Abstract
Autobiographical memory in amnestic Mild Cognitive Impairment (aMCI) is characterized by impaired retrieval of episodic memories, but relatively preserved personal semantic knowledge. This study aimed to identify (via FDG‐PET) the neural substrates of impaired episodic specificity of autobiographical memories in 35 aMCI patients compared with 24 healthy elderly controls. Significant correlations between regional cerebral activity and the proportion of episodic details in autobiographical memories from two life periods were found in specific regions of an autobiographical brain network. In aMCI patients, more than in controls, specifically episodic memories from early adulthood were associated with metabolic activity in the cuneus and in parietal regions. We hypothesized that variable retrieval of episodic autobiographical memories in our aMCI patients would be related to their variable capacity to reactivate specific sensory‐perceptual and contextual details of early adulthood events linked to reduced (occipito‐parietal) visual imagery and less efficient (parietal) attentional processes. For recent memories (last year), a correlation emerged between the proportion of episodic details and activity in lateral temporal regions and the temporo‐parietal junction. Accordingly, variable episodic memory for recent events may be related to the efficiency of controlled search through general events likely to provide cues for the retrieval of episodic details and to the ability to establish a self perspective favouring recollection. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: autobiographical memory, episodic memory, self, attention, mild cognitive impairment, neuroimaging
INTRODUCTION
Autobiographical memory (ABM) corresponds to personally relevant memories that include personal semantic knowledge and memories for specific events (episodic ABMs). ABM is thought to be organized hierarchically [Conway and Pleydell‐Pearce, 2000]: the most general level includes lifetime periods (i.e., general knowledge regarding an extended period of time, such as the school years), followed by general events (i.e., repeated events, such as attending French classes). The most specific level corresponds to unique episodic memories (e.g., the Day one recited a particular poem). Episodic ABMs are often characterized by a rich emotional content and vivid sensory‐perceptual details (mostly visuospatial) and give rise to the subjective feeling of re‐experiencing the event [Conway, 2009].
ABMs are complex multifaceted representations, the retrieval of which depends on various processes, including episodic memory, semantic memory, visual imagery, emotion, self‐reflection and executive functions [Cabeza and St Jacques, 2007; Conway and Pleydell‐Pearce, 2000]. Consistent with this view, neuroimaging studies of ABM have highlighted the involvement of a large core brain network, involving the hippocampus and parahippocampal gyrus, the medial and ventrolateral prefrontal cortex, the precuneus, the retrosplenial/posterior cingulate cortex, the lateral temporal cortex and the temporo‐parietal junction [Cabeza and St Jacques, 2007; Maguire, 2001; Schacter et al., 2007; Svoboda et al., 2006]. Additionally, areas involved in sensory‐perceptual (occipital) and emotional (amygdala) processes are also recruited during ABM retrieval [Cabeza and St Jacques, 2007; Svoboda et al., 2006]. According to Schacter et al. (2007), this autobiographical core brain network allows the retrieval of episodic memories and the flexible recombination of these memories to simulate future events. An alternative account proposes that it supports the construction, maintenance and visualization of scenes [Hassabis and Maguire, 2007].
The constructive view of episodic ABM retrieval is based on a dynamic model in which the brain regions of the core network interact to bring back to mind the memory of a specific episode [Cabeza and St Jacques, 2007; Conway and Pleydell‐Pearce, 2000]. Thus, the retrieval of episodic ABMs depends on effortful search processes (mediated by lateral prefrontal cortices) through semantic knowledge (supported by the lateral temporal cortices) to generate cues that will lead to vivid mental imagery of reactivated sensory‐perceptual event‐specific details (mainly in occipital regions and the precuneus). Moreover, given the personal nature of ABMs, self‐referential processing, related to medial prefrontal and posterior cortices functioning, forms an integral part of the operations. The reactivation of event‐specific details also involves the hippocampus, although it remains unclear whether its role is limited to recent memories or is permanent [Alvarez and Squire, 1994; Moscovitch et al., 2005]. Finally, the hypothetical role of the temporo‐parietal junction (and more generally parietal areas) has been discussed in terms of top‐down and bottom‐up attentional processes [Cabeza, 2008; Ciaramelli et al., 2008].
The exploration of ABM in memory‐impaired populations provides valuable information regarding the crucial mechanisms involved in the retrieval of episodic ABMs and their neural substrates. This is the case, for instance, of amnestic Mild Cognitive Impairment (aMCI), which designates the existence of some memory complaints corroborated by a significant anterograde memory deficit in elderly people in the absence of dementia [Petersen and Negash, 2008]. Recently, episodic ABMs have also been shown to decline in aMCI [Irish et al., 2010; Leyhe et al., 2009; Murphy et al., 2008]. In particular, in Murphy et al.'s study, patients were given the Autobiographical Interview [Levine et al., 2002] in which they were asked to report episodes they had personally experienced at a specific time and space in four remote periods (early childhood, teenage years, early adulthood, middle adulthood) and a recent period (past year). Memories were scored according to the protocol developed by Levine et al. which allows one to distinguish information specific to the remembered event (internal or episodic) from information common to other memories (external or semantic) within each narrative. Murphy et al. found that aMCI patients' ABMs contained fewer episodic details and more semantic details than those of healthy controls. Moreover, the decrease in the episodicity of autobiographical narratives was observed for all life periods, without any temporal gradient. The authors hypothesized that the episodic ABM impairment in aMCI may result from a dysfunction of the medial temporal lobe. Indeed, atrophy of the hippocampus and entorhinal cortex is the predominant structural cerebral change in aMCI [Masdeu et al., 2005]. Moreover, greater hippocampal atrophy correlates with poorer episodic memory performance in these patients [Chételat et al., 2003; Stoub et al., 2006].
Alternatively, the decline in episodic ABM in aMCI may be due to a dysfunction of other regions within the autobiographical core brain network. Consistent with this hypothesis, neuroimaging studies of episodic anterograde memory in MCI have shown the involvement of specific structures of the core network. For instance, an association has been found between the metabolism of the posterior cingulate cortex and episodic memory performance in MCI [Bastin et al., 2010; Chételat et al., 2003]. Furthermore, controlled retrieval of episodic information in MCI patients has been related to metabolic activity in the dorsomedial prefrontal cortex [Bastin et al., 2010].
The main objective of the current study was to further explore the mechanisms underlying the retrieval of episodic ABMs in a population of patients with impaired episodic memory (aMCI). Therefore, the study was designed to confirm findings of impaired episodic ABM in aMCI, and to identify the neural substrates underlying episodic ABM performance in aMCI. Using an adaptation of the TEMPau questionnaire [Piolino et al., 2002, 2003], aMCI patients and healthy controls were required to report memories for specific events in as much detail as possible for two time periods: the period between the age of 18 and 30 years old and the last 12 months. These two periods were chosen to maximize the availability of episodic details as, in older adults, the memories usually accessed in these two periods are known to have high episodic quality [Piolino et al., 2002, 2008]. The contribution of episodic and semantic information to the ABMs recounted by the participants was assessed by means of Levine et al.'s (2002) scoring protocol.
Brain metabolic activity at rest was measured by the 18Ffluorodeoxyglucose method and positron emission tomography (FDG‐PET). We adopted the cognitive‐metabolic approach which has been widely used to reveal the cerebral dysfunction that underlies memory impairments in Alzheimer's disease and MCI by pointing to regions whose metabolic activity is related to the variability of memory performance among patients [Salmon et al., 2008]. The specific cerebral regions whose dysfunction subserves the impairment in episodic ABM in aMCI were identified by correlations between regional (voxel‐based) metabolic activity and a fine‐grained measure of the extent to which memories contained episodic information (the proportion of internal/episodic details contained in the memories). We expected that the ability to retrieve episodic ABMs would be related to the level of activity of regions within the core network. The specific region involved should provide information about the constructive process the functional integrity of which is related to the ability to access event‐specific details in aMCI: attention to memory search, selection of semantic knowledge, cue specification, self‐referential processes or reactivation of sensory‐perceptual details [Cabeza and St Jacques, 2007; Ciaramelli et al., 2008; Conway and Pleydell‐Pearce, 2000].
METHODS
Participants
The research group consisted of 35 elderly participants (12 women) with episodic memory difficulties, who met the criteria for amnestic MCI single domain [Petersen and Negash, 2008]. They were referred by neurologists working in memory clinics and were selected on the basis of a general examination, neurological and neuropsychological assessments, laboratory evaluation and structural neuroimaging (showing mild to moderate atrophy and leukoaraiosis consistent with aging and degenerative processes). They did not experience difficulties in their daily activities and they did not fulfil the criteria for dementia. A control group of 24 healthy elderly participants (18 women) also participated in the study. Subjects in this group had no cognitive or psychiatric problems, were free of medication that could affect cognitive functioning, and reported being in good health.
The demographic and clinical characteristics of both groups are presented in Table I. The aMCI and control groups were matched in terms of age, education, vocabulary abilities and scores on the Geriatric Depression Scale [Yesavage et al., 1983]. The patients had a poorer score on the Mattis Dementia Rating Scale [Mattis, 1973] than the controls, t(57) = 4.1, P < 0.001.
Table I.
Demographic and clinical characteristics, and neuropsychological scores of the aMCI and control groups
| aMCI | Controls | |
|---|---|---|
| Age | 73.9 (6.6) | 73.2 (7.2) |
| Women/men | 12/23a | 18/6 |
| Education (years) | 13.0 (3.5) | 12.5 (2.8) |
| Mattis DRS | 128.9 (9.5)b | 137.8 (6.0) |
| Geriatric depression scale | 3.2 (2.5) | 3.0 (1.3) |
| Mill Hill vocabulary (max. 33)c | 24.8 (6.2) | 24.5 (7.4) |
| Episodic memory: | ||
| Cued recall | 3.1 (3.8)b | 7.1 (5.1) |
| Recognition (remember/know/guess) | 12.7 (3.5)b (4.0b/5.6b/3.1) | 15.6 (4.3) (9.9/3.4/2.3) |
| Continuous recognition | 0.49 (0.28)b | 0.71 (0.21) |
| Working memory: Reading span | 12.7 (6.8) | 15.4 (5.4) |
| Semantic memory: | ||
| Cued recall | 7.0 (3.7) | 7.8 (4.2) |
| Recognition | 12.8 (3.3) | 14.2 (2.9) |
| Executive function: | ||
| Hayling test (errors) | 10.3 (5.5) | 8.2 (5.1) |
| Unawareness of memory function | 3.9 (8.0)b | −0.4 (4.8) |
Standard deviations appear in parentheses.
χ2 = 9.4, P < 0.05.
Significant difference between groups.
(Deltour, 1993).
The data reported here were part of a multidisciplinary longitudinal evaluation of self‐awareness (including questionnaires about behavioural and cognitive functioning) and cognitive functioning (including measures of autobiographical memory, verbal episodic memory and executive function) in mild cognitive impairment. The whole evaluation was performed in two sessions of about 2 hours, with a FDG‐PET scan after the first session and a structural MRI after the second session. The ABM task described in the present study was administered at the beginning of the second session. Both groups performed several neuropsychological tests (Table I). Verbal episodic memory was first assessed by means of a word‐pair learning task. After studying 20 word‐pairs, participants had to recall the second word when the first word was used as a cue. In a subsequent recognition phase, subjects chose from among five alternatives the target word that was presented during the encoding phase. When choosing a word, participants were further asked to indicate whether they recollected the encoding context of the word (Remember responses), they merely felt it was familiar without recollecting anything (Know responses) or they chose it by chance (Guess responses) and were also asked to justify each Remember response [Gardiner et al., 1996]. The scores were the number of correctly recalled words (cued recall) and the number of correctly recognized words (global accuracy and number of correct Remember/Know/Guess responses). A second measure of verbal episodic memory was a continuous recognition memory test in which participants had to identify when certain words were repeated in a continuous series (adapted from Treyer et al., 2006). The measure was the corrected proportion of hits minus false alarms. General knowledge in semantic memory was assessed by asking participants to give the word corresponding to a definition (20 definitions). In a second (recognition) part, each definition was presented with five possible alternative words. The numbers of words correctly recalled and recognized were taken as measures of semantic memory performance. The reading span test [Daneman and Carpenter, 1980; Desmette et al., 1995] was administered to evaluate working memory. Participants read series of 2 to 6 sentences and had to remember the last word of each sentence. The subjects' performance corresponded to the total number of words correctly recalled. The Hayling test [Andrés and Van der Linden, 2000; Burgess and Shallice, 1996] evaluated the ability to inhibit a predominant semantic answer. The number of errors for part B (inhibition) of the task was measured. Finally, the aMCI patients' degree of awareness regarding their memory difficulties was assessed by means of the Memory Awareness Rating scale [Clare et al., 2002]. The discrepancy score between the patient's rating and a close relative's rating was the measure of memory unawareness.
The comparison of the two groups showed that aMCI patients had impaired verbal episodic memory, characterized by impaired recollection and increased reliance on familiarity, as indicated by Remember/Know responses. Awareness of memory problems was also affected in aMCI patients, as they underestimated their problems as compared to controls. In contrast, they showed preserved working memory, executive function and ability to retrieve information from semantic memory.
According to the Declaration of Helsinki, all participants gave their written consent to participate to the study, which was approved by the ethics committee of the University Hospital of Liège.
Materials and Methods
Episodic Autobiographical Memory Questionnaire
The ability to recall personally experienced events in detail was assessed by means of a questionnaire developed on the basis of the TEMPau [Piolino et al., 2003]. Two time periods were explored: 18 to 30 years old (remote) and the last 12 months (recent). For the remote period, participants had to recall a personal event concerning each of four topics: meeting someone or an event involving a particular person, an event concerning a trip or journey, an event involving the family and an event associated with leisure. For the recent period, four points in time were examined: the summer or the Christmas/New Year period (choosing the most remote from the testing time), last month, last week and yesterday or the day before yesterday. So, in total, participants were asked to report 8 ABMs. The instructions were to recall an event situated in time and space, which lasted less than a day and happened only once, and to provide as many details as possible. An illustrative event (a day by the sea) with a set of examples of episodic details (time and place details, people, activities, conversations, emotion and thoughts…) was given. If the participant failed to retrieve any event, cues were given (e.g., a celebration with the family for the topic “an event involving the family”). If the reported event was too generic, the participant was encouraged to be more specific. If the narrative lacked details, general probes asked for more specific details (e.g., “do you remember more details about this event?”). If the recalled event did not contain at least three specific details (in addition to the specification of the time and place), the examiner continued to administer probes (cueing/encouragements to be specific). If the narrative still lacked details after four probes, the examiner switched to the next topic.
Spontaneous recall and recall after probing were scored following Levine et al.'s (2002) protocol. The transcribed memories were segmented to isolate each detail. Details were scored as being internal or external. Internal details corresponded to information that characterized the main event and were episodic in nature. Subcategories of internal details grouped information regarding (1) the event (happenings, individuals involved, actions and reactions of oneself or other people, details of the environment such as weather conditions); (2) the time (day, month, year, season, etc.); (3) the place (city, street, building, position within the room, etc.); (4) perceptual details (visual information, smells, tactile information, sounds, duration, proprioceptive feelings); and (5) thoughts and emotions. Details were scored as external if they were not specific to the main episodic event and corresponded mainly to semantic information (general facts or generic events). Unsolicited repetitions of information, editorializing and details pertaining to unrelated events were also classified as external. Two independent raters (CB and DF) scored the details. Reliability was established on a randomly selected sample of 20 narratives, each from a different participant (10 aMCI and 10 healthy controls). Intraclass correlation coefficients showed that the two raters scored the narratives in a highly reliable manner (internal details: 0.98; external details: 0.97).
For each period (remote and recent), internal and external details generated spontaneously (spontaneous recall) and after complete probing (recall after probing) were summed across the four memories (total number of internal and external details). To assess the level of episodicity of the ABMs, the measure of interest was the ratio of internal details to total details (hereafter referred to as the proportion of internal details). This measure represents the degree to which memories contained episodic details independently of the overall fluency of the participants. Thus, for each group, there were four proportions, representing the proportion of internal details in spontaneous narratives and in narratives after probing for both live periods.
Brain Metabolic Measure
PET images were acquired on a Siemens/CTI (Knoxville, TN) ECAT HR+ scanner (3D mode; 63 image planes; 15.2 cm axial field of view; 5.6 mm transaxial resolution and 2.4 mm slice interval) during quiet wakefulness with eyes closed and ears unplugged after intravenous injection of 2‐[18F]fluoro‐2‐deoxy‐D‐glucose (152–290 MBq) [Lemaire et al., 2002]. Images of tracer distribution in the brain were used for analysis: scan start time was 30 min after tracer injection and scan duration was 20 min. Images were reconstructed using filtered backprojection including correction for measured attenuation and scatter using standard software. FDG‐PET image analyses were performed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK). The PET data were subjected to an affine and nonlinear spatial normalization onto the SPM8 PET brain template. Then, images were smoothed with a 12‐mm full‐width at half‐maximum filter. To test hypotheses about region‐specific effects, parameters were estimated according to the general linear model at each voxel.
Structural MRI Acquisition
A high‐resolution T1‐weighted image (3D MDEFT) was acquired on a 3T Siemens Allegra scanner for each participant using the following parameters: TR/TE/TI = 7.92/2.4/910 ms, FA = 15°, FOV = 256 × 240 × 176 mm2, 1 mm isotropic spatial resolution [Deichmann et al., 2004].
Procedure
Participants were tested individually in two sessions, with an interval of 1 week between them. The first session included the Reading span test, the word‐pair learning task, the general knowledge test, the Hayling task, the MARS questionnaire and the Geriatric Depression Scale, followed by the PET exam. The second session comprised the TEMPau questionnaire, the Mattis DRS, the Mill Hill vocabulary test, following by the structural MRI. For the TEMPau questionnaire, half of the participants first reported memories for the remote period, then memories for the recent period, whereas the other half started with recent memories.
Statistical Analyses
Behavioral analyses
Analyses of variance (ANOVAs) were used to assess between‐group differences on the mean total number of internal and external details and on the proportions of episodic details out of the total number of details for the remote and recent periods. Statistical analyses on these scores were carried out separately for spontaneous recall and recall after probing, as scores after probing represent a cumulative measure including scores for spontaneous recall. To assess whether specific cognitive variables can predict aMCI patients' ability to retrieve episodic ABMs, each proportion of internal details was entered in a multiple linear regression with the neuropsychological scores, assessing episodic (Remember responses) and semantic memory (cued recall of words from a definition), working memory (Reading span), attention (attention subscale from the Mattis DRS), executive function (Hayling test), and the measure of memory unawareness (discrepancy scores) as predictors. The regression analyses used a forward stepwise method which retains the most significant predictors one at a time. A threshold of F > 1 was required for variables to enter the model.
Cerebral metabolic analyses
As a first step, PET images of the patients were compared with those of controls using proportional scaling by cerebral global mean values to control for individual variation in global FDG uptake, and with gender as nuisance variable as both groups differed in the men/women ratio. Correlations between cerebral metabolism and episodic ABM were then performed with multiple regression analyses in SPM8 where individual PET images were entered as independent variables for each group (aMCI and controls) and the proportion of internal details was used as covariate of interest. Age, gender and Mattis DRS scores served as nuisance variables. Four separate analyses were conducted in controls and aMCI patients, considering the proportion of internal details during spontaneous recall and after probing in the two investigated periods (remote and recent). A first contrast looked at regions where activity correlated with the proportion of internal details in both groups (conjunction analyses). Then, contrasts isolated positive correlations in each group. More importantly, to specifically demonstrate brain functioning in aMCI, our target contrast searched for correlations characteristic of the aMCI group by comparison with the control group (correlation in aMCI>controls, with inclusive masking by aMCI at P < 0.05). For all statistical analyses, positive correlations were assessed using a statistical threshold of P < 0.05 corrected for multiple comparisons or P < 0.001 uncorrected at voxel‐level for regions with a priori hypotheses (i.e., the core brain network), with a threshold for minimum spatial extent of 30 contiguous voxels.
Structural MRI analyses
To assess whether the correlations between the metabolism of specific regions with episodic ABMs in aMCI reflected greater atrophy of these regions, structural MR images were analysed with the VBM8 toolbox in SPM8 (http://dbm.neuro.uni-jena.de/vbm.html). The image of one control participant had to be discarded because of poor quality. First, regional grey matter density, extracted with default parameters, were compared between the aMCI and control groups by including gender and total intracranial volume as confounding variables. Second, SPM analyses examined correlations between grey matter density and the proportion of internal details.
RESULTS
Episodic Autobiographical Memory
Figure 1 presents the mean total number of internal and external details generated by the aMCI and control groups for each time period as a function of the presence or absence of probing. Figure 2 shows the proportions of episodic details out of the total number of details produced spontaneously and after general probes for the remote and recent periods by aMCI and control participants.
Figure 1.
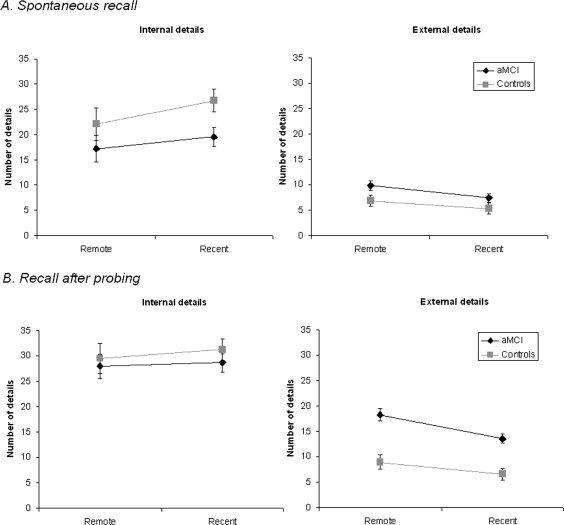
Number of internal and external details contained in autobiographical memories narrated by aMCI patients and healthy controls either spontaneously (A) or after general probing (B) for both time periods.
Figure 2.
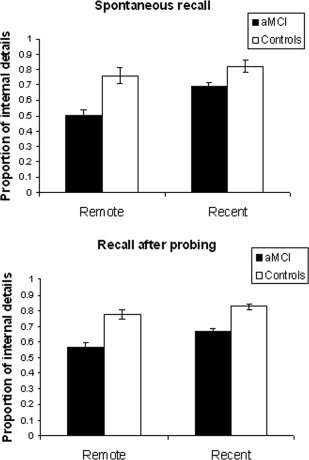
Proportion of internal details in the production of autobiographical memories by patients with aMCI and control participants.
Spontaneous recall
A 2 (group: aMCI, controls) by 2 (period: remote, recent) by 2 (details: internal, external) ANOVA showed that both groups produced memories of similar length in terms of the total number of details given, F(1, 57) = 0.87, P > 0.35, MSE = 204.60. All participants also gave (as required by the instructions) more internal than external details, F(1, 57) = 107.63, P < 0.001, MSE = 104.80. The significant group by details interaction, F(1, 57) = 9.86, P < 0.01, MSE = 104.80, showed that aMCI patients recalled fewer internal details than controls (post hoc Tukey tests, P < 0.05), but as many external details (P > 0.71). The significant period by details interaction, F(1, 57) = 8.31, P < 0.01, MSE = 51.50, was driven by a marginally greater number of internal details for the recent than for the remote period (P < 0.075). No other effect was significant.
The numbers of internal and external details from each subcategory for each period and each group are presented in Supporting Information Figures S1 and S2. For internal details, both groups gave more event details than any other subcategories, F(4,228) = 120.38, P < 0.001, MSE = 15.99, the next most frequent categories being time and place details. There was no group by subcategories interaction, F(4,228) = 1.68, P > 0.15, MSE = 15.99. For external details, the main effect of subcategories indicated that most external details were semantic, F(3,171) = 133.01, P < 0.001, MSE = 5.36. The significant group by subcategories interaction showed that, despite the absence of global difference in external details between groups, patients gave more semantic details than controls, F(3,171) = 5.14, P < 0.01, MSE = 5.36. This pattern is consistent with previous research in normal aging and aMCI [Levine et al., 2002; Murphy et al., 2008].
With regard to the proportion of internal details, on spontaneous recall, 2 aMCI patients did not produce any memory, so the proportion could not be calculated and the following analysis includes 33 aMCI patients. A mixed‐design ANOVA on these scores showed a main effect of group, F(1, 55) = 15.41, P < 0.001, MSE = 0.07, indicating that the memories recalled by aMCI patients contained a smaller proportion of episodic details than the memories of control participants. The proportion of internal details was also greater for the recent than for the remote period, F(1, 55) = 11.14, P < 0.01, MSE = 0.03. Finally, the group by period interaction was not significant, F(1, 55) = 2.91, P > 0.09, MSE = 0.03.
Recall after probing
The mean number of probes given per memory did not differ between the groups, F(1, 57) = 2.43, P > 0.12, MSE = 21.23. However, both groups needed more prompting to provide episodic details for the remote period (M = 2.49) than for the recent one (M = 2.15, F(1, 57) = 8.23, P < 0.01, MSE = 6.42). There was no group by period interaction for the mean number of probes.
After probing, aMCI patients gave on the whole more details than healthy controls, F(1, 57) = 4.19, P < 0.05. Again, the productions contained more internal than external details, F(1, 57) = 127.95, P < 0.001, MSE = 135.52. There was a significant group by details interaction, F(1, 57) = 10.89, P < 0.01, MSE = 135.52, which indicated that aMCI patients produced as many internal details as controls (P > 0.77) but significantly more external details (P < 0.01). The significant period by details interaction, F(1, 57) = 4.02, P < 0.05, MSE = 78.52, reflected the fact that the predominance of internal details over external details was slightly larger in the recent period than in the remote period, although there was a significant effect of the type of details for each period (Ps < 0.001). There was no other significant effect.
When considering the different subcategories of details (see Supporting Information Figs. S1 and S2), one observed a predominance of event details, F(4,228) = 285.77, P < 0.001, MSE = 13.68, and no other effect. Moreover, the increase in external details in aMCI patients compared to controls appeared to be due to a specific increase of semantic details, F(3,171) = 24.60, P < 0.001, MSE = 9.95.
The proportion of internal details was entered into an ANOVA with group as a between‐subject variable and period as a repeated measure. The significant main effect of group showed that the aMCI patients' ABMs were proportionally less episodic than those of controls, F(1, 57) = 29.62, P < 0.001, MSE = 0.03. Memories from the recent period contained a greater proportion of internal details than memories from the remote period, F(1, 57) = 8.11, P < 0.01, MSE = 0.02. The group by period interaction was not significant, F(1, 57) = 0.84, P > 0.36.
Between‐Group Comparison of Cerebral Metabolism and Grey Matter Density
Cerebral metabolism
Patients with aMCI showed significantly reduced metabolic activity in the posterior cingulate cortex mainly (MNI coordinates: x = 6, y = −56, z = 26, Z = 4.11, k = 574, p FWE‐corrected < 0.065 at cluster level), and in a small cluster in the right superior parietal lobule (x = 42, y = −62, z = 52, Z = 3.29, k = 35, P < 0.001 uncorrected at voxel level).
Grey matter density
Significant atrophy in aMCI patients was found in the hippocampus bilaterally (peak MNI coordinates: x = −35, y = −22, z = −12; x = −26, y = −39, z = −2, and x = 39, y = −15, z = −17, Z = 3.87, 3.35, and 3.72, respectively, k = 305, 114, and 357, p uncorrected < 0.001 voxel‐level).
Posterior cingulate hypometabolism and hippocampal atrophy form the typical pattern of brain changes associated with MCI [Chételat et al., 2002, 2003; de Leon et al., 2004; Guedj et al., 2009; Masdeu et al., 2005; Stoub et al., 2006].
Metabolic Correlates of Episodic Autobiographical Memory (Proportion of Internal Details)
The conjunction analyses did not reveal any region to be significantly correlated with the proportion of internal details in both the aMCI and control groups.
Correlations in healthy controls
The results of the correlations are presented in Table II.
-
Spontaneous recall
For the remote period memory, a significant correlation emerged in the left precuneus. For the recent period, the proportion of internal details was associated with activity in the right superior temporal cortex.
-
Recall after probing
For the recall of the remote period and for memory from the last 12 months, there was no significant correlation.
Table II.
Peak MNI coordinates of brain regions showing a positive correlation with the proportion of internal details in control participants (P < 0.001 uncorrected)
| Regions | BA | x | y | z | Z score | Cluster size | |
|---|---|---|---|---|---|---|---|
| Remote period | |||||||
| Spontaneous recall | Left superior parietal/precuneus | 7 | −12 | −78 | 52 | 3.47 | 45 |
| Recall after probing | Nil | ||||||
| Recent period | |||||||
| Spontaneous recall | Right superior temporal | 42 | 72 | −20 | 6 | 3.77 | 32 |
| Recall after probing | Nil | ||||||
BA, Brodmann area.
Correlations characteristically observed in aMCI patients (versus control participants)
In the aMCI group, the proportion of episodic details correlated with activity in large clusters of regions, including medial and lateral parietal regions and occipital regions for remote memories and temporo‐parietal regions for recent memories (see Table III). To clarify the neural correlates of episodic ABMs characteristic of aMCI patients, the contrast aMCI > controls, with inclusive masking by aMCI, showed the regions where metabolic activity was positively correlated with the proportion of internal details in the aMCI patients more than in control participants. The results are presented in Table IV and Figure 3.
-
Spontaneous recall
For the memory from the remote period, significant correlations were found in the left superior parietal cortex and the right occipital cortex (cuneus). For the recent period memory, the results showed a correlation in the right middle temporal cortex.
-
Recall after probing
For the remote period recall, a higher level of episodicity in ABM was associated with higher activity in occipital regions bilaterally and the right inferior parietal cortex. For the recent period, correlations emerged in the right occipital cortex, in two clusters at the right temporo‐parietal junction as well as in the right lateral temporal cortex.
Table III.
Peak MNI coordinates of brain regions showing a positive correlation with the proportion of internal details in aMCI patients (P < 0.001 uncorrected)
| Regions | BA | x | y | z | Z score | Cluster size | |
|---|---|---|---|---|---|---|---|
| Remote period | |||||||
| Spontaneous recall | Right superior parietal/precuneusa | 7 | 10 | −54 | 64 | 4.34 | 2249 |
| Left cuneus | 18 | −4 | −84 | 24 | 4.01 | 545 | |
| Recall after probing | Left occipitalb | 18 | −8 | −86 | 24 | 4.28 | 939 |
| Right occipital | 18 | 16 | −88 | 20 | 3.80 | ||
| Right inferior parietal | 40 | 32 | −54 | 56 | 4.08 | 499 | |
| Precuneus | 7 | −18 | −64 | 52 | 3.57 | 121 | |
| Right postcentral | 40 | 40 | −34 | 58 | 3.46 | 87 | |
| Recent period | |||||||
| Spontaneous recall | Right superior temporal | 22 | 64 | −52 | 6 | 3.70 | 312 |
| Recall after probing | Right temporo‐parietal junctionb | 37 | 52 | −52 | −16 | 4.17 | 1775 |
| Left supramarginal gyrus | 40 | −50 | −52 | 28 | 3.39 | 36 | |
| Left middle temporal | 21 | −64 | −40 | −12 | 3.34 | 37 | |
P ≤ 0.054 FWE‐corrected at voxel level.
P < 0.05 FWE‐corrected at cluster level.
BA, Brodmann area.
Table IV.
Peak MNI coordinates of brain regions showing a positive correlation with the proportion of internal details in aMCI patients (contrast: aMCI > controls, with inclusive masking by aMCI at P < 0.05; P< 0.001 uncorrected)
| Regions | BA | x | y | z | Z score | Cluster size | |
|---|---|---|---|---|---|---|---|
| Remote period | |||||||
| Spontaneous recall | Left superior parietal | 7 | −40 | −68 | 56 | 4.04 | 59 |
| Right cuneus | 19 | 8 | −88 | 26 | 3.90 | 129 | |
| Recall after probing | Right inferior parietal | 40 | 44 | −40 | 40 | 3.72 | 60 |
| Right cuneus | 19 | 6 | −88 | 26 | 3.78 | 227 | |
| Left lingual | 18 | −6 | −94 | −14 | 3.43 | 37 | |
| Recent period | |||||||
| Spontaneous recall | Right middle temporal | 21 | 72 | −28 | −18 | 3.91 | 54 |
| Recall after probing | Right temporo‐parietal junction/supramarginal gyrus | 40 | 46 | −50 | 32 | 4.02 | 135 |
| Right temporo‐parietal junction/middle temporal | 21 | 56 | −56 | 6 | 3.50 | 119 | |
| Right middle temporal | 20 | 70 | −28 | −20 | 3.52 | 42 | |
| Right occipital | 19 | 36 | −78 | 18 | 3.46 | 112 | |
BA, Brodmann area.
Figure 3.
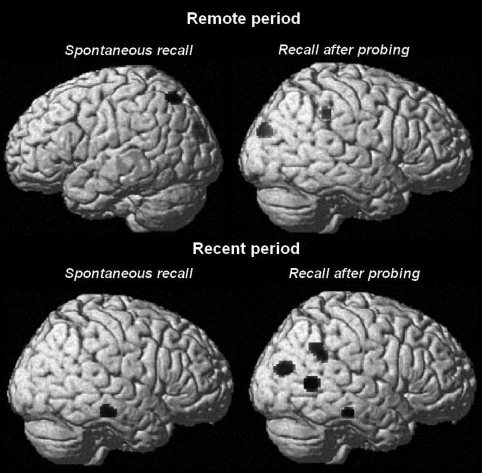
Results of the SPM analyses showing metabolic regional activity significantly correlated (P < 0.001 uncorrected) with the proportion of internal details during spontaneous recall and after general probing in the two time periods in aMCI patients (contrast: aMCI > controls, with inclusive masking by aMCI at P < 0.05) rendered on a single subject brain.
Correlations between atrophy and episodic autobiographical memory
There was no significant correlation between grey matter density and the proportion of internal details in aMCI patients, suggesting that the results of the cognitive‐metabolic correlations were not driven by atrophy.
Neuropsychological predictors of the proportion of internal details in aMCI
-
Spontaneous recall
For remote memories, a significant regression model included the Remember score and the attention subscale of the Mattis DRS (R 2 = 0.36, P < 0.01), with the Remember score as the only significant predictor (beta = 0.51, P < 0.01). For recent memories, the regression model retained the error score on the Hayling test as the only significant predictor (R 2 = 0.22, beta = −0.47, P < 0.01).
-
Recall after probing
For remote memories, the best predictor was again the Remember score (R 2 = 0.36, beta = 0.51, P < 0.01). For recent memories, the regression model including errors on the Hayling test (beta = −0.27), Remember responses (beta = 0.25) and memory unawareness (beta = −0.23) was significant (R 2 = 0.35, P < 0.01), but none of the predictors made a significant contribution on its own.
DISCUSSION
This study explored episodic ABM in aMCI patients, a population characterized by impaired episodic memory. Following up on studies that reported a deficit affecting the retrieval of event‐specific details in aMCI [Irish et al., 2010; Leyhe et al., 2009; Murphy et al., 2008], our results further provide insight into the cerebral bases of this deficit by looking at the cerebral metabolic correlates of specifically episodic ABMs in a relatively large sample of aMCI patients.
Using a questionnaire (adapted from the TEMPau, Piolino et al., 2002, 2003) which shares with the Autobiographical Interview used by Murphy et al. (2008) the emphasis on the retrieval of richly detailed personally experienced episodes, our study confirmed that ABM in aMCI is characterized by an impoverished episodic quality of the retrieved memories compared to healthy elderly participants. More specifically, the memories spontaneously retrieved by aMCI and control participants contained a similar number of external details, but aMCI patients recalled fewer episodic/internal details than controls. In particular, for the remote period (18–30 years old), episodic details constituted only half of aMCI patients' narratives. Patients accessed slightly more episodic details for memories of recent events, but still fewer than controls. However, the memories spontaneously recalled by both groups were often brief and participants needed prompting to reveal all that they might remember. General probing encouraged greater retrieval efforts aiming at the elaboration of event‐specific memories. After probing, aMCI patients recalled on average the same number of internal details as healthy controls, but these were swamped by an excessively large number of external details. Thus, even after probing, ABMs remained proportionally less episodic in aMCI than in controls. The elevated number of external (mostly semantic) details in the current aMCI sample may appear to contradict evidence of deficient personal semantic memory in aMCI [Irish et al., 2010; Leyhe et al., 2009]. However, the procedure stressed the importance of reporting episodic details and did not allow an appropriate exploration of personal semantic memory. It is more likely that aMCI patients compensated for the difficulty to retrieve episodic details by giving external details while healthy controls more successfully restricted themselves to internal details. Nevertheless, this suggests a minima that aMCI patients have access to some semantic knowledge. In more severe conditions of cognitive decline, such as Alzheimer's disease, there is some decline in the production of external details embedded in the narration of episodic events, but much less striking that the impairment in internal details [Addis et al., 2009b].
As in Murphy et al.'s study (2008), the ability of aMCI patients to recall strictly episodic memories was decreased for both remote and recent events. Moreover, aMCI patients as healthy controls were able to report proportionally more episodic details for recent than remote memories. This diverges from Leyhe et al.'s (2009) findings of a decline in the recall of episodic autobiographical memories only for the most recent period in aMCI. This discrepancy may be due to the use of different procedures. Contrary to the Autobiographical Memory Interview [Kopelman et al., 1989] used by Leyhe et al. which assesses separately the access to episodic and semantic ABMs, our procedure and scoring focused on the part of episodic details within the narration of an episodic autobiographical events. In other words, we estimated the degree of episodicity of each memory more than the general ability to retrieve episodes.
The cerebral correlates of episodic ABM in aMCI patients were explored via measures of brain regional metabolism at rest. Although correlation does not mean causality, the correlation analyses of resting brain metabolism identified the crucial regions where variation in metabolic activity is associated with the ability to generate specifically episodic ABMs. As expected, the results yielded correlations in different regions within an autobiographical core brain network [Cabeza and St Jacques, 2007; Schacter et al., 2007; Svoboda et al., 2006]. In aMCI patients more than in healthy controls, the proportion of episodic details in ABMs was related to the metabolic activity in parietal and occipital regions for remote memories, and to activity in temporo‐parietal and occipital regions for recent memories. We will discuss the functional significance of these regions in the process of episodic ABM retrieval, and make some tentative proposals about how variability in the integrity of these processes may contribute to the variability in the degree of episodicity of ABMs in aMCI.
There was a correlation between the proportion of episodic details in spontaneous retrieval of ABMs from the remote period and the level of resting‐state activity in the left precuneus in healthy controls, and aMCI patients showed even more correlation in the left superior parietal and occipital cortex. Activity in the occipital cortex was also related to the proportion of episodic details in memories generated by aMCI after probing for the two time periods. The precuneus, the superior parietal cortex and occipital regions have been claimed to be related to visual mental imagery [Cui et al., 2007; Fletcher et al., 1995; Gardini et al., 2006; Ishai et al., 2000]. Interestingly, in young adults, individual differences in visual imaging abilities affect the amount of sensory‐perceptual and contextual details retrieved in ABMs [D'Argembeau and Van der Linden, 2006]. Consistently, the current findings may suggest that healthy elderly and aMCI participants with a high capacity for visual imagery, supported by a properly functioning precuneus in the former and by a larger cluster of regions comprising superior parietal and occipital cortices in the latter, retrieved more episodic details about past events. Conversely, as the ability to form mental images seems affected in aMCI [Borg et al., 2010], some patients may fail to retrieve sensory‐perceptual details due to deficient visual imagery.
The level of episodicity of aMCI patients' ABMs from the remote period was particularly associated with the left superior parietal region (BA 7) for spontaneous recall and with the right inferior parietal cortex (BA 40) for recall after probing, while the temporo‐parietal junction was associated with recent episodic memories retrieved after probing in aMCI. This is consistent with neuropsychological studies showing that patients with parietal lesions reported ABMs that lacked episodic richness [Berryhill et al., 2007; Davidson et al., 2008]. With regard to the role of the parietal cortex in episodic memory, the current findings can be interpreted in light of the dual attentional processes hypothesis [Cabeza, 2008; Ciaramelli et al., 2008], which distinguishes between the role of dorsal and ventral parietal regions. More specifically, it proposes that the dorsal parietal region mediates the allocation of attention controlling episodic retrieval depending on the rememberer's goal, providing top‐down attentional support for strategic retrieval processes. In contrast, the ventral parietal region supports stimulus‐driven detection of relevant information retrieved from episodic memory. With regard to the retrieval of episodic ABMs, Conway (2009) suggests that the details of one particular episodic memory (e.g., a wedding ceremony) can be accessed either intentionally via a cue from the contextual frame that encompasses the different event‐specific details (e.g., the spatial context: the church) or incidentally via one detail (e.g., the register one signed). With reference to these frameworks, we hypothesize that, for the remote period, aMCI patients' ability to spontaneously retrieve episodic details depends on their ability to engage top‐down attentional processes – supported by the superior/dorsal parietal regions – serving the retrieval of contextual information that will cue the retrieval of specific sensory‐perceptual details. When general probes are given to encourage participants to elaborate on the initial episodes they have retrieved, the ability to bring more specific details back to mind may depend on the spread of activation from the few details already retrieved to all the other details in the memory representation. The automatic mnemonic detection process, subserved by the inferior/ventral parietal regions and the temporo‐parietal junction would facilitate this operation.
This interpretation of the association between parietal regions and episodic ABMs in terms of attention facilitating cued retrieval in episodic memory is coherent with the finding that the best predictor of the proportion of episodic details for remote memories in aMCI patients was the Remember score in the word‐pair memory task. In this task, when participants recognized the test item, most Remember responses corresponded to the retrieval of the word associated with this item at encoding. Thus, in Remember responses, the test word acted as a cue leading to the reactivation of the associated word, which also reinstates the context (pair) in which the word was studied. Similarly, in remote ABMs, we propose that access to specific details also depends on attention to the retrieval of contextual information and other associated specific details.
An association between the amount of episodic details retrieved for recent memories and activity in right lateral temporal regions was also found for spontaneous recall in healthy controls and an even greater correlation for both spontaneous recall and recall after probing in aMCI patients. The lateral temporal regions belong to the core brain network supporting retrieval of ABMs, but have frequently been related to the retrieval of generic events or general semantic knowledge [Addis et al., 2004; Graham et al., 2003; Piolino et al., 2007; Svoboda and Levine, 2009; Svoboda et al., 2006]. Nevertheless, several lines of evidence indicate that the integrity of the lateral temporal lobes is crucial for accessing episodic ABMs. First, neuropsychological studies have indicated that right‐sided or bilateral temporal lobe lesions lead to deficits in episodic ABMs [Kopelman and Kapur, 2001]. Second, neuroimaging studies isolating memory for event‐specific details have reported the involvement of a temporal region at coordinates very close to those observed in the aMCI group [Addis et al., 2009a; Henson et al., 1999; Piolino et al., 2008]. In particular, using the TEMPau questionnaire, Piolino et al. (2008) found a correlation between the number of Remember responses given by older adults for autobiographical events and activity in the right middle temporal gyrus. Finally, a recent report indicates that, for memories of the last 12 months, the best predictors of the phenomenological characteristics typical of episodic ABMs (e.g., emotional intensity, autonoetic consciousness, mental image quality) in healthy aging were activations in frontal and lateral temporal regions of interest [Viard et al., 2010].
Altogether, the association of episodic ABMs with lateral temporal regions in previous research as well as in the current study likely emphasize the role of semantic knowledge in accessing specific personal episodes. Indeed, in hierarchical models of ABM, the higher level of semantic knowledge gives access to the lower level of sensory‐perceptual episodic memories [Conway and Pleydell‐Pearce, 2000]. In fact, Conway proposed that general events are the preferred level of access to ABM [Conway, 1996]. Thus, participants are assumed to shift through general ABMs until they generate sufficiently specific retrieval cues to elicit the ecphory of an episodic ABM [Tulving, 1983]. The importance of controlled access to semantic information in the retrieval of recent episodic ABMs was also demonstrated in the regression analyses showing that the best predictor of the proportion of episodic details in spontaneously recalled recent memories was the number of errors in the Hayling task. These errors designated the completion of a sentence by a related (usually semantically) word while the instruction was to give an unrelated word that makes the sentence nonsense. Success at this task thus depends on the ability to select relevant information and inhibit irrelevant automatically activated representations. In the case of ABM, we propose that participants who can conduct efficient controlled searches though personal semantic memory are able to retrieve more specifically episodic details.
Finally, the proportion of episodic details retrieved by aMCI patients for recent events after general encouragement to be specific was also related to variable activity in the right temporo‐parietal junction. This region forms part of the core brain network activated during ABM [Svoboda et al., 2006], as well as of the default network [Andrews‐Hanna et al., 2010; Buckner et al., 2008]. We have already referred to a possible role of this region in attentional processes for episodic memory (see above). For an alternative (and not mutually exclusive) functional explanation, recent studies of the architecture of the default network have identified different subsystems of intrinsically connected regions: the medial temporal subsystem, the posterior cingulate‐anterior medial prefrontal subsystem and the dorsomedial prefrontal subsystem. The coordinates of the temporo‐parietal region found here correspond to those belonging to the dorsomedial prefrontal subsystem, the general role of which may be to mediate controlled inference about mental states and perspective taking [Andrews‐Hanna et al., 2010]. A related proposal has associated a supramarginal region very close to the present coordinates with a specific mental state promoting access to episodic information: a “listening for recollection” process [Quamme et al., 2010]. With regard to episodic ABM, it is plausible that the ability to retrieve many specific details is related to the ability to establish an internally directed perspective focusing on the search for episodic details.
Even though aMCI patients exhibited deficient episodic ABM for both remote and recent memories, different cerebral correlates were found for remote and recent episodic ABM (with the exception of occipital regions). It should be noted that this may partly be due to differences in the type of retrieval cues contained in the examiner's questions. For the remote period, participants were given relatively specific cues that easily match the life‐changing events that characterize early adulthood [Fitzgerald, 1988; Robinson, 1992]. For example, when asked to report a specific event concerning “meeting someone,” most participants spontaneously told about their first meeting with their future spouse. In contrast, for the recent period, asking to report on an event that happened last month or last week did not single out any particular event, so the ability to come up with a specific event relied on individual differences in the efficiency of the retrieval search through semantic knowledge, as suggested by the association with metabolism of the lateral temporal regions. This limitation should be overcome in future work by using similar cues for both periods.
In conclusion, the findings confirmed that ABM in aMCI is characterized by a decreased episodic quality. Moreover, our results showed that the degree to which aMCI patients were able to retrieve event‐specific details for ABMs was associated with metabolic activity in specific regions within the autobiographical core brain network. The clusters of correlations were larger in aMCI than in control participants, demonstrating that patients' performance depends on large‐scale functional networks. This included parietal and occipital regions in relation to remote ABM, and temporo‐parietal and occipital regions in relation to recent ABM. Future studies should further examine the role of cognitive processes related to these regions (such as visual imagery, attentional support to memory retrieval, access to personal semantic knowledge and self perspective) in the ability of aMCI patients to reconstruct episodic ABMs.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Suppporting Information Figure 1.
Suppporting Information Figure 2.
Acknowledgements
The authors thank Prof. Pascale Piolino for her very useful information on the TEMPau. They are also grateful to Prof. Brian Levine who provided detailed instructions on the scoring of internal and external details in autobiographical narratives.
REFERENCES
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP ( 2004): Characterizing spatial and temporal features of autobiographical memory retrieval networks: A partial least square approach. NeuroImage 23: 1460–1471. [DOI] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu M‐A, Laiser N, Schacter DL ( 2009a): Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia 47: 2222–2238. [DOI] [PubMed] [Google Scholar]
- Addis DR, Sacchetti DC, Ally BA, Budson AE, Schacter DL ( 2009b): Episodic simulation of future events is impaired in mild Alzheimer's disease. Neuropsychologia 47: 2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Squire LR ( 1994): Memory consolidation and the medial temporal lobe: A simple network model. Proc Natl Acad Sci USA 91: 7041–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés P, Van der Linden M ( 2000): Age‐related differences in supervisory attentional system functions. J Gerontol: Psychol Sci 55B: 373–380. [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL ( 2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65: 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin C, Kerrouche N, Lekeu F, Adam S, Guillaume B, Lemaire C, Aerts J, d'Ydewalle G, Collette F, Salmon E ( 2010): Controlled memory processes in questionable Alzheimer's disease: A view from neuroimaging research. J Alzheimer's Dis 20: 547–560. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR ( 2007): Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. J Neurosci 27: 14415–14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg C, Thomas‐Anterion C, Bogey S, Davier K, Laurent B ( 2010): Visual imagery processing and knowledge of famous names in Alzheimer's disease and MCI. Aging Neuropsychol Cogn 17: 603–14. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network. Ann NY Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T ( 1996): Confabulation and the control of recollection. Memory 4: 359–411. [DOI] [PubMed] [Google Scholar]
- Cabeza R ( 2008): Role of parietal regions in episodic memory retrieval: The dual attentional processes hypothesis. Neuropsychologia 46: 1813–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R St Jacques P ( 2007): Functional neuroimaging of autobiographical memory. Trend Cogn Sci 11: 219–227. [DOI] [PubMed] [Google Scholar]
- Chételat G, Desgranges B, De La Sayette V, Viader F, Berkouk K, Landeau B, Lalevée C, Le Doze F, Dupuy B, Hannequin D, Baron JC, Eustache F. ( 2003): Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain 126: 1955–1967. [DOI] [PubMed] [Google Scholar]
- Chételat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC ( 2002): Mapping gray matter loss with voxel‐based morphometry in mild cognitive impairment. NeuroReport 13: 1939–1943. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. ( 2008): Top‐down and bottom‐up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia 46: 1828–1851. [DOI] [PubMed] [Google Scholar]
- Clare L, Wilson BA, Carter G, Roth I, Hodges JR ( 2002): Assessing awareness in early‐stage Alzheimer's disease: Development and piloting of the memory awareness rating scale. Neuropsychol Rehabil 12: 341–362. [Google Scholar]
- Conway MA ( 1996): Autobiographical memories and autobiographical knowledge In: Rubin DC, editor. Remembering Our Past: Studies of Autobiographical Memories. Cambridge, England: Cambridge University Press; pp 67–93. [Google Scholar]
- Conway MA ( 2009): Episodic memories. Neuropsychologia 47: 2305–2313. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell‐Pearce CW ( 2000): The construction of autobiographical memories in the self‐memory system. Psychol Rev 107: 261–288. [DOI] [PubMed] [Google Scholar]
- Cui X, Jeter CB, Yang D, Montague PR, Eagleman DM ( 2007): Vividness of mental imagery: Individual variability can be measured objectively. Vision Res 47: 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Van der Linden M ( 2006): Individual differences in the phenomenology of mental time travel: The effect of vivid visual imagery and emotion regulation strategies. Conscious Cogn 15: 342–350. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA ( 1980): Individual differences in working memory and reading. J Verbal Learning Verbal Behav 19: 450–466. [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim ASN, Murphy KJ, Troyer AK, Moscovitch M, Levine B ( 2008): Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia 46: 1743–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, Desanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, Clark C, Kerkman D, Debernardis J, Li J, Lair L, Reisberg B, Tsui W, Rusinek H( 2004): MRI and CSF studies in the early diagnosis of Alzheimer's disease. J Inter Med 256: 205–223. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R ( 2004): Optimisation of the 3D MDEFT sequence for anatomical brain imaging: Technical implications at 1.5 and 3 T. NeuroImage 21: 757–767. [DOI] [PubMed] [Google Scholar]
- Desmette D, Hupet M, Van der Linden M ( 1995): Adaptation en langue française du “Reading span test” de Daneman et Carpenter (1980). Annee Psychol 95: 459–482. [Google Scholar]
- Fitzgerald JM ( 1988): Vivid memories and the reminiscence phenomenon: The role of self narrative. Hum Dev 31: 261–273. [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RSJ, Dolan RJ ( 1995): The mind's eye: Precuneus activation in memory‐related imagery. NeuroImage 2: 195–200. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Java RI, Richardson‐Klavehn A ( 1996): How level of processing really influences awareness in recognition memory. Can J Exp Psychol 50: 114–122. [Google Scholar]
- Gardini S, Cornoldi C, De Beni R, Venneri A ( 2006): Left mediotemporal structures mediate the retrieval of episodic autobiographical mental images. NeuroImage 30: 645–655. [DOI] [PubMed] [Google Scholar]
- Graham KS, Lee ACH, Brett M, Patterson K ( 2003): The neural basis of autobiographical and semantic memory: New evidence from three PET studies. Cogn Affect Behav Neurosci 3: 234–254. [DOI] [PubMed] [Google Scholar]
- Guedj E, Barbeau EJ, Didic M, Felician O, de Laforte C, Ranjeva JP, Poncet M, Cozzone PJ, Mundler O, Ceccaldi M ( 2009): Effects of medial temporal lobe degeneration on brain perfusion in amnestic MCI of AD type: Deafferentation and functional compensation? Eur J Nucl Med Mol Imaging 36: 1101–1112. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA ( 2007): Deconstructing episodic memory with construction. Trend Cogn Sci 11: 299–306. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Dolan RJ ( 1999): Right prefrontal cortex and episodic memory retrieval: A functional MRI test of the monitoring hypothesis. Brain 122: 1367–1681. [DOI] [PubMed] [Google Scholar]
- Irish M, Lawlor BA, O'Mara SM, Coen RF ( 2010): Exploring the recollective experience during autobiographical memory retrieval in amnestic mild cognitive impairment. J Int Neuropsychol Soc 16: 546–555. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV ( 2000): Distributed neural systems for the generation of visual images. Neuron 28: 979–990. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Kapur N ( 2001): The loss of episodic memories in retrograde amnesia: Single‐case and group studies. Philos Trans R Soc London Ser B 356: 1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD ( 1989): The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. J Clin Exp Neuropsychol 11: 724–744. [DOI] [PubMed] [Google Scholar]
- Lemaire C, Bamhaut P, Lauricella B, Mosdzianowski C, Morelle JL, Monclus M, Van Naemen J, Mulleneers E, Aerts J, Plenevaux A, Brihaye C, Luxen A ( 2002): Fast [18 F]FDG synthesis by alkaline hydrolysis on a low polarity solid phase support. J Labelled Compd Radiopharm 45: 435–447. [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M ( 2002): Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol Aging 17: 677–689. [PubMed] [Google Scholar]
- Leyhe T, Müller S, Milian M, Eschweiler GW, Saur R ( 2009): Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer's disease. Neuropsychologia 47: 2464–2469. [DOI] [PubMed] [Google Scholar]
- Maguire EA ( 2001): Neuroimaging studies of autobiographical event memory. Philos Trans R Soc London Ser B 356: 1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masdeu JC, Zubieta JL, Arbizu J ( 2005): Neuroimaging as a marker of the onset and progression of Alzheimer's disease. J Neurol Sci 236: 55–64. [DOI] [PubMed] [Google Scholar]
- Mattis S ( 1973): Dementia Rating Scale. Windsor, England: NFER‐Nelson. [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L( 2005): Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account based on multiple trace theory. J Anatomy 207: 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, Troyer AK, Levine B, Moscovitch M ( 2008): Episodic, but not semantic, autobiographical memory is reduced in amnestic mild cognitive impairment. Neuropsychologia 46: 3116–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Negash S ( 2008): Mild cognitive impairment: An overview. CNS Spectr 13: 45–53. [DOI] [PubMed] [Google Scholar]
- Piolino P, Chételat G, Matuszweski V, Landeau B, Mé zenge F, Viader F, De La Sayette V, Eustache F, Desgranges B ( 2007): In search of autobiographical memories: A PET study in the frontal variant of frontotemporal dementia. Neuropsychologia 45: 2730–2743. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Belliard S, Matuszweski V, Lalevée C, De La Sayette V, Eustache F ( 2003): Autobiographical memory and autonoetic consciousness: Triple dissociation in neurodegenerative diseases. Brain 126: 2203–2219. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Benali K, Eustache F ( 2002): Episodic and semantic remote autobiographical memory in ageing. Memory 10: 239–257. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Hubert V, Bernard FA, Matuszweski V, Chételat G, Baron JC, Eustache F ( 2008): Reliving lifelong episodic autobiographical memories via the hippocampus: A correlative resting PET study in healthy middle‐aged subjects. Hippocampus 18: 445–459. [DOI] [PubMed] [Google Scholar]
- Quamme JR, Weiss DJ, Norman KA ( 2010): Listening for recollection: A multi‐voxel pattern analysis of recognition memory retrieval strategies. Front Hum Neurosci 4: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JA ( 1992): First experience memories: Contexts and function in personal histories In: Conway MA, Rubin DC, Spinnler H, Wagenaar WA, editors. Theoretical Perspectives on Autobiographical Memory. Dordrecht: Kluwer; pp 223–240. [Google Scholar]
- Salmon E, Lekeu F, Bastin C, Garraux G, Collette F ( 2008): Functional imaging of cognition in Alzheimer's disease using positron emission tomography. Neuropsychologia 46: 1613–1623. [DOI] [PubMed] [Google Scholar]
- Salmon E, Perani D, Herholz K, Marique P, Kalbe E, Holthoff V, Delbeuck X, Beuthien‐Baumann B, Pelati O, Lespagnard S, Collette F, Garraux G( 2006): Neural correlates of anosognosia for cognitive impairment in Alzheimer's disease. Hum Brain Mapping 27: 588–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL ( 2007): Remembering the past to imaging the future: The prospective brain. Nat Rev Neurosci 8: 657–661. [DOI] [PubMed] [Google Scholar]
- Stoub TR, deToledo‐Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC ( 2006): Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci 103: 10041–10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, Levine B ( 2009): The effects of rehearsal on the functional neuroanatomy of episodic autobiographical and semantic remembering: A functional magnetic resonance imaging study. J Neurosci 29: 3073–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B ( 2006): The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia 44: 2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treyer V, Buck A, Schnider A ( 2006): Selection of currently relevant words: An auditory verbal memory study using positron emission tomography. NeuroReport 17: 323–327. [DOI] [PubMed] [Google Scholar]
- Tulving E ( 1983): Elements of Episodic Memory. Oxford: Oxford University Press. [Google Scholar]
- Viard A, Lebreton K, Chételat G, Desgranges B, Landeau B, Young A, De La Sayette V, Eustache F, Piolino P ( 2010): Patterns of hippocampal‐neocortical interactions in the retrieval of episodic autobiographical memories across the entire life‐span of aged adults. Hippocampus 20: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum V, Huang V, Adey M, Leier O ( 1983): Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: 37–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Suppporting Information Figure 1.
Suppporting Information Figure 2.


