Abstract
Goal‐directed behavior requires cognitive control to regulate the occurrence of conflict. The dorsal anterior cingulate cortex (dACC) has been suggested in detecting response conflict during various conflict tasks. Recent findings, however, have indicated not only that two distinct subregions of dACC are involved in conflict processing but also that the conflict occurs at both perceptual and response levels. In this study, we sought to examine whether perceptual and response conflicts are functionally dissociated in dACC. Thirteen healthy subjects performed a version of the Stroop task during functional magnetic resonance imaging (fMRI) scanning. We identified a functional dissociation of the caudal dACC (cdACC) and the rostral dACC (rdACC) in their responses to different sources of conflict. The cdACC was selectively engaged in perceptual conflict whereas the rdACC was more active in response conflict. Further, the dorsolateral prefrontal cortex (DLPFC) was coactivated not with cdACC but with rdACC. We suggest that cdACC plays an important role in regulative processing of perceptual conflict whereas rdACC is involved in detecting response conflict. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: anterior cingulate cortex, dorsolateral prefrontal cortex, cognitive control, conflict monitoring, perceptual conflict, response conflict, functional MRI
INTRODUCTION
Goal‐directed behavior often requires humans to overcome interference caused by distraction. The Stroop task [MacLeod, 1991; Stroop, 1935] is one of the most frequently employed paradigms in studying human ability to control cognition in the face of interference. In a typical Stroop task, subjects are asked to name the color of the ink when a word naming a color is printed in an incongruent color of ink (e.g., “RED” printed in blue ink). Subjects must select the appropriate feature of the stimulus (e.g., the blue ink), while ignoring the word (e.g., “RED”). Since processing written words is rather automatic, some effort is required to accomplish this task. Response time is increased when naming the ink color of an incongruent stimulus compared to a neutral stimulus (e.g., “XXXX” printed in green ink). This is referred to as the Stroop interference effect.
Functional neuroimaging studies have investigated the neural mechanisms underlying how humans control the interference from processing the word. The current most dominant theory, the conflict monitoring theory, suggests that the dorsal anterior cingulate cortex (dACC) plays a role in detecting the conflict in information processing during a conflict task, such as the Stroop task and the flanker task, and the dorsolateral prefrontal cortex (DLPFC) is engaged in resolving the conflict [Botvinick et al. 2001, 2004]. Specifically, this theory suggests that the dACC responds to the occurrence of response conflict and that the signal from the dACC triggers increased top‐down signals from DLPFC, which serves to resolve the conflict. An important aspect of this theory is that the conflict is caused at the response level, such as when overriding prepotent responses, selecting underdetermined responses, or generating errors [Botvinick et al., 2004]. Even though there is a possibility that dACC might function as a general monitoring system as pointed out [Botvinick et al., 2004], the theory has placed the focus on the response level and a number of studies have also focused on the response conflict.
Recent neuroimaging studies, however, have suggested that the conflict might occur at both pre‐response and response levels and that dACC is functionally dissociated by the source of conflict. van Veen and Carter [ 2005] performed a study using a version of the Stroop task, in which they compared the semantic and the semantic‐and‐response incongruent conditions to the congruent condition. They found not only rostral dACC (rdACC) activation in comparing the incongruent semantic‐and‐response condition with the incongruent semantic condition, which they interpreted as response conflict, but also caudal dACC (cdACC) activation in a comparison of the incongruent semantic condition to the congruent condition, which they attribute to semantic conflict. They suggested that dACC activity is caused by the conflict at both semantic (i.e., pre‐response) and response levels and that subregions of dACC are sensitive to the different sources of the conflict. A similar finding was reported by Milham and Banich [ 2005] in which rdACC showed conflict‐specific activity whereas cdACC was not related to conflict but rather was specific to perceptual competition between color information. Braver et al. [ 2001] also suggested that rdACC is involved in response inhibition whereas cdACC is engaged in target detection under a condition that targets appear in low‐frequency (i.e., an oddball condition). These studies suggest that cdACC and rdACC are functionally specialized based on the source of conflict; in which cdACC is specific to preresponse conflict whereas rdACC is specialized in response conflict.
Other neuroimaging studies found that two distinct regions within rostral and caudal dACC are involved in conflict processing but the functions of the two subregions were not differentiated in those studies [Barch et al., 2001; Braver et al., 2001; Liu et al., 2006; Milham et al., 2002]. Also, a recent review found that both of the subregions are involved in conflict processing [Laird et al., 2005]. We localized the coordinates of dACC activations reported in the previous studies in Figure 1. We identified two distinctive subregions of dACC, which correspond to posterior rostral cingulate zone and anterior rostral cingulate zone [Picard and Strick 1996].
Figure 1.
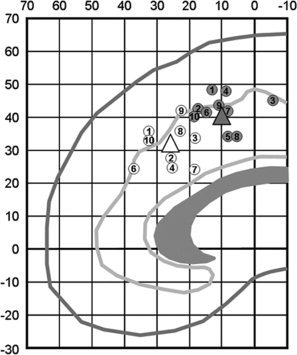
Localization of dACC activations during the Stroop or Stroop‐like tasks found in selected studies. The white circles are located within rdACC and the gray circles are located in cdACC. Two triangles indicate the average coordinates of the white circles and of the gray circles. Each location indicates Talairach coordinates [Talairach and Tournoux, 1988] showing the highest peak value in each study. The numbers indicate references; 1 [Milham and Banich, 2005], 2 [van Veen and Carter, 2005], 3 [Braver et al., 2001], 4 [Orr and Weissman, 2009], 5 [Weissman et al., 2003], 6 [Laird et al., 2005], 7 [Barch et al., 2001], 8 [Liu et al., 2006], 9 [Milham et al., 2001], 10 [Milham et al., 2002]. Four of these studies (Refs. 1–4) found both cdACC activity sensitive to preresponse conflict and rdACC activity sensitive to response conflict, one study (Ref. 5) found cdACC activity sensitive to both preresponse conflict and response conflict, and others (Refs. 6–10) found two subregions within dACC sensitive to conflict processing with no functional dissociation. The average coordinates of white circles are x, y, z = 2, 26, 31, and those of gray circles are x, y, z = 1, 10, 42.
By contrast, some studies have failed to find evidence of a dACC dissociation corresponding to perceptual and response conflict. For example, van Veen et al. used a version of the flanker task to test whether perceptual or response conflict occurs [van Veen et al., 2001]. They found that only response conflict was associated with dACC activation, suggesting that dACC is involved only in response conflict. The presentation rate of the task condition used to measure semantic conflict, however, was not equivalent to other conditions, which might have resulted in a bias. Indeed, their following study using a version of the Stroop task found dissociation within dACC as described above [van Veen and Carter, 2005].
Based upon the previous literature, we assumed that rdACC and cdACC are involved in conflict tasks and are dissociated by the source of conflict: perceptual conflict recruits cdACC whereas response conflict recruits rdACC. The purpose of this study was to test this assumption that the subregions of dACC are functionally specialized for the source of conflict (i.e., perceptual conflict and response conflict). We expected that the conflict is caused at both perceptual and response levels in which the subregions of dACC are specialized to the nature of conflict. Specifically, according to the previous studies, we expected that rdACC is sensitive to the response conflict whereas cdACC is involved in perceptual conflict.
MATERIALS AND METHODS
Subjects
Thirteen healthy right‐handed volunteers (Aged 19–32; five females) with normal or corrected to normal vision without color blindness participated in this study. All subjects completed informed consent forms approved by the Brain Science Research Center at KAIST in Korea. None had any history of neurological or psychiatric problems.
Cognitive Tasks
We employed a version of the Stroop matching task to measure perceptual conflict and response conflict separately, which was used in a previous behavioral study [Sugg and McDonald, 1994]. The task stimuli consisted of a sample and a response set with two alternative cues (see Fig. 2). The response set was either composed of two rectangles with different colors (color‐response condition: CResp) or two words in black ink (word‐response condition: WResp). This set was presented along with an incongruent or neutral sample. In CResp, subjects were required to match the color of a sample with a response cue in a screen without word reading, in which they could select the correct answer by perceptual comparison. In this condition, the translation of color information into a verbal response was not required in selecting the correct answer. In other words, response conflict was minimized or eliminated even in incongruent trials, and thus if an interference effect is observed, it is assumed to result from perceptual conflict at the preresponse level. On the contrary, in WResp, response conflict was maximized in the incongruent condition in which subjects were to translate the ink color of the colored word into a verbal representation to respond correctly, inhibiting the prepotent word reading processing [Sugg and McDonald, 1994]. Thus, if there is an interference effect in WResp, this is caused not only by the incongruent sample (i.e., perceptual conflict) but also by the word response cue (i.e., response conflict).
Figure 2.
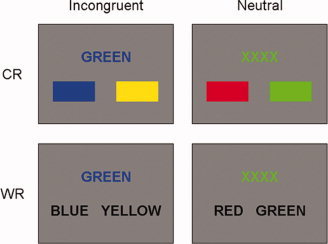
Illustration of task conditions. Subjects were required to respond to the color of a sample and select a left or right response cue. Four different conditions including incongruent CResp (iCResp), neutral CResp (nCResp), incongruent WResp (iWResp), and neutral WResp (nWResp) were presented in randomized order during the task. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
A sample and a set of two alternative response cues were presented in a screen (see Fig. 2). All words of samples and response cues were presented in Korean. A sample was presented in either incongruent (e.g., “BLUE” printed in red ink) or neutral (e.g., “XXXX” printed in green ink) color in a gray screen. Four kinds of colors (red, green, blue, and yellow) were used in the task. Experimental conditions were composed of two sample types (incongruent and neutral trials) and two response types (CResp and WResp), and thus four types of trials were included; incongruent CResp (iCResp), neutral CResp (nCResp), incongruent WResp (iWResp), and neutral WResp (nWResp). The iCResp and nCResp included two rectangles as a response cue whereas iWResp and nWResp had two color names in words. In the iCResp, the task required subjects to identify the color of the sample and select the corresponding color between two response cues by pressing buttons using index and middle fingers. One of the response cues was the correct answer, and the other was a distracter which was not assigned in the word of the sample and was a randomly selected color. Thus, if a sample was “RED” in green colored ink and the response cues included two rectangles, then the one colored in green ink was the correct answer and the other in either blue or yellow ink was a distracter. The sample of the iWResp condition was same as the iCResp sample, but the response cues differed in that the two alternatives were presented in the form of words (e.g., “BLUE” and “YELLOW”). All stimuli continued for 2 s and inter‐stimulus‐intervals (ISIs) averaging 2.9 s followed. Null events with a fixation cross in the middle of the screen were included with the same stimulus duration and ISIs as other stimuli. All trial types were replicated 48 times in mixed random order.
Image Acquisition
fMRI data was acquired using a 3‐T MRI system (Oxford magnet, Varian console magnet built by ISOL, fMRI center at KAIST in Daejeon, South Korea) with a quadrature headcoil. T2*‐weighted gradient echo planner images (EPI) composed of 20 interleaved slices were acquired for the functional images (TR = 2 s, TE = 35 ms, FA = 85°, matrix = 64 × 64, in‐plane resolution = 3.44 mm, thickness = 5 mm). Functional scans were composed of two runs (294 volumes per run). The first six volumes of each run were added for signal equilibration and discarded when analyzing the data. T1‐weighted images for all subjects were also acquired.
Image Analysis
SPM5 (Statistical Parametric Mapping; Wellcome department of Cognitive, Institute of Neurology, UCL, London, UK) was used for image preprocessing and statistical analysis. Temporal disparity between slices was corrected for the functional images and then the images were spatially realigned to the first slice for motion correction. The images were normalized to a standard MNI‐305 T1‐weighted image and accordingly resampled by isotropic 3‐mm voxels. The images were then spatially smoothed with an 8‐mm FWHM Gaussian kernel. These were high‐pass filtered with a 128 s cutoff. Each condition was included in constructing a general linear model using a canonical hemodynamic response function (HRF). Contrast images were constructed by comparison of experimental conditions (i.e., iCResp, nCResp, iWResp, and nWResp) with a null condition. The contrast images were submitted to group‐level random‐effects analysis, in which a threshold was applied at P < 0.05 for multiple comparisons using the false discovery rate [Genovese et al., 2002]. Only correct trials were included in the imaging analysis. We first employed paired‐sample t‐tests for comparisons of incongruent vs. neutral conditions of both WResp and CResp (i.e., [iWResp–nWResp] and [iCResp–nCResp]). The comparison in CResp represents perceptual conflict, but the comparison in WResp shows both perceptual and response conflicts. Thus we performed a two‐way ANOVA to identify the interaction effect between the conflict (i.e., incongruent vs. neutral) and the response modality (i.e., WResp vs. CResp) on neural activity. Namely, the interaction effect (i.e., [iWResp–nWResp]—[iCResp–nCResp]) would show the effect of response conflict in WResp. For further analyses of regions of interest (ROIs), BOLD signal changes were extracted from dACC subregions activated in comparisons of [iWResp–nWResp] and [iCResp–nCResp], in which the ROIs were defined as radial spheres with 8 mm.
RESULTS
Behavioral Data
Hit rates of each subject were higher than 96% in all conditions. The result of a two‐way repeated‐measure ANOVA showed that they were not significantly different in either main effects (P = 0.753 for the response type and P = 0.190 for the sample type) or the interaction effect between the response type and the sample type (P = 0.549). Response time (RT) was also analyzed in the same way. As shown in Figure 3, the results showed significant main effects of both the sample type (F (1,12) = 43.222, MSe = 0.013, P = 0.000) and the response type (F (1,12) = 38.747, MSe = 0.059, P = 0.000), and showed the significant interaction effect as well (F (1,12) = 18.086, MSe = 0.005, P = 0.001). Specifically, the RTs of WResp were slower than of CResp and the RTs of incongruent condition were slower than of neutral condition. In addition, the interference effect was greater in WResp (284 ms) than in CResp (125 ms). This difference does not mean that the response conflict is greater than the perceptual conflict since the response cues of the iWResp induces response conflict but the sample of iWResp also includes perceptual conflict. For the same reason, even though there is a significant positive correlation between interference effects of two conditions (pearson's r = 0.650, P = 0.016), it does not mean that this is the evidence of the relationship between perceptual conflict and response conflict.
Figure 3.
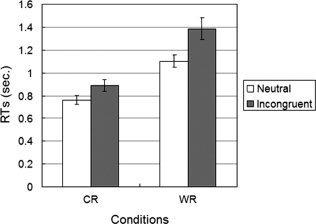
Behavioral performance on the Stroop tasks. Each bar represents the mean of RTs of each condition. Error bars indicate mean ± S.E.M.
Thus, we calculated the response interference effect by subtraction of nWResp from iWResp (i.e., 159 ms). Even though this estimated response interference effect is slightly higher than the perceptual effect, there was no significant difference between the sizes of the response interference effect and the perceptual interference effect (t(12) = 0.933, P = 0.369). Also, there was no significant correlation between the two values (r = 0.285, P = 0.345). These results do indicate, however, that the Stroop interference effect is caused by both perceptual conflict and response conflict.
Imaging Data
We compared incongruent trials with neutral trials for CResp and WResp separately. These analyses were conducted to identify neural responses to the perceptual conflict and to both perceptual and response conflicts, respectively (Table I and Fig. 4a). The results showed that when subjects performed CResp, cdACC activity (peaked at y = 16) was greater on incongruent than on neutral trials. Namely, cdACC showed selective activation to the perceptual conflict. In contrast, both cdACC and rdACC (peaked at y = 16 and at y = 33, respectively) showed greater activity on iWResp than on nWResp. As described above, however, the comparison of iCResp with nCResp includes only perceptual conflict, whereas the comparison of iWResp with nWResp includes both perceptual and response conflicts. Thus we analyzed the same data using a two‐way ANOVA to measure perceptual and response conflicts separately (see Table II and Fig. 4b). We found a positive main effect of the conflict in cdACC (peaked at y = 5), representing perceptual conflict. In contrast, an interaction effect (i.e., [iWResp–nWResp]—[iCResp–nCResp]) was significant in rdACC (peaked at y = 27). That is, rdACC showed high involvement in response conflict, whereas cdACC was highly engaged in perceptual conflict. The main effect of response modality (i.e., [WResp–CResp]) was not significant in any subregion of dACC.
Table I.
Brain regions significantly activated by the Stroop interference effect in WResp and CResp using paired‐sample t‐tests
| Anatomical region | L/R | BA | Coordinates (mm) | z | mm3 | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| WResp | |||||||
| Precentral gyrus | L | 6/9 | −36 | −1 | 39 | 5.37 | 11,421 |
| Middle frontal gyrus | R | 9 | 48 | 13 | 27 | 4.09 | 3,456 |
| Precentral gyrus | R | 44 | 48 | 7 | 13 | 3.4 | 243 |
| Anterior cingulate | L | 24 | 0 | 33 | 15 | 4.81 | 891 |
| Cingulate gyrus | R | 32 | 9 | 16 | 32 | 4.68 | 3,915 |
| Precuneus | L | 7 | −30 | −44 | 44 | 4.28 | 4,509 |
| Inferior parietal lobule | R | 40 | 45 | −36 | 40 | 3.8 | 1,323 |
| R | 40 | 36 | −53 | 44 | 3.7 | 1,809 | |
| L | 40 | −48 | −33 | 38 | 3.52 | 1,107 | |
| Precuneus | R | 7 | 21 | −60 | 33 | 3.5 | 270 |
| Caudate | L | −15 | 9 | 11 | 3.83 | 1,350 | |
| Thalamus | L | −3 | −26 | 1 | 3.6 | 351 | |
| R | 12 | −20 | −1 | 3.25 | 270 | ||
| Insula | R | 13 | 36 | 12 | −1 | 3.32 | 297 |
| Lentiform nucleus | R | 18 | −2 | 14 | 3.3 | 270 | |
| Culmen of vermis | L | −3 | −61 | 1 | 3.77 | 270 | |
| CResp | |||||||
| Cingulate gyrus | L | 32 | −6 | 16 | 38 | 3.8 | 432 |
| L | 31 | −24 | −42 | 38 | 4.14 | 405 | |
| Parahippocampal gyrus | L | 30 | −15 | −38 | 7 | 4.27 | 486 |
| Lentiform nucleus | L | −18 | 3 | 3 | 4.31 | 2,997 | |
| Caudate | R | 12 | 15 | 10 | 4.31 | 1,296 | |
| Thalamus | R | 15 | −5 | 14 | 3.89 | 459 | |
Figure 4.
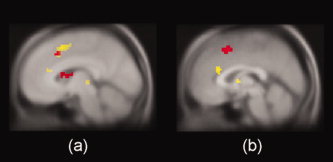
Significant activation within dACC. (A) The conflict effect observed in only cdACC (red) for CResp but in both cdACC and rdACC (yellow) for WResp. (B) A main effect of the sample type (incongruent vs. neutral) showed significant activation in cdACC (red). Peak activity was observed at y = 5 mm in the cdACC. A positive interaction effect ([iWResp–nWResp]—[iCResp–nCResp]) was significant in rdACC (yellow). Peak activity was observed at y = 27 mm in the rdACC. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Brain regions significantly activated in a two‐way ANOVA
| Anatomical region | L/R | BA | Coordinates (mm) | z | mm3 | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Incongruent‐neutral | |||||||
| Medial frontal gyrus | L | 6/32 | −3 | 14 | 46 | 4.25 | 2,700 |
| Inferior frontal gyrus | L | 9 | −42 | 7 | 25 | 4.14 | 2,403 |
| Medial frontal gyrus | L | 6 | −21 | 8 | 49 | 3.69 | 405 |
| Superior parietal lobule | L | 7 | −30 | −50 | 41 | 4.2 | 1,755 |
| Inferior parietal lobule | R | 40 | 42 | −33 | 40 | 3.46 | 270 |
| Thalamus | R | 15 | −2 | 11 | 4.11 | 2,214 | |
| Caudate | L | −12 | 4 | 14 | 4.06 | 567 | |
| WResp‐CResp | |||||||
| Cerebellum | L | −3 | −61 | −2 | 4.64 | 270 | |
| Interaction | |||||||
| Middle frontal gyrus | L | 46 | −42 | 19 | 21 | 3.28 | 648 |
| Precentral gyrus | R | 6 | 36 | 2 | 30 | 4.53 | 15,417 |
| L | 6 | −50 | −2 | 17 | 4.17 | 5,778 | |
| Medial frontal gyrus | L | 9 | −15 | 28 | 29 | 3.64 | 1,242 |
| Anterior cingulate | R | 24 | 6 | 27 | 18 | 4.01 | 756 |
| Parahippocampal gyrus | R | 30 | 24 | −52 | 5 | 3.53 | 432 |
| Postcentral gyrus | R | 2 | 39 | −28 | 29 | 3.88 | 1,107 |
| L | 2 | −42 | −22 | 29 | 3.79 | 5,940 | |
| Inferior parietal lobule | L | 40 | −53 | −37 | 27 | 3.41 | 1,512 |
| Precuneus | R | 7 | 30 | −50 | 47 | 3.33 | 1,134 |
| L | 7 | −15 | −44 | 52 | 3.21 | 459 | |
| Superior temporal gyrus | R | 22 | 56 | −43 | 8 | 4.42 | 5,022 |
| Cuneus | L | 30 | −18 | −69 | 12 | 3.42 | 540 |
| R | 30 | 21 | −69 | 12 | 3.35 | 1,134 | |
| Insula | R | 42 | 6 | 0 | 3.19 | 675 | |
| Thalamus | L | −6 | −26 | 1 | 3.23 | 297 | |
| R | 12 | −29 | −1 | 3.4 | 810 | ||
| Lentiform nucleus | L | −21 | 17 | −1 | 3.19 | 459 | |
| R | 18 | −3 | 3 | 3.29 | 513 | ||
We depicted BOLD signal changes of each trial type in the ROIs within both cdACC and rdACC in Figure 5. All four conditions showed active patterns in the cdACC region, in which incongruent trials were more active than neutral trials. In comparisons of peak activation between iCResp and nCResp and between iWResp and nWResp using paired‐sample t‐tests, the peak activation was higher in iCResp than in nCResp (M = 0.18% and 0.07%; t(12) = 2.360, P = 0.036) and in iWResp than in nWResp (M = 0.21 and 0.08%; t(12) = 3.303, P = 0.006). In contrast, in the rdACC region, the peak activation was higher in iWResp than in nWResp (M = 0.16 and 0.04%; t(12) = 2.963, P = 0.012), whereas iCResp and nCResp were not different (M = 0.04 and 0.02%; t(12) = 0.442, P = 0.666). These results confirmed that both perceptual and response conflicts recruit cdACC activity but only response conflict involves rdACC.
Figure 5.
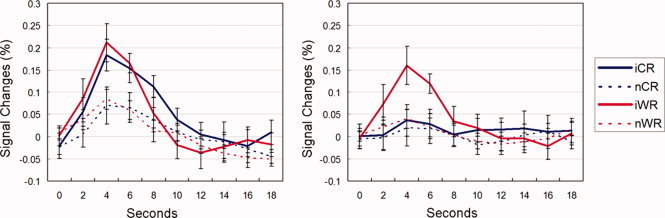
BOLD signal changes of each trial type with cdACC (the left) and rdACC (the right). Error bars indicate mean ± S.E.M. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To test the relationship between neural activity (i.e., the conflict effect) and behavioral responses (i.e., the interference effect), we calculated conflict effects of each subject by subtracting the peak value of the BOLD signal changes in each neutral condition from those in incongruent condition (i.e., iWResp–nWResp and iCResp–nCResp) in both cdACC and rdACC. That is, the perceptual conflict effect was calculated by subtraction of nCResp from iCResp while the response conflict effect was calculated by the subtraction of nWResp from iWResp. The results showed that the positive correlation between the interference effect observed in CResp and the perceptual conflict effect was significant in cdACC (r = 0.623, P = 0.023) but not in rdACC (r = 0.402, P = 0.173). These two correlation values, however, were not significantly different (t(10) = 0.666, P = 0.260, one‐tailed). In contrast, the interference effect in WResp and the response conflict effect showed a significant positive relationship in rdACC (r = 0.740, P = 0.004) but not in cdACC (r = 0.508, P = 0.076), in which the difference between these correlations were not significant (t(10) = 1.303, P = 0.111, one‐tailed). The estimated response interference effect was highly correlated with rdACC activity (r = 0.748, P = 0.003) but not with cdACC activity (r = 0.424, P = 0.148). The difference between these correlations were significant (t(10) = 1.857, P = 0.042, one‐tailed).
An additional important finding was observed in DLPFC (see Table I). The comparison of the incongruent condition with the neutral condition showed enhanced neural activity of DLPFC in WResp but not in CResp. In other words, DLPFC was coactivated with rdACC but not with cdACC. This coactivation of DLPFC and rdACC was also observed in the two‐way ANOVA as the positive interaction effect which reflects response conflict (see Table II). Even though the main effect of conflict also showed DLPFC activation, this was mainly caused by iWResp since no activation of DLPFC was observed in comparing incongruent with neutral trials in CResp.
DISCUSSION
The purpose of this study was to identify the functional dissociation of the subregions of dACC during conflict processing. Specifically, we designed a version of the Stroop task so that perceptual and response conflicts could be separately measured. We hypothesized that both perceptual and response conflicts contribute to the Stroop interference effect. Behavioral results demonstrated that the interference effects were evoked by both perceptual and response conflicts, consistent with previous cognitive psychological studies [De Houwer, 2003; Luo, 1999; Nassauer and Halperin, 2003; Sugg and McDonald, 1994].
Imaging results showed a functional dissociation of conflict processing within dACC, in which response conflict recruits rdACC whereas perceptual conflict involves cdACC. This dissociation is in accordance with previous studies which found that rdACC is specialized for response conflict whereas cdACC is involved in preresponse conflict [Braver et al., 2001; Milham and Banich, 2005; van Veen and Carter, 2005]. The coordinates within each subregion of dACC are similar to the mean coordinates of the previous studies (see Fig. 1). The following ROI analysis confirmed this dissociation, in which response conflict results in greater activation in rdACC. In addition, the level of cdACC activation was highly related to the Stroop interference effect caused by perceptual conflict but not by response conflict.
Consistent with our findings, cortical activation accompanying conflict processing has been found within rdACC [Botvinick et al., 1999; Fan et al., 2003; Milham and Banich, 2005; van Veen and Carter, 2005]. In contrast, other studies have shown that cdACC is involved in conflict processing [Egner and Hirsch, 2005; Kerns et al., 2004; MacDonald et al., 2000]. It might be argued that these findings are incompatible with our results. One possible interpretation of the different findings is that these studies did not separate response‐specific conflict from perceptual conflict and thus the cdACC activation observed might represent an accumulated effect of conflict of both types. Another possibility is that the studies have employed a predefined ROI within cdACC [Mitchell, 2006]. Another potential interpretation is that a large region across dACC was activated by both preresponse and response conflicts. For example, Weissman et al. [ 2003] performed a study which employed a cued global/local attention task, in which they found that the peak activation within cdACC to response conflict as well as preresponse (i.e., perceptual or semantic) conflict which was caused by global distracters. Subsequently they suggested that the conflict occurs at both preresponse and response levels and that cdACC region plays a role in processing of both kinds of conflict. As described above, however, they found a large region across dACC activated by both preresponse and response conflict.
Other studies have proposed that dACC is sensitive to response conflict but not to preresponse conflict [Liu et al., 2006; Milham et al., 2001]. In these studies, however, two distinct subregions in dACC (one in rostral area and the other in caudal area) were activated by inducing response conflict. We observed the same pattern of activations in the comparison of iWResp vs. nWResp. That is, even if a task condition measured response conflict, it is possible that the condition includes additional brain activation resulting from perceptual conflict. Thus, cdACC activation in their study might represent perceptual conflict as well as response conflict.
Our findings are also supported by a neuropsychological study [Swick and Jovanovic, 2002], in which two patients with lesions in either cdACC or rdACC showed different performance on three different variations of the Stroop task: a patient with lesions in cdACC showed a normal interference effect but a reduced facilitation effect, whereas the other patient who had lesions in rdACC showed an increase in the interference effect. The reduced facilitation suggests that the patient with lesions in cdACC might be impaired in perceptual selection and that subregions of rostral and caudal dACC make separate contribution to the conflict processing.
If two subregions of dACC are dissociated by the nature of the conflict, then it is possible that error processing is also specific to rdACC. Two studies have dissociated error processing and conflict processing. Garavan et al. [ 2003] found that cdACC close to pre‐SMA was specific to the occurrence of conflict but not to error processing, while rdACC showed error‐related activation. The conflict in their study might have been perceptual conflict since the same region of rdACC has been associated with both inhibiting responses and generating errors whereas cdACC has been involved in detecting targets [Braver et al., 2001]. Thus, these studies have presented evidence supporting our findings.
An important result was also found in DLPFC, which was coactivated with dACC only when response conflict occurred. This is consistent with previous studies in which DLPFC plays an important role in cognitive control by resolving prepotent responses [Botvinick et al., 1999; MacDonald et al., 2000]. Even though the interference effect was observed in our behavioral results and perceptual conflict activated cdACC, no activation was observed in DLPFC when no response conflict occurred. These results suggest that DLPFC is only involved in resolving response conflict so that one can override prepotent responses as predicted by the conflict monitoring theory. Even though no DLPFC activation in response to perceptual conflict was observed in our study, previous studies reported coactivation of DLPFC, which they found cdACC activation to preresponse conflict such as semantic conflict [van Veen and Carter, 2005] and target detection [Braver et al., 2001]. One possible interpretation of this disagreement is that the task conditions of those studies might require additional top‐down processing from DLPFC. For example, when subjects were to detect a target presented in low frequency [Braver et al., 2001], subjects were required to change their strategy of response selection during the low‐frequency condition and thus DLPFC activation might be due to the change of response selection [Huettel and McCarthy, 2004].
Inconsistent with the current finding, some studies have suggested that dACC is associated only with response conflict [Liston et al., 2006; van Veen et al., 2001]. Specifically, van Veen et al. found that dACC was associated with response conflict but not with stimulus conflict in the flanker task [van Veen et al., 2001]. In their study, however, stimulus presentation rates were low, which might result in reduced attentional demands as other studies have pointed out [van Veen and Carter, 2005; Weissman et al., 2003]. Interestingly, however, the location of dACC activation for response conflict (y = 32) observed in their results is very close to rdACC activation found in the current study (y = 33 or y = 27). This suggests that their task design was not sufficient to measure neural activation specific to perceptual conflict. In another study using a task switching paradigm, response conflict activated dACC, but stimulus conflict was associated with enhanced posterior parietal cortex activation [Liston et al., 2006]. Posterior parietal cortex activation in their study, however, might reflect attentional demands in detecting motion [Liu et al., 2003].
Taken together, we suggest that distinct subregions of dACC are involved in conflict processing in which cdACC is engaged in perceptual conflict whereas rdACC is involved in response conflict. This supports the conflict monitoring theory [Botvinick et al., 2001], but it might be possible to add a perceptual conflict module to the current conflict monitoring theory as a mediator. This regulative model would provide a more general account of the conflict monitoring system. With the current results, however, it is difficult to conclude how perceptual conflict could be resolved. One potential suggestion is that cdACC plays a role in regulative processing at a perceptual level even if no response conflict occurs [Roelofs et al., 2006].
CONCLUSION
In conclusion, we found perceptual and response conflicts during the Stroop task, in which the perceptual conflict recruits cdACC whereas the response conflict involves rdACC. DLPFC activation was not engaged by the perceptual conflict but was engaged by the response conflict. Our findings support the conflict monitoring hypothesis in which dACC plays a crucial role in detecting conflict and DLPFC is involved in overriding the conflict. We suggest, however, that only rdACC is specialized for response conflict whereas cdACC is involved in perceptual conflict. Also, DLPFC activity is specific to the response conflict suggesting that only the response conflict requires top‐down cognitive control processing to override prepotent responses and that cdACC might be involved in regulative processing of the perceptual conflict.
REFERENCES
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A ( 2001): Anterior cingulate cortex and response conflict: Effects of response modality and processing domain. Cereb Cortex 11: 837–848. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom LE, Fissell K, Carter CS, Cohen JD ( 1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD ( 2001): Conflict monitoring and cognitive control. Psychol Rev 108: 624–652. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS ( 2004): Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci 8: 539–546. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A ( 2001): Anterior cingulate cortex and response conflict: Effects of frequency, inhibition and errors. Cereb Cortex 11: 825–836. [DOI] [PubMed] [Google Scholar]
- De Houwer JAN ( 2003): On the role of stimulus‐response and stimulus‐stimulus compatibility in the Stroop effect. Mem Cogn 31: 353–359. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J ( 2005): The neural correlates and functional integration of cognitive control in a Stroop task. NeuroImage 24: 539–547. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI ( 2003): Cognitive and brain consequences of conflict. NeuroImage 18: 42–57. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA ( 2003): A midline dissociation between error‐processing and response‐conflict monitoring. NeuroImage 20: 1132–1139. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Huettel SA, McCarthy G ( 2004): That is odd in the oddball task?: Prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia 42: 379–386. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS ( 2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT ( 2005): A comparison of label‐based review and ALE meta‐analysis in the Stroop task. Hum Brain Mapp 25: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ ( 2006): Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task‐switching paradigm. Neuron 50: 643–653. [DOI] [PubMed] [Google Scholar]
- Liu T, Slotnick SD, Serences JT, Yantis S ( 2003): Cortical mechanisms of feature‐based attentional control. Cereb Cortex 13: 1334–1343. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL ( 2006): Functional dissociation of attentional selection within PFC: Response and non‐response related aspects of attentional selection as ascertained by fMRI. Cereb Cortex 16: 827–834. [DOI] [PubMed] [Google Scholar]
- Luo CR ( 1999): Semantic competition as the basis of stroop interference: Evidence from color‐word matching tasks. Psychol Sci 10: 35–40. [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS ( 2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- MacLeod CM ( 1991): Half a century of research on the stroop effect: An integrative review. Psychol Bull 109: 163–203. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT ( 2005): Anterior cingulate cortex: An fMRI analysis of conflict specificity and functional differentiation. Hum Brain Mapp 25: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF ( 2001): The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn Brain Res 12: 467–473. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ ( 2002): Attentional control in the aging brain: Insights from an fMRI study of the stroop task. Brain Cogn 49: 277–296. [DOI] [PubMed] [Google Scholar]
- Mitchell RLC ( 2006): Anterior cingulate activity and level of cognitive conflict: Explicit comparisons. Behav Neurosci 120: 1395–1401. [DOI] [PubMed] [Google Scholar]
- Nassauer KW, Halperin JM ( 2003): Dissociation of perceptual and motor inhibition processes through the use of novel computerized conflict tasks. J Int Neuropsychol Soc 9: 25–30. [DOI] [PubMed] [Google Scholar]
- Orr JM, Weissman DH ( 2009): Anterior cingulate cortex makes 2 contributions to minimizing distraction. Cereb Cortex 19: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL ( 1996): Motor areas of the medial wall: A review of their location and functional activation. Cereb Cortex 6: 342–353. [DOI] [PubMed] [Google Scholar]
- Roelofs A, van Turennout M, Coles MGH ( 2006): Anterior cingulate cortex activity can be independent of response conflict in Stroop‐like tasks. Proc Natl Acad Sci USA 103: 13884–13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR ( 1935): Studies of interference in serial verbal reactions. J Exp Psychol 28: 643–662. [Google Scholar]
- Sugg MJ, McDonald JE ( 1994): Time course of inhibition in color‐response and word‐response versions of the Stroop task. J Exp Psychol Hum Percept Perform 20: 647–675. [DOI] [PubMed] [Google Scholar]
- Swick D, Jovanovic J ( 2002): Anterior cingulate cortex and the Stroop task: Neuropsychological evidence for topographic specificity. Neuropsychologia 40: 1240–1253. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Coplanar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers. [Google Scholar]
- van Veen V, Carter CS ( 2005): Separating semantic conflict and response conflict in the Stroop task: A functional MRI study. NeuroImage 27: 497–504. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS ( 2001): Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage 14: 1302–1308. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG ( 2003): Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. NeuroImage 19: 1361–1368. [DOI] [PubMed] [Google Scholar]


