Abstract
Bodily abnormalities in other persons often evoke an uneasy feeling, even disgust. Here, we studied the brain basis of such perceptual salience by presenting static pictures of distorted hand postures to healthy subjects during functional magnetic resonance imaging. Cortical activation sensitive to distorted (vs. natural) finger postures was found—with right‐hemispheric dominance—in the primary motor cortex, postcentral somatosensory areas, amygdala, and insula, and bilaterally in the putamen. This activation pattern suggests that the instantaneous “gut feelings” during the observation of bodily distortions in others are related to embodied percepts that also involve affect‐related brain areas. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: embodied perception, motor cortex, somatosensory areas, amygdala, insula, putamen
INTRODUCTION
Young infants, while practicing important skills of visuomotor coordination, spend long hours just by inspecting their own hands, and adults pay considerable attention to hand postures of other people. Finger postures (as part of hand gestures and postures), although quite infrequently studied in neuroscience, can reveal an individual's feelings and intentions and automatically attract the viewer's attention [Mataric and Pomplun, 1998; Avikainen et al., 2003]. Perceptual salience is even higher to abnormal finger postures, to the point that the observer may instantaneously feel uneasy or disgusted. How do such embodied visceral percepts, “gut reactions,” arise? Could the intense and immediate response reflect an involvement of the observer's own sensorimotor system (see e.g. Varela et al. [ 2001]), most likely complemented by activation of affect‐related brain areas? Such involvement could be predicted from the seamless and omnipresent correspondence of action and perception, both within an individual, and importantly also between individuals during social interaction (for a review, see Hari and Kujala [ 2009]).
To test the degree of embodiment in the perception of bodily abnormalities that induce negative emotions (such as disgust) in the observer, we showed images of distorted finger postures (see Fig. 1) to healthy subjects whose brains were scanned with functional magnetic resonance imaging (fMRI). In a research area as topical as embodied cognition, it comes as no surprise that definitions of central terms vary [Wilson, 2002; Goldman and de Vignemont, 2009]. For the purposes of this study, we refer to perception as “embodied” (1) when the perceptual processes are shaped by the subject's experience of her own motor actions (or postures) and the concomitant sensory feedback, or by internal simulation of actions as it occurs during mental imagery or action observation (for a review, see Jeannerod [ 2001]) and (2) when the perceptual processes can be modeled as if they relied on signals related to body state, either arising in the body periphery or in the brain's representation of the body; such a mechanism is suggested in the still controversial somatic‐marker hypothesis (for reviews, see Damasio [ 1996], Craig [ 2002], and Dunn et al. [ 2006]).
Figure 1.

Examples of visually presented hand postures. Left column: Distorted postures, presented to subjects as computer‐generated color images. Right column: Natural postures. Panels with light gray background show stimuli as presented during the MR experiment. Panels with dark gray background show stimuli superimposed with “hotspots” of fixation (identified by eye tracking) which are radically different for distorted versus natural postures (tracking with a Tobii 1750 eye tracker, Tobii Technology AB, Sweden, several months after the fMRI sessions and analyzed with the manufacturer‐supplied ClearView software). Eye tracking figures show averages across 20 subjects, only four of whom had earlier taken part in the fMRI experiment (recordings by M. Kaksonen, V.‐M. Saarinen, and R. Hari).
Our study applied stimuli that mimic the immediate effect of observed bodily abnormalities—as they may occur congenitally or after an accident. We presented postures as still images rather than showing videos of biologically impossible movement [Costantini et al., 2005; Romani et al., 2005]. Specifically, we wondered whether the images would activate the observer's own sensorimotor areas, analyzed here as regions of interest (ROIs) located in the primary motor and primary somatosensory cortices. An additional ROI analysis served to establish whether the distorted finger images would activate areas of the amygdala that respond to pictures of emotional faces and of emotional whole‐body postures [Pessoa et al., 2002; Hadjikhani and de Gelder, 2003]. Further ROIs were defined on the basis of previous literature in (1) insula, which is activated in subjects who are either themselves exposed to disgusting odorants or observe the facial expression of disgust in others [Wicker et al., 2003], (2) putamen and globus pallidus, where disgusting pictures induce activations that vary with the individual disgust sensitivity [Calder et al., 2007; Mataix‐Cols et al., 2008], and (3) anterior cingulate that served as an area to indicate affective–emotional activation, for example related to pain [Raij et al., 2005; for a review see Tracey, 2008]. We expected the emotional valence of the distorted finger postures (with all pictures showing right hands) to be reflected as a right‐hemisphere‐dominant activation, similar to previous findings for fearful faces [Adolphs et al., 1996].
Fifteen subjects were scanned while they viewed computer‐generated static images of natural and distorted (not naturally attainable) finger postures of the right hand (see Fig. 1). The stimuli mimicked occasional encounters in everyday life, namely body postures in abnormal spatial configuration that evoke a composite emotional reaction (including disgust). Indeed, a separate eye‐gaze tracking study showed that the distorted fingers effectively captured the viewer's gaze (see Fig. 1). Because motor‐cortex modulation during action viewing is stronger when the hands that the subject sees correspond to her own hand orientation [Maeda et al., 2002], hand postures were shown in two different perspectives (see Fig. 1): Half of the images were presented in third‐person perspective—as if the fingers belonged to the hand of another person facing the subject. The other half were shown in first‐person perspective, that is in a position that the subject's own right hand could have taken. However, such a view could also have occurred during observation of another person, for example, when an adult is reading to a child sitting on her lap, when a couple or a group of people are moving toward the same direction, and when some complex handicraft skills are taught, with the master and apprentice positioned side‐by‐side, rather than opposite, to each other.
SUBJECTS AND METHODS
Subjects
Fifteen participants without history of neurologic or psychiatric disease took part after giving written informed consent (seven males, eight females, mean ± SEM age 27.1 ± 1.0 years, 14 subjects right‐handed with Oldfield's laterality quotients [Oldfield, 1971] between 66.7 and 100, one subject ambidextrous with laterality quotient −33.3, overall mean ± SEM laterality quotient 83.0 ± 8.6). The study had prior approval by the Ethics Committee of the Helsinki and Uusimaa Hospital District.
Stimuli
Pictures of hand postures (see Fig. 1) were produced with the Poser software (Version 4, MetaCreations, Florida, CA). All stimuli were obtained as computer‐generated postures, with either physiologically possible (“natural”) or impossible (“distorted”) configurations of the finger joints. The fingers were always at the center of the picture. The hand was presented in either of two configurations:
-
1
“First‐person perspective”: Volar side of the hand (distal from the wrist) at the lower right of the picture and fingers pointing to the center of the picture, just as the subject's own right hand would have appeared to the subject lying in the scanner, with her gaze directed to the feet. This set of stimuli comprised 30 natural and 30 distorted postures.
-
2
“Third‐person perspective”: A similar hand but now the wrist at the upper left corner of the image and fingers pointing to the center of the picture, i.e., a position that the subject could not have perceived as her own right hand's position while lying in the scanner. These stimuli were obtained by 180° rotation of the 60 first‐person perspective stimuli.
All stimuli showed right‐hand postures. Left‐hand postures were not presented to limit the duration of the fMRI sessions (see below) and thereby to minimize fatigue and discomfort in the subjects (for a systematic study of brain activation in response to natural right‐ and left‐hand movement in first‐ vs. third‐person perspective, see Shmuelof and Zohary [ 2008]).
Stimuli were presented in two successive fMRI sessions of 7 min duration (separated by a short rest of ∼1 min), both of which comprised 60 different postures, each shown for 5 s. In the first session, 4 × 15 stimuli (natural vs. distorted, first‐person vs. third‐person perspective) and 24 episodes of gray screen (serving as null events) were presented in random order. The duration of null events varied between 2.5 and 10.0 s (10 × 2.5 s, 9 × 5.0 s, 4 × 7.5 s, 1 × 10.0 s). Stimuli and null events followed a schedule determined by the optseq tool (http://surfer.nmr.mgh.harvard.edu). The second session relied on the same schedule and the remaining 4 × 15 stimuli. The subject was instructed to watch the stimuli attentively, without further instructions.
After the scanning session, stimuli were replayed to the subjects who were asked to report how unpleasant they found the stimuli, rating them on a scale of 0–10 (minimally to maximally unpleasant). Accidentally, these data were not recorded for the first subject of the sample and one subject's data were lost, leaving behavioral data for 13 out of 15 subjects. The purpose of this questionnaire was to demonstrate that distorted postures were actually perceived as unpleasant (see the beginning of Results) and to search for differences within the set of distorted postures that would be reflected in brain activation (see the end of Results). In‐scanner ratings were not feasible because (1) amygdalar responses to emotional stimuli are known to be weaker when subjects have a cognitive task as opposed to no task [Taylor et al., 2003] and (2) button presses in the scanner would have interfered with motor‐cortex activation related to internal simulation of finger postures.
MR Acquisition
Functional MRI data were acquired with a General Electrics Signa 3T scanner at Advanced Magnetic Imaging Centre, Helsinki University of Technology, using a gradient‐echo (GRE) planar imaging (EPI) sequence with the following parameters: flip angle (FA) 90°, time of repetition (TR) 2500 ms, time to echo (TE) 32 ms, field of view (FOV) 220 mm, matrix 64 × 64, number of excitations (NEX) 1, slice thickness 3.5 mm, no between‐slice spacing, and interleaved slice acquisition. Altogether 37 oblique axial slices covered the whole head. The resulting voxel size was 3.5 × 3.5 × 3.5 mm3.
Anatomical MRI volumes were acquired immediately after the functional scans using a 3‐D fast spoiled gradient echo sequence (inversion‐recovery prepared) with the following parameters: TR 9 ms, TE 1.9 ms, FA 15°, matrix 256 × 256, FOV 240–260 mm, NEX 1, and sagittal slice thickness 1.3 (or 1.4) mm. The resulting voxel size was 1.0 × 1.0 × 1.3 (or 1.4) mm3.
fMRI Evaluation—Preprocessing in SPM2
Preprocessing in SPM2 [Wellcome Department of Imaging Neuroscience, London, UK; Friston et al., 1995] included realignment, acquisition time correction (with the lowermost slice, acquired first, as a reference—for optimal precision with respect to amygdalar activation), and normalization with subsampling to voxels of 1.75 × 1.75 × 1.75 mm3, followed by “minimal” smoothing with a 3.5 × 3.5 × 3.5 mm3 kernel (full width at half maximum corresponded to the original voxel size), to minimize smoothing‐related spread of activation in the analysis of cortical areas. To study activation in hypothesis‐driven subcortical ROIs, an additional analysis relied on normalization which kept the original voxel size, followed by “liberal” smoothing with a 7.0 × 7.0 × 7.0 mm3 kernel to address subject‐to‐subject differences in anatomical location.
fMRI Evaluation—Statistics in SPM2
First, exploratory whole‐brain analysis served to define parameters for the subsequent search for activations in hypothesis‐driven cortical and subcortical ROIs.
Next, cortical activation was studied in minimally smoothed data. Because initial exploratory data analysis of precentral activation suggested most robust condition‐dependent differences in the onset phase of the hemodynamic responses, finite impulse response models were used, with 10 time points (2.5 s apart) per event. Parameter estimates for time points 2.5 s and 5.0 s were processed in a single t‐contrast per subject that was used for random‐effects analysis. Results were subjected to small‐volume correction to detect clusters of activation within hypothesis‐driven ROIs (precentral gyri; Fig. 3A1,A2). To assess whether hemispheric differences were statistically significant, contrast images were compared (original vs. flipped in the x plane) in within‐subjects group analysis (Table I). To exclude anatomical asymmetry of motor cortices as an explanation of the cluster of right > left precentral activation, a hemisphere‐independent precentral ROI was created by intersecting the volumes of the left precentral gyrus and the flipped volume of the right precentral gyrus as defined in Tzourio‐Mazoyer et al.'s [ 2002] hemisphere‐specific parcellation. Cortical activation in Fig. 3A1,A2 was plotted by means of xjview (http://people.hnl.bcm.tmc.edu/cuixu/xjView; Xu Cui and Jian Li).
Figure 3.
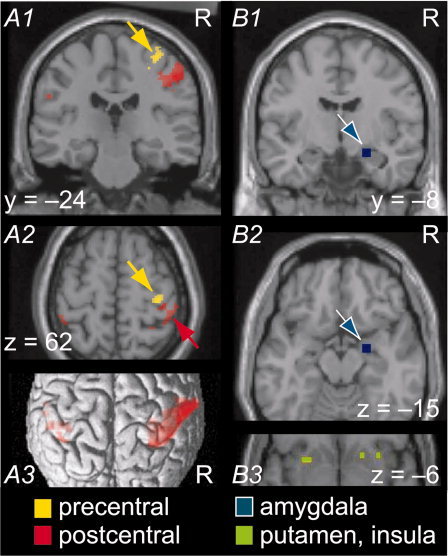
Results of ROI analysis: Sensorimotor, amygdalar, insular, and putamen activation in response to visually presented distorted hand postures. Random‐effects analysis (N = 15 subjects). (A1, A2) Precentral (yellow) and postcentral (red) activation in contrast Distorted – Natural hands (for first‐person perspective). Yellow cluster: 28 voxels in primary motor cortex (each 1.75 × 1.75 × 1.75 mm3, Z max = 3.95, peak voxel at 35 −26 60, P = 0.012 at cluster level with small‐volume correction for right precentral ROI; see Subjects and Methods). No activation in the left precentral ROI analysis. The right > left difference in precentral activation was statistically significant in an 11‐voxel cluster, peak at 33 −26 65 in MNI coordinates (anatomical asymmetry of motor cortices was ruled out as a confound; see Subjects and Methods and Table I). Red clusters: postcentral gyrus portions of large parietal activations (obtained by intersecting activation shown in Fig. 2C with postcentral ROI and thresholding at 20 voxels, submaxima listed in Supporting Information Table S1). Peak voxel of the right postcentral portion at 56 −21 46, Z max = 4.80. Asymmetry of activation (see Subjects and Methods) was statistically significant (right > left) in the postcentral gyrus (peak voxels for most robust asymmetry: ±60 −18 46, ±51 −23 46) and in the posterior wall of the central sulcus (±54 −12 39); see Table I. (A3) Postcentral activation in contrast Distorted — Natural Hands (for first‐person perspective; same intersection as in panel A2). Except for voxel‐level thresholds, analysis parameters for cortical ROIs in (A1) to (A3) were as for Figure 2C (i.e., minimally smoothed data for optimal cortical resolution and evaluation of first two sampling points after stimulus onset to assess between‐condition differences in onsets of hemodynamic responses). (B1, B2) Amygdalar activation (blue) in contrast Distorted — Natural hands (regardless of perspective). Cluster of 4 voxels of 3.5 × 3.5 × 3.5 mm3, Z max = 4.73, P = 0.006 at cluster level with small‐volume correction for right amygdalar ROI (see Subjects and Methods), peak voxel at 24 −7 −14. (B3) Right anterior insula and bilateral putamen activations (green) in contrast “First 10 distorted postures”–“Last 10 distorted postures” (out of 30 distorted postures in total in Session 1). Activation in this contrast was also found in the right amygdalar ROI (see Table III). To account for anatomical variation across subjects, the results of (B1) to (B3) were obtained on liberally smoothed data (kernel of 2 × original voxel size), assessing the entire time range of the hemodynamic response (canonical model). Activations in all panels superimposed on SPM2 single‐subject template brain.
Table I.
Clusters of statistically significant left versus right hemisphere differences in activation for the Distorted – Natural hands contrast (for first‐person perspective; complementary to results shown in Fig. 3)
| Voxel count | L > R | L < R | Z max | MNI | Anatomical location |
|---|---|---|---|---|---|
| 327 | X | 5.21 | ±7 −80 −9 | Lingual gyrus and calcarine sulcus | |
| 11 | X | 4.54 | ±33 −26 65 | Precentral gyrusa | |
| 20 | X | 4.33 | ±47 −60 −7 | Inferior temporal gyrus | |
| 96 | X | 4.32 | ±60 −18 46 | Postcentral gyrus and inferior parietal lobule | |
| 10 | X | 4.09 | ±2 44 −5 | Superior frontal gyrus and medial orbital | |
| 9 | X | 3.64 | ±44 −54 37 | Angular gyrus | |
| 10 | X | 3.53 | ±51 −37 28 | Supramarginal gyrus |
The table shows all clusters exceeding an extremely lenient cluster‐level threshold of 3.5 × 3.5 × 3.5 mm3 (1 voxel in original resolution = 8 subsampled voxels), with the exception of two clusters at 21 −28 40 (14 voxels, white matter) and −37 −21 16 (10 voxels, left hippocampus and left lateral ventricle). Note the left > right activation in visual cortex (327 voxels, cf Supporting Information Fig. S1, Panel B1) corresponding to the images in first‐person perspective that occupied the center and lower right quadrant of the visual field (cf Fig. 1).
Voxel count refers to voxels of 1.75 × 1.75 × 1.75 mm3, MNI refers to MNI coordinates of local maximum, and anatomical location was defined in AAL toolbox (Tzourio‐Mazoyer et al., 2002). L refers to the left hemisphere and R to the right hemisphere.
This cluster was within the hemisphere‐independent precentral ROI (see Subjects and Methods), thereby excluding anatomical asymmetry of motor cortices as an explanation of asymmetric activation.
Finally, subcortical activation—in amygdala, insula, putamen, globus pallidus, and anterior cingulate ROIs—was searched for in liberally smoothed data, using models with canonical hemodynamic response functions. This analysis step relied on small‐volume correction to detect clusters of activation in hypothesis‐derived ROIs—anatomically defined as in Tzourio‐Mazoyer et al. [ 2002].
Cluster locations were identified in MNI coordinates. Putative Brodmann areas and anatomical regions were found in the AAL toolbox [Tzourio‐Mazoyer et al., 2002] and in a brain atlas [Talairach and Tournoux, 1988], after conversion of coordinates (mni2tal procedure, Matthew Brett, http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml). Probabilities for cytoarchitectonic areas were obtained by means of the SPM Anatomy toolbox, version 2004 [Eickhoff et al., 2005].
Results that are of immediate interest to the topic of this study, namely brain activation evoked by distorted (relative to natural) hand postures, are reported in the next section; for other results, see Supporting Information online.
RESULTS
Behavioral Data
On the 0–10 scale (minimally to maximally unpleasant), the ratings for distorted hands, 6.20 ± 0.52 (mean ± SEM), were much higher than those for natural hands, 0.57 ± 0.27 (t = 10.07; df = 12; P < 0.001). Ratings for distorted stimuli in first‐ and third‐person perspectives did not differ (6.21 ± 0.54 vs. 6.19 ± 0.54; t = 0.18; df = 12; P = 0.858).
Exploratory Analysis of fMRI Data
The finger images elicited robust activation in the visual thalamus and in striate and extrastriate visual cortices (Supporting Information Fig. S1). The contrast of Distorted – Natural postures (Fig. 2A) showed a bilateral, right‐hemisphere dominant pattern of strong activation in the extrastriate visual cortex and in large parietal clusters (comprising portions of postcentral gyrus, inferior parietal lobule, and supramarginal gyrus). A further cluster exceeded the threshold with small‐volume correction for the right amygdalar ROI (see legend to Fig. 2 where lacking activation in reverse contrast is also reported).
Figure 2.

Results of exploratory data analysis of minimally smoothed data (random effects, N = 15 subjects). (A) Clusters of stronger activation in the Distorted – Natural hands contrast (regardless of first‐ or third‐person perspective). Extrastriate (occipito‐temporal) and postcentral activation, with larger and more robust clusters in the right hemisphere, shown here—for display purposes—with a very lenient cluster‐level threshold (volume of 1 voxel of 3.5 mm × 3.5 mm × 3.5 mm in original resolution = 8 subsampled voxels in this figure). Out of these clusters, the following exceeded the threshold for the whole‐brain search volume: parietal clusters (comprising portions of postcentral gyrus, inferior parietal lobule, and supramarginal gyrus bilaterally), right 574 voxels of 1.75 × 1.75 × 1.75 m3, Z max = 5.57, peak voxel 65 −18 37 (right postcentral gyrus, highlighted by cursor) and left 147 voxels, Z max = 4.90, peak voxel −46 −26 30 (inferior parietal lobule); extrastriate clusters: 832 voxels, Z max = 4.77, peak voxel 53 −67 −4; 473 voxels, Z max = 4.68, 28 −72 30; 467 voxels, Z max = 4.43, −49 −72 30, 53 voxels, Z max = 3.91, 40 −49 −19. Activation in the right amygdala did not exceed the 8‐voxel threshold (therefore not seen in figure); with small‐volume correction for right amygdalar ROI: P = 0.036 for 3 voxels, Z max = 3.59, 23 −7 −14 and P = 0.062 for 1 voxel, Z max = 3.34, 25 −4 −16. Effect sizes (% BOLD change) are given in Supporting Information Figure S2. No suprathreshold activation in the reverse contrast, Natural–Distorted hands, neither for whole brain as search volume nor for ROIs. (B) Clusters of stronger activation for distorted versus natural postures in first‐person compared with third‐person perspective, displayed with the same lenient threshold as in panel A. Out of these, an 18‐voxel cluster in right precentral gyrus exceeded a small‐volume‐corrected threshold for the right precentral gyrus ROI (Z max = 3.83, peak voxel 33 −25 58, highlighted with a square), with ROI as defined in Tzourio‐Mazoyer et al. (2002). Effect sizes are given in Supporting Information Figure S2. Furthermore, a 65‐voxel occipital cluster exceeded the threshold for the whole‐brain search volume (Z max = 4.35, peak voxel 23 −70 16, area 17, highlighted by cursor). No suprathreshold activations in subcortical ROIs. No suprathreshold activations, neither in whole brain as search volume nor in ROIs, in the reverse contrast. (C) Cortical activation in contrast Distorted – Natural hands (restricted to first‐person‐perspective stimuli) rendered onto SPM2 single subject template brain. Notice the asymmetry between right‐ and left‐parietal clusters (altogether 736 vs. 373 voxels). For details on asymmetry, see text and Table I. The largest clusters of activation in this contrast were in extrastriate occipito‐temporal areas, comprising altogether 1028 voxels in the left hemisphere and 1360 in the right. Whole‐brain analysis with voxel‐level threshold P = 0.001 uncorrected, cluster‐level threshold P = 0.05 corrected (minimum 50 voxels). Otherwise, analysis parameters were as in the subsequent ROI analysis of cortical activations, i.e., to assess between‐condition differences in onsets of hemodynamic responses, the contrast evaluated the first two sampling points (2.5 and 5.0 s) after stimulus onset (finite impulse response model).
The next exploratory step identified brain areas where Distorted – Natural differences were stronger for stimuli in first‐person (compared with third‐person) perspective (Fig. 2B; the legend also reports lacking activation in reverse contrast). Comparison between Figure 2B and Figure 2A shows that activation in the large parietal clusters of the Distorted – Natural contrast did not vary with perspective. Only in one of the cortical and subcortical ROIs, namely in right motor cortex, did the first‐person perspective stimuli elicit stronger Distorted – Natural differences than the stimuli in third‐person perspective (Fig. 2B, highlighted).
Based on the results shown in Figure 2B, the further analysis of cortical Distorted – Natural activation was restricted to stimuli in first‐person perspective; the aim was to capture perspective‐dependent right motor‐cortex activation. For parietal and extrastriate occipital areas, the first‐person‐restricted contrast (Fig. 2C) can be considered as representing the Distorted (all stimuli)–Natural (all stimuli) contrast because Figure 2B gives no indication of perspective‐related differences. Accordingly, the pattern of activation in Figure 2A is faithfully reflected in Figure 2C, with bilateral, right‐hemisphere‐dominant parietal and extrastriate occipital activations. Details of cortical activation will now be reported separately for each ROI.
Cortical Activation in the Distorted – Natural Contrast
In the right motor‐cortex ROI, the most robust differences between distorted versus natural finger postures occurred in the hand knob [Yousry et al., 1997] of the primary motor cortex (Fig. 3A1,A2). The MNI coordinates of the peak voxel (35 −26 60) also supported activation of Brodmann's area 4, both according to an atlas brain [Talairach and Tournoux, 1988] and an atlas of probabilistic cytoarchitectonics [Eickhoff et al., 2005]. No corresponding activation was found in the left hand knob. The hemispheric right > left difference was statistically significant in a subcluster comprising nearly half of the hand knob cluster (see Table I and Fig. 3, legend). Anterior and inferior to the right primary motor‐cortex cluster, activation occurred in the left precentral gyrus (peak voxel −47 −1 43, area 6 in atlas brain) but without statistically significant hemispheric asymmetry.
The right postcentral activation comprised a subcluster immediately adjacent to the motor‐cortex cluster (Fig. 3A2) and further submaxima (see Supporting Information Table S1) in cytoarchitectonic areas 2, 1, and 3b [Eickhoff et al., 2005]. The bilateral activation in the postcentral sulcus extended into the putative Brodmann area 5 (see Fig. 3A3). Clusters also extended into the supramarginal and superior parietal gyri, as well as into the inferior parietal lobule (corresponding to areas 7 and 40), consistent with activation of secondary somatosensory cortex (see Fig. 2C and Table II). Right‐hemisphere dominance was statistically significant in the postcentral gyrus and in the posterior wall of the central sulcus (Table I).
Table II.
Voxel counts in parietal clusters, broken down by regions defined according to AAL toolbox
| Postcentral gyrus | Parietal inferior | Parietal superior | Supramarginal | |
|---|---|---|---|---|
| Left | 31 | 135 | 92 | 109 |
| Right | 537 | 43 | 87 | 53 |
The original clusters were obtained as results of whole‐brain analysis; P < 0.001 uncorrected at voxel level; minimum cluster size 50 voxels of 1.75 × 1.75 × 1.75 mm3.
Subcortical Activations in the Distorted – Natural Contrast
In none of the subcortical ROIs, Distorted – Natural differences varied with stimulus perspective (see Fig. 2B legend). Accordingly, the analysis of subcortical activations relied on the full set of stimuli, pooled across first‐ and third‐person perspectives (see Subjects and Methods for further details on analysis parameters).
Activation of the right amygdala was evident in the Distorted – Natural contrast, regardless of the perspective (Fig. 3B1,B2). Amygdalar activation showed marked decrease within the first session, as was evident in the contrast of first 10–last 10 distorted postures. This contrast also revealed activation in the right anterior insula and bilaterally in the putamen, but not in globus pallidus (Fig. 3B3; for a full list of clusters for this contrast, please see Table III). No precentral nor postcentral clusters were found in this contrast; nor was any suprathreshold activation found in the reverse contrast, last 10–first 10 distorted postures.
Table III.
Clusters resulting from contrast “First 10 distorted postures”–“Last 10 distorted postures”
| Voxel count | Z max | P | MNI | Anatomical location |
|---|---|---|---|---|
| 3 (in ROI) | 3.39 | 0.007 | 32 0 −18 | Right amygdala |
| 4 (in ROI) | 3.80 | 0.030 | 39 11 −11 |
Right insula (shown in Fig. 3B3) |
| 3 (in ROI) | 3.33 | 0.025 | −25 11 −7 |
Left putamen (shown in Fig. 3B3) |
| 3 (in ROI) | 3.27 | 0.026 | 21 14 −11 |
Right putamen (shown in Fig. 3B3) |
| 41 | 4.13 | 0.001 | −35 −77 −11 | Left inferior occipital sulcus |
| 26 | 4.05 | 0.011 | 60 −60 0 | Right middle temporal gyrus |
| 35 | 3.98 | 0.002 | −42 −60 −21 | Left fusiform gyrus |
| 21 | 3.63 | 0.031 | 46 −60 −18 | Right fusiform gyrus |
Each set of 10 postures comprised five in first‐person perspective and five in third‐person perspective out of 30 distorted postures in total in Session 1 only. The table lists results of ROI analysis (top 4 rows, with small‐volume correction for the respective ROIs as defined in AAL toolbox [Tzourio‐Mazoyer et al. 2002]) and all areas where activation exceeded the cluster‐level threshold for whole‐brain analysis (bottom 4 rows).
Voxel count refers to voxels of 3.5 × 3.5 × 3.5 mm3, MNI refers to MNI coordinates of local maximum, and anatomical location was defined in AAL toolbox.
In contradistinction to these activated brain regions, no activation for distorted (relative to natural postures) was seen in the anterior cingulate. Nor did we detect brain activations in the contrast of 20 most unpleasant versus 20 least unpleasant distorted postures (sorted for each subject on the basis of postscanning unpleasantness ratings).
DISCUSSION
We aimed to identify brain correlates of the “gut reactions,” complex emotions including disgust, that occur when humans notice bodily abnormalities in other humans. The brain imaging results in the Distorted – Natural contrast support our hypothesis of embodied perception of abnormal finger postures, involving activation of the primary motor cortex and of primary and secondary somatosensory cortices, as well as amygdala and insula—all of these areas in a right‐hemisphere‐dominant manner—and of bilateral putamen.
Sensitivity of the hand knob area of the primary motor cortex to distorted finger postures resembles magnetoencephalographic motor‐cortex modulation during observation of finger actions [Hari et al., 1998; Caetano et al., 2007]. The stronger primary motor‐cortex response to distorted postures in first‐person versus third‐person perspective stimuli is in line with the literature for action viewing [Maeda et al., 2002]. In this respect, activation of the primary motor cortex in the Distorted – Natural contrast was different from parietal and subcortical clusters where activation did not vary with perspective (discussed below). It is conceivable that the primary motor‐cortex activation reflects a process of internal simulation of hand postures (for a review, see Jeannerod [ 2001]) which could be particularly strong for stimuli in the familiar first‐person perspective. Whether or not this process is actually absent for stimuli in third‐person perspective cannot be stated on the basis of the present results.
Whatever the cause for the perspective‐related difference in the primary motor cortex is, it is noteworthy that corresponding differences were not found in the parietal and extrastriate occipital activations in the Distorted – Natural contrast. Although not too much emphasis can be paid on negative fMRI results, it is intriguing to view the lack of differential activation as reflecting that other persons' hands can appear to an observer in third‐ as well as first‐person perspectives (for example, when an expert demonstrates how to use a hand‐held tool).
Primary motor‐cortex activation was associated with premotor activation located within 1.5 cm from the site activated during observation of hand action in a previous fMRI study [Buccino et al., 2001].
The extension of the parietal activation into area 5 bilaterally (see Fig. 3A2) is in line with activation in the left postcentral sulcus when subjects viewed object‐related hand actions in a movie [Hasson et al., 2004]. Such an activation is understandable because area 5 contributes to processing of the limb positions, supporting the observer's own position sense and sensorimotor guidance [Kalaska et al., 1997]. The parietal clusters extended bilaterally into the intraparietal sulcus—where the small human parietal eye field has been located [Müri et al., 1996]—possibly reflecting the gaze pattern during presentation of distorted postures. The bulk of parietal activation, however, is clearly anterior to eye‐movement‐related sites. Several postcentral submaxima (Supporting Information Table S1) were located in area 2 that receives input from joint receptors, in line with the observation that TMS‐induced virtual lesions to primary somatosensory cortex interfere with the processing of biologically impossible index‐finger movement [Avenanti et al., 2007]. Specifically, virtual lesions abolished the facilitation of motor‐evoked potentials that is normally found during observation of biologically impossible movement. The study was, however, limited to the left hemisphere and to videos of right‐index‐finger movement [Avenanti et al., 2007]. Moreover, left supramarginal activation, found here in the Distorted – Natural contrast (Table II), was also observed when subjects had an explicit motor imagery task for hand movements [Lorey et al., 2009]. This pattern of activation suggests that the highly automatic perception of bodily distortions involves the subject's own proprioceptive and motor circuits, again in agreement with the embodied perception of the distorted fingers. Although our still pictures of finger postures are not comparable to the videos of impossible movement used in an earlier study, there is remarkable agreement in terms of posterior parietal activation [Costantini et al., 2005]. This activation was interpreted as a correlate of a process that determines whether or not an observed movement can be performed [Costantini et al., 2005].
Although all pictures showed right hands, the pre‐ and postcentral activations in the Distorted – Natural contrast depicted statistically significant hemispheric right > left differences that were most robust in the motor‐cortex cluster. This laterality could reflect the emotional valence of the distorted finger postures which the subjects rated as unpleasant. A predominant role of the right hemisphere for the processing of negative emotions is predicted by several theories: (a) by the right hemisphere model, which claims a role of right hemisphere for processing all emotions, regardless of valence; (b) by the valence model and the approach‐withdrawal model, which both claim left‐hemisphere dominance for positive emotions related to approach behavior and right‐hemisphere dominance for negative emotions related to withdrawal behavior [Borod, 1992; Borod et al., 1998; Demaree et al., 2005]. Analogous laterality effects are evident in difficulties that patients with lesions in the somatosensory areas of the right inferior parietal cortex encounter when trying to recognize fearful faces [Adolphs et al., 1996]. The role of the right somatosensory cortex in the processing of fearful faces is also supported by interference effects of transcranial magnetic stimulation [Pourtois et al., 2004]. Furthermore, after right‐hemisphere stroke with anosognosia for hemiparesis, some patients show symptoms of misoplegia, that is, hatred of a body part [Critchley, 1955; Baier and Karnath, 2008]. In contrast to the right‐hemisphere‐dominant activation by the distorted finger postures, emotionally neutral handwritten letters, written by a person unknown to the subject, activate predominantly the left motor cortex [Longcamp et al., 2006]. Only in the right‐hemisphere motor cortex, distorted‐posture‐related activation was different for first‐ versus third‐person perspective (Fig. 2B).
Activation of the right primary motor cortex in response to distorted right‐hand postures might seem surprising at first sight. However, it is well established that the primary motor cortex is activated bilaterally during observation of unilateral hand movements [Hari et al., 1998]. To some extent, our results resemble motor‐cortex activation observed when subjects viewed videos of biologically impossible movement [Romani et al., 2005]; however, that study was limited to the hemisphere contralateral to the (first‐person‐perspective) stimulus hand.
The observed right‐hemisphere‐dominant motor‐cortex activation in response to distorted right‐hand postures might also reflect the emotional valence of the stimuli or the extra demands for visuospatial analysis of distorted finger postures. The latter explanation would be in line with findings in left‐ versus right‐hemisphere‐damaged patients based on tasks as used in limb apraxia diagnostics; the results suggest that the left hemisphere is fully competent for the processing of hand postures, whereas finger postures require an additional right‐hemispheric contribution, possibly because of demands for more complex visuospatial analysis [Goldenberg, 1999, 2001]. However, a later study—although supporting left‐hemisphere dominance for hand postures—found finger‐posture‐specific imitation deficits only in two out of 24 patients with right‐hemisphere lesions [Della Sala et al., 2006]. Furthermore, brain activation related to finger versus hand gestures was not different (in either left or right hemisphere) in an fMRI study of healthy subjects who performed an in‐scanner imitation task [Mühlau et al., 2005]. We therefore cannot at this stage be conclusive about the causes of the right‐hemisphere‐dominant motor‐cortex activation.
The observed right‐sided amygdala activation could reflect the social–emotional valence of the unpleasant distorted fingers. Although different in visual properties, distorted fingers can be considered comparable to emotional faces in terms of emotional valence. Faces, especially portraying fear, activate the amygdala [Whalen et al., 1998; Haxby et al., 2002; Pessoa et al., 2002] in a right‐side‐dominant manner [Noesselt et al., 2005]. Given the social context of the distorted finger postures (as if a conspecific or oneself—after an accident, for example—were affected by a bodily abnormality), the observed amygdalar activation could also reflect social stress—which amygdala‐lesioned monkeys do not experience [Amaral, 2002]. The rapid decrease of amygdalar activation with stimulus repetition (within the first session) is in line with earlier reports for responses to fearful faces [Breiter et al., 1996; Wright et al., 2001].
Our results suggest a synergy between processing of disgust‐provoking hand postures, emotional faces, and whole‐body expressions of fear [Hadjikhani and de Gelder, 2003], in line with the role of the right amygdala in mediating emotional global reactions [Gläscher and Adolphs, 2003]. The observed coactivation of amygdala and extrastriate visual areas is consistent with amygdalar modulation of the extrastriate cortex. Established in patient studies [Vuilleumier et al., 2004], such modulation would explain the observed pattern of fMRI coactivation as well as magnetoencephalographic (MEG) responses that started to differ between pictures of distorted and natural hand postures only 260 ms after stimulus onset and only in the extrastriate, but not in the striate, cortex [Avikainen et al., 2003]. Correspondingly, the present study unravelled occipitotemporal activation for distorted (relative to natural) finger postures in the extrastriate (rather than the striate) cortex: The clusters bilaterally extended into fusiform gyri (see also Fig. 2C and Table III)—in line with the right fusiform activation for angry (compared with neutral) hand movements shown in video clips [Grosbras and Paus, 2006].
Could our bilateral clusters of extrastriate activation (Fig. 2C) overlap with the extrastriate body area [Downing et al., 2001; Pitcher et al., 2009]? Activation in this area is known to be modulated by the emotional content of stimuli [Peelen et al., 2007], with peak voxels for body‐part‐specific activation (−47 −71 3 and 48 −66 3 in Talairach coordinates) well within our clusters of activation in the Distorted – Natural contrast (as reported in Fig. 2C). However, the extrastriate body area is typically found separately in each individual in a localizer experiment—e.g., human body parts versus inanimate objects [Downing et al., 2001]—and since we do not have such data, we cannot go into deeper discussion about the involvement of this area in the processing of distorted finger postures.
The short‐lived activations in right anterior insula and bilaterally in putamen at the beginning of the first fMRI session could be related to processing of disgust‐related stimulus content [Wicker et al., 2003; Calder et al., 2007; Mataix‐Cols et al., 2008]. Remarkably, activation of putamen and insula has been found when subjects viewed hated (as compared with neutral) faces [Zeki and Romaya, 2008].
Insular activation related to disgust‐related processing is more likely than a conceivable activation as part of the pain matrix, given the co‐occurrence of putamen activation, but lack of anterior cingulate activation as it occurs when subjects view painful facial expressions [Saarela et al., 2007] or otherwise become aware of others experiencing pain (for a review, see Hein and Singer [ 2008]). Therefore, empathy for pain is a less likely correlate of the observed brain activation. It is conceivable that this result reflects the isolated appearance of hand and fingers in our stimuli (as opposed to whole‐body stimuli including faces).
In contradistinction to the rapidly habituating activations in amygdala, insula, and putamen, neither pre‐ nor postcentral clusters were observed in the contrast of first 10–last 10 distorted pictures. It is thus possible that somatomotor areas contribute a particular non‐habituating aspect of stimulus evaluation, for example, automatic simulation of postures. Appealing as this interpretation is, it should be treated with caution because it is based on a negative result.
Unpleasantness ratings convincingly demonstrated strong differences between distorted and natural hand postures. However, within the set of distorted postures, the most unpleasant versus least unpleasant stimuli did not differ in terms of brain activation. This result is not surprising because ratings were only obtained when the subjects viewed—for a second time—the stimuli that had been already presented in the scanner. Given the marked habituation of brain activation in response to distorted postures, it is likely that ratings underestimated stimulus‐to‐stimulus differences in unpleasantness during scanning. In‐scanner rating was not feasible due to possible interference with stimulus‐induced activation in the primary motor cortex, one of our ROIs.
The regional differences in perspective‐dependence of the Distorted – Natural differences (present in right motor cortex but absent in amygdala) need to be interpreted with caution, due to the negative result with respect to amygdala. As discussed above, the data raise the possibility that motor‐cortex activation could reflect automatic motor simulation that differs for first‐person versus third‐person perspective—in line with perspective‐dependent differences in motor cortex excitability when subjects viewed videotaped natural hand actions [Maeda et al., 2002]. Amygdalar activation, on the other hand, could be a correlate of perspective‐independent emotional processing—in line with the lack of perspective‐dependence in subjects' estimates of stimulus unpleasantness.
CONCLUSION
Responses to distorted (compared with natural) finger postures were observed in several brain areas. This network comprised at least the precentral motor cortex, the postcentral somatosensory cortex, secondary somatosensory areas, extrastriate visual cortex, the right amygdala, the right anterior insula, and putamen bilaterally. The network could support the embodied percept of observed bodily abnormalities, partly through internal simulation of observed postures and partly through processing of the emotional valence of the stimuli.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
We are grateful to Marita Kattelus for technical assistance during fMRI scanning, Marika Kaksonen for the eye tracking data, and Kristiina Luoma for participation in stimulus editing and pilot recordings.
REFERENCES
- Adolphs R, Damasio H, Tranel D, Damasio AR ( 1996): Cortical systems for the recognition of emotion in facial expressions. J Neurosci 16: 7678–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG ( 2002): The primate amygdala and the neurobiology of social behavior: Implications for understanding social anxiety. Biol Psychiatry 51: 11–17. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bolognini N, Maravita A, Aglioti SM ( 2007): Somatic and motor components of action simulation. Curr Biol 17: 2129–2135. [DOI] [PubMed] [Google Scholar]
- Avikainen S, Liuhanen S, Schürmann M, Hari R ( 2003): Enhanced extrastriate activation during observation of distorted finger postures. J Cogn Neurosci 15: 658–663. [DOI] [PubMed] [Google Scholar]
- Baier B, Karnath HO ( 2008): Tight link between our sense of limb ownership and self‐awareness of actions. Stroke 39: 486–488. [DOI] [PubMed] [Google Scholar]
- Borod JC ( 1992): Interhemispheric and intrahemispheric control of emotion: A focus on unilateral brain damage. J Consult Clin Psychol 60: 339–348. [DOI] [PubMed] [Google Scholar]
- Borod JC, Cicero BA, Obler LK, Welkowitz J, Erhan HM, Santschi C, Grunwald IS, Agosti RM, Whalen JR ( 1998): Right hemisphere emotional perception: Evidence across multiple channels. Neuropsychology 12: 446–458. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR ( 1996): Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17: 875–887. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ ( 2001): Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur J Neurosci 13: 400–404. [PubMed] [Google Scholar]
- Caetano G, Jousmäki V, Hari R ( 2007): Actor's and observer's primary motor cortices stabilize similarly after seen or heard motor actions. Proc Natl Acad Sci USA 104: 9058–9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD ( 2007): Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci 25: 3422–3428. [DOI] [PubMed] [Google Scholar]
- Costantini M, Galati G, Ferretti A, Caulo M, Tartaro A, Romani GL, Aglioti SM ( 2005): Neural systems underlying observation of humanly impossible movements: An FMRI study. Cereb Cortex 15: 1761–1767. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley M ( 1955): Personification of paralyzed limbs in hemiplegics. Br Med J 2: 284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR ( 1996): The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 351: 1413–1420. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Faglioni P, Motto C, Spinnler H ( 2006): Hemisphere asymmetry for imitation of hand and finger movements, Goldenberg's hypothesis reworked. Neuropsychologia 44: 1496–1500. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Everhart DE, Youngstrom EA, Harrison DW ( 2005): Brain lateralization of emotional processing: historical roots and a future incorporating “dominance”. Behav Cogn Neurosci Rev 4: 3–20. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N ( 2001): A cortical area selective for visual processing of the human body. Science 293: 2470–2473. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD ( 2006): The somatic marker hypothesis: A critical evaluation. Neurosci Biobehav Rev 30: 239–271. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gläscher J, Adolphs R ( 2003): Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci 23: 10274–10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G ( 1999): Matching and imitation of hand and finger postures in patients with damage in the left or right hemispheres. Neuropsychologia 37: 559–566. [DOI] [PubMed] [Google Scholar]
- Goldenberg G ( 2001): Imitation and Matching of Hand and Finger Postures. NeuroImage 14: S132–S136. [DOI] [PubMed] [Google Scholar]
- Goldman A, de Vignemont F ( 2009): Is social cognition embodied? Trends Cogn Sci 13: 154–159. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T ( 2006): Brain networks involved in viewing angry hands or faces. Cereb Cortex 16: 1087–1096. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, de Gelder B ( 2003): Seeing fearful body expressions activates the fusiform cortex and amygdala. Curr Biol 13: 2201–2205. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G ( 1998): Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc Natl Acad Sci USA 95: 15061–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Kujala MV ( 2009): Brain basis of human social interaction: From concepts to brain imaging. Physiol Rev 89: 453–479. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R ( 2004): Intersubject synchronization of cortical activity during natural vision. Science 303: 1634–1640. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI ( 2002): Human neural systems for face recognition and social communication. Biol Psychiatry 51: 59–67. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T ( 2008): I feel how you feel but not always: The empathic brain and its modulation. Curr Opin Neurobiol 18: 153–158. [DOI] [PubMed] [Google Scholar]
- Jeannerod M ( 2001): Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage 14: S103–S109. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Scott SH, Cisek P, Sergio LE ( 1997): Cortical control of reaching movements. Curr Opin Neurobiol 7: 849–859. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Tanskanen T, Hari R ( 2006): The imprint of action: Motor cortex involvement in visual perception of handwritten letters. Neuroimage 33: 681–688. [DOI] [PubMed] [Google Scholar]
- Lorey B, Bischoff M, Pilgramm S, Stark R, Munzert J, Zentgraf K ( 2009): The embodied nature of motor imagery: The influence of posture and perspective. Exp Brain Res 194: 233–243. [DOI] [PubMed] [Google Scholar]
- Maeda F, Kleiner‐Fisman G, Pascual‐Leone A ( 2002): Motor facilitation while observing hand actions: Specificity of the effect and role of observer's orientation. J Neurophysiol 87: 1329–1335. [DOI] [PubMed] [Google Scholar]
- Mataix‐Cols D, An SK, Lawrence NS, Caseras X, Speckens A, Giampietro V, Brammer MJ, Phillips ML ( 2008): Individual differences in disgust sensitivity modulate neural responses to aversive/disgusting stimuli. Eur J Neurosci 27: 3050–3058. [DOI] [PubMed] [Google Scholar]
- Mataric MJ, Pomplun M ( 1998): Fixation behavior in observation and imitation of human movement. Brain Res Cogn Brain Res 7: 191–202. [DOI] [PubMed] [Google Scholar]
- Mühlau M, Hermsdörfer J, Goldenberg G, Wohlschläger AM, Castrop F, Stahl R, Röttinger M, Erhard P, Haslinger B, Ceballos‐Baumann AO, Conrad B, Boecker H ( 2005): Left inferior parietal dominance in gesture imitation: An fMRI study. Neuropsychologia 43: 1086–1098. [DOI] [PubMed] [Google Scholar]
- Müri RM, Iba‐Zizen MT, Derosier C, Cabanis EA, Pierrot‐Deseilligny C ( 1996): Location of the human posterior eye field with functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry 60: 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noesselt T, Driver J, Heinze HJ, Dolan R ( 2005): Asymmetrical activation in the human brain during processing of fearful faces. Curr Biol 15: 424–429. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Atkinson AP, Andersson F, Vuilleumier P ( 2007): Emotional modulation of body‐selective visual areas. Soc Cogn Affect Neurosci 2: 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG ( 2002): Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA 99: 11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Charles L, Devlin JT, Walsh V, Duchaine B ( 2009): Triple dissociation of faces, bodies, and objects in extrastriate cortex. Curr Biol 19: 319–324. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Sander D, Andres M, Grandjean D, Reveret L, Olivier E, Vuilleumier P ( 2004): Dissociable roles of the human somatosensory and superior temporal cortices for processing social face signals. Eur J Neurosci 20: 3507–3515. [DOI] [PubMed] [Google Scholar]
- Raij TT, Numminen J, Närvänen S, Hiltunen J, Hari R ( 2005): Brain correlates of subjective reality of physically and psychologically induced pain. Proc Natl Acad Sci USA 102: 2147–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani M, Cesari P, Urgesi C, Facchini S, Aglioti SM ( 2005): Motor facilitation of the human cortico‐spinal system during observation of bio‐mechanically impossible movements. Neuroimage 26: 755–763. [DOI] [PubMed] [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schürmann M, Kalso E, Hari R ( 2007): The compassionate brain: Humans detect intensity of pain from another's face. Cereb Cortex 17: 230–237. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E ( 2008): Mirror‐image representation of action in the anterior parietal cortex. Nat Neurosci 11: 1267–1269. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): A Co‐Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I ( 2003): Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage 18: 650–659. [DOI] [PubMed] [Google Scholar]
- Tracey I ( 2008): Imaging pain. Br J Anaesth 101: 32–39. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Varela F, Thompson E, Rosch E ( 2001): The Embodied Mind: Cognitive Science and Human Experience. Cambridge, MA: MIT Press. [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ ( 2004): Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci 7: 1271–1278. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA ( 1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G ( 2003): Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron 40: 655–664. [DOI] [PubMed] [Google Scholar]
- Wilson M ( 2002): Six views of embodied cognition. Psychon Bull Rev 9: 625–636. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL ( 2001): Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport 12: 379–383. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P ( 1997): Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120: 141–157. [DOI] [PubMed] [Google Scholar]
- Zeki S, Romaya JP ( 2008): Neural correlates of hate. PLoS ONE 3: e3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


