Abstract
Repeated experiences with an event create the expectation that subsequent events will expose an analog structure. These spontaneous expectations rely on an internal model of the event that results from learning. But what happens when events change? Do experience‐based internal models get adapted instantaneously, or is model adaptation a function of the solidity of, i.e., familiarity with, the corresponding internal model? The present fMRI study investigated the effects of model solidity on model adaptation in an action observation paradigm. Subjects were made acquainted with a set of action movies that displayed an altered script when encountered again in the scanning session. We found model adaptation to result in an attenuation of the premotor‐parietal network for action observation. Model solidity was found to modulate activation in the parahippocampal gyrus and the anterior cerebellar lobules, where increased solidity correlated with activity increase. Finally, the comparison between early and late stages of learning indicated an effect of model solidity on adaptation rate. This contrast revealed the involvement of a fronto‐mesial network of Brodmann area 10 and the ACC in those states of learning that were signified by high model solidity, no matter if the memorized original or the altered action model was the more solid component. Findings suggest that the revision of an internal model is dependent on its familiarity. Unwarranted adaptations, but also perseverations may thus be prevented. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: forward model, frontal pole, action observation, adaptation, breach of expectation, fMRI
INTRODUCTION
We don't inspect events without expecting their course. According to the predictive coding account of action observation, action perception triggers an “internal model” [Kilner et al., 2007; Neal and Kilner, 2010] that is run in real time and consists of predictions on the course of action [Schutz‐Bosbach and Prinz, 2007]. Evidently, such predictions save resources [Zacks et al., 2007].
However, it is not only of tremendous importance to establish internal models through experience, but also to attune them to persistent changes, and thus maintain valid predictions. Consider being forced to change your well‐known way to work because of some indiscernible traffic condition at some point of the route. If this happens once, you may surely assume that something like a traffic accident has happened. In all probability, you would not decide to take another way to work on the next day. This is an example of a well‐established and therefore solid internal model being violated. Solidity means that a model has strong connection weights between encompassed events. Events that have through repeated exposure become very well associated with each other elicit implicit prediction of each other. Solidity, i.e., a large strength of association, determines that the deviation is treated as a one‐time occurrence of no further importance for future predictions.
Now consider being on holiday and the road to the beach being blocked on your second day in the unfamiliar countryside. You may start wondering whether you have chosen exactly the way you went the day before and try to reverse the mental map you have created of your surroundings. If you find a new way to the beach and follow it on all occasions thereafter, you may quite forget, or begin to doubt that another way has ever been possible. This form of adaptation seems likely in case of low familiarity, i.e., a weak internal model. The weak internal model is questioned and possibly revised after a one‐time breach of expectation. However, it remains to be experimentally established how an internal model's solidity influences its revision and hence adaptation of predictions. To our knowledge, only a few studies on reversal learning in stimulus–response paradigms [Ghahremani et al., 2009] have dealt with the influence of model solidity on adaptation; no study has addressed the question in an action observation paradigm.
The present fMRI study was designed to investigate the influence of model solidity on its adaptation during iterations of a divergent script. Internal models of different solidity were established by presenting a number of scripts, i.e., movies showing everyday actions (as will be described below in more detail). The concept of solidity is similar to associative strength [McClelland et al., 1995] between components of an internal representation. Thus, solidity pertains to an internal model with constituent events that are highly associated with each other. Thus, in a fixed temporal schedule, each constituent elicits prediction of the next. This prediction is a consequence of statistical learning [Turke‐Brown et al., 2010]. Statistical learning results from repeated pairing of events, i.e., stimulus familiarity, that has been proposed to be critical in extending the persistence of memory [Eichenbaum, 2000]. Concisely, repeated exposure leads to solidity. In a solid model, each event is highly associated with its neighbor. Solidity was expected to affect adaptation rate to subsequent script change. Within the Bayes' theorem framework, the goal of probabilistic learning can be described as the acquisition of appropriate models for inference based on past experience. Events that cooccur persistently shape a solid model. The estimated likelihood of an event is dependent on its base‐rate and how reliably it occurred in the past, given that an associated event had happened. This likelihood is adapted on each iteration of the predictive and the associated event [Fiser et al., 2010]. The more often one event has followed another, the closer is the association between them and the more likely seems the succession. Therefore, within solid models, the likelihood of the respective next event is very high. This tying of prediction to a conditional probability is proposed to result in slower adaptation of more solid models. It takes longer to rewrite, or rather rewire, strong associations. Lastly, we were interested in “biased” adaptation stages at early and advanced stages of learning. In biased stages, the number of iterations of divergent expositions differed considerably from the number of iterations of the respective original script. These states are of specific interest to the validation of predictions. To resurrect the picture outlined above, only a well‐known path blocked instigates maintenance of the original idea, or “shielding” predictions from divergent influences. But previous experiences in a new environment should pale in insignificance to repeatedly coming across a divergence for the creation of an internal script and its predictions.
Functional Neuroanatomy
As a main effect of the factor “adaptation,” we expected adaptation of the internal model to the divergent script to lead to BOLD attenuation in a premotor‐parietal network. The premotor‐parietal network is associated with action observation and prediction of external events [cf., Schubotz, 2007]. Its parietal constituent is associated with coding for object pragmatics and space [Fagg and Arbib, 1998]. The frontal constituent, the lateral premotor cortex, has been suggested to code for transformations underlying both our movements as well as observed events, for example, changes in the position of objects [cf., Schubotz, 2007], and hence contributes to both action planning and action prediction. The concept of prediction refers to “filtering” of anticipated perception as has been described in motor control theories [Wolpert and Flanagan, 2001; cf., Schubotz, 2007]. We therefore expected that repeated exposure of the same action would lead to a decrease of activity in the premotor‐parietal network, signifying adaptation.
As a main effect of the factor “solidity,” we hypothesized higher activity for more solid compared to weaker models in the hippocampal formation. The close proximity of the concept of solidity to associative strength [Eichenbaum, 2000; Kim and Baxter, 2001; McClelland et al., 1995] and probabilistic learning [Kim and Baxter, 2001; Turke‐Brown et al., 2010] points toward an involvement of the hippocampal cortex, revealed in stronger activity for more solid compared to less solid models [Eichenbaum, 2000; Kim and Baxter, 2001; McClelland et al., 1995; Turke‐Brown et al., 2010].
Finally, we expected a significant interaction of the factors “solidity” and “adaptation.” This common‐sense assumption is supported by the fact that habits (also habits of thought), as an example for solid associations, are particularly difficult to unlearn [see Graybiel, 2008 for a review]. Moreover, it has been established that stable environments, which by inference allow shaping solid models, are signified by a slow learning rate [Rushworth and Behrens, 2008]. However, as the neural correlates of an influence of solidity on adaptation have not been investigated so far, the study was explorative concerning the existence and location of an interaction's neural correlates.
Implementation
To test our hypothesis, we familiarized participants previous to the fMRI session with a number of scripts containing everyday life actions, for example, a movie of making a salad. Each script encompassed a number of action steps, for example, taking a bowl, grasping the lettuce, placing it in the bowl, sprinkling vinegar on top, taking salad tongues, tossing the salad. Original scripts were presented three, six, or nine times in a preexperimental exposition session. In the fMRI session, participants encountered some scripts in the same version as before. In some scripts, however, the sequence changed from a certain point on. For example, the salad script now contained the subevents taking the bowl, grasping the lettuce, placing it in the bowl, reaching for the cheese, reaching for a knife, cutting pats of cheese into the bowl. Note that divergent scripts did not contain any action slips but were actions as valid as the original. Each script was shown nine times during the fMRI, either nine times in the original or nine times in the divergent version (no script appeared in two versions during the fMRI). Two main effects and their interaction were calculated:
-
1
To investigate the solidity effect, we contrasted the perception of divergent scripts with a large number (i.e., nine) of preexperimental expositions (factor level “solid”) with the perception of divergent scripts with a low number (i.e., three) of preexperimental expositions (factor level “weak”).
-
2
To test whether adaptation would occur, we contrasted the first (i.e., first three—factor level “first”) with the last (i.e., seventh to ninth) repetitions (factor level “last”) of the divergent scripts pooled over all preexperimental exposition frequencies.
-
3
Finally, we aimed to establish a neuronal network that would reflect the dependence of adaptation rates on model solidity. To this end, we calculated the interaction contrast between the two‐level factors “adaptation” and “solidity.”
METHODS
Subjects
Nineteen right‐handed, healthy participants (seven women, age 22–30 years old, mean age 25.3 years) took part in the study. The participants were right‐handed as assessed with the Edinburgh handedness inventory [Oldfield, 1971]. All participants were health‐screened by a physician and gave written informed consent.
Stimuli and Task
The stimulus material contained 37 different movies of 8‐ to 12‐s length. The movies were shot from the third‐person perspective, not showing the actor's face. They contained every‐day actions taking place at a table. Most movie scripts, e.g., making a sandwich, existed in two versions (a and b). These scripts had an identical beginning, but started to diverge at some individual point, whereafter no commonality existed (Fig. 1). Each version of each script was filmed 18 times. Thus, even though the same script appeared repeatedly during the preexperimental exposition and the experiment, the exact same shot of each script occurred only once. This method was employed to minimize surface‐similarities between the scripts. A subset of 13 scripts was filmed in five different versions.
Figure 1.
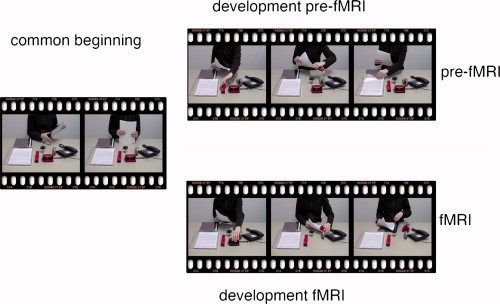
The initial version, that was displayed previous to the fMRI, and the divergent version, that was displayed during the fMRI, had a common beginning, i.e., they started with the same action step(s). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The experiment consisted of a preexperimental exposition of the movie material and an fMRI session starting exactly 15 min after the end of the preexposition. During the preexperimental exposition session, participants were seated in a sound attenuated chamber facing a computer screen. Distance to the screen was adjusted to ensure that the video displayed on the screen did not extend 5° of visual angle. They watched 27 scripts, a third of which was displayed three times, another third six times and the last third nine times in a randomized fashion over the course of the 28‐min lasting session. The participants saw one version of each script; but each repetition was another shot of the same script. Questions concerning whether some action or another was part of the script (e.g., “grasping an apple?”) were posed on average after every fifth script to ensure ongoing attention to the stimulus material. Participants received visual feedback for 400 ms on whether they had answered correctly, incorrectly, or too late. After the preexposition, the participants were transferred directly to the fMRI chamber.
FMRI Session
The fMRI session encompassed display of 36 different scripts. Each script was repeated nine times over the experiment. Nine scripts, that had previously been displayed during the preexposition, returned in the fMRI session in the same version as before (“originals” hereafter). Another nine of the preexperimentally shown scripts were presented in the fMRI session only in their complementary version (“divergents” hereafter) (Figs. 1 and 2). The last nine scripts appeared in five different versions during the fMRI, each being displayed only once (“unpredictables” hereafter). The first third of the originals, the divergents and the unpredictables had previously been displayed three times each, the second third of all three kinds six times each, and the last third nine times each. Additionally, the design encompassed nine scripts that were completely new to the participants (“new originals”) when they were displayed during the fMRI. The latter as well as the unpredictables will not be subject of the present paper, but discussed in detail in a companion paper [Schiffer et al., in preparation]. However, the likely psychological effect of the unpredictables should be taken into account. Their presence and the associated experience of constantly changing scripts should decrease the likelihood of a divergent to be accepted as persistent at first encounter. That means that having seen a divergent only once does not allow the prediction that it returns in the same fashion–it could still turn out unpredictable at the second encounter. Only the second encounter of the same divergent delivers evidence that this script, albeit changed, is “learnable.”
Figure 2.
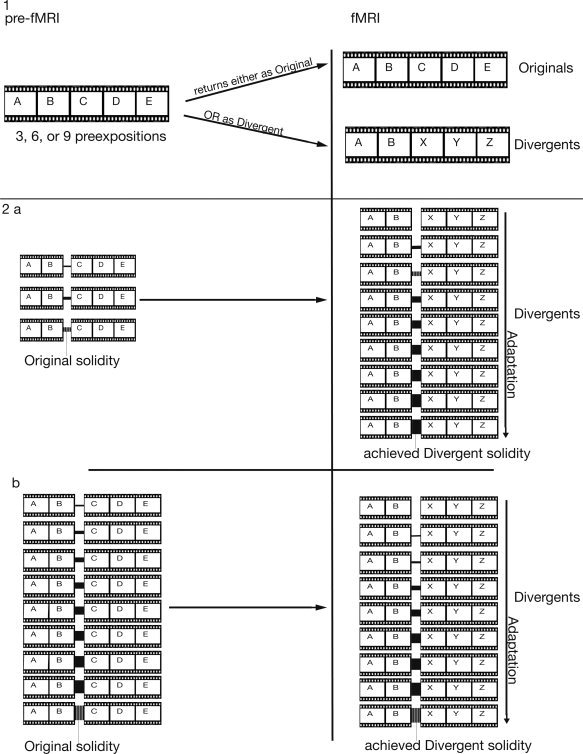
Abstract representation of the script‐structure. Letters refer to action steps. (1) Movies were preexposed three, six, or nine times in one version. A third of the movies reappeared in the fMRI in the same version as before “original”. Another third appeared in a “divergent” version. This version started exactly as the original version had, but developed differently thereafter. (2a) Movies that were preexposed three times returned nine times as divergents during the fMRI. Strength of the indicated link reflects solidity; only the solidity of the transition of importance is indicated; each transition has the same assumed solidity in the beginning. (2b) Movies that were preexposed nine times similarly returned nine times as divergents during the fMRI. Again, only the solidity of the relevant, i.e., later breached transition is graphically indicated.
The randomization distributed scripts of the same function, for instance, the first presentation of the divergent version, evenly across the session. Thus, the temporal correlation between the function of a script and experiment duration, as well as the accumulation of identical functions during a specific period was minimized.
During the fMRI session, participants lay supine on the scanner bed. Their head and arms were stabilized using form‐fitting cushioning and their hands rested on a rubber foam tablet. On the right hand side, a response panel was mounted on the tablet and fixed with tape. With their right hand index and middle finger resting on two response buttons, participants could answer the 32 intermittent questions concerning the content within the same response‐contingencies as in the preexposition (Fig. 3). Participants had three seconds to answer the question. Feedback on whether a response had been registered or not was displayed on the screen for 400 ms. The participants wore earplugs and headphones to attenuate scanner noise. Participants saw a reflection of the screen in a mirror built into the head‐coil and adjusted individually to allow for comfortable view of the entire screen. The movies did not extend further than 5° of visual angle in the mirror image of the computer screen. Sixteen null‐events of 10‐s length were displayed, consisting of display of the gray background on the screen. Participants were instructed to relax during null‐events.
Figure 3.
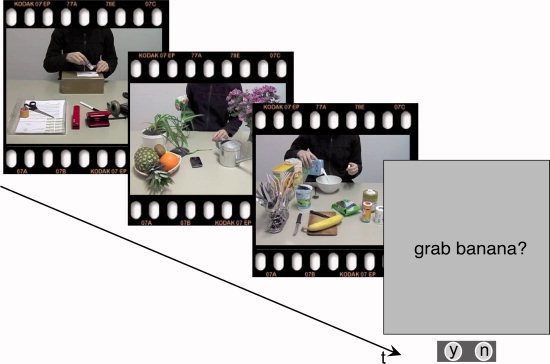
During the fMRI session, participants watched divergents and originals in a randomized fashion and had to answer content‐related questions on average after every 5th script. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Data Acquisition
The functional imaging session took place in a 3T Siemens Magnetom Trio scanner (Siemens, Erlangen, Germany). In a separate session, prior to the functional MRI, high‐resolution 3D T‐1 weighted whole‐brain MDEFT sequences were recorded for every participant (128 slices, field of view 256 mm, 256 × 256 pixel matrix, thickness 1 mm, spacing 0.25 mm).
The functional session engaged a single‐shot gradient echo‐planar imaging (EPI) sequence sensitive to blood oxygen level‐dependent contrast (28 slices, parallel to the bicommisural plane, echo time 30‐ms, flip angle 90°; repetition time 2,000 ms; serial recording). Following the functional session immediately, a set of T1‐weighted 2D‐FLASH images was acquired for each participant (28 slices, field of view 200 mm, 128 × 128 pixel matrix, thickness 4 mm, spacing 0.6 mm, in‐plane resolution 3 × 3 mm2).
FMRI Data Analysis
Functional data were offline motion‐corrected using the Siemens motion protocol PACE (Siemens, Erlangen, Germany). Further processing was conducted with the LIPSIA software package [Lohmann, et al., 2001]. Cubic‐spline interpolation was used to correct for the temporal offset between the slices acquired in one scan. To remove low‐frequency signal changes and baseline drifts, a 1/110 Hz filter was applied. The matching parameters (six degrees of freedom, three rotational, three translational) of the T1‐weighted 2D‐FLASH data onto the individual 3D MDEFT reference set were used to calculate the transformation matrices for linear registration. These matrices were subsequently normalized to a standardized Talairach brain size [x = 135 mm, y = 175 mm, z = 120 mm; Talairach and Tournoux, 1988] by linear scaling. The normalized transformation matrices were then applied to the functional slices to transform them using trilinear interpolation and align them with the 3D reference set in the stereotactic coordinate system. The generated output had thus a spatial resolution of 3 × 3 × 3 mm3.
The statistical evaluation was based on a least‐square estimation using the general linear model (GLM) for serially autocorrelated observations [Worsley and Friston, 1995]. Temporal Gaussian smoothing (4 s FWHM) was applied to deal with temporal autocorrelation and determine the degrees of freedom [Worsley and Friston, 1995]. A spatial Gaussian filter of FWHM 5 mm was applied. The design matrix was generated by hemodynamic modeling using a γ‐function and encompassed the first derivate. The onset vectors in the design matrix were modeled in a time‐locked event‐related fashion.
All contrasts were drawn from one design matrix. The first contrast accounted for the effect of model “solidity.” The second contrast accounted for the overall adaptation effect. The third contrast targeted the interaction between model solidity and adaptation. To ensure that the activation from the interaction contrast was rooted in an orthogonal interaction, we also calculated the conjunction analysis that accounted for the same proposed interaction effect. The onset vectors were modeled to the point in time when the divergent was recognizable as divergent (hereupon “breach,” Fig. 1). This breach had previously been visually timed to the moment when movement trajectories revealed that either the manipulation or the reached‐for object was different from that in the originals. All divergents as well as the null‐events were added as conditions of no‐interest into the design matrix.
Main effect Solidity
This effect was calculated as (solid / first ∩ solid / last) > (weak / first ∩ weak / last). Factor level “solid” refers to models that had been preexposed nine times; factor level “weak” refers to models that had been preexposed three times. Factor level “first” refers to the first three presentations of a divergent; factor level “last” refers to its last three presentations (Fig. 4).
Figure 4.
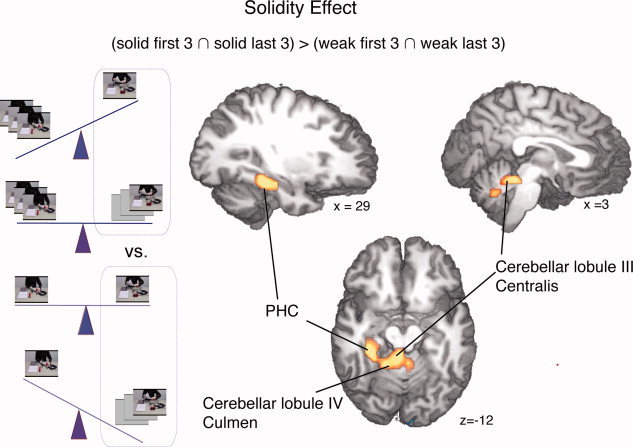
The effect of model solidity was calculated contrasting the 1st to 3rd and 7th to 9th iteration of scripts that had been preexposed nine times with the 1st to 3rd and 7th to 9th iteration of scripts that had been preexposed three times. PHC: Parahippocampal cortex.
Main effect adaptation
This effect was calculated as (solid / first ∩ weak / first) > (solid / last ∩ weak / last). Please refer to the above explanation of the factor levels (Fig. 5).
Figure 5.
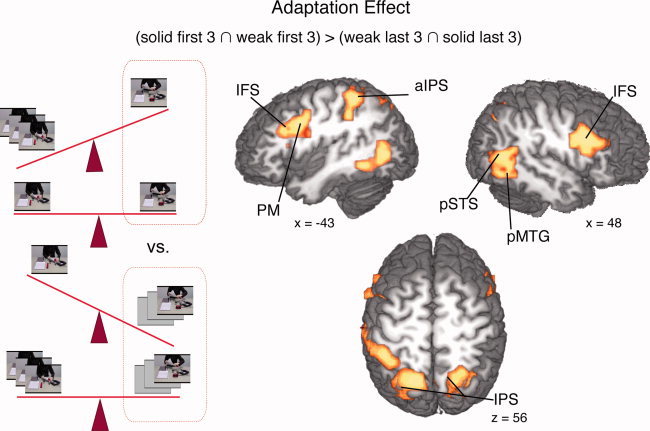
The effect of model adaptation effect was calculated contrasting the 1st to 3rd iteration of scripts that had been preexposed either three or nine times with the 7th to 9th iteration of scripts that had been preexposed either three or nine times. (a) IPS: (anterior) intraparietal sulcus; IFS: inferior frontal sulcus; pMTG: posterior middle temporal gyrus; pSTS: posterior superior temporal sulcus; PM: premotor cortex.
Interaction solidity by adaptation
The interaction contrast signifies the interaction between the two two‐level factors “solidity” and “adaptation,” and is thus derived from the crossing of the respective levels. Hence, it was calculated as contrast (solid / first > weak / first) > (solid / last > weak / last). Please refer to the above explanation of the factor levels (Fig. 6).
Figure 6.
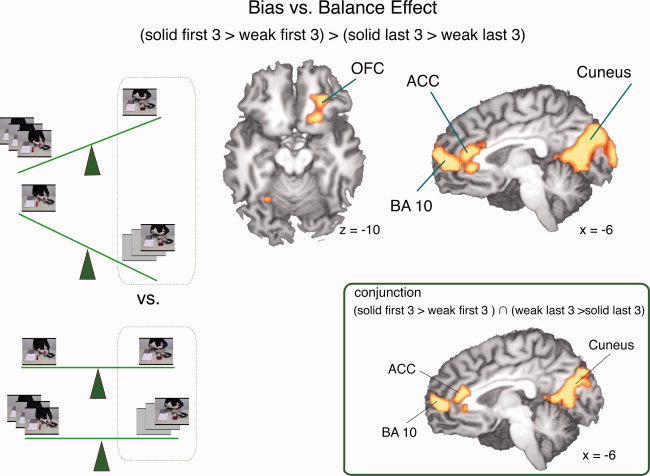
The biased vs. balanced effect was calculated contrasting the 1st to 3rd iteration of scripts that had been preexposed nine times and the 7th to 9th iteration of scripts that had been preexposed three times with the 1st to 3rd iteration of scripts that had been preexposed three times and 7th to 9th iteration of scripts that had been preexposed nine times. ACC: anterior cingulate cortex; BA 10: Brodmann area 10; OFC: orbitofrontal cortex.
To enable an interpretation of the significant effects derived from this interaction contrast, it was important to ensure that all significant voxels reflected the same direction of the effect (this rationale applies to all interaction contrasts in fMRI). Therefore, we additionally calculated the conjunction of the contrasts (weak / first > weak / last) and (solid / first > solid / last).
All contrast images were fed into a second‐level random effects analysis. The group analysis consisted of one‐sample t tests across all contrast images to analyze whether the observed differences between conditions were significantly deviant from zero. Acquired t‐values were transformed to z‐scores. A two‐step correction for false positive results based on a Monte‐Carlo simulation was performed. In a first step, an initial z‐threshold of 2.33 (P < 0.05, one‐tailed) was applied to the simulated voxels. Afterward, based on the remaining clusters, statistical thresholds were calculated to correct for false positives at a significance level of P = 0.05. Cluster size as well as cluster value were taken into account at thresholding in a compensatory matter to prevent neglecting true positive activations in small anatomical structures [Lohmann et al., 2008]. Hence, all reported activations were significantly activated at P ≤ 0.05, corrected for multiple comparisons at cluster level.
Pilot Study
Previous behavioral results support the validity of the described contrasts. A preceding pilot study in another group of participants had provided behavioral evidence for the influence of solidity on adaptation. In the study, participants viewed each movie first three, six, or nine times in the original version, followed by three, six, or nine divergent displays and eventually one or two original presentations. Meanwhile, they had to constantly indicate whether the version that was on display at the moment was identical to the last display, or represented a change in script. We measured reaction times (RT) for the responses and conducted repeated measures ANOVA on the RTs of all correct responses to repetitions of divergents. The repeated measures ANOVA thus included two factors, the two‐level factor original presentations (levels: three original presentations, nine original presentations) and eight‐level factor divergent iteration (levels: 2nd iteration, 3rd iteration, …, 9th iteration). The first divergent was not included in the analysis, as it demanded a different response (indication of change) than the ensuing divergents (indication of repetition). The interaction effect between number of original presentations and iteration of the divergent approached significance at P = 0.07 (Greenhouse‐Geisser corrected). To disentangle what effect carried the interaction, we correlated the RT for each iteration with the number of previous originals. The correlation between RT of the divergents that had been displayed three times as original and their iterations was not significant (r = 0.081, P = 0.3). In contrast, the correlation between RT of the divergents that had been displayed nine times as original and their iterations approached significance (r = −0.157, P = 0.06). This marginal correlation reveals a continuous decrease in reaction times that we take to reflect ongoing adaptation to the divergents that had previously been shown nine times in their original version. Taken together, these results reflect a difference in adaptation rate dependent on the number of preexpositions.
RESULTS
Behavioral Results
The participants answered on average 87% of the 32 questions correctly (>27 questions). Standard deviation was 7%. In the postexperimental questionnaire, participants were asked whether all movies had returned as before and no participant indicated that all movies had. Six of the 19 participants reported spontaneously to the open question whether they wished to report anything whatsoever, that some movies were different than before. This behavioral measure fosters the argument that the participants were aware that some movies were altered versions of what they had seen preexperimentally, instead of believing that the different movies (divergents) were not related to the initial version.
FMRI Results
The model “solidity contrast” (solid / first ∩ solid / last) > (weak / last ∩ weak / first) yielded activity in the right parahippocampal cortex, and also in the right cerebellar Lobule III (centralis) and bilaterally in the Lobule IV (culmen) of the cerebellum (Table I) (Fig. 4).
Table I.
Solidity contrast: Anatomical specification, Talairach coordinates (x,y,z) and maximal Z‐scores of significantly activated voxels for model solidity: divergents with high (nine preexpositions) or weak (three preexpositions) model solidity
| Localization | Talairach coordinates | Z‐values, local maxima | ||
|---|---|---|---|---|
| x | y | z | ||
| Parahippocampal cortex | 32 | −32 | −12 | 3.43 |
| Cerebellum, Lobule III, Centralis | 4 | −38 | −9 | 5.16 |
| Cerebellum, Lobule IV, Culmen | −8 | −47 | −18 | 4.8 |
The model “adaptation contrast” (solid / first ∩ weak / first) > (solid / last ∩ weak / last) yielded bilateral activity in the inferior frontal sulcus (IFS), the left premotor cortex (PM), the left superior parietal lobe (SPL), and intraparietal sulcus (IPS), extending into anterior IPS in the left hemisphere. The posterior middle temporal gyrus (MTG) was activated bilaterally (Table II) (Fig. 5).
Table II.
Adaptation contrast: Anatomical specification, Talairach coordinates (x,y,z) and maximal Z‐scores of significantly activated voxels for the model adaptation: first vs. last presentations of divergents
| Localization | Talairach coordinates | Z‐values, local maxima | ||
|---|---|---|---|---|
| X | y | z | ||
| Superior parietal lobule | −14 | −59 | −57 | 3.67 |
| Intraparietal sulcus | 32 | −62 | 45 | 4.18 |
| −20 | −65 | 39 | 3.74 | |
| −40 | −41 | 54 | 3.6 | |
| Intraparietal sulcus, anterior segment | −58 | −23 | 42 | 2.9 |
| Premotor cortex | −46 | 10 | 24 | 3.69 |
| Inferior frontal gyrus | 46 | 16 | 30 | 3.72 |
| −44 | 22 | 24 | 3.16 | |
| Posterior middle temporal gyrus | 44 | −56 | 15 | 3.72 |
| 40 | −47 | −3 | 3.81 | |
| −46 | −65 | 12 | 3.25 | |
| −40 | −50 | −6 | 3.4 | |
The solidity by adaptation interaction contrast (solid / first > weak / first) > (solid / last > weak / last) showed significant activation of the frontopolar cortex, comprising mesial Brodmann Area (BA) 10 and right lateral BA10. Further activations were in the anterior cingulate cortex (ACC), right orbitofrontal cortex (OFC), the right striatum, right posterior superior temporal gyrus (pSTS), cuneus, and the left fusiform gyrus (Table III; Fig. 6). The second approach to this analysis, the conjunction analysis (iii‐a), i.e., (weak / last > weak / first) ∩ (solid / first > solid / last), yielded activity in the mesial and the lateral BA10, ACC, and cuneus, and in the right fusiform gyrus (Table IV).
Table III.
Interaction contrast: Anatomical specification, Talairach coordinates (x,y,z) and maximal Z‐scores of significantly activated voxels for biased vs. balanced states: the first divergents of a solid internal model and the last divergents of a weak internal model vs. the first divergents of a weak internal model and the last divergents of a solid internal model
| Localization | Talairach coordinates | Z‐values, local maxima | ||
|---|---|---|---|---|
| X | y | z | ||
| Frontal pole, BA10 | −10 | 61 | 12 | 4.31 |
| 14 | 52 | 9 | 3.33 | |
| Anterior cingulate gyrus, BA24 | 2 | 34 | 15 | 2.85 |
| −4 | 31 | 15 | 2.79 | |
| Orbitofrontal gyrus | 22 | 31 | −9 | 3.14 |
| Cuneus | 8 | −77 | 18 | 3.81 |
| Posterior superior temporal sulcus | 56 | −32 | 9 | 3.8 |
| Fusiform gyrus | −26 | −56 | −6 | 3.19 |
| Striatum | 20 | 19 | −3 | 4.1 |
Table IV.
Conjunction analysis: Anatomical specification, Talairach coordinates (x,y,z) and maximal Z‐scores of significantly activated voxels for biased vs. balanced states: the first divergents of a solid internal model vs. the first divergents of a weak internal model and the last divergents of a weak internal model vs. and the last divergents a solid internal model
| Localization | Talairach coordinates | Z‐values, local maxima | ||
|---|---|---|---|---|
| X | y | z | ||
| Frontal pole, BA10 | 6 | 43 | 3 | 2.40 |
| −4 | 49 | 3 | 2.91 | |
| Anterior cingulate gyrus, BA24 | 2 | 31 | 15 | 3.54 |
| 2 | 34 | −3 | 2.16 | |
| −4 | 28 | 0 | 3.89 | |
| Cuneus | −2 | −71 | 21 | 2.83 |
| Fusiform gyrus | 16 | −53 | −6 | 2.46 |
DISCUSSION
Internal models of an action encompass expectations on the development of this action [Bar, 2009; Jeannerod, 1995]. Valid predictions make perception more efficient and are beneficial to fast reactions [Wolpert and Flanagan, 2001]. The present fMRI study investigated the neural correlates of the influence of the solidity of the original internal model of an action on subsequent adaptation of the internal model to a divergent script. To that end, participants watched movies that familiarized them with the original scripts and thus established an internal model. In the fMRI, they were confronted with divergent versions of the previously learnt scripts.
We found a persistent effect of preexperimental exposition frequency (main effect of solidity) in the right parahippocampal cortex as implied by the concept's proximity to associative strength. There was also an effect of solidity bilaterally in the anterior cerebellum. This result stresses the importance of previous experience to expectation, especially in the face of new information. As hypothesized, divergent experiences incited adaptation in fronto‐parietal motor regions, i.e., left PMv, bilateral IFS and IPS. Moreover, the adaptation effect was evident in the posterior MTG and in the left SPL. Finally, the exciting finding of a network dealing with a solidity bias, i.e., stages where solidity of one script surpasses that of another (solidity by adaptation interaction), supports the notion of a lasting influence of possible alternatives. The activity that was found for this interaction, located in the left frontomedian cortex (FMC), i.e., BA 10 and the ACC, as well as right striatum and right OFC, suggests a continuous processing of divergent information in these areas, be it current or past.
Solidity Exerts Prolonged Influence
Activity in the solidity contrast reflects an ongoing response to divergent scripts that is more pronounced for solid than for weaker original internal models. The cerebellar activity was in a classical motor region [Marvel and Desmond, 2010], in Lobules III and IV [Schmahman et al., 1999]. Working memory function, proposed for cerebellar Lobules VI/Crus I [Marvel and Desmond, 2010], is rather an unlikely explanation for this anterior activity. Hence, we take it to reflect continuing mismatch between the internal motor model's expectations and perception, which is increased if the original internal model was highly habituated. The parahippocampal cortex has been associated with topographical learning [Aguirre et al., 1996], scene processing [Epstein and Kanwisher, 1998] and the association of scenes and locations with objects [Bar et al., 2008; Sommer et al., 2005]. Here, we propose that parahippocampal activity signifies the revision of associations [Eichenbaum, 2000; McClelland et al., 1995] between scenes and actions or action‐relevant objects. The present data allow no decision between these alternatives, as the divergent script sometimes included the use of a different object than the original script did, but sometimes only entailed an altered manipulation of the same object.
Adaptation in the Cortical Motor Network
The adaptation contrast (ii) yielded activity in the left PM(v), the bilateral IPS and the left posterior MTG, a network that is not only relevant for action execution, but also prominent in action observation [Jeannerod, 1995]. The adaptation contrast tested whether the hypothesized fronto‐parietal motor regions would be sensitive to violated expectations and would show an adaptation to the new action script.
During the first encounters of the divergent script, perception was assumed to deviate from the internal model. An increase of neuronal activity at this stage reflects a breach of expectation signal that incites learning [Summerfield et al., 2008]. This signal can also be understood as a correlate of the processing of unexpected (salient) objects or manipulations [Keysers and Perret, 2004]. These functions can be seen as two sides of the same coin. Accordingly, the original script acts like a filter that minimizes processing demands of all according perceptions. Divergent perceptions, however, are not filtered, rendering them more salient than prefiltered perceptions. The resulting increased activation is a “breach of expectation signal” and incites learning. As soon as the divergent script has been learnt, it can serve as a filter for all according perceptions again.
Adapting the internal model to account for the divergent script is a learning or relearning process, and in a stable environment, strong evidence should be required to motivate learning [Rushworth and Behrens, 2008]. Otherwise, assembling and memorizing experiences would be pointless, as they would loose their capacity to guide successful behavior as soon as a one‐time breach of expectation had occurred. Hence, the divergent perception should not cause instantaneous adaptation of the internal model; accordingly, a process of adaptation is revealed by diminution of the neural correlate of divergence over a large number of iterations [Friston et al., 2006; Grill‐Spector et al., 2006; Majdanžić et al., 2009] as targeted in the adaptation contrast (ii). It has previously been established that the cortical motor network is capable of predicting the ongoing course of action [Jeannerod, 1995]. The current study furthers our understanding thereof, suggesting that the network is sensitive to salient violations of its predictions and shows appropriately slow adaptation. A detailed account of the proposed functions of the constituents adapting in this process will be supplied below.
The SPL has been discussed as a potential site of spatial priority maps, which designate relevant object locations and can be internally guided or externally cued [Molenberghs et al., 2007; Nobre et al., 2004]; one of the SPL's functions seems to be constructing and changing these spatial priority maps [Chiu and Yantis, 2009; Molenberghs et al., 2007]. Activity in the adaptation contrast is evidence for the remapping of spatially guided attention in SPL; this remapping or changing of weights in the priority map [Molenberghs et al., 2007] becomes important to action emulation as suddenly relevant objects demand attention, while previously used objects loose their significance for the action sequence.
Activity in the posterior MTG is taken to reflect increased processing of the movements of the actor and the actions associated with suddenly relevant objects [Beauchamp and Martin, 2007; Beauchamp et al., 2002]. Divergent scripts encompassed use (and accordingly motion) of different objects or different use of the same object as the original scripts. Encounter of the first presentations of the divergent script entailed a mismatch between emulated associations and valid, but unpredicted perceived use. Activity in the posterior MTG has been discussed in association with the frontoparietal motor network [Beauchamp and Martin, 2007; Johnson‐Frey, 2004]. The role of this frontoparietal network of IPS and PM in goal‐directed object manipulation and internal modeling thereof has been researched extensively [Grèzes and Decety, 2001; Jeannerod, 1995; Johnson‐Frey, 2004 for reviews]. The anterior IPS has been proposed to provide the ventral premotor cortex with information on object pragmatics [Fagg and Arbib, 1998; Schubotz and von Cramon, 2008]. Attenuation of its activity has previously been interpreted as a teaching signal that allows model adaptation [Tunik et al., 2007]. Medial IPS has previously been reported to be crucial for the online control of goal‐directed precision movement [Grefkes and Fink, 2005 for a review]. Online correction relies on the detection of mismatch between internal emulation and sensorimotor information [Wolpert and Flanagan, 2001]. We suggest that the activity along IPS reflects a decreasing mismatch between the internal model's emulated action and the currently perceived action. The closely linked [Geyer et al., 2000] PMv, which is assumed to store action knowledge and object function, shows increased activity when new scripts have to be learnt [see Schubotz and von Cramon, 2003 for review]. Activity in premotor cortex is increased when prediction [Schubotz and von Cramon, 2003], or simulation [Grèzes and Decety, 2001], and planning of movements [Johnson‐Frey, 2004] is involved. Against this backdrop, PMv activation during the first encounters of unpredicted divergences can be regarded as further evidence of this area's involvement in compiling complex actions.
Initial bias toward the original script
Activity in IFS has been suggested to modulate the bias between competing representations [Badre et al., 2005; Kuhl et al., 2007; Wurm and Schubotz, 2011]. This fits well with an influential model of prefrontal cortex function that suggests that prefrontal cortex is involved in activating and supporting relevant but unfavored or weak associations [Miller and Cohen, 2001 ]. The present study delivers new evidence for the assumption that the IFS supports weak models: attenuation of IFS activity points to its involvement in supporting the new divergent internal model and its associations during the first encounters of the divergent script. Each iteration of this divergent script should solidify its representation, diminishing IFS activity as a balanced state of competition between original and divergent internal model is approached and the bias runs eventually in favor of the new internal model [Schubotz and von Cramon, 2008].
Bias vs. Balance–Prefrontally Mediated Integration of Incompatible Models
The activation of the FMC, occipital areas, as well as the pSTS in the solidity–adaptation interaction contrast revealed these areas' involvement in processing information when the solidity of one internal model surpasses that of another. Strikingly, this network was found to be involved not only when this bias ran in favor of the original script (and thus against the currently perceived one), but also when the bias was already toward the actually presented action (and thus against the former original script). The underlying analysis was explorative concerning the areas that would be involved in the interaction of solidity and adaptation. However, the interesting results help to explain previous puzzling findings [Frank et al., 2005] and enhance our understanding of a conundrum in the EEG‐centered conflict‐monitoring literature:
FMC activity spread from the ACC into BA10. The ACC is understood to be responsive to bias, especially in decision and stimulus–response paradigms [Bunge et al., 2004; Miller and Cohen, 2001]. It is supposed to convey this bias to the dorsolateral prefrontal cortex [Miller and Cohen, 2001]. Classic bias‐related responses recorded in the ACC focus on conflict [see Botvinick et al., 2004; van Veen and Carter, 2002 for review]. Conflict is often understood as bias running against the necessary association, demanding PFC to support or maintain activation of a “weaker” association [Kuhl et al., 2007; Miller and Cohen, 2001]. This “conflict solving,” triggered by the ACC, could also mean suppression of an unlikely target [Kuhl et al., 2007], apart from the classic conception as fostering a weaker alternative [Miller and Cohen, 2001]. The current study, in contrast, revealed that the ACC is active for both biased states, even when perception is in accordance with the currently more solid internal representation. This latter form of bias, however, is not signified by what is often understood as conflict, i.e., the need to resolve competition in favor of the weaker alternative. Consequently, IFS activation is diminished at this stage, as apparent in the adaptation contrast and discussed above, while it is present when bias does run against the presented model at the beginning of adaptation. The proposed bias account is in line with an account of ACC function that integrates conflict monitoring and more general evaluative computation [Botvinick et al., 2004]. Conflict would then mean the activation of the representations of two incompatible (action) models [Botvinick et al., 2004]. The present results seem to singularly underpin a point in the EEG literature of conflict monitoring with fMRI‐derived results. Yeung et al. [2004] argue that the N2 component in correct trials and ERN component following errors is elicited when evidence for one representation outweighs that for the other—with the N2 preceding correct responses and the ERN being a posterror correlate of surmounting evidence for the (discarded) correct response. This aspect of “outweighing” the competing alternative, or bias, has however not always been taken into consideration in the conflict monitoring literature, even though one study [Frank et al., 2005] found that in a forced choice task a higher discrepancy between the respective reward values of two options resulted in a higher ERN than a more equal distribution of reward. Our study reveals that activity in the FMC is stronger if evidence is biased in favor of one of the incompatible representations, indicating in this case a higher predictive capacity for one model than the other. The study thus contributes to the clarification of the EEG centered conflict monitoring debate [Botvinick et al., 2004], corroborating a bias‐related definition of conflict, as opposed to the notion of equally strong competitors.
The ACC is closely linked to BA10 [Allman et al., 2002]. A special kind of neuron, the spindle neurons in the ACC, have been proposed to convey the motivation to adapt to changes to BA10 [Allman et al., 2002]. More generally, the frontopolar area is part of the hippocampal‐cortical memory system [Vincent et al., 2008]. Moreover, BA10 is taken to be responsible for the integration of separate cognitive operations [see Ramnani and Owen, 2004 for review]. One example is episodic retrieval and success monitoring, a process that can be understood in terms of comparing an internal representation to an outcome [Ramnani and Owen, 2004]. We propose that only the biased states entailed suppression of either the original or the divergent internal model, respectively. The deterministic nature of the paradigm suggested solidifying the divergent internal model, thus the biased and balanced states both encompassed a need to register and to encode the divergent internal model. But the biased states also suggested suppression of either the original or the divergent. If there were no suppression of the divergent internal model in the beginning, learning would be instantaneous. This was not the case. If the diversion was not registered, accumulating evidence would not be tracked and learning would never set in. Once evidence for the validity of the divergent internal model outweighs that for the original, suppression of the neglected alternative is regarded as efficient [Kuhl et al., 2007] and guides expectations toward the most likely outcome. A coupling of the ACC and BA10 during suppression has previously been reported by Kuhl et al. [ 2007]. In the balanced states, evidence for neither internal model outweighs evidence for the other and suppression could be regarded as too persistent (for the divergent internal model) or too premature (for the original internal model), respectively.
Activity of the OFC in the interaction contrast complements the emerging picture [Ghahremani et al., 2009]. Biased states necessarily have one strong or solid component, like a prepotent response or well practiced forward model. As discussed above, this strong component can trigger suppression of alternatives as it allows generation of hypotheses. Both, hypothesis generation and suppression have been discussed as potential OFC functions [Elliott et al., 2000; Ghahremani et al., 2009; Vartanian and Goel, 2005]. Hypothesis generation and suppression can be reframed as evaluation or weight changes as a result of evaluation, which itself is a function ascribed to the OFC [Wallis, 2007]. A steady environment, as signified by the existence of one solid internal model, makes it worthwhile to track contingencies and integrate outcome histories into learning [Rushworth and Behrens, 2008]. Responses to contingency differences, another type of evaluation, have similarly been allocated in the OFC [Windmann et al., 2006]. We propose that the activity increase in the OFC during a state of bias is indicative of the evaluation of the current forward model [Schubotz and von Cramon, 2008] against the backdrop of one solid and one weak or paling internal model. Closely linked to the OFC in its evaluative function is the striatum that was similarly active in the interaction contrast [Grinband et al., 2006; Oenguer et al., 2003; Schoenbaum et al., 2009].
To sum up, the similarities the networks display during the beginning and during an advanced state of learning single model solidity bias out as the determinant factor, as opposed to conflict between equally strong representations. It is likely that there is only consolidation in the balanced state, but an integration of consolidation of one and suppression of the other internal model in the biased states. Thus, bias incites the same operation in different situations, i.e., suppression of the divergent internal model in the beginning and suppression of the original internal model in the end. In the beginning, the divergent script stands in stark contrast to a solid internal model with identical onset phases; hence, it demands attention [Summerfield, 2008], possibly against a backdrop of previous suppression. In the end, even though the old original internal model has not been valid for a large number of iterations, it still exerts an influence on predictions. The emergence of significant bias‐related activations suggests that the opposite, i.e., a state of balance or ambiguity, is reached when the number of expositions of the divergent script matches the number of previous expositions of the original script. This finding is indicative of a slower adaptation rate for a solid, compared to a weak internal model and supported by the data from the pilot study (see Pilot Study section).
Concluding Remarks
In a dynamic environment, it is particularly important not only to set up internal models, but also to keep them up to date. Hence, expectations must be revised if they do not accord to our last experiences. However, unwarranted revision should be prevented to not loose the gain of experience. The current study provided evidence for the notion that familiarity with an event influences the adaptation rate of according expectations.
REFERENCES
- Aguirre GK, Detre JA, Alsop DC, D'Esposito M ( 1996): The parahippocampus subserves topographical learning in man. Cereb Cortex 6: 823–829. [DOI] [PubMed] [Google Scholar]
- Allman J, Hakeem A, Watson K ( 2002): Book review: Two phylogenetic specializations in the human brain. Neuroscientist 8: 335–346. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Parè‐Blagoev EJ, Insler RZ, Wagner AD ( 2005): Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 47: 907–918. [DOI] [PubMed] [Google Scholar]
- Bar M ( 2009): The proactive brain: Memory for predictions. Philos Trans R Soc B Biol Sci 364: 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Schacter DL ( 2008): Scenes unseen: The parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J Neurosci 28: 8539–8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Martin A ( 2007): Grounding object concepts in perception and action: Evidence from FMRI studies of tools. Cortex (a journal devoted to the study of the nervous system and behavior) 43: 461–468. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A ( 2002): Parallel visual motion processing streams for manipulable objects and human movements. Neuron 34: 149–159. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS ( 2004): Conflict monitoring and anterior cingulate cortex: An update. TrendsCogn Sci 8: 539–546. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD ( 2004): Prefrontal and hippocampal contributions to visual associative recognition: Interactions between cognitive control and episodic retrieval. BrainCogn 56: 141–152. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB ( 2010): ALE meta‐analysis of action observation and imitation in the human brain. NeuroImage 50: 1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y‐C, Yantis S ( 2009): A domain‐independent source of cognitive control for task sets: Shifting spatial attention and switching categorization rules. J Neurosci 29: 3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H ( 2000): A cortical‐hippocampal system for declarative memory. Nat Rev Neurosci 1: 41–50. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD ( 2000): Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cereb Cortex 10: 308–317. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N ( 1998): A cortical representation of the local visual environment. Nature 392: 598–601. [DOI] [PubMed] [Google Scholar]
- Fagg AH, Arbib MA ( 1998): Modeling parietal‐premotor interactions in primate control of grasping. Neural Netw 11: 1277–1303. [DOI] [PubMed] [Google Scholar]
- Fiser J, Berkes P, Orbán G, Lengyel M ( 2010): Statistically optimal perception and learning: From behavior to neural representations. Trends Cogn Sci 14: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Woroch BS, Curran T ( 2005): Error‐related negativity predicts reinforcement learning and conflict bias. Neuron 47: 495–451. [DOI] [PubMed] [Google Scholar]
- Friston K, Kilner J, Harrison L ( 2006): A free energy principle for the brain. J Physiol Paris 100: 70–87. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K ( 2000): Functional neuroanatomy of the primate isocortical motor system. Anat Embryol 202: 443–474. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA ( 2009): Neural components underlying behavioral flexibility in human reversal learning. Cereb Cortex 20:1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM ( 2008): The basal ganglia: Learning new tricks and loving it. Curr Opin Neurobiol 15: 638–644. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Decety J ( 2001): Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta‐analysis. Hum Brain Mapp 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K, Henson R, Martin A ( 2006): Repetition and the brain: Neural models of stimulus‐specific effects. Trends Cogn Sci 10: 14–23. [DOI] [PubMed] [Google Scholar]
- Grinband J, Hirsch J, Ferrera VP ( 2006): A neural representation of categorization uncertainty in the human brain. Neuron 49: 757–763. [DOI] [PubMed] [Google Scholar]
- Jeannerod M ( 1995): Mental imagery in the motor context. Neuropsychologia 33: 1419–1432. [DOI] [PubMed] [Google Scholar]
- Johnson‐Frey SH ( 2004): The neural bases of complex tool use in humans. Trends Cogn Sci 8: 71–78. [DOI] [PubMed] [Google Scholar]
- Keysers C, Perrett DI ( 2004): Demystifying social cognition: A Hebbian perspective. Trends Cogn Sci 11: 501–507. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD ( 2007): Predictive coding: An account of the mirror neuron system. Cogn Process 8: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Baxter MG ( 2001): Multiple brain‐memory systems: The whole does not equal the sum of its parts. Trends Neurosci 24: 324–330. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Dudukovic NM, Kahn I, Wagner AD ( 2007): Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat Neurosci 10: 908–914. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Mueller K, Bosch V, Mentzel H, Hessler S, Chen L, Zysset S, Yves von Cramon D( 2001): Lipsia—A new software system for the evaluation of functional magnetic resonance images of the human brain. Comput Med Imaging Graphics 25: 449–457. [DOI] [PubMed] [Google Scholar]
- Majdanžić J, Bekkering H, van Schie HT, Toni I ( 2009): Movement‐specific repetition suppression in ventral and dorsal premotor cortex during action observation. Cereb Cortex 19: 2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel C, Desmond J ( 2010): Functional topography of the cerebellum in verbal working memory. Neuropsychol Rev 20: 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC ( 1995): Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102: 419–457. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RRC ( 2007): Remapping attentional priorities: Differential contribution of superior parietal lobule and intraparietal sulcus. Cereb Cortex 17: 2703–2712. [DOI] [PubMed] [Google Scholar]
- Neal A, Kilner JM ( 2010): What is simulated in the action observation network when we observe actions? Eur J Neurosci 32: 1765–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Maquet P, Frith CD, Vandenberghe R, Mesulam MM ( 2004): Orienting attention to locations in perceptual versus mental representations. J Cogn Neurosci 16: 363–373. [DOI] [PubMed] [Google Scholar]
- Öngür D, Ferry AT, Price JL ( 2003): Architectonic Subdivision of the Human Orbital and Medial Prefrontal Cortex, Vol. 460 New York, NY: ETATS‐UNIS, Wiley‐Liss. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM ( 2004): Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat Rev Neurosci 5: 184–194. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ ( 2008): Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci 11: 389–397. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK ( 2009): A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci 10: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI ( 2007): Prediction of external events with our motor system: Towards a new framework. Trends Cogn Sci 11: 211–218. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY ( 2003): Functional‐anatomical concepts of human premotor cortex: Evidence from fMRI and PET studies. NeuroImage 20 ( Suppl 1): S120–S131. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY ( 2008): The case of pretense: Observing actions and inferring goals. J Cogn Neurosci 21: 642–653. [DOI] [PubMed] [Google Scholar]
- Schutz‐Bosbach S, Prinz W ( 2007): Prospective coding in event representation. Cogn Process 8: 93–102. [DOI] [PubMed] [Google Scholar]
- Sommer T, Rose M, Gläscher J, Wolbers T, Büchel C ( 2005): Dissociable contributions within the medial temporal lobe to encoding of object‐location associations. Learn Mem 12: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh EH, Monti JM, Mesulam MM, Egner T ( 2008): Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci 11: 1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Rice NJ, Hamilton A, Grafton ST ( 2007): Beyond grasping: Representation of action in human anterior intraparietal sulcus. NeuroImage 36 ( Suppl 2): T77–T86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turke‐Brown NB, Scholl BJ, Johnson MK, Chun M ( 2010): Implicit perceptual anticipation triggered by statistical learning. J Neurosci 30: 11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS ( 2002): The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav 77: 477–482. [DOI] [PubMed] [Google Scholar]
- Vartanian O, Goel V ( 2005): Task constraints modulate activation in right ventral lateral prefrontal cortex. NeuroImage 27: 927–933. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL ( 2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100: 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmann S, Kirsch P, Mier D, Stark R, Walter B, Guentuerkuen O, et al. ( 2006): On framing effects in decision making: Linking lateral versus medial orbitofrontal cortex activation to choice outcome processing. J Cogn Neurosci 18: 1198–1211. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR ( 2001): Motor prediction. Curr Biol 11: 729–732. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ ( 1995): Analysis of FMRI time series revisited—again. Neuroimage 2: 173–181. [DOI] [PubMed] [Google Scholar]
- Wurm MF, Schubotz RI ( 2011): Squeezing lemons in the bathroom: Contextual information modulates action recognition. NeuroImage. doi:10.1016/j.neuroimage.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Speer NK, Swallow KM, Braver TS, Reynolds JR ( 2007): Event perception: A mind‐brain perspective. Psychol Bull 133: 273–293. [DOI] [PMC free article] [PubMed] [Google Scholar]


