Abstract
Evidence for an anterior‐posterior gradient of age‐related volume reduction along the hippocampal longitudinal axis has been reported in normal aging, but functional changes have yet to be systematically investigated. The current study applied an advanced brain mapping technique, large deformation diffeomorphic metric mapping (LDDMM), automatically delineating the hippocampus into the anterior and posterior segments based on anatomical landmarks. We studied this anterior‐posterior gradient in terms of structural and functional MRI in 66 participants aged from 19 to 79 years. The results showed age‐related structural volume reduction in both anterior and posterior hippocampi, with greater tendency for anterior decrease. FMRI task contrasts that robustly activated the anterior (associative/relational processing) and posterior (novelty) hippocampus independently, showed only significant reduction of activation in the anterior hippocampus as age increased. Our results revealed positive correlation between structural atrophy and functional decrease in the anterior hippocampi, regardless of task performance in normal aging. These findings suggest that anatomy and functions related to the anterior hippocampus may be more vulnerable to aging, than previously thought. Hum Brain Mapp 33:2415–2427, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: hippocampus, longitudinal axis, fMRI, aging, novelty and relational processing
INTRODUCTION
Converging data from postmortem, structural, and functional imaging studies suggest that aberrant hippocampal morphology and function play important roles in the pathophysiology of aging. Evidence from neuropathology showed neuronal loss, reduced long‐term potentiation and decrease of dendritic growth in the hippocampus in older adults [Grady and Craik,2000]. In previous structural and functional neuroimaging studies, age‐related reductions in the structural hippocampal volumes [Driscoll et al.,2009; Raz et al.,2010] were more consistently reported than age‐related functional changes in the hippocampal activations to episodic memory tasks, relevant to encoding and retrieval processes [Daselaar et al.,2003; Grady and Craik,2000; Grady et al.,1995; Miller et al.,2008; Sperling et al.,2003b; Trivedi et al.,2008]. This discrepancy seen among the functional studies might lie in the task of choice and the contrast of comparison, as well as the functional segregation of the hippocampus per se. Both human and animal studies have suggested a functional segregation along the longitudinal axis of the hippocampus [Colombo et al.,1998; Fanselow and Dong,2010; Fernandez et al.,1998; Lepage et al.,1998; Strange et al.,1999]. The ventral or temporal pole of the hippocampus, which corresponds to the anterior hippocampus in humans, has been reported to engage relational/associative and to a greater extent, encoding processes, as well as to modulate emotional and affective processes. This is opposed to the dorsal or septal pole of the hippocampus, which corresponds to the posterior hippocampus in humans, has been thought to be mainly engaged in cognitive processes with an emphasis on information retrieval, although novelty encoding has been reported to activate the subiculum [Preston et al.,2010] a structure that has greater involvement with the posterior regions of the hippocampus. A convergence of the above lines of investigations has gained much interest in recent years, and paved the way to study aging processes of the hippocampal morphology and functions along its longitudinal axis.
Age‐related vulnerabilities of the anterior and posterior hippocampi have thus far been studied using MRI volumetric analysis and behavioral assessment. MRI volumetric analysis has generally indicated a greater vulnerability of the posterior hippocampus to aging than the anterior hippocampus [Driscoll et al.,2003; Kalpouzos et al.,2009; Malykhin et al.,2008; Raz,2000]. For example, Driscoll et al. [2003] compared 16 young and 16 elderly adults in terms of the hippocampal volume and their performance on hippocampus‐dependent task. The findings suggested a greater age‐related volume reduction of the posterior than the anterior hippocampal volume. Moreover, both anterior and posterior hippocampal normalized volumes were significantly correlated with the behavioral performances of hippocampal‐related tasks [Driscoll et al.,2003]. Malykhin et al. [2008] found volumetric reductions to be progressively more severe from hippocampal head to tail in 28 younger compared with 39 older subjects. In a study that combined voxel‐based morphometry with resting‐state 18FDG‐PET in 45 subjects (20–83 years), the anterior hippocampal region was found to be least affected by age [Kalpouzos et al.,2009]. In contrast, other studies with larger sample sizes [Chen et al.,2010; Hackert et al.,2002; Jack et al.,1997] revealed a discrepant finding of age‐related anterior hippocampal vulnerability in terms of volumes in older subjects. Material specific hippocampal laterality was also reported independent of age, gender, education, and speed of processing: the right hippocampal tail volume correlated with nonverbal learning and left hippocampal body volume was associated with delayed verbal memory [Chen et al.,2010]. Thus, it appears that the effect of aging along the longitudinal axis of the hippocampus is far from clear. In addition, the association of structural changes to behavioral performance and resting‐state activity is an indirect inference. Hence, how age‐related structural changes in the hippocampus along the longitudinal axis would be presented in its associated cognitive functions remains uncertain. Clarifying this relationship between structural and functional changes of the hippocampus in aging would help understand pathological developments and cognitive decline. One possible way to directly examine the functional age‐related changes is by employing task‐activated fMRI. A recent study [Persson et al., 2010] applied an episodic face‐name paired‐associates task during fMRI to 16 young and 20 older subjects, but found no difference in the activation of the hippocampus between the groups. However, the authors noted that the null finding could be due to weak statistical power. The results were not reported based on the longitudinal axis of the hippocampus. To our knowledge, fMRI investigations that apply cognitive tasks which robustly activate anterior and posterior hippocampi independently have yet to be conducted for normal aging.
The main aim of this study is to investigate whether the anterior hippocampus is more resistant to aging in terms of structural volume and functional activation than the posterior hippocampus or vice versa by segregating the hippocampal anatomy and functional activations along the hippocampal longitudinal axis. Unlike previous studies focusing on comparisons between young and elderly groups [Driscoll et al.,2003; Malykhin et al.,2008; Raz,2000] or older subjects only [Chen et al.,2010; Hackert et al.,2002; Jack et al.,1997], the present study examined subjects aged from 19 to 79 years old. This is somewhat analogous to the age range in the Kalpouzous et al.'s study [2009]. In our anatomical analysis, we employed an advanced brain mapping technique, large deformation diffeomorphic metric mapping (LDDMM) [Miller and Qiu,2009], to automatically delineate the hippocampus from the structural MR images and divide it into the anterior and posterior segments based on anatomical landmarks. Several functional studies showed that the LDDMM mapping increased statistical power in detecting regional functional activations when compared to SPM or FSL [Kirwan et al.,2007; Miller et al.,2005]. We then applied an fMRI incidental encoding protocol adapted from [Binder et al.,2005] and originally designed to segregate anterior and posterior hippocampal activity for presurgical evaluation of patients with temporal lobe epilepsy. We revised the protocol using our own stimuli, condensed the length of presentation duration, and made it suitable for administration in the elderly. This task combined two rather different task contrasts that have been reported to produce hippocampal activity. The first contrast emphasized stimulus novelty and contrasted novel to repeating scenes that are either indoor or outdoor. For both conditions, the stimuli are meaningful and required recognition of indoor or outdoor scenes, and differ only in the novelty of the stimuli. In regards to the hippocampus, this contrast has been observed to typically elicit activation more posterior of the hippocampus, including the subiculum [Binder et al.,2005; Gabrieli et al.,1997; Golby et al.,2001; Preston et al.,2010]. The second contrast emphasized relational processing by using a contrast between novel scenes and nonsense stimuli, and both conditions are novel and differ only in the virtue of meaningfulness. The association of indoor or outdoor responses to the novel scenes is emphasized in this contrast. The degree to which the stimuli encouraged association or relational processing, has been thought to increase the engagement of the hippocampus in preparation for later retrieval [Alvarez and Squire,1994; McClelland et al.,1995; O'Reilly and Rudy,2001]. This is supported by empirical studies that observed greater hippocampal activation for meaningful/associative relative to meaningless/nonsemantic stimuli in the anterior hippocampus [Davachi and Wagner,2002; Henke et al.,1997; Small et al.,2001; Zeineh et al.,2003]. Numerous investigations have also reported that medial temporal lobe activations, including the hippocampus, tend to be more anterior when the difference in degree of relational processing is emphasized (see meta‐analysis by [Schacter et al.,1999]) and findings from [Binder et al.,2005; Giovanello et al.,2009]). Hence, these two functional contrasts facilitated our investigation of age‐related structural and functional changes of the anterior and posterior hippocampus, as well as their interaction.
As the age distribution of our sample size is similar to that of Kalpouzos et al. [2009], we expect to replicate their finding showing the posterior hippocampus to have more reduction in volume than the anterior hippocampus. Age‐related reductions in hippocampal activation should exist after controlling for overall hippocampal volume atrophy. This is based on previous studies that found decreased hippocampal activation in the elderly compared with the young during novel encoding tasks [Daselaar et al.,2003; Sperling et al.,2003b; Trivedi et al.,2008]. Based on these findings, we hypothesized that age‐related reduction in fMRI activation would be found in the posterior hippocampus as elicited by the novelty processing. As previous studies of associative processing did not find age‐related changes in hippocampal activation [Miller et al.,2008; Sperling et al.,2003b], we would not expect to find significant age‐related reduction in the anterior hippocampal activation for the relational processing contrast.
METHODS
Participants
Sixty‐six participants were recruited through advertisements posted in the National Taiwan University Hospital (NTUH) and the surrounding community. 29 males and 37 females ranged from 19 to 79 years old (mean age of 40.3 ± 15.5 years) participated in the study. A health screening questionnaire along with informed consent approved by the NTUH Institutional Review Board in accord with the Helsinki Declaration was acquired from each participant. Any participant with a history of psychological, neurological disorder, or surgical implantation that was not MR compatible was excluded from the study. Subjects with vascular risk factors of hypertension, diabetes, and cardiac abnormalities, as well as, those on medications, other than supplements, were excluded. All participants were administered the Edinburgh Handedness Inventory [Oldfield,1971] and only right‐handers were recruited. A Mini Mental Status Examination (MMSE) was also administered to each participant to rule out possible cognitive impairments. The mean MMSE score for the young and middle‐aged participants (19–55 years) was 29.74 (SD = 0.65) with a range of 27 to 30; for elderly (greater than 55 years) the mean MMSE score was 28.92 (SD = 1.12) with a range of 26 to 30.
MRI Acquisition
All subjects were scanned in a 3.0T Trio at the National Taiwan University Hospital (Siemens, Erlangen, Germany). T2‐weighted (FOV 192 × 192 mm, 34 slices, slice thickness = 3 mm, voxel size = 1 mm × 1 mm × 3 mm) images were acquired to verify proper slice selection before functional imaging and later coregistration of anatomical structures with functional activations. A three‐dimensional MPRAGE T1‐weighted scan (FOV 256 × 256 mm, TR = 2,530 ms, TE = 2.64 ms, flip angle = 7°, matrix size = 256 × 256, isotropic voxels of 1 mm3) was acquired. Functional images were acquired using single‐shot echo‐planar imaging (EPI) with 39 ascending 3 mm (no gap) axial slices parallel to the AC‐PC plane (FOV = 192 × 192 mm; TR = 2,000 ms, TE = 24 ms; flip angle = 90°; matrix = 64 × 64, slice thickness = 3 mm, in‐plane resolution = 3 mm × 3 mm, total number of volumes = 150).
FMRI and Behavioral Experiments
The fMRI and behavioral experiments were designed by adapting the tasks described in [Binder et al.,2005]. As illustrated in Figure 1, the fMRI session included one run of a blocked design task that contained five cycles and lasted 6 min. Each cycle contained three 24 second‐blocks that respectively corresponded to the conditions of novel pictures, repeated pictures, and scrambled pictures. Either indoor or outdoor pictures were displayed in the novel and repeated conditions, while pictures made of pixilated mosaic from the scenic pictures were displayed in the scrambled conditions. The task was presented using E‐prime (Psychology Software Tools, Pittsburgh, PA) through a back projection screen. Prior to the fMRI session, all participants went through practice trials to ensure their understanding of the experimental tasks. During the scan, the participants made a task irrelevant judgment of whether the scene presented on the screen was indoor or outdoor, or whether the two halves of the scrambled scenes were identical. The participants responded to the stimuli by a button press with their right hand. After the fMRI scan, a recognition test was administered within the scanner without image acquisition to ensure the subjects were attending to the tasks.
Figure 1.

FMRI experimental design. FMRI task of novel, repeated, and scrambled scenes presented in 24 second‐blocks with five cycles adapted from Binder et al. [2005]. Subjects judged if scenes were indoor or outdoor for the novel and repeated scenes conditions, and for the scrambled scenes they decided if the two halves of the scrambled scene were identical.
In the postexperimental recognition task, the participants were asked to judge if the picture has been shown in the previous experimental condition. The recognition stimuli included both indoor/outdoor and scrambled pictures.
Anatomical and Functional MRI Analysis
A hippocampal template with the labels of four areas (left‐anterior, right‐anterior, left‐posterior, right‐posterior) was manually traced respectively on a normalized T1‐weighted image in MRIcro in MNI space [Rorden and Brett,2000]. Inter‐rater reliability ([Kappa] = 0.74; see Fig. 2) was obtained from one experienced rater, a neuroradiologist, and a novice rater. We followed the anatomical definitions of the anterior and posterior hippocampi provided by Binder [Binder et al.,2005]. In particular, the uncus was first identified in the image as a boundary between the anterior and posterior hippocampi. The first section when the uncus appeared was the first section labeled as the anterior hippocampus. All sections posterior to it were labeled as the posterior hippocampus. The anterior hippocampus was traced forward till the slice when the temporal horn has moved completely from a lateral position to a medial position and lay completely beneath the amygdala. We identified the alveus as the superior boundary, the white matter of the parahippocampal gyrus as the inferior boundary, the temporal horn of the lateral ventricle as the lateral boundary, and the ambient cistern as the medial boundary.
Figure 2.
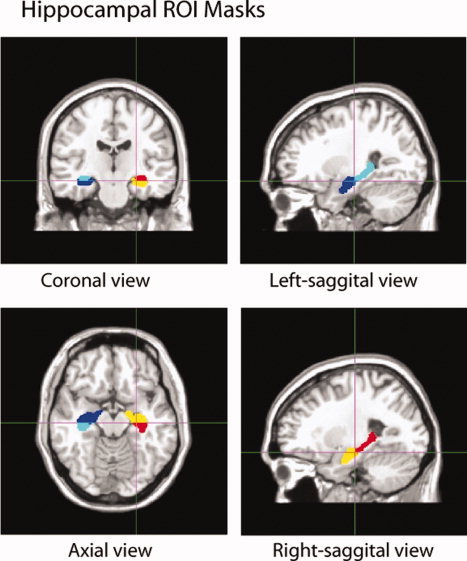
Hippocampal masks delineating the four regions (left‐anterior, right‐anterior, left‐posterior, right‐posterior) traced on a T1‐weighted template in standard space.
To delineate the anterior and posterior hippocampi from each individual subject, we first automatically segmented the entire hippocampus from the intensity‐inhomogeneity corrected T1‐weighted MR images [Sled et al.,1998] using a Markov random field model and then separated it into the anterior and posterior segments by translating the labels of the hippocampal template via large deformation diffeomorphic metric mapping (LDDMM) [Qiu and Miller,2008]. In details, the Markov random field model was first applied to label each voxel in the image volume as the hippocampus and others [Fischl et al.,2002]. Due to lack of constraint on the hippocampal shape, this labeling process introduced irregularities and topological errors (e.g. holes) at the hippocampal boundary. This may increase volume variation and thus reduce statistical power to detect group difference in volumes. To avoid this issue, we generated the hippocampal volumes of each individual subject with properties of smoothness and correct topology by injecting the template shape into them using LDDMM [Qiu and Miller,2008]. Then, the template's hippocampal binary mask was registered and deformed to the hippocampal mask of each individual by affine registration followed by LDDMM diffeomorphic map. The labels of the anterior and posterior segments of the template were then transferred to the subject's hippocampus mask. The volumes of the anterior and posterior hippocampi were computed as the number of voxels in the corresponding masks. In addition, the intracranial volume (ICV) was also computed as a sum of cerebral white and gray matter, cerebellum, ventricular systems, and brainstem.
Within individual subjects, the functional volumes were first corrected for motion artifacts and temporal offsets among slices and then temporally low‐pass filtered using SPM5 (Wellcome Trust Centre for Neuroimaging). Finally, the functional volumes were aligned to their corresponding anatomical image using rigid transformation found by maximizing cross‐correlation between the anatomical image and the mean fMRI volume.
Statistical Analysis
Behavior data
Linear regression with a main factor of age and covariates of gender and years of education was performed on the behavioral data, including reaction time and response accuracy, to reveal age effects on behavioral response in the fMRI experiment and post recognition task. The gender was considered as a covariate in all statistical tests since our sample has slightly more females than males (29 males and 38 females).
Hippocampal volumes
We investigated age effects on total hippocampal volumes, volumes of the anterior and posterior hippocampal segments using linear regression with a main factor of age and covariates of gender, ICV, and years of education. Using Student's t‐test, we further examined whether the volume atrophy rate of the anterior hippocampus was the same as that of the posterior hippocampus.
Hippocampal fMRI activation
Within individual subjects, linear regression was used to model functional temporal data at each voxel of the hippocampus. Novel, repeated, and scrambled blocks were modeled by a boxcar function convolved with a canonical hemodynamic response function. Contrasts between relevant stimulus types (novel vs. repeated; novel vs. scrambled) were made to generate t‐statistic maps for testing novelty and relational processing, respectively. For each contrast, activated volumes in the anterior and posterior segments of the hippocampus were counted as voxels with t‐values greater than a threshold, where the threshold was determined as the mean t‐value among voxels above 95th percentile of the t‐statistics and divided by 2, in the entire hippocampal volume [Fernandez et al.,2001].
Within each hippocampal ROIs, age effects on the two functional contrasts (novel vs. repeated; novel vs. scrambled) were examined by modeling activated volumes using linear regression with a main factor of age and covariates of gender, entire hippocampal volume, years of education, and behavioral data that showed significant age effects. Student's t‐test was used to further examine whether the functional activations were reduced at the same rate in both anterior and posterior hippocampi.
Hippocampal Structural Volumes and Functional Activation
To examine the relationship between structure and function of the hippocampus, we applied partial correlation to the volumetric size of the hippocampus and the number of activated voxels within the hippocampal ROIs controlling for gender and years of education. This was conducted for each of the contrasts of novelty and relational processing.
RESULTS
Behavioral Data
Figure 3A,B illustrates the distributions of accuracy rate and reaction time for each task (novel, repeated, and scrambled) measured during the fMRI experiment. The means and standard deviations of each behavioral measure are listed in Table I. Linear regression revealed no significant effects of age on reaction time of all tasks after controlling for gender and years of education (novel: t 62 = −0.918, P = 0.362; repeated: t 62 = −0.860, P = 0.393; scrambled: t 62 = −0.589, P = 0.558). In addition, linear regression revealed no significant age effects on accuracy rate in all the three tasks after controlling for gender and years of education (novel: t 62 = −0.024, P = 0.981; repeated: t 62 = 0.399, P = 0.691; scrambled: t 62 = −1.416, P = 0.162).
Figure 3.
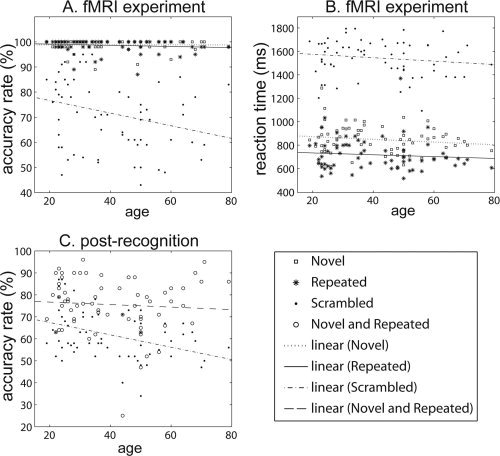
Distributions of behavioral data with respect to age. (A and B) The distributions of accuracy rate and reaction time measured during the fMRI experiment. Squares, asterisks, and dots respectively denote measures for novel, repeated, and scrambled tasks, while dotted, solid, dash‐dot lines are linear fits with respect to age for novel, repeated, and scrambled tasks, respectively. (C) The distribution of accuracy rate for each task measured during the post recognition examination. Circles and dots respectively denote measures for novel and repeated task as well as scrambled task, while dashed and dash‐dot lines are linear fits with respect to age for novel and repeated task as well as scrambled tasks, respectively.
Table I.
Mean and standard deviation of behavioral measures in each task
| Task | Mean | Standard deviation | |
|---|---|---|---|
| Accuracy rate (%) | Novel | 98.675 | 2.335 |
| Repeated | 98.528 | 2.679 | |
| Scrambled | 71.406 | 14.771 | |
| Reaction time (msec) | Novel | 853.100 | 118.334 |
| Repeated | 721.944 | 138.938 | |
| Scrambled | 1549.767 | 158.644 | |
| Postrecognition accuracy rate (%) | Novel and repeated | 75.621 | 12.451 |
| Scrambled | 61.660 | 11.065 |
During the postrecognition examination, the accuracy rate was used to measure subjects' ability in encoding each stimulus that had been shown in the fMRI experiment. Figure 3C illustrates its distribution for each task with respect to age. Table I lists the means and standard deviations for each task. Linear regression revealed no significant age effects on recognition accuracy of novel and repeated tasks (t 62 = −0.211, P = 0.833) but significant negative effect of age on recognition accuracy in scrambled task (t 62 = −3.264, P = 0.002) after controlling for gender and years of education.
Hippocampal Volumes
Among all subjects, the mean ICV is 1549.3 cm3 (±183.6). Linear regression revealed significant effects of age on ICV after controlling for gender and years of education (t 62 = −4.144, P < 0.001).
Linear regression revealed a significant reduction in the total volume of the left hippocampus but a marginally significant reduction in the total volume of the right hippocampus with age after controlling for gender, years of education, and ICV (left: t 61 = −2.641, P = 0.010; right: t 61 = −1.890, P = 0.064). Figure 4 illustrates the distributions of the anterior and posterior hippocampal volumes with respect to age. After controlling for gender, years of education, and ICV, bilateral anterior hippocampal volumes significantly reduced as age increased (left anterior (LA): t 61 = −3.123, P = 0.003; right anterior (RA): t 61 = −2.038, P = 0.046). But no significant effects of age on the volume were found in the bilateral posterior hippocampi (left posterior (LP), t 61 = −1.224, P = 0.225; right posterior (RP), t 61 = −1.154, P = 0.253). Student's t‐test revealed no statistically significant difference in the rates of the volume reductions between the anterior and posterior hippocampi with respect to age (left: t 122 = −1.497, P = 0.068; right: t 122 = −0.582, P = 0.281), although a trend for a greater vulnerability of the anterior hippocampal volume to aging was noted. In the application of a Bonferroni Correction for multiple comparisons, age‐related effects were still significant for the left anterior hippocampus (P < 0.008).
Figure 4.
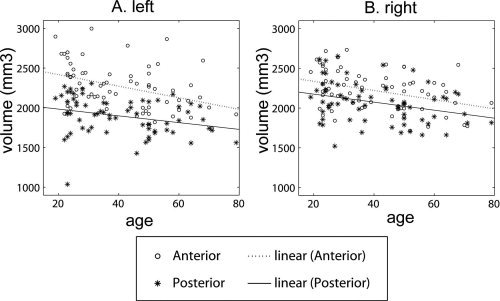
(A and B) The distributions of the anterior (circles) and posterior (asterisks) hippocampal volumes with respect to age for the left and right hemispheres, respectively. Dotted and solid lines are respectively linear fits of the anterior and posterior hippocampal volumes with respect to age.
Functional Activations
Novelty contrast (novel vs. repeated)
Linear regression controlling for gender, years of education, and structural hippocampal volume revealed significant reduction in bilateral hippocampal volumes that were activated by novelty as age increased (left: t 61 = −3.379, P = 0.001; right: t 61 = −2.882, P = 0.005). Figure 5A,B illustrates the distributions of the anterior and posterior hippocampal volumes that responded to novelty with respect to age. The activated volumes in the bilateral anterior hippocampi also significantly reduced as age increased (LA: t 61 = −3.714, P < 0.001; RA: t 62 = −2.769, P = 0.007). However, there were no significant effects of age on the activated volumes in bilateral posterior hippocampi (LP: t 61 = −1.515, P = 0.135; RP: t 61 = −0.832, P = 0.409). Student's t‐test revealed greater age effects on functional activation in the novelty contrast for the bilateral anterior than posterior hippocampus (left: t 122 = −2.072, P = 0.020; right: t 122 = −1.667, P = 0.049). However, this difference did not reach significance with the stringent Bonferroni Correction for multiple comparisons (P < 0.008), while the activated volumes in the bilateral anterior hippocampi remained significantly reduced as age increased.
Figure 5.
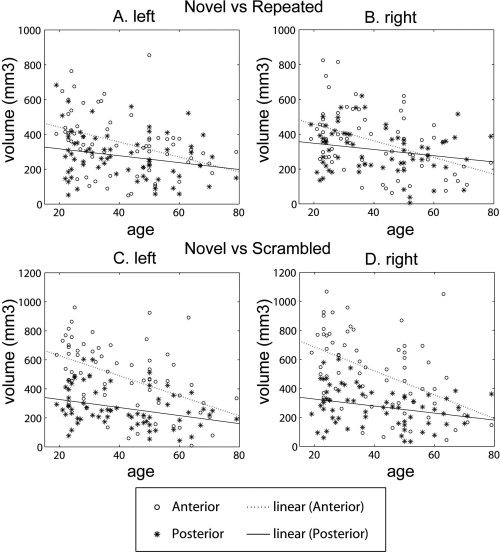
(A and B) The distributions of the anterior (circles) and posterior (asterisks) hippocampal volumes activated to the contrast between the novel and repeated tasks (novelty) with respect to age for the left and right hemispheres, respectively. (C and D) The distributions for the contrast between the novel and scrambled tasks (relational processing). Dotted and solid lines are respectively linear fits of the anterior and posterior hippocampal volumes with respect to age.
Relational processing (novel vs. scrambled)
After controlling for gender, years of education, and the structural hippocampal volume, linear regression revealed a significant reduction in the activation of bilateral hippocampi as age increased (left: t 61 = −4.972, P < 0.001; right: t 61 = −4.601, P < 0.001). Figure 5C,D illustrates the distributions of the anterior and posterior functional hippocampal volumes activated in relational processing. The activation in the bilateral anterior hippocampi also significantly reduced as age increased (LA: t 61 = −5.431, P < 0.001; RA: t 61 = −4.651, P < 0.001) when structural hippocampal volume was controlled for. However, no significant effects of age on the activation in the bilateral posterior hippocampi were found (LP, t 61 = −1.372, P = 0.175; RP, t 61 = −0.789, P = 0.433). Student's t‐test further revealed greater age effects on the relational processing activation in the anterior than posterior hippocampus (left: t 122 = −3.862, P < 0.001; right: t 122 = −3.603, P < 0.001). This age‐related reduction in activation remained significant with the Bonferroni Correction (P < 0.008).
Structure and Function
After controlling for gender and years of education, partial correlation analysis revealed significant positive correlations of the functional hippocampal activations in the novelty contrast to the structural hippocampal volumes (left: r 62 = 0.504, P < 0.001; right: r 62 = 0.474, P < 0.001). We further found that the functional volumes in the anterior and posterior hippocampi were significantly correlated with their corresponding structural hippocampal volumes (LA: r 62 = 0.302, P = 0.015; RA: r 62 = 0.362; P = 0.003; LP: r 62 = 0.347, P = 0.005; RP: r 62 = 0.248; P = 0.049) for this contrast.
Partial correlation analysis controlling for gender and years of education, revealed significant positive correlations between structural and functional activation of hippocampal volumes in relational processing (left: r 62 = 0.318, P = 0.010; right: r 62 = 0.319, P = 0.010). We further found that the functional volumes in the anterior hippocampi were significantly correlated with their corresponding structural hippocampal volumes (LA: r 62 = 0.300, P = 0.016; RA: r 62 = 0.325; P = 0.009) but the functional volumes in the posterior hippocampi were marginally significantly correlated with their corresponding structural hippocampal volumes (LP: r 62 = 0.218, P = 0.084; RP: r 62 = 0.220; P = 0.081).
DISCUSSION
To the best of our knowledge, this is the first study to examine age‐related influences on the structural volumes and functional activations to novelty and relational processing in the anterior and posterior hippocampi. Our results showed age‐related reductions in both anterior and posterior hippocampal volumes and in functional activation of the anterior hippocampus for novelty and relational processing. Moreover, we found greater age‐related reduction in the anterior than the posterior hippocampus in terms of functional activation and our data evidenced a trend for this in structural volumes. This highly suggests that the structure and function of the hippocampus have similar vulnerability to aging.
Anatomical Findings
Our findings were in line with results from previous studies, i.e. the volumes of the anterior and posterior hippocampi decreased with normal aging [Driscoll et al.,2003; Kalpouzos et al.,2009; Malykhin et al.,2008; Raz,2000; Raz et al.,2010]. We found a trend to suggest that the anterior hippocampus to have greater vulnerability to age‐related deterioration than the posterior hippocampus, and this is partly consistent with previous studies with larger samples (N = 147; N = 511; N = 126) of older adults (age range: 55–83 yrs; 60–90 yrs; 51–89 yrs) [Chen et al.,2010; Hackert et al.,2002; Jack et al.,1997], respectively. However, our findings did not support previous reports of the anterior hippocampus to be more resistant to aging [Driscoll et al.,2009; Kalpouzos et al.,2009; Malykhin et al.,2008]. The discrepant findings were thought to reflect the underlying biological heterogeneity of normal aging in the population, and supported the observation that age‐related anatomical findings could differ depending on whom, among the sample, is carrying the variance [Buckner,2004]. In addition, variability of the anterior hippocampus across all age ranges highlighted by Raz et al., Lupien et al. [2007] could contribute to this inconsistency. One possible contribution to this contrary finding is the difference in the age range and size of our sample. Malykin et al. [2008] and Driscoll et al. [2003] had smaller groups of 28 young (22–50 yrs) and 27 old (65–84 yrs) and 16 young (20–39 yrs) and 16 old (60–85 yrs), respectively. However, Kalpouzos et al. [2009] had 45 subjects ranging from 20 to 83 years that was closer to our pool of 66 subjects but still about one third less. Therefore, differences in sample size could have contributed to the contrary finding, but not entirely.
Previous studies that included either young and elderly subjects [Malykhin et al.,2008] or older subjects [Chen et al.,2010; Hackert et al.,2002; Jack et al.,1997] suggested a linear relationship for age and hippocampal volumes. The inclusion of the middle‐aged subjects in our study allowed us to investigate whether age‐related curvilinear changes exist in hippocampal volumes, or if hippocampal atrophy accelerated with age [Raz et al.,2004]. By incorporating the second‐order term of age in our statistical model, we did not find significant curvilinear relationship between age and the hippocampal volumes either. However, in a large scale aging study with over 300 subjects a curvilinear reduction of hippocampal shape and volume was evident [Yang et al., 2011].
Functional Findings and Relationship With Anatomical Findings
Functional segregation along the longitudinal axis of the hippocampus has been indicated previously, and it is plausible that differential age‐related functional changes could be detected in the anterior and posterior hippocampi for different cognitive tasks based on the volumetric findings. Binder et al. [2005] found that relational processing and novel scenes respectively elicited functional activations in the anterior and posterior hippocampi. We replicated this finding in our group where the anterior and posterior hippocampi were differentially activated by relational processing and novelty, respectively (see Supporting Information). In addition, our functional findings were consistent with those reported by previous functional hippocampus‐dependent studies, where increasing age was associated with reduced activity in the hippocampus when the hippocampus was considered as a ROI [Cabeza et al.,2004; Daselaar et al.,2003; Sperling,2007; Trivedi et al.,2008]. Our study was the first to analyze hippocampal activations separately for anterior and posterior sections, which allowed better understanding of the changes in the hippocampus along its longitudinal axis in normal aging. Our functional findings suggested that there is greater age‐related reduction in activations for bilateral anterior than posterior hippocampi. This was an unexpected finding that did not support our hypotheses that greater reductions should be seen in the posterior hippocampi for novel scenes and no age‐related functional changes should be observed for relational processing. However, this interesting finding would have implications for vulnerability of cognitive functions, such as associative encoding and relational processing, emphasized in the anterior hippocampus [De Vogelaere et al.,2010; Jackson and Schacter,2004; Prince et al.,2005; Sperling,2007; Sperling et al.,2003a]. Indeed, a recent meta‐analytic study showed that associative encoding is affected during the course of aging [Old and Naveh‐Benjamin,2008] providing indirect support to our finding. Interestingly, post hoc analyses of our current data showed significant positive correlation between postscan recognition accuracy for scramble condition and bilateral posterior but not anterior hippocampus activation. That is the posterior hippocampus appears to be sensitive to the recognition of perceptual information where meaningfulness is minimized. This finding is consistent with the posterior hippocampus role in retrieval that is sensitive to the exact perceptual match between the meaningless scrambled images and what is learned during the scan [Giovanello et al.,2009]. The fact that we found a tendency for age‐related functional reductions in the anterior hippocampi for novel versus repeated, a contrast that engaged more of the posterior hippocampus in the group as a whole, further supported the functional vulnerability of the anterior hippocampus in aging. This finding also suggests that perceptual matching and novelty processing may remain intact in healthy aging.
There are a number of studies that suggested a possible negative association between hippocampal functional activity and its anatomical structure in demented elderly patients serving as a compensatory response to pathology (e.g. [Dickerson et al.,2005; Sandstrom et al.,2006]). However, such an association has never been examined with normal aging, especially in the context of the anterior and posterior hippocampi. Our findings did not support this compensation theory to hold in normal aging. Instead, our results suggested a positive correlation between volume atrophy and functional decrease in the whole, anterior and posterior hippocampi, regardless of performance. This is also congruent with results from studies that looked at grey matter cortical atrophy in aging and functional changes in relation to task performance independently [Cabeza et al.,2004; Dickerson et al.,2009]. This relationship might indicate a structural change that was significant enough to interfere with the functional performance of the hippocampus, which could have implications regarding the possible decline in functions related to the anterior hippocampus in the aging brain. Nevertheless, functional compensation could be manifested in other neocortical regions, which was not a focus of evaluation in the current study.
Anterior‐Posterior Shift in Aging for the Hippocampus
A number of previous studies have supported an age‐related reduction along the longitudinal axis in the direction of posterior‐to‐anterior hippocampus for structural volumetric studies, as well as, resting‐state function [Driscoll et al.,2003; Kalpouzos et al.,2009; Malykhin et al.,2008; Raz,2000]. However, both our structural and functional findings supported an age‐related reduction in the anterior‐to‐posterior direction of the hippocampus. There may be a number of factors that could contribute to the contradictory finding. As mentioned earlier, the age‐related findings could differ depending on whom, among the sample, is carrying the variance [Buckner,2004]. Moreover, the conclusion of the age‐related functional changes in the anterior and posterior hippocampi could be dependent on the way that the hippocampus is sectioned. Previous anatomical studies [Chen et al.,2010; Driscoll et al.,2003; Hackert et al.,2002; Jack et al.,1997; Malykhin et al.,2008] have divided the hippocampus into head, body, and tail, but the delineation of these sections has been variable. In our study, we sectioned the hippocampus only into anterior and posterior sections based on the study where the fMRI paradigm was adapted from [Binder et al.,2005] for consistency. However, the results from our structural data were consistent with the previous studies with larger sample size in older adults. Hence, different approaches of segregating the hippocampus should be investigated. In addition, segregations such as suggested by Malykhin et al. [2010] and La Joie et al. [2010] using high‐resolution 3T MR sequence where the hippocampus was segregated into three different subfields: subiculum, cornu ammonis (CA1‐3), dentate gyrus, and examined along the longitudinal axis of head, body, and tail should be investigated. Consistent anatomical definitions are thus needed for across‐studies comparisons.
Nevertheless, our finding of volume and functional loss in the hippocampus, indirectly supports one of the explanations provided for the phenomenon of posterior‐anterior shift in aging (PASA) seen in functional neuroimaging studies [Davis et al.,2008], where an age‐related reduction in occipital activity was coupled with increased frontal activity. For older adults, high levels of inferior frontal activation were associated with low levels of parahippocampal activation, suggesting that the frontal activity may be compensatory for decreased medial temporal activations [Davis et al.,2008; Gutchess et al.,2005]. This could be further ascertained in analyzing whole brain activations which was not a focus of our current study.
CONCLUSION
Our current study shows an anterior‐posterior gradient for age‐related reduction in both hippocampal structure and activation. In other words, the posterior hippocampus appears to be more resilient to aging than the anterior hippocampus, and functions associated with the anterior hippocampus may be more vulnerable to aging than previously thought. This finding may have clinical implications regarding the possible pathological changes of hippocampus in aging brains. As the current study is limited by the cross‐sectional sampling, we would need to investigate this with larger longitudinal samples in the future.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors thank Nicholas Trost, MD, for helping to delineate the anterior and posterior hippocampus mask.
All authors disclose no actual or potential conflicts of interest with other people and organizations related to this present work. This research protocol involving human subjects was approved by the Institutional Review Board of the National Taiwan University Hospitals, Taipei, Taiwan in accord with the Helsinki Declaration.
Contributor Information
Shen‐Hsing Annabel Chen, Email: annabelchen@ntu.edu.sg.
Anqi Qiu, Email: bieqa@nus.edu.sg.
REFERENCES
- Alvarez P, Squire LR ( 1994): Memory consolidation and the medial temporal lobe: A simple network model. Proc Natl Acad Sci USA 91: 7041–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Bellgowan PS, Hammeke TA, Possing ET, Frost JA ( 2005): A comparison of two FMRI protocols for eliciting hippocampal activation. Epilepsia 46: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Buckner RL ( 2004): Three Principles for Cognitive Aging Research In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford University Press. [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L ( 2004): Task‐independent and task‐specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex 14: 364–375. [DOI] [PubMed] [Google Scholar]
- Chen KH, Chuah LY, Sim SK, Chee MW ( 2010): Hippocampal region‐specific contributions to memory performance in normal elderly. Brain Cogn 72: 400–407. [DOI] [PubMed] [Google Scholar]
- Colombo M, Fernandez T, Nakamura K, Gross CG ( 1998): Functional differentiation along the anterior‐posterior axis of the hippocampus in monkeys. J Neurophysiol 80: 1002–1005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C ( 2003): Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain 126: 43–56. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD ( 2002): Hippocampal contributions to episodic encoding: Insights from relational and item‐based learning. J Neurophysiol 88: 982–990. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R ( 2008): Que PASA? The posterior‐anterior shift in aging. Cereb Cortex 18: 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vogelaere F, Santens P, Achten E, Boon P, Vingerhoets G ( 2010): Hippocampal activation during face‐name associative memory encoding: Blocked versus permuted design. Neuroradiology 52: 25–36. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, Buckner RL ( 2009): Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging 30: 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand‐Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA ( 2005): Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM ( 2009): Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 72: 1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ ( 2003): The aging hippocampus: Cognitive, biochemical and structural findings. Cereb Cortex 13: 1344–1351. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW ( 2010): Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, de Greiff A, von Oertzen J, Reuber M, Lun S, Klaver P, Ruhlmann J, Reul J, Elger CE ( 2001): Language mapping in less than 15 minutes: Real‐time functional MRI during routine clinical investigation. Neuroimage 14: 585–594. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Weyerts H, Schrader‐Bolsche M, Tendolkar I, Smid HG, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR, Heinze HJ ( 1998): Successful verbal encoding into episodic memory engages the posterior hippocampus: A parametrically analyzed functional magnetic resonance imaging study. J Neurosci 18: 1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM ( 2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33: 341–355. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH ( 1997): Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 276: 264–266. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer D, Verfaellie M ( 2009): Distinct hippocampal regions make unique contributions to relational memory. Hippocampus 19: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, Gabrieli JD ( 2001): Material‐specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain 124: 1841–1854. [DOI] [PubMed] [Google Scholar]
- Grady CL, Craik FI ( 2000): Changes in memory processing with age. Curr Opin Neurobiol 10: 224–231. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV ( 1995): Age‐related reductions in human recognition memory due to impaired encoding. Science 269: 218–221. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC ( 2005): Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial‐temporal activity. J Cogn Neurosci 17: 84–96. [DOI] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM ( 2002): Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage 17: 1365–1372. [DOI] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG ( 1997): Human hippocampus establishes associations in memory. Hippocampus 7: 249–256. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E ( 1997): Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology 49: 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson O III, Schacter DL ( 2004): Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage 21: 456–462. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chetelat G, Baron JC, Landeau B, Mevel K, Godeau C, Barre L, Constans JM, Viader F, Eustache F, Desgranges B ( 2009): Voxel‐based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging 30: 112–124. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CE ( 2007): High‐resolution fMRI investigation of the medial temporal lobe. Hum Brain Mapp 28: 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R, Fouquet M, Mezenge F, Landeau B, Villain N, Mevel K, Pelerin A, Eustache F, Desgranges B, Chetelat G ( 2010): Differential effect of age on hippocampal subfields assessed using a new high‐resolution 3T MR sequence. Neuroimage 53: 506–514. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E ( 1998): Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus 8: 313–322. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, Pruessner JC ( 2007): Hippocampal volume is as variable in young as in older adults: Implications for the notion of hippocampal atrophy in humans. Neuroimage 34: 479–485. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Bouchard TP, Camicioli R, Coupland NJ ( 2008): Aging hippocampus and amygdala. Neuroreport 19: 543–547. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Lebel RM, Coupland NJ, Wilman AH, Carter R ( 2010): In vivo quantification of hippocampal subfields using 4.7 T fast spin echo imaging. Neuroimage 49: 1224–1230. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC ( 1995): Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102: 419–457. [DOI] [PubMed] [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C ( 2005): Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proc Natl Acad Sci USA 102: 9685–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Qiu A ( 2009): The emerging discipline of computational functional anatomy. Neuroimage 45: S16–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA ( 2008): Age‐related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA 105: 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW ( 2001): Conjunctive representations in learning and memory: Principles of cortical and hippocampal function. Psychol Rev 108: 311–345. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh‐Benjamin M ( 2008): Differential effects of age on item and associative measures of memory: A meta‐analysis. Psychol Aging 23: 104–118. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Persson J, Kalpouzos G, Nilsson LG, Ryberg M, Nyberg L. (2010): Preserved hippocampus activation in normal aging as revealed by fMRI. Hippocampus. DOI:10.1002/hipo.20794 [DOI] [PubMed]
- Preston AR, Bornstein AM, Hutchinson JB, Gaare ME, Glover GH, Wagner AD ( 2010): High‐resolution fMRI of content‐sensitive subsequent memory responses in human medial temporal lobe. J Cogn Neurosci 22: 156–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R ( 2005): Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. J Neurosci 25: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Miller MI ( 2008): Multi‐structure network shape analysis via normal surface momentum maps. Neuroimage 42: 1430–1438. [DOI] [PubMed] [Google Scholar]
- Raz N ( 2000): Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings In: Craik FIM, Salthouse TA, editor. The Handbook of Aging and Cognition. Mahwah: Lawrence Erlbaum Associates; pp 1–90. [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U ( 2010): Trajectories of brain aging in middle‐aged and older adults: Regional and individual differences. Neuroimage 51: 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD ( 2004): Differential aging of the medial temporal lobe: A study of a five‐year change. Neurology 62: 433–438. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M ( 2000): Stereotaxic display of brain lesions. Behav Neurol 12: 191–200. [DOI] [PubMed] [Google Scholar]
- Sandstrom CK, Krishnan S, Slavin MJ, Tran TT, Doraiswamy PM, Petrella JR ( 2006): Hippocampal atrophy confounds template‐based functional MR imaging measures of hippocampal activation in patients with mild cognitive impairment. AJNR Am J Neuroradiol 27: 1622–1627. [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Curran T, Reiman EM, Chen K, Bandy DJ, Frost JT ( 1999): Medial temporal lobe activation during episodic encoding and retrieval: A PET study. Hippocampus 9: 575–581. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC ( 1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17: 87–97. [DOI] [PubMed] [Google Scholar]
- Small SA, Nava AS, Perera GM, DeLaPaz R, Mayeux R, Stern Y ( 2001): Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nat Neurosci 4: 442–449. [DOI] [PubMed] [Google Scholar]
- Sperling R ( 2007): Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann NY Acad Sci 1097: 146–155. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand‐Giovannetti E, Poldrack R, Schacter DL, Albert M ( 2003a): Putting names to faces: Successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage 20: 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS ( 2003b): fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry 74: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ ( 1999): Segregating the functions of human hippocampus. Proc Natl Acad Sci USA 96: 4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Hess TM, Fitzgerald ME, Atwood CS, Rowley HA, Asthana S, Sager MA, Johnson SC ( 2008): fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: Risk factors for Alzheimer's disease. Neuropsychologia 46: 1667–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY ( 2003): Dynamics of the hippocampus during encoding and retrieval of face‐name pairs. Science 299: 577–580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


