Abstract
To investigate whether personality traits affect the rate of decline of gray matter volume, we analyzed the relationships between personality traits and the annual rate of changes of gray matter volume in 274 healthy community dwelling subjects with a large age range by applying a longitudinal design over 6 years, using brain magnetic resonance images (MRI) and the Revised NEO Personality Inventory (NEO‐PI‐R) at baseline. Brain MRI data were processed using voxel‐based morphometry with a custom template by applying the DARTEL diffeomorphic registration tool. For each subject, we used NEO‐PI‐R to evaluate the five major personality traits, including neuroticism, extraversion, openness, agreeableness, and conscientiousness. The results show that the annual rate of change in regional gray matter volume in the right inferior parietal lobule was correlated significantly and negatively with a personality of openness, which is known to be related to intellect, intellectual curiosity, and creativity adjusting for age, gender, and intracranial volume. This result indicates that subjects with a personality trait of less openness have an accelerated loss of gray matter volume in the right inferior parietal lobule, compared with subjects with a personality trait of more openness. Because the right inferior parietal lobule is involved in higher cognitive function such as working memory and creativity, a personality trait of openness is thought to be important for preserving gray matter volume and cognitive function of the right inferior parietal lobule in healthy adults. Hum Brain Mapp 34:3347–3353, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: gray matter, magnetic resonance imaging, voxel‐based morphometry, longitudinal, personality, NEO‐PI‐R, big five
INTRODUCTION
Personality traits are thought to be stable, enduring, and important aspects of individuals [Funder, 2001]. Recent studies have focused on personality traits using the “five‐factor model” or the “big five” [Costa and McCrae, 1992; Funder, 2001; Hu et al., 2011]. The five factors generally consist of neuroticism, extraversion, openness, agreeableness, and conscientiousness. Neuroticism is linked to the tendency to experience negative emotions [Costa and McCrae, 1992; DeYoung et al., 2010], and includes several traits such as anxiety, self‐consciousness, and irritability. Extraversion is “linked to the tendency to experience positive emotions, which are typically derived from experience of reward or the promise of reward” [Costa and McCrae, 1992; DeYoung et al., 2010]. In addition, extraversion “encompasses an array of traits, such as assertiveness, sociability, and talkativeness” [DeYoung et al., 2010]. Openness appears to “reflect the tendency to process abstract and perceptual information flexibly and effectively” [DeYoung et al., 2010], and includes traits such as intellectual engagement and aesthetic interest [DeYoung et al., 2005]. In addition, Openness is the only big five trait that is consistently and positively associated with intelligence [DeYoung et al., 2005]. Agreeableness appears to “identify the collection of traits related to altruism” [DeYoung et al., 2010], which are related to one's concern for the needs, desires, and rights of others. The positive role of agreeableness describes prosocial traits such as cooperation, compassion, and politeness [DeYoung et al., 2010]. Conscientiousness is thought to “reflect the ability and tendency of individuals to inhibit or constrain impulses to follow rules or pursue nonimmediate goals” [DeYoung et al., 2010]. This trait is linked to “both academic and occupational success, as well as to behavior that promotes health and longevity” [Ozer and Benet‐Martinez, 2006]. Thus, big‐five personality traits are often applied to understand individual differences in behavior, emotion, motivation, and cognition. To analyze the big‐five personality traits, the Revised NEO Personality Inventory (NEO‐PI‐R) is widely used [Costa and McCrae, 1992]. NEO‐PI‐R is a self‐reported questionnaire that can be applied to subjects within a wide age range, from young adults to the elderly.
Recently, neuroimaging studies have identified a correlation between personality traits and brain structures. One study has shown that extraversion is associated with the medial orbitofrontal cortex, which is related to processing reward information, and that neuroticism is associated with several gray matter regions such as the medial temporal lobe and dorsomedial prefrontal cortex, which are related to threat and punishment [DeYoung et al., 2010]. Another study has also shown that neuroticism is related to the regional gray matter in the anterior cingulate cortex [Blankstein et al., 2009]. In addition, another study has shown that agreeableness is associated with the cerebellum, and that openness is associated with the orbitofrontal cortex [Hu et al., 2011]. Although these studies have shed light on the correlation between personality traits and gray matter volume using a cross‐sectional design, it is not known whether personality traits affect the rate of decline of gray matter volume. In other words, it is not known whether some specific personality traits accelerate or preserve gray matter volume loss in specific regions. Because regional gray matter volume in several regions is associated with several cognitive functions including attention, executive function, and semantic memory [Kramer et al., 2007; Taki et al., 2011b; Zimmerman et al., 2006], it is important to investigate an association between the rate of decline of gray matter volume with personality traits in healthy subjects. Therefore, if some personality traits are associated with a rate of decline in gray matter volume in specific regions, it may be possible to anticipate which cognitive functions decline in relation to each personality trait.
Therefore, to investigate whether personality traits affect a decline in gray matter volume, we analyzed whether there is a correlation between personality traits and an annual rate of gray matter volume change in 274 healthy community‐dwelling subjects within a large age range (21–80 years) by applying a longitudinal design over 6 years using brain magnetic resonance images (MRI) and NEO‐PI‐R. We applied voxel‐based morphometry (VBM) [Ashburner and Friston, 2000] in MR image analysis. VBM is an established automated neuroimaging technique that enables the global analysis of brain structure without a priori identification of a region of interest. VBM is not biased toward any specific brain region, and permits the identification of unsuspected potential brain structural differences or abnormalities. We hypothesized that the gray matter regions that show a significant correlation with personality traits, such as extraversion in the study mentioned above [DeYoung et al., 2010], also showed a significant correlation with the annual rate of gray matter volume change in the same region.
METHODS
Subjects
All subjects were Japanese and recruited from our previous brain‐imaging project, a part of the Aoba Brain Imaging Project [Sato et al., 2003]. Recruitment of subjects was described previously [Taki et al., 2011a]. Briefly, from an initial 1,604 potential participants, we selected individuals who lived in the city of Sendai (the center of the previous project) at the time of the previous study. Then, eligible people were contacted by mail and telephone and invited to participate in the longitudinal follow‐up, and 442 completed the follow‐up study. Screening criteria applied to the follow‐up sample were the same as those that had been used to determine eligibility at entry. Persons who reported a history of any malignant tumor, head trauma with loss of consciousness for >5 min, cerebrovascular disease, epilepsy, any psychiatric disease, or claustrophobia were excluded from the study. Of the 442 subjects who completed the follow‐up, 54 (12.2%) were excluded from this study because they no longer met the health screening criteria. All subjects were screened for dementia using the Mini‐Mental State Examination (MMSE) [Folstein et al., 1975], using a cutoff of 26. Two subjects were excluded from the study based on the MMSE (one male and one female, who each scored 25). Blood pressure in the right brachial artery was measured in the sitting position after a 10‐min rest. An experienced neuroradiologist examined the MR scans for any tumors and cerebrovascular disease. Follow‐up MRI data on an additional eight subjects (1.8%) was unsuitable for the longitudinal analysis (four subjects showed brain tumors and four subjects showed cerebral infarcts). Following these steps, the sample consisted of 381 subjects. In addition, because we did not collect the NEO‐PI‐R data from the first 447 of the 1,604 subjects for technical reasons, the final sample consisted of 274 subjects (113 men and 161 women). The mean ± SD follow‐up interval was 7.42 ± 0.55 years (range: 6.2–9.0). The mean ± SD age of the participants at baseline was 51.2 ± 11.8 years (range: 21–80 years). We collected personality traits using NEO‐PI‐R in the baseline scan. Characteristics of the subjects are shown in Table 1.
Table 1.
Characteristics of the subjects
| Factor | Men (n = 113) | Range | Women (n = 161) | Range | P |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| Age (years)a | 55.7 (11.9) | 24–75 | 53.1 (12.4) | 24–75 | 0.076 |
| Neutoticismb | 97.07 (17.8) | 37–146 | 97.45 (19.9) | 48–152 | 0.871 |
| Extraversonb | 101.15 (17.54) | 57–138 | 101.47 (18.70) | 38–153 | 0.886 |
| Opennessb | 107.30 (13.50) | 71–137 | 108.14 (14.70) | 74–144 | 0.633 |
| Agreeablenessb | 113.78 (13.68) | 65–151 | 113.07 (15.13) | 51–152 | 0.694 |
| Conscientiousnessb | 104.14 (17.72) | 50–144 | 107.20 (19.31) | 38–164 | 0.183 |
Mean between baseline and follow‐up scans.
Scores derived from NEO‐PI‐R in the baseline scan.
SD: standard deviation
Written informed consent according to the Declaration of Helsinki (1991) was obtained from each subject prior to MR image scanning after a full explanation of the purpose and procedures of the study was provided. Approval for these experiments was obtained from the institutional review board of Tohoku University.
Image Acquisition
All images were collected using the same 0.5‐T MR scanner (Signa contour; GE‐Yokogawa Medical Systems, Tokyo, Japan) for both baseline and follow‐up images. The scanner was routinely calibrated using the same standard GE phantom between baseline and follow‐up. During this period, no major hardware upgrade occurred. At baseline and follow‐up, all subjects were scanned with identical pulse sequences: 124 contiguous, 1.5‐mm‐thick axial planes of three‐dimensional T1‐weighted images (spoiled gradient recalled acquisition in steady state: repetition time, 40 ms; echo time, 7 ms; flip angle, 30; voxel size, 1.02 × 1.02 × 1.5 mm).
Image Analysis
We applied VBM to conduct the image analyses. Specifically, we used Statistical Parametric Mapping 8 software (SPM8, Wellcome Department of Cognitive Neurology, London, UK) for the structural segmentation, longitudinal registration, and group statistics, and the diffeomorphic anatomical registration using exponentiated Lie algebra (DARTEL) [Ashburner, 2007] deformation framework for intersubject spatial normalization. Because DARTEL produces more accurate registration [Klein et al., 2009], it allows for improved sensitivity in identifying correlations between regional gray matter volume and age. The overall processing, which is further detailed below, consisted of three main steps. First, the first time‐point images were segmented, and their gray matter volume was warped to Montreal Neurological Institute (MNI) space by creating DARTEL deformation fields. Second, the follow‐up scan images were registered to their baseline scan using high‐dimensional warping, and their gray matter volume was warped accordingly. Then, the images were further warped to the MNI space using the deformation fields computed previously. Last, a weighted‐difference image of the (smoothed and modulated) gray matter tissues from the two time points was computed so that the group analysis could be conducted using the general linear model framework.
Image analysis of the baseline scan
First, the “New Segmentation” algorithm from SPM8 was applied to every T1‐weighted MRI image from the baseline scan to extract tissue maps corresponding to gray matter, white matter, and cerebrospinal fluid (CSF) space. This algorithm, which is an improvement on “Unified Segmentation” [Ashburner and Friston, 2005], uses a Bayesian framework to perform iteratively the probabilistic tissue classification and the spatial deformation to normalized space. Although we were only interested in the probabilistic tissue segmentation, the new Bayesian segmentation‐and‐warping algorithm, which includes an improved set of tissues priors [Ashburner and Friston, 2009] for regularization, increased the robustness and accuracy of the segmentation compared with previous standard VBM algorithms. This step allowed us to obtain probability maps for the three aforementioned tissues of every subject and to have them all rigidly registered to a common space. The temporary common space of rigidly‐registered tissues is necessary as a starting point for the DARTEL algorithm. Next, the segmented tissues maps were used to create a custom template and associated warping fields using the DARTEL template‐creation tool [Ashburner, 2007]. This tool estimates a best set of smooth, nonlinear deformations from every subject's tissues to their common average, applies the deformations to create a new average, then reiterates until convergence. Smoothness and reversibility of the deformation are obtained from the diffeomorphic property of DARTEL transformations and ensures the accuracy of the mapping between the original and final spaces. The resulting template space was matched to the MNI space using an affine‐only registration, which enabled us to match our image custom coordinate space to the more standard MNI space [Bergouignan et al., 2009]. The final affine registration was provided and conducted by SPM by minimizing a multiclass divergence metric from our tissue set to its own bundled MNI‐space tissue set derived from the ICBM Tissue Probabilistic Atlas. At the end of that process, each subject's gray matter map was warped using its corresponding smooth, reversible deformation parameters to the custom template space and then to the final MNI standard space. To preserve the amount of gray matter in the process, we applied modulation by locally multiplying gray matter values by the determinant of the Jacobian of the (reverse) warping field, which has the effect of compensating for the volume change induced by the warping [Good et al., 2001]. Finally, the warped gray matter images were smoothened by convolving an 8‐mm full‐width at half maximum (FWHM) isotropic Gaussian kernel.
Image analysis of the follow‐up scan
For every subject, the follow‐up scan image was also initially segmented using the “New Segmentation” algorithm of SPM8, a process that yielded individual segmented tissue probability maps in native space. Then, using the “Coregister” algorithm in SPM8, a rigid spatial transformation was estimated to match every image of the follow‐up scan to its corresponding image of the baseline scan. This rigid transformation was also applied to tissues maps. Next, a fine‐grained, nonlinear, high‐frequency warping field was computed using the “High Dimensional Warping” tool in SPM8 [Ashburner et al., 2000], which estimates possible local changes of the image at one time point relative to another time point. The high‐resolution warping field was then used to spatially transform the gray matter tissue map from the second time point to match that from the first time point. Here again, tissue volume modulation was used to correct the scaling effect of the warping. Then, for each subject's data, the DARTEL flow field computed on the baseline images was applied to the registered follow‐up scan image, again using modulation, to produce a final gray matter amount image in the MNI space, which we smoothed at 8 mm FWHM. Finally, to calculate the annual rate of regional gray matter volume change for each subject, we subtracted the normalized, modulated, smoothed gray matter image of the follow‐up scan from that of the baseline scan and then divided the result by the interval between baseline and follow‐up (in years).
Statistical Analysis
To investigate whether personality traits affect a decline in gray matter volume decline, we performed a multiple regression analysis using age, gender, intracranial volume, and the NEO‐PI‐R score of five personality traits (neuroticism, extraversion, openness, agreeableness, and conscientiousness) as independent variables, with the annual rate of the regional gray matter volume change as a dependent variable. The intracranial volume was calculated by summing the gray matter volume, white matter volume, and CSF space volume derived in the above mentioned image preprocess. We used data from five personality traits at baseline scan in the analysis. We inserted all the five personality traits in one regression analysis. Additionally, as a supplementary analysis, we performed a cross‐sectional analysis, in which age, gender, intracranial volume, and the NEO‐PI‐R score of the five personality traits were used as independent variables, and regional gray matter volume at baseline was used as a dependent variable. We set the significance level at P < 0.05, corrected for family wise error rate.
RESULTS
The annual rate of change in regional gray matter volume in the right inferior parietal lobule was significantly negatively correlated with the score of openness, adjusting for age, gender, and intracranial volume (x, y, z = 45, −42, 33; t = 4.90, P = 0.042; Fig. 1). The result indicates that subjects with a personality trait of less openness showed an accelerated loss of gray matter volume in the right inferior parietal lobule compared with subjects with a personality trait of more openness. If the statistical threshold was set to more liberal condition (P < 0.001, uncorrected), the annual rate of change in regional gray matter volume in the right dorsolateral prefrontal cortex was also negatively correlated with the score of openness, adjusting for age, gender, and intracranial volume. No other personality traits were significantly correlated with the annual rate of change in regional gray matter volume. Regarding the supplementary cross‐sectional analysis, there was no significant correlation between regional gray matter volume at baseline and any personality trait, adjusting for age, gender, and intracranial volume.
Figure 1.
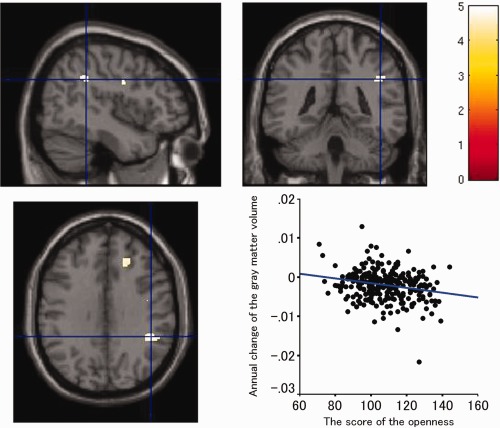
Gray matter regions where the annual rate of volume change showed a significant negative correlation with the score of the openness personality trait derived from NEO‐PI‐R adjusted for age, gender, intracranial volume, and the other four personality traits. The results of the correlation were superimposed onto structural MR images. The left side of the image represents the left side of the brain. The color scale indicates the t‐score. To clarify the extent of the regions, we showed data, the significance level of which was set at P < 0.00001, for multiple comparisons, uncorrected.
DISCUSSION
This study provides the first longitudinal findings showing a correlation between the rate of change in regional gray matter volume and personality traits by applying a longitudinal design over 6 years using brain MR images in healthy, community‐dwelling subjects with a large age range. Inconsistent with our primary hypothesis, we found that the annual rate of change in regional gray matter volume in the right inferior parietal lobule was significantly negatively correlated with the score for openness. As described in a recent study [DeYoung et al., 2005], although there is considerable disagreement about what is associated with the openness trait, several studies have shown that the openness trait is associated with traits of intellect such as intellectuality and intelligence, traits of imagination, unconventionality, and interest in art, and traits for which either label would be appropriate such as curiosity and creativity [DeYoung et al., 2005; John and Srivastava, 1999; McCrae and Costa, 1997; Saucier, 1992]. As for the functions of the right inferior parietal lobule, recent neuroimaging studies have also shown that the right inferior parietal lobule is related with fluid intelligence [Gray et al., 2003], which refers to the ability to reason and solve novel problems [Gray et al., 2003]. In addition, the right inferior parietal lobule has been reported to be related to creativity [Takeuchi et al., 2010b]. Actually, a recent neuroimaging study has shown that there is a substantial positive correlation between the score of the openness trait and the regional gray matter volume of the right inferior parietal lobule [DeYoung et al., 2010]. Thus, because the openness trait is related with several traits such as intelligence and creativity, as described above, we think it is reasonable that the annual rate of change in regional gray matter volume in the right inferior parietal lobule showed a significant negative correlation with the score of openness.
Our finding that the annual rate of change in regional gray matter volume in the right inferior parietal lobule showed a significant negative correlation with the score of openness is thought to be important because the right inferior parietal lobule is associated with several higher cognitive functions such as fluid general intelligence [Gray et al., 2003] and working memory [Takeuchi et al., 2010a]. Because our results indicate that subjects with a personality trait of less openness had an accelerated loss of gray matter in the right inferior parietal lobule compared with subjects with a personality trait of more openness, it is possible that people with a personality trait of less openness show a greater decline in several of those cognitive functions. In this regard, because personality traits are thought to be stable [Caver and Scheier, 2004; Funder, 2001], it may be difficult to change these personality traits. Our results suggest that continuing to have a personality trait of openness such as curiosity may help to preserve the regional gray matter volume of the right inferior parietal lobule and higher cognitive functions such as fluid intelligence and working memory.
Except for openness, from this longitudinal study, we did not find any other correlation between the annual rate of change in regional gray matter volume and the score of personality traits. These results indicate that no personality trait, except openness, showed an accelerated loss or preservation of gray matter. Additionally, we showed that there was no significant correlation between regional gray matter volume at baseline and the score for any personality trait. However, a recent cross‐sectional neuroimaging study has shown that several personality traits are correlated with several regions of gray matter volume [DeYoung et al., 2010]. For example, a personality of extraversion is related to regional gray matter volume of the medial orbitofrontal cortex, which is related to processing reward information [Depue and Collins, 1999]. In addition, neuroticism is related to several regional gray matter regions such as the medial temporal lobe, which is related to threats and negative affects [Gray and McNaughton, 2000]. This difference between the recent study [DeYoung et al., 2010] and our study is thought to be due to differences in the setting of statistical significance (the recent study, cluster level; our study, and voxel level) and/or whether the intracranial volume was used as an independent variable (the recent study, not including; our study, including). At least, we can conclude that only the openness personality trait was related with the rate of decline in gray matter volume.
This study had some limitations. First, regarding the MR scanner, it is possible that scanner drift may result in signal changes and altered image quality over time, although the MR scanner that we used was the same for the baseline and follow‐up images, and the scanner was routinely calibrated using the same standard GE phantom at both baseline and follow‐up, and was appropriately maintained. Second, also related to the MR scanner, tissue contrast at 0.5 tesla was substantially lower than that of higher field strength magnets, so that the use of a less powerful scanner may have affected the results of the tissue segmentation process. However, it is also beneficial that we used the same MR scanner for the baseline and follow‐up images because a different MR scanner or a different pulse sequence of MR image acquisition could have affected the tissue segmentation process. Third, regarding the subjects, selection biases may have occurred in that subjects who were concerned about their medical or psychological condition remained in the follow‐up study. Fourth, regarding image processing, the possibility of misclassification during tissue segmentation, such as the classification of white matter hyperintensities as gray matter, cannot be dismissed. Although we cannot rule out misclassification in tissue segmentation using our fully automated method, to reduce the possibility of tissue misclassification, we used not only voxel intensity itself but also a priori knowledge of the normal location of gray matter, white matter, and CSF to instruct the segmentation process. Considering these limitations, a fully automated method of MR image processing was important in dealing with a large quantity of data objectively and efficiently.
In summary, using a longitudinal design over 6 years with 274 community‐dwelling healthy individuals, we analyzed the correlations between personality and the annual rate of change in gray matter volume using NEO‐PI‐R. As a result, the annual rate of change in regional gray matter volume in the right inferior parietal lobule was significantly and negatively correlated with a personality of openness, which is known to be related to intellect, intellectual curiosity and creativity adjusting for age, gender, and intracranial volume. Because the right inferior parietal lobule is involved in higher cognitive function such as working memory and creativity, a personality trait of openness is thought to be important for preserving gray matter volume and cognitive functions of the right inferior parietal lobule in healthy adults.
ACKNOWLEDGMENTS
The authors thank K. Inoue and K. Okada for their insightful comments and K. Inaba, K. Saito, N. Ishibashi, and H. Masuyama for their technical help in collecting data. All authors have no conflict of interest to declare.
REFERENCES
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry‐the methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2009): Computing average shaped tissue probability templates. Neuroimage 45:333–341. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Andersson JL, Friston KJ (2000): Image registration using a symmetric prior‐in three dimensions. Hum Brain Mapp 9:212–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergouignan L, Chupin M, Czechowska Y, Kinkingnehun S, Lemogne C, Le Bastard G, Lepage M, Garnero L, Colliot O, Fossati P (2009): Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage 45:29–37. [DOI] [PubMed] [Google Scholar]
- Blankstein U, Chen JY, Mincic AM, McGrath PA, Davis KD (2009): The complex minds of teenagers: Neuroanatomy of personality differs between sexes. Neuropsychologia 47:599–603. [DOI] [PubMed] [Google Scholar]
- Caver CS, Scheier MF (2004): Perspectives on Personality.Boston:Allyn and Bacon. [Google Scholar]
- Costa PT Jr., McCrae RR (1992): The Revised NEO Personality Inventory (NEO PI‐R) and NEO Five Factor Inventory: Professional ManualPsychological Assessment Resources.Florida:Odessa. [Google Scholar]
- Depue RA, Collins PF (1999): Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci 22:491–517. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Peterson JB, Higgins DM (2005): Sources of openness/intellect: cognitive and neuropsychological correlates of the fifth factor of personality. J Pers 73:825–858. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR (2010): Testing predictions from personality neuroscience. Brain structure and the big five. Psychol Sci 21:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Funder DC (2001): Personality. Annu Rev Psychol 52:197–221. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ (2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14:21–36. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N (2000):The neuropsychology of anxiety: An enquiry into the functions of the septo‐hippocampal system.New York:Oxford University Press. [Google Scholar]
- Gray JR, Chabris CF, Braver TS (2003): Neural mechanisms of general fluid intelligence. Nat Neurosci 6:316–322. [DOI] [PubMed] [Google Scholar]
- Hu X, Erb M, Ackermann H, Martin JA, Grodd W, Reiterer SM (2011): Voxel‐based morphometry studies of personality: Issue of statistical model specification‐effect of nuisance covariates. Neuroimage 54:1994–2005. [DOI] [PubMed] [Google Scholar]
- John OP, Srivastava S (1999): The Big Five Trait taxonomy: History, measurement, and theoretical perspectives In: Pervin LA, John OP, editors.Handbook of personality: Theory and research.New York:Guilford Press; pp102–138. [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV (2009): Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46:786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC (2007): Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology 21:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Costa PT (1997): Conceptions and correlates of openness to experience In: Hogan R, Johnson J, Briggs S, editors.Handbook of personality psychology.Boston:Academic Press. [Google Scholar]
- Ozer DJ, Benet‐Martinez V (2006): Personality and the prediction of consequential outcomes. Annu Rev Psychol 57:401–421. [DOI] [PubMed] [Google Scholar]
- Sato K, Taki Y, Fukuda H, Kawashima R (2003): Neuroanatomical database of normal Japanese brains. Neural Networks 16:1301–1310. [DOI] [PubMed] [Google Scholar]
- Saucier G (1992): Openness versus intellect: Much ado about nothing? Eur J Pers 6:381–386. [Google Scholar]
- Takeuchi H, Taki Y, Kawashima R (2010a)Effects of working memory training on cognitive functions and neural systems. Rev Neurosci 21:427–449. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2010b)White matter structures associated with creativity: Evidence from diffusion tensor imaging. Neuroimage 51:11–18. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H (2011a)A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiol Aging 32:907–915. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Goto R, Wu K, Kawashima R, Fukuda H (2011b)Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn 75:170–176. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Cohen RA, Aloia MS, Williams LM, Clark CR, Whitford TJ, Gordon E (2006): The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry 14:823–833. [DOI] [PubMed] [Google Scholar]


