Abstract
It is well established that the insular cortex processes noxious information. We have previously shown that noxious inputs from the arm and leg are coarsely organized somatotopically within the dorsal posterior insula. The same has been shown for inputs from C tactile afferents, which mediate affective touch, and it has been suggested that the insula may be responsible for the localization of some somatosensory stimuli. Knowing the degree of spatial detail may have significant implications for the potential role of the dorsal posterior insula in the processing of noxious stimuli. Using high‐resolution functional magnetic resonance imaging (fMRI), we compared insula activation patterns in 13 subjects during muscle pain induced by injection of hypertonic saline (5%) into three muscles within the same limb: shoulder (deltoid), forearm (flexor carpi radialis), and hand (first dorsal interosseous). Mapping the maximally activated voxels within the contralateral dorsal posterior insula in each individual subject during each pain stimulus revealed a clear somatotopy of activation within the contralateral dorsal posterior insula. Shoulder pain was represented anterior to forearm pain and medial to hand pain. This fine somatotopic organization may be crucial for pain localization or other aspects of the pain experience that differ depending on stimulation site. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: insular cortex, functional magnetic resonance imaging, localization
INTRODUCTION
It is well established that the insula cortex is involved in the processing of noxious stimuli. It has been proposed that the dorsal posterior part of the insular cortex receives noxious information via direct, somatotopically organized projections from a pain‐specific region of the thalamus; the posterior portion of the ventromedial thalamic nucleus (VMpo) [Blomqvist et al.,2000; Craig,2003; Craig and Zhang,2006; Craig et al.,1994]. Indeed, we and others have recently shown that the insula is, at least crudely, somatotopically organized for noxious inputs, with noxious stimulation of the forearm evoking activation that was located immediately anterior and lateral to the region activated by noxious stimuli applied to the leg, although an area of overlap was also evident [Brooks et al.,2005; Henderson et al.,2007; Hua le et al.,2005].
Interestingly, it has recently been revealed that the dorsal posterior insula also displays a somatotopic organization for the processing of gentle touch, mediated by C tactile afferents [Bjornsdotter et al.,2009]. Indeed, the organization of functional activation evoked by gentle touch applied to the forearm and thigh was almost identical to that reported in our earlier investigation into noxious processing [Henderson et al.,2007]. In addition, the authors report that in a subject lacking large‐diameter afferents, insula somatotopy is preserved, suggesting that insula organization may underlie the patient's ability to correctly localize gentle touch; a previous study from this group had shown that C tactile afferent activation does not evoke signal increases in the primary somatosensory cortex [Olausson et al.,2008b]. Although it was suggested that the dorsal posterior insula may contain only a crude somatotopic map, it is possible that there is a preservation of afferent fiber organization from the body, through the VMpo, to the dorsal posterior insula.
Knowing the degree of spatial detail within the insula may have significant implications for the potential role of the dorsal posterior insula in the processing of noxious stimuli. For example, it is well established that the primary somatosensory cortex (SI) has a fine somatotopic organization and it is thought that this detail allows for the precise localization of somatosensory stimuli [Penfield and Boldrey,1937; Penfield and Rasmussen,1950]. Indeed, we have recently shown that within SI the degree of perceived referred pain following an intramuscular injection of hypertonic saline is represented by the extent of spread of SI activation [Macefield et al.,2007]. In contrast to SI, it has been shown in rodents that in phylogenetically older brain regions, such as the midbrain periaqueductal gray matter, noxious afferent inputs are also somatotopically organized, although this organization appears to be rather crude compared to SI [Keay and Bandler,2001]. Indeed, this relatively crude body representation of sensory input is presumably all that is required for the body to organize and direct the relatively gross behavioral responses to pain such as fight and flight. If a similar crude map exists within the dorsal posterior insula then it may be the case that this region is not involved in processing aspects of the pain experience that require precise stimulus localization, but instead is involved in processing more global aspects of the pain experience, such as the direction of behavioral responses.
The aim of this investigation was to use high‐resolution functional magnetic resonance imaging to determine if a fine somatotopic organization of noxious stimuli applied to muscles within a single limb (the arm) exists within the dorsal posterior insular cortex. Furthermore, we reanalyzed earlier data to create a whole‐body map of noxious inputs arising from skeletal muscles within the dorsal posterior insula.
MATERIALS AND METHODS
Subjects
Thirteen healthy subjects (nine males) aged 19–48 years participated in this study. All procedures were carried out with the understanding and written informed consent of each subject. All procedures were approved by local institutional Human Research Ethics Committees (University of Western Sydney and the University of Sydney) and were conducted in accordance with the conditions established by the Declaration of Helsinki.
Stimulus and MR Imaging
With subjects in a supine position, a fine plastic cannula (23 gauge), attached to a 1 ml syringe containing sterile hypertonic (4.5%) saline was inserted deep into the central belly of the left deltoid muscle, another into the belly of the left flexor carpi radialis muscle (FCR), and a third into the belly of the left first dorsal interosseus muscle (FDI). Continuous series of gradient echo image sets using Blood Oxygen Level Dependent (BOLD) contrast were then collected using a 3 Tesla, Siemens Magnetom Trio scanner (32 axial slices, TR = 4 s, TE = 40 ms, flip angle = 90 degrees, FOV = 220 × 220, raw voxel size = 1.96 × 1.96 × 4 mm3 thick, 0.4 mm interslice gap). Following a 70 volume baseline period, subjects received an intramuscular injection of hypertonic saline (1.0 ml) into one of the three muscles. A further 218 volumes were then collected for a total scanning period of 288 volumes (70 volume baseline, 218 volume challenge). Each subject received injections into each of the three different muscles during subsequent fMRI scans. Prior to scanning, the order of injections was selected randomly by the experimenter. Furthermore, subjects were not made aware of when the injection was to be administered or in which order it was to be presented. Each injection was separated by at least 20 min and was only performed after the pain from the previous injection had subsided to 0 and had remained at 0 for at least 10 min. During each scan, subjects were instructed to press a button with their right thumb to indicate when they (i) felt the onset of pain, (ii) the pain began to subside from its peak, and (iii) the pain had ceased. Immediately following each fMRI scan and while still inside the scanner, subjects were read a linear 10 point pain intensity scale (0 = no pain, 10 = maximum imaginable pain) and were asked to indicate the maximum pain intensity. Furthermore, each subject was presented with a standard picture of the arm on a clipboard and with the help of the standard MRI mirror, was asked to draw an outline of the area of perceived pain. A 3‐D T1 weighted anatomical scan (voxel size = 0.8 × 0.8 × 0.8 mm3) was also collected. During a separate scanning session, in two subjects, the same procedures were repeated but the hypertonic saline injections were made into the right arm.
MRI Analysis
Using SPM5 [Friston et al.,1995], all functional image sets were motion corrected and only subjects with movement parameters less than 1 mm in the X, Y, and Z planes were used for analysis. Following realignment, individual's functional images were spatially normalized to the Montreal Neurological Institute (MNI) template, global signals removed using the technique described by Macey et al., [2004] and spatially smoothed with a 6 mm full‐width‐at‐half‐maximum Gaussian filter.
Significant changes in signal intensity were determined on a voxel‐by‐voxel basis using a box‐car model (convolved with a haemodynamic delay) which approximated to the period of perceived pain (30 volume baseline, 30 volumes on). One‐sample t‐tests were performed to determine significant signal intensity increases and decreases during each of the three stimulation paradigms (P < 0.05 random effects, corrected for multiple comparisons, minimum cluster size 10 voxels). The resulting statistical maps were then overlaid onto a T1‐anatomical image set.
In addition, individual subject analyses were performed that were restricted to the contralateral posterior insula. The most significantly activated voxel within the contralateral posterior insula was determined in each subject for each of the three muscle pain stimuli. The co‐ordinates of these most significantly activated voxels, in MNI space, were determined and the mean (±SEM) values plotted onto an individual subject's T1‐weighted anatomical image. Significant differences in these MNI co‐ordinates during each of the three paradigms were determined (P < 0.05, two‐tailed t‐test). The X and Y co‐ordinates in each subject, for each of the three stimulation paradigms, were plotted and the 3‐dimensional distances between each of the maximally activated voxels during each stimulation paradigm determined using Euclidean geometry. In the two subjects in which hypertonic saline injections were made into both the left and right upper limbs, the activation maps within the dorsal posterior insular during all 6 noxious stimulation paradigms were overlaid onto their T1‐weighted anatomical images and the changes in signal intensities plotted over time.
Finally, in previous investigations we used fMRI to determined the patterns of brain activity evoked by hypertonic saline injections into the leg (tibialis anterior muscle, n = 19) and face (masetter muscle, n = 17) [Henderson et al.,2007; Nash et al.,2009]. We performed an identical processing and statistical analysis procedure on these previously obtained data and determined the maximally activated voxels within the contralateral dorsal posterior insula in each subject.
RESULTS
Pain Perception and Spread
In all subjects, injection of hypertonic saline evoked pain which began within 5–10 s of the injection began, reached a peak within ∼30 s, and remained above baseline for at least 4 min. The mean (±SE) maximum intensities of pain, rated on a 0–10 point visual analogue scale (VAS), were: deltoid: 5.0 ± 0.5, FCR: 5.9 ± 0.5 and FDI: 5.9 ± 0.6. There was no significant difference between the maximum pain intensities during each of the three paradigms (t‐test; P > 0.05). It can be seen in Figure 1 that, in the vast majority of subjects, injections of hypertonic saline into the deltoid and FDI muscles resulted in a relatively restricted pattern of perceived pain spread, i.e., immediately surrounding the injection site. In contrast, in a number of subjects, hypertonic saline injection into FCR muscle resulted in pain that was perceived as radiating distally, sometimes extending into the wrist and hand.
Figure 1.
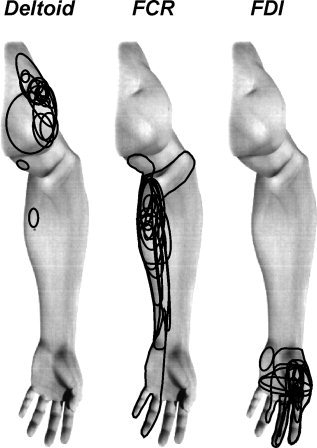
Individual pain referral patterns overlaid onto a standard picture of the arm following intramuscular hypertonic saline (5%) injection into the deltoid, flexor carpi radialis (FCR) and first dorsal interosseus (FDI) muscles.
Signal Intensity Changes
Group analysis
Group analysis revealed that muscle pain was associated with significant increases in signal intensity in a number of brain regions. Following hypertonic saline injection into the deltoid, FCR, and FDI muscles, significant increases in signal intensity occurred bilaterally in the anterior cingulate, anterior insular, dorsal posterior insular, cerebellar and secondary somatosensory cortices (see Fig. 2). Overall, the pattern of brain activation was similar during all three sources of muscle pain.
Figure 2.
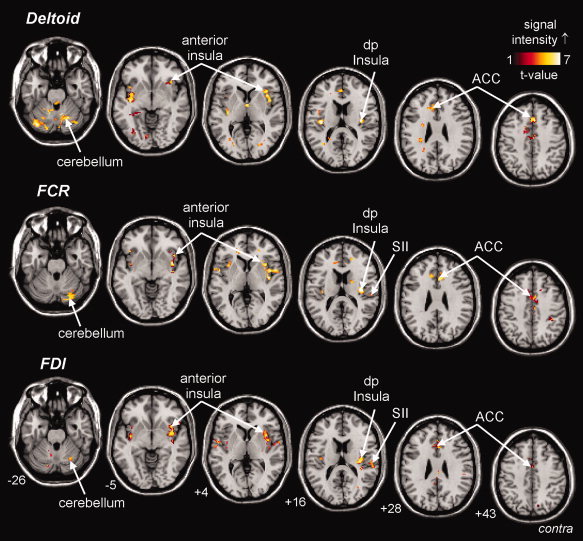
Cortical and subcortical regions which show increases (hot color scale) in signal intensity during pain in the deltoid (top row), flexor carpi radialis (FCR; middle row). and first dorsal interosseus (FDI; lower row) muscles. The slice locations in Montreal Neurological Institute (MNI) space are indicated in the bottom left of each image in the lower row. ACC, anterior cingulate cortex; SII, secondary somatosensory cortex; dp Insula, dorsal posterior insula.
Individual subject analysis
Within the contralateral posterior insula, an analysis of individual subject data revealed a clear somatotopy. Hypertonic saline injection in the deltoid muscle evoked signal intensity increases that were located medial to those evoked by FDI injections, and anterior to those evoked by FCR injections (Table I; Fig. 3). This somatotopic organization was confirmed in the two subjects in whom hypertonic saline was administered to both the left and right arms (on different days). That is, in three of four comparisons, deltoid pain activated a region medial to that activated by FDI pain and, in all four comparisons, deltoid pain activated a region anterior to that activated by FCR pain (see Fig. 4).
Table 1.
Mean (±SEM) X, Y, and Z co‐ordinates in Montreal Neurological Institute space within the contralateral dorsal posterior insular cortex following injection of hypertonic saline (5%) into the masetter, deltoid, flexor carpi radialis, first dorsal interossous, and tibilais anterior muscles
| X (±SEM) | Y (±SEM) | Z (±SEM) | |
|---|---|---|---|
| Face [masetter muscle: (Nash et al.,2009)] | 37.3 ± 0.8 | −15.4 ± 0.8 | 12.2 ± 0.8 |
| Shoulder (deltoid muscle) | 37.1 ± 0.6 | −15.5 ± 0.8 | 15.5 ± 1.0 |
| Forearm (flexor carpi radialis muscle) | 37.7 ± 0.6 | −18.6 ± 0.9 | 15.7 ± 0.9 |
| Hand (first dorsal interosseous muscle) | 38.6 ± 0.5 | −17.1 ± 0.8 | 14.6 ± 1.1 |
| Leg [tibialis anterior muscle; (Henderson et al.,2007)] | 36.1 ± 0.6 | −18.5 ± 1.0 | 14.7 ± 0.8 |
Images used for the analysis of masetter and tibialis anterior muscle pain were collected in previous studies.
Figure 3.
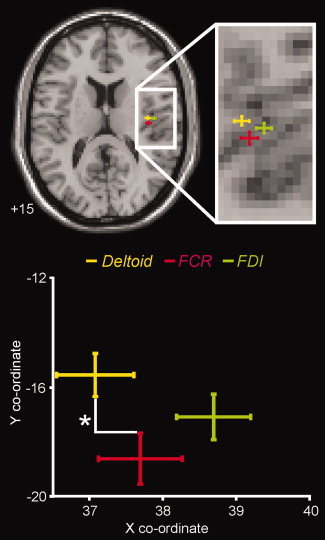
Plot of the mean (±SEM) locations of the most significantly activated voxels within the contralateral dorsal posterior insula, following injection of hypertonic saline into the deltoid, flexor carpi radialis (FCR), and first dorsal interosseous (FDI) muscles. The slice location in MNI space is indicated in the bottom left of the image. Below is a plot of the mean (±SEM) X and Y co‐ordinates in MNI space of these activated voxels. Considering only the X and Y planes, the mean location during deltoid pain is located anterior to the mean location during FCR pain (P < 0.05).
Figure 4.
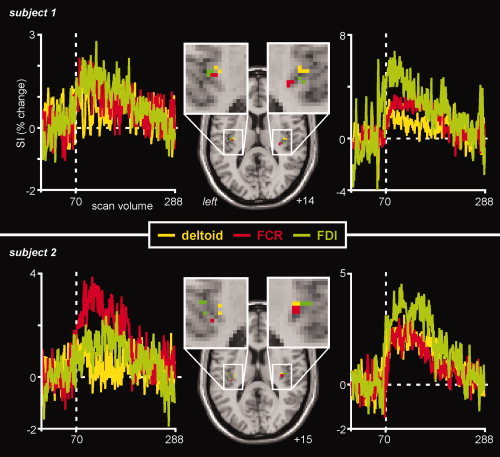
Locations of significant signal intensity increases in the dorsal posterior insula cortex in two subjects in which hypertonic saline injections were made into the left deltoid, flexor carpii radialis (FCR), and first dorsal interosseus muscles (FDI) during one session and then the right deltoid, FCR and FDI in a second session. Activations on the left side occurred during injections made into the right side of the body and vice versa. The slice locations in MNI space are indicated in the bottom right of each image. To the left and right are plots of the percentage signal intensity changes over time, relative to the baseline period, for each significantly activated region. The vertical dashed lines indicate the scan at which each hypertonic saline injection was delivered. Note that within the contralateral dorsal posterior insula, deltoid pain activated a region medial to that activated by FDI pain and anterior and medial to that activated by FCR pain.
Scatter plots of the differences in X and Y co‐ordinates of maximally activated voxels revealed that, in the vast majority of subjects, hypertonic saline injection into the FCR resulted in a mean maximal increase in signal intensity that had either the same X and Y co‐ordinates (n = 5), or was located posterior to the location of signal intensity increases evoked by injection into the deltoid (n = 7) (see Fig. 5). Furthermore, in only three subjects did deltoid and FCR injections evoke maximal signal increases in the same location, with a mean distance between the two loci of 4.4 ± 0.9 mm. Similarly, in the majority of subjects, hypertonic saline injection into FCR evoked maximal increases in signal intensity that were located posterior and medial to the location of signal intensity increases evoked by injection into FDI (n = 7). In only one subject did FDI and FCR injections evoke maximal signal intensity increases in the same location, with a mean distance between the two signal increases of 5.1 ± 0.9 mm. Finally, in the majority of subjects, hypertonic saline injection into the deltoid resulted in maximal signal intensities that were located lateral to the location of signal intensity increases evoked by injection into FDI (n = 9). Furthermore, in only two subjects FDI and deltoid injections evoked maximal signal intensity increases in the same location, with a mean distance between the two signal increases of 5.9 ± 1.1 mm.
Figure 5.
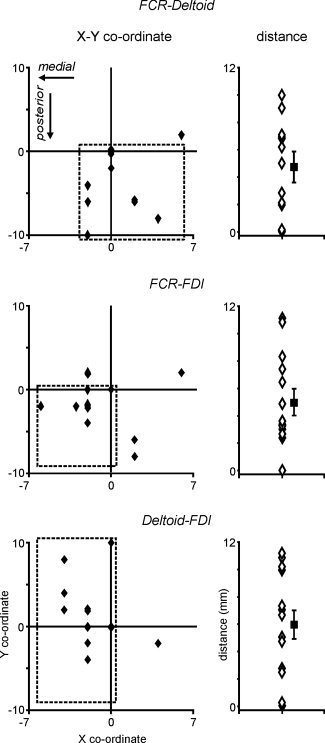
Left column: Two‐dimensional scatter plots of differences in the X and Y direction (millimeters) of the most significantly activated voxel during flexor carpii radialis (FCR) relative to deltoid pain (upper panel), FCR relative to first dorsal interosseous (FDI) pain (middle panel), and deltoid relative to FDI pain (lower panel). The dashed squares indicate the region in which most individual subjects are located. Note that in most subjects FCR pain activated a region posterior to that activated by deltoid pain and posterior and medial to that activated by FDI pain. Further, in the vast majority of subjects, deltoid pain activated a region medial to that activated during FDI pain. Right column: Open triangles represent the 3‐dimensional distances between the most significantly activated voxel during FCR relative to deltoid pain (upper panel), FCR relative to FDI pain (middle panel), and deltoid relative to FDI pain (lower panel). Filled squares represent the mean (±SEM) 3‐D distances for all subjects.
In addition to determining the locations of maximally activated voxels within the contralateral dorsal posterior insula during muscle pain applied to the upper limb, we have previously mapped activity within the dorsal insula during muscle pain applied to the face (masseter) and leg (tibialis anterior). Using these images we found that the mean (±SEM) X, Y, and Z co‐ordinates evoked by leg pain (tibialis anterior muscle) were 36.1 ± 0.6, −18.5 ± 1.0, 14.7 ± 0.8 and face pain (masseter muscle) were 37.3 ± 0.8, −15.4 ± 0.8, 12.2 ± 0.8. Furthermore, on average, masseter pain evoked signal intensity increases within the dorsal posterior insula that were 3.1 mm, 4.7 mm, 3.2 mm, and 4.2 mm away from deltoid, FCR, FDI and tibialis anterior pain, respectively. Similarly, tibialis anterior pain evoked signal intensity increases that were 3.2 mm, 1.9 mm, and 2.9 mm away from deltoid, FCR, and FDI pain, respectively.
DISCUSSION
Using high‐resolution fMRI, we have shown, for the first time, that within the dorsal posterior insular cortex muscle pain applied to different muscles of a single limb evokes somatotopically organized increases in signal intensity. Signal intensity increases evoked by deltoid pain were located medial and anterior to those evoked by muscle pain in the forearm and hand, respectively. Combined with our previous data, it appears that, in humans, the dorsal posterior insular cortex contains a whole‐body somatotopic map of noxious inputs arising from skeletal muscles. Furthermore, this somatotopic representation cannot be considered coarse.
We have previously used fMRI to determine the patterns of brain activity evoked by hypertonic saline injections into the leg and forearm [Henderson et al.,2007]. In this previous study we demonstrated that pain originating in muscle or skin of the leg evoked increases in signal intensity in the contralateral dorsal posterior insula that were located medial and posterior to those evoked by forearm pain. Using the images collected in this and another study [Henderson et al.,2007; Nash et al.,2009], we performed an identical processing and statistical analysis procedure to that of employed in the current investigation and determined the maximally activated voxels within the contralateral dorsal posterior insula in each subject. Despite the fact that these images were collected using a different MRI scanner, following spatial normalization we found excellent coregistration between functional images collected on different scanners in the same individual (n = 3). Further, since we did not compare the magnitudes of signal intensity changes but instead restricted our analysis to the maximally activated voxels, we are confident that the whole body map shown in Figure 6 is accurate. Indeed, this combined analysis reveals that the body schema within the contralateral dorsal posterior insula is not as crude as has been previously suggested, but instead contains separate representations for muscles within a single limb.
Figure 6.
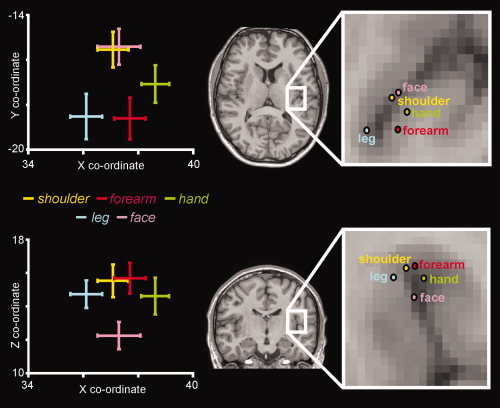
Plots of the mean (±SEM) X, Y, and Z co‐ordinates of the most significantly activated voxels within the contralateral dorsal posterior insula, during shoulder (deltoid), forearm (flexor carpii radialis; FCR), and hand (first dorsal interosseous; FDI) pain assessed in this investigation. Also included are the X, Y, and Z co‐ordinates of the most significantly activated voxels during leg (tibialis anterior; TA) and face (masetter) muscle pain determined using data from two previous investigations (Henderson et al.2007; Nash et al.2009).
It has been previously shown by us and others that the dorsal posterior region of the insular cortex is activated by noxious cutaneous and noxious muscle stimuli [Brooks et al.,2005; Henderson et al.,2007; Vogel et al.,2003]. Although not universally accepted, it has been proposed that the dorsal posterior insula receives noxious information via direct, somatotopically organized, inputs from the ventromedial thalamic nucleus (VMpo) [Craig,2003,2004; Craig and Blomqvist,2002]. In addition to noxious stimuli, a recent human brain imaging investigation by Bjornsdotter et al. [2009] revealed that crude light touch, mediated by low‐threshold C fibres (C tactile afferents), also activates the dorsal posterior insula in a somatotopic fashion. Indeed, the authors report a seemingly identical organization of arm and leg activation patterns to that which we previously reported during noxious forearm and leg cutaneous and muscle pain, i.e., leg medial and posterior to arm [Henderson et al.,2007]. Further, this somatotopically organized activation also occurred in an individual lacking large‐diameter myelinated afferents, suggesting that, in healthy individuals, dorsal posterior insula activation during crude light touch results from activation of C tactile (CT) afferents [Bjornsdotter et al.,2009].
It has been reported that in two individuals who lack A‐beta fibres that light crude touch can be localized [Olausson et al.,2008a]. Since light crude touch in A‐beta deficient subjects does not evoke signal intensity increases within the primary or secondary somatosensory cortices, rather causing decreases [Olausson et al.,2008b], it was proposed that the dorsal posterior insula provides the neural substrate via which light crude touch is localized. Although it is possible that the dorsal posterior insula may contain only a very crude body map for light touch, our data reveals that it contains a rather fine within‐limb representation for skeletal muscle pain. Furthermore, in a recent rodent investigation, it was reported that low‐threshold mechanoreceptor and nociceptive afferents converge within the dorsal horn [Andrew,2010]. If a similar convergence within the dorsal horn also occurs in humans, it is possible that the within‐limb somatotopic map reported here results from the activation of wide‐dynamic range neurons and would be revealed by either light touch or muscle nociceptor activation. That is, the same fine somatotopic map would result from both muscle nociceptor activation and light touch. The presence of a fine somatotopic map suggests that the dorsal posterior insula is involved in the accurate localization of muscle pain and possibly other somatosensory stimuli such as light touch.
The idea that the dorsal posterior insula is involved in the accurate localization of painful stimuli is also supported by the results of investigations by Mazzola et al. [2006,2009], in which they report that stimulation of a single posterior insula site can evoke painful sensations restricted to face, upper limb or lower limb. Similar to our results, they found that the face representation was anterior to that of the upper and lower limbs and that the upper limb was represented dorsal to the lower limb. Using laser‐evoked potentials, Baumgartner et al., [2006] also reported a similar somatotopic organization to noxious laser stimuli within the posterior insula of the monkey. That is, the leg, arm and face are represented in a posterior to anterior pattern. Although we and others suggest that the somatotopic organization of the posterior insula is involved in noxious stimulus localization, this remains to be established. Interestingly, in reports which investigate the effects of insula lesions on pain processing in humans, although increases in pain thresholds are consistently reported, the effect of the lesion on the ability of the patient to localize the noxious stimulus is not [Berthier et al.,1988; Greenspan et al.,1999]. One would assume that their ability to localize the noxious stimulus has remained intact. It is possible that the dorsal posterior insula acts in concert with the primary and secondary somatosensory cortices to provide an individual with an ability to accurately localize noxious stimuli that arise from deep body structures such as muscle (and perhaps viscera).
Alternatively, the somatotopic organization within the dorsal posterior insula may provide a means by which an individual directs appropriate behaviors to noxious and non‐noxious (pleasant touch) stimuli. For example, different motor and autonomic patterns are evoked by noxious stimuli originating in different body parts and different tissues in the same body region [Bandler et al.,2000; Lewis,1942]. Cutaneous pain evokes active coping responses such as flight and fight, whereas muscle and visceral pain evokes a passive coping response characterized by quiescence and hyporeactivity [Bandler et al.,2000]. In primates, the dorsal insula sends somatotopically organized inputs to regions of the striatum which contain nociceptive responsive neurons, receive primary sensory and motor inputs and are involved in stimulus‐response associations and novelty [Chikama et al.,1997; Chudler and Dong,1995]. Furthermore, it has been reported that the putamen is activated in a somatotopic fashion during noxious cutaneous stimuli; it was suggested that this organization reflects pain‐related motor responses [Bingel et al.,2004]. An earlier report by Berthier et al. [1988] supports the idea that the insula may play a critical role in directing appropriate motor behaviors. They found in six patients with lesions encompassing the posterior insula, that neither superficial nor deep painful stimuli elicited a withdrawal response. In fact, one patient exhibited a reaction of “approach to the painful stimuli, i.e., he directed his limb towards the noxious stimuli.” This observation suggests that the dorsal posterior insula is critical in driving the behavioral responses to noxious stimuli in humans.
In addition to mediating motor responses, the results of investigations into the insular representation of light touch suggest that, in addition to stimulus localization, the dorsal posterior insula is involved in processing the emotional component of somatosensory stimuli. The activation of CT afferents by light touch evokes a pleasant emotional response and activates the dorsal posterior insula [Bjornsdotter et al.,2009]. Similarly, it is thought that the emotional component of noxious stimuli is processed within the insular cortex since lesions encompassing the insula can result in pain asymbolia, a condition in which the intensity and quality of noxious stimuli are preserved, but patients appear “unable to recognize the disagreeable nature of painful or threatening stimuli” [Berthier et al.,1988]. Indeed, it has been hypothetized that the dorsal posterior insula is “interoceptive” cortex; that is, a region that monitors the internal state of the body [Craig,2002]. These previous reports, in combination with the data presented here, suggest that the dorsal posterior insular cortex may process the emotional component of both non‐noxious and noxious stimuli.
CONCLUSIONS
We have shown for the first time that the contralateral dorsal posterior insula receives noxious inputs from muscles in the upper limb in a fine somatotopic fashion, arguing for a role of the dorsal posterior insula in stimulus localization.
Acknowledgements
All MRI scanning was conducted at the MRI Clinical Imaging Department at Blacktown Hospital. We are grateful to Patrick Wong for his assistance in the scanning.
REFERENCES
- Andrew D ( 2010): Quantitative characterization of low‐threshold mechanoreceptor inputs to lamina I spinoparabrachial neurons in the rat. J Physiol 588 ( Part 1): 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Price JL, Keay KA ( 2000): Brain mediation of active and passive emotional coping. Prog Brain Res 122: 333–349. [DOI] [PubMed] [Google Scholar]
- Baumgartner U, Tiede W, Treede RD, Craig AD ( 2006): Laser‐evoked potentials are graded and somatotopically organized anteroposteriorly in the operculoinsular cortex of anesthetized monkeys. J Neurophysiol 96: 2802–2808. [DOI] [PubMed] [Google Scholar]
- Berthier M, Starkstein S, Leiguarda R ( 1988): Asymbolia for pain: A sensory‐limbic disconnection syndrome. Ann Neurol 24: 41–49. [DOI] [PubMed] [Google Scholar]
- Bingel U, Glascher J, Weiller C, Buchel C ( 2004): Somatotopic representation of nociceptive information in the putamen: An event‐related fMRI study. Cereb Cortex 14: 1340–1345. [DOI] [PubMed] [Google Scholar]
- Bjornsdotter M, Loken L, Olausson H, Vallbo A, Wessberg J ( 2009): Somatotopic organization of gentle touch processing in the posterior insular cortex. J Neurosci 29: 9314–9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist A, Zhang ET, Craig AD ( 2000): Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the posterior portion of the ventral medial nucleus, in the human thalamus. Brain 123 ( Part 3): 601–619. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I ( 2005): Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage 27: 201–209. [DOI] [PubMed] [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN ( 1997): Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci 17: 9686–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudler EH, Dong WK ( 1995): The role of the basal ganglia in nociception and pain. Pain 60: 3–38. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2003): Pain mechanisms: Labeled lines versus convergence in central processing. Annu Rev Neurosci 26: 1–30. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2004): Distribution of trigeminothalamic and spinothalamic lamina I terminations in the macaque monkey. J Comp Neurol 477: 119–148. [DOI] [PubMed] [Google Scholar]
- Craig AD, Blomqvist A ( 2002): Is there a specific lamina I spinothalamocortical pathway for pain and temperature sensations in primates? J Pain 3: 95–101; discussion 113–114. [DOI] [PubMed] [Google Scholar]
- Craig AD, Zhang ET ( 2006): Retrograde analyses of spinothalamic projections in the macaque monkey: Input to posterolateral thalamus. J Comp Neurol 499: 953–964. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC, Zhang ET, Blomqvist A ( 1994): A thalamic nucleus specific for pain and temperature sensation. Nature 372: 770–773. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley K, Poline J‐B, Frith CD, Frackowiak RS ( 1995): Statistic parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Greenspan JD, Lee RR, Lenz FA ( 1999): Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain 81: 273–282. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Gandevia SC, Macefield VG ( 2007): Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: A single‐trial fMRI study. Pain 128: 20–30. [DOI] [PubMed] [Google Scholar]
- Hua le H, Strigo IA, Baxter LC, Johnson SC, Craig AD ( 2005): Anteroposterior somatotopy of innocuous cooling activation focus in human dorsal posterior insular cortex. Am J Physiol Regul Integr Comp Physiol 289: R319–R325. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R ( 2001): Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 25: 669–678. [DOI] [PubMed] [Google Scholar]
- Lewis T ( 1942): Pain. New York: MacMillan. [Google Scholar]
- Macefield VG, Gandevia SC, Henderson LA ( 2007): Discrete changes in cortical activation during experimentally induced referred muscle pain: A single‐trial fMRI study. Cereb Cortex 17: 2050–2059. [DOI] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM ( 2004): A method for removal of global effects from fMRI time series. Neuroimage 22: 360–366. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Mauguiere F ( 2006): Somatosensory and pain responses to stimulation of the second somatosensory area (SII) in humans. A comparison with SI and insular responses. Cereb Cortex 16: 960–968. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Peyron R, Guenot M, Mauguiere F ( 2009): Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain 146: 99–104. [DOI] [PubMed] [Google Scholar]
- Nash PG, Macefield VG, Klineberg IJ, Murray GM, Henderson LA ( 2009): Differential activation of the human trigeminal nuclear complex by noxious and non‐noxious orofacial stimulation. Hum Brain Mapp 30: 3772–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Cole J, Rylander K, McGlone F, Lamarre Y, Wallin BG, Krämer H, Wessberg J, Elam M, Bushnell MC, Vallbo ÅB. ( 2008a): Functional role of unmyelinated tactile afferents in human hairy skin: Sympathetic response and perceptual localization. Exp Brain Res 184: 135–140. [DOI] [PubMed] [Google Scholar]
- Olausson HW, Cole J, Vallbo A, McGlone F, Elam M, Kramer HH, Rylander K, Wessberg J, Bushnell MC ( 2008b) Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci Lett 436: 128–132. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E ( 1937): Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443. [Google Scholar]
- Penfield W, Rasmussen T ( 1950): The Cerebral Cortex of Man: A Clinical Study of Localization of Function. NewYork: MacMillan. [Google Scholar]
- Vogel H, Port JD, Lenz FA, Solaiyappan M, Krauss G, Treede RD ( 2003): Dipole source analysis of laser‐evoked subdural potentials recorded from parasylvian cortex in humans. J Neurophysiol 89: 3051–3060. [DOI] [PubMed] [Google Scholar]


