Abstract
Memory deficits are highly prevalent in multiple sclerosis (MS). As the hippocampus is crucial to memory processing, a functional magnetic resonance imaging (fMRI) task was used to investigate changes in hippocampal function in MS patients with and without cognitive decline. Fifty patients with MS, (34 cognitively preserved (CP) and 16 cognitively impaired (CI)) and 30 healthy controls completed an episodic memory fMRI task (encoding and retrieval) that was used to specifically activate the hippocampus. During encoding of correctly remembered items, increased brain activation was seen in the parahippocampal areas bilaterally and in the left anterior cingulate gyrus in the CP patients compared to the controls (unclustered, Z ≥ 3.1, P ≤ 0.001). No brain areas showed less activation. In CI patients the right (para)hippocampal areas and the prefrontal cortex showed less brain activation compared to controls (cluster‐corrected, P < 0.05). The posterior cingulate gyrus and the left precuneus showed increased activation in CI patients when compared to controls (unclustered Z ≥ 3.1, P ≤ 0.001). No significant differences were found on structural MRI measures between the CP and CI patients. These results suggest the presence of functional adaptation in the memory network before cognitive decline becomes evident in MS, as displayed by the increased brain activation in the hippocampal‐cingulate memory system in CP patients. Interestingly, CI patients showed less activation in the hippocampal network during correct encoding. These findings are important for future cognitive therapeutic studies, since cognitive intervention might be most effective before cognitive impairment is present and when adaptive changes of the brain are most prominent. Hum Brain Mapp 33:2268–2280, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: multiple sclerosis, cognition, functional magnetic resonance imaging, brain adaptation, hippocampus
INTRODUCTION
Multiple sclerosis (MS) is a chronic, demyelinating, and neurodegenerative disease of the central nervous system affecting mostly young adults. Besides the well known neurological symptoms, such as deficits in motor and sensory function, cognitive deficits are frequently reported as well. Thirty to sixty‐five percent of all patients with MS suffer from cognitive impairment [Peyser et al.,1980; Rao et al.,1991a], which is independent of physical disability and can occur at all stages of the disease [Pelosi et al.,1997; Piras et al.,2003]. The presence of cognitive impairment has a great functional influence on daily and social life and leads to a significant decrease in quality of life [Amato et al.,2010; Rao et al.,1991b]. Not all cognitive domains are commonly impaired in MS, the most frequent deficits are found in processing speed, visual memory, and verbal memory (for reviews see [Benedict et al.,2008; Chiaravalloti and DeLuca,2008]).
So far, the pathobiological underpinnings of cognitive impairment in MS are unknown. However, the hippocampus plays a crucial role in both visual and verbal memory and is therefore an important target to study memory impairment in MS. Postmortem studies showed that the hippocampus is vulnerable to MS pathology resulting in extensive demyelination and atrophy [Geurts et al.,2007; Papadopoulos et al.,2009]. Furthermore, in vivo magnetic resonance imaging (MRI) studies demonstrated the presence of focal hippocampal lesions on double inversion recovery (DIR) images [Roosendaal et al.,2008] as well as hippocampal atrophy [Anderson et al.,2010; Sicotte et al.,2008]. Atrophy of the hippocampus was associated with a poorer verbal memory performance [Sicotte et al.,2008]. In a recent functional MRI (fMRI) study, it was found that patients with MS with intact spatial memory already showed decreased functional connectivity within the hippocampal network (connections between the hippocampus, anterior cingulate cortex, and prefrontal cortex) as measured with resting state fMRI. This decreased functional connectivity was more pronounced in patients with MS who had hippocampal atrophy. However, functional connectivity changes were also found in patients with MS who did not have hippocampal atrophy [Roosendaal et al.,2010a].
Task‐specific fMRI studies on working memory and attention showed that patients with MS undergo functional adaptation which can consist of either functional enhancement (i.e., increased activation in a brain area which is also activated in healthy controls; [Audoin et al.,2008; Sweet et al.,2006]) or the recruitment of alternative brain areas [Forn et al.,2007; Staffen et al.,2002]. In one study, recruitment as well as enhancement was reported [Mainero et al.,2004].
Although visuospatial and verbal memory are among the most frequently affected cognitive domains in MS [Chiaravalloti and DeLuca,2008], visuospatial memory has never been investigated with fMRI before in MS and only two fMRI studies were performed investigating verbal memory. These verbal memory fMRI studies showed either no differences in brain activation patterns for healthy controls and patients with MS during the encoding phase [Morgen et al.,2007] or both increased and decreased brain activation in patients with MS [Bobholz et al.,2006]. In the latter study, the right middle frontal gyrus and the left lingual gyrus showed increased activation while decreased activation was seen in the right anterior cingulate gyrus. All these activations correlated with increased lesion volume. Importantly, none of the studies focused on the hippocampus specifically. The hippocampus is one of the main brain areas involved in episodic memory function and is known to be structurally influenced by MS pathology. Thus, it is important to know how this structure functions in MS. In the current study, the function of the hippocampus was tested with an episodic memory fMRI paradigm that has been proven to specifically activate the hippocampus [Van Der Werf et al.,2009]. The purpose of our study was to find out whether there is altered activation within the hippocampal memory system in response to MS pathology, and whether different brain regions take over its function. To address possible effects of functional brain adaptation, we examined two groups of patients with MS; cognitively preserved (CP) and cognitively impaired (CI). We hypothesized that hippocampal function would be decreased in both MS groups compared to healthy controls. The CP patients will show subtle reductions in hippocampal functioning without clinical effects. We expected to find more pronounced functional hippocampal changes in the CI patients which might be related to the presence of overt cognitive deficits.
METHODS
Participants
The study protocol was approved by the institutional ethics review board and all subjects gave written informed consent prior to participation. Patients were recruited from several channels; a clinical MS database from our MS center, an advertisement in a magazine from the Dutch MS research foundation, and via the treating neurologists. All patients were diagnosed with clinically definite MS [Polman et al.,2005]. Disease severity of all patients was measured on the day of scanning with a questionnaire based on the Expanded Disability Status Scale (EDSS) [Lechner‐Scott et al.,2003]. All patients had sufficient visual acuity to perform the fMRI task. Age‐ and sex‐matched healthy controls were included in this study.
Exclusion criteria for all subjects were the presence or history of psychiatric or neurological disease (for patients: other than MS), claustrophobia, and contra‐indications for undergoing MRI investigation. Patients were not allowed to enroll if they received treatment with corticosteroids in the 6 weeks prior to the investigation.
Neuropsychological Examination
All subjects underwent an extensive neuropsychological test battery specifically designed to assess memory function. Verbal memory and learning was assessed with the verbal learning and memory task (Verbale Leer‐ en Geheugen Taak, VLGT) [Mulder et al.,1996]. The VLGT is the Dutch equivalent of the California Verbal Learning Test [Delis et al.,1987]. In the learning phase, 16 items were verbally presented to the subjects in five consecutive trials followed by immediate recall. The learning phase was followed by an interference trial during which 16 new items were verbally presented once, after which the subjects had to repeat the first list, both during free recall and cued recall (four categories: “clothes,” “machinery,” “fruits,” and “herbs and spices”). After approximately 15 min long‐term delayed recall was assessed, again both free and cued recall, as well as recognition.
The Letter Digit Substitution Test (LDST) [Jolles et al.,1995] was included to assess processing speed of visual information and was verbally administered for 90 s.
In the test battery, spatial memory was assessed with the Location Learning Test (LLT) [Bucks and Willison,1997]. The learning phase consists of five consecutive trials and generates a total displacement score (LLT total score). A score of zero indicates a perfect performance, the more errors one made the higher the score. Approximately 15 min after the learning phase, a delayed recall trial was assessed which generated a measure for “rapid forgetting” (LLT delay score).
Digit span (both forward and backward) is a subtest of the Wechsler Adult Intelligence Scale [Wechsler,1997] and was used to test working memory ability (and to a lesser extent concentration, attention, and mental control).
A semantic word fluency test was used to investigate word knowledge, access to semantic memory, and long‐term verbal memory [Ruff et al.,1997]. Subjects had 60 s to generate as many words as possible that belong to the category “Animals,” “Professions,” and “M‐words” (four letter words beginning with the letter M) [Lezak et al.,2004].
Scores on all test parameters were converted to Z scores by comparison with the mean and standard deviation of the healthy control group. Patients were defined as being CI when their score was at least 2 SD below that of the healthy controls on a minimum of 2 out of 5 tests, corresponding to a probability of 5% to fall in the normal population for each test. Otherwise, patients were considered to be CP. It should be noted that this classification is made based on the abovementioned neuropsychological test battery which predominantly focused on memory function and relatively lacks in‐depth information on cognitive domains like psychomotor speed and executive functioning.
Symptoms of depression, anxiety, and fatigue are known nuisance factors when assessing cognition. The Hospital Anxiety and Depression Scale (HADS‐A and HADS‐D) was used to investigate the presence of these symptoms [Zigmond and Snaith,1983]. Fatigue was assessed by the Checklist of Individual Strength (CIS‐20) questionnaire [Vercoulen et al.,1994]. Pre‐morbid intelligence was measured using the Dutch version of the New Adult Reading Test (DART) [Nelson and O'Connell,1978; Schmand et al.,1991]. Educational level was assessed with a scoring system that comprised a 7‐point scale, ranging from having not finished primary education (level 1) to university educated (level 7) [Verhage,1964]. The Edinburgh Handedness Scale was used to evaluate right or left handedness [Oldfield,1971].
Structural and Functional MRI Acquisitions
MR imaging was performed on a 1.5T whole‐body scanner (Siemens Sonata, Erlangen, Germany) using an eight‐channel phased‐array head coil. 3DT1‐weighted magnetization prepared rapid acquisition gradient‐echo (MPRAGE) images (TR 2700 ms, TE 5 ms, TI 950 ms; 176 sagittal slices with 1.3 mm thickness; 248 × 330 mm2 field‐of‐view [FOV] and 1.3 × 1.3 mm2 in plane resolution; acquisition time 4.9 min) were obtained using parallel imaging with an acceleration factor of 2.
For white matter (WM) lesion detection a turbo spin‐echo proton density (PD) and T2‐weighted images (TR 3130 ms, TE 24/85 ms, 46 axial slices with 3 mm thickness; 192 × 256 mm2 FOV and 1.0 × 1.0 mm2 in plane resolution) were obtained, as well as spin‐echo T1‐weighted images (TR 485 ms, TE 12 ms, 46 axial slices with 3 mm thickness; 192 × 256 mm2 FOV and 1.0 × 1.0 mm2 in plane resolution).
3D‐DIR images were acquired to detect grey matter pathology (TR 2350 ms, TE 355 ms, TI 350 ms, 120 sagittal slices, 192 × 256 mm2 FOV and 1.2 × 1.2 mm2 in plane resolution; acquisition time 10 min).
A localizer of 7 slices was made to accurately identify the hippocampus for the fMRI sequence. During the fMRI task 208 volumes of echo planar images (EPI) were acquired (TR 2220 ms, TE 60 ms; 28 axial slices with 3 mm thickness, 3.3 × 3.3 mm2 in plane resolution and 211 × 211 mm2 FOV; acquisition time 7.7 min).
fMRI Paradigm
An episodic memory encoding paradigm [Van Der Werf et al.,2009] was used for fMRI measurements. This particular fMRI paradigm was chosen because of the reliable and robust activation of the hippocampus in healthy controls [Van Der Werf et al.,2009]. During the encoding phase, 50 different novel landscape images were presented to the subjects. Each picture was presented for 5 s in which the participants were asked to decide whether the images were “tropical” or “non‐tropical” by pressing a button with either their left or their right index finger (Photon Control, Burnaby, BC, Canada). This ensures the encoding by requiring the subjects to attend to details in the images, which has been shown to enhance hippocampal activation and subsequent recall [Daselaar et al.,2003]. The novel landscape images appeared in a pre‐randomized order and were intermixed with 20 control images (a previously familiarized landscape image with a centrally positioned arrow pointing left or right, indicating which button to press). Thirty minutes following encoding, the retrieval phase was initiated. Here, a total of 100 landscape images were shown, 50 of which were novel and another 50 of which were old (i.e., already presented during the encoding phase), presented in a random order, intermixed with the same (arrow) control images. Participants had to indicate whether they had seen the pictures before, again by pressing the left or the right button. E‐prime 1.1 software with service pack 3 (Psychology Software Tools, Pittsburgh) was used to present the images and to record all responses.
Functional MRI Image Analysis
All imaging processing steps and statistical fMRI analyses were performed using FSL 4.0 (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl). For each subject, all non‐brain tissue was removed from the images (BET) and a motion correction (MCFLIRT) was applied. A highpass filter cutoff of 50 s was used, as well as spatial smoothing with full‐width‐at‐half‐maximum set at 6 mm. Thereafter, the functional images were aligned to the subject's high resolution T1‐weighted image using an affine registration (FLIRT) using six degrees of freedom and subsequently to the MNI152 standard brain using non‐linear registration (FNIRT, warp resolution: 10 mm). The functional data were modeled using FEAT with a double‐gamma hemodynamic response function, contrasting correctly remembered items with the control images for every single subject in an event‐related design using a univariate general linear model. For all the subjects, the correctly remembered items (as measured during the retrieval) were selected from the encoding phase (5 s per item) and modeled in the analyses. This was based on the assumption that when someone remembered a landscape picture correctly, the encoding had been successful. The brain activation that is found therefore reflects correct performance.
Group‐analysis was carried out, contrasting the three different subject groups to each other with age and sex added to the general linear model as covariates.
Firstly, the results that survived cluster correction were inspected. Cluster correction allows for a correction for multiple comparisons by taking into account the activation of associated voxels into clusters of voxels. Differences between groups within clusters were considered significant at P < 0.05. Secondly, subtle changes between groups (i.e., CP patients vs. healthy controls) might stay undetected this way, especially when looking for small differences in activation patterns. Small numbers of activated voxels might not survive cluster correction and possibly informative and robust results might as such disappear. To explore these subtle differences, results were also explored without cluster correction using a conservative P‐value (Z ≥ 3.1, P ≤ 0.001) as an alternative method which still assures significant activation. Brain areas that showed enhanced activation during the task compared to the healthy controls, as well as recruitment of new brain areas were considered to be functional adaptation.
To specifically investigate activation differences within the hippocampus, a region‐of‐interest analysis was performed. For each subject, hippocampal masks (see structural MRI) were co‐registered to the individual's functional MR images. The co‐registered hippocampal masks were kept conservative by applying an intensity threshold of >0.6 to ensure that only hippocampal tissue was included in the mask. The individual masks were overlaid onto the subject specific Z‐statistic map of activation during the encoding of correctly remembered items as obtained from the single‐subject analysis. The averaged Z‐values in the left and right hippocampus during fMRI were extracted for all subjects separately.
Structural MRI Image Analysis
For each subject, the whole brain volume (as well as grey and white matter volume separately) was measured using the MPRAGE images and SIENAX [Smith et al.,2002]. Hippocampal masks were drawn manually according to a standardized operating procedure based on previous reports using an in‐house developed software program [Jack,1994]. All volumetric measures were corrected for head size.
WM lesions were marked and manually outlined on the PD‐ and T1‐weighted images using a local‐threshold technique. Cortical lesions and hippocampal lesions were scored and counted on the 3D‐DIR images according to recently developed consensus guidelines [Geurts et al.,2011]. Cortical lesions were scored on axial slices, the hippocampal lesions on coronal slices with the images reformatted perpendicular to the rostral‐caudal axis of the hippocampus.
Statistical Analysis
Statistical analyses on the demographical, clinical, and volumetric variables were performed using Statistical Package for the Social Sciences version 15.0 (SPSS, Chicago, IL). Comparisons were made between the three different groups: CP patients, CI patients, and healthy controls. When the variables were normally distributed, a multivariate general linear model was used with age and sex included as covariates. If a variable displayed a significant effect of group, a post‐hoc analysis using Bonferroni confidence interval adjustment was performed. When variables were not normally distributed the Mann–Whitney (two groups) or the Kruskal–Wallis test (three groups) was used. P‐values of <0.05 were considered statistically significant.
RESULTS
Subject Descriptives
A total of 50 patients were included in the study with ages between 24 and 61 years (mean age 47.4 ± 8.4), of whom 35 were females. Thirty‐six patients had a relapsing remitting disease type with a mean disease duration of 10.8 years (±7.1). Fourteen patients had a secondary progressive disease type with a mean disease duration of 13.9 years (±5.7). Thirty age‐ and sex‐matched healthy controls (mean age 44.5 ± 8.8; 19 females) were included in the study.
According to the definition for cognitive impairment (2SD below the mean score of the healthy controls on at least 2 out of 5 tests), 34 patients with MS were defined as CP and 16 patients with MS as CI.
Table I summarizes the demographical and clinical data of the subjects included in this study. Patients and controls did not differ significantly with regard to age, sex, handedness, premorbid IQ, and educational level. The MS specific characteristics; mean disease duration, disease type, and mean EDSS score were not significantly different between CP and CI patients. As expected, both patient groups differed significantly from the healthy controls regarding fatigue, anxiety, and depression measures (see Table I). CP patients did, however, not differ from the CI patients on any of these measures.
Table I.
Demographic and clinical measures of healthy controls (HC), cognitively preserved (CP), and cognitively impaired (CI) MS patients
| HC (n = 30) | CP (n = 34) | CI (n = 16) | P‐value | |
|---|---|---|---|---|
| Age (in years) | 44.5 (8.8) | 46.0 (9.2) | 50.3 (5.6) | 0.086 |
| Sex (female/male) | 19/11 | 27/7 | 8/8 | 0.051 |
| Handedness (R/L/M) | 26/3/1 | 32/2/0 | 13/3/0 | 0.449 |
| Premorbid IQ | 103.8 (10.8) | 105.1 (12.3) | 96.7 (12.4) | 0.066 |
| Educational level | 5.7 (0.9) | 5.8 (0.7) | 5.2 (1.0) | 0.401 |
| Disease type (RRMS/ SPMS) | — | 27/7 | 9/7 | 0.138 |
| Disease duration (in years) | — | 11.32 (6.6) | 12.50 (7.3) | 0.573 |
| EDSS | — | 4.1 (1.3) | 4.3 (1.5) | 0.404 |
| HADS‐A | 3.0 (2.0–6.0) | 5.0 (4.0–8.0) | 6.5 (4.0–9.5) | <0.001a |
| HADS‐D | 1.0 (0–2.3) | 4.0 (2.5–6.3) | 4.0 (3.0–5.8) | <0.01a |
| CIS‐20 | 25.0 (16.8–46.0) | 72.5 (49.8–90.3) | 80.5 (48.0–88.8) | <0.001a |
HADS: Hospital Anxiety and Depression Scale; A: anxiety; D: depression; CIS‐20: Checklist individual strength, fatigue questionnaire.
Data are means and (standard deviation) for normally distributed variables, variables EDSS, HADS‐A, HADS‐D, and CIS‐20 were not normally distributed and therefore median (interquartile range) are provided.
Significant differences were found between both patient groups and the healthy controls, the CP and CI patients did not differ significantly from each other.
Cognitive Impairment
The neuropsychological test scores for each cognitive task are presented in Table II for the healthy controls, the CP patients, and the CI patients. Of the 34 CP patients, 22 patients displayed no impairment at all. Twelve patients were impaired on one test. On the location learning task (spatial memory) most impaired scores were found (four patients), followed by digit span backwards (three patients), and the letter digit substitution task (processing speed; three patients). Two patients showed impairment on semantic word fluency. Of the 16 CI patients, seven patients were impaired on two tests, five patients on three tests, and four patients on four tests of the total test battery. The location learning task was most often impaired (12 patients), followed by the letter digit substitution task (11 patients) and the verbal learning and memory task (10 patients). Nine patients were impaired on the digit span backwards, while almost none of the patients were impaired on digit span forwards (one patient) and semantic word fluency (two patients).
Table II.
Neuropsychological test data of healthy controls (HC), cognitively preserved (CP), and cognitively impaired (CI) MS patients
| Measure | HC (n = 30) | CP (n = 34) | CI (n = 16) |
|---|---|---|---|
| VLGT | |||
| Total no. of correct items | 62.00 (52.00–69.00) | 59.00 (53.25–63.25) | 38.50 (32.55–45.00) |
| LDST | |||
| No. of substitutionsa | 63.50 (9.57) | 54.09 (9.21) | 43.19 (10.86) |
| LLT | |||
| Total no. displacements | 7.50 (2.00–15.00) | 11.00 (7.75–19.50) | 41.50 (26.25–56.25) |
| Digit span | |||
| Forward | 10.00 (8.00–12.00) | 9.00 (8.00–11.00) | 7.00 (7.00–8.00) |
| Backward | 7.50 (7.00–8.25) | 7.00 (5.75–8.25) | 4.00 (3.25–5.00) |
| WLG‐Animals | 24.00 (20.75–29.00) | 24.00 (19.75–27.25) | 19.50 (15.25–22.50) |
| WLG‐Professionsa | 18.30 (5.41) | 18.03 (5.10) | 13.88 (4.80) |
| WLG‐M‐words | 10.00 (7.00–13.25) | 9.00 (7.75–14.00) | 6.50 (3.50–10.00) |
VLGT = verbal learning and memory task, LDST = Letter Digit Substitution Task, LLT = Location Learning Task, WLG = Word List Generation.
Median and interquartile range are displayed for not normally distributed data, except for a where because of normal distribution mean and standard deviation are provided.
Early Functional Changes During Memory Encoding
The number of correctly remembered items during the retrieval phase was marginally lower in the CP patients (median 38, inter quartile range (IQR) 30–40) and substantially lower in the CI patients (median 32, IQR 19–35) compared to the healthy controls (median 40, IQR 35–42, P = 0.05 and P < 0.01, respectively). Furthermore, the number of correctly remembered items was significantly lower in the CI patients compared to the CP patients (P = 0.01).
During the encoding of correctly remembered items (as measured during the retrieval) CP patients showed significantly increased activation within the left anterior cingulate gyrus, the left hippocampus, and the left and right parahippocampal gyrus compared to healthy controls. Activation was also seen in the right cerebellum and occipital cortex bilaterally (unclustered, Z ≥ 3.1, P ≤ 0.001; see Fig. 1 and Table III). There were no brain areas in the CP patients with less activation compared to the healthy controls.
Figure 1.
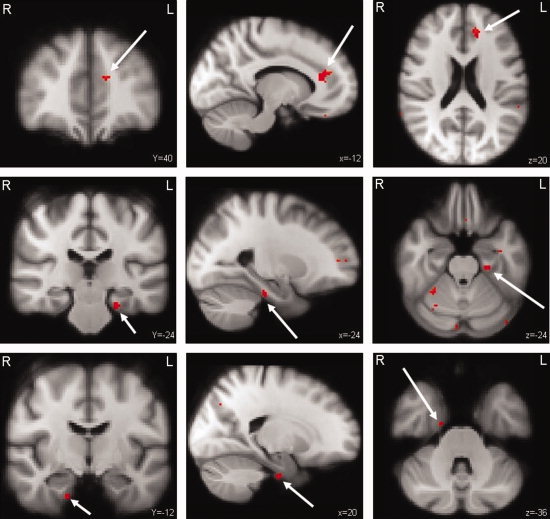
CP patients versus healthy controls. Differences in brain activation between CP patients and healthy controls during encoding of successfully recalled items. The areas in red were increased active in the CP patients. Upper panel: left anterior cingulate gyrus (arrow); Middle panel: left hippocampus (arrow) and parahippocampal area as well as cerebellar activation. Lower panel: right parahippocampal area (arrow) (Unclustered, Z ≥ 3.1, P ≤ 0.001).
Table III.
Clusters of significant activation during memory encoding, differences between the subject groups
| Clustera | Z max | x | y | z | Area |
|---|---|---|---|---|---|
| CP > HC | |||||
| 1 | 4.38 | −12 | 40 | 20 | Anterior (para)cingulate gyrus (left) |
| 2 | 4.22 | 18 | −84 | 10 | Occipital cortex (right) |
| 3 | 4.19 | 32 | −48 | −24 | Cerebellum (right) |
| 4 | 4.11 | 28 | −74 | 40 | Occipital/parietal cortex (right) |
| 5 | 3.89 | −28 | −72 | 40 | Occipital/parietal cortex (left) |
| 6 | 3.67 | 20 | −12 | −36 | Parahippocampal gyrus (right) |
| 7 | 3.66 | −24 | −26 | −24 | Parahippocampal gyrus and hippocampus (left) |
| CI > HC | |||||
| 1 | 4.22 | 8 | −54 | 36 | Precuneus (right) |
| 2 | 3.44 | 8 | −40 | 26 | Posterior cingulate gyrus (right) |
| CI < HC | |||||
| 1 | 6.07 | 12 | −84 | −16 | Occipital cortex/ ventral stream (right) |
| 2 | 5.47 | −10 | −100 | 20 | Occipital cortex/ ventral stream (left) |
| 3 | 4.65 | 34 | −36 | −20 | Hippocampal formation (right) |
| 4 | 4.15 | −18 | −68 | 12 | Parietal occipital cortex (left) |
| 5 | 4.03 | 2 | 34 | −24 | Cingulate cortex and medial frontal cortex (left) |
| CI < CP | |||||
| 1 | 6.89 | −14 | −102 | 16 | Occipital pole (bilaterally) |
| 2 | 4.59 | −24 | 30 | −24 | Frontal medial cortex (left) |
| 3 | 4.03 | −50 | 48 | 2 | Frontal pole (left) |
| 4 | 3.81 | 12 | −14 | 10 | Thalamus (right), Nucleus accumbens (right) |
Z max: maximal z‐value of the cluster/activated area; x, y, z: MNI‐space coordinates of the Z max.
For the contrasts CP>HC and CI>HC the results from the unclustered analyses are shown (Z ≥ 3.1, P < 0.001) and cluster numbers resemble brain areas with significantly increased activation, in the other contrasts (CI<HC and CI<CP) the results from the cluster corrected analyses are shown.
The CI patients showed significant differences in brain activation patterns compared to the healthy controls (see Fig. 2 and Table III). Increased brain activation was seen in the right precuneus as well as the posterior part of the cingulate gyrus (unclustered, Z ≥ 3.1, P ≤ 0.001). In the right hippocampus and parahippocampal areas and in the ventral visual stream, significantly less activation was seen. Also, in the left anterior paracingulate gyrus extending into frontal brain areas significantly less brain activation was seen in the CI patients compared to healthy controls (cluster‐corrected, P < 0.05).
Figure 2.
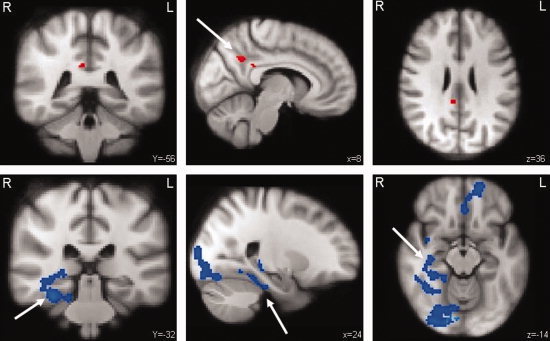
CI patients versus healthy controls. Differences in brain activation between CI patients and healthy controls during encoding of successfully recalled items. The areas in red display increased activation while the areas in blue display less activation in the CI patients. Upper row: activation in the precuneus (arrow) and the posterior cingulate gyrus (unclustered, Z ≥ 3.1, P ≤ 0.001). Bottom row: extensive brain areas with less activation, the right hippocampus (arrow) and parahippocampal areas, right ventral visual stream and the left paracingulate gyrus extending into left frontal brain areas (cluster‐corrected, Z ≥ 2.3, P ≤ 0.05).
Patient groups were also compared to each other. Areas with significantly increased activation in CI patients compared to the CP patients were not found. Increased brain activation, however, could be seen in the precuneus and posterior cingulate gyrus (also seen in CI patients compared to healthy controls) at Z ≥ 2.3, P > 0.05, which indicates a trend. The CI patients did show significantly less brain activation throughout the brain (see Fig. 3 and Table III). Less brain activation was seen in the right and left thalamus, the right nucleus accumbens, the hippocampus and parahippocampal gyri bilaterally, the posterior part of the cingulate gyrus, left frontal brain areas, left brain stem, and the cerebellum. Less activation was found in the ventral and dorsal stream of visual memory processing and in the temporal gyrus at the right side (cluster‐corrected, P < 0.05; see Fig. 3 and Table III).
Figure 3.
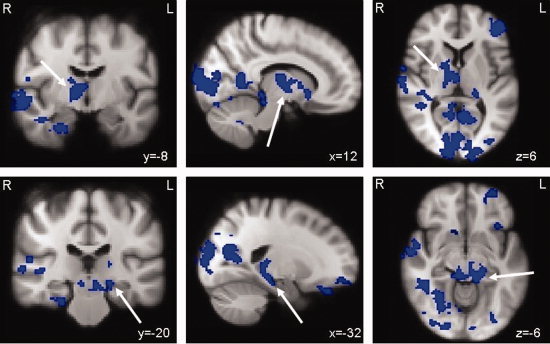
CI patients versus CP patients. Differences in brain activation between CI and CP patients during encoding of successfully recalled items. The areas in blue display less activation in the CI patients. Upper panel: brain regions with reduced activation were the left thalamus and right thalamus (arrow) as well as the ventral visual system, right superior and middle temporal gyrus, frontal areas, cerebellum, and the right parahippocampal gyrus. Lower panel: less activation was found as well in the left hippocampus (arrow), right parahippocampal gyrus, the right nucleus accumbens, and areas within the brain stem (cluster‐corrected, Z ≥ 2.3, P ≤ 0.05)
The region‐of‐interest analysis showed the average activation in the left and right hippocampus for each subject and was corrected for hippocampal volume. There was a significant effect of group for the activation of the right hippocampus (F = 5.9, P = 0.004; see Fig. 4). Post‐hoc Bonferroni‐corrected analyses revealed significantly lower activation in the CI patients compared to the CP patients (P = 0.003) as well as compared to the healthy controls (P = 0.03). No significant differences were found between the CP patients and the healthy controls. There was no significant effect of group for the activation of the left hippocampus (F = 2.9, P = 0.06; see Fig. 4). Important to note is that there is a reduced activation measured in the hippocampus in the CI patients compared to the healthy controls, although on average all subjects had positive activation values.
Figure 4.
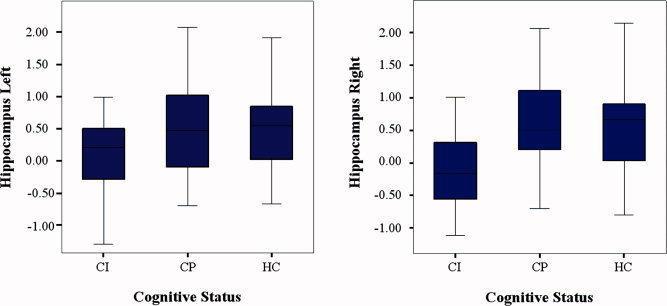
Region‐of‐interest analysis of the average activation in the hippocampus. Left Panel: the average activation of the left hippocampus during encoding of successfully recalled items. No significant differences were found between the subject groups. Right panel: the average activation of the right hippocampus during encoding of successfully recalled items. Significant differences were seen between the CP and CI patients, as well as between the healthy controls and the CI patients (corrected for hippocampal volume, P = 0.003 and P = 0.03, respectively). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
No Significant Differences Between CP and CI Patients With Regard to Brain Structure
In Table IV, the results from the structural MRI analyses are provided. There was a significant effect of group for normalized brain volume (NBV; F = 8.7, P < 0.001), normalized grey matter volume (NGMV; F = 4.7, P = 0.01), and normalized white matter volume (NWMV; F = 8.3, P = 0.001). Post‐hoc Bonferroni‐corrected analyses revealed significant reductions in NBV for both CP and CI patients compared to healthy controls (P = 0.003 and P = 0.002, respectively). NBV was not significantly different between CP and CI patients. The same pattern was seen for NGMV (CP patients vs HC: P = 0.04; CI vs HC: P = 0.03) and for NWMV (CP vs HC: P = 0.004; CI vs HC: P = 0.002). Both CP and CI patients did not differ significantly from each other for NGMV and NWMV. There was no significant effect of group for hippocampus volume (left hippocampus volume F = 2.6, P = 0.08; right hippocampus volume F = 2.2, P = 0.11). However, when patients with MS as a whole group were compared to healthy controls, the analysis showed a significant reduction in the volume of the left hippocampus (F = 4.7, P = 0.033) and a trend towards a reduction in the volume of the right hippocampus (F = 3.8, P = 0.056). The Mann–Whitney test displayed no significant differences between the patient groups on T2 hyperintense and T1 hypointense lesion volume (P = 0.23 and P = 0.34, respectively) neither on the number of cortical and hippocampal lesions as seen on the DIR (P = 0.81 and P = 0.86, respectively).
Table IV.
Structural MRI measures of healthy controls (HC), cognitively preserved (CP), and cognitively impaired (CI) MS patients
| HC (n = 30) | CP (n = 34) | CI (n = 16) | |
|---|---|---|---|
| NBV (L)a | 1.47 (0.06) | 1.40 (0.07) | 1.38 (0.09) |
| NGMV (L)b | 0.77 (0.04) | 0.75 (0.04) | 0.73 (0.05) |
| NWMV (L)a | 0.69 (0.04) | 0.66 (0.04) | 0.65 (0.05) |
| NHV left (mL) | 4.31 (0.46) | 4.07 (0.49) | 4.11 (0.41) |
| NHV right (mL) | 4.33 (0.44) | 4.10 (0.49) | 4.15 (0.61) |
| T2 lesion volume (mL)† | — | 4.30 (2.52–6.70) | 6.83 (2.85–9.86) |
| T1 lesion volume (mL)† | — | 1.71 (0.74–3.34) | 2.84 (0.63–5.62) |
| Number cortical lesions† | — | 5 (3.0–8.5) | 7 (2.0–13.0) |
| Number hippocampal lesions† | — | 1 (0–2) | 1 (0–1) |
Data are means (standard deviation), except for † where because of non‐normal distribution median and interquartile range are provided.
NBV: normalized brain volume; NGMV: normalized gray matter volume; NWMV: normalized white matter volume; NHV: normalized hippocampal volume, L: liters, mL: milliliters.
Significant differences between groups: a CP & CI vs. controls (P < 0.01); b CP & CI vs. controls (P < 0.05).
DISCUSSION
The current study focused on the hippocampal memory system of MS patients with and without cognitive impairment (predominantly memory impairment) using a specific fMRI episodic memory paradigm to study hippocampal function as well as a comprehensive set of structural MRI measures and a detailed neuropsychological assessment. Our results showed that the activity of the hippocampal memory system of CP MS patients is increased compared to healthy controls during the encoding of correctly remembered items. In the CI MS patients relatively few extra‐limbic brain areas showed increased activation, whereas most limbic areas displayed less activation, possibly underlying the cognitive deficits that were clearly measurable at this stage.
Changes in the Hippocampal Memory System
In CP MS patients, increased brain activation was found in the left anterior cingulate gyrus, left hippocampus, and in the parahippocampal gyri bilaterally compared to healthy controls. In the literature, lateralization of some hippocampus‐dependent memory functions has been described. The right hippocampus is thought to be preferentially involved in visuospatial memory and the right parahippocampal gyrus in the processing of spatial scenes while the left hippocampus is involved in context‐dependent episodic memory or verbal memory [Burgess et al.,2002]. In addition to the hippocampus, the cingulate gyrus is more active in CP MS patients. The anterior cingulate gyrus is indirectly connected to the hippocampus via the thalamus, subiculum, and parahippocampal gyrus and has been implicated in both verbal [Shallice et al.,1994] as well as visuospatial episodic memory [Roland and Gulyas,1995]. We assume that the additional recruitment of the cingulate gyrus (besides the increased activity within the (para)hippocampal areas), in CP patients might be necessary to maintain normal memory encoding capacity. Outside the hippocampal memory network, increased activation was also seen in the cerebellum of CP MS patients. Several studies have shown that the cerebellum is involved in the modulation of cognitive tasks [Baillieux et al.,2008; Timmann and Daum,2007].
The MS patients with cognitive impairment showed an almost opposite pattern of brain activation. In the CI MS patients, less activation was found in the hippocampal memory system as well as in the medial prefrontal area which is thought to be involved in self‐referential or introspectively oriented mental activity [McGuire et al.,1996]. The right precuneus and the posterior cingulate gyrus showed increased activity in the CI patients and are known to be involved in visuospatial and episodic memory [Cavanna and Trimble,2006].
The CI patients displayed significantly less activation compared to the CP patients in many brain areas. The areas with less activation were mostly comparable to what was found in comparison to the healthy controls (i.e., right hippocampus, parahippocampal gyrus, medial frontal cortex, and ventral visual system). It is important to note that the left and right thalamus and the left hippocampus also showed less activity in the CI patients compared to the CP patients. The thalamus plays an important role in attention [Van Der Werf et al.,2002] and in patients with MS thalamic atrophy has been related to cognitive performance [Houtchens et al.,2007].
A Functional Adaptive Process
Our hypothesis was that the CP MS patients would already show less hippocampal activation preceding overt cognitive impairment. However, CP MS patients only showed increased brain activation compared to healthy controls which might be associated with the presence of a functionally adaptive mechanism within the hippocampal memory network of patients with MS.
It has been reported that during a simple motor task, the location of brain activation in patients with benign MS resembles that of healthy controls; however, increased activation in these areas was observed. Interestingly, in patients with secondary progressive MS, additional brain areas are being recruited [Rocca et al.,2010a]. During resting state fMRI it was found that clinically isolated syndrome (CIS) patients showed increased synchronization in several resting state networks, while this was not seen in patients with relapsing remitting MS with more overt structural damage [Roosendaal et al.,2010b]. This increased synchronization in CIS patients was suggested to be early functional reorganization of resting state networks. Another resting state study showed decreased synchronization in the default mode network (DMN; the DMN is frequently deactivated during cognitive tasks) in patients with progressive MS which was associated with a lower cognitive performance [Rocca et al.,2010b]. Decreased synchronization was especially found in the anterior cingulate, prefrontal, and precentral cortices. Recently, the hypothesis was put forward [Schoonheim et al.,2010] that in the early stages of MS, relatively little structural damage in the brain may trigger a functional adaptive mechanism. To preserve cognitive function, hyperactivation of task‐related and additional brain structures, might be induced. Over time, structural damage will increase together with the level of cognitive impairment. Still in this phase, functional adaptive mechanisms are expected to mask cognitive deterioration to a certain extent. When structural damage has become too extensive, functional adaptation will be naturally limited, as underlined by the observed differences between benign and secondary progressive MS patients [Rocca et al.,2010a]. Finally, the functional adaptive mechanism is no longer effective, resulting in hypoactivation on fMRI and overt, measurable cognitive deficits [Rocca et al.,2010b]. The ability of the brain to functionally adapt in response to structural damage may differ between people. Future (longitudinal) research is needed to investigate the complex interplay between brain adaptive properties and ongoing structural damage in more detail, as well as to investigate the time frame in which adaptive processes take place.
In our task fMRI study such patterns were indeed observed for the hippocampal memory system. The increased brain activation seen in the CP MS patients may reflect a functional adaptive process to prevent cognitive deficits, supporting the hypothesis mentioned above [Schoonheim et al.,2010]. When this adaptive mechanism becomes exhausted, the functionality of the hippocampal memory system will deteriorate, which might be related to decreased brain activation as measured by fMRI as well as the appearance of cognitive deficits. In the current study, we cannot verify the order of events due to the cross‐sectional design. Therefore, future studies will have to investigate the exact timeframe in which hyper‐ and hypo‐activation of brain structures related to cognition occur in MS. In a recent study by O'Brien et al. [2010] a longitudinal associative memory study in elderly subjects without dementia was performed. They showed that over time, clinical decline in elderly subjects is accompanied by significant loss of hippocampus activation. The subjects with the highest hippocampal activation at baseline (hyperactive hippocampus) demonstrated the greatest clinical decline over time, as well as the greatest loss of hippocampal function over 2 years. This possibly illustrates the occurrence of hyperactivation of the hippocampus in preclinical stages of cognitive impairment. Unfortunately, we were not able to provide longitudinal information in the current study.
A potential confounder of cognitive performance is the influence of depression, anxiety, and fatigue, that are frequently reported in patients with MS [Weingartner et al.,1981]. Previous studies have shown that cognitive deficits in MS are present independently of depression [Krupp et al.,1994]. In the current study, both patients with and patients without cognitive impairment suffered equally from these factors, indicating that cognitive impairment may indeed present independently from problems in one (or more) of these domains. Additionally, it is very unlikely that the differences found were due to other clinical characteristics inherent to MS (disease duration, or EDSS score) since the two groups did not significantly differ on these variables.
The male/female ratio as well as age differed slightly between the groups (not statistically significant). To prevent an unwanted influence of these parameters on the main study outcome, all these variables were added as covariates in the fMRI analyses. Disease type was not significantly different between the CP and CI MS patients (Table I; P = 0.138). Relatively, however, the CI group contained more secondary progressive patients. This, although disease duration and disability did not differ (Table I; P = 0.573 and P = 0.404, respectively) between the CI and CP groups, might theoretically have influenced the main outcome measure. In other words, despite a stringent matching procedure, we cannot completely exclude that factors other than cognitive impairment alone might have partly played a role.
Regarding structural MRI measures, the two patient groups were not significantly different. We expected that the hippocampal volume of CI patients would be reduced compared to CP patients and healthy controls; however this was not confirmed in the current study. Hippocampal volume was reduced in patients with MS as a whole group compared to healthy controls, which is consistent with the literature [Roosendaal et al.,2010a; Sicotte et al.,2008]. The relatively small number of CI patients (n = 16) and the greater variance in hippocampal volume in this particular patient group (compared to the CP patients and healthy controls) might be the reason why no significant differences were found between the CP and CI MS patients concerning hippocampal volume. Since we corrected the signal in the hippocampus for hippocampal volume, it is very unlikely that the results of our study will be influenced by hippocampal atrophy.
Additionally, other more sensitive MRI measures, such as diffusion tensor imaging, could have picked up more subtle structural differences that precede the functional changes. More work needs to be done to investigate the role of this subtle structural damage on hippocampal function.
In the current study, less activity of the hippocampal memory system in CI patients was found during the encoding of subsequently correctly recalled items. The CI patient group recalled fewer items on average than the healthy controls, but was still able to correctly recall items although extensive brain areas related to memory function showed less activation. The increased activation in the posterior cingulate gyrus and the precuneus found in the CI patients might explain why patients could still remember some of the items and might be a late phenomenon of functional adaptation.
CONCLUSION
This is the first fMRI study that specifically investigated hippocampal function in MS patients with and MS patients without cognitive deficits. Changes in the hippocampal memory system were detected for both patient groups compared to the healthy controls. Activity in the hippocampal memory system was increased when memory function was not impaired (CP patients). We proposed that this reflects a functional adaptive process to prevent cognitive deficits from developing. In a later phase, where cognitive impairment has already set in, functional adaptation may no longer be possible. This coincides with our findings of reduced brain activation in the hippocampal memory system of CI patients. In the current study, patients were investigated on one time point only; therefore, these results should now be further investigated in a longitudinal setting to better understand the temporal dynamics of structural brain damage, functional adaptive processes, and cognitive decline. This information may then be used as an input for studies working towards cognitive therapeutic strategies.
Acknowledgements
The authors would like to thank A.R. Hammerschlag for helping collecting the neuropsychological data; W.M.A. Jonker for outlining WM lesions, F.C. van Dommelen and M.M.M. Pronk for the measurements of hippocampal volumes.
REFERENCES
- Amato MP, Portaccio E, Goretti B, Zipoli V, Hakiki B, Giannini M, Pasto L, Razzolini L ( 2010): Cognitive impairment in early stages of multiple sclerosis. Neurol Sci Suppl 2: S227–S230. [DOI] [PubMed] [Google Scholar]
- Anderson V, Fisniku L, Khaleeli Z, Summers M, Penny S, Altmann D, Thompson A, Ron M, Miller D ( 2010): Hippocampal atrophy in relapsing‐remitting and primary progressive MS: A comparative study. Mult Scler 16: 1083–1090. [DOI] [PubMed] [Google Scholar]
- Audoin B, Reuter F, Duong MV, Malikova I, Confort‐Gouny S, Cherif AA, Cozzone PJ, Pelletier J, Ranjeva JP ( 2008): Efficiency of cognitive control recruitment in the very early stage of multiple sclerosis: A one‐year fMRI follow‐up study. Mult Scler 14: 786–792. [DOI] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Marien P ( 2008): Cerebellar neurocognition: Insights into the bottom of the brain. Clin Neurol Neurosurg 110: 763–773. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Shucard JL, Zivadinov R, Shucard DW ( 2008): Neuropsychological impairment in systemic lupus erythematosus: A comparison with multiple sclerosis. Neuropsychol Rev 18: 149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobholz JA, Rao SM, Lobeck L, Elsinger C, Gleason A, Kanz J, Durgerian S, Maas E ( 2006): fMRI study of episodic memory in relapsing‐remitting MS: Correlation with T2 lesion volume. Neurology 67: 1640–1645. [DOI] [PubMed] [Google Scholar]
- Bucks R, Willison J ( 1997): Development and validation of the location learning test (LLT): A test of visuo‐spatial learning designed for use with older adults and in dementia. Clin Neuropsychol 11: 273–286. [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J ( 2002): The human hippocampus and spatial and episodic memory. Neuron 35: 625–641. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J ( 2008): Cognitive impairment in multiple sclerosis. Lancet Neurol 7: 1139–1151. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C ( 2003): Deep processing activates the medial temporal lobe in young but not in old adults. Neurobiol Aging 24: 1005–1011. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA ( 1987): California Verbal Learning Test. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Forn C, Barros‐Loscertales A, Escudero J, Benlloch V, Campos S, Antonia PM, Avila C ( 2007): Compensatory activations in patients with multiple sclerosis during preserved performance on the auditory N‐back task. Hum Brain Mapp 28: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts JJ, Bo L, Roosendaal SD, Hazes T, Daniels R, Barkhof F, Witter MP, Huitinga I, van d V ( 2007): Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol 66: 819–827. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Roosendaal SD, Calabrese M, Ciccarelli O, Agosta F, Chard DT, Gass A, Huerga E, Moraal B, Pareto D, Rocca MA, Wattjes MP, Yousry TA, Uitdehaag BM, Barkhof F ( 2011): Consensus recommendations for MS cortical lesion scoring using double inversion recovery MRI. Neurology 76: 418–424. [DOI] [PubMed] [Google Scholar]
- Houtchens MK, Benedict RH, Killiany R, Sharma J, Jaisani Z, Singh B, Weinstock‐Guttman B, Guttmann CR, Bakshi R ( 2007): Thalamic atrophy and cognition in multiple sclerosis. Neurology 69: 1213–1223. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr ( 1994): MRI‐based hippocampal volume measurements in epilepsy. Epilepsia 35 ( Suppl 6): S21–S29. [DOI] [PubMed] [Google Scholar]
- Jolles J, Houx PJ, van Boxtel MPJ, Ponds RWHM ( 1995): Maastricht Aging Study: Determinants of Cognitive Ageing. Maastricht, The Netherlands: Neuropsych Publishers. [Google Scholar]
- Krupp LB, Sliwinski M, Masur DM, Friedberg F, Coyle PK ( 1994): Cognitive functioning and depression in patients with chronic fatigue syndrome and multiple sclerosis. Arch Neurol 51: 705–710. [DOI] [PubMed] [Google Scholar]
- Lechner‐Scott J, Kappos L, Hofman M, Polman CH, Ronner H, Montalban X, Tintore M, Frontoni M, Buttinelli C, Amato MP, Bartolozzi ML, Versavel M, Dahlke F, Kapp JF, Gibberd R ( 2003): Can the Expanded Disability Status Scale be assessed by telephone? Mult Scler 9: 154–159. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW ( 2004): Neuropsychological Assessment. New York: Oxford University Press. [Google Scholar]
- Mainero C, Caramia F, Pozzilli C, Pisani A, Pestalozza I, Borriello G, Bozzao L, Pantano P ( 2004): fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage 21: 858–867. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Paulesu E, Frackowiak RS, Frith CD ( 1996): Brain activity during stimulus independent thought. Neuroreport 7: 2095–2099. [PubMed] [Google Scholar]
- Morgen K, Sammer G, Courtney SM, Wolters T, Melchior H, Blecker CR, Oschmann P, Kaps M, Vaitl D ( 2007): Distinct mechanisms of altered brain activation in patients with multiple sclerosis. Neuroimage 37: 937–946. [DOI] [PubMed] [Google Scholar]
- Mulder JL, Dekker PH, Dekker R ( 1996): Verbale Leer‐ en Geheugentest. Lisse, The Netherlands: Swets and Zeitlinger. [Google Scholar]
- Nelson HE, O'Connell A ( 1978): Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex 14: 234–244. [DOI] [PubMed] [Google Scholar]
- O'Brien JL, O'Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, Sperling RA ( 2010): Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology 74: 1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R ( 2009): Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol 19: 238–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi L, Geesken JM, Holly M, Hayward M, Blumhardt LD ( 1997): Working memory impairment in early multiple sclerosis. Evidence from an event‐related potential study of patients with clinically isolated myelopathy. Brain 120 ( Part 11): 2039–2058. [DOI] [PubMed] [Google Scholar]
- Peyser JM, Edwards KR, Poser CM, Filskov SB ( 1980): Cognitive function in patients with multiple sclerosis. Arch Neurol 37: 577–579. [DOI] [PubMed] [Google Scholar]
- Piras MR, Magnano I, Canu ED, Paulus KS, Satta WM, Soddu A, Conti M, Achene A, Solinas G, Aiello I ( 2003): Longitudinal study of cognitive dysfunction in multiple sclerosis: Neuropsychological, neuroradiological, and neurophysiological findings. J Neurol Neurosurg Psychiatry 74: 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O'Connor PW, Sandberg‐Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS ( 2005): Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58: 840–846. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Bernardin L, Unverzagt F ( 1991a): Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41: 685–691. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F ( 1991b): Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 41: 692–696. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Ceccarelli A, Rodegher M, Misci P, Riccitelli G, Falini A, Comi G, Filippi M ( 2010a): Preserved brain adaptive properties in patients with benign multiple sclerosis. Neurology 74: 142–149. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P, Rossi P, Falini A, Comi G, Filippi M ( 2010b): Default‐mode network dysfunction and cognitive impairment in progressive MS. Neurology 74: 1252–1259. [DOI] [PubMed] [Google Scholar]
- Roland PE, Gulyas B ( 1995): Visual memory, visual imagery, and visual recognition of large field patterns by the human brain: Functional anatomy by positron emission tomography. Cereb Cortex 5: 79–93. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Moraal B, Vrenken H, Castelijns JA, Pouwels PJ, Barkhof F, Geurts JJ ( 2008): In vivo MR imaging of hippocampal lesions in multiple sclerosis. J Magn Reson Imaging 27: 726–731. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Hulst HE, Vrenken H, Feenstra HE, Castelijns JA, Pouwels PJ, Barkhof F, Geurts JJ ( 2010a): Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology 255: 595–604. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Schoonheim MM, Hulst HE, Sanz‐Arigita EJ, Smith SM, Geurts JJ, Barkhof F ( 2010b): Resting state networks change in clinically isolated syndrome. Brain 133: 1612–1621. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Light RH, Parker SB, Levin HS ( 1997): The psychological construct of word fluency. Brain Lang 57: 394–405. [DOI] [PubMed] [Google Scholar]
- Schmand B, Bakker D, Saan R, Louman J ( 1991): The Dutch Reading Test for Adults: A measure of premorbid intelligence level. Tijdschr Gerontol Geriatr 22: 15–19. [PubMed] [Google Scholar]
- Schoonheim MM, Geurts JJ, Barkhof F ( 2010): The limits of functional reorganization in multiple sclerosis. Neurology 74: 1246–1247. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ ( 1994): Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature 368: 633–635. [DOI] [PubMed] [Google Scholar]
- Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, Bookheimer SY ( 2008): Regional hippocampal atrophy in multiple sclerosis. Brain 131: 1134–1141. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N ( 2002): Accurate, robust, and automated longitudinal and cross‐sectional brain change analysis. Neuroimage 17: 479–489. [DOI] [PubMed] [Google Scholar]
- Staffen W, Mair A, Zauner H, Unterrainer J, Niederhofer H, Kutzelnigg A, Ritter S, Golaszewski S, Iglseder B, Ladurner G ( 2002): Cognitive function and fMRI in patients with multiple sclerosis: Evidence for compensatory cortical activation during an attention task. Brain 125: 1275–1282. [DOI] [PubMed] [Google Scholar]
- Sweet LH, Rao SM, Primeau M, Durgerian S, Cohen RA ( 2006): Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp 27: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D, Daum I ( 2007): Cerebellar contributions to cognitive functions: A progress report after two decades of research. Cerebellum 6: 159–162. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Witter MP, Groenewegen HJ ( 2002): The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev 39: 107–140. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Altena E, Schoonheim MM, Sanz‐Arigita EJ, Vis JC, De Rijke W, Van Someren EJ ( 2009): Sleep benefits subsequent hippocampal functioning. Nat Neurosci 12: 122–123. [DOI] [PubMed] [Google Scholar]
- Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G ( 1994): Dimensional assessment of chronic fatigue syndrome. J Psychosom Res 38: 383–392. [DOI] [PubMed] [Google Scholar]
- Verhage F ( 1964): Intelligentie en leeftijd: onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar. Assen: Van Gorkum. [Google Scholar]
- Wechsler D ( 1997): Wechsler Adult Intelligence Scale—Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weingartner H, Cohen RM, Murphy DL, Martello J, Gerdt C ( 1981): Cognitive processes in depression. Arch Gen Psychiatry 38: 42–47. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP ( 1983): The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361–370. [DOI] [PubMed] [Google Scholar]


