Abstract
Neural foundations of syntactic gender processing remain poorly understood. We used electrostimulation mapping in nine right‐handed awake patients during surgery for a glioma within the left hemisphere, to study whether the cortico‐subcortical structures involved in naming versus syntactic gender processing are common or distinct. In French, the article determines the grammatical gender. Thus, the patient was asked to perform a picture naming task and to give the appropriate article for each picture, with and without stimulation. Cortical stimulation elicited reproducible syntactic gender disturbances in six patients, in the inferior frontal gyrus (three cases), and in the posterior middle temporal gyrus (three cases). Interestingly, no naming disorders were generated during stimulation of the syntactic sites, while cortical areas inducing naming disturbances never elicited grammatical gender errors when stimulated. Moreover, at the subcortical level, stimulation of the white matter lateral to the caudate nucleus induced gender errors in three patients, with no naming disorders. Using cortico‐subcortical electrical mapping in awake patients, we demonstrate for the first time (1) a double dissociation between syntactic gender and naming processing, supporting independent network model rather than serial theory, (2) the involvement of the left inferior frontal gyrus, especially the pars triangularis, and the posterior left middle temporal gyrus in grammatical gender processing, (3) the existence of white matter pathways, likely a sub‐part of the left superior longitudinal fasciculus, underlying a large‐scale distributed cortico‐subcortical circuit which might selectively sub‐serve syntactic gender processing, even if interconnected with parallel sub‐networks involved in naming (semantic and phonological) processing. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: grammatical gender, syntactic processing, language, naming, awake electrical mapping
INTRODUCTION
Although many lesion and functional neuroimaging studies have tried to better understand neural basis of syntactic gender processing in the past decade [Badecker et al., 1995; Bordag et al., 2006; Comrie and Helm, 1997; Eddington, 2002; Friederici et al., 1999; Friedmann and Biran, 2003; Heim, 2008; Heim et al., 2002, 2005; Hofmann et al., 2007; Huber et al., 2004; Miceli et al., 2002; Padovani et al., 2005; Vigliocco and Nicol, 1998], the mechanisms underlying this complex function remain poorly known. Different neurocognitive models have been proposed, especially at the word level. One postulates that the phonological form of the target word becomes activated only after the corresponding abstract lexical representation (the “lemma”) has been selected [Levelt et al., 1999; Roelofs, 1992]. In an alternative model, semantic representations can directly activate word forms, without assuming an intervening lemma code [Caramazza and Miozzo, 1997]. Despite recent advances in noninvasive tools which enable to perform more accurate anatomo‐functional correlations and to build probabilistic maps [Vigneau et al., 2006], the debate is still open.
Interestingly, during brain surgery for resection of intrinsic cerebral tumors, it has become common clinical practice to awake patients to assess the functional role of both cortical and subcortical structures, so that the surgeon can maximize the extent of resection without inducing permanent neurological worsening [Duffau, 2005; Duffau et al., 2008a]. Patients perform cognitive tasks, such as picture naming, while the surgeon temporarily inactivates restricted cerebral regions by means of electrical stimuli (the brain has no receptors for pain) [Berger and Hadjipanayis, 2007; Duffau et al., 2005a]. As a consequence, intraoperative electrostimulation mapping represents a unique opportunity to investigate the neural foundations of cognitive functions such as language [Duffau, 2008; Duffau et al., 2002, 2003a, 2005b, 2009; Plaza et al., 2008; Thiebaut de Schotten et al., 2005]. However, although intraoperative language assessment mainly focused on articulation, phonology, and semantics, syntactic processing has received less attention.
Here, for the first time to our knowledge, we used the method of awake mapping to study whether the cortico‐subcortical areas involved in syntactic processing versus lexical access and speech production (i.e., disrupted by electrostimulation during a grammatical gender task and a picture naming, respectively) are common or distinct. In other word, the question asked was: is it possible to elicit a transient syntactic gender error without disturbing the lexical access and the production of the target word? It is worth noting that we focused on grammatical gender, because it allows drawing conclusion about syntactic features that go beyond gender.
MATERIALS AND METHODS
Patients
Nine French right‐handed adults were operated while awake for a glioma involving the left dominant hemisphere using electrical language mapping (Table I).
Table I.
Clinical, radiological, and surgical characteristics of the nine patients
| Patient | Age | Sex | Presenting symptoms | Preoperative DO 80 | Glioma location | “Syntactic” sites detected by stimulation | “Naming” sites detected by stimulation | Preoperative DO 80 (3 months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 37 | M | Seizures | 80/80 | Left parietal lobe (SMG, AG) | pMTG | pSTG, SMG, AG | 80/80 |
| 2 | 27 | F | Seizures | 80/80 | Left frontal lobe (SMA) | Subcortical white matter around the caudate | DLPMC | 80/80 |
| 3 | 30 | M | Headache | 80/80 | Left insula | Pars opercularis IFG | Pars opercularis IFG | 80/80 |
| 4 | 26 | M | Seizures | 80/80 | Left frontal lobe (DLPMC) | Pars triangularis IFG + Subcortical white matter | Pars opercularis IFG | 80/80 |
| 5 | 43 | M | Seizures | 80/80 | Left temporal lobe (pMTG) | pMTG | pSTG | 80/80 |
| 6 | 47 | F | Seizures | 80/80 | Left frontal lobe | Pars orbitaris IFG | Pars opercularis IFG, DLPMC | 80/80 |
| 7 | 37 | F | Seizures | 80/80 | Left frontal lobe (including Broca's area) | Pars triangularis IFG | Pars orbitaris IFG | 80/80 |
| 8 | 33 | M | Seizures | 80/80 | Left frontal lobe (including Broca's area) | Subcortical white matter around the caudate | DLPMC | 80/80 |
| 9 | 27 | M | Seizures | 80/80 | Left parietal lobe | pMTG | pSTG | 80/80 |
Abbreviations: M = Male; F = Female; pSTG = posterior Superior Temporal Gyrus; pMTG = posterior Middle Temporal Gyrus; SMG = Supramarginal Gyrus; AG = Angular Gyrus; SMA = Supplementary Motor Area; DLPMC = Dorsolateral Premotor Cortex; IFG = Inferior Frontal Gyrus
This series included six men and three women, ranging in age from 27 to 47 years (mean age 34 years). All patients were right‐handed as assessed by the Edinburgh inventory [Oldfield, 1971]. Presenting symptoms were seizures in eight cases and headaches in one patient.
All patients had normal preoperative neurological and language examinations. Presurgical performances on the “Boston Diagnostic Aphasia Examination” [Goodglass and Kaplan, 1983] as well as on the picture‐naming test “DO 80” [Metz‐Lutz et al., 1991] were normal.
The topography of the tumor was accurately analyzed on a preoperative MRI (T1‐weighted images after gadolinium enhancement in all orthogonal planes, and FLAIR‐weighted axial images).
Surgical Procedure: Intraoperative Electrostimulation Mapping
All patients underwent surgery under local anesthesia so that functional (especially language) cortical and subcortical mapping could be carried out using direct electrical stimulation. This method, including the electrical parameters, was previously described by the authors [Duffau et al., 2002, 2005a, 2008a]. A bipolar electrode with 5‐mm spaced tips delivering a biphasic current (pulse frequency of 60 Hz, single pulse phase duration of 1 ms, amplitude from 2 to 6 mA—Nimbus*, Hemodia) was applied to the brain of awake patients. Tumor boundaries were also systematically obtained using repeated ultrasonography.
Before starting with the resection, cortical mapping was performed. The current intensity adapted to each patient was determined by progressively increasing the amplitude in 1 mA increments from a baseline of 2 mA until a functional response was elicited, with 6 mA as the upper limit, with the goal of avoiding the generation of seizures. The patient was asked to perform counting (regularly from 1 to 10) until speech arrest was induced at the level of the ventral premotor cortex [Duffau et al., 2002, 2003a, 2005b, 2008a]. Thereafter, picture naming was used to identify the crucial language sites known to be inhibited by stimulation [Ojemann et al., 1989]. We used the DO 80, which consists of 80 black and white pictures selected according to variable such as frequency, familiarity, age of acquisition, and level of education [Metz‐Lutz et al., 1991]. In this subgroup of patients, we did not apply other language tasks, because of limitation of time, due to the fact that the tumors were voluminous (mean volume: 60 mL).
The patient was never informed when the brain was stimulated. The stimulation started with picture onset, and was continued until the good answer was given by the patient or up to 4 s (beyond this delay, another item was presented to the patient). At least one picture presentation without stimulation separated each stimulation, and no site was stimulated twice in succession, to avoid seizures. Each cortical site (size 5 × 5 mm2, due to the spatial resolution of the probe) of the entire cortex exposed by the bone flap was tested three times. Indeed, it is agreed nowadays, since the seminal publication of Ojemann et al. [1989], that three trials are sufficient to assure if an area is crucial for language, by generating disturbances during its three stimulations, with normalization of language as soon as the stimulation is stopped. This limitation of trials and task is required by the timing of the surgical procedure, because the patient is awake.
The type of language disturbances was detailed by a speech therapist, who was always present in the operating room during the functional mapping. She used a classification previously detailed, that is, speech arrest, anomia, dysarthria (disorders of the articulatory realization from one to several phonemes), phonemic paraphasia (disorders of the phonological form of the word), semantic paraphasia (disorders of the meaning of the word), slowness with initiation disturbances, perseveration (repetition of the previous item while the next item is presented to the patient), and hesitation [Duffau et al., 2005b, 2008a; Gatignol et al., 2004; Gil Robles et al., 2005].
Mapping of Syntactic Gender Processing
It is worth noting that, in the naming task, the picture was preceded by a short sentence to read, “ceci est …,” namely the French translation of “this is …,” to check that there were no seizures generating complete speech arrest if the patient was not able to name the picture. Interestingly, the grammatical article was not written on the screen between the sentence and the picture. In French, the article determines the grammatical gender, namely “un” for masculine noun and “une” for feminine noun. However, there is no correlation between gender and the semantic or phonological characteristics of the noun. In other words, in French, the form of the indefinite article tested does not depend on the phonology of the following words, as it does in some languages. Therefore, the patient was asked to give the appropriate article for each picture, and any disturbance in syntactic gender selection elicited by stimulation (i.e., “un” for “une” or vice‐versa) was also detailed by the speech therapist in addition to the naming task itself.
Each eloquent area was marked using a sterile number tag on the brain surface, and its location was correlated with the anatomical landmark (sulci/gyri/tumor boundaries) previously identified on ultrasonography studies. A photograph of the cortical map was always made before resection.
During a second surgical stage, the tumor was removed by alternating resection and subcortical stimulation. The functional pathways were followed progressively from the cortical eloquent sites already mapped, to the depth of the resection. The patient had to continue naming and providing the appropriate grammatical article when the resection became close to the subcortical language structures, which were also identified by functional inhibition during stimulation as at the cortical level [Duffau et al., 2002, 2003b, 2005b, 2008a; Mandonnet et al., 2007]. Again, the type of language disturbance was detailed by a speech therapist throughout the resection. To perform the best possible tumor removal without damaging functional areas, all resections were pursued until eloquent pathways were encountered around the surgical cavity, then followed according to functional boundaries. Thus, there was no margin left around the cortico‐subcortical eloquent areas.
Postoperative Course
Postoperative functional outcome was assessed by the team as preoperatively, by using the same DO 80 and BDAE tasks for language as before surgery, 3 months after surgery. A control MRI was performed in all cases immediately and at 3 months after surgery. The imaging studies allowed us first to evaluate the extent of tumor removal [Berger et al., 1994]. Second, postoperative MRI also enabled us to analyze the anatomical location of language pathways; that is, at the periphery of the cavity, where the resection was stopped, a methodology that we have extensively reported [Duffau et al., 2002, 2003b, 2005b, 2008a, 2008b, 2009; Gil Robles et al., 2005; Mandonnet et al., 2007].
RESULTS
Preoperative Results
Results of preoperative DO 80 were normal in all patients, with neither naming deficit nor grammatical gender error, i.e., the appropriate article was given in 100% of cases.
According to the preoperative MRI, five patients had a tumor within the left frontal lobe; two patients had a tumor within the left parietal lobe; one patient had a left insular tumor; and one patient had a left temporal glioma (for more detailed information see Table I; for the item list of the DO 80 see Table II).
Table II.
Item list of the DO 80
| French (English) | ||
|---|---|---|
| Cloche (Bell) | Chat (Cat) | Tabouret (Stool) |
| Fauteuil (Armchair) | Arrosoir (Watering can) | Croix (Cross) |
| Train (Train) | Grillage (Wire mesh) | Balance (Scales) |
| Eléphant (Elephant) | Kangourou (Kangaroo) | Coq (Rooster) |
| Canard (Duck) | Main (Hand) | Flèche (Arrow) |
| Lit (Bed) | Ciseaux (Chisel) | Cendrier (Ashtray) |
| Aspirateur (Vacuum cleaner) | Papillon (Butterfly) | Botte (Boot) |
| Chien (Dog) | Brosse (Brush) | Escargot (Snail) |
| Poire (Pear) | Chaise (Chair) | Hache (Axe) |
| Bougie (Candle) | Ecureuil (Squirrel) | Soleil (Sun) |
| Accordéon (Accordion) | Sabot (Clog) | Tortue (Turtle) |
| Cheval (Horse) | Pipe (Pipe) | Champignon (Mushroom) |
| Sapin (Fir) | Bouteille (Bottle) | Bureau (Desk) |
| Etoile (Star) | Coeur (Heart) | Girafe (Giraffe) |
| Canon (Cannon) | Vache (Cow) | Canne (Walking stick) |
| Serpent (Snake) | Rose (Rose) | Fourchette (Fork) |
| Brouette (Wheelbarrow) | Peigne (Comb) | Poisson (Fish) |
| Pied (Foot) | Corde à sauter (Skipping rope) | Marteau (Hammer) |
| Fraise (Strawberry) | Ours (Bear) | |
| Rhinocéros (Rhinoceros) | Casserole (Pan) | |
| Chapeau (Hat) | Cadenas (Padlock) | |
| Tambour (Drum) | Zèbre (Zebra) | |
| Paon (Peacock) | Louche (Ladle) | |
| Téléphone (Phone) | Seau (Bucket) | |
| Citron (Lemon) | Masque (Mask) | |
| Avion (Plane) | Hélicoptère (Helicopter) | |
| Drapeau (Flag) | Poule (Hen) | |
| Lion (Lion) | Banc (Bench) | |
| Couteau (Knife) | Lapin (Rabbit) | |
| Balai (Broom) | Commode (Chest of drawers) | |
| Père Nöel (Santa Claus) | Parapluie (Umbrella) | |
Operative Findings
All items processed without stimulation were correctly performed (see Fig. 1).
Figure 1.
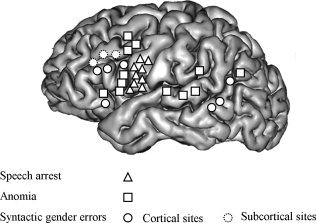
Summary of language interference sites identified by direct electrostimulation during counting and picture naming in nine patients with a glioma involving the left dominant hemisphere, with special emphasis on the site locations of syntactic gender errors (both at cortical and subcortical levels). Thirty‐one eloquent sites have been identified: 9 cortical sites eliciting speech arrest during counting (one in each patient); 12 cortical sites eliciting anomia (one in 7 patients, two in one patient, three in one patient); 7 cortical sites eliciting syntactic disorders in 7 patients; and 3 subcortical sites eliciting syntactic disorders in 3 patients (see Results, operative findings, pages 7 and 8).
In contrast, at the cortical level, electrostimulation allowed the detection of language sites in all cases, with no negative mapping.
First, reproducible speech arrest was elicited by stimulating the ventral premotor cortex (i.e., the lateral part of the precentral gyrus) in the nine patients during counting.
Second, stimulation induced reproducible anomia or phonemic paraphasia or semantic paraphasia for at least one cortical site in each patient (two and three naming sites identified in two patients, respectively): within the pars opercularis of the inferior frontal gyrus in three cases, the pars orbitaris of the inferior frontal gyrus in one case, the dorsolateral prefrontal cortex in three cases (posterior part of the middle frontal gyrus), the supramarginal gyrus in one case, the angular gyrus in one case, and the posterior part of the superior temporal gyrus in three cases. There has been never syntactic gender error generated during stimulation of the naming sites. It is worth noting that the words that induced anomia but correct article retrieval had no phonological characteristics (e.g., words endings) that correlated with gender. Thus, the article was not correctly retrieved on the basis of the partial availability of phonological information. In addition, in cases of semantic paraphasia, the patients were able to say the correct article of a name whose meaning was not known by him or her.
Third, cortical stimulation also elicited reproducible syntactic gender disturbance in six patients, with a wrong selection of the article “un/une” (see Fig. 2): in three cases by stimulating the inferior frontal gyrus—pars opercularis in one case (Fig. 2A), pars triangularis in two cases, pars orbitaris in one case (Fig. 2B)—and in three cases by stimulating the posterior part of the middle temporal gyrus (Fig. 2C). There were never naming disturbances generated during stimulation of the syntactic sites.
Figure 2.
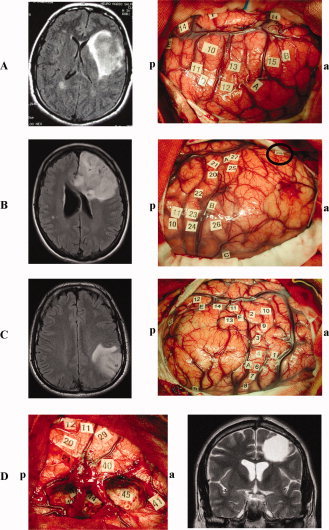
A: Patient 3, Left: Preoperative axial fluid attenuation inversion recovery (FLAIR)‐weighted MRI showing a left insular glioma. Right: Intraoperative view before resection and location of specific disturbances following electrical stimulation. The letter tags demarcate the tumor boundaries identified using intrasurgical ultrasonography. Complete speech arrest was produced with stimulation in the ventral premotor cortex (i.e., the lateral part of the precentral gyrus) (10), anomia but no gender errors with stimulation in the pars opercularis of the inferior frontal gyrus (13), and syntactic gender error but no naming disturbances with stimulation in the pars opercularis of the inferior frontal gyrus (12). The other letter tags correspond to sensory‐motor sites or areas generating transient hesitation when stimulated during naming task without speech arrest, anomia or syntactic gender disorders. a = anterior; p = posterior. B: Patient 6, Left: Preoperative axial FLAIR‐weighted MRI showing a left frontal glioma. Right: Intraoperative view before resection and location of specific disturbances following electrical stimulation. The letter tags demarcate the tumor boundaries identified using intrasurgical ultrasonography. Complete speech arrest was produced with stimulation in the ventral premotor cortex (20/21), anomia but no gender errors with stimulation in the pars opercularis of the inferior frontal gyrus (27/25) as well as in the dorsolateral prefrontal cortex (i.e. posterior part of the middle frontal gyrus) (26), and syntactic gender error but no naming disturbances with stimulation in the pars orbitaris of the inferior frontal gyrus (28). The other letter tags correspond to sensory‐motor sites or areas generating transient hesitation when stimulated during naming task without speech arrest, anomia or syntactic gender disorders. a = anterior; p = posterior. C: Patient 9, Left: Preoperative axial FLAIR‐weighted MRI showing a left parietal glioma. Right: Intraoperative view before resection and location of specific disturbances following electrical stimulation. The letter tags demarcate the tumor boundaries identified using intrasurgical ultrasonography. Complete speech arrest was produced with stimulation in the ventral premotor cortex (1), anomia but no gender errors with stimulation in the posterior part of the superior temporal gyrus (14), and syntactic gender errors but no naming disturbances with stimulation in the posterior part of the middle temporal gyrus (12). The other letter tags correspond to sensory‐motor sites or areas generating transient hesitation when stimulated during naming task without speech arrest, anomia or syntactic gender disorders. a = anterior; p = posterior. D: Patient 4, Left: Intraoperative view after resection of a left frontal glioma and location of specific disturbances following electrical stimulation at both cortical and subcortical levels. Complete speech arrest was produced with stimulation in the ventral premotor cortex (11/12), anomia but no gender errors with stimulation in the pars opercularis of the inferior frontal gyrus (29), and syntactic gender error but no naming disturbances with stimulation in the pars triangularis of the inferior frontal gyrus (40). Interestingly, subcortical stimulation of the white matter around the head of the caudate nucleus, below the cortical site 40, also elicited reproducible syntactic gender error without naming disorders (anomia was specifically generated in 45). The other letter tags correspond to sensory‐motor sites or areas generating transient hesitation when stimulated during naming task without speech arrest, anomia or syntactic gender disorders. a = anterior; p = posterior. Right: Postoperative coronal T2‐weighted MRI confirming that the surgical cavity was into the contact of white matter around the head of the caudate nucleus, in particular with pathways coming for the inferior frontal gyrus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Finally, at the subcortical level, stimulation of the white matter lateral to the head of the caudate nucleus induced reproducible gender errors in three patients (Fig. 2D), with no naming disorders.
Both at cortical and subcortical levels, a region was classified as “syntactic site” if the article was incorrectly produced 3/3 times. There was no preference for some gender (feminine versus masculine noun) when these errors occurred.
Postoperative Results
Three months after surgery, neurological and language examinations were normal in the nine patients. In particular, there were neither naming deficit nor grammatical gender error using DO 80 task, i.e., the appropriate article was given in 100% of cases, as during the presurgical period. The nine patients were able to resume a normal life.
Pathological examination diagnosed a glioma in all cases (seven WHO Grade II gliomas and two WHO Grade III gliomas). Postoperative MRI showed a total or subtotal resection in six cases and a partial resection in three cases.
DISCUSSION
This is the first report of a transient and reproducible induction of syntactic gender disorders during intraoperative direct stimulation of the left dominant inferior frontal gyrus, the posterior part of the middle temporal gyrus, and/or the white matter lateral to the head of the caudate, with no associated picture naming disturbances, showing a double dissociation between both processing.
Models of Syntactic Gender Processing: The Controversy
Grammatical gender is a feature present in many languages (e.g., Spanish, Catalan, Portuguese, French, Italian, Hebrew, German, Dutch, Polish, and Russian), in which it plays an important syntactic role. It is a property of nouns used in these languages to signify syntactic agreement, for instance between nouns and their determiners or nouns and adjectives [Comrie and Helm, 1997]. Despite its relevance in numerous languages, syntactic gender has not explicitly been included in most neurocognitive models of language processing. Moreover, for a long time, these models mainly focus on syntactic frames at the sentence level rather than on syntactic gender at the word level. Interestingly, in recent years, syntactic gender processing has attracted the interest of a growing number of researchers. Several psycholinguistic studies on grammatical gender have been published [Friederici et al., 2002; Grodzinsky and Friederici, 2006; Schriefers and Teruel, 2000; van Berkum, 1997]. Nonetheless, different conflicting models about the functional architecture of the language processing systems have been proposed [Caramazza and Miozzo, 1997; Levelt, 1999].
First, a large number of experimental studies support the independent representation of grammatical gender information [Schriefers and Teruel, 2000]. Indeed, the performances of aphasic patients [Avila et al., 2001; Badecker et al., 1995; Henaff Gonon et al., 1989] and those of neurologically intact speakers experiencing the “TOT phenomenon” [Caramazza and Miozzo, 1997; Miozzo and Caramazza, 1997; Vigliocco and Nicol, 1998] are consistent with the distinction between gender and phonological information. Given the autonomy of grammatical gender from semantics and phonology in many languages, most prominent psycholinguistic models postulate that gender information is stored as a property of nouns at a representational level different from those specifying the corresponding conceptual and phonological information. Nonetheless, the “WEAVER model,” originally proposed by Roelofs [1992] and refined by Levelt et al. [1999], assumes three main layers. Whereas the top layer describes the words meaning through a network of conceptual connections and the third layer specifies the words phonological forms (lexeme), the intermediate layer contains the abstract lexical representation (lemma), which is connected to nodes representing the words syntactic properties, such as grammatical gender. The lemma stratum mediates between conceptual and phonological lexical information. The phonological form of the target word becomes activated only after the corresponding lemma has been selected, which in turn is activated by its corresponding conceptual node. Then, according to this model, the retrieval of syntactic information is necessary for the establishment of the morphologic code. In their alternative model, called the “independent network,” Caramazza and Miozzo [1997] also distinguish three separated networks representing lexical‐semantic, syntactic, and phonological information. However, in this other model, semantic representations can activate word forms directly, without assuming an intervening lemma node. Therefore, the controversy is not yet solved.
Double Dissociation Between Syntactic Gender and Naming Processing Showed by Stimulation
Here, we used the reliable technique of direct cortico‐subcortical electrostimulation during awake surgery in language areas [Duffau, 2005; Duffau et al., 2003a, 2005b, 2009; Gil Robles et al., 2005; Plaza et al., 2008; Thiebaut de Schotten et al., 2005]. This method, which induces a transient virtual lesion, gives a unique opportunity to clarify this disputed topic of the psycholinguistic literature.
The occurrence of grammatical gender errors in the absence of naming disturbances (i.e., with both preservation of lexical access and word production) during focal stimulation, as well as the induction of naming disorders without gender errors during stimulation of other brain areas, constitutes a strong argumentation in favor of a segregated functional organization of language processing. This double dissociation support the theory suggesting an independency between syntactic, semantic, and phonological information, organized in parallel networks, even if likely interconnected [Caramazza and Miozzo, 1997]. Our data contradict a strictly serial model in which it would not be possible to obtain a gender grammatical error with no phonological disturbances, considering the unidirectional transmission of the information from the semantic to the morpho‐phonological level via the “lemma” [Levelt, 1999]. Indeed, according to this theory, the processing of each type of information (semantic, syntactic, or phonological) must be accomplished before starting with the next level. Thus, to our knowledge, our results showed for the first time the specific and reproducible occurrence of grammatical gender errors without lexical access and phonological disorders during a picture naming task, demonstrating that the retrieval of syntactic information is not necessary for the establishment of the morphologic code.
Furthermore, we have to acknowledge that grammatical gender errors were not systematically noted during the intraoperative mapping in the initial period of our experience. As a consequence, it is very likely that this transient deficit occurred in several other patients, but that the data were lost. In addition, it is worth noting that the observed break down of “un/une” naming and the nonbreak down of noun naming could also be interpreted as reflecting the difference between open and closed class words (known to differ in many respects, especially in neural representation), independently of syntactic processing. Nonetheless, this hypothesis is very unlikely. Indeed, if the stimulation had elicited disruption in closed classed words, it would have induced the same kind of errors that observed when eliciting disruption in open words (nouns), that is, anomia or replacement of one noun by another noun. Interestingly, it was not the case with regard to the grammatical article. The patients never said no article, or never replaced “un/une” by another closed class word, like for instance “le, la, les, des ….” During stimulation over a crucial area, the patients systematically said “un” for “une” or vice versa, supporting the fact that it was really a disturbance of the syntactic processing. We also have to insist on the fact that in the DO 80 in French, there is no link of meaning and gender like in German, and no link of phonology and gender like in Spanish and Italian. Moreover, there is no link of morphology and gender, especially no “‐ment” words, known to be masculine in French. Finally, since stimulation is not restricted to 0–250 ms [estimated speech planning time, see Sahin et al., 2009], it is worth noting that not only planning but also monitoring of syntax could have been effected by the stimulation. However, using intraoperative stimulation, our results support parallel rather than serial processing, even if using local field potential, Sahin et al. suggested serial rather than parallel processing.
Neural Basis of Syntactic Gender Processing
In the recent years, an increasing number of lesion and functional neuroimaging studies [Bordag et al., 2006; Eddington, 2002; Friedmann and Biran, 2003; Hofmann et al., 2007; Huber et al., 2004; Miceli et al., 2002; Padovani et al., 2005] have been carried out to assess how the brain processes syntactic gender information, but several aspects of this processing have not yet been addressed. Levelt et al. [1998] were among the first ones to investigate brain areas involved in syntactic gender processing during language production. Based on a comprehensive review, Indefrey and Levelt [2004] suggested that the middle portion of the left middle temporal gyrus was a potential candidate for lemma selection and retrieval processes—which in some psycholinguistic models include syntactic gender [Levelt et al., 1999]. Interestingly, in a specific stimulus‐onset asynchrony (SOA) design between distractors and stimuli matters, depending on the SOA and the triggered location one might disrupt different processes [Indefrey and Levelt, 2004; Schuhmann et al., 2009]. Although our results using intraoperative mapping do not support the serial model, as detailed above, it is nonetheless worth noting that stimulation of the posterior part of the left middle temporal gyrus elicited reproducible syntactic gender errors in three patients, pleading in favor of a key role of this cortical area in syntactic processing.
Furthermore, functional imaging studies have also found an implication of Broca's area in active retrieval and processing of gender information [Heim, 2008; Miceli et al., 2002]. The dorsal part of BA 44 would be more implied in phonological processing, whereas the ventral portion might be related with syntactic processing [Friederici et al., 2002; Hagoort, 2005]. Indeed, BA 45 activation occurred when the subject internally generated morphological cues for subsequent gender decisions [Heim, 2005]. However, gender decisions about visually presented nouns in German [Heim et al., 2002, 2005] and in Spanish [Hernandez et al., 2004; Hernandez et al., 2007] yielded an effect in the left BA 44. Interestingly, using intraoperative electrical mapping, we showed that stimulation of the left inferior frontal gyrus, especially the pars triangularis (BA 45) (in two patients in the present series), as well as the pars opercularis (BA 44)—and even the pars orbitaris—can generate reproducible syntactic gender errors. Therefore, our findings confirm that Broca's area is also an angular stone for grammatical gender processing. Interestingly, syntactic gender comprehension has been localized in similar networks in fMRI studies [e.g., Hammer et al., 2007], suggesting a common syntax processing network for production and comprehension.
Finally, it is important to insist on the fact that the subcortical connectivity underlying syntactic processing has received less attention in the literature. Interestingly, direct electrostimulation of the white matter within the left frontal lobe reproducibly elicited selective grammatical gender disorders with no naming errors (in particular no phonological disturbances) in three patients. We have to acknowledge that we did not perform diffusion tensor tractography in these patients. Nevertheless, postoperative anatomical MRI showed that the white matter pathways where the resection was stopped (because intraoperative stimulation induced syntactic disturbances) were located around the head of the caudate nucleus (Fig. 2D). Using this reliable method of anatomo‐functional correlation combining intrasurgical functional mapping and post‐surgical MRI, that we extensively validated in previous studies [Duffau et al., 2002, 2003b, 2005b, 2008b, 2009; Gil Robles et al., 2005; Mandonnet et al., 2007], we can hypothesize that at least a sub‐part of the left superior longitudinal fasciculus, known to run laterally to the striatum and to go to Broca's area [Duffau, 2008; Duffau et al., 2002b], might subserve syntactic processing.
Therefore, we argue that grammatical gender processing is actually underlined by a large‐scale cortico‐subcortical network involving the inferior frontal gyrus, the posterior left middle temporal gyrus, and the superior longitudinal fasciculus. Interestingly, on the basis of our findings, evidence on the time course of information access might be reinterpreted. The observed 40‐ms delay from gender to phonological access was hypothesized as to be related to either serial/cascading processing [van Turennout et al., 1997]. In fact, it could reflect the involvement of different processing pathways.
CONCLUSIONS
Using the method of cortico‐subcortical electrical mapping in awake patients, we demonstrate for the first time (1) a double dissociation between syntactic gender and naming processing, supporting independent network model rather than serial model, (2) the involvement of the left inferior frontal gyrus, especially the pars triangularis, as well as the posterior part of the left middle temporal gyrus in grammatical gender processing, (3) the existence of white matter pathways, likely a subpart of the left superior longitudinal fasciculus, underlying a large‐scale distributed cortico‐subcortical circuit which might selectively sub‐serve syntactic gender processing, even if interconnected with parallel sub‐networks involved in naming (semantic and phonological) processing.
REFERENCES
- Avila C, Lambon Ralph MA, Parcet MA, Geffner D, Gonzalez‐Darder JM ( 2001): Implicit word cues facilitate impaired naming performance: Evidence from a case of anomia. Brain Lang 79: 185–200. [DOI] [PubMed] [Google Scholar]
- Badecker W, Miozzo M, Zanuttini R ( 1995): The two‐stage model of lexical retrieval: Evidence from a case of anomia with selective preservation of grammatical gender. Cognition 57: 193–216. [DOI] [PubMed] [Google Scholar]
- Berger MS, Hadjipanayis CG ( 2007): Surgery of intrinsic cerebral tumors. Neurosurgery 61: 279–304. [DOI] [PubMed] [Google Scholar]
- Berger MS, Deliganis AV, Dobbins J, Keles E ( 1994): The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer 74: 1784–1791. [DOI] [PubMed] [Google Scholar]
- Bordag D, Opitz A, Pechmann T ( 2006): Gender processing in first and second languages: The role of noun termination. J Exp Psychol Learn Mem Cogn 32: 1090–1101. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Miozzo M ( 1997): The relation between syntactic and phonological knowledge in lexical access: Evidence from the “tip‐of‐the‐tongue” phenomenon. Cognition 64: 309–343. [DOI] [PubMed] [Google Scholar]
- Comrie JD, Helm JM ( 1997): Common feeding problems in the intensive care nursery: Maturation, organization, evaluation, and management strategies. Semin Speech Lang 18: 239–260. [DOI] [PubMed] [Google Scholar]
- Duffau H ( 2005): Lessons from brain mapping in surgery for low‐grade glioma: Insights into associations between tumour and brain plasticity. Lancet Neurol 4: 476–486. [DOI] [PubMed] [Google Scholar]
- Duffau H ( 2008): The anatomo‐functional connectivity of language revisited: New insights provided by electrostimulation and tractography. Neuropsychologia 46: 927–934. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Sichez N, Lopes M, Sichez JP, Bitar A, Fohanno D ( 2002): Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo‐functional study. Brain 125: 199–214. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Denvil D, Gatignol P, Sichez N, Lopes M, Sichez JP, van Effenterre R ( 2003a): The role of dominant premotor cortex in language: A study using intraoperative functional mapping in awake patients. Neuroimage 20: 1903–1914. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Denvil D, Lopes M, Capelle L ( 2003b): The articulatory loop: Study of the subcortical connectivity by electrostimulation. Neuroreport 14: 2005–2008. [DOI] [PubMed] [Google Scholar]
- Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, Van Effenterre R, Capelle L ( 2005a): Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985‐96) and with (1996‐2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry 76: 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio‐Mazoyer N, Capelle L ( 2005b): New insights into the anatomo‐functional connectivity of the semantic system: A study using cortico‐subcortical electrostimulations. Brain 128: 797–810. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Capelle L, Taillandier L ( 2008a): Contribution of intraoperative subcortical stimulation mapping of language pathways: A consecutive series of 115 patients operated on for a WHO grade II glioma in the left dominant hemisphere. J Neurosurg 109: 461–471. [DOI] [PubMed] [Google Scholar]
- Duffau H, Leroy M, Gatignol P ( 2008b): Cortico‐subcortical organization of language networks in the right hemisphere: An electrostimulation study in left‐handers. Neuropsychologia 46: 3197–3209. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Moritz‐Gasser S, Mandonnet E ( 2009): Is the left uncinate fasciculus essential for language? A cerebral stimulation study. J Neurol 256: 382–389. [DOI] [PubMed] [Google Scholar]
- Eddington D ( 2002): Dissociation in Italian conjugations: A single‐route account. Brain Lang 81: 291–302. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Steinhauer K, Frisch S ( 1999): Lexical integration: Sequential effects of syntactic and semantic information. Mem Cognit 27: 438–453. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Hahne A, Saddy D ( 2002): Distinct neurophysiological patterns reflecting aspects of syntactic complexity and syntactic repair. J Psycholinguist Res 31: 45–63. [DOI] [PubMed] [Google Scholar]
- Friedmann N, Biran M ( 2003): When is gender accessed? A study of paraphasias in Hebrew anomia. Cortex 39: 441–463. [DOI] [PubMed] [Google Scholar]
- Gatignol P, Capelle L, Le Bihan R, Duffau H ( 2004): Double dissociation between picture naming and comprehension: An electrostimulation study. Neuroreport 15: 191–195. [DOI] [PubMed] [Google Scholar]
- Gil Robles S, Gatignol P, Capelle L, Mitchell MC, Duffau H ( 2005): The role of dominant striatum in language: A study using intraoperative electrical stimulations. J Neurol Neurosurg Psychiatry 76: 940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E ( 1983): Boston Diagnostic Aphasia Examination. San Antonio (TX): The Psychological Corporation. [Google Scholar]
- Grodzinsky Y, Friederici AD ( 2006): Neuroimaging of syntax and syntactic processing. Curr Opin Neurobiol 16: 240–246. [DOI] [PubMed] [Google Scholar]
- Hagoort P ( 2005): On Broca, brain, and binding: A new framework. Trends Cogn Sci 9: 416–423. [DOI] [PubMed] [Google Scholar]
- Hammer A, Goebel R, Schwarzbach J, Münte TF, Jansma BM ( 2007): When sex meets syntactic gender on a neural basis during pronoun processing. Brain Res 1146: 185–198. [DOI] [PubMed] [Google Scholar]
- Heim S ( 2005): The structure and dynamics of normal language processing: Insights from neuroimaging. Acta Neurobiol Exp 65: 95–116. [DOI] [PubMed] [Google Scholar]
- Heim S ( 2008): Syntactic gender processing in the human brain: A review and a model. Brain Lang 106: 55–64. [DOI] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici AD ( 2002): Broca's area in the human brain is involved in the selection of grammatical gender for language production: Evidence from event‐related functional magnetic resonance imaging. Neurosci Lett 328: 101–104. [DOI] [PubMed] [Google Scholar]
- Heim S, Alter K, Friederici AD ( 2005): A dual‐route account for access to grammatical gender: Evidence from functional MRI. Anat Embryol (Berl) 210: 473–483. [DOI] [PubMed] [Google Scholar]
- Henaff Gonon MA, Bruckert R, Michel F ( 1989): Lexicalization in an anomic patient. Neuropsychologia 27: 391–407. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Kotz SA, Hofmann J, Valentin VV, Dapretto M, Bookheimer SY ( 2004): The neural correlates of grammatical gender decisions in Spanish. Neuroreport 15: 863–866. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Hofmann J, Kotz SA ( 2007): Age of acquisition modulates neural activity for both regular and irregular syntactic functions. Neuroimage 36: 912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MJ, Stenneken P, Conrad M, Jacobs AM ( 2007): Sublexical frequency measures for orthographic and phonological units in German. Behav Res Methods 39: 620–629. [DOI] [PubMed] [Google Scholar]
- Huber JE, Stathopoulos ET, Sussman JE ( 2004): The control of aerodynamics, acoustics, and perceptual characteristics during speech production. J Acoust Soc Am 116: 2345–2353. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ ( 2004): The spatial and temporal signatures of word production components. Cognition 92: 101–144. [DOI] [PubMed] [Google Scholar]
- Levelt WJ ( 1999): Models of word production. Trends Cogn Sci 3: 223–232. [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Praamstra P, Meyer AS, Helenius P, Salmelin R ( 1998): An MEG study of picture naming. J Cogn Neurosci 10: 553–567. [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS ( 1999): A theory of lexical access in speech production. Behav Brain Sci 22: 1–38. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H ( 2007): Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain 130: 623–629. [DOI] [PubMed] [Google Scholar]
- Metz‐Lutz MN, Kremin H, Deloche G ( 1991): Standardisation d'un test de dénomination orale: Contrôle des effets de l'âge, du sexe et du niveau de scolarité chez les sujets adultes normaux. Rev Neuropsychol 1: 73–95. [Google Scholar]
- Miceli G, Turriziani P, Caltagirone C, Capasso R, Tomaiuolo F, Caramazza A ( 2002): The neural correlates of grammatical gender: An fMRI investigation. J Cogn Neurosci 14: 618–628. [DOI] [PubMed] [Google Scholar]
- Miozzo M, Caramazza A ( 1997): Retrieval of lexical‐syntactic features in tip‐of‐the‐tongue states. J Exp Psychol Learn Mem Cogn 23: 1410–1423. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M ( 1989): Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg 71: 316–326. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Padovani R, Calandra‐Buonaura G, Cacciari C, Benuzzi F, Nichelli P ( 2005): Grammatical gender in the brain: Evidence from an fMRI study on Italian. Brain Res Bull 65: 301–308. [DOI] [PubMed] [Google Scholar]
- Plaza M, Gatignol P, Cohen H, Berger B, Duffau H ( 2008): A discrete area within the left dorsolateral prefrontal cortex involved in visual‐verbal incongruence judgment. Cereb Cortex 18: 1253–1259. [DOI] [PubMed] [Google Scholar]
- Roelofs A ( 1992): A spreading‐activation theory of lemma retrieval in speaking. Cognition 42: 107–142. [DOI] [PubMed] [Google Scholar]
- Sahin NT, Pinkers S, Cash SS, Shomer D, Halgren E ( 2009): Sequential processing of lexical, grammatical, and phonological information within Broca's area. Science 326: 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriefers H, Teruel E ( 2000): Grammatical gender in noun phrase production: The gender interference effect in German. J Exp Psychol Learn Mem Cogn 26: 1368–1377. [DOI] [PubMed] [Google Scholar]
- Schuhmann T, Schiller NO, Goebel R, Sack AT ( 2009): The temporal characteristics of functional activation in Broca's area during overt picture naming. Cortex 45: 1111–1116. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Lévy R, Dubois B, Bartolomeo P. ( 2005): Direct evidence for a parietal‐frontal pathway subserving spatial awareness in humans. Science 309: 2226–2228. [DOI] [PubMed] [Google Scholar]
- van Berkum JJ ( 1997): Syntactic processes in speech production: the retrieval of grammatical gender. Cognition 64: 115–152. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Hagoort P, Brown CM ( 1997): Electrophysiological evidence on the time course of semantic and phonological processes in speech production. J Exp Psychol Learn Mem Cogn 23: 787–806. [DOI] [PubMed] [Google Scholar]
- Vigliocco G, Nicol J ( 1998): Separating hierarchical relations and word order in language production: Is proximity concord syntactic or linear? Cognition 68: 13–29. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio‐Mazoyer N ( 2006): Meta‐analyzing left‐hemisphere language areas: Phonology, semantics and sentence processing. Neuroimage 30: 1414–1432. [DOI] [PubMed] [Google Scholar]


