Abstract
Intracortical evoked potentials to nonnoxious Aβ (electrical) and noxious Aδ (laser) stimuli within the human primary somatosensory (S1) and motor (M1) areas were recorded from 71 electrode sites in 9 epileptic patients. All cortical sites responding to specific noxious inputs also responded to nonnoxious stimuli, while the reverse was not always true. Evoked responses in S1 area 3b were systematic for nonnoxious inputs, but seen in only half of cases after nociceptive stimulation. Nociceptive responses were systematically recorded when electrode tracks reached the crown of the postcentral gyrus, consistent with an origin in somatosensory areas 1–2. Sites in the precentral cortex also exhibited noxious and nonnoxious responses with phase reversals indicating a local origin in area 4 (M1). We conclude that a representation of thermal nociceptive information does exist in human S1, although to a much lesser extent than the nonnociceptive one. Notably, area 3b, which responds massively to nonnoxious Aβ activation was less involved in the processing of noxious heat. S1 and M1 responses to noxious heat occurred at latencies comparable to those observed in the supra‐sylvian opercular region of the same patients, suggesting a parallel, rather than hierarchical, processing of noxious inputs in S1, M1 and opercular cortex. This study provides the first direct evidence for a spinothalamic related input to the motor cortex in humans. Hum Brain Mapp 34:2655–2668, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: human, somatosensory cortex, motor cortex, evoked potentials, pain
INTRODUCTION
Since the description of the “parietal sensory syndrome” at the turn of the 20th century [Dejerine, 1914; Verger, 1900], it is widely acknowledged that lesions involving exclusively the primary somatosensory cortex (S1), while creating impressive discriminative sensory loss (stereognosis, position sense, two‐point discrimination) do not entail sizeable deficits in pain and temperature sensations [e.g., Kim, 2007]. Still today, the role of S1 in human pain perception remains controversial. By 1995–2000 the detection of significant pain‐related S1 activity concerned a rough half of functional imaging studies [Peyron et al., 2000], but this increased to more than 2/3rds of cases in later years [Apkarian et al., 2005]. In parallel, correlations between activity in S1 and other pain‐related regions, such as the operculo‐insular cortex, could be demonstrated even when subtraction analyses failed to demonstrate significant S1 activation [Petrovic et al., 2002]. Accumulating evidence from electrophysiological studies in humans indicates S1 responsiveness following stimulations that activate selectively A‐delta and C nociceptors, such as laser pulses [Kanda et al., 2000; Nakata et al., 2004; Ploner et al., 1999a; Timmermann et al., 2001] or intraepidermal electrical stimuli [Inui et al., 2002, 2003a, 2003b; Ogino et al., 2005].
Although evidence points to the existence of pain‐related activity in S1, the variability of pain‐induced S1 responses across studies and experimental conditions raises several questions. Should pain be actually represented in the S1 area, it would be difficult to understand why focal S1 injury does not entail significant deficits in pain sensation [Dejerine, 1914] or why direct S1 electrical stimulation rarely, if ever, evoke painful sensations [Penfield and Rasmussen, 1955; Mazzola et al., 2012]. S1 is an anatomically very complex area, very close to M1, and including four cytoarchitectonically distinct subdivisions, namely areas 3a, 3b, 1, and 2 [Kaas and Pons, 1988]. Studies in primates support a possible nociceptive role for areas 3a and 1, which in humans lay, respectively in the depth and the crown of the postcentral gyrus [Kenshalo et al., 2000; Tommerdahl et al., 1996; Whitsel et al., 2009]. In humans, nociceptive responses originating in areas 1–2 have been suggested from MEG and subdural EEG data [Inui et al., 2002, 2003a, 2003b; Kanda et al., 2000; Ogino et al., 2005; Ploner et al., 1999a]. A single‐case study using one intracranial electrode directly implanted in S1 suggests that area 3b located in the posterior bank of the central sulcus does not respond to specific noxious laser stimuli [Valeriani et al., 2004]. Two other studies using subdural recordings over cortical surface in a small number of patients show contradictory results concerning the involvement of S1 area 3b in pain responses [Baumgärtner et al., 2011; Kanda et al., 2000].
In this study we shed light on the issue of human S1 nociceptive responses by recording intracerebral responses to both innocuous and noxious stimuli recorded in epileptic patients from 31 different S1 sites. In addition, by recording nociceptive responses from 40 sites in M1, we specifically addressed the issue of possible local responses in the motor areas, which has been suggested in animals [Padel and Relova, 1991, Dum et al., 2009] but never demonstrated previously in humans.
MATERIALS AND METHODS
Patients
Nine patients (five women and four men; mean age 24 years, range: 20–28 years) were included in this study; all of them suffered from partial refractory epileptic seizures suspected to originate from the temporal lobe but with a rapid spread to supra‐sylvian frontal and/or parietal cortex. Invasive recordings were carried out using stereotactically implanted intracerebral electrodes (Stereo‐Electro‐Encephalography or SEEG) before functional neurosurgery. Among other sites, these patients had electrodes chronically implanted in the pre‐ and postcentral gyri, supra‐sylvian opercular and insular cortices for the recording of their seizures [see Isnard et al., 2000, 2004 for a complete description of the rationale of electrode implantation]. The recording of spontaneous seizures is routinely completed by the functional mapping of potentially eloquent cortical areas using evoked potentials recordings and cortical electrical stimulation before epilepsy surgery in patients implanted with depth electrodes [for a description of the stimulation procedure see Ostrowsky et al., 2002; Mazzola et al., 2006]. In agreement with French regulations relative to invasive investigations with a direct individual benefit, patients gave their consent after being fully informed about electrode implantation, SEEG, evoked potentials recordings, and cortical stimulation procedures used to localize the epileptogenic and eloquent cortical. The YAP laser stimulation paradigm was submitted to, and approved by, the local Ethics Committee.
Evoked potential recordings were performed at the end of the SEEG monitoring period, which lasted a maximum of 2 weeks. At the time of the SEEG procedure antiepileptic treatment had been tapered down, so that all patients were under therapy with one or two of the major anti‐epileptic drugs (carbamazepine, phenytoin, valproate, lamotrigine, or topiramate) with daily dosages at, or slightly under the minimum of their usual therapeutic range.
Electrode Implantation
Intracerebral electrodes were implanted using the Talairach's stereotactic frame. As a first step, a cerebral angiography was performed in stereotactic conditions using an X‐ray source located 4.85 m away from the patient's head. This eliminates the linear enlargement due to X‐ray divergence, so that the films could be used for measurements without any correction. In a second step, the relevant targets were identified on the patient's MRI, previously enlarged at scale one‐to‐one. As MR and angiographic images were at the same scale, they could easily be superimposed, allowing to avoid cerebral vessels during electrode implantation and thus to minimize the risk of hemorrhages. The electrodes were orthogonally implanted using the Talairach's stereotactic grid; each electrode had 10–15 contacts, each of 2 mm length, separated by 1.5 mm, and could be left in place chronically up to 15 days. Because of the physical characteristics of the contacts (stainless steel), MRI could not be performed with electrodes in place. Scale 1:1 skull radiographies superimposed to scale 1:1 angiographies were used to perform the implantation within the stereotactic frame of Talairach and Tournoux 1988. The electrode tracks and the contacts of each electrode could be plotted onto the appropriate MRI slices of each patient [MRIcro® software; Rorden and Brett, 2000]. Each contact was then localized in the Talairach space using its stereotactic coordinates: x for the lateral medial axis, with x = 0 being the coordinate of the sagittal interhemispheric plane; y for the rostro‐caudal (anterior–posterior) axis, y = 0 being the coordinate of the vertical anterior commissure (VAC) plane and z for the inferior–superior axis, z = 0 being the coordinate of the horizontal AC‐PC plane orthogonal to the mid‐sagittal vertical plane and passing through the anterior and posterior commissures [see Frot et al., 2007, 2008].
Postcentral and Precentral Cortex Exploration
In a given patient, averages of responses recorded in referential and bipolar modes obtained from each contact located in a given region were considered. According to this procedure, a total of 71 pre‐ and postcentral sites (31 contacts in S1 and 40 in M1) were explored. Then the contact exhibiting the largest peak‐to‐peak amplitude for the largest component in referential mode was retained to perform the grand‐average, representative of the response obtained in a given structure.
Thus, altogether a total of 21 contacts located in S1 and M1 areas were considered. The locations of these contacts and their mean Talairach coordinates are shown in Figure 1. Identification of the different subareas (1–2 and 3b) within S1 was done considering the morphological aspect of gyral and sulcal structures on the brain MRI of each patient, as well as the phase reversal of somatosensory evoked potentials (N20/P20 potentials) across the central sulcus, which characterizes responses originating from area 3b [Allison et al., 1989; Mauguière et al., 1983; Wood et al., 1988]. Within S1, eight contacts were analyzed in area 3b, and 4 contacts in the crown of the postcentral gyrus (areas 1–2). Nine other contacts were selected in area 4 of the precentral gyrus (M1).
Figure 1.
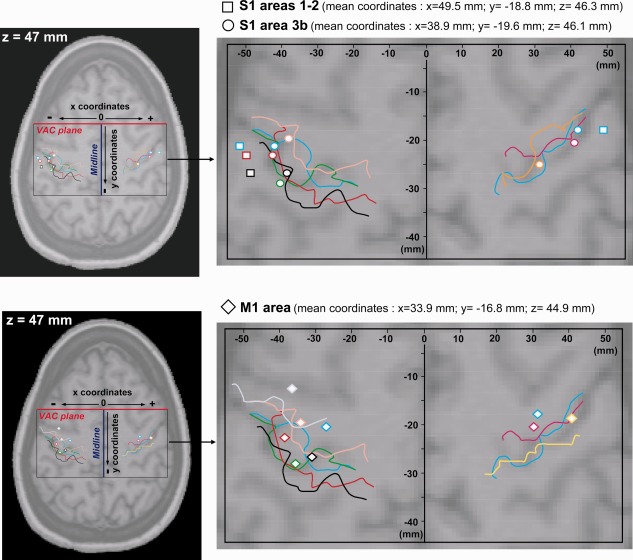
Localization of intracerebral contacts exploring the pre‐ and postcentral gyri. The position of each contact in a given patient was determined in three dimensions using his/her own 3D‐MRI. Then, positions of all contacts were registered in the Talairach and Tournoux referential and plotted together on a horizontal MRI slice, with z coordinate corresponding to the mean z coordinate of contacts in S1/M1 (z = 47 mm). Colored lines illustrate the position of the central sulcus in each patient. The same color was used to represent the central sulcus and the electrode contact positions in a given subject. Contacts located in S1 areas 1–2 are represented by squares, contacts located in S1 area 3b by circles and contacts located in M1 by diamonds. All the contacts are located according to their x (distance between each contact and the midline plane) and y coordinates (distance between each contact and the Vertical Anterior Commissure (VAC) plane).
Supra‐Sylvian Opercular and Insular Cortices Exploration
In addition to S1/M1, seven patients had also electrodes implanted in the supra‐sylvian operculum (including S2) and insular cortices that were explored by a total of 44 contacts. To analyze evoked potentials and perform a grand‐average of the responses, we also selected the recording site where the response amplitude was the largest among the peak to peak voltages recorded from all contacts in referential recording mode. Thus, evoked potentials issued from six contacts located in the supra‐sylvian operculum and eight in the insula were analyzed. The locations of these contacts and their mean Talairach coordinates are summarized in Figure 2.
Figure 2.
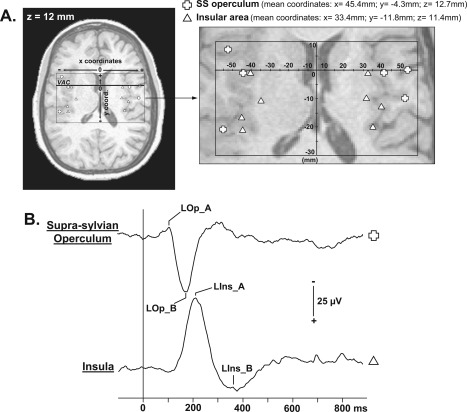
Localization of contacts exploring supra‐sylvian opercular and insular cortices and LEPs recorded in these areas. A: According to the same procedure described in Figure 1, positions of all contacts recorded in operculum (open cross) and insula (open triangle) are represented on a horizontal MRI slice (z = 12 mm). B: Supra‐sylvian opercular and insular LEPs grand average from all the patients (referential recording mode; positivity: downward). VAC: Vertical Anterior Commissure plane. SS operculum: Supra‐sylvian operculum.
Stimulation Procedure, Recording, and Signal Averaging
Evoked potential recordings were performed between 10 and 15 days after electrode implantation. During the recordings, the patients lay down on a bed in a quiet room.
Nociceptive Stimulation and LEPs
Laser evoked potentials (LEPs) were recorded in response to nociceptive stimuli applied with a Nd:YAP laser (Yttrium Aluminium Perovskite; wavelength 1.34 μm, El‐En®), which delivered brief radiant heat pulses of 5‐ms duration. The laser beam was transmitted from the generator to the stimulating probe via an optical fibre of 10 m length. Two separate runs of 12 to 15 stimulations applied to the skin in the superficial radial nerve territory on the dorsum of the hand were delivered contralateral to the implanted electrodes. The interstimulus interval varied randomly between 10 and 25 s. The laser beam was slightly moved between two successive stimuli to avoid habituation and especially peripheral nociceptor fatigue [Schwarz et al., 2000]. The intensity was set up according to subjects' subjective reports, rated on a visual numerical scale. The printed scales consisted of 10‐cm horizontal lines where the left extreme was labelled “no sensation” and the right extreme “maximal pain,” anchored level 4 corresponding to pain threshold. Stimulation intensity was kept stable for any given patient during the whole recording time, 20% above the pain threshold determined before the experimental session. The subjects had to provide pain ratings after each run of stimulation. For all patients, pain threshold was obtained with a beam diameter of 4–5 mm and beam energy of 1J, i.e., 50–79 mJ mm−2, well in accordance with previous work using this type of stimulus [Leandri et al., 2006; Perchet et al., 2008]. The mean intensity rating was 5.8 ± 0.9, described as “painful but tolerable” by all patients.
Online recordings were performed using a sampling frequency of 256 Hz and a band pass filter of (‐3 dB) 0.03–200 Hz (Micromed®).
Nonnociceptive Stimulation and SEPs
Median nerve somatosensory evoked potentials (SEPs) were recorded to obtain the N20‐P20 response known to originate from the posterior bank of the postcentral gyrus and thus to identify contacts located in the area 3b [Allison et al., 1991]. Electrical stimuli of 200 μs were delivered at 2 Hz by skin electrodes to the median nerve at the wrist contralateral to the intracerebral electrodes. Two runs of 200 stimuli were performed. The stimulus intensity was set at motor threshold eliciting a twitch in thenar muscles (intensity 7–15 mA) and was not painful. The subjects provided an intensity rating using the same visual numerical scale as for the nociceptive stimuli. The mean intensity rating was 2.3 ± 0.5, described as not painful by all patients. Online recordings were performed using a sampling frequency of 512 Hz and a band pass filter (‐3 dB) of 0.03–200 Hz (Micromed®).
The skull reference contact was chosen individually according to the implantation scheme, which was itself determined by the location of the epileptogenic area. In all patients this reference contact was the most superficial one along an electrode track situated at distance of the central cortical region and was located in the skull outside the cortex. At this location, the amplitude of the EEG activity is extremely reduced with respect to the one recorded directly in the gray matter. Although the chosen reference changed from patient to patient, its location was always the same for the LEPs and SEPs recordings in a given patient.
Data Analysis
Epoching of the EEG, selective averaging and record analysis were performed off‐line using Neuroscan® software. For the LEPs analysis each epoch started 100 ms before and ended 900 ms after the stimulus. For SEPs, each epoch started 100 ms before and ended 350 ms after the electrical stimulus. A prestimulus baseline correction was performed before averaging. Epochs presenting epileptic transient activities were rejected from analysis. Averaging was performed to reduce the background EEG noise so as to facilitate analysis of stimulus‐locked activity (evoked potentials). Finally, the two runs of laser or electrical stimulation were pooled after having checked that the averaged waveforms were reproducible. For illustration purposes we performed grand‐averages of LEPs and SEPs recorded in the same regions; however, all statistical analyses were based on amplitudes and latencies measured in each patient in referential recordings.
The different response components were determined according to the usual guidelines used for evoked potentials analyses that is according to their polarities and peak latencies [Cruccu et al., 2008]. Response components were labeled according to their location, the type of stimulus, and numbered “A” to “E” in the order of increasing peaking latencies; for example “LM1_A, LM1_B” corresponded to two laser evoked response components recorded in M1, with LM1_A being the earliest one. This nomenclature was applied for all the components of the LEPs and SEPs recorded in S1 and M1 except for the earliest response recorded after the electrical stimulation, which was named N20‐P20 according to the conventional nomenclature. Recordings in both referential and bipolar montages were considered. In the text and tables, mean latencies and amplitudes are given together with ±1 SD.
RESULTS
Mean latencies and amplitudes of LEPs and SEPs are given in Tables 1 and 2. Although all SEPs components are described in Table 2, only the N20‐P20 response was analyzed to identify contacts located in the area 3b; the later components were not be discussed in this article.
Table 1.
Mean latencies and amplitudes of LEPs recorded in S1, M1, supra‐sylvian operculum, and insula
| S1 3b | S1 1_2 | M1 | SS operculum | Insula | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LS1_C | LS1_D | LS1_A | LS1_B | LS1_C | LS1_D | LM1_A | LM1_B | LM1_C | LOp_A | LOp_B | LIns_A | LIns_B | |
| LEPs | |||||||||||||
| Latencies (ms) | 141.6 ± 5.9 | 195.3 ± 10.1 | 101.6 ± 3.2 | 128.9 ± 3.2 | 145.8 ± 2.3 | 188.8 ± 9 | 115.6 ± 10.9 | 171.6 ± 16.2 | 249.4 ± 35.4 | 126.4 ± 18.5 | 186.1 ± 9.7 | 217.8 ± 21.3 | 328.4 ± 31.4 |
| Amplitudes (μV) | 16.2 ± 6.7 | 59.9 ± 30 | 8.4 ± 5 | 18.9 ± 10 | 15.2 ± 9.5 | 53.4 ± 17.4 | 32.8 ± 26.8 | 109.8 ± 101 | 68.5 ± 71.3 | 20.7 ± 28.7 | 35.6 ± 4.3 | 66.4 ± 26.2 | 33.3 ± 24.2 |
Table 2.
Mean latencies and amplitudes of SEPs recorded in S1 and M1 areas
| S1 3b | S1 1_2 | M1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N20 | sS1_C | sS1_D | sS1_E | N20 | sS1_A | sS1_B | sS1_E | P20 | sM1_A | sM1_B | |
| SEPs | |||||||||||
| Latencies (ms) | 21.5 ± 1.6 | 44.5 ± 1.8 | 52.3 ± 1.6 | 62.5 ± 2 | 22.1 ± 1.1 | 29.9 ± 1.1 | 37.7 ± 1.1 | 61.9 ± 3 | 22 ± 2.1 | 31.8 ± 2.5 | 53.3 ± 2.2 |
| Amplitudes (μV) | 52.5 ± 64.4 | 55.8 ± 40.2 | 59.2 ± 26.9 | 83.5 ± 58 | 46.8 ± 66.9 | 23.1 ± 19 | 23.7 ± 8.1 | 31.1 ± 22.8 | 57 ± 55.1 | 44.3 ± 34.8 | 111.5 ± 96.7 |
Evoked Potentials (EPs) Recorded From S1
3b area
EPs from S1 area 3b were analyzed on eight contacts (Fig. 1). At all these sites a somatosensory N20 response to nonnoxious electrical stimulation was recorded with a phase reversal (P20) across the central sulcus in referential recordings, thus confirming that these contacts were implanted within the area 3b (Fig. 3).
Figure 3.
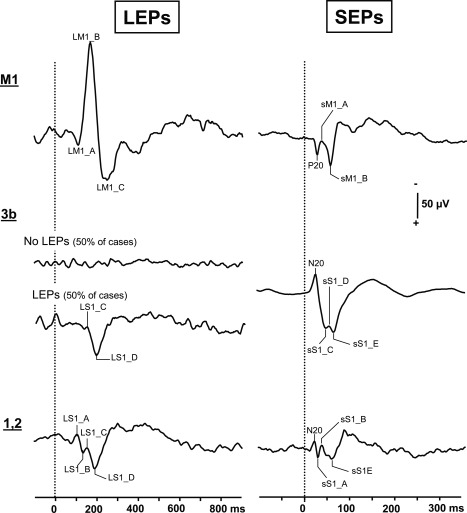
LEPs and SEPs grand averages recorded in M1 and S1 cortices. These grand averages are based on EPs recorded in referential mode on the contacts showing the highest amplitudes in M1 and S1 cortices (M1: nine contacts for LEPs and SEPs; S1 area 3b: four contacts for LEPs and eight for SEPs; S1 areas 1,2: four contacts for LEPs and SEPs). Positivity: downwards.
Whereas SEPs with a phase‐reversal across the central sulcus were systematically obtained, LEPs to thermo‐nociceptive stimuli were recorded only by half of the contacts implanted in area 3b. The responses consisted of a biphasic waveform with two components, (LS1_C and LS1_D) recorded at 141.6 ± 5.9 ms and 195.3 ± 10.1 ms, respectively (Table 1). A phase reversal across the central sulcus in referential recordings was obtained in two cases only (P1 and P2 in Fig. 4) suggesting that these LEPs might have a local generator in the 3b area, as the N20 SEP (Fig. 4). In two other patients, LEPs recorded by contacts in area 3b did not show any phase reversal in bipolar or referential recordings. In four remaining patients with contacts in area 3b, no local potential at all could be obtained within this area in response to nociceptive laser stimuli (Fig. 3).
Figure 4.
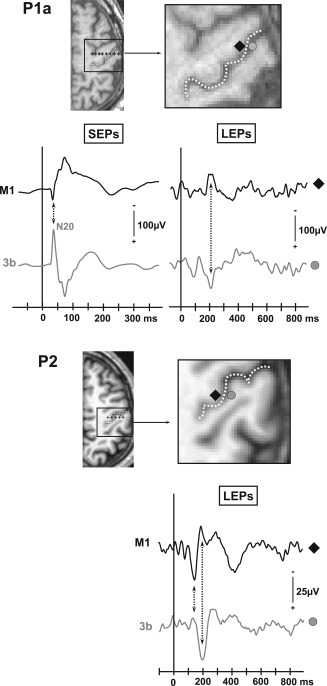
Phase reversals recorded in referential mode across the central sulcus in two patients (P1a, P2). Positions of the selected electrode contacts are plotted on the patient's MRI slices (black shapes: contacts located in the anterior bank of the central sulcus; gray shapes: contacts located in the posterior bank of the central sulcus). The location of the central sulcus is enlightened by a white dotted line. No SEP could be obtained in patient P2 because corrupted recording files.
Areas 1–2 (crown of the postcentral gyrus)
EPs from the crown of the postcentral gyrus were analyzed on 4 contacts (Fig. 1). SEPs were recorded from all these contacts, with no observable phase reversal in bipolar or referential recordings. The amplitude of the first component to nonnoxious stimuli (SEPs), had the same peaking latency as the N20 recorded in area 3b (see Table 2) and tended to decrease on the more external contacts, suggesting that this response was volume‐conducted from area 3b, rather than locally generated in areas 1–2.
LEPs recorded on the contacts implanted in this region were composed of four components, (LS1_A, LS1_B, LS1_C, and LS1_D) (Fig. 3). The two first components peaked at latencies (101.6 ± 3.2 ms and 128.9 ± 3.2 ms) slightly shorter than those of the earliest responses recorded in area 3b and with no phase reversal in referential or bipolar recordings. Such early responses were never obtained at deeper contacts located in area 3b, even in patients who were recorded simultaneously from both locations, so that one can assume that their sources are likely to be located within areas 1 or 2. Conversely, the later components (LS1_C and LS1_D) recorded in areas 1–2 had latencies identical to those of the LEPs in area 3b but did not show any phase reversal across the central sulcus. The fact that these latter components at 140–190 ms showed a phase reversal in area 3b (see above), but not in areas 1–2, suggests that they originate from 3b and were volume‐conducted to areas 1–2.
EPs Recorded From Supra‐Sylvian Opercular and Insular Cortices
EPs from the supra‐sylvian opercular and insular cortices were analyzed on six and eight contacts, respectively (Fig. 2). Characteristics of LEPs obtained in these two regions were those commonly encountered in previous studies [Frot and Mauguière, 2003; Frot et al., 2007, 2008; Ohara et al., 2004]. LEPs were recorded on all sites within the supra‐sylvian operculum, and consisted of two components (LOp_A and LOp_B) recorded at 126.4 ± 18.5 ms and 186.1 ± 9.7 ms, respectively (Fig. 2 and Table 1). In the insular cortex, we recorded biphasic LEPs with two components (LIns_A and LIns_B) at 217.8 ± 21.3 ms and 328.4 ± 31.4 ms after the noxious stimulus (Fig. 2 and Table 1).
EPs Recorded From Area 4 of the Precentral Gyrus (M1 Area)
EPs from the precentral gyrus (primary motor cortex, Brodmann area 4) were analyzed on nine contacts (Fig.1). All these contacts recorded reproducible responses to both laser and electrical stimuli (Fig. 3).
After nonnoxious stimuli polarity reversal of the N20 response recorded in the postcentral gyrus was recorded by all contacts implanted in M1. This EP, named P20, appeared at a latency of 22 ± 2.1 ms (Fig. 3 and Table 2).
The morphology of LEPs in M1 was remarkably similar across all the patients, and was composed of three components which we labeled LM1_A, LM1_B, and LM1_C (Fig. 3 and Table 1). In one patient (case P3 in Fig. 5), these LEPs exhibited a clear phase‐reversal in referential recording mode across two adjacent electrode contacts inside M1 area 4 thus suggesting a local origin in the precentral gyrus. The local origin of a laser EP generator in M1 was strongly supported by phase reversals observed in bipolar recordings along six other electrode trajectories in M1 (see an example in Fig. 5, case P1b).
Figure 5.
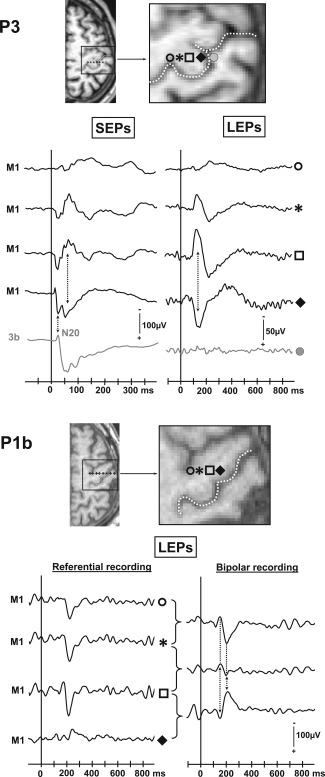
Phase reversals recorded in M1 cortex in two patients (P3, P1b). In patient 3 SEPs and LEPs are represented in referential recording mode. In patient 1b only LEPs are illustrated both in referential and bipolar recording modes.
Along the two remaining electrode tracks there was no observable phase reversal of the M1 LEPs in bipolar or referential recordings. We assumed, however, that the LEPs generator may have been close to the M1 contacts since LEPs of highest amplitudes were always recorded on the contacts located close to the anterior bank of the central sulcus, with progressive amplitude decrease with increasing distance from the sulcus. Also, adjacent contacts located either in the post‐central cortex or anteriorly, in area 6, never recorded high amplitude LEPs in these patients. All these reasons supported the hypothesis of a local M1 origin of the LEPs such as those illustrated in Figure 5, even in the absence of phase reversal.
In summary, SEPs and LEPs were systematically obtained whenever the electrode track explored the M1 area 4. LEPs exhibited a clear phase reversal between two contacts in seven cases, and a local steep gradient in the other 2, strongly suggesting a local origin in M1.
Statistical Analyses on LEPs Latencies
The latencies of all LEPs components are illustrated on a same graph in Figure 6. Because these latencies were not normally distributed, a nonparametric Wilcoxon test was performed. The latency difference between two different components was considered significant for P < 0.05.
Figure 6.
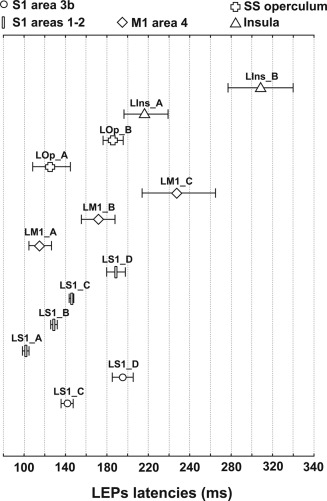
Mean latency values (±1 SD) of all LEPs components recorded in S1 (3b; 1–2), M1 (Area 4), supra‐sylvian operculum (SS operculum) and insula. In each of these areas all responses recorded presented the same pattern of components. Responses in S1 Areas 1–2 (rectangles), M1 Area 4 (diamonds), SS operculum (crosses), and insula (triangles) were systematically present on all contacts located in these regions. Responses in S1 Area 3b (circles) were recorded in only 50% of cases. Response components were labelled according to their location, the type of stimulus, and numbered “A” to “D” in the order of increasing peaking latencies; for example “LM1_A, LM1_B” corresponded to two laser evoked response components recorded in M1, with LM1_A being the earliest one. For detailed latency and amplitude values of each evoke response components see Table 1.
There were no significant differences between the latencies of all the S1 components, and the M1 and S2 components. In the same way, there were no significant differences between all the components of M1 LEPs and the opercular responses. However LM1_A was recorded significantly earlier than LIns_A and, in agreement with our previous observation [Frot and Mauguière, 2003], supra‐sylvian opercular LEPs were obtained earlier than insular LEPs. Statistical analyses to test the differences between S1 components latencies and insular LEPs were impossible due to the too small number of data.
DISCUSSION
Timing of Laser Evoked Potentials in S1 Area
The majority of LEPs recorded in S1 peaked at latencies ranging in the same time window as those recorded from S2 area in the same subjects. The pioneering EEG dipole‐modeling study by Tarkka and Treede 1993 suggested that S1 LEP sources became active later than opercular (S2) generators, whereas most recent studies by the same group have reported concomitant initial activation for S2 and S1 source equivalent dipoles [Baumgartner et al., 2011]. Source localization MEG studies have also reported simultaneous activation in S1 and S2 areas after nociceptive stimulation, culminating around 125 ms for intraepidermal stimuli [Ogino et al., 2005], 170 ms for solid‐state lasers [Ploner et al., 1999b, 2002; Raij et al., 2003] and surprisingly 210 ms for CO2‐laser stimuli [Kanda et al., 2000]. Three studies using subdural recordings on the cortical surface in single‐subjects also reported S1 activities simultaneous to S2/opercular responses [Baumgartner et al., 2011; Kanda et al., 2000; Ohara et al., 2004]. Moreover, in a recent study using dynamic causal modelling of fMRI data, Liang et al. 2011 showed that the neural activities elicited by nociceptive laser stimuli were best explained by models in which the fMRI responses in both S1 and the opercular region depend on direct thalamocortical projections, suggesting a parallel processing of nociceptive information to these areas.
Our results support the simultaneity of opercular and S1 nociceptive responses, which for the first time was confirmed by intracortical recordings within both areas in the same subjects. This suggests therefore that the early cortical processing of pain information is largely parallel in S1 and the opercular cortex, in accordance with the parallel thalamo‐cortical projections of the spino‐thalamic tract to these two regions in primates [Dum et al., 2009]. This is in contrast to the early cortical processing of nonnoxious somatosensory stimuli in anthropoid primates, including humans in whom a serial activation from S1 to the opercular area [Allison et al., 1989, 1991, 1992; Garraghty et al., 1991] has been demonstrated with a delay between S1 and supra‐sylvian opercular responses evaluated at 40–60 ms by human intracranial EEG [Allison et al., 1992; Frot et al., 2001] and MEG recordings [Hari et al., 1993; Mauguière et al., 1997].
Localization of Evoked Responses in S1 Area
While area 3b exhibited early and consistent SEPs to nonnoxious Aβ inputs, responses specific to noxious heat in this area were much less frequent and recorded by only four of the eight 3b contacts. Clear phase reversals of SEPs to nonnoxious electrical stimuli (N20/P20) were recorded across the central sulcus in every instance, in accordance with previous intracranial data [Allison et al., 1989, 1991; Balzamo et al., 2004; Barba et al., 2008; Valeriani et al., 2004], whereas a similar phase reversal to noxious laser pulses were obtained in only two out of the four LEPs recorded in 3b [thus in only 2/8 (25%) of all recordings from this area]. Although the two other LEPs recorded in area 3b did not show any phase reversal across the central sulcus, their morphologies, latencies and amplitudes were similar to 3b LEPs showing polarity reversal. It is therefore possible that the source of these LEPs might have well been located in area 3b and the lack of reversal due to electrode tracks not being oriented orthogonal to the sulcus. A polarity reversal across the central sulcus, as recorded by subdural electrodes posed on the surface of the hemisphere or located at a short distance of the sources in the neighboring white matter, simply indicates that the dipolar source is orthogonal to this sulcus without providing any direct clue to decide whether it is located in the anterior (M1‐ area 4) or posterior (S1‐ area 3b) bank of the sulcus. Interpretation of polarity reversal across the central sulcus in terms of source localization, even when using intracortical contacts as we did in our study, is also complicated by the 3D orientation complexity of the central sulcus in humans and by our finding that distinct LEP sources with opposite dipolar orientations located in the posterior and anterior bank of the central fissure can be simultaneously activated. As shown for SEPs in monkeys [Nicholson‐Petersen et al., 1995] the potential field resulting from simultaneous activities of M1 and S1 sources, even when recorded at a short distance of the generator by intracerebral contacts, is then an algebraic summation of potentials generated in S1 (negativity backward) and in M1 (positivity backward). Therefore, the localization in 3b of LEPs recorded in that area and showing a polarity reversal across the central sulcus mostly stems from an analogy with the N20‐P20 SEP component for which an origin in area 3b is widely accepted not only because of its field distribution but also because this component was found to be selectively abolished with persisting prerolandic responses in S1 lesions causing tactile anesthesia [Mauguière et al., 1983], an argument that is lacking for S1 LEPs. The instability of LEPs recorded in area 3b suggests that the density of nociceptive units might be small or heterogeneously distributed in this area, and may explain why two previous intracranial studies in single patients failed to report any nociceptive response from this region [Kanda et al., 2000; Valeriani et al., 2004]. Nevertheless, in a recent study, local field potential source analysis of LEPs obtained from subdural grids over sensori‐motor cortex showed a dipole that could be located in area 3b, with the limitations mentioned above regarding the difficulty of modeling sources of potentials generated close to the central fissure from surface recordings [Baumgärtner et al., 2011]. It is noteworthy that, although four spinothalamic recipient structures in the thalamus (ventro‐posterior lateral, ventro‐posterior inferior, anterior pulvinar and central lateral nuclei) send nociceptive afferents to area 3b [Cusick and Gould, 1990; Darian‐Smith and Darian‐Smith, 1993; Dum et al., 2009; Jones and Leavitt, 1974; Minciacchi et al., 1995; Padberg et al., 2009], S1 receives quantitatively very limited nociceptive inputs [Dum et al., 2009; Gingold et al., 1991]. In accordance with the paucity of local 3b nociceptive responses observed in the present work, neurophysiological studies in animals have shown that only a limited number of neurons responding to noxious thermal and mechanical stimuli are present in this area [Kenshalo and Isensee, 1983; Kenshalo and Willis, 1991; Kenshalo et al., 1988, 2000].
Contrasting with area 3b, LEP responses to nociceptive input could be recorded systematically when electrode tracks reached the crown of the postcentral gyrus, corresponding to the localization of areas 1 and 2. This region has been pointed out by electrophysiological (MEG) source analysis as the most likely source of noxious thermal responses in humans [Inui et al., 2003b; Kanda et al., 2000; Ogino et al., 2005; Ploner et al., 2000], and nociceptive neurons have been indeed identified in monkey area 1 [Kenshalo et al., 1988, 2000; Tommerdahl et al., 1996].
Distribution of Nonnoxious and Nociceptive Responses in S1
All electrode sites recording LEPs in our patients also recorded SEPs, and all sites showing phase reversals for LEPs also exhibited such reversals for SEPs. This suggests that neuronal populations responding to noxious and nonnoxious stimuli, although of different density, are interspersed or colocalized in S1 subareas, at least at the macroscopic discrimination level. When different modal types are mixed in a S1 subarea, they are arranged in separate mode‐specific columns [Mountcastle, 2005], the respective activity of which cannot be discriminated with the resolution of implanted macroelectrodes. Spatial convergence of noxious and non‐noxious responses was also shown in the human operculo‐insular cortex [Frot et al., 2001] and might reflect the above limitations, as well as the intrinsic wide‐dynamic range nature of most nociceptive‐responding units, which are activated both to non‐noxious and noxious thermal stimuli. This is the case of most of the S1 nociceptive neurons tagged in primates, especially in areas 3b and 1 [Kenshalo and Isensee, 1983; Kenshalo and Willis, 1991; Kenshalo et al., 1988, 2000]. Although the primary somatosensory cortex might also contain some nociceptive‐specific neurons [Whitsel et al., 2009], their functional significance in terms of pain processing remains questionable, considering that the number of S1 neurons receiving a spinothalamic related input may be <4% of the total spinothalamic tract‐driven projections to cortex [Dum et al., 2009], and that, in humans, even massive lesions of S1 do not entail significant deficits of nociceptive sensation [Dejerine, 1914; Kim, 2007].
Nociceptive‐Evoked Responses in M1‐Area 4
Although motor and premotor cortical areas are often activated by heat pain in functional imaging studies [e.g., Gelnar et al., 1999], these activations have been considered as related to pain epiphenomena, such as “suppression of movement, or actual pain‐evoked movements themselves” [Apkarian et al., 2005]. In contrast, our results show that nociceptive specific stimuli elicit local responses in the primary motor area, where the earliest LEP components peaked at a latency similar to the earliest S1 responses, and showed local phase reversals within the M1 gray matter suggesting a local origin within this area. This is coherent with thalamic afferent projections to M1 issued from the anterior segment of the ventral posterior lateral nucleus, the caudal portion of the ventral lateral nucleus [Craig, 2008; Jones et al., 1979; Matelli et al., 1989; Shindo et al., 1995] and to a lesser extent from the intralaminar (central lateral and centre median) nuclei [Darian‐Smith et al., 1990; Huffman and Krubitzer, 2001; Kultas‐Ilinsky et al., 2003; Leichnetz, 1986], which in monkeys receive spinothalamic input from superficial and deep dorsal horn layers [Apkarian and Hodge, 1989a, 1989b; Craig, 2006]. Extra‐ and intracellular recordings in the cat motor cortex have also supported the existence of a spinothalamic related input to M1 [Padel and Relova, 1991], which is also consistent with recent transneuronal tracing data in nonhuman primates [Dum et al., 2009]. Indeed, both electrical and natural stimulation of the limbs elicited short‐latency responses in motor cortex neurons, even after any transit of exteroceptive information via the cerebellum, the somatosensory cortex and the dorsal column nuclei was prevented, leaving intact only the afferent inflow ascending in the spinothalamic tract [Padel and Relova, 1991; Relova and Padel, 1989]. Although projections exist from S1 to M1 [Burton and Fabri, 1995; Jones et al., 1978; Künzle, 1978; Leichnetz, 1986], the concomitance of S1 and M1 LEPs in our patients suggests that, at least, the earliest M1 responses to noxious laser stimuli reflect a spinothalamic‐related input, rather than an activation issued from a hierarchical processing originating in S1.
There is some controversy concerning the sites of termination of spinothalamic cortical afferents in primates, which target preferentially superficial cortical layers in Old World monkeys [Rausell and Jones, 1991], while they may equally access superficial and deep layers in New World monkeys [Shi et al., 1993]. Given the location of human M1 largely within the central sulcus a preferential STT projection to its deep layers is expected to be reflected initially as a posterior positive field and an anterior negative counterpart. Although further recordings are of course necessary to confirm the consistency of these observations, such field distribution was indeed observed in our study, where the contact situated immediately anterior to the rolandic fissure, close to the M1 surface, picked up a positivity whereas that located more anterior picked up a negativity (Fig. 5), thus supporting an initial depolarization of M1 deep layers IV/V.
Functionally speaking, one can speculate that this very early spinothalamic input to M1 is able to engage motor reactions to interrupt nociceptive stimulations in a very quick and effective way. More practically, the disruption of pain processing by the motor cortex using repetitive electrical stimulation might explain why this procedure shows some efficacy in the treatment of neuropathic pain (André‐Obadia et al., 2008; Fonoff et al., 2011; Hansen et al., 2011).
ACKNOWLEDGMENTS
Grateful thanks are due to Dr M. Guenot (Department of Functional Neurosurgery) for stereotactic electrode implantation and to Dr J. Isnard for useful discussions of these data. Thanks also to Drs C. Fischer and P. Ryvlin for the opportunity to study their patients.
REFERENCES
- Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD (1989): Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short‐latency activity. J Neurophysiol 62:694–710. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC, Jones SJ (1991): Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. Brain 114:2465–2503. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC (1992): The relationship between human long‐latency somatosensory evoked potentials recorded from the cortical surface and from the scalp. Electroenceph Clin Neurophysiol 84:301–314. [DOI] [PubMed] [Google Scholar]
- André‐Obadia N, Mertens P, Gueguen A, Peyron R, Garcia‐Larrea L (2008): Pain relief by rTMS: differential effect of current flow but no specific action on pain subtypes. Neurology 71:833–840. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hodge CJ (1989a): Primate spinothalamic pathways: I. A quantitative study of the cells of origin of the spinothalamic pathway. J Comp Neurol 288:447–473. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hodge CJ (1989b): Primate spinothalamic pathways: III. Thalamic terminations of the dorsolateral and ventral spinothalamic pathways. J Comp Neurol 288:493–511. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484. [DOI] [PubMed] [Google Scholar]
- Balzamo E, Marquis P, Chauvel P, Régis J (2004): Short‐latency components of evoked potentials to median nerve stimulation recorded by intracerebral electrodes in the human pre‐ and postcentral areas. Clin Neurophysiol 115:1616–1623. [DOI] [PubMed] [Google Scholar]
- Barba C, Valeriani M, Colicchio G, Mauguière F (2008): New depth short‐latency somatosensory evoked potential (SEP) component recorded in human SI area. Neurosci Lett 432:179–183. [DOI] [PubMed] [Google Scholar]
- Baumgärtner U, Vogel H, Ohara S, Treede RD, Lenz F (2011): Dipole source analyses of laser evoked potentials obtained from subdural grid recordings from primary somatic sensory cortex. J Neurophysiol 106:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Fabri M (1995): Ipsilateral intracortical connections of physiologically defined cutaneous representations in areas 3b and 1 of macaque monkeys: Projections in the vicinity of the central sulcus. J Comp Neurol 355:508–538. [DOI] [PubMed] [Google Scholar]
- Craig AD (2006): Retrograde analyses of spinothalamic projections in the macaque monkey: Input to ventral posterior nuclei. J Comp Neurol 499:965–978. [DOI] [PubMed] [Google Scholar]
- Craig AD (2008): Retrograde analyses of spinothalamic projections in the macaque monkey: Input to the ventral lateral nucleus. J Comp Neurol 508:315–328. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, Rossini PM, Treede RD, Garcia‐Larrea L (2008): Recommendations for the clinical use of somatosensory‐evoked potentials. Clin Neurophysiol 119:1705–1719. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Gould HJ III (1990): Connections between area 3b of the somatosensory cortex and subdivisions of the ventroposterior nuclear complex and the anterior pulvinar nucleus in squirrel monkeys. J Comp Neurol 292:83–102. [DOI] [PubMed] [Google Scholar]
- Darian‐Smith C, Darian‐Smith I (1993): Thalamic projections to areas 3a, 3b, and 4 in the sensorimotor cortex of the mature and infant macaque monkey. J Comp Neurol 335:173–199. [DOI] [PubMed] [Google Scholar]
- Darian‐Smith C, Darian‐Smith I, Cheema SS (1990): Thalamic projections to sensorimotor cortex in the macaque monkey: Use of multiple retrograde fluorescent tracers. J Comp Neurol 299:17–46. [DOI] [PubMed] [Google Scholar]
- Dejerine JJ (1914): Sémiologie des Affections du Système Nerveux. Paris: Masson. [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL (2009): The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 29:14223–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonoff ET, Hamani C, Ciampi de Andrade D, Yeng LT, Marcolin MA, Jacobsen Teixeira M (2011): Pain relief and functional recovery in patients with complex regional pain syndrome after motor cortex stimulation. Stereotact Funct Neurosurg 89:167–172. [DOI] [PubMed] [Google Scholar]
- Frot M, Mauguière F (2003): Dual representation of pain in the operculo‐insular cortex in humans. Brain 126:438–450. [DOI] [PubMed] [Google Scholar]
- Frot M, Garcia‐Larrea L, Guénot M, Mauguière F (2001): Responses of the supra‐sylvian (SII) cortex in humans to painful and innocuous stimuli. A study using intra‐cerebral recordings. Pain 94:65–73. [DOI] [PubMed] [Google Scholar]
- Frot M, Magnin M, Mauguière F, Garcia‐Larrea L (2007): Human SII and insula differently encode thermal stimuli. Cereb Cortex 17:610–620. [DOI] [PubMed] [Google Scholar]
- Frot M, Mauguière F, Magnin M, Garcia‐Larrea L (2008): Parallel processing of nociceptive A‐delta inputs in SII and mid‐cingulate cortex in humans. J Neurosci 28:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraghty PE, Florence SL, Tenhula WN, Kaas JH (1991): Parallel thalamic activation of the first and second somatosensory areas in prosimian primates and tree shrews. J Comp Neurol 311:289–299. [DOI] [PubMed] [Google Scholar]
- Gelnar PA, Krauss BR, Sheehe PR, Szeverenyi NM, Apkarian AV (1999): A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. Neuroimage 10:460–482. [DOI] [PubMed] [Google Scholar]
- Gingold SI, Greenspan JD, Apkarian AV (1991): Anatomic evidence of nociceptive inputs to primary somatosensory cortex: Relationship between spinothalamic terminals and thalamocortical celles in squirrell monkeys. J Comp Neurol 308:467–490. [DOI] [PubMed] [Google Scholar]
- Hansen N, Obermann M, Poitz F, Holle D, Diener HC, Antal A, Paulus W, Katsarava Z (2011): Modulation of human trigeminal and extracranial nociceptive processing by transcranial direct current stimulation of the motor cortex. Cephalalgia 31:661–670. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L (2001): Thalamo‐cortical connections of areas 3a and M1 in marmoset monkeys. J Comp Neurol 435:291–310. [DOI] [PubMed] [Google Scholar]
- Inui K, Tran TD, Qiu Y, Wang X, Hoshiyama M, Kakigi R (2002): Pain‐related magnetic fields evoked by intra‐epidermal electrical stimulation in humans. Clin Neurophysiol 113:298–304. [DOI] [PubMed] [Google Scholar]
- Inui K, Tran TD, Qiu Y, Wang X, Hoshiyama M, Kakigi R (2003a): A comparative magnetoencephalographic study of cortical activations evoked by noxious and innocuous somatosensory stimulations. Neuroscience 120:235–248. [DOI] [PubMed] [Google Scholar]
- Inui K, Wang X, Qiu Y, Nguyen BT, Ojima S, Tamura Y, Nakata H, Wasaka T, Tran TD, Kakigi R (2003b) Pain processing within the primary somatosensory cortex in humans. Eur J Neurosci 18:2859–2866. [DOI] [PubMed] [Google Scholar]
- Isnard J, Guénot M, Ostrowsky K, Sindou M, Mauguière F (2000): The role of the insular cortex in temporal lobe epilepsy. Ann Neurol 48:614–623. [PubMed] [Google Scholar]
- Isnard J, Guénot M, Sindou M, Mauguière F (2004): Clinical manifestations of insular lobe seizures: A stereo‐electroencephalographic study. Epilepsia 45:1079–1090. [DOI] [PubMed] [Google Scholar]
- Jones EG, Leavitt RY (1974): Retrograde axonal transport and the demonstration of non‐specific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. J Comp Neurol 154:349–377. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Hendry SH (1978): Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol 181:291–347. [DOI] [PubMed] [Google Scholar]
- Jones EG, Wise SP, Coulter JD (1979): Differential thalamic relationships of sensory‐motor and parietal cortical fields in monkeys. J Comp Neurol 183:833–881. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Pons TP (1988): The somatosensory system of primates In: Stelikis H, editor. Comparative Primate Biology: Neurosciences, Vol. 4 New York: Liss; pp 421–468. [Google Scholar]
- Kanda M, Nagamine T, Ikeda A, Ohara S, Kunieda T, Fujiwara N, Yazawa S, Sawamoto N, Matsumoto R, Taki W, Shibasaki H (2000): Primary somatosensory cortex is actively involved in pain processing in human. Brain Res 853:282–289. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR Jr, Isensee O (1983): Responses of primate SI cortical neurons to noxious stimuli. J Neurophysiol 50:1479–1496. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Willis WD (1991): The role of the cerebral cortex in pain sensation In: Jones EG, Peters A, editors. Cerebral Cortex, Normal and Altered States of Function, Vol. 9 New York: Plenum; pp 153–212. [Google Scholar]
- Kenshalo DR Jr, Chudler EH, Anton F, Dubner R (1988): SI nociceptive neurons participate in the encoding process by which monkeys perceive the intensity of noxious thermal stimulation. Brain Res 454:378–382. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Iwata K, Sholas M, Thomas DA (2000): Response properties and organization of nociceptive neurons in area 1 of monkey primary somatosensory cortex. J Neurophysiol 84:719–729. [DOI] [PubMed] [Google Scholar]
- Kim JS (2007): Patterns of sensory abnormality in cortical stroke: Evidence for a dichotomized sensory system. Neurology 68:174–180. [DOI] [PubMed] [Google Scholar]
- Kultas‐Ilinsky K, Sivan‐Loukianova E, Ilinsky IA (2003): Reevaluation of the primary motor cortex connections with the thalamus in primates. J Comp Neurol 457:133–158. [DOI] [PubMed] [Google Scholar]
- Künzle H (1978): Cortico‐cortical efferents of primary motor and somatosensory regions of the cerebral cortex in Macaca fascicularis . Neuroscience 3:25–39. [Google Scholar]
- Leandri M, Saturno M, Spadavecchia L, Iannetti GD, Cruccu G, Truini A (2006): Measurement of skin temperature after infrared laser stimulation. Neurophysiol Clin 36:207–218. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR (1986): Afferent and efferent connections of the dorsolateral precentral gyrus (area 4, hand/arm region) in the macaque monkey, with comparisons to area 8. J Comp Neurol 254:460–492. [DOI] [PubMed] [Google Scholar]
- Liang M, Mouraux A, Iannetti G (2011): Parallel processing of nociceptive and non‐nociceptive somatosensory information in the human primary and secondary somatosensory cortices: Evidence from dynamic causal modeling of functional magnetic resonance imaging data. J Neurosci 31:8976–8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Fogassi L, Rizzolatti G (1989): Thalamic input to inferior area 6 and area 4 in the macaque monkey. J Comp Neurol 280:468–488. [DOI] [PubMed] [Google Scholar]
- Mauguière F, Desmedt JE, Courjon J (1983): Astereognosis and dissociated loss of frontal or parietal components of somatosensory evoked potentials in hemisphere lesions. Brain 106:271–311. [DOI] [PubMed] [Google Scholar]
- Mauguière F, Merlet I, Forss N, Vanni S, Jousmäki V, Adeleine P, Hari R (1997): Activation of a distributed somatosensory cortical network in the human brain. A dipole modelling study of magnetic fields evoked by median nerve stimulation. Part I: Location and activation timing of SEF sources. Electroencephalogr Clin Neurophysiol 104:281–289. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Mauguière F (2006): Somatosensory and pain responses to stimulation of the second somatosensory area (SII) in humans. A comparison with SI and insular responses. Cereb Cortex 16:960–968. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Peyron R, Mauguière F (2012): Stimulation of the human cortex and the experience of pain: Wilder Penfield's observations revisited. Brain 135:631–640. [DOI] [PubMed] [Google Scholar]
- Minciacchi D, Granato A, Antonini A, Tassinari G, Santarelli M, Zanolli L, Macchi G (1995): Mapping subcortical extrarelay afferents onto primary somatosensory and visual areas in cats. J Comp Neurol 362:46–70. [DOI] [PubMed] [Google Scholar]
- Nakata H, Inui K, Wasaka T, Tamura Y, Tran TD, Qiu Y, Wang X, Nguyen TB, Kakigi R (2004): Movements modulate cortical activities evoked by noxious stimulation. Pain 107:91–98. [DOI] [PubMed] [Google Scholar]
- Nicholson‐Petersen N, Schroeder CE, Arezzo J (1995): Neural generators of early cortical somatosensory evoked potentials in the awake monkey. Electroenceph Clin Neurophysiol 96:248–260. [DOI] [PubMed] [Google Scholar]
- Ogino Y, Nemoto H, Goto F (2005): Somatotopy in human primary somatosensory cortex in pain system. Anesthesiology 103:821–827. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Treede RD, Lenz FA (2004): Amplitudes of laser evoked potential recorded from primary somatosensory, parasylvian and medial frontal cortex are graded with stimulus intensity. Pain 110:318–328. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guénot M, Mauguière F (2002): Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cereb Cortex 12:376–385. [DOI] [PubMed] [Google Scholar]
- Padberg J, Cerkevich C, Engle J, Rajan AT, Recanzone G, Kaas J, Krubitzer L (2009): Thalamocortical connections of parietal somatosensory cortical fields in macaque monkeys are highly divergent and convergent. Cereb Cortex 19:2038–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padel Y, Relova JL (1991): Somatosensory responses in the cat motor cortex. I. Identification and course of an afferent pathway. J Neurophysiol 66:2041–2058. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T (1955): The Cerebral Cortex of Man. New York: MacMillan. [Google Scholar]
- Perchet C, Godinho F, Mazza S, Frot M, Legrain V, Magnin M, Garcia‐Larrea L (2008): Evoked potentials to nociceptive stimuli delivered by CO2 or Nd:YAP lasers. Clin Neurophysiol 119:2615–2622. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Hansson P, Ingvar M (2002): A regression analysis study of the primary somatosensory cortex during pain. Neuroimage 16:1142–1150. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia‐Larrea L (2000): Functional imaging of brain responses to pain. A review and meta‐analysis. Neurophysiol Clin 30:263–288. [DOI] [PubMed] [Google Scholar]
- Ploner M, Freund HJ, Schnitzler A (1999a): Pain affect without pain sensation in a patient with a postcentral lesion. Pain 81:211–214. [DOI] [PubMed] [Google Scholar]
- Ploner M, Schmitz F, Freund HJ, Schnitzler A (1999b): Parallel activation of primary and secondary somatosensory cortices in human pain processing. J Neurophysiol 81:3100–3104. [DOI] [PubMed] [Google Scholar]
- Ploner M, Schmitz F, Freund HJ, Schnitzler A (2000): Differential organization of touch and pain in human primary somatosensory cortex. J Neurophysiol 83:1770–1776. [DOI] [PubMed] [Google Scholar]
- Ploner M, Gross J, Timmermann L, Schnitzler A (2002): Cortical representation of first and second pain sensation in humans. Proc Natl Acad Sci USA 99:12444–12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell E, Jones EG (1991): Chemically distinct compartments of the thalamic VPM nucleus in monkeys relay principal and spinal trigeminal pathways to different layers of the somatosensory cortex. J Neurosci 11:226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relova JL, Padel Y (1989) Short latency somaesthetic responses in motor cortex, transmitted through the spino‐thalamic system, in the cat. Exp Brain Res 75:639–643. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M (2000): Stereotaxic display of brain lesions. Behav Neurol 12:191–200. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Greffrath W, Büsselberg D, Treede RD (2000) Inactivation and tachyphylaxis of heat‐evoked inward currents in nociceptive primary sensory neurones of rats. J Physiol 528:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Stevens RT, Tessier J, Apkarian AV (1993): Spinothalamocortical inputs nonpreferentially innervate the superficial and deep cortical layers of SI. Neurosci Lett 160:209–213. [DOI] [PubMed] [Google Scholar]
- Shindo K, Shima K, Tanji J (1995): Spatial distribution of thalamic projections to the supplementary motor area and the primary motor cortex: A retrograde multiple labeling study in the macaque monkey. J Comp Neurol 357:98–116. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Coplanar Stereotaxic Atlas of the Human Brain. 3‐Dimensional Proportional System: An approach to Cerebral Imaging. Stuttgart: Georg Thieme Verlag. [Google Scholar]
- Tarkka IM, Treede RD (1993): Equivalent electrical source analysis of pain‐related somatosensory evoked potentials elicited by a CO2 laser. J Clin Neurophysiol 10:513–519. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Ploner M, Haucke K, Schmitz F, Baltissen R, Schnitzler A (2001) Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol 86:1499–1503. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Delemos KA, Vierck CJ, Favorov OV, Whitsel BL (1996). Anterior parietal cortical response to tactile and skin‐heating stimuli applied to the same skin site. J Neurophysiol 75:2662–2670. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Barba C, Le Pera D, Restuccia D, Colicchio G, Tonali P, Gagliardo O, Treede RD (2004): Different neuronal contribution to N20 somatosensory evoked potential and to CO2 laser evoked potentials: An intracerebral recording study. Clin Neurophysiol 115:211–216. [DOI] [PubMed] [Google Scholar]
- Verger H (1900) : Sur les troubles de la sensibilité générale consécutifs aux lésions des hémisphères cérébraux chez l'Homme. Arch Gen de Med N S, 4:513–582, 641–714. [Google Scholar]
- Whitsel BL, Favorov OV, Li Y, Quibrera M, Tommerdahl M (2009): Area 3a neuron response to skin nociceptor afferent drive. Cereb Cortex 19:349–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CC, Spencer DD, Allison T, McCarthy G, Williamson PD, Goff W (1988): Localization of human sensorimotor cortex during surgery by cortical surface recording of somatosensory evoked potentials. J Neurosurg 68:99–111. [DOI] [PubMed] [Google Scholar]


