Abstract
This study aimed to quantify dynamic structural changes in the brain after subcortical stroke and identify brain areas that contribute to motor recovery of affected limbs. High‐resolution structural MRI and neurological examinations were conducted at five consecutive time points during the year following stroke in 10 patients with left hemisphere subcortical infarctions involving motor pathways. Gray matter volume (GMV) was calculated using an optimized voxel‐based morphometry technique, and dynamic changes in GMV were evaluated using a mixed‐effects model. After stroke, GMV was decreased bilaterally in brain areas that directly or indirectly connected with lesions, which suggests the presence of regional damage in these “healthy” brain tissues in stroke patients. Moreover, the GMVs of these brain areas were not correlated with the Motricity Index (MI) scores when controlling for time intervals after stroke, which indicates that these structural changes may reflect an independent process (such as axonal degeneration) but cannot affect the improvement of motor function. In contrast, the GMV was increased in several brain areas associated with motor and cognitive functions after stroke. When controlling for time intervals after stroke, only the GMVs in the cognitive‐related brain areas (hippocampus and precuneus) were positively correlated with MI scores, which suggests that the structural reorganization in cognitive‐related brain areas may facilitate the recovery of motor function. However, considering the small sample size of this study, further studies are needed to clarify the exact relationships between structural changes and recovery of motor function in stroke patients. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: ischemic stroke, post‐stroke recovery, plasticity, volumetric MRI, motor cortex
INTRODUCTION
Motor disability is the most common deficit after ischemic stroke. Following initial damage, stroke patients can usually recover to some extent, but the mechanism of spontaneous recovery of motor function is not completely understood. Many researchers have studied the process of spontaneous recovery after stroke using functional neuroimaging techniques. Stroke patients initially show extensive activation of bilateral motor areas and recruitment of sensory and secondary motor structures that are not normally involved in motor tasks [Enzinger et al.,2008; Luft et al.,2004; Weder et al.,1994; Weiller et al.,1992,1993]. Patients then show a general trend of focusing these activation changes toward the ipsilesional primary motor cortex as time elapses [Feydy et al.,2002; Marshall et al.,2000; Nelles et al.,1999; Tombari et al.,2004], although a few patients still show persistent recruitment of other motor‐related structures [Calautti et al.,2001; Feydy et al.,2002]. Stroke patients also demonstrate a shift in the peak location of activation in the ipsilesional primary motor cortex [Calautti and Baron,2003; Pineiro et al.,2001; Weiller et al.,1993] and altered functional or effective connectivity [Carter et al.,2010; Sharma et al.,2009; van Meer et al.,2010; Wang et al.,2010] and network properties [Nomura et al.,2010; Wang et al.,2010]. Some of the functional changes, such as recovered activation of the ipsilesional primary motor cortex, are thought to be compensatory and to contribute to the recovery of motor function of the affected limbs [Loubinoux et al.,2007; Rehme et al.,2011; Tombari et al.,2004]; however, other functional changes have been suggested to be maladaptive and to impede the recovery process [Dafotakis et al.,2008; Enzinger et al.,2008]. To date, longitudinal structural changes following a motor pathway subcortical stroke have seldom been studied, and therefore, their contribution to the recovery of motor function of affected limbs is far from clear.
Our current understanding of structural changes after stroke involving motor pathways includes: (1) several brain areas that anatomically connect with lesions have been shown to have delayed brain atrophy after acute ischemic stroke and a simultaneous improvement in motor function [Kraemer et al.,2004]; (2) structural plasticity has been shown to be co‐localized with brain areas that exhibit functional plasticity after stroke [Schaechter et al.,2006]; and (3) constraint‐induced movement therapy has been shown to induce structural plasticity in motor‐ and cognitive‐related areas in chronic stroke patients [Gauthier et al.,2008]. However, these structural MRI studies adopted either a retrospective, cross‐sectional or interventional design in which the dynamics of the structural changes after stroke were not assessable. Moreover, highly variable lesion sites across stroke patients might increase the difficulties in clarifying relationships between observed structural changes and recovery of motor function.
In the current study, 10 subcortical stroke patients with relatively homogeneous lesion locations in the left hemisphere motor pathway were recruited, and a longitudinal study design was adopted to quantify the dynamic gray matter value (GMV) changes in the brain after stroke and to identify both brain areas with structural changes from the acute to the chronic phase of stroke and their relationship to the recovery of motor function.
SUBJECTS AND METHODS
Subjects
Ten right‐handed patients (nine males and one female; mean age: 48.7 years; range: 41–55 years) with subcortical ischemic stroke in the left hemisphere motor pathway were recruited from the inpatient services at the Xuanwu Hospital of Capital Medical University (Beijing, China). All patients were first‐onset stroke patients who showed motor deficits in both the upper and lower extremities. None of them had a history of neurological or psychiatric disorders, nor did any of them experience subsequent symptomatic stroke. Conventional magnetic resonance images (MRI) did not identify any abnormalities in the patients other than the infarct lesion. Eight age‐matched healthy controls (eight males; mean age: 47.6; range: 41–53 years) were also recruited for comparison.
The motor function of each patient was assessed using the Motricity Index (MI) [Demeurisse et al.,1980]. This scale measures motor abilities including hand‐grasp, elbow flexion, shoulder abduction, ankle dorsiflexion, knee extension and hip flexion in the limbs on the affected side. The validity [Bohannon,1999; Cameron and Bohannon,2000] and reliability [Collin and Wade,1990] of this scale have been confirmed. Stroke patients were scanned and neurologically assessed 5 times after the stroke, that is, within 1 week, at 2 weeks, 1 month, 3 months, and 1 year after stroke onset. The clinical characteristics of these stroke patients are summarized in Table I. Control subjects were scanned once to establish the population‐specific gray matter (GM) template. The Ethics Committee of Xuanwu Hospital approved this study, and written informed consent was obtained from each patient or control subject.
Table I.
Clinical and demographic data of stroke patients
| Patient No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (year) | 42 | 48 | 53 | 52 | 51 | 43 | 50 | 55 | 41 | 52 |
| Gender | M | M | M | M | M | M | M | M | M | F |
| LV (mL) | 20.1 | 17.9 | 13.3 | 11.0 | 4.4 | 3.5 | 4.2 | 2.3 | 31.1 | 4.3 |
| Lesion locations | IC | IC | IC | IC | IC | IC | IC | IC | IC | IC |
| CR | CR | CR | CR | CR | CR | CR | CR | |||
| BG | BG | BG | BG | |||||||
| Past medical history | – | HT | – | HT | HT | HT | HT | HT | DT | HT |
| HL | DT | |||||||||
| No. of scans | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 5 | 3 |
| TP1 (days) | 4 | 1 | 2 | 0 | 4 | 1 | – | 6 | 4 | 2 |
| TP2 (days) | 13 | 12 | 16 | 14 | – | 9 | 11 | 12 | 13 | 12 |
| TP3 (days) | 32 | 35 | 34 | 30 | 27 | – | 33 | 31 | 29 | – |
| TP4 (days) | 147 | 88 | 97 | 92 | 93 | 49 | 93 | 91 | 111 | 115 |
| TP5 (days) | 354 | 301 | 350 | 369 | 411 | 300 | 432 | – | 375 | – |
| MI 1 | 16.5 | 0 | 7 | 70.5 | 7 | 14 | – | 18.5 | 0 | 7 |
| MI 2 | 44 | 7 | 29 | 91.5 | – | 23.5 | 43 | 26.5 | 7 | 14 |
| MI 3 | 65 | 9.5 | 44 | 99 | 23.5 | – | 69 | 45.5 | 16.5 | – |
| MI 4 | 95 | 41 | 56.5 | 99 | 44 | 63.5 | 89.5 | 51 | 39 | 49.5 |
| MI 5 | 95 | 47.5 | 56.5 | 99 | 58 | 65 | 91.5 | – | 41.5 | – |
TP 1–5 represent the specific MR acquisition time (days after stroke) of each time point, and MI 1–5 represent the specific MI score for each patient at each time point.
BG, basal ganglia; CR, corona radiate; DT, diabetes; F, female; HL, hyperlipidemia; HT, hypertension; IC, internal capsule; LV, lesion volume; M, male; MI, Motricity Index (0–100); TP, time point.
Data Acquisition
Structural MRI data were acquired on a 3.0 Tesla MR scanner (Trio system; Siemens, Erlangen, Germany) using a T1‐weighted (T1W) sagittal 3D‐MPRAGE (magnetization prepared rapid acquisition gradient echo) sequence: echo time (TE) = 2.6 ms; inversion time (TI) = 800 ms; repetition time (TR) = 1,600 ms; flip angle (FA) = 9°; field of view (FOV) = 256 mm × 224 mm; matrix size = 512 × 448; slice thickness = 1 mm; voxel dimension = 1 mm × 1 mm × 1 mm. T2‐weighted images (T2WI) were acquired for constructing lesion tracings using a turbo‐spin‐echo (TSE) sequence: 20 axial slices, thickness/gap = 5/1.5 mm, matrix = 512 × 416, TR = 4,140 ms, TE = 92 ms, FA = 150°, FOV = 187 mm × 230 mm. For each patient, a variable number of scans were performed after their stroke, and a total number of 44 acquisitions (up to five scanning sessions per subject) were collected (Table I).
Optimized VBM Analysis
The T1W data were analyzed using the optimized voxel‐based morphometry (VBM) technique implemented with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) [Ashburner and Friston,2000; Good et al.,2001]. A customized GM template was constructed from the control group based on the GM probability map of each subject [Shen et al.,2005,2007]. Then, a GMV map was generated in Montreal Neurological Institute (MNI) space for each scan in each patient and smoothed by a Gaussian filter with a full‐width at half‐maximum (FWHM) smoothing kernel of 8 mm [Draganski et al.,2004,2006]. Details about the optimized VBM method can be found in the Supporting Information. To eliminate the adverse impact of lesions on the accuracy of segmentation and spatial normalization [Mehta et al.,2003], lesions were manually outlined using MRIcron software (http://www.sph.sc.edu/comd/rorden/mricron/) and were masked out during the segmentation and normalization procedures [Crinion et al.,2007; Stebbins et al.,2008].
Statistical Analysis
To quantify the dynamic changes in GMV after stroke, a mixed‐effects model was employed in the present study. In contrast with repeated measures analysis of variance, the mixed‐effects model allowed us to use all available data for each patient, even if some time points were missing [Pineiro et al.,2002]. The random intercept term accounts for the correlation due to repeated measurements within a single patient [Gibbons et al.,1988]. For the mixed‐effects model used in our study, all patients were assumed to possess a common slope (fixed effect), and the intercepts allowed for variations across different patients (random effect):
| (1) |
where Y ij represents the GMV of the ith subject from the jth scan (up to five scans), μ is the constant term common to all subjects, b i is a random intercept allowing a unique intercept for each patient, X ij is the time interval (i.e., days after stroke), β1 is the scalar of fixed effect (i.e., the common slope), β2 is the quadratic term, Nis the number of subjects, and εij is the residual error of the model. This model could simultaneously evaluate both the linear (β1) and nonlinear (β2) changes. For each voxel of interest, the mixed model was estimated by the restricted maximum likelihood (REML) method [Lerch et al.,2005]. A one‐sample t‐test was then performed on β1 and β2 voxel by voxel to test if there was a significant change in GMV after stroke. Significance levels for the t statistics were set at q < 0.01, FDR (false discovery rate) corrected for multiple comparisons, and cluster size > 100 voxels. The q‐value is defined to be the FDR analogue of the P‐value, and the q‐value of an individual hypothesis test is the minimum FDR at which the test may be called significant [Genovese et al.,2002]. To demonstrate the dynamic changes in GMV after stroke, the GMV at a location with the most significant β1 was plotted against the corresponding time intervals. Considering lesion volume as a potential contributing variable, the same statistical analysis was repeated using the lesion volume as a covariate. To confirm the stability of the results using the analysis strategy, we also analyzed the GMV differences in eight healthy older subjects given two structural MRI scans across a 1.5‐year interval using a paired t‐test and did not find any significant GMV changes using the same threshold.
To analyze the relationship between the structural changes and motor function after stroke, the mixed‐effects model was also employed on brain regions showing significant changes in GMV after stroke. The mixed‐effects model was the same as the first model (1), but here, X i was the normalized MI score (calculated by subtracting the subject‐specific mean value from the score of each session). Because both changes in GMV and MI scores were correlated with the number of days after stroke, we investigated correlations between GMVs and MI scores with days after stroke as the nuisance covariate. Significance levels for the t statistics were set at P < 0.001 (two‐tailed, uncorrected) and cluster size > 100 voxels.
RESULTS
Clinical and Demographic Characteristics of Stroke Patients
The clinical and demographic data of the stroke patients are listed in Table I. The mean intervals between stroke onset and each scan were 2.7 ± 1.9 days, 12.4 ± 1.9 days, 31.4 ± 2.7 days, 97.6 ± 24.7 days, and 361.5 ± 46.7 days, respectively. The MI scores of each time point were obtained from each patient (Table I). As shown in Figure 1, all patients experienced a faster initial recovery followed by a slower asymptotic pattern. On the basis of the five patients who completed all five neurological assessments and MRI examinations, the repeated‐measures analysis of variance on the MI scale demonstrated a significant functional recovery (P < 0.001). The lesion location of each patient was demonstrated on axial MR images (Fig. 2), and the lesion volume (11.21 ± 9.46 mL) was determined by manual tracing on T2WI.
Figure 1.
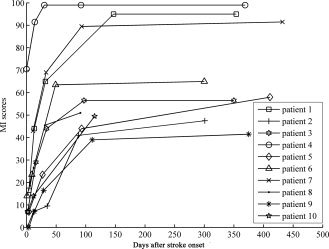
Post‐stroke recovery curves of stroke patients. The x‐axis denotes days after stroke onset; y‐axis denotes MI scores (ranging from 0 to 100, 100 represents complete recovery).
Figure 2.
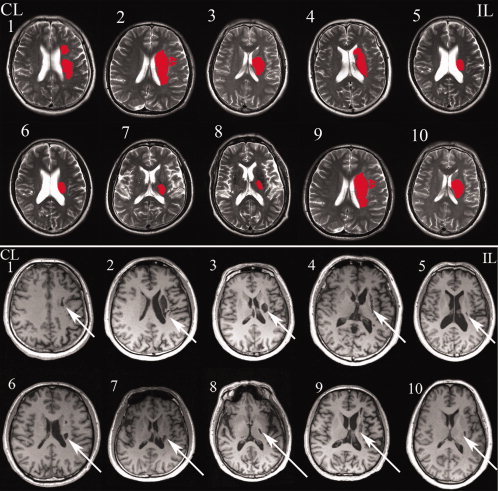
Lesion locations of stroke patients are shown on axial slices of the T2‐weighted and T1‐weighted MRI scans. CL, contralesional hemisphere; IL, ipsilesional hemisphere. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Whole Brain Analysis of Dynamic GMV Changes After Stroke
In the whole brain analysis, there were no significant nonlinear changes in GMVs in any brain areas after stroke; however, brain areas with significant linear changes in GMV are shown in Table II and Figure 3. Increased GMV following stroke was observed ipsilesionally in the mid‐cingulate cortex, lingual gyrus and cerebellum lobule VI, contralesionally in the hippocampus and bilaterally in the precuneus. Significant decreases in GMV after stroke were found in the sensorimotor cortex of the ipsilesional frontal and parietal lobes and in the contralesional cerebellum. Small clusters with decreased GMV were also found in the ipsilesional hippocampus and cingulate cortex and in the contralesional frontal, parietal, temporal and occipital lobes. In view of the effect of lesion volume on longitudinal analysis results [Smith et al.,2007], the same statistical analysis was repeated using lesion volume as a covariate, and similar results were obtained.
Table II.
Brain regions with significant changes in gray matter volume after stroke
| Location | Hemisphere | Local maximum | Cluster size (voxel) | |||
|---|---|---|---|---|---|---|
| Z‐value | x | y | z | |||
| Increased | ||||||
| Mid‐cingulate cortex | IL | 6.02 | −6 | −28 | 33 | 577 |
| Hippocampus | CL | 5.73 | 24 | −31 | −2 | 313 |
| Precuneus lobe | CL | 5.54 | 10 | −48 | 13 | 591 |
| Precuneus lobe | IL | 5.36 | −15 | −49 | 9 | 175 |
| Lingual gyrus | IL | 5.17 | −17 | −80 | −5 | 316 |
| Cerebellum VI | IL | 5.16 | −18 | −74 | −21 | 331 |
| Decreased | ||||||
| Precentral gyrus | IL | 5.57 | −55 | −7 | 43 | 8,684 |
| Precentral gyrus | CL | 5.20 | 0 | −32 | 56 | 2,508 |
| Postcentral gyrus | IL | 5.37 | −54 | −11 | 40 | 9,492 |
| Postcentral gyrus | CL | 4.29 | 18 | −37 | 71 | 410 |
| Premotor cortex | IL | 5.38 | −53 | −6 | 44 | 6,564 |
| Mid‐cingulate cortex | IL | 5.92 | −5 | −40 | 55 | 4,282 |
| Mid‐cingulate cortex | CL | 5.09 | 5 | 3 | 30 | 1,624 |
| Middle frontal gyrus | IL | 4.45 | −25 | 64 | 11 | 427 |
| Posterior cingulate cortex | IL | 4.44 | −4 | 59 | 3 | 124 |
| Posterior cingulate cortex | IL | 4.97 | −3 | −41 | 17 | 122 |
| Hippocampus | IL | 4.94 | −31 | −40 | −2 | 4,328 |
| Parahippocampus | CL | 4.62 | 36 | −41 | −3 | 211 |
| Caudate | CL | 4.39 | 14 | 5 | 10 | 255 |
| Cuneus lobe | CL | 4.28 | 19 | −82 | 44 | 359 |
| Superior frontal gyrus | IL | 4.22 | −25 | 46 | 12 | 342 |
| Middle frontal gyrus | IL | 3.79 | −23 | 16 | 41 | 162 |
| Superior frontal gyrus | IL | 3.78 | −16 | 43 | 46 | 209 |
| Middle temporal gyrus | CL | 3.96 | 45 | −66 | 18 | 201 |
| Superior temporal gyrus | CL | 4.20 | 49 | −49 | 19 | 826 |
| Angular gyrus | CL | 3.92 | 51 | −63 | 35 | 282 |
| Cuneus lobe | CL | 3.90 | 32 | −90 | 25 | 140 |
| Inferior parietal lobule | CL | 3.90 | 37 | −42 | 42 | 271 |
| Cerebellum anterior lobe | CL | 4.95 | 8 | −51 | −38 | 463 |
| Cerebellum VI | CL | 5.70 | 38 | −53 | −27 | 11,517 |
| Cerebellum VIII | CL | 4.64 | 9 | −66 | −42 | 404 |
| Cerebelum_Crus1 | CL | 4.37 | 10 | −87 | −23 | 223 |
x, y, z represent peak‐value voxel location in the standard MNI space. Z‐value is the maximum Z score of the cluster demonstrating significant longitudinal changes in gray matter volume after stroke.
CL, contralesional hemisphere; IL, ipsilesional hemisphere.
Figure 3.
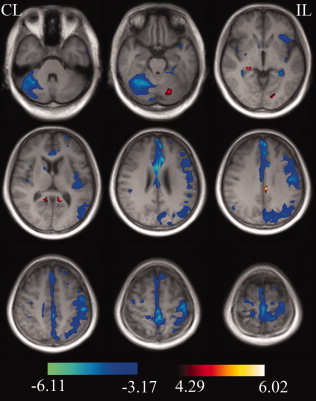
Whole brain analysis shows brain regions with significant changes in gray matter volumes after stroke. These brain areas are displayed on the representative slices of the average T1‐weighted images of all stroke patients with q < 0.01, false discovery rate (FDR) corrected for multiple comparisons, and cluster size >100 voxels. CL, contralesional hemisphere; IL, ipsilesional hemisphere. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Correlation Analysis Between GMV and Motor Function
A mixed‐effects model was used to analyze relationships between structural changes and motor function (MI scores) after stroke in brain regions showing significant GMV changes in the whole brain analysis. As shown in Figure 4, when controlling for the effect of days after stroke, significant correlations (P < 0.001, uncorrected) between the GMVs and MI scores were found only contralesionally in the hippocampus and precuneus.
Figure 4.
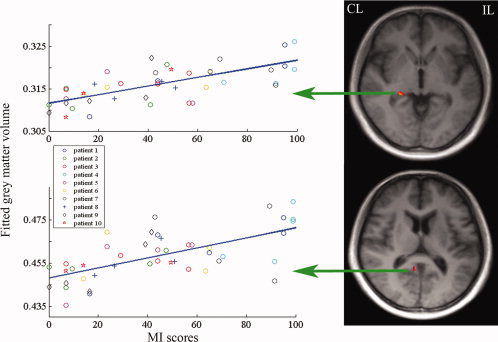
Brain regions with significant correlation between gray matter volume and MI scores. These brain areas are overlaid on slices of the averaged T1‐weighted images of all stroke patients, including the contralesional hippocampus (peak voxel x = 25, y = −34, z = −3, cluster size 135 voxels, z = 4.97, P < 0.001, uncorrected) and the contralesional precuneus (peak voxel x =7, y = −50, z = 11, cluster size 143 voxels, z = 4.14, P < 0.001, uncorrected). For the correlation map, the x‐axis represents MI scores at each time point after stroke, and the y‐axis denotes the mean gray matter volume of each cluster. CL, contralesional hemisphere; IL, ipsilesional hemisphere. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Increased GMV After Stroke
The most important finding of this study is the increased GMVs in the contralesional hippocampus and precuneus after stroke, and these increases in GMVs are positively correlated with the recovery of motor function. This finding suggests that the structural plasticity of these two brain areas is involved in the spontaneous recovery of motor function in stroke patients, which is consistent with a previous VBM study that emphasized the contribution of the structural plasticity of the hippocampus to the therapy‐induced recovery of motor function in stroke patients after constraint‐induced movement therapy [Gauthier et al.,2008]. Although the exact mechanism needs to be further investigated, we think the recruitment of cognitive resources might be a candidate since both the hippocampus and precuneus are known to be involved in a variety of cognitive functions, such as learning and memory [Cavanna and Trimble,2006; Howland and Wang,2008]. The importance of cognitive condition or ability for the recovery of motor function has been extensively reported [Leung et al.,2010; Oneş et al.,2009]. Moreover, cognitive strategy‐based interventions have been shown to have beneficial effects on the recovery of motor function in stroke patients [Cirstea et al.,2006; McEwen et al.,2009]. Taken all together, the plastic changes in cognitive‐related brain areas may facilitate the recovery of motor function in stroke patients, which calls for further development of cognitive strategy‐based interventions to improve long‐term stroke outcomes.
In the present study, we also found increased GMVs following stroke in several secondary motor‐related brain areas, such as the ipsilesional cingulate motor area and the cerebellum lobule VI. However, the GMVs of these areas were not correlated with the MI scores after controlling for the time effect. Considering the rather small sample size used in the present study, we cannot exclude the contribution of these secondary motor‐related brain areas to the recovery of motor function in stroke patients. As for the secondary motor‐related areas in the ipsilesional cerebral hemisphere, several previous studies have consistently reported that the functional plasticity in these areas contributed to both the spontaneous recovery and therapy‐induced improvement of motor function in stroke patients [Enzinger et al.,2009; Fridman et al.,2004; Johansen‐Berg et al.,2002]. Nevertheless, the ipsilesional cerebellum is a component of the unaffected motor network whose role in the recovery of motor function of the affected limbs is a matter of debate. On the one hand, the recruitment of the unaffected motor network is reported to be a temporary phenomenon [Feydy et al.,2002; Marshall et al.,2000; Nelles et al.,1999; Tombari et al.,2004] and is sometimes suggested to be a predictor of a negative outcome [Dafotakis et al.,2008; Enzinger et al.,2008; Loubinoux et al.,2003]. On the other hand, a substantial contribution by the unaffected motor network to the recovery of motor function of affected limbs has also been documented [Enzinger et al.,2009; Gauthier et al.,2008; Riecker et al.,2010; Serrien et al.,2004]. Our finding of the increased GMV in the ipsilesional cerebellum and lack of association between the increase in GMV and the improvement in motor function prevent us to draw a definite conclusion on its contribution to the functional recovery of the affected limbs. Besides compensatory mechanisms for the recovery of motor function in the affected limbs, other mechanisms may also be related to the increased GMV in the ipsilesional cerebellum after stroke. One mechanism is activity‐dependent plasticity; since stroke patients used their unaffected arms 3–6 times more than their affected ones [Vega‐González and Granat,2005]. Another mechanism is a compensatory change contributing to the recovery of dexterity in the unaffected hand. In stroke patients, reduced dexterity of the unaffected hand is a common clinical phenomenon during the initial stage, which later recovers, at least to some extent [Chestnut and Haaland,2008; Noskin et al.,2008; Nowak et al.,2007a; Sunderland,2000; Sunderland et al.,1999; Wetter et al.,2005]. The cerebellum also plays an important role in controlling the dexterity of the ipsilateral hand [Ehrsson et al.,2002; Imamizu et al.,2003; Nowak et al.,2007b].
Decreased GMV After Stroke
Another finding of this study is the extensively decreased GMVs bilaterally in brain areas that are directly or indirectly connected with the lesions, especially in the affected hemisphere, which is consistent with a previous VBM study that reported diffusive atrophy in the bilateral sensorimotor areas at chronic stage of stroke [Kraemer et al.,2004]. Many factors (axonal degeneration, compromised blood flow and metabolism) may be related to the atrophied “healthy” brain tissues in stroke patients. Among them, secondary axonal degeneration is the most important because almost all of these atrophied brain areas are directly or indirectly connected with the lesion site, and axonal degeneration in “healthy” white matter fiber tracts in stroke patients has been revealed by diffusion tensor imaging [Yu et al.,2009]. Our finding of decreased GMV in the ipsilesional primary sensorimotor area seems inconsistent with the structural [Schaechter et al.,2006] and functional plasticity [Calautti and Baron,2003; Enzinger et al.,2009; Feydy et al.,2002; Loubinoux et al.,2007; Pariente et al.,2001; Schaechter et al.,2006] in this area in stroke patients. The discrepancy in results between Schaechter et al. [2006] and ours may be caused by the difference in experimental design. The former employed a cross‐sectional design and a small sample size [Schaechter et al.,2006], which lends itself to false positive results. In the present study, we employed a longitudinal design, which lends strength to our data regardless of the small sample size. Moreover, our results are consistent with previous work [Kraemer et al.,2004]. Regarding the decreased GMV and increased activation in the same brain areas, our explanation is that the degenerated and plastic brain regions are differentially located and exert different roles in the recovery of motor function. The former is located in brain areas connected with the lesions, and the process of degeneration will last for several months or years in these areas. The degeneration process will be related to the damage and bad outcome of motor function [Yu et al.,2009] but cannot affect the recovery of motor function. However, the latter is located in brain regions near the degenerated areas, and their structural and functional plasticity will contribute to the recovery of motor function.
Limitations
Although this is a longitudinal designed study, the relatively small sample size prevents us from providing conclusive evidence for structural changes in the brain after stroke. Longitudinal studies with large sample sizes should be performed to validate our findings. The non‐uniformity of motor deficits in stroke patients is another limitation of our study. Although all 10 stroke patients had motor deficits in both the upper and lower extremities, they showed varying degrees across individuals. The recruited stroke patients were also relatively young (mean age: 48.7 years), and future studies must address the structural changes in older stroke patients.
CONCLUSION
To our knowledge, this is the first longitudinal study to investigate dynamic structural changes in the motor‐related network after motor pathway subcortical stroke in humans. We found decreased GMVs in brain areas connected to the lesions, and the GMV changes were not associated with the improvement of motor function, which suggests structural damage in “healthy” brain tissues. We also found increased GMVs in cognitive‐related brain areas that were positively correlated with the recovery of motor function, which indicates the contribution of structural plasticity in cognitive‐related brain areas to stroke recovery. Some secondary motor‐related areas also showed increased GMVs that were not correlated with the recovery of motor function, which suggests the functional significance of these changes requires further investigation.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
REFERENCES
- Ashburner J, Friston KJ ( 2000): Voxel‐based morphometry: The methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Bohannon RW ( 1999): Motricity index scores are valid indicators of paretic upper extremity strength following stroke. J Phys Ther Sci 11: 59–61. [Google Scholar]
- Calautti C, Baron JC ( 2003): Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke 34: 1553–1566. [DOI] [PubMed] [Google Scholar]
- Calautti C, Leroy F, Guincestre JY, Baron JC ( 2001): Dynamics of motor network overactivation after striatocapsular stroke: A longitudinal PET study using a fixed‐performance paradigm. Stroke 32: 2534–2542. [DOI] [PubMed] [Google Scholar]
- Cameron D, Bohannon RW ( 2000): Criterion validity of lower extremity Motricity Index scores. Clin Rehabil 14: 208–211. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M ( 2010): Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol 67: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioral correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Chestnut C, Haaland KY ( 2008): Functional significance of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil 89: 62–68. [DOI] [PubMed] [Google Scholar]
- Cirstea CM, Ptito A, Levin MF ( 2006): Feedback and cognition in arm motor skill reacquisition after stroke. Stroke 37: 1237–1242. [DOI] [PubMed] [Google Scholar]
- Collin C, Wade D ( 1990): Assessing motor impairment after stroke: A pilot reliability study. J Neurol Neurosurg Psychiatry 53: 576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K ( 2007): Spatial normalization of lesioned brains: Performance evaluation and impact on fmri analyses. Neuroimage 37: 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafotakis M, Grefkes C, Eickhoff SB, Karbe H, Fink GR, Nowak DA ( 2008): Effects of rTMS on grip force control following subcortical stroke. Exp Neurol 211: 407–412. [DOI] [PubMed] [Google Scholar]
- Demeurisse G, Demol O, Robaye E ( 1980): Motor evaluation in vascular hemiplegia. Eur Neurol 19: 382–389. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A ( 2004): Neuroplasticity: Changes in gray matter induced by training. Nature 427: 311–312. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A ( 2006): Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 26: 6314–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Kuhtz‐Buschbeck JP, Forssberg H ( 2002): Brain regions controlling nonsynergistic versus synergistic movement of the digits: a functional magnetic resonance imaging study. J Neurosci 22: 5074–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzinger C, Johansen‐Berg H, Dawes H, Bogdanovic M, Collett J, Guy C, Ropele S, Kischka U, Wade D, Fazekas F, Matthews PM ( 2008): Functional MRI correlates of lower limb function in stroke victims with gait impairment. Stroke 39: 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzinger C, Dawes H, Johansen‐Berg H, Wade D, Bogdanovic M, Collett J, Kischka U, Ropele S, Fazekas F, Matthews PM ( 2009): Brain activity changes associated with treadmill training after stroke. Stroke 40: 2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby‐Brami A, Bussel B, Cazalis F, Pierot L, Burnod Y, Maier MA ( 2002): Longitudinal study of motor recovery after stroke: Recruitment and focusing of brain activation. Stroke 33: 1610–1617. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG ( 2004): Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127: 747–758. [DOI] [PubMed] [Google Scholar]
- Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G ( 2008): Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke 39: 1520–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Waternaux C, Davis JM ( 1988): Random regression models: A comprehensive approach to the analysis of longitudinal psychiatric data. Psychopharmacol Bull 24: 438–443. [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS ( 2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14: 21–36. [DOI] [PubMed] [Google Scholar]
- Howland JG, Wang YT ( 2008): Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res 169: 145–158. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Kuroda T, Miyauchi S, Yoshioka T, Kawato M ( 2003): Modular organization of internal models of tools in the human cerebellum. Proc Natl Acad Sci USA 100: 5461–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM ( 2002): Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 125: 2731–2742. [DOI] [PubMed] [Google Scholar]
- Kraemer M, Schormann T, Hagemann G, Qi B, Witte OW, Seitz RJ ( 2004): Delayed shrinkage of the brain after ischemic stroke: Preliminary observations with voxel‐guided morphometry. J Neuroimaging 14: 265–272. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC ( 2005): Focal decline of cortical thickness in alzheimer's disease identified by computational neuroanatomy. Cereb Cortex 15: 995–1001. [DOI] [PubMed] [Google Scholar]
- Leung AW, Cheng SK, Mak AK, Leung KK, Li LS, Lee TM ( 2010): Functional gain in hemorrhagic stroke patients is predicted by functional level and cognitive abilities measured at hospital admission. NeuroRehabilitation 27: 351–358. [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Carel C, Pariente J, Dechaumont S, Albucher JF, Marque P, Manelfe C, Chollet F ( 2003): Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage 20: 2166–2180. [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Dechaumont‐Palacin S, Castel‐Lacanal E, De Boissezon X, Marque P, Pariente J, Albucher JF, Berry I, Chollet F ( 2007). Prognostic value of FMRI in recovery of hand function in subcortical stroke patients. Cereb Cortex 17: 2980–2987. [DOI] [PubMed] [Google Scholar]
- Luft AR, Waller S, Forrester L, Smith GV, Whitall J, Macko RF, Schulz JB, Hanley DF ( 2004): Lesion location alters brain activation in chronically impaired stroke survivors. Neuroimage 21: 924–935. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL ( 2000): Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke 31: 656–661. [DOI] [PubMed] [Google Scholar]
- McEwen SE, Huijbregts MP, Ryan JD, Polatajko HJ ( 2009): Cognitive strategy use to enhance motor skill acquisition post‐stroke: A critical review. Brain Inj 23: 263–277. [DOI] [PubMed] [Google Scholar]
- Mehta S, Grabowski TJ, Trivedi Y, Damasio H ( 2003): Evaluation of voxel‐based morphometry for focal lesion detection in individuals. Neuroimage 20: 1438–1454. [DOI] [PubMed] [Google Scholar]
- Nelles G, Spiekramann G, Jueptner M, Leonhardt G, Müller S, Gerhard H, Diener HC ( 1999): Evolution of functional reorganization in hemiplegic stroke: A serial positron emission tomographic activation study. Ann Neurol 46: 901–909. [DOI] [PubMed] [Google Scholar]
- Nomura EM, Gratton C, Visser RM, Kayser A, Perez F, D'Esposito M ( 2010): Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci USA 107: 12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noskin O, Krakauer JW, Lazar RM, Festa JR, Handy C, O'Brien KA, Marshall RS ( 2008): Ipsilateral motor dysfunction from unilateral stroke: implications for the functional neuroanatomy of hemiparesis. J Neurol Neurosurg Psychiatry 79: 401–406. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Küst J, Karbe H, Fink GR ( 2007a): Dexterity is impaired at both hands following unilateral subcortical middle cerebral artery stroke. Eur J Neurosci 25: 3173–3184. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Timmann D, Hermsdörfer J ( 2007b): Dexterity in cerebellar agenesis. Neuropsychologia 45: 696–703. [DOI] [PubMed] [Google Scholar]
- Oneş K, Yalçinkaya EY, Toklu BC, Caǧlar N ( 2009): Effects of age, gender, and cognitive, functional and motor status on functional outcomes of stroke rehabilitation. NeuroRehabilitation 25: 241–249. [DOI] [PubMed] [Google Scholar]
- Pariente J, Loubinoux I, Carel C, Albucher JF, Leger A, Manelfe C, Rascol O, Chollet F ( 2001): Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol 50: 718–729. [DOI] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury S, Johansen‐Berg H, Matthews PM ( 2001): Functional MRI detects posterior shifts in primary sensorimotor cortex activation after stroke: evidence of local adaptive reorganization? Stroke 32: 1134–1139. [DOI] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury S, Johansen‐Berg H, Matthews PM ( 2002): Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke 33: 103–109. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C ( 2011): Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage 55: 1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Gröschel K, Ackermann H, Schnaudigel S, Kassubek J, Kastrup A ( 2010): The role of the unaffected hemisphere in motor recovery after stroke. Hum Brain Mapp 31: 1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM ( 2006): Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain 129: 2722–2733. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Strens LH, Cassidy MJ, Thompson AJ, Brown P ( 2004): Functional significance of the ipsilateral hemisphere during movement of the affected hand after stroke. Exp Neurol 190: 425–432. [DOI] [PubMed] [Google Scholar]
- Sharma N, Baron JC, Rowe JB ( 2009): Motor imagery after stroke: Relating outcome to motor network connectivity. Ann Neurol 66: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Sterr A, Szameitat A ( 2005): A template effect study on voxel‐based morphometry in statistic parametric mapping. Conf Proc IEEE Eng Med Biol Soc 3: 3051–3054. [DOI] [PubMed] [Google Scholar]
- Shen S, Szameitat AJ, Sterr A ( 2007): VBM lesion detection depends on the normalization template: A study using simulated atrophy. Magn Reson Imaging 25: 1385–1396. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR ( 2007): Age and gender effects on human brain anatomy: A voxel‐based morphometric study in healthy elderly. Neurobiol Aging 28: 1075–1087. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Nyenhuis DL, Wang C, Cox JL, Freels S, Bangen K, deToledo‐Morrell L, Sripathirathan K, Moseley M, Turner DA, Gabrieli JD, Gorelick PB ( 2008): Gray matter atrophy in patients with ischemic stroke with cognitive impairment. Stroke 39: 785–793. [DOI] [PubMed] [Google Scholar]
- Sunderland A ( 2000): Recovery of ipsilateral dexterity after stroke. Stroke 31: 430–433. [DOI] [PubMed] [Google Scholar]
- Sunderland A, Bowers MP, Sluman SM, Wilcock DJ, Ardron ME ( 1999): Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke 30: 949–955. [DOI] [PubMed] [Google Scholar]
- Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, Cassol E, Chollet F ( 2004): A longitudinal fMRI study: In recovering and then in clinically stable sub‐cortical stroke patients. Neuroimage 23: 827–839. [DOI] [PubMed] [Google Scholar]
- van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, Viergever MA, Berkelbach van der Sprenkel JW, Dijkhuizen RM ( 2010): Recovery of sensorimotor function after experimental stroke correlates with restoration of resting‐state interhemispheric functional connectivity. J Neurosci 30: 3964–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega‐González A, Granat MH ( 2005): Continuous monitoring of upper‐limb activity in a free‐living environment. Arch Phys Med Rehabil 86: 541–548. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C ( 2010): Dynamic functional reorganization of the motor execution network after stroke. Brain 133: 1224–1238. [DOI] [PubMed] [Google Scholar]
- Weder B, Knorr U, Herzog H, Nebeling B, Kleinschmidt A, Huang Y, Steinmetz H, Freund HJ, Seitz RJ ( 1994): Tactile exploration of shape after subcortical ischaemic infarction studied with PET. Brain 117: 593–605. [DOI] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS ( 1992): Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol 31: 463–472. [DOI] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS ( 1993): Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol 33: 181–189. [DOI] [PubMed] [Google Scholar]
- Wetter S, Poole JL, Haaland KY ( 2005): Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil 86: 776–781. [DOI] [PubMed] [Google Scholar]
- Yu C, Zhu C, Zhang Y, Chen H, Qin W, Wang M, Li K ( 2009): A longitudinal diffusion tensor imaging study on wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage 47: 451–458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


