Abstract
A novel method of automated MRI volumetry was used to study regional atrophy and disease progression in repeated MRI measurements of patients with frontotemporal lobar degeneration (FTLD). Fifty‐nine structural MRI data sets of 17 clinically diagnosed FTLD patients were acquired over up to 30 months in intervals of 6 months and compared with data of 30 age‐matched healthy controls. Patients were further subgrouped into behavioral variant FTLD (bvFTLD), progressive nonfluent aphasia (PNFA), and semantic dementia (SemD). Gray matter (GM) volumes of frontal lobes (FL) and temporal lobes (TL) were determined by voxel‐based volumetry based on SPM5 algorithms and a probabilistic brain atlas. MRI volumetry revealed frontal and temporal GM atrophy across FTLD patients, with further progression over time. Significant side asymmetry of TL volumes was found in SemD. The ratio of TL to FL volumes was significantly reduced in SemD and increased in bvFTLD. Using this ratio, 6/7 SemD patients and 5/6 bvFTLD patients could be correctly differentiated. TL/FL ratios in bvFTLD and SemD further diverged significantly over a time span of only 6 months. Rates of temporal GM loss per 6 months were 3–4% in SemD, and 2.5% for frontal GM loss in bvFTLD, and thereby clearly exceeded published cerebral volume loss in healthy elderly subjects. The study presents a fully automated, observer‐independent volumetric assessment of regional atrophy which allows differentiation of FTLD subgroups. Its sensitivity for atrophy progression—even in such short intervals like 6 months—might benefit future clinical trials as treatment outcome measure. Hum Brain Mapp, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: dementia, frontotemporal lobar degeneration, MRI, postprocessing, volumetry
INTRODUCTION
Atrophy of the cerebral cortex is a structural hallmark of primary degenerative dementia. MRI provides the opportunity of assessing structural changes of the brain in vivo at high spatial resolution. A multitude of quantitative MRI studies have investigated cortical atrophy in dementia [e.g., Du et al.,2007; Rohrer et al.,2010; Schroeter et al.,2009]. They can be dichotomized along two axes: (1) studies making inferences at the group level [Rosen et al.,2002] versus those allowing for characterization of single patients [Davies et al.,2009], and (2) studies employing manual volumetry or visual rating scales [Fukui and Kertesz,2000; van de Pol et al.,2006] versus those using automated, observer‐independent methods [Du et al.,2007]. With respect to objectivity, reproducibility, and clinical relevance, automated unbiased assessment tools allowing for single patient analysis appear to be the most favorable combination. In the current study, a recently developed method for automated voxel‐based MRI volumetry, which provides plain volume results of various cerebral structures in individual subjects [Huppertz et al.,2010] has been applied to study patients with clinically diagnosed frontotemporal lobar degeneration (FTLD).
FTLD is the second most common neurodegenerative cause of dementia before 65 years of age [Ratnavalli et al.,2002] and is also likely to be a significant, yet underdiagnosed, cause in patients beyond 65 years of age [Hodges et al.,2010]. Clinically, FTLD is usually defined according to consensus criteria by Neary et al. [1998] and is generally further subtyped into three main clinical variants behavioral variant FTLD (bvFTLD), progressive nonfluent aphasia (PNFA), and semantic dementia (SemD). Typical signs and symptoms of bvFTLD are deficits in social interpersonal conduct, loss of insight, and executive dysfunction. SemD patients typically present with anomia and loss of word meaning, but fluent speech, while PNFA patients show apraxia of speech and/or agrammatism. The language variants SemD and PNFA are subsumed under the clinical syndrome of primary progressive aphasia [Mesulam,2001].
Prevalence of FTLD (about 15 per 100,000 [Ratnavalli et al.,2002]) is lower than that of AD; reports in the literature are consequently much sparser. While clinical presentation, demographic background, and cognitive deficits are well‐characterized, in vivo brain imaging data are more limited. In particular, only a few studies report longitudinal imaging data [Brambati et al.,2007; Chan et al.,2001; Knopman et al.,2009; Krueger et al.,2010; Whitwell et al.,2004]. The latter studies demonstrated longitudinal atrophy in FTLD of the whole brain [Chan et al.,2001; Knopman et al.,2009], the frontal and temporal lobes [Krueger et al.,2010; Whitwell et al.,2004], or the cingulate cortex [Brambati et al.,2007; Whitwell et al.,2004]. These studies investigated atrophy progression at group level over 12 months using not fully automated and observer‐independent techniques. In contrast, in the current study MR images of FTLD patients were acquired at short intervals of six months for up to 30 months and submitted to fully automated volumetry at single patient level. The study aimed at testing if the method of automated MRI volumetry presented here is able to detect specific patterns of atrophy progression in FTLD subgroups and to disclose whole brain and lobar atrophy for such short time intervals at group and at single patient level. Demonstration of the latter would be an important step toward using MRI read‐outs in future clinical trials as a treatment outcome measure.
METHODS
Subjects
Patients were recruited from a specialized memory clinic at the Center of Geriatrics and Gerontology (ZGGF) of the University of Freiburg, Germany. Seventeen patients with clinically diagnosed FTLD according to published criteria [Neary et al.,1998] were included in this study: seven patients with SemD, six with bvFTLD, and four with PNFA (Table I). Clinical diagnoses were based on multiprofessional consensus taking into account assessments of behavior and cognition. In detail, the multiprofessional team included a consultant in geriatric psychiatry, a consultant in neurology, a neuropsychologist and—if performed—a specialist in nuclear medicine for the interpretation of PET or SPECT data. Tc99‐SPECT (N = 6) or FDG‐PET (N = 3) were carried out to further support the diagnoses. Structural MR or CT imaging was used to exclude alternative causes for cognitive deficits, but not for clinical syndrome classification. Baseline (T0) MRI data were obtained from all 17 patients and additional 30 healthy elderly controls (CON). Age and sex distribution were not significantly different between CON and patient groups (pairwise two‐sample T‐tests; chi‐square test). Fifteen patients returned for MR scanning for up to five times every 6 months (T6, T12, T18, T24, and T30), so that in total 59 MRI patient data sets were available. Mean time interval between consecutive scans was 187 days (SD: 25). Clinical symptom onset was estimated from interviews of patients' caregivers. Except for one female PNFA patient with a disease duration of 10 years at first assessment, disease stages were rather early, with a median duration of clinical symptoms of 2 years at baseline MR scanning.
Table I.
Demographic data, MMSE, and CDR scores, whole brain as well as frontal and temporal gray matter volumes (TIV‐corrected) of controls and patients at baseline, and atrophy progression rates
| CON | bvFTLD | PNFA | SemD | |
|---|---|---|---|---|
| Age (years) | 62.9 (7.7) | 64.2 (9.2) | 69.2 (7.6) | 63.8 (9.8) |
| Disease duration (years) | N/A | 2.0 (1.5) | 4.0 (4.0) | 3.0 (1.0) |
| Sex (F/M) | 17/13 | 4/2 | 1/3 | 3/4 |
| MMSE score (/30) | 29.1 (1.1)a | 23.3 (4.2) | 24.3 (5.6) | 25.7 (2.1) |
| CDR score (median) | N/A | 0.75 | 0.5 | 0.5 |
| CDR sum of boxes (median) | N/A | 3.75 | 2.25 | 2.0 |
| Absolute volumes at baseline (TIV‐corrected) | ||||
| Brain (ml) | 1126.0 (67.0) | 917.2 (69.7)b | 1031.1 (73.2)c | 1048.7 (132.3) |
| Left TL (ml) | 64.5 (5.7) | 52.0 (7.0)b | 54.6 (9.4)b | 47.9 (6.8)b |
| Right TL (ml) | 65.0 (5.3) | 53.3 (4.3)b | 58.0 (3.3)c | 56.5 (8.8)b |
| Left FL (ml) | 84.4 (7.9) | 61.8 (13.6)b | 70.1 (6.9)b | 76.3 (13.9) |
| Right FL (ml) | 87.3 (8.1) | 64.4 (10.6)b | 74.6 (8.0)b | 81.2 (15.0) |
| Atrophy progression rates (per 6 months) | ||||
| Brain (%) | N/A | 1.41 (1.13) | 1.94 (0.74) | 2.04 (1.52) |
| Left TL (%) | N/A | 1.56 (2.06) | 2.75 (1.25) | 4.41 (2.07) |
| Right TL (%) | N/A | 1.35 (1.44) | 2.46 (1.52) | 3.38 (3.24) |
| Left FL (%) | N/A | 2.64 (1.64) | 3.06 (1.15) | 2.74 (2.25) |
| Right FL (%) | N/A | 2.38 (1.56) | 3.42 (3.65) | 2.54 (2.27) |
Arithmetic mean (SD), except as indicated.
MMSE scores were determined in 15/30 healthy control participants.
Significance at P < 0.01.
Significant difference between diagnostic group and CON (P < 0.05; two‐sample T‐test).
CON, controls; bvFTLD, behavioral variant FTLD; PNFA, progressive nonfluent aphasia; SemD, semantic dementia; TIV, total intracranial volume; TL, temporal lobe; FL, frontal lobe.
Data were acquired from 2007 to 2010 at the University of Freiburg as part of a larger project investigating longitudinal structural and functional changes in dementia, funded by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF). Written informed consent was obtained from each participant. The study was performed according to the Declaration of Helsinki of 1964 and approved by the Ethics Committee of the University of Freiburg.
MRI Acquisition
High‐resolution T1‐weighted volume data sets of the whole head were acquired at a 3 T scanner (TIM‐Trio; Siemens, Erlangen, Germany) using a magnetization‐prepared rapid gradient echo (MPRAGE) sequence with TR/TE = 2,200/2.15 ms, 12° flip angle, and 1 mm isotropic voxel size. The field of view was 256 × 256 mm2 in plane, 176 sagittal slices were acquired.
MRI Data Processing and Volumetry
The MRI data processing and volumetry have been described in detail elsewhere [Huppertz et al.,2010]. The method is based on SPM5 (statistical parametric mapping software, Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm), and masks derived from a probabilistic brain atlas provided by the Laboratory of Neuroimaging (LONI) at the University of California, Los Angeles, CA (LONI Probabilistic Brain Atlas (LPBA40); http://www.loni.ucla.edu/Atlases). The analysis is fully automated by use of a MATLAB® batch script and requires about 1 h per MRI scan on an AMD Opteron 2.0 GHz PC.
In short, each T1‐weighted volume dataset was normalized to the standard brain of the Montreal Neurological Institute (MNI) included in the SPM5 distribution and segmented into different brain compartments, i.e., gray matter (GM), white matter (WM), and cerebrospinal fluid. This was done by using the “unified segmentation” tool of SPM5 with its default settings. The segmentation resulted in “modulated” and “unmodulated” images for the different tissue compartments. Modulation compensates for dilatation or shrinkage during spatial normalization and has the effect of preserving the total amount of signal from the respective tissue class in the normalized partitions [Ashburner and Friston,2000].
To determine the volume of a specific brain structure of interest the corresponding binary mask (e.g. for frontal or temporal lobes) derived from the LPBA40 atlas was multiplied with the modulated image of the desired tissue class. The values of all voxels in the resulting image were summed up and divided by 1,000 to get the volume of the investigated structure in milliliter units. Because of modulation of the tissue images, the effect of normalization (i.e., extension or shrinkage of the investigated structure) was compensated for so that the computed volume represented the volume of the original structure in native space (see Fig. 1). Substructures of the LPBA40 atlas were combined in the following way to form lobes:
 |
 |
The composition of lobar masks instead of smaller ones (e.g., gyral masks which could be principally achieved with the LPBA40 atlas) was motivated by the observation from previous studies that smaller structures tend to show a higher variability of volumetric results which could obscure the small volume changes expected from short measurement intervals [Huppertz et al.,2010]. Furthermore, the risk of misregistration between the investigated brain and the probabilistic brain atlas increases for smaller cerebral structures. It has to be noted that the structure labeled as “hippocampus” in the LPBA40 atlas apparently comprises hippocampus and amygdala.
Figure 1.
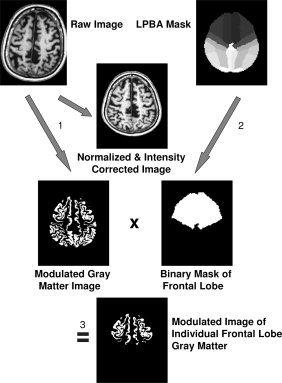
Image processing and volume determination shown exemplarily for the frontal lobe gray matter: (1) Unified segmentation of SPM5 (i.e., normalization, segmentation, and intensity correction) is performed on a T1‐weighted volume data set. (2) A binary frontal lobe mask is derived from the LONI Probabilistic Brain Atlas (LPBA40) by setting all voxels of the maximum likelihood map belonging to the frontal lobe to a value of “one” while all other voxels are set to zero. (3) The modulated gray matter image resulting from unified segmentation is multiplied with the frontal lobe mask. This results in a modulated image of the individual frontal lobe gray matter. Because of modulation of the gray matter image, the effect of normalization (i.e., extension or shrinkage of the investigated structure) is compensated for so that the computed volume represents the volume of the original structure in native space. The analysis is fully automated and requires about 1 h per MRI scan on an AMD Opteron 2.0 GHz PC.
Each dataset was processed independently from other datasets with the same, fully automated protocol, regardless of representing a baseline or follow‐up dataset. Processing of follow‐up scans did not require coregistration to baseline scans.
Statistical Analyses
While in principle, all regions anatomically labeled in the LPBA40 atlas could be measured and subjected to statistical analyses, the focus of this study was on GM volumes of left and right temporal lobes (TL) and frontal lobes (FL). These anatomical regions were chosen a priori as they have been described as primary regions of GM atrophy in FTLD [Davies et al.,2009; Rosen et al.,2002]. In addition, whole brain volumes (GM + WM) as well as total intracranial volumes (TIV = GM + WM + CSF) were determined. Apart from absolute volume results the following ratios were calculated:
“TL symmetry” (i.e., GM volume of left TL divided by that of right TL, in %)
“TL/FL ratio” (i.e., total (= bilateral) TL GM volume divided by total FL GM volume, in %).
For cross‐sectional comparisons the absolute volumes of whole brain as well as frontal and temporal GM were corrected by individual TIV and standardized to the mean TIV of healthy controls (i.e., standardized V structure of interest = absolute V structure of interest/individual TIV × mean TIV of healthy controls). For volume ratios this was not necessary. For longitudinal evaluations, this was not done since the measurement variability of the CSF compartment included in the TIV is known to be high [Huppertz et al.,2010] and probably larger than the subtle volume loss of lobar GM within a 6 months interval. Statistical tests were performed using SPSS 17 (http://www.spss.com). Results of all statistical analyses were regarded as significant if P < 0.05.
Cross‐sectional comparison of volumes at baseline (T0)
TIV‐corrected brain as well as temporal and frontal GM volumes were compared between groups using a multivariate analysis of covariance (MANCOVA) with factor “group” (CON–bvFTLD–PNFA–SemD) including sex and age as covariates. Post‐hoc two‐sample T‐tests were applied to examine which patient groups differed from CON. In addition, lobar GM volumes were analyzed with respect to separation of single patients from controls.
Cross‐sectional group comparison of lobar volume ratios at baseline (T0)
A MANCOVA on the two ratios TL symmetry and TL/FL ratio with factor group (CON–bvFTLD–PNFA–SemD) and covariates age and sex was performed in order to test for a significant group effect. Post‐hoc two‐sample T‐tests were applied to reveal significant, pairwise differences.
Longitudinal changes of lobar volume ratios in patient subgroups (T0–T6)
Repeated measures analyses of covariance (rmANCOVA) were carried out on the variables TL symmetry (at T0 and T6) and TL/FL ratio (at T0 and T6) with sex and age as covariates. In these analyses, we included all six bvFTLD patients and those six SemD patients who had both a T0 and a T6 scan. We did not include PNFA patients in this analysis as only three of them had repeated MRI scans. Of primary interest here were the interaction effects of factors “time” and “patient group.”
Rates of atrophy progression (T0–T30)
To quantify the rate (in %) of atrophy progression per 6 months for whole brain as well as GM volumes of left and right TL and FL separately, volume differences between consecutive time points were calculated for each of the fifteen patients with multiple assessments, resulting in one to five difference measures per patient and region. In detail, 14 volume differences per region were calculated from 20 bvFTLD MRI datasets, 7 from 11 PNFA datasets, 21 from 28 SemD MRI datasets. The resulting atrophy rates (i.e., volume differences in relation to volume at baseline) were averaged for each patient independently, representing the best estimates of individual atrophy progression rates. Afterward, individual rates were averaged across all members of the respective diagnostic group. Between‐group differences in atrophy rates were assessed with pairwise two‐sample T‐tests.
RESULTS
-
1
The MANCOVA testing for baseline group differences, adjusted for age and sex, demonstrated a significant main effect of factor group regarding TIV‐corrected whole brain and lobar GM volumes (whole brain, left TL, right TL, left FL, right FL, P < 0.01). Post‐hoc T‐tests showed that bvFTLD patients had significantly smaller lobar GM and whole brain volumes than CON (P < 0.01). The same was true for PNFA patients (whole brain, left TL, left FL, right FL, P < 0.01; right TL, P < 0.05). SemD patients had significantly less left and right TL GM volumes than CON (P < 0.01). Notably, TL GM volumes at baseline were not statistically different between bvFTLD and SemD patients, whereas FL GM as well as whole brain volumes were significantly smaller in bvFTLD compared with SemD (right FL, P < 0.05) or showed a tendency toward significance (left FL, P = 0.085; whole brain, P = 0.052; Table I). A clear separation of single patients from controls based on whole brain volumes was not possible (Fig. 2, left). However, each SemD and two of four PNFA patients fell below the CON range of left TL GM volumes, and each bvFTLD patient and two of four PNFA patients fell below the CON range of left FL GM volumes (Fig. 2, right).
-
2
Assessment of TL symmetry and TL/FL ratios at baseline revealed a significant main effect of factor group (P < 0.01, regarding both TL/FL ratio and TL symmetry; MANCOVA). Post‐hoc pairwise two‐sample T‐tests showed that TL symmetry was significantly altered only in SemD (P < 0.01, SemD < CON; P < 0.05, SemD < bvFTLD; cf. Fig. 3, left). Five of seven SemD patients showed left‐lateralized temporal atrophy, whereas it was rather bilateral in the remaining two SemD patients, with respect to the normal range in CON (mean ± 1 SD: 99.2% ± 3.3%). In contrast, TL atrophy was left‐lateralized in only 2/6 bvFTLD and 1/4 PNFA patients. Notably, the five SemD patients with left‐lateralized temporal atrophy had lower TL symmetry measures than any CON. TL/FL ratios exhibited a significant increase in bvFTLD compared with CON (P < 0.01), and a decrease in SemD patients (P < 0.01, SemD < CON and SemD < bvFTLD; P < 0.05, SemD < PNFA; cf. Fig. 3, right). Taking the mean TL/FL ratio of the control group (76%) as a classification boundary between bvFTLD and SemD, 6/7 SemD patients had lower, and 5/6 bvFTLD patients had higher ratios. When using the range of CON mean ± 1 SD for differentiation, 6/7 SemD patients had lower, and 4/6 bvFTLD patients had higher ratios. The full range of CON TL/FL ratios, however, overlapped with ratios from both bvFTLD as well as SemD patients—only 2/6 bvFTLD fell above, 4/7 SemD fell below the full CON range at baseline.
-
3
The rmANCOVA concerning TL/FL ratios revealed a significant interaction between factors time (T0 vs. T6) and group (bvFTLD vs. SemD), i.e., the discrepancy between bvFTLD and SemD patients already observed at baseline significantly increased further over time (P < 0.05; see Fig. 3, right). This effect was present in 5/6 bvFTLD and 5/6 SemD patients. TL symmetry was rather stable within groups and did not exhibit a significant group × time interaction.
-
4
At single patient level, a decrease of whole brain volumes was recognizable in each of the 15 patients with at least two MRIs, for each available 6 months interval (cf. Fig. 2, left). Generally, the same was true for lobar GM volumes, except for two patients with bvFTLD and one SemD patient, who showed increasing lobar GM volumes in at least one 6 months interval (cf. Fig. 4). Rates of lobar atrophy progression expressed as percentage loss of GM volume per diagnostic group within 6 months ranged from a group mean (SD) of 4.41% (2.07%) for left TL in SemD to 1.35% (1.44%) for right TL in bvFTLD (Table I). Pairwise comparisons revealed that left TL atrophy rates were significantly larger in SemD than bvFTLD (P < 0.05). Lobar atrophy rates tended to be more marked than atrophy rates of the whole brain, which were lowest in bvFTLD (1.41%; SD = 1.13% per 6 months), and almost identical in PNFA (1.94%; SD = 0.74%) and SemD (2.04%; SD = 1.52%). In each individual SemD patient, atrophy rate was largest in the left or right temporal lobe, whereas in bvFTLD patients it was largest in the left or right frontal lobe. Group averaged progression rates are shown in Table I and Figure 5.
Figure 2.
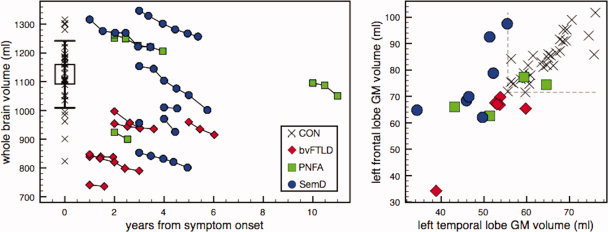
Left subfigure: whole brain volumes (uncorrected for total intracranial volumes) in relation to disease duration. Chains represent individual patients. Black error bar indicates controls' mean ± 1 SD. Right subfigure: TIV‐corrected gray matter volumes of left FL in relation to left TL at baseline per group. Dotted lines represent CON range lower bounds. Black crosses indicate individual control data. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
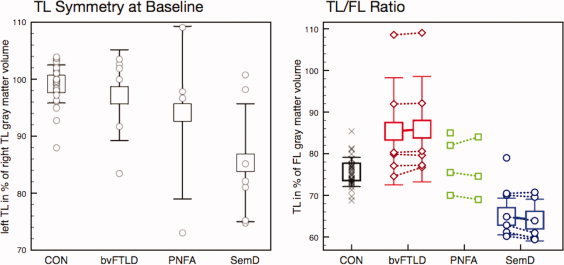
Left subfigure: TL atrophy is left‐lateralized in SemD and differs significantly from healthy elderly controls. Right subfigure: temporal in relation to frontal lobe gray matter volumes are increased in bvFTLD and decreased in SemD, further diverging over 6 months time. Error bars indicate mean ± 1 SD. Symbols represent individual values. Please note that in this right subfigure, patients with missing follow‐up MRI were neither included in the calculation of group means and SD nor in the statistical analysis that revealed significantly different longitudinal TL/FL ratio development between bvFTLD and SemD. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 4.
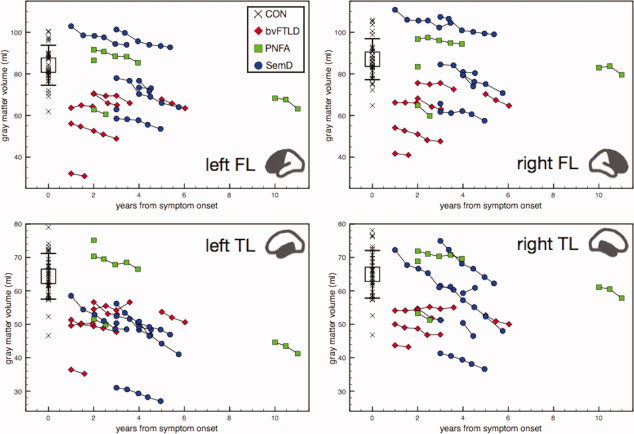
Lobar gray matter volumes (uncorrected for total intracranial volumes) in relation to disease duration. Chains represent individual patients. Black error bar indicates controls' mean ± 1 SD, black crosses indicate individual control data. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 5.
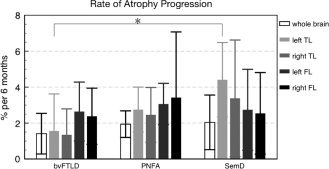
Rates of gray matter atrophy progression over 6 months (in %), averaged per patient group (mean ± 1 SD). Asterisk indicates significant group difference.
DISCUSSION
In the current study, longitudinal MRI data of FTLD patients acquired over up to 30 months with closely spaced sampling intervals (6 months) and analyzed by a novel method of MRI volumetry are presented.
At group level and compared with age‐matched controls, whole brain as well as lobar volumes were significantly reduced already at baseline in bvFTLD and PNFA patients, whereas in SemD only temporal GM volumes were reduced. While absolute TL volumes were similarly reduced in both bvFTLD and SemD—not allowing for differentiation between the two subgroups—TL/FL ratios were increased in bvFTLD and decreased in SemD compared with healthy elderly controls, reflecting the predominant atrophy of frontal versus temporal lobes, respectively, in the patients studied. Moreover, the difference in TL/FL ratios between bvFTLD and SemD increased further in longitudinally studied patients over 6 months.
Significant left versus right TL asymmetry was found in SemD, but not in the other clinical FTLD subgroups. This is in line with results in the literature [Davies et al.,2009; Rohrer et al.,2010; Rosen et al.,2002]. TL asymmetry in SemD did not increase further during the observation period, consistent with previous reports of bilateral atrophy progression of both the primarily affected and the contralateral hemisphere [Brambati et al.,2009; Rohrer et al.,2008]. Previously reported asymmetry of atrophy in PNFA [Gorno‐Tempini et al.,2004; Rohrer et al.,2009] could not be demonstrated in this study, possibly due to the small number of PNFA patients in this study and insufficient statistical power to detect the more subtle asymmetry in PNFA compared with SemD [Rohrer et al.,2010].
A complete separation of patients and healthy controls at baseline was not observed, possibly due to rather short disease durations in this study. Nevertheless, 15 of 17 patients (i.e., each SemD and bvFTLD patient and 2 of 4 PNFA patients in this study) fell below the ranges of left temporal or left frontal GM volumes in the age‐matched controls (cf. Fig. 2, right). This suggests that the combination of volumetric results from different cerebral structures, for example, as input for more sophisticated classification methods like support vector machines [Desikan et al.,2009; Kloppel et al.,2008], might allow for a better differentiation of single patients from controls.
At single patient level, a steady decrease of whole brain volumes was observed in each patient for each 6 months interval. This was also true for lobar GM volumes, with only a few exceptions (i.e., three patients showed apparently increasing lobar GM volumes in at least one 6 months interval), possibly due to higher measurement variances in smaller substructures [Huppertz et al.,2010]. Furthermore, patients' averaged lobar atrophy progression rates in the current study (about 2–8% per year across groups; please note that atrophy rates principally depend on the absolute volumes at first MRI measurement and that 6 months‐atrophy rates are reported in Table I) were 4–16 times larger than published cerebral volume loss in healthy elderly subjects. Fjell et al. [2009] reported frontal and temporal atrophy of only about 0.5% per year. Decline of the same magnitude has been shown for global brain volume [Chan et al.,2001; Fotenos et al.,2005; Gordon et al.,2010; Resnick et al.,2003]. In addition, the notably steep decline of temporal GM volume in SemD of about 8% per year, observed in the current study, remarkably well replicated the figures published by Rohrer et al. [2008].
Although this could not be directly demonstrated in the current study—as longitudinal data from control subjects were not available—these differences in atrophy progression rates could in principle also be used for differentiation of patients and controls. The results of this study suggest that already two MRI measurements with an interval of only 6 months could be sufficient to fully separate FTLD patients from healthy controls. Even in subjects in which absolute lobar volumes were still within normal range (e.g., at onset of clinical symptoms), the calculation of an atrophy progression rate from two subsequent MRI measurements could unequivocally demonstrate the degenerative process. Differences in atrophy rates of various lobar volumes might also be helpful to better understand the regional progression in various clinical subtypes of FTLD. However, in this study, bvFTLD and SemD patients could already be distinguished considerably well by their TL/FL ratios based on a single observation at baseline.
PNFA patients were included in this study, although their number was presumably too small to reveal statistically significant differences compared with healthy controls or the other FTLD subgroups. Therefore, they were excluded from some of the analyses, but nevertheless presented for the interested reader, as volumetric data from PNFA patients in the literature are very rare.
The study presented has a number of limitations. An obvious limitation is the small number of patients enrolled (N = 15 with longitudinal data) and the need of subdividing the patient population even further into subtypes based on clinical presentation. Not surprisingly, therefore the power to detect group difference in this study was limited, not allowing for instance for TL side asymmetries in PNFA patients to be demonstrated. In addition, there is evidence that clinical presentations like bvFTLD may have distinct anatomical substrates [Whitwell et al.,2009]. An analysis based on a priori defined large ROIs like frontal or temporal lobes may therefore result in an under‐appreciation of such regional variability in atrophy rates. On the other hand, the fact that the present small study (N = 17 and 15, respectively) was able to demonstrate statistically significant group difference speaks both to the robustness of the changes in FTLD as well as to the power of the method used.
The present results stress the fact that the rate of change in predefined anatomical regions in individual patients may add certainty in diagnosis in early stages of FTLD and confirms the impression that the annualized rate of regional atrophy is larger in FTLD than in other dementias [Whitwell et al.,2007] thus making FTLD a neurodegenerative disorder with a more readily measurable MRI biomarker and therefore a prime candidate for therapeutical studies.
Given the well‐documented etiological heterogeneity of clinical subtypes of FTLD [e.g., Baker et al.,2006; Neumann et al.,2006], potential therapeutical trials aiming at modifying the underlying biology of the neurodegenerative processes will in all likelihood yield responders and non‐responders. Therefore, biomarkers like MRI read‐outs to support and extend clinical trial findings need to allow for an analysis at the level of individual patients. The method presented here does meet this requirement and demonstrates that changes can be measured in single patients thus allowing to assess individual treatment responses.
CONCLUSION
The volumetric method applied in the current study allows for observer‐independent, objective and time‐efficient volumetric evaluation of group as well as individual patient data. The results are plain volume measures which can be directly compared with results for reference groups. In this study, simple ratios combining temporal and frontal lobe GM volumes showed a high sensitivity for differentiation between subgroups of FTLD patients. This might aid diagnostics in the clinical setting. Compared with more sophisticated classification techniques like support vector machines [Desikan et al.,2009; Kloppel et al.,2008], the current approach not only provides a means of classifying individual patients into diagnostic groups, but allows for quantification of longitudinal volume loss. In this study, the technique was sensitive enough for the detection of volume changes within half a year, which indicates that the volumetric results could be used as potential biomarker in clinical trials and future disease‐modifying studies.
REFERENCES
- Ashburner J, Friston KJ ( 2000): Voxel‐based morphometry—The methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering‐Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M ( 2006): Mutations in progranulin cause tau‐negative frontotemporal dementia linked to chromosome 17. Nature 442: 916–919. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Renda NC, Rankin KP, Rosen HJ, Seeley WW, Ashburner J, Weiner MW, Miller BL, Gorno‐Tempini ML ( 2007): A tensor based morphometry study of longitudinal gray matter contraction in FTD. Neuroimage 35: 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, Miller BL, Ashburner J, Gorno‐Tempini ML ( 2009): Atrophy progression in semantic dementia with asymmetric temporal involvement: A tensor‐based morphometry study. Neurobiol Aging 30: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Fox NC, Jenkins R, Scahill RI, Crum WR, Rossor MN ( 2001): Rates of global and regional cerebral atrophy in AD and frontotemporal dementia. Neurology 57: 1756–1763. [DOI] [PubMed] [Google Scholar]
- Davies RR, Scahill VL, Graham A, Williams GB, Graham KS, Hodges JR ( 2009): Development of an MRI rating scale for multiple brain regions: Comparison with volumetrics and with voxel‐based morphometry. Neuroradiology 51: 491–503. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, Schmansky NJ, Greve DN, Salat DH, Buckner RL, Fischl B ( 2009): Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain 132: 2048–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno‐Tempini ML, Rankin K, Miller BL, Weiner MW ( 2007): Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain 130: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema‐Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM ( 2009): One‐year brain atrophy evident in healthy aging. J Neurosci 29: 15223–15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL ( 2005): Normative estimates of cross‐sectional and longitudinal brain volume decline in aging and AD. Neurology 64: 1032–1039. [DOI] [PubMed] [Google Scholar]
- Fukui T, Kertesz A ( 2000): Volumetric study of lobar atrophy in Pick complex and Alzheimer's disease. J Neurol Sci 174: 111–121. [DOI] [PubMed] [Google Scholar]
- Gordon E, Rohrer JD, Kim LG, Omar R, Rossor MN, Fox NC, Warren JD ( 2010): Measuring disease progression in frontotemporal lobar degeneration: A clinical and MRI study. Neurology 74: 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno‐Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL ( 2004): Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 55: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Mitchell J, Dawson K, Spillantini MG, Xuereb JH, McMonagle P, Nestor PJ, Patterson K ( 2010): Semantic dementia: Demography, familial factors and survival in a consecutive series of 100 cases. Brain 133: 300–306. [DOI] [PubMed] [Google Scholar]
- Huppertz HJ, Kroll‐Seger J, Kloppel S, Ganz RE, Kassubek J ( 2010): Intra‐ and interscanner variability of automated voxel‐based volumetry based on a 3D probabilistic atlas of human cerebral structures. Neuroimage 49: 2216–2224. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, Chute DJ, Roberson ED, Pace‐Savitsky C, Neumann M, Chow TW, Rosen HJ, Forstl H, Kurz A, Miller BL ( 2005): Frontotemporal lobar degeneration: Demographic characteristics of 353 patients. Arch Neurol 62: 925–930. [DOI] [PubMed] [Google Scholar]
- Kloppel S, Stonnington CM, Chu C, Draganski B, Scahill RI, Rohrer JD, Fox NC, Jack CRJ, Ashburner J, Frackowiak RS ( 2008): Automatic classification of MR scans in Alzheimer's disease. Brain 131: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Jack CRJ, Kramer JH, Boeve BF, Caselli RJ, Graff‐Radford NR, Mendez MF, Miller BL, Mercaldo ND ( 2009): Brain and ventricular volumetric changes in frontotemporal lobar degeneration over 1 year. Neurology 72: 1843–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger CE, Dean DL, Rosen HJ, Halabi C, Weiner M, Miller BL, Kramer JH ( 2010): Longitudinal rates of lobar atrophy in frontotemporal dementia, semantic dementia, and Alzheimer's disease. Alzheimer Dis Assoc Disord 24: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM ( 2001): Primary progressive aphasia. Ann Neurol 49: 425–432. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF ( 1998): Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 51: 1546–1554. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM ( 2006): Ubiquitinated TDP‐43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314: 130–133. [DOI] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR ( 2002): The prevalence of frontotemporal dementia. Neurology 58: 1615–1621. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C ( 2003): Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci 23: 3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, McNaught E, Foster J, Clegg SL, Barnes J, Omar R, Warrington EK, Rossor MN, Warren JD, Fox NC ( 2008): Tracking progression in frontotemporal lobar degeneration: Serial MRI in semantic dementia. Neurology 71: 1445–1451. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, Ourselin S, Fox NC ( 2009): Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology 72: 1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, Mead S, Beck J, Mummery C, Ourselin S, Warrington EK, Rossor MN, Warren JD ( 2010): Progressive logopenic/phonological aphasia: Erosion of the language network. Neuroimage 49: 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno‐Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL ( 2002): Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 58: 198–208. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Stein T, Maslowski N, Neumann J ( 2009): Neural correlates of Alzheimer's disease and mild cognitive impairment: A systematic and quantitative meta‐analysis involving 1351 patients. Neuroimage 47: 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pol LA, Hensel A, van der Flier WM, Visser PJ, Pijnenburg YA, Barkhof F, Gertz HJ, Scheltens P ( 2006): Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer's disease. J Neurol Neurosurg Psychiatry 77: 439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Anderson VM, Scahill RI, Rossor MN, Fox NC ( 2004): Longitudinal patterns of regional change on volumetric MRI in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 17: 307–310. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CRJ, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Ferman TJ, Dickson DW, Josephs KA ( 2007): Rates of cerebral atrophy differ in different degenerative pathologies. Brain 130: 1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, Senjem ML, Shiung MM, Boeve BF, Knopman DS, Parisi JE, Dickson DW, Petersen RC, Jack CRJ, Josephs KA ( 2009): Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: A cluster analysis study. Brain 132: 2932–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]


