Abstract
Visuospatial neglect is a multicomponent syndrome, and one dissociation reported is between neglect for near (peripersonal) and far (extrapersonal) space. Owing to patient heterogeneity and extensive lesions, it is difficult to determine the precise neural mechanisms underlying this dissociation using clinical methodology. In this study, transcranial magnetic stimulation was used to examine the involvement of three areas in the undamaged brain, while participants completed a conjunction search task in near and far space. The brain areas investigated were right posterior parietal cortex (rPPC), right frontal eye field (rFEF), and right ventral occipital cortex (rVO), each of which has been implicated in visuospatial processing. The results revealed a double dissociation, whereby rPPC was involved for search in near space only, whilst rVO only became necessary when the task was completed in far space. These data provide clear evidence for a dorsal and ventral dissociation between the processing of near and far space, which is compatible with the functional roles previously attributed to the two streams. For example, the involvement of the dorsal stream in near space reflects its role in vision for action, because it is within this spatial location that actions can be performed. The results also revealed that rFEF is involved in the processing of visual search in both near and far space and may contribute to visuospatial attention and/or the control of eye‐movements irrespective of spatial frame. We discuss our results with respect to their clear ramifications for clinical diagnosis and neurorehabilitation. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: spatial processing, transcranial magnetic stimulation, visual search
INTRODUCTION
Visuospatial perception involves understanding the location of visual items, which is important for successful interaction with the environment. Some situations involve having to search for objects which are nearby; for example, looking for a pen on a desk in front of you, whilst other scenarios involve searching further afield, such as for a friend in a busy room. Accordingly, space is not a unitary concept. Peripersonal, or near space, is defined as the immediate space around the body in which arm and hand actions can be used [Rizzolatti et al., 1983]. Extrapersonal, or far space, refers to that which extends beyond this and which requires walking in order to reach a target. Evidence from neuropsychology suggests that the perception of near and far space may involve separable neural processes [e.g., Berti and Rizzolatti, 2002]. This work investigates this issue in the undamaged brain.
Neglect is a disorder characterized by a deficit in the ability to orient attention toward the contralesional (usually the left) side of space [Heilman et al., 1983]. It is widely considered to be a multicomponent syndrome [Bisiach and Vallar, 2000; Halligan et al., 2003; Husain and Rorden, 2003], and, as such, patients can experience impairments in visual, auditory, tactile, and motor abilities [Bisiach et al., 1984; Laplane and Degos, 1983; Pierson‐Savage et al., 1988] as well as perceptual or representational space [Bisiach and Luzzatti, 1978]. Furthermore, some individuals present with neglect that is restricted to near space [Berti and Frassinetti, 2000; Halligan and Marshall, 1991; Mennemeier et al., 1992], whilst others show the reverse pattern [Cowey et al., 1994, 1999; Vuilleumier et al., 1998]. However, there are also patients who present with neglect in the absence of any distance modulation effects [Pizzamiglio et al., 1989]. For a review of near and far space dissociations in neglect, see Berti and Rizzolatti [ 2002].
Neglect is most frequently (although not exclusively) associated with lesions to right posterior parietal cortex [rPPC; Driver and Mattingley, 1998; Halligan et al., 2003; Mort et al., 2003], which is one of the primary components of the frontoparietal network of attention [Corbetta, 1998; Corbetta and Shulman, 2002; Gitelman et al., 1999]. However, brain areas such as the superior temporal gyrus have also been implicated in neglect [Karnath et al., 2001, 2004]. More specifically, dorsal visual system areas such as rPPC are associated with near space neglect [Halligan and Marshall, 1991; Previc, 1990]. However, patients with neglect often present with extensive lesions, and there is a high degree of lesion heterogeneity across this population. Furthermore, patients may develop compensatory behaviors. Consequently, it can be difficult to determine precisely which brain areas are involved in the processing of near and far space on the basis of clinical investigation alone.
In addition to rPPC being implicated in neglect, Cowey et al. [ 1994] reported that at least three of their five patients who demonstrated greater neglect for far space had damage to the right frontal eye field (rFEF), an area which when ablated in monkeys results in attentional deficits that are more severe in far space than they are in near space [Rizzolatti et al., 1983]. This suggests a role for rFEF in the processing of attention within far space. However, it is unlikely to be the sole contributing area for far space processing, because areas within the ventral visual stream, such as right ventral occipital cortex (rVO), have also been identified as important in attention to far space [Bjoertomt et al., 2002; Vuilleumier et al., 1998; Weiss et al., 2000].
A second problem with the neuropsychological work is that the majority of studies examining visual attention within near and far space have focused on line bisection (or its variants), despite reports that it is not the most reliable clinical predictor of neglect [Ferber and Karnath, 2001; Halligan et al., 1989]. Indeed, different tasks that assess visuospatial attention draw on a variety of cognitive and neural resources [Ellison et al., 2004], and patients with neglect can show deficits on some tasks (i.e., item cancellation) and not others [i.e., line bisection; Binder et al., 1992]. Therefore, one question to consider is whether or not the dissociation between near and far is consistent across tasks. Vuilleumier et al.'s [ 1998] patient presented with neglect for far but not near space on six different tasks, including line bisection, reading, and item cancellation. However, such task consistency was not supported by the findings of Keller et al. [ 2005], whose patients presented with more severe neglect in far space when assessed using a line bisection task, but who did not demonstrate any distance effects on an item cancellation task. If a task requires a directional motor response (like line bisection or item cancellation tasks do), then perceptual and motor effects may be confounded [Bisiach, 1993; Bisiach et al., 1990]; an error could reflect an impairment in orienting visual attention toward contralesional space or in making movements toward such locations. Much research has been conducted to try and dissociate the contribution of perceptual and motor effects [Bisiach et al., 1990; Harvey et al., 2002a], and various tasks have been designed to try and overcome this confounding such as landmark and pulley device tasks (see Harvey [ 2004] for a review). We feel that it is important to examine perceptual visuospatial processes using a task that does not rely on a potentially confounding motor response, and visual search is one such task.
The classic visual search paradigm [Treisman and Gelade, 1980], in addition to not requiring a directional response, is a valid and reliable measure of visuospatial attention that requires naturalistic scanning behavior. Visual search tasks involve bottom‐up perceptual processes as well as top‐down components such as target memory. The additional advantages that such tasks can offer include being able to use a wide variety of arrays and the ability to measure both speed and accuracy. Consequently, visual search may be more sensitive to subtle changes in visuospatial attention than alternatives such as line bisection or the purely perceptual landmark version of the task used by Bjoertomt et al. [ 2002]. Given these issues and the fact that line bisection and visual search involve at least partly different neural processes [Ellison et al., 2004], it is likely that the performance of patients with neglect on line bisection tasks in near and far space do not necessarily predict their performance on visual search tasks across the two spatial domains. It is therefore important to examine the neural mechanisms with regard to this particular paradigm.
Patients with neglect can show impaired performance on visual search, with reduced accuracy and slower responses and clear left/right asymmetries in search behavior [Behrmann et al., 2004; Harvey et al., 2002b]. Although there is some disagreement regarding the ability of patients with neglect to perform feature search, in which the target and distractors differ with respect to a single feature, there is a general consensus that such patients are impaired at conjunction search, whereby the target is defined by a combination of features [Aglioti et al., 1997; Eglin et al., 1989; Esterman et al., 2000; Harvey et al., 2002b; Pavlovskaya et al., 2002]. Neuroimaging studies have consistently revealed both rPPC and rFEF to be involved in visual search [Donner et al., 2000, 2002; Nobre et al., 2003], and transcranial magnetic stimulation (TMS) has demonstrated the importance of both areas in the control of conjunction search [Ellison et al., 2003; Lane et al., in press; Muggleton et al., 2003, 2008]. However, to date, this has only been investigated for near space, typically with the search arrays presented on a computer monitor.
To summarize, rPPC, rFEF, and rVO have all been implicated in the control of visuospatial attention to some extent, but it remains unclear how these different areas specifically contribute to this process. As described earlier, the clinical results are inconsistent across different behavioral tasks, some paradigms fail to distinguish between perceptual and visuomotor problems, and the reliance on clinical studies means that the critical brain areas cannot always be identified. The aim of the present study was therefore to examine the involvement of the three brain areas using the same behavioral paradigm, namely visual search, for stimuli presented in near and far space. This was achieved by using TMS to briefly disrupt underlying cortex, thereby allowing the necessary involvement of these brain regions to be assessed in a controlled manner. Although the involvement of rPPC and rFEF has previously been demonstrated for near visual search [Ellison et al., 2003; Muggleton et al., 2003, 2008], this study extends previous work by investigating whether processing differences emerge between the two areas when this task is performed in far space. Given that rFEF has been associated with attention processing in far space [Rizzolatti et al., 1983], it is possible that TMS may also impair performance in the far space condition. The specific involvement of rVO in visual search has not previously been examined. As rVO is an area in the ventral stream, evaluating the effect of TMS to this area allows us to investigate the proposal that the ventral visual stream is selectively concerned with attentional processes involving far space [Previc, 1990].
MATERIALS AND METHODS
Participants
Thirty‐six neurologically healthy participants (16 males and 20 females) aged between 18 and 52 years (median: 26 years) participated. All had normal or corrected‐to‐normal vision. They gave their signed informed consent in accordance with the Declaration of Helsinki and with the approval of Durham University Ethics Advisory Committee and could withdraw at any point. Participant selection complied with the current guidelines for repetitive TMS research [Rossi et al., 2009]. Each of the three experimental conditions (in which TMS was delivered to a different stimulation site) was carried out by 12 participants. Stimulation site was chosen as a between‐subjects variable in order to reduce the effects of practice. The participants in the different groups did not differ significantly with regards to sex [χ2(2, N = 36) = 0.90, P = 0.638] with six males and six females in both the rFEF and rVO groups and four males and eight females in the rPPC group. The mean age of the participants was 25 years (SD = 4) for the rPPC group, 32 years (SD = 11) for the rFEF group, and 32 years (SD = 8) for the rVO group. There was no significant difference in the age of participants across the three groups [F (2,33) = 1.65, P = 0.207].
Stimuli and Procedure
The task involved participants deciding as quickly and accurately as possible whether the target stimulus was present in the search array (see Fig. 1). E‐Prime (Psychology Software Tools, Pittsburgh, PA) was used to program the visual search displays and to remotely trigger the TMS. Participants were instructed to fixate a white central cross (0.5° × 0.5° of visual angle), which was presented for 500 ms at the start of each trial. This was followed immediately by the presentation of the search array, which remained present until the participant made a button‐press response. Participants were asked to respond with their right hand, which was ipsilateral to the stimulation sites, using their index and middle fingers for the two choices (present and absent, respectively). The intertrial interval was 4,000 ms, during which time a blank black screen was presented.
Figure 1.
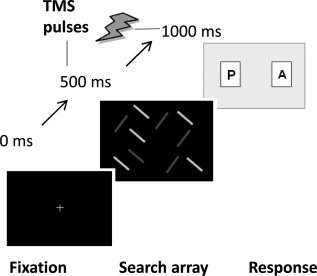
Diagram depicting the trial progression. A central fixation cross was presented for 500 ms, followed immediately by the 10‐item search array. This array remained present until the participant responded with a button‐press (Present or Absent), which could be at any time after display onset. TMS was delivered at 10 Hz for 500 ms from the beginning of array onset.
The search array consisted of 10 items: nine distractors plus the target (target‐present condition) or 10 distractors (target‐absent condition). The display area, which subtended ∼32° × 20° of visual angle, comprised a 10 × 6 virtual grid. Each item (target and distractors) was ∼2.5° of visual angle in length, and the items were presented at random locations within the virtual grid. It was only possible for each position to be occupied by one item in order to prevent overlap. The target was a green backward‐slash (\), and the distractors were green forward‐slashes (/) and red backward‐slashes. All stimuli were presented against a black background and matched for photometric luminance within and between items across the display. The target was present in 50% of the trials, and there was never more than one target. On each trial, the search items were equally distributed across the two hemifields, with the target appearing in each hemifield equally often. Participants were free to move their eyes whilst searching.
The search task was completed under two conditions of distance: near and far. In the near condition, the search arrays were presented on a CRT computer monitor with a 75 Hz refresh rate, which was positioned ∼57 cm away from where the participants were seated. In the far condition, the stimuli were presented onto a blank white screen using a Epson EMP‐74 projector, and the participants were seated ∼172 cm from the display. The displays in both conditions subtended the same visual angle to ensure that their retinal size was identical irrespective of viewing distance. In each condition, the participant's head and trunk sagittal midline was aligned with the center of the display. Participants were encouraged to remain as still as possible in order to maintain a stable viewing distance.
There were three conditions for TMS site: TMS was applied to rFEF, rPPC, or rVO. Participants completed one site condition each to minimize the effect of practice. Each participant completed eight blocks of trials, with 40 trials per block; 20 target‐present and 20 target‐absent. These eight blocks included four blocks of trials in the near condition and four in the far condition, half of which was completed with TMS and the other half with sham‐TMS. The TMS and sham‐TMS blocks were interleaved, with half of the participants starting with TMS. The testing session for each individual lasted no longer than 1.5 h.
A pilot study (n = 6) was conducted in which the visual search task was performed at the two distances (near and far) and with three different set‐sizes (4, 8, and 12 items). This allowed the slope of the response time (RT) function over the display size to be calculated (RT slope), which can be used as a measure of the involvement of attention. According to the Feature Integration Theory [Treisman and Gelade, 1980], search is defined as parallel when the RT slope is shallow (<10 ms/item), which indicates that the number of distractors have a minimal effect on target identification/localization. Parallel search is therefore considered to be conducted in a preattentive manner. Conversely, in serial search, the RT increases with the number of distractors resulting in search rates >10 ms/item. Treisman and Gelade suggested that as identifying a target in a conjunction search requires combining different visual features, an attentional process must be performed for each item sequentially until the target is located. The pilot data confirmed that the task was always performed in a serial manner [the mean search functions were 28.36 ms/item (SD = 17.64) for near and 29.86 ms/item (SD = 16.33) for far space]. Importantly, the search rates in near and far space were not significantly different [t (5) = −0.53, P = 0.620], indicating comparable attentional demands in each condition.
TMS and Site Localization
Five pulses of transcranial magnetic stimulation (TMS) were delivered at 10 Hz to either rPPC, rFEF, or rVO at visual array onset using a Magstim™ Rapid (Magstim, Whitland, Carmarthenshire, UK) at 65% of the maximum machine output (i.e., 1.3 T). TMS was applied over one area of interest (rPPC, rFEF, or rVO; Fig. 2). Each participant's skull was co‐registered with their own MRI brain scan using BrainSight™ frameless stereotaxic software (Rogue Research, Montreal, Quebec, Canada) to confirm the anatomical locus of the stimulation. The rFEF site was located anatomically and determined as the intersection of the precentral and superior frontal sulci, a location that has repeatedly been used with TMS and confirmed as a functional locus [Grosbras and Paus, 2002; Paus, 1996; Ro et al., 1999]. The rVO site was determined using the averaged scalp co‐ordinates reported by Bjoertomt et al. [ 2002], who also used this same site to examine near and far space processing. They stated that for an inion–nasion distance of 35 cm, VO is located 1.5 cm dorsal and 2.25 cm lateral to the inion. As the parietal region is large, and the precise locus of involvement varies across subjects, in the case of rPPC, we used a method of localization that examined functional effects (see Sack et al. [ 2009] for a discussion of the relative merits of localization methodologies). The rPPC sites were then verified using frameless stereotaxy. We therefore functionally localized this site using the conjunction search hunting procedure first described by Ashbridge et al. [ 1997]. This meant that the area of cortex stimulated was the region within rPPC that was specifically involved in the processing of conjunction search, which was the experimental task. Briefly, the procedure involved 10 trials of TMS being given to each site in a 3 × 3 matrix, with each adjacent point 1 cm apart. The central point was located 9 cm dorsal to the mastoid inion and 6 cm lateral. The selected site was the one which demonstrated an ∼100 ms increase in RT relative to no‐TMS trials. The anatomical location of this site was confirmed as being consistently located in the angular gyrus using BrainSight. For each brain area, once the site was established, the position was recorded and marked with a sticker on a tightly fitting lycra swimming‐cap.
Figure 2.
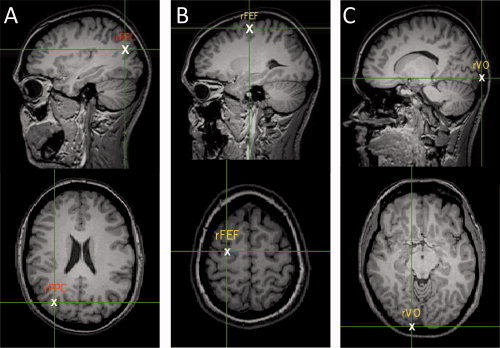
Diagram showing the averaged location of each of the stimulated sites: rPPC (MNI co‐ordinates: x = 30, y = −70, z = 28 mm), rFEF (x = −493, y = 183, z = −34 mm), and rVO (x = 28, y = −95, z = 5 mm). The position was verified using each participants' MRI scan co‐registered to their skull co‐ordinates using BrainSight™ software. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For rPPC stimulation, a 70‐mm figure‐of‐eight coil was placed tangential to the skull, with the handle pointing backward, parallel to the mid‐sagittal plane. A 50‐mm figure‐of‐eight branding iron coil was used to stimulate rFEF and rVO, and angle/orientation of the coil was adjusted for each individual to prevent any unwanted peripheral nerve stimulation or eye‐blinks. The coil was held in place by the experimenter. In the sham‐TMS blocks, a discharging coil was placed in close proximity to the participant, whilst an inactive coil was positioned over the relevant site. Therefore, the subjective sensation of coil position and auditory effects was comparable to those experienced in the TMS blocks, but no stimulation was delivered.
Statistical Analyses
The mean target‐present RT was subjected to a 2 (Distance: near vs. far) × 2 (TMS: TMS vs. sham‐TMS) × 3 (Site: rPPC, rFEF, and rVO) mixed‐design ANOVA, with stimulation site as the between‐subjects factor. To further examine the interaction effects revealed by this ANOVA, 2 (Distance) × 2 (TMS) ANOVAs were conducted for each site separately, and paired‐samples t‐tests were then performed as appropriate. These t‐tests were adjusted for multiple comparisons using a Bonferroni correction, resulting in a corrected α‐level of 0.025.
RESULTS
Analysis was only concerned with RT for the target present trials, where the decision to respond is initiated by locating the target. Target absent trials involve an added cognitive and neurological component related to the decision to terminate the search [Van Zandt and Townsend, 1993]. Therefore, to examine the involvement of our regions of interest in the response to targets, uncomplicated by extra substrates (see Ashbridge et al. [ 1997]), only target present responses were analyzed. Incorrect responses accounted for less than 5% of the data (mean accuracy was 95.11%), and these trials were removed from the RT analyses. Preliminary analyses revealed no significant interaction effects between the TMS and target location (hemifield), and the data were therefore combined across the two sides of target presentation in order to increase statistical power. Friedman tests were performed on the accuracy data for each of the conditions and no significant effects were found (P > 0.322). Consequently, any effects for RT were not associated with a speed‐accuracy trade‐off.
The initial 2 × 2 × 3 mixed‐design ANOVA revealed a significant main effect of TMS [F (1, 33) = 21.03, P < 0.001], such that RT was increased in the TMS condition relative to sham‐TMS. The three‐way interaction effect between the within‐subjects variables of Distance and TMS and the between‐subjects variable Site was also significant [F (2, 33) = 6.87, P = 0.003]. This indicates that the effect of TMS on performance when the task is completed at different viewing distances is modulated by the site of the stimulation. Further analyses are reported below which examine this interaction. All other main effects and interactions revealed by this ANOVA were nonsignificant (P > 0.068). Importantly, the main between‐subjects effect of Site was nonsignificant [F (2,33) = 0.12, P = 0.890], thus showing that the performance of participants across the groups was not significantly different.
rPPC
The results of the 2 × 2 repeated‐measures ANOVA revealed that the main effect of Distance was nonsignificant [F (1,11) = 3.15, P = 0.104]; performance was comparable across the two distances. However, there was a significant main effect of TMS [F (1,11) = 7.10, P = 0.022]: mean RT was significantly greater in the TMS condition relative to the sham‐TMS condition (Fig. 3a). Of particular importance is the finding that the interaction between the variables Distance and TMS was significant [F (1,11) = 5.98, P = 0.033]. A significant increase in mean RT with TMS (M = 934.56, SD = 40.11) relative to sham‐TMS (M = 870.96, SD = 35.26) was found for the near space condition [t (11) = −3.43, P = 0.006]. When the task was performed in far space, there was no significant difference in mean RT between the TMS (M = 887.61, SD = 38.72) and sham‐TMS conditions [M = 870.83, SD = 34.72; t (11) = −0.98, P = 0.350].
Figure 3.
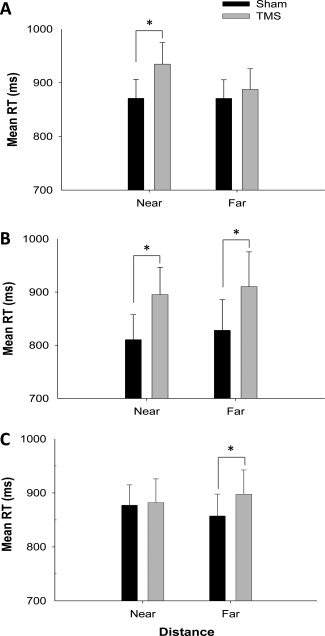
Graphs showing the mean RT (in ms) for each condition of TMS and distance. There are different graphs for each of the three stimulation sites: rPPC (A), rFEF (B), and rVO (C). Error bars represent the SEM across participants and an asterisk indicates a significant difference (P < 0.025).
rFEF
The results of the 2 × 2 ANOVA showed that there was no significant main effect of Distance [F (1,11) = 0.73, P = 0.411]: RT was not different when the task was completed in both near and far space. The main effect of TMS was however significant [F (1,11) = 10.63, P = 0.008], with the mean RT being longer in the TMS relative to the sham‐TMS condition (Fig. 3b). The interaction effect between distance and TMS was not significant [F (1,11) = 0.03, P = 0.871], indicating that the disruptive effect of the TMS on performance was comparable in both conditions of distance (see Fig. 3b). The mean RT for the TMS condition (M = 895.11, SD = 51.43) was significantly greater than in the sham‐TMS condition (M = 810.60, SD = 46.92) for near space [t (11) = −3.008, P = 0.012]. Similarly, mean RT was also significantly increased in the TMS condition (M = 910.32, SD = 65.34) relative to the sham‐TMS condition (M = 828.24, SD = 57.51) for far space [t (11) = −3.292, P = 0.007].
rVO
The 2 × 2 repeated‐measures ANOVA for the stimulation site of rVO revealed nonsignificant main effects of both Distance [F (1,11) = 0.05, P = 0.835] and TMS [F (1,11) = 3.81, P = 0.077]. There was however a significant interaction effect between Distance and TMS [F (1,11) = 7.77, P = 0.018]. Further investigation revealed that the difference in RT between the sham‐TMS (M = 877.25, SD = 130.34) and TMS conditions (M = 881.89, SD = 152.75) was not significant for near space [t (11) = −0.31, P = 0.766], whereas, in far space, TMS (M = 897.47, SD = 156.22) significantly increased mean RT relative to sham‐TMS [M = 857.39, SD = 139.80; t (11) = −3.79, P = 0.003; Fig. 3c].
DISCUSSION
Our aim was to investigate if there are separate neural processes for near (peripersonal) space and for far (extrapersonal) space. TMS was used to examine the involvement of three different brain areas (rPPC, rFEF, and rVO) in the processing of a conjunction visual search task when it was presented in both near and far space. The observation that rFEF is involved in near space conjunction visual search is in accordance with previous neuroimaging [Donner et al., 2000, 2002] and TMS studies (Ellison et al., 2003; Muggleton et al., 2003, 2008]. Our finding that this involvement in search processing in neurologically healthy participants extends to cover far space is novel. It does however support previous research, which reported on the basis of surgical ablations in monkeys, that rFEF was necessary for orientation within far space [Rizzolatti et al., 1983]. The results here furthermore revealed a double dissociation between rPPC and rVO: rPPC was specifically involved in visual search presented in near but not far space, whilst disrupting the processing of rVO with TMS impaired performance only in the far condition. These findings provide confirmatory evidence for the dissociation between the dorsal and ventral visual systems, such that the former is involved in processing attention for near space with the latter processing far space [Bjoertomt et al., 2002; Previc, 1990; Vuilleumier et al., 1998; Weiss et al., 2000].
To our knowledge, this is the first study demonstrating the important involvement of rVO in conjunction visual search in far space and also that the involvement of rPPC to visual search is limited to near space. Previous research has primarily focused on the role of different brain areas in near and far space with regards to line bisection tasks; for example, a neuroimaging study revealed increased activation within rVO (alongside other areas that form the ventral visual stream) for far space line bisection [Weiss et al., 2000]. Furthermore, Bjoertomt et al. [ 2002] examined the involvement of both rVO and rPPC (amongst other brain areas) in perceptual line bisection (i.e., a form of landmark task) and reported that in neurologically healthy participants, TMS over rPPC induced a rightward shift in the perceived midpoint of a line in near space, whilst TMS over rVO induced this effect for far space judgments. The present findings confirm this same dorsal/ventral dissociation for a conjunction search task. In contrast to tasks involving directional response indicators, in the present study, TMS affected reaction times to targets with nondirectional response indicators appearing in the left and right hemifields equally, an issue previously investigated by Schindler et al. [ 2008].
It is the potential ability that the viewer has to interact with their environment that distinguishes near and far space; viewers can only directly act upon items that are presented within near space. Because the dorsal visual system has been attributed the role of perception for action [Goodale and Milner, 1992; Milner and Goodale, 1995], it might seem logical to assume that this stream has a preference for processing near actionable space. The results not only confirm the role of rPPC in visual search in near space [Ellison et al., 2003; Muggleton et al., 2008] but also show that rPPC is not involved in the same task when the stimuli are beyond arm's reach. The findings are also in accordance with electrophysiological research that has revealed activation within the parietal cortex of monkeys in response to stimuli that are close to the monkey, but not when they are more than 1 m away [Leinonen et al., 1979].
One proposed role for rPPC is in the coding and processing of visuospatial attention [Driver and Vuilleumier, 2001]. Previously, we demonstrated a significant involvement of rPPC in feature search only when an explicit motor response was required [Lane et al., 2011], suggesting that this area is necessary for resolving spatial ambiguity to enable successful interaction with the environment. Here, we find that the same brain area is necessary for purely perceptual conjunction search, meaning that there are no explicit or directional motor demands that would have otherwise explained the involvement of rPPC. Our finding that the involvement of rPPC within near space is not dependent upon any motor components is in line with previous reports [Pitzalis et al., 2001; Vuilleumier et al., 1998; Weiss et al., 2003]. It appears that the role of rPPC is in the orienting of visuospatial attention within actionable space, even if motor action is not required.
The ventral visual stream is associated with recognition and the representation of objects and scenes [Goodale and Milner, 1992; Milner and Goodale, 1995]. As the present study shows, rVO has a preference for processing far space information (see also Bjoertomt et al. [ 2002] and Weiss et al. [ 2000]). Ventral stream areas may be more important for far space where people are more reliant on the visual modality for recognition, because tactile cues are not available to assist with this process. However, the visual search task used was solely perceptual, meaning not only were motor responses not required but there was no tactile or proprioceptive feedback either this therefore cannot account for the differences seen between the dorsal and ventral streams in near and far space.
Importantly, we controlled for viewing angle across the near and far conditions in this study; the items always subtended the same visual angle, and thus effects could be compared across each condition. This thereby avoided problems that made the interpretation of some past studies more difficult [Butler et al., 2004, 2009]. By controlling for viewing angle, we can exclude the possibility that the near‐far dissociations between rPPC and rVO are caused by differences in target salience, search area, or item density for example, factors that can influence search performance in both healthy individuals and those with hemispatial neglect [Drury and Clement, 1978; Eglin et al., 1994]. Similarly, matching visual angle meant that the search arrays extended into the upper and lower portions of the visual field to an equivalent extent in both conditions, and, thus, the upper field bias previously reported for visual search [Previc, 1996; Previc and Blume, 1993] should not have influenced the results.
Because the viewing angle was the same in both the near and far space conditions, the same saccade metrics would be required to search the array and locate the target in both. This could explain why rFEF appears to be equally involved for near and far space. Previous studies using TMS have demonstrated that it is possible to interfere with the preparation of eye‐movements by stimulating rFEF [Müri et al., 1991; Thickbroom et al., 1996]. Because rFEF is involved in the production of saccades, a process that can (although does not have to) dissociate from the process of shifting attention [Juan et al., 2004, 2008; Schall, 2004; Wardak et al., 2006], it is possible that the TMS interfered with the production of searching eye‐movements or the saccadic localization of the target that might precede identification in visual search regardless of viewing distance.
An alternative explanation for the role of rFEF in visual search is that it is involved in controlling spatial attention [Grosbras and Paus, 2002; Smith et al., 2005; Szczepanski et al., 2010]. Related to this explanation is the possibility that rFEF is necessary for computing the salience of items within the search array [Thompson and Bichot, 2005], which may be crucial for target selection. The results presented here suggest that perhaps rFEF's salience map does not distinguish near and far. Juan and colleagues [ 2008] reported early and late stages of FEF involvement, with visual selection preceding saccade preparation. In this study, a relatively long TMS duration was used (500 ms), and therefore it is possible that stimulation disrupted both aspects: visual selection and saccade production.
However, while one cannot conclusively exclude the possibility that the effects of TMS over rFEF were due to eye‐blinks, the risk that this had a functional effect is miniscule. Recent research using high‐speed video recording has revealed that stimulation over the occipital pole can induce blinking and full covering of the pupil by the eyelids without participant awareness of this [Corthout et al., 2011]. Such effects have not been directly reported with rFEF, and, furthermore, Corthout and colleagues used much higher stimulation intensity (1.8–2 T) when compared with this study (1.3 T). Despite this, the stimulation used could be sufficient to induce eye‐blinks that may then interfere with the task performance and would have an effect across both distance conditions. We did attempt to prevent this however: the orientation of the coil was adjusted on an individual basis in order to minimize unwanted peripheral nerve stimulation or blinking. Also, none of the participants reported experiencing any such effects, although neither did the participants in the Corthout et al. [ 2011] study, and so we are aware that this does not negate the possibility of blinking occurring to some extent. Because we did not monitor this behavior explicitly, it is possible that minor facial twitching, including small eye‐blinks, could have influenced the results to a minor degree.
Both rPPC and rFEF have been connected with orienting of attention, as discussed earlier, yet the two areas dissociate with regard to their involvement in far space processing. This could be associated with how the spatial information processed is used to guide interaction with the visual environment. The two brain regions may act as attention orienting systems that contribute to different motor control systems: upper limb and eye, respectively [Sakata and Kusunoki, 1992]. Whilst arm or hand movements can only be used in near space, there is no limit to the space in which eye‐movements are useful.
Our results highlight the importance of considering space when both interpreting experimental data and conducting patient assessments. Research examining visual perception is frequently conducted using tasks in near space, but this may not reveal the full extent of the neural mechanisms involved in such processing. Similarly, clinicians may fail to identify patients with visuospatial deficits if performance is not routinely assessed for both near and far space or may fail to appreciate if a patient presents with such a dissociation. Clearly, it is important to recognize the particular deficits that a patient has in order to tailor their rehabilitation.
With regards to future rehabilitation, it may be possible to identify novel means of compensation using the intact pathways responsible for near or far space processing for a particular task; for example, by manipulating the boundaries of near and far space. There is evidence that the boundary between near and far space is flexible. If the reach of a patient is extended by getting them to hold a tool (i.e., a stick), then this can introduce spatial deficits into far space situations, which are otherwise restricted to the near space condition when a tool that does not physically extend reach (i.e., a light pen) is used to point [Berti and Frassinetti, 2000; Maravita et al., 2001]. Such findings that tool use can affect perceptual judgments have also been observed in neurologically healthy individuals [Longo and Lourenco, 2006]. Furthermore, adding weights to the arms of participants during pointing can “shrink” their near space perception [Lourenco and Longo, 2009]. Keller et al. [ 2005] observed that the extent of patients' neglect was not only influenced by the space the task was performed in (near or far) but also by the frame of reference required, namely allocentric or egocentric. Patients were more impaired on a line bisection task when it was performed in far space compared to when it was completed in near space. However, this distance dissociation was not observed in an item cancellation task: their performance was equally poor in both spatial domains. Keller and colleagues proposed that the two tasks rely on different frames of reference. It could be argued that line bisection requires an allocentric frame of reference (i.e., judgments are made relative to another item in the visual array, namely, the two halves of the line) while the cancellation task requires egocentric processing since the location of the items to be crossed out are coded relative to the observer [Burgess et al., 2004; Rains, 2002]. This suggests that if the cancellation task was to require allocentric instead of egocentric processing then the patients' deficits may be reduced for this task in near space.
These studies indicate that space can be instantly remapped according to the use of additional sources of information. Therefore, perhaps, redefining space would be beneficial for understanding the neural processes underlying perception: rather than considering near/far space, space could be defined according to the action possibilities. Likewise, the proposal that vision for perception relies on allocentric coding and vision for action relies on egocentric coding [Galati et al., 2000; Milner and Goodale, 1995, 2006] means that the frame of reference that a particular task requires should also be taken into account. This is in line with the evidence suggesting that both visual streams are capable of processing attention relating to near and far space, with the systems being biased by the current potential for interaction.
It is likely that there are many more brain areas involved in the control of visuospatial attention within near and far space than rFEF, rPPC, and rVO. Numerous other areas have been associated with attention and neglect, including, amongst others, the right superior temporal gyrus [Karnath et al., 2001, 2004], right temporal‐parietal junction [TPJ; Corbetta et al., 2005], and right ventral frontal cortex [VFC; Corbetta et al., 2005; Rengachary et al., 2011]. It would be worthwhile examining the involvement of these additional areas in visuospatial attention in near and far space in future research, and we are aware that researchers in other laboratories are currently investigating the role of areas such as VFC and TPJ in spatial processing. Given the possible role for right VFC in mediating dorsal–ventral interactions [He et al., 2007; Rengachary et al., 2011], then this area would be of particular interest to investigate with regards to the issue of near and far space.
CONCLUSION
In conclusion, our findings demonstrate that different brain regions are responsible for the processing of a classic conjunction visual search task when it is performed in near and far space. One area, rPPC, was preferentially involved in directing visual attention within near space, whilst conversely rVO was important for the far space condition only. The double dissociation between rPPC and rVO is in accordance with previous data, suggesting a dorsal/near and ventral/far dissociation, and the current findings extend this idea to the processing of conjunction search. The two visual streams are capable of processing visuospatial attention, but there appears to be a bias depending on whether or not stimuli are presented within actionable space. A further area examined, rFEF, lacked such specificity, being involved in the task regardless of distance. This area is important for the performance of conjunction search and may play a role in either the orientation of attention or the control of saccades.
REFERENCES
- Aglioti S, Smania N, Barbieri C, Corbetta M ( 1997): Influence of stimulus salience and attentional demands on visual search patterns in hemispatial neglect. Brain Cogn 34: 388–403. [DOI] [PubMed] [Google Scholar]
- Ashbridge E, Walsh V, Cowey A ( 1997): Temporal aspects of visual search studied by transcranial magnetic stimulation. Neuropsychologia 35: 1121–1131. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Ebert P, Black SE ( 2004): Hemispatial neglect and visual search: A large scale analysis. Cortex 40: 247–263. [DOI] [PubMed] [Google Scholar]
- Berti A, Frassinetti F ( 2000): When far becomes near: Remapping of space by tool use. J Cogn Neurosci 12: 415–420. [DOI] [PubMed] [Google Scholar]
- Berti A, Rizzolatti G ( 2002): Coding near and far space In Karnath H‐O, Milner D, Vallar G, editors. The Cognitive and Neural Bases of Spatial Neglect. Oxford, UK: Oxford University Press; pp 119–129. [Google Scholar]
- Binder J, Marshall R, Lazar R, Benjamin J, Mohr JP ( 1992): Distinct syndromes of hemineglect. Arch Neurol 49: 1187–1194. [DOI] [PubMed] [Google Scholar]
- Bisiach E ( 1993): Mental representation in unilateral neglect and related disorders: The twentieth Bartlett Memorial Lecture. Q J Exp Psychol A 46: 435–461. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Cornacchia L, Sterzi R, Vallar G ( 1984): Disorders of perceived auditory lateralization after lesions of the right hemisphere. Brain 107: 37–52. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Geminiani G, Berti A, Rusconi ML ( 1990): Perceptual and premotor factors of unilateral neglect. Neurology 40: 1278–1281. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Luzzatti C ( 1978): Unilateral neglect of representational space. Cortex 14: 129–133. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Vallar G ( 2000): Unilateral neglect in humans In Boller F, Grafman J, editors. Handbook of Neuropsychology, 2nd ed. Amsterdam, the Netherlands: Elsevier; Vol. 1, pp 459–502. [Google Scholar]
- Bjoertomt O, Cowey A, Walsh V ( 2002): Spatial neglect in near and far space investigated by repetitive transcranial magnetic stimulation. Brain 125: 2012–2022. [DOI] [PubMed] [Google Scholar]
- Burgess N, Spiers HJ, Paleologou E ( 2004): Orientational manoeuvres in the dark: Dissociating allocentric and egocentric influences on spatial memory. Cognition 94: 149–166. [DOI] [PubMed] [Google Scholar]
- Butler BC, Eskes GA, Vandorpe RA ( 2004): Gradients of detection in neglect: Comparison of peripersonal and extrapersonal space. Neuropsychologia 42: 346–358. [DOI] [PubMed] [Google Scholar]
- Butler BC, Lawrence M, Eskes GA, Klein R ( 2009): Visual search patterns in neglect: Comparison of peripersonal and extrapersonal space. Neuropsychologia 47: 869–878. [DOI] [PubMed] [Google Scholar]
- Corbetta M ( 1998): Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proc Natl Acad Sci USA 95: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A ( 2005): Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci 8: 1603–1610. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Corthout E, Hallett M, Cowey A ( 2011): TMS‐induced blinking assessed with high‐speed video: Optical disruption of visual perception. Exp Brain Res 210: 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey A, Small M, Ellis S ( 1994): Left visuo‐spatial neglect can be worse in far than in near space. Neuropsychologia 32: 1059–1066. [DOI] [PubMed] [Google Scholar]
- Cowey A, Small M, Ellis S ( 1999): No abrupt change in visual hemineglect for near to far space. Neuropsychologia 37: 1–6. [DOI] [PubMed] [Google Scholar]
- Donner T, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt S ( 2000): Involvement of the human frontal eye field and multiple parietal areas in covert visual selection during conjunction search. Eur J Neurosci 12: 3407–3414. [DOI] [PubMed] [Google Scholar]
- Donner T, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt S ( 2002): Visual feature and conjunction searches of equal difficulty engage only partially overlapping frontoparietal networks. Neuroimage 15: 16–25. [DOI] [PubMed] [Google Scholar]
- Driver J, Mattingley JB ( 1998): Parietal neglect and visual awareness. Nat Neurosci 1: 17–22. [DOI] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P ( 2001): Perceptual awareness and its loss in unilateral neglect and extinction. Cognition 79: 39–88. [DOI] [PubMed] [Google Scholar]
- Drury CG, Clement MR ( 1978): The effect of area, density and number of background characters on visual search. Hum Factors 20: 597–602. [DOI] [PubMed] [Google Scholar]
- Eglin M, Robertson LC, Knight RT ( 1989): Visual search performance in the neglect syndrome. J Cogn Neurosci 1: 372–385. [DOI] [PubMed] [Google Scholar]
- Eglin M, Robertson LC, Knight RT, Brugger P ( 1994): Search deficits in neglect patients are dependent on size of the visual scene. Neuropsychology 8: 451–463. [Google Scholar]
- Ellison A, Rushworth M, Walsh V ( 2003): The parietal cortex in visual search ‐ a visuomotor hypothesis. Clin Neurophysiol 56: 321–330. [DOI] [PubMed] [Google Scholar]
- Ellison A, Schindler I, Pattison L, Milner AD ( 2004): An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain 127: 2307–2315. [DOI] [PubMed] [Google Scholar]
- Esterman M, McGlinchey‐Berroth R, Milberg W ( 2000): Preattentive and attentive visual search in individuals with hemispatial neglect. Neuropsychology 14: 599–611. [DOI] [PubMed] [Google Scholar]
- Ferber S, Karnath HO ( 2001): How to assess spatial neglect—Line bisection or cancellation tasks? J Clin Exp Neuropsychol 23: 599–607. [DOI] [PubMed] [Google Scholar]
- Galati G, Lobel E, Vallar G, Berthoz A, Pizzamiglio L, Le Bihan D ( 2000): The neural basis of egocentric and allocentric coding of space in humans: A functional magnetic resonance study. Exp Brain Res 113: 156–164. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim Y‐H, Meyer JR, Mesulam MM ( 1999): A large‐scale distributed network for covert spatial attention: Further anatomical delineation based on stringent behavioural and cognitive controls. Brain 122: 1093–1106. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD ( 1992): Separate visual pathways for perception and action. Trends Neurosci 15: 20–25. [DOI] [PubMed] [Google Scholar]
- Grosbras M‐H, Paus T ( 2002): Transcranial magnetic stimulation of the human frontal eye field: Effects on visual perception and attention. J Cogn Neurosci 14: 1109–1120. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Fink GR, Marshall JC, Vallar G ( 2003): Spatial cognition: Evidence from visual neglect. Trends Cogn Sci 7: 125–133. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Marshall JC ( 1991): Left neglect for near but not far space in man. Nature 350: 498–500. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Marshall JC, Wade DT ( 1989): Visuospatial neglect: Underlying factors and test sensitivity. Lancet 334: 908–911. [DOI] [PubMed] [Google Scholar]
- Harvey M ( 2004): Perceptual and premotor neglect: Is there an ideal task to categorise patients? Cortex 40: 323–328. [DOI] [PubMed] [Google Scholar]
- Harvey M, Krämer‐McCaffery T, Dow L, Murphy PJS, Gilchrist ID ( 2002a): Categorisation of ‘perceptual’ and ‘premotor’ neglect patients across different tasks: Is there strong evidence for a dichotomy? Neuropsychologia 40: 1387–1395. [DOI] [PubMed] [Google Scholar]
- Harvey M, Olk B, Muir K, Gilchrist ID ( 2002b) Manual responses and saccades in chronic and recovered hemispatial neglect: A study using visual search. Neuropsychologia 40: 705–717. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M ( 2007): Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 53: 905–918. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E, Damasio AR ( 1983): Localization of lesions in neglect In: Kertesz A, editor. Localization in Neuropsychology. New York: Academic Press; pp 471–492. [Google Scholar]
- Husain M, Rorden C ( 2003): Non‐spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci 4: 26–36. [DOI] [PubMed] [Google Scholar]
- Juan C‐H, Muggleton N, Tzeng OJ, Hung DL, Cowey A, Walsh V ( 2008): Segregation of visual selection and saccades in human frontal eye fields. Cereb Cortex 18: 2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan C‐H, Shorter‐Jacobi SM, Schall JD ( 2004): Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci USA 101: 15541–15544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M ( 2001): Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature 411: 950–953. [DOI] [PubMed] [Google Scholar]
- Karnath H‐O, Berger MF, Küker W, Rorden C ( 2004): The anatomy of spatial neglect based on voxelwise statistical analysis: A study of 140 patients. Cereb Cortex 14: 1164–1172. [DOI] [PubMed] [Google Scholar]
- Keller I, Schindler I, Kerkhoff G, von Rosen F, Golz D ( 2005): Visuospatial neglect in near and far space: Dissociation between line bisection and letter cancellation. Neuropsychologia 43: 724–731. [DOI] [PubMed] [Google Scholar]
- Lane AR, Smith DT, Schenk T, Ellison A ( 2011): The involvement of posterior parietal cortex in feature and conjunction visuomotor search. J Cogn Neurosci 23: 1964–1972. [DOI] [PubMed] [Google Scholar]
- Lane AR, Smith DT, Schenk T, Ellison A: The involvement of posterior parietal cortex and frontal eye fields in spatially primed visual search. Brain Stimul. doi:10.1016/j.brs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Laplane D, Degos JD ( 1983): Motor neglect. J Neurol Neurosurg Psychiatry 46: 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen L, Hyvärinen J, Nyman G, Linnankoski I ( 1979): I. Functional properties of neurons in lateral part of associative area 7 in awake monkeys. Exp Brain Res 34: 299–320. [DOI] [PubMed] [Google Scholar]
- Longo MR, Lourenco SF ( 2006): On the nature of near space: Effects of tool use and the transition to far space. Neuropsychologia 44: 977–981. [DOI] [PubMed] [Google Scholar]
- Lourenco SF, Longo MR ( 2009): The plasticity of near space: Evidence for contraction. Cognition 112: 451–456. [DOI] [PubMed] [Google Scholar]
- Maravita A, Husain M, Clarke K, Driver J ( 2001): Reaching with a tool extends visual‐tactile interactions into far space: Evidence from cross‐modal extinction. Neuropsychologia 39: 580–585. [DOI] [PubMed] [Google Scholar]
- Mennemeier M, Wertman E, Heilman KM ( 1992): Neglect of near peripersonal space. Brain 115: 37–50. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA ( 1995): The Visual Brain in Action. Oxford, UK: Oxford University Press. [Google Scholar]
- Milner AD, Goodale MA ( 2006): The Visual Brain in Action, 2nd ed. Oxford, UK: Oxford University Press. [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M ( 2003): The anatomy of visual neglect. Brain 126: 1986–1997. [DOI] [PubMed] [Google Scholar]
- Muggleton N, Cowey A, Walsh V ( 2008): The role of the angular gyrus in visual conjunction search investigated using signal detection analysis and transcranial magnetic stimulation. Neuropsychologia 46: 2198–2202. [DOI] [PubMed] [Google Scholar]
- Muggleton N, Juan C‐H, Cowey A, Walsh V ( 2003): Human frontal eye fields and visual search. J Physiol 89: 3340–3343. [DOI] [PubMed] [Google Scholar]
- Müri RM, Hess CW, Meienberg O ( 1991): Transcranial stimulation of the human frontal eye field by magnetic pulses. Exp Brain Res 86: 219–223. [DOI] [PubMed] [Google Scholar]
- Nobre A, Coull J, Walsh V, Frith C ( 2003): Brain activations during visual search: Contributions of search efficiency versus feature binding. Neuroimage 18: 91–103. [DOI] [PubMed] [Google Scholar]
- Paus T ( 1996): Location and function of the human frontal eye field: A selective review. Neuropsychologia 34: 475–483. [DOI] [PubMed] [Google Scholar]
- Pavlovskaya M, Ring H, Groswasser Z, Hochstein S ( 2002): Searching with unilateral neglect. J Cogn Neurosci 14: 745–756. [DOI] [PubMed] [Google Scholar]
- Pierson‐Savage JM, Bradshaw JL, Bradshaw JA, Nettleton NC ( 1988): Vibrotactile reaction times in unilateral neglect. The effects of hand location, rehabilitation and eyes open/closed. Brain 111: 1531–1545. [DOI] [PubMed] [Google Scholar]
- Pitzalis S, Di Russo F, Spinelli D, Zoccolotti P ( 2001): Influence of the radial and vertical dimensions on lateral neglect. Exp Brain Res 136: 281–294. [DOI] [PubMed] [Google Scholar]
- Pizzamiglio L, Cappa S, Vallar G, Zoccolotti P, Bottini G, Ciurli P, Antonucci G ( 1989): Visual neglect for far and near extra‐personal space in humans. Cortex 25: 471–477. [DOI] [PubMed] [Google Scholar]
- Previc FH ( 1990): Functional specialization in the lower and upper visual fields in humans: Its ecological origins and neurophysiological implications. Behav Brain Sci 13: 519–542. [Google Scholar]
- Previc FH ( 1996): Attentional and oculomotor influences on visual field anisotropies in visual search performance. Vis Cogn 3: 277–302. [Google Scholar]
- Previc FH, Blume JL ( 1993): Visual search asymmetries in three‐dimensional space. Vis Res 33: 2697–2704. [DOI] [PubMed] [Google Scholar]
- Rains GD ( 2002): Principles of Human Neuropsychology. Boston, MA: McGraw‐Hill. [Google Scholar]
- Rizzolatti G, Matelli M, Pavesi G ( 1983): Deficits in attention and movement following the removal of postarcuate (Area 6) and prearcuate (Area 8) cortex in macaque monkeys. Brain 106: 655–673. [DOI] [PubMed] [Google Scholar]
- Rengachary J, He BJ, Shulman GL, Corbetta M ( 2011): A behavioral analysis of spatial neglect and its recovery after stroke. Front Hum Neurosci 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro T, Cheifet S, Ingle H, Shoup R, Rafal R ( 1999): Localization of the human frontal eye fields and motor hand area with transcranial magnetic stimulation and magnetic resonance imaging. Neuropsychologia 37: 225–231. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual‐Leone A. The Safety of TMS Consensus Group ( 2009): Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120: 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT, Kadosh RC, Schuhmann T, Moerel M, Walsh V, Goebel R ( 2009): Optimizing functional accuracy of TMS in cognitive studies: A comparison of methods. J Cogn Neurosci 21: 207–221. [DOI] [PubMed] [Google Scholar]
- Sakata H, Kusunoki M ( 1992): Organization of space perception: Neural representation of three‐dimensional space in the posterior parietal cortex. Curr Opin Neurobiol 2: 170–174. [DOI] [PubMed] [Google Scholar]
- Schall JD ( 2004): On the role of frontal eye field in guiding attention and saccades. Vision Res 44: 1453–1467. [DOI] [PubMed] [Google Scholar]
- Schindler I, Ellison A, Milner AD ( 2008): Contralateral visual search deficits following TMS. J Neuropsychol 2: 501–508. [DOI] [PubMed] [Google Scholar]
- Smith DT, Jackson SR, Rorden C ( 2005): Transcranial magnetic stimulation of the left human frontal eye fields eliminates the cost of invalid endogenous cues. Neuropsychologia 43: 1288–1296. [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S ( 2010): Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci 30: 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW, Stell R, Mastaglia FL ( 1996): Transcranial magnetic stimulation of the human frontal eye field. J Neurol Sci 144: 114–118. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP ( 2005): A visual salience map in the primate frontal eye field. Prog Brain Res 147: 249–262. [DOI] [PubMed] [Google Scholar]
- Treisman A, Gelade G ( 1980): A feature integration theory of attention. Cogn Psychol 12: 97–136. [DOI] [PubMed] [Google Scholar]
- Van Zandt T, Townsend JT ( 1993): Self‐terminating versus exhaustive processes in rapid visual and memory search: An evaluative review. Percept Psychophys 53: 563–580. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Valenza N, Mayer E, Reverdin A, Landis T ( 1998): Near and far visual space in unilateral neglect. Ann Neurol 43: 406–410. [DOI] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel J‐R, Olivier E ( 2006): Contribution of the monkey frontal eye field to covert visual attention. J Neurosci 26: 4228–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss PH, Marshall JC, Wunderlich G, Tellmann L, Halligan PW, Freund H‐J, Fink GR ( 2000): Neural consequences of acting in near versus far space: A physiological basis for clinical dissociations. Brain 123: 2531–2541. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Marshall JC, Zilles K, Fink GR ( 2003): Are action and perception in near and far space additive or interactive factors? Neuroimage 18: 837–846. [DOI] [PubMed] [Google Scholar]


