of self‐control [Mason et al.,1995].
Potential participants were recruited from a database of healthy volunteers (MindSearch, Institute of Psychiatry; n ≥ 500) and by circular emails sent to the staff and students of King's College London. Inclusion criteria were as follows: (i) IQ > 90, estimated as >5 correct responses on the National Adult Reading Test [Nelson and Wilson,1991], (ii) right‐handed, (iii) 18–45 years age range, (iv) normal‐to‐corrected vision, and (v) normal hearing. Exclusion criteria were as follows: (i) Beck Depression Inventory (BDI) [Beck et al.,1996] score > 30, (ii) a history of mental disorder, brain injury, neurological disorder, learning disabilities, or loss of consciousness for more than 5 min, and (iii) a history of alcohol or drug abuse within the last 12 months. Parental socioeconomic status was classified as follows: 1, professional (doctor/lawyer); 2, intermediate (manager/teacher/nurse); 3, skilled (secretary/bus driver); 4, semi‐skilled (shop assistant); and 5, manual (cleaner, laborer).
Study procedures were approved by the King's College London Research Ethics Committee (CREC/07/08‐66). Participants provided written informed consent to their participation and were compensated for their time and travel.
fMRI Paradigm and Procedure
Rejection‐acceptance task: Stimulus selection
Images depicting social rejection, acceptance, and neutral scenes were sourced from the International Affective Pictures System [Lang et al.,1999] or purchased from a web‐based company (http://www.jupiterimages.co.uk) supplying stock photographic images for professional use. Images of different types of rejection and acceptance situations (parental, partner, or peer) were sourced. One hundred and sixty‐four images (35 rejection, 49 acceptance, and 80 neutral) were obtained. Six doctoral or postdoctoral level psychology researchers were asked to rate the images blind to the emotional content of the image on two indices: rejection level (rejection‐acceptance) and valence (negative‐positive) on 11‐point Likert scales from −5 to +5. Fifteen images from each category were chosen based on the means and S.D. of the six researchers' ratings of each image on rejection level (rejected‐accepted) and valence (negative‐positive). In the rejection category, 15 images with a mean score nearest to −5 on rejection level and valence and the lowest S.D. were chosen (summary statistics of the six raters' scores are provided in Appendix A). In the acceptance category, 15 images with a mean score nearest to +5 on rejection level and valence and lowest S.D. were chosen. In the neutral category, 15 images with a mean score nearest to 0 on rejection level and valence and lowest S.D. were chosen. Across the three categories, images were matched for the number of people in the scene, their gender and their ethnicity.
On‐line task
Participants were presented with the rejection‐acceptance task projected onto a screen at the end of the scanner bed. During this task, the three types of images (rejection, acceptance, neutral) were shown in 15 s blocks of three images each, with each image being presented for 5 s (Fig. 1). Immediately after each block, the participant had to respond to the question, “How do you feel right now?” within a 5 s time period. The participant responded on an 11‐point visual analog scale (VAS) ranging from −5 (sad) to +5 (happy) by pressing left or right with their right hand on a button‐box. The order of rejection, acceptance, and neutral blocks was pseudorandomized. Each 20 s block (15 s to view the images plus 5 s to make a response) was separated by a 10 s rest block (blue blank screen). Two stimulus playlists were used in a random manner (using a randomization list) to counterbalance for whether the participant viewed images of rejection or acceptance in the first block.
Figure 1.
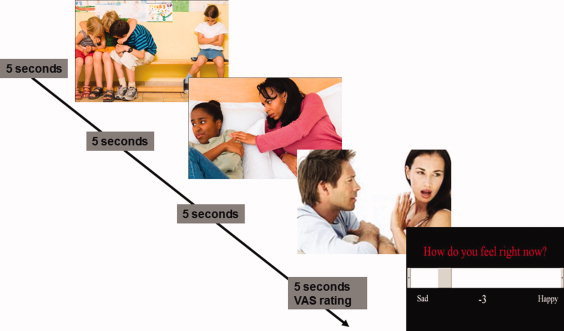
A representation of a 20‐s rejection block from the rejection‐acceptance task.
Off‐line task
After the scanning session, the participants once again viewed the rejection‐acceptance task images on a laptop computer. Each image appeared for 5 s in the same sequence as during fMRI. Participants rated each image on 11‐point VASs on the following three themes: rejection‐acceptance [rejected (−5) to accepted (+5)], affect [sad (−5) to happy (+5)], and arousal [low (0) to high (10)] taking as long as they wished. Participants were not restricted in the time they took to respond to each question. The task usually took approximately 25 min to complete.
Participants completed the Positive and Negative Affect scale (PANAS, Watson et al.,1988]—moment subscale before the scanning session and the full scale after the scanning session, but before performing the off‐line task. The PANAS contains 10 positive (e.g., interested, proud) and 10 negative (e.g., ashamed, irritable) mood descriptors and six time points, namely moment, today, past few days, past few weeks, year, and general. Participants rated all subscales; only ratings of the moment subscale before scanning and general subscale were taken for statistical analyses in the present study.
Participants were also asked to complete the Beck Anxiety Inventory (BAI) [Beck and Steer,1993] and the Adult Rejection Sensitivity Scale (RSS) [Downey and Feldman,1996] before performing the off‐line task to measure anxiety and rejection sensitivity traits. As mentioned earlier, participants completed the BDI at the time of their screening and were included only if they did not have depression scores at a clinically important level.
Image acquisition
Echo‐planar T2*‐weighted MR images of the brain were acquired using a 1.5 Tesla GE Signa HDx scanner (General Electric, Milwaukee, WI) at the Centre for Neuroimaging Sciences, Institute of Psychiatry, King's College London. Head movements were minimized using foam padding. A localizer scan for placing the volume of interest and a high‐resolution structural scan for image co‐registration were first acquired. An eight‐channel radio frequency head coil working in parallel mode was used to acquire images from each of 36 near‐axial noncontiguous planes parallel to the intercommissural plane. These MR images depicting BOLD contrast were acquired with an echo time (TE) = 40 ms, repetition time (TR) = 2.5 s, field of view (FOV) = 24 cm, flip angle = 85°, in‐plane resolution = 3.75 mm, slice thickness = 3 mm, interslice gap = 0.3 mm. Four dummy scans followed by 210 volumes were acquired (total scan time 8 min and 55 s). An inversion recovery prepared fast 3D SPGR was acquired (TR = 11.1 ms, TE = 4.9 ms, TI = 300 ms, acquisition matrix 256 × 160, 150 locations, slice thickness 1.1 mm in‐plane resolution 1.094 mm flip angle = 18 degrees, scan time 6 min and 4 s).
Statistical Analysis
Demographic, behavioral characteristics, and ratings of the rejection‐acceptance task in the HS and LS groups
A Chi‐squared test for group differences in gender was performed. For continuous variables with no heterogeneity of variance, namely O‐LIFE cognitive disorganization, introvertive anhedonia and impulsive non‐conformity subscales, BDI, RSS and PANAS moment positive and general positive and negative subscales, analysis of variance (ANOVA) was performed with group as the independent variable, and for variables with heterogeneity of variance (parental socioeconomic status, O‐LIFE UE subscale, BAI, and PANAS moment negative), Mann–Whitney U‐test was performed. Effect size (Cohen's d) was also calculated to determine whether the size of the difference in continuous variables was small (Cohen's d < 0.5), medium (≥0.5 and <0.8), or large (≥0.8).
Two (group) × three (rejection, acceptance, and neutral conditions) repeated‐measures ANOVAs were performed on each off‐line task rating scale (rejection–acceptance, sad–happy, and high–low arousal).
Statistical significance was set a priori at P level <0.05; analyses of behavioral data were carried out using Statistical Package for the Social Sciences (SPSS, version 16).
fMRI analysis
fMRI preprocessing
For each participant, the 210 volume functional time series images were motion corrected, transformed into stereotactic space (Montreal Neurological Institute, MNI), smoothed with an 8 mm FWHM Gaussian filter and band pass filtered using statistical parametric mapping software (SPM, version 5‐1782, 2008; http://www.fil.ion.ucl.ac.uk/spm).
Statistical inferences
Data were analyzed using the general linear model within SPM. At the single‐subject level, contrast maps of each of the three conditions (rejection, acceptance, and neutral) were created, covarying for motion parameters, with the resting period as the implicit baseline. This analysis was carried out by modeling each condition at each voxel using a boxcar function which incorporates the delay inherent in the hemodynamic response. The resulting maps were entered into a random‐effects procedure at the second level to investigate task condition‐related activation differences (rejection vs. neutral, acceptance vs. neutral, and rejection vs. acceptance) (i) across all participants using one sample t‐tests (height threshold P < 0.001 and cluster corrected P < 0.05), and (ii) between HS and LS groups using SPM ANOVA (height threshold P < 0.005, cluster corrected P < 0.05).
Because of a slight difference in gender distribution and significant difference in PANAS moment negative scores between HS and LS groups, subject‐specific average activation values from clusters showing significant differences between groups were extracted using the MarsBaR toolbox (http://marsbar.sourceforge.net/projects/marsbar), entered into SPSS and analysed using analyses of covariance with subject‐specific average activation values as the dependent variable, group as the between‐subjects variable and gender or PANAS moment negative scores as the covariate.
Correlation between neural response to rejection‐acceptance task and ratings of the off‐line task and mood
Subject‐specific average activation values from clusters showing significant task condition differences (rejection vs. neutral, acceptance vs. neutral, and rejection vs. acceptance) (total number of clusters = 7) across all participants were extracted using MarsBar and entered into SPSS. Correlations between these subject‐specific activation values and the three post‐fMRI off‐line task ratings and mood (PANAS moment and general subscales) were evaluated first using Pearson's correlations and then using partial correlations controlling for gender. For the correlation between the rejection versus acceptance contrast activation values and off‐line rejection‐acceptance and sad‐happy ratings, the off‐line ratings of the rejection and acceptance images were combined by calculating the difference between the ratings for two image types, the assumption being that the difference in ratings between the two image types would give an estimate of the emotional range across image type. Because of the large number of correlations, correlations with P ≤ 0.01 were considered significant.
RESULTS
Demographic and Behavioral Characteristics of Groups
In both groups, participants were mostly female (Table I). The HS group scored higher than the LS group on the cognitive disorganization and impulsive nonconformity subscales of the O‐LIFE and reported more negative current mood than the LS group (P < 0.05; large effect size) (Table I). The HS group also had higher self‐reported anxiety and rejection sensitivity (P > 0.05 but medium effect sizes) (Table I).
Table I.
Demographic and behavioral characteristics of high schizotypy (HS) and low schizotypy (LS) groups
| Characteristic | HS (n = 12) | LS (n = 14) | Test | χ2 or z (df) | P | Effect size (Cohen's d) |
|---|---|---|---|---|---|---|
| Gender: male/female (n) | 3/9 | 2/12 | χ2 | 0.478 (1) | 0.490 | — |
| Parental socio‐economic status | M‐W U | 0.287 (1) | 0.820 | — | ||
| Professional | 6 | 6 | ||||
| Intermediate | 5 | 7 | ||||
| Skilled | 1 | 1 | ||||
| mean, s.d. | mean, s.d. | F or z (df) | ||||
| Age in years | 30.00 (10.58) | 28.64 (6.07) | ANOVA | 0.167 (1,24) | 0.686 | 0.157 |
| Years in education | 16.67 (3.05) | 17.28 (1.68) | ANOVA | 0.426 (1,24) | 0.520 | 0.247 |
| O‐LIFE | ||||||
| Unusual experiences | 8.17 (2.33) | 1.00 (0.96) | M‐W U | 4.381 (1) | <0.001 | 4.024 |
| Cognitive disorganization | 3.07 (2.97) | 4.35 (2.87) | ANOVA | 5.782 (1,24) | 0.024 | 0.438 |
| Introverted anhedonia | 1.00 (1.13) | 1.28 (0.91) | ANOVA | 0.509 (1,24) | 0.482 | 0.273 |
| Impulsive non‐conformity | 4.50 (2.47) | 2.71 (1.94) | ANOVA | 4.268 (1,24) | 0.050 | 0.806 |
| Total | 18.92 (5.81) | 8.07 (5.01) | ANOVA | 26.138 (1,24) | <0.001 | 2.000 |
| BDI | 7.75 (6.70) | 5.93 (5.93) | ANOVA | 0.541 (1,24) | 0.469 | 0.287 |
| BAI | 10.50 (9.93) | 5.36 (5.21) | M‐W U | 0.878 (1) | 0.380 | 0.648 |
| RSS | 11.44 (4.12) | 8.88 (3.80) | ANOVA | 2.721 (1,24) | 0.112 | 0.646 |
| PANAS moment | ||||||
| Positive | 30.50 (10.71) | 29.43 (8.64) | ANOVA | 0.080 (1,24) | 0.780 | 0.110 |
| Negative | 14.50 (4.52) | 11.36 (1.60) | M‐W U | 2.314 (1) | 0.023 | 0.926 |
| PANAS general | ||||||
| Positive | 35.17 (7.87) | 32.57 (7.26) | ANOVA | 0.764 (1,24) | 0.939 | 0.343 |
| Negative | 16.17 (5.29) | 16.00 (5.60) | ANOVA | 0.006 (1,24) | 0.391 | 0.031 |
BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; M‐W U: Mann–Whitney U‐test; O‐LIFE: Oxford and Liverpool Inventory of Feelings and Experiences; PANAS: Positive and Negative Affect Scale: RSS: Rejection Sensitivity Scale.
Group Differences in Ratings of the Rejection‐Acceptance Task
There was no significant difference between groups in the off‐line ratings of the three types of images (Table II).
Table II.
Participant ratings of the rejection‐acceptance task
| Image type | HS (n = 12) | LS (n = 14) | Test | F (df) | P | Effect size (Cohen's d) |
|---|---|---|---|---|---|---|
| Rejection level rating [−5 (rejected) to +5 (accepted)] | ||||||
| Rejection images | −1.58 (1.20) | −1.45 (0.96) | ANOVA | 0.001 | 0.972 | 0.119 |
| Acceptance images | 2.73 (1.59) | 2.47 (1.28) | 0.180 | |||
| Neutral images | 0.08 (0.32) | 0.24 (0.80) | 0.263 | |||
| Affect level rating [−5 (sad) to +5 (happy)] | ||||||
| Rejection images | −1.59 (1.23) | −1.58 (0.79) | ANOVA | 0.149 | 0.703 | 0.009 |
| Acceptance images | 2.80 (1.39) | 2.85 (1.07) | 0.040 | |||
| Neutral images | 0.07 (0.29) | 0.19 (0.70) | 0.224 | |||
| Arousal level rating [0 (low) to 10 (high)] | ||||||
| Rejection images | 1.14 (1.43) | 1.90 (1.67) | ANOVA | 1.517 | 0.230 | 0.489 |
| Acceptance images | 2.25 (2.36) | 3.15 (2.30) | 0.386 | |||
| Neutral images | 0.52 (0.58) | 0.99 (1.33) | 0.458 | |||
fMRI Results
All participants
Rejection versus neutral
Across both groups, greater activation in the left middle occipital/middle temporal gyri, left pre/postcentral gyri, right cerebellum, and right superior/middle temporal gyri during rejection relative to neutral images was observed (Table III and Fig. 2). No area showed greater activity during neutral, relative to rejection, images.
Table III.
Brain regions showing differences in activation between task conditions (rejection, acceptance, and neutral images) across all participants (n = 26) at height threshold P = 0.001
| Region | BA | Cluster size | Cluster P corrected | Side | MNI coordinates | Voxel T | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Rejection > neutral | ||||||||
| Middle occipital gyrus | 19 | 1,151 | <0.001 | Left | −44 | −78 | 2 | 6.23 |
| Middle temporal gyrus | 39 | Left | −50 | −64 | 8 | 5.83 | ||
| Middle occipital gyrus | 19 | Left | −52 | −76 | 2 | 5.80 | ||
| Postcentral gyrus | 1 | 684 | <0.001 | Left | −52 | −22 | 54 | 5.36 |
| Postcentral gyrus | 2 | Left | −36 | −36 | 60 | 5.09 | ||
| Precentral gyrus | 4 | Left | −34 | −20 | 48 | 4.31 | ||
| Cerebellum | 431 | 0.002 | Right | 12 | −48 | −22 | 5.13 | |
| Superior temporal gyrus | 22 | 549 | 0.001 | Right | 58 | −46 | 12 | 4.75 |
| Middle temporal gyrus | 37 | Right | 44 | −64 | 0 | 4.73 | ||
| Middle temporal gyrus | 39 | Right | 50 | −66 | 10 | 4.67 | ||
| Acceptance > neutral | ||||||||
| Medial frontal gyrus | 10 | 389 | 0.021 | Left | −4 | 54 | 0 | 5.57 |
| Postcentral gyrus | 1 | 342 | 0.033 | Left | −40 | −28 | 58 | 5.37 |
| Rejection > acceptance | ||||||||
| Lingual gyrus | 18 | 988 | <0.001 | Left | −8 | −82 | 2 | 5.68 |
| Lingual gyrus | 19 | Right | 18 | −68 | −2 | 4.51 | ||
| Lingual gyrus | 18 | Right | 4 | −78 | 0 | 4.31 | ||
Figure 2.
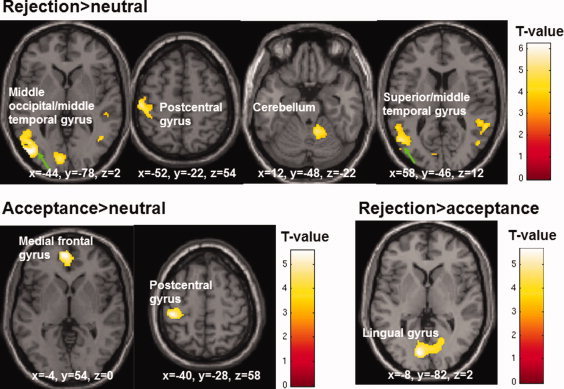
Activation maps showing differences between rejection, acceptance and neutral conditions across all participants (maps thresholded at P = 0.001; displayed clusters corrected for multiple comparisons, P = 0.05).
Acceptance versus neutral
Participants activated the left medial frontal gyrus and left postcentral gyrus during acceptance relative to neutral images. No area showed greater activity during neutral, relative to acceptance, images.
Rejection versus acceptance
Participants activated the lingual gyrus bilaterally during rejection compared to acceptance images. No area showed greater activity during acceptance, relative to rejection, images.
High versus low schizotypy groups
Groups differed when viewing rejection, compared to neutral, images in the activation of the dACC bilaterally, right superior frontal gyrus (SFG), and left ventrolateral prefrontal cortex (VLPFC)/ventromedial prefrontal cortex (VMPFC) (Table IV and Fig. 3). A plot of the percentage fMRI signal change showed that the LS group activated, but the HS group deactivated these areas (Fig. 3).
Table IV.
Brain areas showing differences between low (LS) relative to high (HS) groups in the rejection > neutral activation contrast at height threshold P = 0.005
| Region | BA | Cluster size | Cluster P corrected | Side | MNI coordinates | Voxel T | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Dorsal ACC | 32 | 2,920 | <0.001 | Right | 2 | 4 | 48 | 4.96 |
| Dorsal ACC | 24 | Left | −4 | −8 | 42 | 4.67 | ||
| Dorsal ACC | 32 | Right | 12 | 6 | 54 | 4.48 | ||
| Superior frontal gyrus | 10 | 696 | 0.014 | Right | 22 | 66 | 4 | 4.52 |
| Superior frontal gyrus | 10 | Right | 12 | 64 | 22 | 4.48 | ||
| Superior frontal gyrus | 10 | Right | 18 | 66 | 12 | 3.92 | ||
| Ventrolateral PFC | 47 | 517 | 0.052 | Left | −48 | 24 | −6 | 4.50 |
| Ventromedial PFC | 11 | Left | −12 | 48 | −12 | 3.79 | ||
| Ventromedial PFC | 10 | Left | −8 | 54 | 0 | 3.64 | ||
ACC, anterior cingulate cortex; BA, Brodmann area; PFC, prefrontal cortex.
Figure 3.
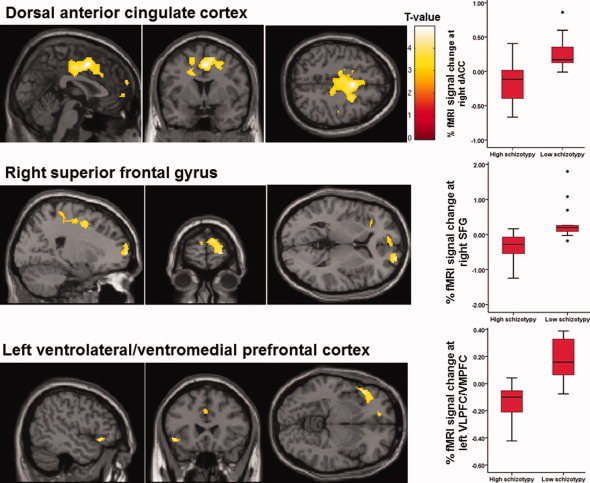
Activation maps and boxplots of percentage fMRI signal showing differences between high and low schizotypy groups in the rejection > neutral activation contrast (maps thresholded at P = 0.005; displayed clusters corrected for multiple comparisons, P = 0.05).
The effect of schizotypy on activity changes between task conditions remained significant with comparable significance values after co‐varying for gender (Table V). The effect of schizotypy also remained significant in all clusters after covarying for PANAS moment negative scores though the effect was slightly reduced (F value reduced from 16.09 to 11.46) in the dACC cluster (Table V).
Table V.
The main effect of group in subject‐specific activations (ANOVAs) with gender and PANAS moment negative as covariates (ANCOVAs)
| Region | ANOVA df = 1,24 | ANCOVA with gender as a covariate df = 1,23 | ANCOVA with PANAS moment negative as a covariate df =1, 23 |
|---|---|---|---|
| Dorsal ACC | F = 16.09, P = 0.001 | F = 15.77, P = 0.001 | F = 11.46, P = 0.003 |
| Right superior frontal gyrus | F = 14.36, P = 0.001 | F = 13.16, P = 0.001 | F = 13.15, P = 0.001 |
| Left ventrolateral/ventromedial PFC | F = 29.00, P < 0.001 | F = 29.30, P < 0.001 | F = 24.04, P < 0.001 |
In all analyses, F < 1 (nonsignificant) for gender effect.
Relation between neural response to rejection‐acceptance task and ratings of the off‐line task and mood
Greater bilateral activation of the lingual gyrus in the rejection>acceptance contrast across all participants was correlated with smaller emotional range between ratings (i.e. range of scores on the rejection‐acceptance VAS) of acceptance and rejection images (r = −0.488, P = 0.011; partial correlation controlling for gender, r = −0.497, P = 0.012), lower arousal ratings of rejection images (r = −0.521, P = 0.006; partial correlation controlling for gender, r = −0.492, P = 0.012), and lower arousal ratings of acceptance images (r = −0.555, P = 0.003; partial correlation controlling for gender, r = −0.551, P = 0.004).
Greater activation of the right superior temporal gyrus in the rejection > neutral contrast across all participants was correlated with higher ratings of negative mood on the PANAS general subscale (r = 0.493, P = 0.011; partial correlation controlling for gender, r = 0.507, P = 0.010).
DISCUSSION
The study aimed to determine whether activity in the dACC, which is normally activated during experiences of social rejection, differs between HS and LS individuals when viewing scenes of social rejection. It was hypothesized that HS individuals would show decreased activation of the dACC during social rejection scenes relative to LS individuals similar to patients in remission from depression when listening to maternal criticisms [Hooley et al.,2005,2009]. We expected this pattern on the basis that HS individuals may have altered ways of perceiving rejection cues [Torgersen et al.,2002] to minimize distress and that HS individuals may downregulate salience to rejection cues, similarly to what was observed in recovered depressed patients [Hooley et al.,2009]. As hypothesized, the HS group deactivated, while the LS group activated the dACC bilaterally during social rejection compared to neutral conditions. In addition, the HS group deactivated, while the LS group activated the right SFG and left VLPFC/VMPFC during social rejection compared to neutral conditions. The findings suggest that the mental processes that are involved in perceiving social rejection differ between HS and LS individuals. This might be due to different ways of coping with stress‐provoking situations.
Activation Differences Between HS and LS Groups in Response to Rejection Compared to Neutral Conditions
The LS group activated the dACC bilaterally, right SFG and left VLPFC/VMPFC, whereas the HS group deactivated these areas during social rejection compared to neutral conditions. Studies of the neural response to rejection in healthy individuals have shown increased activation in the dACC (Burklund et al.,2007; Eisenberger et al.,2003; Kross et al.,2007; Masten et al.,2009; Somerville et al.,2006], as well as the VLPFC and SFG [Kross et al.,2007] during experiences of social rejection. Greater activation of a frontal lobe network comprising the dACC bilaterally, left VMPFC/VLPFC and right SFG in the LS group may reflect the LS individuals' ability to attend to and process rejection cues without being anxious about the consequences of social rejection. The LS group may effectively engage the dACC and VLPFC in conflict detection and emotional decision‐making (Bechara et al.,2000; Carter et al.,2001; Somerville et al.,2006; Walton et al.,2004,2007] and the SFG and VLPFC/VMPFC to empathize with others [Hooker et al.,2010b; Kramer et al.,2010; Sommer et al.,2010] and regulate emotion (Hooker et al.,2010a; Mak et al.,2009] in response to perceived rejection. A recent study [Hooker et al.,2010a] showed that healthy individuals activate the left VLPFC when viewing their partners' negative facial expressions and that left VLPFC activation is associated with the occurrence of interpersonal conflicts between participant and their partner in predicting weaker negative mood and stronger positive mood.
The neural response to rejection in our group of participants with elevated schizotypal personality traits, however, showed deactivation of this frontal lobe network. Our HS group tended to have a higher level of RS than the LS group (P = 0.1, medium effect size), supporting an earlier study [Torgersen et al.,2002] where individuals with a schizotypal personality disorder had greater RS than healthy individuals. The HS group, selected on the basis of a high level of unusual experiences, also had a higher level of cognitive disorganization, impulsive nonconformity, momentary negative affect and on average higher anxiety than the LS group. HS individuals with a greater‐than‐normal propensity for these schizotypal personality traits and low mood may adopt alternative ways of dealing with social rejection compared to LS individuals, for instance by downregulating their responses to rejection cues by distancing themselves from the rejection scenes [Koenigsberg et al.,2010]. This may explain why, in the present study, the HS group did not differ from the LS group in the off‐line rejection‐acceptance ratings of the images. When participants are expected to mentalize a given emotional state but also to see their own emotional state as distinct from the observed emotion, they may express a more neutral mood [Polivy and Doyle,1980]. Low RS individuals activated, while high RS individuals deactivated the VLPFC and SFG when perceiving social rejection [Kross et al.,2007], which suggested that these areas may be important for interpreting rejection‐related events in ways that minimize personal distress. HS individuals, like high RS individuals [Kross et al.,2007], who deactivate these regions during social rejection may do so in order to distance themselves from the observed emotional state, i.e. social rejection, rather than engage themselves in meaningful interpretation of the event. HS individuals, who have greater anxiety and RS levels due to their greater propensity for unusual experiences, may find it more beneficial to distance themselves from, rather than engage in, stress‐provoking situations.
Patients with a past history of major depression who had been symptom free for more than 6 months deactivated the dACC compared to healthy participants on hearing maternal criticisms [Hooley et al.,2009]. Hooley et al. [2009] discussed that increased dACC activity in healthy individuals when listening to maternal criticisms may reflect increased attention to emotionally salient stimuli, while the previously depressed patients may be able to reduce attention to such stimuli as a protective strategy and consequently “turn off” the dACC. In this study, a difference in current negative mood between HS and LS groups seemed to contribute towards some—but not all—of the variation in dACC activation, suggesting that negative affect may play a similar role in individuals with a schizotypal personality to that observed in patients with depression. Individuals with a schizotypal personality and patients with depression may have similar ways of responding to negative expressed emotion in the form of rejection or criticism, as positive schizotypy is associated with depression [Lewandowski et al.,2006]. A high level of relative's expressed emotion in the form of criticism is associated with a greater likelihood of relapse in patients with a major depressive disorder (see Wearden et al.,2000 for a review of the literature). Altered responses to perceived rejection in individuals with a schizotypal personality who are rejection sensitive may also be due to higher levels of relative's expressed emotion. Such an explanation needs to be tested in future studies on expressed emotion in schizotypy.
Greater Lingual Gyral Activation During Rejection Compared to Acceptance Conditions Across All Participants
The lingual gyrus was activated bilaterally to a greater extent during rejection than acceptance conditions across all participants. The lingual gyrus is frequently associated with the identification of facial emotional expressions (Keightley et al.,2007; Kitada et al.,2010; Scheuerecker et al.,2007], but also when individuals simulate other people's facial expressions [Kim et al.,2007]. The lingual gyrus is also activated when experiencing a form of rejection, i.e. when a person is immersed in a social interaction with his/her partner and learns that his/her partner has failed to reciprocate cooperation [Rilling et al.,2008]. Our findings confirm that the lingual gyrus is involved in social cognition, but more specifically in discriminating between rejection and acceptance, and in this regard the HS group showed a normal neural response to social rejection. In addition, greater activation of the lingual gyrus was associated with a more restricted emotional range (as measured by the VAS ratings) between rejection and acceptance conditions, and with lower arousal ratings of rejection and acceptance images. These relationships may suggest an association between more effortful use of the lingual gyrus to discriminate between rejection and acceptance conditions and possibly cognitive control (top‐down processing) of emotions. These relationships may also suggest an attenuated ability to interpret rejection and acceptance scenes as extremely rejecting and accepting, respectively, and an increased ability to regard these scenes in a more neutral way. Kross et al. [2007] found that a stronger neural response to rejection scenes was associated with lower subsequent distress ratings of rejection images.
Greater activation of the right superior temporal gyrus across all participants during rejection compared to neutral conditions was associated with greater negative mood in general. Recent research has shown that the superior temporal gyrus is associated with the regulation of negative emotions (Koenigsberg et al.,2010; Mak et al.,2009; Winecoff et al., in press]. In recent studies [Koenigsberg et al.,2010; Winecoff et al., in press], healthy participants activated the superior temporal gyrus when reappraising negative images using a response style that involved detaching themselves from the image. Our results suggest that greater use of the right superior temporal gyrus when viewing rejection scenes may cause individuals to experience more negative mood. Conversely, individuals who do not show this neural response to rejection are able to feel less general negative mood.
Limitations and Future Research
First, the groups did not differ in the behavioral response to the task stimuli. The demand characteristics of the situation, viz. favoring the prototypical expression of how a person should feel after exposure to such scenes, may have minimized differences in subjective ratings of LS and HS individuals. This does not necessarily preclude the presence of a group difference in the neural response to the stimuli [Wilkinson and Halligan,2004]; the neural response can be used to inform some of the cognitive processes engaged in behaviors that are less well understood or in need of further explanation [Wilkinson and Halligan,2004]. Second, our HS group had only a marginally higher level of RS than the LS group (P = 0.1, medium effect size). The small sample sizes would have made it difficult for some of the group differences to have reached a statistically significant level. The study's findings therefore need to be replicated in a larger sample. Third, given the association between positive schizotypy and depression [Lewandowski et al.,2006], it is possible that the observed neural response to rejection scenes in the HS group may be further moderated by depression. However, the HS and LS groups in our study did not differ in the level of depression as measured by the BDI because we excluded those with high levels of depression. The neural response to perceived rejection may be stronger in individuals with a high level of depression and schizotypal personality. Future studies may consider the role of depression in behavioral and neural response styles to rejection in individuals with a schizotypal personality. Fourth, the RSS was administered after participants performed the RS on‐line task, but before the RS off‐line task, that may have temporarily altered their responses on the RSS. Fifth, this study was not powered to investigate potential sex‐specific effects of schizotypy in response to social rejection. Finally, the rejection scenes may not have had personal relevance to the participants and therefore not engaged the participants optimally. It is possible that schizotypal individuals are able to regulate their subjective and neural responses to social rejection only when the scenes are not personally relevant.
CONCLUSIONS
Schizotypal individuals deactivate a dorso‐ventral frontal lobe network, consisting of the dACC, right SFG, and left VLPFC/VMPFC. The neural and behavioral response to rejection stimuli suggests that schizotypal individuals may use strategies that help them to distance themselves from and minimize the salience attached to rejection‐provoking stimuli.
Table Appendix A.
Average of the ratings on rejection level and valence provided by six doctoral or postdoctoral researchers of the 15 rejection, 15 acceptance, and 15 neutral images used in the rejection‐acceptance task
| Image type | Rejection level | Valence |
|---|---|---|
| Rejection images | ||
| Mean | −2.83 | −2.34 |
| Minimum | −3.50 | −3.33 |
| Maximum | −2.50 | −1.50 |
| Acceptance images | ||
| Mean | 4.03 | 3.82 |
| Minimum | 3.67 | 3.50 |
| Maximum | 4.50 | 4.33 |
| Neutral images | ||
| Mean | 0.00 | 0.66 |
| Minimum | −0.50 | 0.26 |
| Maximum | 0.50 | 1.21 |
Rejection level rated from −5 rejected to +5 accepted; valence rated from −5 negative to +5 positive.
REFERENCES
- Bailer J, Rist F, Brauer W, Rey ER ( 1994): Patient Rejection Scale: Correlations with symptoms, social disability and number of rehospitalizations. Eur Arch Psychiatry Clin Neurosci 244: 45–48. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR ( 2000): Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10: 295–307. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA ( 1993): Manual for the Beck Anxiety Inventory. San Antonio, Texas: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, Brown GK ( 1996): Manual for the Beck Depression Inventory, 2nd ed. San Antonion, Texas: Psychological Corporation. [Google Scholar]
- Bollini AM, Compton MT, Esterberg ML, Rutland J, Chien VH, Walker EF ( 2007): Associations between schizotypal features and indicators of neurological and morphological abnormalities. Schizophr Res 92: 32–40. [DOI] [PubMed] [Google Scholar]
- Burklund LJ, Eisenberger NI, Lieberman MD ( 2007): The face of rejection: Rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Soc Neurosci 2: 238–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd , Ross LL, Stenger VA ( 2001): Anterior cingulate cortex activity and impaired self‐monitoring of performance in patients with schizophrenia: An event‐related fMRI study. Am J Psychiatry 158: 1423–1428. [DOI] [PubMed] [Google Scholar]
- Cochrane M, Petch I, Pickering AD ( 2010): Do measures of schizotypal personality provide non‐clinical analogues of schizophrenic symptomatology? Psychiatry Res 176: 150–154. [DOI] [PubMed] [Google Scholar]
- Downey G, Feldman S ( 1996): Implications of rejection sensitivity for intimate relationships. J Pers Soc Psychol 70: 545–560. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD ( 2003): Does rejection hurt? An FMRI study of social exclusion. Science 302: 290–292. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Crawford TJ, Flak V, Sharma T, Davis RE, Corr PJ ( 2005): Saccadic eye movements, schizotypy, and the role of neuroticism. Biol Psychol 68: 61–78. [DOI] [PubMed] [Google Scholar]
- Fanous A, Gardner C, Walsh D, Kendler KS ( 2001): Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms in nonpsychotic relatives. Arch Gen Psychiatry 58: 669–673. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Neale MC, Gardner CO, Webb BT, Straub RE, O'Neill FA, Walsh D, Riley BP, Kendler KS ( 2007): Significant correlation in linkage signals from genome‐wide scans of schizophrenia and schizotypy. Mol Psychiatry 12: 958–965. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Matts CW, Rollmann EA ( 2006): Sustained attention deficits in relation to psychometrically identified schizotypy: Evaluating a potential endophenotypic marker. Schizophr Res 82: 27–37. [DOI] [PubMed] [Google Scholar]
- Grant PM, Beck AT ( 2009): Evaluation sensitivity as a moderator of communication disorder in schizophrenia. Psychol Med 39: 1211–1219. [DOI] [PubMed] [Google Scholar]
- Heresco‐Levy U, Brom D, Greenberg D ( 1992): The Patient Rejection Scale in an Israeli sample: Correlations with relapse and physician's assessment. Schizophr Res 8: 81–87. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Gyurak A, Verosky SC, Miyakawa A, Ayduk O ( 2010a): Neural activity to a partner's facial expression predicts self‐regulation after conflict. Biol Psychiatry 67: 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D'Esposito M ( 2010b): Neural activity during social signal perception correlates with self‐reported empathy. Brain Res 1308: 100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Scott LA, Hiller JB, Yurgelun‐Todd DA ( 2005): Activation in dorsolateral prefrontal cortex in response to maternal criticism and praise in recovered depressed and healthy control participants. Biol Psychiatry 57: 809–812. [DOI] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Parker HA, Guillaumot J, Rogowska J, Yurgelun‐Todd DA ( 2009): Cortico‐limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Res 172: 83–91. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Chiew KS, Winocur G, Grady CL ( 2007): Age‐related differences in brain activity underlying identification of emotional expressions in faces. Soc Cogn Affect Neurosci 2: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Kim JW, Kim JJ, Jeong BS, Choi EA, Jeong YG, Kim JH, Ku J, Ki SW ( 2007): The neural mechanism of imagining facial affective expression. Brain Res 1145: 128–137. [DOI] [PubMed] [Google Scholar]
- Kitada R, Johnsrude IS, Kochiyama T, Lederman SJ ( 2010): Brain networks involved in haptic and visual identification of facial expressions of emotion: An fMRI study. Neuroimage 49: 1677–1689. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise K, Pizzarello S, Dorantes C, Tecuta L, Guerreri S, Goodman M, New A, Flory J, Siever LJ ( 2010): Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia 48: 1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer UM, Mohammadi B, Donamayor N, Samii A, Munte TF ( 2010): Emotional and cognitive aspects of empathy and their relation to social cognition—An fMRI‐study. Brain Res 1311: 110–120. [DOI] [PubMed] [Google Scholar]
- Kreisman D, Blumenthal R, Borenstein M, Woerner M, Kane J, Rifkin A, Reardon G ( 1988): Family attitudes and patient social adjustment in a longitudinal study of outpatient schizophrenics receiving low‐dose neuroleptics: The family's view. Psychiatry 51: 3–13. [DOI] [PubMed] [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, Downey G ( 2007): Neural dynamics of rejection sensitivity. J Cogn Neurosci 19: 945–956. [DOI] [PubMed] [Google Scholar]
- Kuipers E, Onwumere J, Bebbington P ( 2010): Cognitive model of caregiving in psychosis. Br J Psychiatry 196: 259–265. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN ( 1999): International Affective Picture System (IAPS): Instruction Manual and Affective Ratings. The Center for Research in Psychophysiology. Gainsville, Florida: University of Florida. [Google Scholar]
- Langens TA, Schuler J ( 2005): Written emotional expression and emotional well‐being: The moderating role of fear of rejection. Pers Soc Psychol Bull 31: 818–830. [DOI] [PubMed] [Google Scholar]
- Leff J, Vaughn CE ( 1985): The expressed emotion scales In: Leff J, Vaughn CE, editors. Expressed Emotion in Families: Its Significance for Mental Illness. London: Guidlford Press; 37–63. [Google Scholar]
- Lemay EP, Clark MS ( 2008): “Walking on eggshells”: How expressing relationship insecurities perpetuates them. J Pers Soc Psychol 95: 420–441. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Barrantes‐Vidal N, Nelson‐Gray RO, Clancy C, Kepley HO, Kwapil TR ( 2006): Anxiety and depression symptoms in psychometrically identified schizotypy. Schizophr Res 83: 225–235. [DOI] [PubMed] [Google Scholar]
- Mak AK, Hu ZG, Zhang JX, Xiao ZW, Lee TM ( 2009): Neural correlates of regulation of positive and negative emotions: An fmri study. Neurosci Lett 457: 101–106. [DOI] [PubMed] [Google Scholar]
- Mason O, Claridge G, Jackson M ( 1995): New scales for the assessment of schizotypy. Pers Individ Differ 18: 7–13. [Google Scholar]
- Mason O, Linney Y, Claridge G ( 2005): Short scales for measuring schizotypy. Schizophr Res 78: 293–296. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M ( 2009): Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Soc Cogn Affect Neurosci 4: 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian A, Ksionzky S ( 1974): A Theory of Affiliation. Toronto, Canada: Lexington Books. [Google Scholar]
- Nelson HE, Wilson J ( 1991): National Adult Reading Test Manual, 2nd ed. Windsor: NFER‐Nelson. [Google Scholar]
- Polivy J, Doyle C ( 1980): Laboratory induction of mood states through the reading of self‐referent mood statements: Affective changes or demand characteristics? J Abnorm Psychol 89: 286–290. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Goldsmith DR, Glenn AL, Jairam MR, Elfenbein HA, Dagenais JE, Murdock CD, Pagnoni G ( 2008): The neural correlates of the affective response to unreciprocated cooperation. Neuropsychologia 46: 1256–1266. [DOI] [PubMed] [Google Scholar]
- Rosenfarb IS, Goldstein MJ, Mintz J, Nuechterlein KH ( 1995): Expressed emotion and subclinical psychopathology observable within the transactions between schizophrenic patients and their family members. J Abnorm Psychol 104: 259–267. [DOI] [PubMed] [Google Scholar]
- Rosenfarb IS, Nuechterlein KH, Goldstein MJ, Subotnik KL ( 2000): Neurocognitive vulnerability, interpersonal criticism, and the emergence of unusual thinking by schizophrenic patients during family transactions. Arch Gen Psychiatry 57: 1174–1179. [DOI] [PubMed] [Google Scholar]
- Rusch N, Corrigan PW, Wassel A, Michaels P, Olschewski M, Wilkniss S, Batia K ( 2009): A stress‐coping model of mental illness stigma. I. Predictors of cognitive stress appraisal. Schizophr Res 110: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Brown GW ( 1966): The reliability and validity of measures of family life and relationships in families containing a psychiatric patient. Soc Psychiatry 1: 38–53. [Google Scholar]
- Scheuerecker J, Frodl T, Koutsouleris N, Zetzsche T, Wiesmann M, Kleemann AM, Bruckmann H, Schmitt G, Moller HJ, Meisenzahl EM ( 2007): Cerebral differences in explicit and implicit emotional processing—An fMRI study. Neuropsychobiology 56: 32–39. [DOI] [PubMed] [Google Scholar]
- Sokolowski K, Schmalt HD, Langens TA, Puca RM ( 2000): Assessing achievement, affiliation, and power motives all at once: The Multi‐Motive Grid (MMG). J Pers Assess 74: 126–145. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM ( 2006): Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci 9: 1007–1008. [DOI] [PubMed] [Google Scholar]
- Sommer M, Sodian B, Dohnel K, Schwerdtner J, Meinhardt J, Hajak G ( 2010): In psychopathic patients emotion attribution modulates activity in outcome‐related brain areas. Psychiatry Res 182: 88–95. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Edvardsen J, Oien PA, Onstad S, Skre I, Lygren S, Kringlen E ( 2002): Schizotypal personality disorder inside and outside the schizophrenic spectrum. Schizophr Res 54: 33–38. [DOI] [PubMed] [Google Scholar]
- Vollema MG, van den Bosch RJ ( 1995): The multidimensionality of schizotypy. Schizophr Bull 21: 19–31. [DOI] [PubMed] [Google Scholar]
- Vorauer JD, Cameron JJ, Holmes JG, Pearce DG ( 2003): Invisible overtures: Fears of rejection and the signal amplification bias. J Pers Soc Psychol 84: 793–812. [DOI] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF ( 2004): Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci 7: 1259–1265. [DOI] [PubMed] [Google Scholar]
- Walton ME, Croxson PL, Behrens TE, Kennerley SW, Rushworth MF ( 2007): Adaptive decision making and value in the anterior cingulate cortex. Neuroimage 36 ( Suppl 2): T142–T154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A ( 1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wearden AJ, Tarrier N, Barrowclough C, Zastowny TR, Rahill AA ( 2000): A review of expressed emotion research in health care. Clin Psychol Rev 20: 633–666. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Halligan P ( 2004): The relevance of behavioural measures for functional‐imaging studies of cognition. Nat Rev Neurosci 5: 67–73. [DOI] [PubMed] [Google Scholar]
- Winecoff A, Labar KS, Madden DJ, Cabeza R, Huettel SA: Cognitive and neural contributors to emotion regulation in aging. Soc Cogn Affect Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]


