Abstract
Sentence processing problems form a common consequence of left‐hemisphere brain injury, in some patients to such an extent that their pattern of language performance is characterized as “agrammatic”. However, the location of left‐hemisphere damage that causes such problems remains controversial. It has been suggested that the critical site for syntactic processing is Broca's area of the frontal cortex or, alternatively, that a more widely distributed network is responsible for syntactic processing. The aim of this study was to identify brain regions that are required for successful sentence processing. Voxel‐based lesion‐symptom mapping (VLSM) was used to identify brain regions where injury predicted impaired sentence processing in 50 native speakers of Icelandic with left‐hemisphere stroke. Sentence processing was assessed by having individuals identify which picture corresponded to a verbally presented sentence. The VLSM analysis revealed that impaired sentence processing was best predicted by damage to a large left‐hemisphere temporo‐parieto‐occipital area. This is likely due to the multimodal nature of the sentence processing task, which involves auditory and visual analysis, as well as lexical and syntactic processing. Specifically impaired processing of noncanonical sentence types, when compared with canonical sentence processing, was associated with damage to the left‐hemisphere anterior superior and middle temporal gyri and the temporal pole. Anterior temporal cortex, therefore, appears to play a crucial role in syntactic processing, and patients with brain damage to this area are more likely to present with receptive agrammatism than patients in which anterior temporal cortex is spared. Hum Brain Mapp 34:2715–2723, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: stroke, agrammatism, syntactic comprehension, aphasia, anterior temporal
INTRODUCTION
It is well‐established that language deficits are common following damage to the left hemisphere. However, at a more fine‐grained level of language analysis, the anatomical basis for sentence processing remains under dispute, with evidence presented from functional imaging studies, as well as from lesion investigations of individuals who present with agrammatism, i.e., particular problems with the production and comprehension of functional categories and complex syntactic structures. Although most researchers in this field would agree that sentence processing, and even more fine‐grained syntactic processing, are ultimately functions of a distributed network of cortical and subcortical centers, some argue that this diffusion inherently means that no functional role can be identified for subparts within this network. According to the latter holistic view, agrammatism as an aphasic symptom does not associate with any particular focal injury, nor does it distinctly dissociate from other forms of aphasia. Particularly with respect to receptive agrammatic performance, our aim was to examine these claims using new measures of syntactic processing and high‐resolution medical imaging in a large group of individuals with acute injury.
Much of the discussion about localization of areas critical to syntactic processing revolves around Broca's area [Grodzinsky, 2000; Rogalsky and Hickock, 2011]. Traditionally, this part of left‐hemisphere inferior frontal cortex, including the pars triangularis and pars opercularis, has been viewed as crucial for speech production [Broca, 1861] and, more specifically, for motor‐speech planning [see Hillis et al., 2004]. However, since Caramazza and Zurif's [1976] seminal paper, Broca's area has also been recognized to play a role in syntactic processing [see also Heilman and Scholes, 1976]. Caramazza and Zurif observed that patients with Broca's aphasia, a disorder characterized by nonfluent, agrammatic speech production, also have difficulty comprehending grammatically complex sentences, in particular those with noncanonical word order, i.e. other than subject–verb–object in English. Language comprehension and production have thus been inferred to utilize the same syntactic functions [Grodzinsky, 2000; Swinney and Zurif, 1995; Zurif et al., 1993], while the association between the syndrome of Broca's aphasia and presumed damage to Broca's area led to localization of such functions in inferior frontal cortex.
Several studies appear to provide supporting evidence for the suggestion that Broca's area underlies syntactic processing, even if not exclusively [see Embick et al., 2000]. For example, Caplan, Hildebrandt and Makris [1996] examined the CT scans of 18 individuals with left hemisphere injury and suggested that syntactic processing involves Broca's area as well as an extensive neural network rooted in the left peri‐sylvian cortex. Furthermore, numerous functional neuroimaging studies have demonstrated a general role of the left peri‐sylvian cortex in syntactic processing in healthy speakers [e.g., Bornkessel et al., 2005; Friederici et al., 2003; Stromswold et al., 1996]. As to the nature of the role of Broca's area in syntactic processing, it has been claimed to support specific structure‐building or linearization operations [Bornkessel‐Schlesewsky et al., 2009; Grodzinsky and Friederici, 2006; Santi and Grodzinsky, 2007], more general cognitive processes of representational conflict resolution [Novick et al., 2005] or the integration/unification of different types of information into the sentence context [Hagoort, 2005]. Broca's area has also been claimed to support a working memory component underlying processes such as those listed above [Fiebach et al., 2005; Kaan and Swaab, 2002].
Other areas that are often implicated in syntactic functions are posterior temporal cortex, in particular posterior superior temporal sulcus (pSTS), and anterior temporal cortex [Den Ouden et al., 2012]. Activation in left‐hemisphere posterior superior temporal cortex has been shown to increase with syntactic complexity in sentence processing, [e.g., Just et al., 1996]. In line with observed effects of verb representation complexity, the role of this area in the syntactic network may well be one of thematic role assignment [Bornkessel et al., 2005; Den Ouden et al., 2009; Grewe et al., 2007; Shetreet et al., 2007; Thompson, et al., 2007, 2010].
Left anterior temporal cortex has also been suggested to play a role in sentence processing [see Kaan and Swaab, 2002]. Dronkers et al. [1994] found lesion damage to this area (anterior BA 22) to be associated with lower scores on morphosyntactic tests in patients with aphasia. Functional imaging studies also point to anterior middle and/or superior temporal gyri underlying morphosyntactic processing [Newman et al., 2010], or sentence structure analysis [e.g., Caplan et al., 2008; Humphries et al., 2005].
The notion that grammar is a modular function of the human brain remains controversial. Wilson and Saygun [2004] interpret data from 21 stroke patients as suggesting that the areas “important for syntax” are distributed throughout the left perisylvian region. Caplan et al. [2007] used acquired structural (MRI) and functional (positron emission tomography) data from 31 left hemisphere patients and inferred that syntax processing was localized in different regions in different individuals. A review by Dick and colleagues [2001] argues that grammar is a distributed function, suggesting “it is no more appropriate to refer to a participating region as a language zone or a grammar zone than it would be to refer to the elbow as a tennis organ”. With respect to the latter argument, it is important to note that to point out an area's crucial involvement in the execution of a certain function is by no means the same as to say that the area “has this function,” or that it is “the function” of this area to be thus involved. Even if elbows are not tennis organs, they do contribute something to the game that is quite relevant. Likewise, it may still be worthwhile to chart the role of specific regions in language processing, even if one recognizes that the role played is not their “function”.
While functional neuroimaging studies in neurologically healthy adults may provide important data to inform the brain‐syntax relationship in humans, they cannot identify areas that are crucial for grammatical processing, as opposed to areas that are involved in the execution of a grammatical task but may not be necessary for successful task completion. The general relation between left hemisphere damage and agrammatic language performance has been established, with further support from neuroimaging studies in normal subjects showing left‐hemisphere involvement in syntactic processing. Lesion‐symptom mapping can provide complementary data to findings from functional neuroimaging studies in normal subjects, revealing areas that may be crucially involved in certain types of processing.
It is conceivable that damage to any of the regions mentioned above might lead to agrammatic patterns of language behavior. A complicating factor in this is that the definition of agrammatism is not uncontroversial, nor indeed, is its status as a “syndrome,” autonomous or not from the similarly ill‐defined and controversial “Broca's aphasia”. In this study, therefore, we have chosen to focus on patterns of task performance in a nonselective group of patients with left‐hemisphere stroke and concomitant aphasia. Rather than separating them by binary diagnosis, we have correlated their scores on a sentence comprehension task with their lesion information, narrowing down to specific problems with noncanonical sentences, relative to canonical sentence structures.
This study examined localized brain damage associated with impaired sentence processing and more specifically with impaired processing of noncanonical syntactic structures. Fifty Icelandic stroke patients with acute left‐hemisphere injury were studied to determine brain damage associated with reduced sentence processing ability, as assessed by the comprehension of spoken sentences matched with pictures. Icelandic is structurally interesting in its own right (e.g., it is morphologically more complex than other Germanic languages), but it is also similar enough to other Germanic languages to allow for detailed comparison. Therefore, syntactic processing in Icelandic speakers may offer a new perspective on language comprehension in general [see also Magnúsdóttir, 2000].
METHOD
Participants
A total of 68 stroke patients admitted to the Neurology ward at Landspitali University Hospital in Reykjavik, Iceland, were recruited for this study. All participants had incurred a single ischemic stroke to the left hemisphere and gave informed consent for study inclusion. Patients were excluded from the study sample based on the following criteria: (1) non‐native speaker of Icelandic, (2) clinical history of stroke, (3) history of dementia, alcoholism, or major psychiatric illness, (4) contraindication for MRI examination, and (5) inability to complete the neuropsychological language battery. Out of 68 patients, three were unable to complete MRI examination, nine were unable to complete the neuropsychological battery, and three more were excluded based on severe auditory comprehension impairment. Three additional patients were excluded from further analysis after MRI scanning: one patient had no detectable brain lesion, and two patients were found to have right hemisphere lesions. Therefore, data from a total of 50 patients (24 female) were used in the final statistical analyses described below. The mean patient age was 63.8 (SD = 11.5 years), with a range of 34–85 years. Most patients received MRI and behavioral examination within three days of hospital admission. However, a handful of patients were examined as late as 20 days following stroke due to several factors such as fatigue and other stroke related issues. Behavioral and MRI examinations were always completed within 24‐hours of each other.
General language impairment was evaluated with the Bedside Evaluation Screening Test, 2nd edition [BEST‐2; Fitch‐West, Sands, and Ross‐Swain, 1998]. Based on the BEST‐2 overall aphasia severity scale, five patients had moderate language impairment, four had mild impairment, and the remaining 41 presented with no language impairment.
Behavioral Testing
All participants completed a sentence‐picture‐matching task, in which the experimenter spoke the target sentence and the participant was asked to point to a picture that corresponded to this sentence [Magnúsdóttir, 2005]. Stimulus materials consisted of line drawings, arranged horizontally in a three‐picture choice format [Magnúsdóttir, 2000]. Each set of three pictures consisted of a picture depicting the target sentence, a reverse version of the target sentence, and a lexical foil. The test consisted of 45 reversible test sentences, divided into nine groups of different sentence types, containing five sentences each (see 1–9).
In our description, (a) provides the Icelandic version of the sample sentence and (b) the proposed syntactic structure to give an idea of the nature of the complexity involved. Here “t i” and “t j” indicate the position of the relevant “gaps” (i.e., the site of moved or deleted constituents) to be associated with the preceding coindexed element [Chomsky, 1981, 1995]. When a finite verb has supposedly moved from its basic position inside a verb phrase (VP), this movement is indicated by a “t v” in the basic position(s). The Icelandic sentences used are followed by an English word by word gloss and an idiomatic translation. Case marking of the Icelandic noun phrases and agreement features are also indicated in the translation, using standard linguistic abbreviations (e.g. N, nominative; A, accusative; m, masculine, sg, singular, etc.) In a previous study [Magnúsdóttir, 2000], it was shown that some Icelandic agrammatic patients could use this morphological information as grammatical clues to the structure.

As these examples and their annotations show, in all sentence types, knowing who did what to whom involves associating the gaps with the appropriate antecedents. The total number of incorrect items (out of 45 total items) on the sentence‐picture‐matching paradigm was used as the dependent variable in the lesion‐symptom analysis described further below.
In separate analyses, we compared performance on sentences with canonical word order, i.e., subject‐verb‐object (C: sentence Types 1, 4, and 8), to that on noncanonical sentence types (NC: 2, 5, and 9), separately and in direct contrast. In this comparison, we excluded truncated sentences of Type 3, because these do not include an overt object (only a theme, but no agent), as well as the topicalized sentence Types 6 and 7, because their noncanonical syntactic structure cannot be directly compared with a canonical counterpart. We first calculated the main effects, using error scores on canonical and noncanonical sentence types separately. We then investigated the lesions associated with the relative performance on these sentence types by using the difference scores between noncanonical and canonical sentence processing as dependent variables, in both directions (NC‐C and C‐NC). Although we had a similar interest in even more fine‐grained analysis, lack of statistical power withheld us from comparing individual syntactic sentence types.
Neuroimaging Data
All participants underwent a 1.5T MRI workup (using a Siemens scanner) that included the following sequences: T1‐weighted imaging (3D GR/IR sequence, TR = 1,160 ms, TI = 600 ms, TE = 4.24 ms, flip angle = 15°, the 256 × 256 matrix was reconstructed at 512 × 512, yielding a 0.45 × 0.45 mm2 in axial‐plane resolution, with 192 0.9mm slices), diffusion‐weighted imaging (three scans with B0 = 0, 500, and 1,000; TR = 3,808 ms, TE = 89 ms, flip angle = 90°, Nx = 4, 192 × 192 matrix, 1.2 × 1.2 mm2 in axial plane, 24 slices each 5 mm thick with 1.5‐mm gap), and FLAIR (TR = 9,000 ms, TI = 2,500 ms, TE = 112 ms, flip angle = 15°, 280 × 320 matrix with 0.72 × 0.72 mm2 in axial plane resolution, 24 slices each 5 mm thick with 1.5‐mm gap). Only those who had an infarct seen on the FLAIR or DWI were included in the final data sample. Images were converted from DICOM to NIfTI format using dcm2nii (http://www.mricro.com), which preserves spatial coordinates (yielding a good starting estimate for the subsequent co‐registration of the T1 image to the T2 scan).
Brain lesions were demarcated on DWI images by a trained neurologist (LB) with extensive experience with lesion‐symptom mapping, using FLAIR and T1 images to help guide lesion boundaries. Spatial processing was conducted using SPM5. Initially, DWI images were co‐registered to the individual's T1 scan. This transform was applied to the lesion map. Finally, the T1 image was warped to standard MNI space using SPM5′s unified segmentation and normalization algorithm, which has proved robust even in the case of large lesions [Crinion et al., 2007]. Each subject's lesion mask was smoothed at 8 mm FWHM, and used as a cost‐function mask to decrease the effect of abnormal tissue in the computation of normalization parameters [Andersen et al., 2010; Brett et al., 2001]. The resulting transforms were applied to both the T1 scans and lesion maps for each patient, with the resulting images resliced to an isotropic 2 mm in standard MNI space. This normalization procedure effectively aligns the shape and size of each individual's brain to the same stereotaxic spacing, allowing voxelwise statistical analysis across individuals.
Voxelwise lesion‐symptom mapping (VLSM) was carried out using NPM [non‐parametric mapping; Rorden et al., 2007], a software package available from http://www.mricro.com. For every voxel, a t‐test was computed contrasting behavioral performance in patients with a lesion at that location to those without a lesion [cf. Bates et al., 2003]. Voxels which were damaged in less than 5% of the sample were excluded from the analysis. The resulting statistical maps were corrected for multiple comparisons using the false‐discovery rate to ensure a false‐positive rate of P < 0.05 [Genovese et al, 2002]. Corrected Z‐thresholds were 2.23 for the full analysis, 2.35 and 2.38 for the main effects contrasts (canonical and noncanonical, respectively), and 2.69 for the syntactic contrast. Additionally, a cluster threshold of 10 voxels was applied to each statistical map to exclude any remaining single‐voxel false positives. To ensure adequate detection of lesion‐behavior relationships, a power analysis was conducted [see Glascher et al., 2009, for details, also see Kimberg et al., 2007, for further discussion], the results of which are illustrated in Figure 1b. The minimum Z‐score from the power analysis was 3.15, such that all voxels examined had adequate power (Power‐Z > Threshold‐Z) to be able to detect correspondence between brain damage and behavior.
Figure 1.
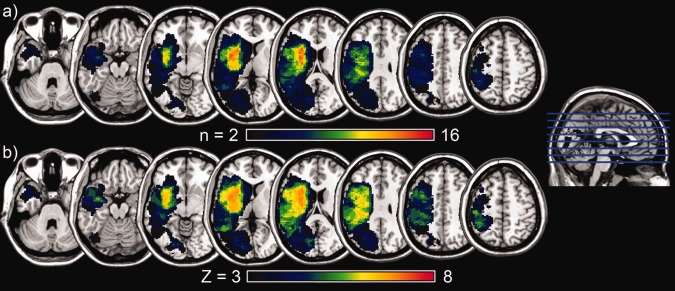
Lesion information. A: Lesion overlay map. This shows the lesion overlap from the fifty subjects included in the study. The maximum site of overlap is in the insular region, where up to sixteen subjects had shared regions of damage. B: Power map. This shows the maximum possible Z‐score, given the number of subjects with damage to each region.
RESULTS
Of the 50 patients who were retained in the final analysis, 12 achieved a perfect score (45/45) on the syntactic processing test. The mean number of correct trials among those who made one or more errors was 33.6 (SD = 10.1) with a range of 12–44. With the exception of one patient, all 50 participants scored better than chance.
The lesion overlap illustration presented in Figure 1a shows that the combined lesions of the group of participants cover almost the entire extent of the left hemisphere, excluding only prefrontal and superior frontal cortex and underlying white matter. Insular cortex and underlying white matter are affected in most of the patients in this sample.
The VLSM analysis revealed that impaired sentence processing in the auditory–visual sentence picture matching task was best predicted by damage to a large left‐hemisphere temporo‐parieto‐occipital area, with a total volume of 91.21 cc. This region encompasses middle and superior temporal gyri, inferior parietal cortex, angular gyrus, superior to inferior occipital cortex and fusiform gyrus, and extends inferiorly to the medial temporal lobe (see Table 1 and Fig. 2a). Table 1 lists the areas for each contrast for which the set of correlated voxels encompassed a proportion greater than 0.1 of the total cortical/white matter. As to the main effects of scores on canonical and noncanonical sentence types separately, there was relatively greater superior and posterior involvement for the canonical sentence types (60.92 cc total volume), with greater inferior and anterior involvement for the noncanonical types (68.76 cc total volume), particularly in the temporal and frontal lobes (see Table 1 and Fig. 2b).
Table 1.
Cortical areas and white matter tracts associated with sentence processing performance for various sentence types
| Sentence type | Total volume | Lesioned areas (left hemisphere) |
|---|---|---|
| All sentences (1‐9) | 91.21cc. | Cortical: |
| Amygdala; Angular; Heschl; Inferior frontal – opercularis and triangularis; Inferior parietal; Inferior, middle and superior temporal; Middle occipital; Middle and superior temporal pole; Rolandic operculum | ||
| Subcortical: | ||
| Anterior and posterior corona radiata; Posterior thalamic radiation; Retrolenticular internal capsule; Sagittal stratum/Inferior longitudinal/fronto‐occipital fasciculi; Superior longitudinal fasciculus; Tapetum; Uncinate fasciculus | ||
| Canonical (1, 4 and 8) | 60.92 cc. | Cortical: |
| Angular; Middle occipital; Middle temporal Inferior Parietal | ||
| Subcortical: | ||
| Anterior and posterior corona radiata; Posterior thalamic radiation; Retrolenticular internal capsule; Superior longitudinal fasciculus; Tapetum; Uncinate fasciculus | ||
| Noncanonical (2, 5, and 9) | 68.76 cc. | Cortical: |
| Amygdala; Heschl; Inferior frontal – opercularis and triangularis; Inferior occipital; Inferior, middle and superior temporal; Middle and superior temporal pole; Parahippocampal; Rolandic operculum | ||
| Subcortical: | ||
| Posterior corona radiata; Posterior thalamic radiation; Retrolenticular internal capsule; Sagittal stratum/Inferior longitudinal/fronto‐occipital fasciculi; Superior longitudinal fasciculus; Tapetum; Uncinate fasciculus | ||
| Canonical>Noncanonical (1, 4, and 8) vs. (2, 5, and 9) | 28.94 cc | Cortical: |
| Amygdala; Parahippocampal; | ||
| Middle and superior temporal; Middle and superior temporal pole | ||
| Subcortical: | ||
| Fornix/Stria terminalis; Retrolenticular internal capsule; Sagittal stratum/Inferior longitudinal/fronto‐occipital fasciculi |
Listed are all areas for which the set of voxels for a contrast encompassed a proportion > 0.1 of the total cortical/white matter area. Area names are given according to aal naming conventions [Tzourio‐Mazoyer et al., 2002]. Note the greater posterior involvement for the canonical sentence types, with greater temporal and frontal involvement for the noncanonical types.
Figure 2.
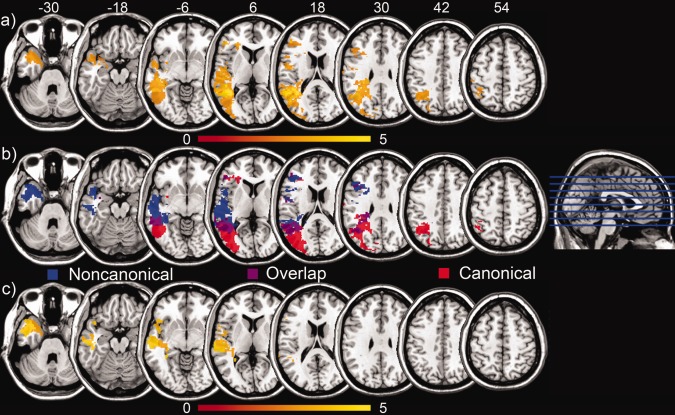
Lesion symptom mapping results. A: A large temporal‐occipital‐parietal region was found, where structural damage was correlated with decreased overall performance on the sentence comprehension battery. Regions with highest t‐values are shown in yellow. B: Canonical sentence types were matched to equivalent noncanonical types, and patterns of deficit were examined for each. Posterior and superior damage was associated with difficulty on canonical sentences (as seen in red), while anterior and inferior lesions were associated with difficulty on non‐canonical sentences (as seen in blue). Areas shown in purple exhibit overlap between both sentence types. C: Directly contrasting performance on the canonical and noncanonical sentences, regions were found in the superior and middle temporal lobes, through the temporal pole, where damage was associated with increased difficulty with noncanonical sentence types.
Specifically impaired processing of noncanonical sentence types, when directly compared with canonical sentence processing, was associated with damage to the left‐hemisphere anterior superior and middle temporal gyri and the temporal pole (see Fig. 2c and Table 1). The total volume of this area was 28.94 cc. The opposite contrast, of better performance on noncanonical structures over canonical sentence types, was not associated with damage to a particular area. These findings were robust, surviving a false discovery rate (FDR) threshold of P < 0.05 [Genovese et al., 2002].
DISCUSSION
The present results show that damage to temporo‐parieto‐occipital cortex and the underlying white matter predicts impaired performance on an auditory–visual sentence processing task in Icelandic adult stroke patients. A similar, though slightly more lateral cluster was also found in a recent VLSM study of sentence comprehension, using English‐speaking stroke patients [Thothathiri et. al., 2011]. The extent of this region is likely due to the multimodal nature of the sentence processing task, which involves auditory and visual analysis, as well as lexical and syntactic processing. In the first analysis, no differentiation was made between sentence types, so a wide array of factors and functional deficits may underlie difficulties with the sentence processing task, as such.
A follow‐up analysis examined specific problems with complex syntactic processing, by comparing scores on sentences with canonical word order with those on sentences with noncanonical word order. There was an inferior, anterior bias for lesioned areas correlated with impaired noncanonical sentence processing, versus a superior, posterior bias for areas associated with impaired canonical sentence processing. Thothathiri and colleagues [2011] also found canonical processing to be associated with more superior areas than noncanonical processing, though the effects were largely restricted to the parietal lobe in that study. Patients who use a default strategy in the parsing of reversible sentences, for example by assigning the thematic role of agent to the first verb argument they encounter, will make more picture selection errors on the noncanonical sentences, in which the first argument may be a theme, or a goal. Patients with an intact syntactic deconstruction mechanism are not likely to show this differential pattern between canonical and noncanonical sentence processing. There is no theoretical reason to expect certain patients to have more difficulty with canonical sentences than with noncanonical sentences.
The areas in which lesions were correlated with impaired syntactic processing (C>NC task performance) were found primarily in anterior middle and superior temporal cortex, including the temporal pole. It is interesting to note that this area encompasses the area found by Dronkers et al. [1994] to be involved in morphosyntactic processing in a group of English‐speaking patients with agrammatic aphasia, so these results converge. The difference between noncanonical and canonical sentence types, for example subject clefts versus object clefts, is not resolved through word order analysis alone in Icelandic, but crucially also through morphosyntactic analysis of case marking (stelpan vs. stelpuna for “girl,” nominative vs. accusative). Looking at main effects of noncanonical sentence performance, we also observe lesion correlations in Broca's area, and we find a very small part of Broca's area (less than the 0.1 proportion for areas listed in Table 1) to be correlated with the C>NC behavioral pattern. This confirms the role that inferior frontal cortex appears to play in syntactic processing. The level of power obtained with the current task and sentence set does not allow us to tease apart effects of word order from more morphosyntactically driven effects, but this might be a fruitful path of further study. For comparision, Thothathiri [2011] examined syntactic processing as well, but found no regions significantly associated with syntax, either in Broca's area, or in the temporal lobe. This was possibly due to a smaller set of sentences (30 vs 45) and sentence types (6 vs 9) in that study than in the current one, leaving less behavioral variance to be explained by the presence or absence of regional brain damage.
Interestingly, Caplan's [2001] thorough compilation of results from different PET and fMRI studies in normal subjects does not only point to great involvement of Broca's area in the processing of complex sentences but also to increased activation in other parts of the left perisylvian association cortex. More specifically, functional neuroimaging studies suggest that, compared to parsing simpler sentence structures, grammatically complex sentences such as passives, object clefts, topicalization sentences, and wh‐questions induce greater activity in Broca's area as well as posterior regions such as the superior temporal gyrus. Although these most posterior regions are not associated specifically with impaired syntactic processing, they do appear to underlie performance on a sentence processing task, perhaps as neural substrates for other (types of) processes. As is the case with all activation studies in normal subjects, the results summarized by Caplan cannot reveal what areas are crucial for sentence parsing. Thus, the results of the present study add an important piece to the puzzle as they suggest that damage to anterior middle and superior temporal cortex is likely to result in impaired syntactic comprehension.
Several studies have also stressed the importance of using information about cerebral perfusion, in addition to structural scans, when defining regions to be examined and related to behavioral peformance [Fridriksson et al., 2012; Hillis et al., 2004, Richardson et al., 2011]. For example, Hillis et al. [2004] found that in some acute cases, perfusion deficits predicted behavioral deficits in cases where structural deficits did not. Additionally, the relationship between areas damaged structurally and areas with resulting hypoperfusion was not always straightforward. In any case, relying solely on structural measures to infer lesion location probably leads to additional variability in our measures. It is certainly possible that we would have found additional regions associated with syntactic processing problems, had we also examined perfusion in these patients. For the same reason, it is conceivable that the areas which we have identified play an indirect role in our question of interest, in that they may mediate hypoperfusion in regions which play a more direct role in syntactic processing.
We have identified brain areas in which lesions are correlated with impaired syntactic processing in Icelandic speakers with aphasia. This study is unable to definitively determine whether these patterns generalize to other languages, let alone make claims about lesions associated with more extensive clusters of symptoms, such as recognized in agrammatism. Clearly, Icelandic is a morphologically complex language involving case marking of nouns, subject agreement marking on verbs, and agreement marking on participles (e.g. in passives). Furthermore, while our results clearly demonstrate that lesions in anterior temporal cortex are robust predictors of impaired syntactic processing, this does not necessarily mean that other locations do not play a role in syntax processing in some individuals [as suggested by Caplan, 2007]. Such regions would not survive our conservative statistical thresholding.
This study did not consider the specific production deficits associated with agrammatism, deliberately focusing only on sentence comprehension scores. Our conclusions, therefore, only relate to receptive agrammatic behavior. While impaired syntactic processing is a vital characteristic of agrammatism, it is quite possible that an additional analysis quantifying agrammatical output may yield more detailed information on the neural damage underlying agrammatism as a “syndrome” or a set of symptoms.
In conclusion, these findings suggest that impaired syntactic processing is associated with anterior middle and superior temporal cortical brain lesions in Icelandic stroke patients. Similar studies in different languages should help determine which, if any, of our results are specific for morphologically complex languages like Icelandic.
REFERENCES
- Andersen SM, Rapcsak SZ, Beeson PM (2010): Cost function masking during normalization of brains with focal lesions: Still a necessity? Neuroimage 53:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF (2003): Voxel‐based lesion‐symptom mapping. Nat Neurosci 6:448–450. [DOI] [PubMed] [Google Scholar]
- Bornkessel I, Zysset S, Friederici AD, von Cramon DY, Schlesewsky M (2005): Who did what to whom? The neural basis of argument hierarchies during language comprehension. NeuroImage 26:221–233. [DOI] [PubMed] [Google Scholar]
- Bornkessel‐Schlesewsky I, Schlesewsky M, von Cramon DY (2009): Word order and Broca's region: Evidence for a supra‐syntactic perspective. Brain Lang 111:125–139. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J (2001)Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 14:486–500. [DOI] [PubMed] [Google Scholar]
- Broca P (1861): Remarques sur le siége de la faculté du langage articulé, suivies d'une observation d'aphémie (perte de la parole). Bulletins de la Societe anatomique (Paris) 2e series 6:330–357. [Google Scholar]
- Caplan D (2001): Functional neuroimaging studies of syntactic processing. J Psycholinguistic Res 30:297–320. [DOI] [PubMed] [Google Scholar]
- Caplan D, Hildebrandt H, Makris N (1996): Location of lesions in stroke patients with deficits in syntactic processing in sentence comprehension. Brain 119:933–949. [DOI] [PubMed] [Google Scholar]
- Caplan D, Waters G, Kennedy D, Alpert N, Makris N, Dede G, Michaud J, Reddy A (2007): A study of syntactic processing in aphasia. II. Neurological aspects. Brain Lang 101:151–177. [DOI] [PubMed] [Google Scholar]
- Caplan D, Stanczak L, Waters G (2008): Syntactic and thematic constraint effects on blood oxygenation level dependent signal correlates of comprehension of relative clauses. J Cogn Neurosci 20:643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB (1976): Dissociation of algorithmic and heuristic processes in language comprehension: Evidence from aphasia. Brain Lang 3:572–582. [DOI] [PubMed] [Google Scholar]
- Chomsky N (1981).Lectures on Government and Binding.Dordrecht:Foris; 413 p. [Google Scholar]
- Chomsky N (1995).The Minimalist Program.Cambridge, MA:MIT Press; 427 p. [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K (2007): Spatial normalization of lesioned brains: Performance evaluation and impact on fMRI analyses. NeuroImage 37:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Ouden DB, Fix S, Parrish TB, Thompson CK (2009): Argument structure effects in action verb naming in static and dynamic conditions. J Neurolinguistics 22:196–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Ouden DB, Saur D, Mader W, Schelter B, Lukic S, Wali E, Timmer J, Thompson CK (2012): Network modulation during complex syntactic processing. Neuroimage 59:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick F, Bates E, Wulfeck B, Utman JA, Dronkers N, Gernsbacher MA (2001): Language deficits, localization, and grammar: evidence for a distributive model of language breakdown in aphasic patients and neurologically intact individuals. Psychol Rev 108:759–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD Jr., Redfern BB, Jaeger JJ (1994): A reconsideration of the brain areas involved in the disruption of morphosyntactic comprehension. Brain Lang 47:461–463. [Google Scholar]
- Embick D, Marantz A, Miyashita Y, O'Neil W, Sakai KL (2000): A syntactic specialization for Broca's area. Proc Natl Acad Sci USA 97:6150–6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD (2005): Revisiting the role of Broca's area in sentence processing: Syntactic integration versus syntactic working memory. Hum Brain Mapp 24:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch‐West J, Sands ES, Ross‐Swain D.1998Bedside Evaluation Screening Test (BEST‐2), 2nd EdAliMed® Inc. [Google Scholar]
- Friederici AD, Rüschemeyer SA, Hahne A, Fiebach CJ (2003): The role of left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cerebral Cortex 13:170–177. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Fillmore P, Cai B (2012): Left hemisphere plasticity and aphasia recovery. Neuroimage 60:854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C, Lazar N, Nichols T (2002): Thresholding of statistical maps in neuroimaging using the false discovery rate. NeuroImage 15:870–878. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, Grabowski T, Damasio H, Adolphs R (2009): Lesion mapping of cognitive abilities linked to intelligence. Neuron 61:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe T, Bornkessel‐Schlesewsky I, Zysset S, Wiese R, von Cramon DY, Schlesewsky M (2007): The role of the posterior superior temporal sulcus in the processing of unmarked transitivity. Neuroimage 35:343–352. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y (2000): The neurology of syntax. Language use without Broca's area. Behav Brain Sci 23:1–71. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y, Friederici AD (2006): Neuroimaging of syntax and syntactic processing. Curr Opin Neurobiol 16:240–246. [DOI] [PubMed] [Google Scholar]
- Hagoort P (2005): On Broca, brain, and binding: A new framework. Trends Cogn Sci 9:416–423. [DOI] [PubMed] [Google Scholar]
- Heilman K, Scholes RJ (1976): The nature of comprehension errors in Broca's conduction and Wernicke's aphasics. Cortex 12:258–265. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K (2004): Reexamining the brain regions crucial for orchestrating speech articulation. Brain 127:1479–1487. [DOI] [PubMed] [Google Scholar]
- Humphries C, Love T, Swinney D, Hickok G (2005): Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Hum Brain Mapp 26:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR (1996): Brain activation modulated by sentence comprehension. Science 274:114–116. [DOI] [PubMed] [Google Scholar]
- Kaan E, Swaab TY (2002): The brain circuitry of syntactic comprehension. Trends Cogn Sci 6:350–356. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF (2007): Power in voxel‐based lesion‐symptom mapping. J Cogn Neurosci 19:1067–1080. [DOI] [PubMed] [Google Scholar]
- Magnúsdóttir S (2000): On grammatical knowledge in agrammatism. Evidence from Icelandic. Unpublished doctoral dissertation. Boston University.
- Magnúsdóttir, S .2005. Setningafræðipróf (Test of Syntax). Landspítali ‐ University Hospital, Reykjavík, Iceland.
- Newman AJ, Supalla T, Hauser P, Newport EL, Bavelier D (2010): Dissociating neural subsystems for grammar by contrasting word order and inflection. Proc Natl Acad Sci USA 107:7539–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson‐Schill SL (2005): Cognitive control and parsing: Reexamining the role of Broca's area in sentence comprehension. Cogn Affect Behav Neurosci 5:263–281. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Baker JM, Morgan PS, Rorden C, Bonilha L, Fridriksson J (2011): Cerebral perfusion in chronic stroke: Implications for lesion‐symptom mapping and functional MRI. Behav Neurol 24:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Hickok G (2011): The role of Broca's area in sentence comprehension. J Cogn Neurosci 23:1664–1680. [DOI] [PubMed] [Google Scholar]
- Rorden C, Fridriksson J, Karnath HO (2008): An evaluation of traditional and novel tools for lesion behavior mapping. NeuroImage 44:1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L (2007): Improving lesion‐symptom mapping. J Cogn Neurosci 19:1081–1088. [DOI] [PubMed] [Google Scholar]
- Santi A, Grodzinsky Y (2007): Working memory and syntax interact in Broca's area. NeuroImage 37:8–17. [DOI] [PubMed] [Google Scholar]
- Shetreet E, Palti D, Friedmann N, Hadar U (2007): Cortical representation of verb processing in sentence comprehension: number of complements, subcategorization, and thematic frames. Cereb Cortex 17:1958–1969. [DOI] [PubMed] [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rausch S (1996): Localization of syntactic comprehension by positron emission tomography. Brain Lang 52:452–473. [DOI] [PubMed] [Google Scholar]
- Swinney D, Zurif E (1995): Syntactic processing in aphasia. Brain Lang 50:225–239. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Bonakdarpour B, Fix SC, Blumenfeld HK, Parrish TB, Gitelman DR, Mesulam MM (2007): Neural correlates of verb argument structure processing. J Cogn Neurosci 19:1753–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Bonakdarpour B, Fix SF (2010): Neural mechanisms of verb argument structure processing in agrammatic aphasic and healthy age‐matched listeners. J Cogn Neurosci 22:1993–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thothathiri W, Kimberg D, Schwartz M (2012): The neural basis of reversible sentence comprehension: Evidence from voxel‐based lesion symptom mapping in aphasia. J Cogn Neurosci 24:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer T, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP (2004): Grammaticality judgment in aphasia: Deficits are not specific to syntactic structures, aphasic syndromes, or lesion sites. J Cogn Neurosci 16:238–252. [DOI] [PubMed] [Google Scholar]
- Zurif E, Swinney D, Prather P, Solomon J, Bushell C (1993): An on‐line analysis of syntactic processing in Broca's and Wernicke's aphasia. Brain Lang 45:448–464. [DOI] [PubMed] [Google Scholar]


