Abstract
Stress has a powerful impact on memory. Corticosteroids, released in response to stress, are thought to mediate, at least in part, these effects by affecting neuronal plasticity in brain regions involved in memory formation, including the hippocampus and prefrontal cortex. Animal studies have delineated aspects of the underlying physiological mechanisms, revealing rapid, nongenomic effects facilitating synaptic plasticity, followed several hours later by a gene‐mediated suppression of this plasticity. Here, we tested the hypothesis that corticosteroids would also rapidly upregulate and slowly downregulate brain regions critical for episodic memory formation in humans. To target rapid and slow effects of corticosteroids on neural processing associated with memory formation, we investigated 18 young, healthy men who received 20 mg hydrocortisone either 30 or 180 min before a memory encoding task in a double‐blind, placebo‐controlled, counter‐balanced, crossover design. We used functional MRI to measure neural responses during these memory encoding sessions, which were separated by a month. Results revealed that corticosteroids' slow effects reduced both prefrontal and hippocampal responses, while no significant rapid actions of corticosteroids were observed. Thereby, this study provides initial evidence for dynamically changing corticosteroid effects on brain regions involved in memory formation in humans. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: corticosteroids, memory, hippocampus, prefrontal cortex, fMRI
INTRODUCTION
Aversive, stressful life experiences are extremely well remembered [Joels et al.,2006; Sandi and Pinelo‐Nava,2007]. Corticosteroids, released in response to stress, are thought to be critically involved in this memory enhancement by affecting neural plasticity [Joels and de Kloet,1989; McEwen,1994]. Recent animal studies on cellular excitability and long‐term potentiation (LTP), the alleged neurobiological substrate of memory formation [Martin and Morris,2002], suggest that corticosteroids alter neural plasticity in a time‐dependent manner. On the one hand, corticosteroids were shown to rapidly enhance hippocampal excitability and LTP via a low‐affinity mineralocorticoid receptor (MR) thought to reside in the plasma membrane [Karst et al.,2005]. These rapid actions of corticosteroids work in concert with (and amplify) the effects of catecholamines [Roozendaal et al.,2006] and are suggested to optimize rapid adaptive behavior by relocating neural resources away from higher‐order cognitive processing regions in the prefrontal cortex (PFC) to the medial temporal lobe (MTL) [Diamond,2007]. On the other hand, the initiation of a corticosteroid‐induced genomic cascade by the binding of intracellular mineralocorticoid and glucocorticoid receptors (GRs) is known to suppress hippocampal LTP several hours later [Pavlides et al.,1995; Wiegert et al.,2005]; this delayed action is considered to promote consolidation of relevant information [de Kloet et al., 2008], possibly by impairing retroactive interference. Although these neurobiological mechanisms are quite well established in rodents, at present, it is unclear if and how they translate to the human brain.
Therefore, we tested the hypothesis that corticosteroids rapidly up regulate and slowly down regulate brain regions critical for episodic memory formation at the human system level. We focused on two brain regions known to be affected by corticosteroids [de Kloet,1991], and critically involved in memory processing [Fernandez and Tendolkar,2001]; the hippocampus and prefrontal cortex. Rather than giving participants corticosteroids at one time point and follow them along the process of memory formation (which would involve rapidly succeeding fMRI sessions, inevitably inducing strong order effects), participants received either placebo, 20 mg hydrocortisone 30 min before the study phase to target the rapid actions of corticosteroids, or 20 mg hydrocortisone 180 min before the study phase to target the slow actions of corticosteroids. We used a double‐blind, placebo‐controlled, counter‐balanced crossover design and invited participants for three study‐test cycles each separated by approximately 1 month, receiving each time a different pharmacological manipulation. In every cycle, participants were instructed to memorize different sets of both emotionally negative and neutral pictures while brain activity was measured using fMRI. The memory for these pictures was tested 24 h later. Moreover, to exclude potential physiological or psychological side‐effects of hydrocortisone administration, heart rate and mood state were assessed throughout the experiment.
MATERIALS AND METHODS
Participants
Eighteen young (ages 18–29, median 23), right‐handed, healthy male volunteers participated in the study after signing written informed consent. Women were excluded from participation since previous research has indicated that they respond differently to hydrocortisone than men, both in behavior [Andreano and Cahill,2006; Bohnke et al.,2010] and brain activation [Merz et al.,2010; Stark et al.,2006]. We presently focused on men, allowing easier comparison with the results from an earlier study in which subjects were exposed to stress [Henckens et al.,2009], a situation that is known to induce a more stable neuroendocrine response in men than in women [Bouma et al.,2009; Kajantie and Phillips,2006; Kirschbaum et al.,1999; Ossewaarde et al.,2010]. Furthermore, individuals who met any of the following criteria were excluded from participation during screening: history of head injury, autonomic failure, history of or current psychiatric, neurological, or endocrine disorders, current periodontitis, acute inflammatory disease, acute peptic or duodenal ulcers, regular use of corticosteroids, treatment with psychotropic medications, narcotics, β‐blockers, steroids, or any other medication that affects central nervous system or endocrine systems, medical illness within the 3 weeks before testing, self reported mental or substance use disorder, daily tobacco or alcohol use, regular night shift work, current stressful episode or major life event, and previous exposure to slides used in the study [i.e., International Affective Picture System; Lang et al.,1999]. The study was executed in accordance with the declaration of Helsinki and approved by the local ethics committee (CMO region Arnhem‐Nijmegen, Netherlands).
Procedure
Screening
After granting informed consent, all participants were invited for an introductory interview, during which they were asked to complete an initial screenings questionnaire, the Beck Depression Inventory [Beck,2002], and NEO‐FFI Personality Inventory [Costa and McCrae,1992]. Further, a T 1‐weighted anatomical scan was made, familiarizing participants with the MRI environment before the study sessions began (see Supporting Information, Fig. S1 for a schematic overview of the complete procedure).
Before arrival
To minimize differences in baseline cortisol levels, we instructed participants not to use any recreational drugs for 3 days and to refrain from drinking alcohol, exercising, and smoking for 24 h before each session. Furthermore, participants were requested not to brush their teeth, floss, or eat and drink anything but water for 1 h before all sessions enabling adequate saliva sampling for cortisol assessment. They were asked to take a light lunch and do so no later than 1 h before arrival; their lunch could not contain any citrus products, coffee, tea, milk, and sweets [Maheu et al.,2005]. Throughout each session, they had no further food intake and had only water to drink.
Arrival
To reduce the impact of diurnal variation in cortisol levels, the experiment started in the afternoon, when hormone levels are relatively stable. After arrival at 12:00 h (±45 min) on the first day, participants rested 30 min before taking the first saliva sample, followed by another sample 15 min later. The average value of these two samples served as baseline cortisol level. To increase familiarity with the procedure and minimize task repetition effects, participants were explicitly informed about all details of the memory experiment. A financial reward was promised proportional to the participant's performance in the recall test to encourage motivation. During the entire period (∼3.75 h) before the encoding task, the participants had to wait in a quiet, isolated room where they were free to conduct any activities except for anything potentially arousing (e.g. video games).
Drug administration
Implementing a double‐blind, placebo‐controlled, counter‐balanced crossover design, each participant underwent three experimental sessions, with an approximate intersession interval of 1 month (mean interval ± SEM; 40 ± 4 days). The whole procedure for individual sessions remained identical except that the drug administration schemes differed from session to session. All drug capsules, containing either 20 mg CORT (hydrocortison CF 20 mg tablets, Centrafarm Services B.V. Etten‐Leur, The Netherlands) or placebo (cellulose) were administered orally. The administration dose of 20 mg was based on previous studies using a similar dose [Buchanan and Lovallo,2001; van Stegeren et al.,2010] showing that this dose elevated cortisol levels to those observed during exposure to severe stress [Morgan et al.,2000]. In order to ensure a double‐blind paradigm and to monitor the time‐dependent effect of cortisol, participants received two capsules at distinct time points; at 180 min before the start of picture encoding (t = 45) and at 30 min before the start of picture encoding (t = 195). At these time points they received either: (1) 1st capsule containing CORT, 2nd placebo—to reveal the slow effect of cortisol; (2) 1st placebo, 2nd CORT—to disclose the rapid cortisol effect; and (3) 1st placebo, 2nd placebo—the control. Timing of administration at 30 min before encoding was based on previous studies in humans showing rather immediate (<15 min) increases in salivary cortisol levels following hydrocortisone intake [van Stegeren et al.,2010] and a high correlation between salivary cortisol levels and serum levels of free (i.e. active) cortisol [Kirschbaum and Hellhammer,1994]. Cortisol is known to pass the blood–brain barrier quite well [Karssen et al.,2001], and rodent studies have shown a strong correlation between plasma and brain corticosteroid levels [Droste et al.,2008], but with a small time delay (∼20 min) between plasma peak corticosteroid levels and those in the brain after exposure to stress. Based on these studies, brain cortisol levels are expected to rise approximately 30 min after hydrocortisone administration. Given that the rapid, non‐genomic corticosteroid effects on the brain are known to occur almost immediately upon brain exposure to elevated corticosteroid levels [Karst et al.,2005], we therefore optimally targeted rapid effects by administering hydrocortisone 30 min before scanning. The slow, genomic effects of corticosteroids were not expected to start earlier than approximately 90 min after corticosteroid administration, and last for hours [Joels and de Kloet,1992,1994; Joels et al.,2003]. The timing for targeting these effects, i.e. 3 hours postadministration, was based on previous work showing suppressed LTP [Pavlides et al.,1995; Wiegert et al.,2005], and strongest corticosteroid effects on hippocampal gene expression at this time‐delay [Morsink et al.,2006].
Scanning
Participants lay supine in the scanner and viewed the screen through a mirror positioned on the head coil. They were asked to lie as still as possible, keep their eyes open, and look directly and continuously at the center of the screen in front of them. Participants were instructed to view each picture for the entire time that it was displayed. Pictures belonged to two categories, either with a neutral or negatively arousing content. Participants were asked to memorize each picture and to rate its aversiveness. Ratings were given with right‐hand button presses, with the index finger for negative and the middle finger for neutral pictures. Pictures were shown in a pseudorandom order (no more than two pictures of the same category consecutively), and all first slides were neutral to avoid ceiling effects in recall that might result from the combined effect of arousal and primacy on memory.
Stimulus materials
Participants viewed a distinct stimulus set during each picture encoding session [Henckens et al.,2009]; resulting in the requirement of three different stimulus sets. Each of these sets consisted of 80 negative and 80 neutral pictures, supplemented with 41 null events (fixation). Pictures were selected from both a standard set of affective pictures (International Affective Picture System (IAPS) [Lang et al.,1999] and an additional set of newly rated pictures. New pictures were previously [Henckens et al.,2009] downloaded from the internet and selected on the authors' assessment of emotionality and similarity to IAPS pictures. New pictures were rated on a scale from 1 to 9 on both arousal and valence using the Self‐Assessment Manikin (SAM) scales [Bradley and Lang,1994] by an additional 20 male volunteers. To assure reliable rating that did not significantly differ from IAPS ratings, and to serve as a reference frame, positive and negative IAPS pictures were added. Negative slides were chosen for their moderate to high arousal quality (mean ± SE; 5.5 ± 0.7), and negative valence (mean ± SE; 3.1 ± 0.7), rated on a 1‐ to 9‐point rating scale as determined by the SAM [Bradley and Lang,1994]. Neutral slides were selected for their relatively low arousal (mean ± SE; 2.5 ± 0.7) and neutral valence (mean ± SE; 5.3 ± 0.3). Used picture sets contained about 50% newly rated neutral and 15% newly rated negative pictures and were matched on chromatic features and complexity, while overlap in contents within one set was minimized. Stimulus sets did not differ in mean arousal and valence ratings. All slides were presented for 6 s with a 4‐ to 8‐s intertrial interval (fixation cross), resulting in a total scanning time of ∼40 min for each session.
Subsequent memory test
To exclude any corticosteroid effects on memory retrieval, participants came back on the day after each encoding session (at 14:15 h (±45 min)) to perform a free and a cued recall test, both lasting 60 min. In both tests, participants were required to write to the utmost detail all the characteristics of the pictures they could remember, so that an outsider would be able to identify the pictures as distinctively recognizable with the information provided [Dolcos et al.,2004]. A short introduction was written to help the participants in listing characteristics. The cued recall test differed from the free recall in that the participant received one‐ or two‐word written cues (of similar arousal to that of the picture) that may facilitate his recall. This cue could e.g. be the negative one “wounded hand”, to which participants could mention the details “left hand, few fingers missing, tendons sticking out, held above a metal bowl, etc”. The cue could also describe a neutral picture, e.g. “bike”, to which participants could write down “pink bike, put against a brick wall, basket on steering wheel, etc”. These written descriptions provided by the participants were evaluated by a researcher blind to the drug condition the participant was in, and only pictures with a description that allowed both identification and discrimination were classified as remembered. Since some pictures that were mentioned during free recall were not described in the cued recall test (due to motivational issues or specifics of the cues), but were obviously remembered, all pictures mentioned in either the free or cued recall test were considered remembered for further analyses. Pictures with no recollection of characteristics were considered forgotten.
Physiological and Behavioral Measures
Saliva collection and analysis
Cortisol levels were measured from saliva at eight time points: baseline measurements at the beginning of the experiment (twice) (t = 30, 45 min), and six samples (t = 75, 105, 135, 195, 225, 285 min) to assess cortisol changes throughout the experiment. Saliva was collected using a commercially available collection device (Salivette®, Sarstedt, Germany). For each sample, the participant first placed the cotton swab provided in each Salivette tube in his mouth and chewed gently on it for 1 min to produce saliva. The swab was then placed back in the salivette tube, and the samples were stored in a freezer at −25°C until assayed. Laboratory analyses were performed at the Department of Biopsychology, TU Dresden, Germany. After thawing, salivettes were centrifuged at 3,000 rpm for 5 min, which resulted in a clear supernatant of low viscosity. Salivary free cortisol concentrations were subsequently measured using a commercially available chemiluminescence‐immuno‐assay (CLIA) with high sensitivity of 0.16 ng/ml (IBL, Hamburg, Germany).
Heart rate
Cardiac rhythm of the participants was measured during scanning using a pulse oximeter placed on their left index finger. Participants were instructed to keep their hands as still as possible during the measurement. Heart rate frequency was calculated using in‐house software. Data of one subject were discarded from analyses, due to excessive artifacts in the recorded signal.
Mood state
Mood state was assessed using the Positive and Negative Affect Schedule questionnaire [Watson et al.,1988] at three time points: at the beginning of the experiment (t = 30 min), just before encoding (t = 225 min), and immediately after encoding (t = 285 min).
Physiological and Behavioral Statistical Analysis
Behavioral and physiological data were analyzed in SPSS 15.0 (SPSS, Inc., Chicago, IL) using repeated measures ANOVAs with drug manipulation (slow CORT vs. rapid CORT vs. placebo), subsequent memory (remembered vs. forgotten), and picture arousal (aversive vs. neutral) as within subject factors, and paired samples t‐test statistics. Although the implemented session order (of the placebo, rapid CORT, and slow CORT sessions) was counterbalanced over participants, we also tested whether this factor still potentially modulated the effects of drug administration. Therefore, we tested whether session order had any influence on the drug effects observed by including it as a covariate in the analyses of the drug effects. This did not change the observed pattern of results, which made us to exclude this factor in all further analyses. Alpha was set at 0.05 throughout.
MRI Acquisition
Participants were scanned in a Siemens (Erlangen, Germany) MAGNETOM Avanto 1.5 T MRI scanner equipped with an eight‐channel head coil. During each of the three scanning sessions, a series of blood oxygenation level‐dependent (BOLD) T ‐weighted gradient echo EPI images were acquired with the following parameters: TR = 2,340 ms, TE = 35 ms, FA = 90°, 32 axial slices approximately aligned with AC‐PC plane, slice matrix size = 64 × 64, slice thickness = 3.5 mm, slice gap = 0.35 mm, FOV = 212 × 212 mm2. High‐resolution anatomical images were acquired using a T 1‐weighted three‐dimensional Magnetization‐Prepared RApid Gradient Echo (MP‐RAGE) sequence with the following parameters: TR = 2,250 ms, TE = 2.95 ms, FA = 15°, orientation: sagittal, FOV = 256 × 256 mm2, voxel size = 1.0 mm isotropic.
fMRI Data Analysis
Data were analyzed using Statistical Parametric Mapping software (SPM5; UCL, London) and in‐house software. The first five EPI‐volumes were discarded to allow for T 1 equilibration. Before fMRI analysis, the images were motion corrected using rigid body transformations and least sum of squares minimization. Subsequently, they were temporally adjusted to account for differences in sampling times across different slices. All functional images were then co‐registered with the high‐resolution T 1‐weighted structural image using normalized mutual information maximization. The anatomical image was subsequently used to normalize all scans into MNI152 (Montreal Neurological Institute) space. All functional images were resampled with a voxel size of 2 mm isotropic. Finally, all images were smoothed with an isotropic 8‐mm full‐width‐at‐half‐maximum Gaussian kernel in order to accommodate residual functional/anatomical variance between subjects.
Subsequently, data were analyzed using a general linear model, in which individual events were modeled based on drug condition (slow CORT vs. rapid CORT vs. placebo), subsequent memory (remembered vs. forgotten), and picture arousal (aversive vs. neutral). Regressors were temporally convolved with the canonical hemodynamic response function of SPM5. The six covariates corresponding to the movement parameters obtained from the realignment procedure for every session were also included in the model. To reduce unspecific differences between scan sessions, global normalization using proportional scaling was applied (see Supporting Information, Fig. S2 for the first level model applied). The single subject parameter estimates of each session and condition obtained from the first‐level analysis were included in subsequent random effects analyses. For the second‐level analysis a factorial ANOVA was used, with drug manipulation, subsequent memory, and picture arousal as within subject factors.
Statistical tests were family‐wise error (FWE) rate corrected (P fwe < 0.05) for multiple comparisons at the cluster‐level using a height threshold of P < 0.001. F‐contrast cluster‐level statistics in SPM were performed by implementing the random field theory (RFT) version of cluster size inference (under stationarity) extended to F‐tests [Ashburner and Friston,2000; Hayasaka et al.,2004]. Correction for multiple comparisons was done across the entire brain, or for the search volume for regions of interest using a small volume correction. Given strong neurophysiological evidence for the locus of CORT receptors [de Kloet,1991], and their known involvement in memory formation [Fernandez and Tendolkar,2001], the hippocampus and PFC were a priori considered regions of interest. The search volumes for these ROIs were anatomically defined using the WFU PickAtlas Tool (version 2.4) toolbox implemented in SPM5 [Maldjian et al.,2003]. The specific masks used were those for the hippocampus (bilaterally) and the frontal lobe.
To test for distributed drug effects on hippocampal activity specifically, mean activity of the anatomically defined hippocampus was extracted and analyzed in SPSS. Visualizations of activations were created using MRIcroN (available at: http://www.sph.sc.edu/comd/rorden/mricron/) by superimposing statistical parametric maps thresholded at P < 0.005 uncorrected and an extended cluster‐size of 500 voxels (to filter out effects that did not reach our statistical threshold corrected for multiple testing), onto a canonical T 1‐weighted image in standard MNI152 space.
RESULTS
Cortisol Level
Twenty mg of hydrocortisone (CORT) was effective in elevating salivary cortisol levels to levels observed during severe stress [Morgan et al.,2000]. Both drug administration conditions increased cortisol levels (peak level rapid CORT vs. placebo: t (17) = 4.45, P < 0.001), peak level slow CORT vs. placebo: t (17) = 8.10; P < 0.001), with levels either peaking during or at 120 min before the study phase, respectively (see Fig. 1). As intended, in the rapid CORT condition cortisol levels during scanning were higher than those in both the placebo (F (1,17) = 21.73; P < 0.001) and the slow CORT condition (F (1,17) = 11.88; P = 0.003). In the slow CORT condition cortisol levels were still slightly, but significantly, elevated compared with placebo during memory encoding (F (1,17) = 32.38; P < 0.001). To correct for any potential effect of this remainder of circulating cortisol in the slow CORT condition, the absolute difference in cortisol levels as compared with placebo was included as a covariate in all comparisons between these drug conditions.
Figure 1.
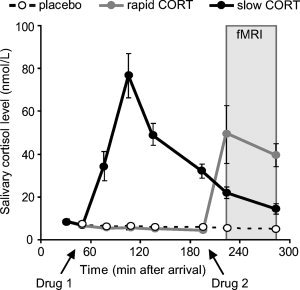
Salivary cortisol levels. Participants received two capsules (Drug 1 and Drug 2) containing either 20 mg hydrocortisone (CORT) or placebo at different time‐points before picture encoding during fMRI scanning. CORT intake significantly elevated salivary cortisol levels to levels observed during severe stress in both CORT administration conditions. Rapid CORT: 20 mg CORT administered 30 min before encoding, slow CORT: 20 mg CORT administered 180 min before encoding, Placebo: mere placebo administered. Error bars represent SEM.
Physiological and Psychological Measures
Hydrocortisone did not have any subjective, noticeable effects. Postexperiment debriefing revealed that participants were not able to identify the substance received. As expected, hydrocortisone administration did not affect autonomic measures of heart rate (main effect of drug: F (2,15) = 2.39; P = 0.125) and heart rate variability (F (2,15) = 1.72; P = 0.213; Supporting Information, Table S1).
Further, hydrocortisone administration did not affect psychological state as assessed by the Positive and Negative Affect Schedule questionnaire [Watson et al.,1988]. A consistent reduction in positive affect over time was observed in all drug conditions (F (2,16) = 18.18; P < 0.001), independently of drug administration (main effect of drug: F (2,16) = 1.56; P = 0.241, drug × time interaction: F (4,14) = 1.54; P = 0.244). Negative affect did not change throughout the experiment (main effect of time: F (2,16) = 2.53; P = 0.111), nor was it affected by drug administration (main effect of drug F (2,16) = 2.68; P = 0.099, drug × time interaction: F (4,14) < 1; Supporting Information, Table S1). Hence, differences in brain activity due to drug administration cannot readily be explained by autonomic or psychological side effects of the drug.
Memory Performance
As intended, about 50% of the pictures were recalled the subsequent day (mean ± SEM; 47.56 ± 1.78 aversive pictures, 36.72 ± 3.08 neutral pictures, see Supporting Information, Table S1 and Fig. S3). Hydrocortisone administration did not induce any significant effects on memory performance (F (2,16) < 1). As expected, we did observe a strong effect of picture arousal, with participants recalling more aversive than neutral items (F (1,17) = 32.11; P < 0.001, Supporting Information, Table S1), but also this effect was not modulated by CORT administration (F (2,16) < 1).
Brain Activation
First, regions supporting successful memory formation were identified. Confirming earlier findings [Brewer et al.,1998; Henckens et al.,2009; Wagner et al.,1998], regions displaying larger neural activity during encoding of subsequently remembered than forgotten pictures included the hippocampus, bilateral inferior temporal gyrus, inferior, middle, and superior frontal gyrus, inferior parietal gyrus, and the mid/superior occipital lobe (Supporting Information, Table S2). Second, brain imaging results revealed strong main effects of picture arousal (aversive > neutral) in the amygdala, hippocampus, insula, cerebellum, brain stem, inferior frontal cortex, and regions associated with visual processing (including the middle temporal gyri) [Henckens et al.,2009; Phan et al.,2002] (Supporting Information, Table S2).
Next, we examined the main question at issue, how CORT affects brain regions involved in memory formation over time. We first tested whether there were any differences in brain activity between all three drug conditions (i.e., the main effect of drug) by performing an ANOVA with three levels of the factor drug. This analysis revealed a large cluster within the middle frontal gyrus [comprising Brodmann areas (BA) 9, 45, 46, and 48] affected by CORT administration [local maximum at (x = 32, y = 28, z = 26), F (2,204) = 13.16; P fwe < 0.001]. Thus, CORT administration clearly modulated prefrontal cortex activity. Next, we wanted to perform follow‐up tests to investigate whether this observed effect for corticosteroids was time‐dependent. However, we were not allowed to extract the data from this activation cluster, since the selection of voxels would have been biased towards differences between the three drug conditions (it would induce circularity arising from a nonindependent selection of voxels [Kriegeskorte et al.,2009]). Therefore, we conducted a new contrast for corticosteroid modulation that was orthogonal (i.e. independent) to the timing effect by contrasting placebo to both drug conditions combined (CORT(rapid + slow) vs. placebo). This analysis revealed again a cluster in the middle frontal gyrus that exhibited a negative CORT effect for this contrast (Table I, Fig. 2A in the manuscript; CORT(rapid + slow) < placebo). The parameter estimates for both CORT conditions were subsequently extracted and their direct comparison showed that the slow CORT condition was characterized by a stronger reduction in prefrontal cortex activity than the rapid CORT condition (F (1,17) = 4.46; P = 0.050, Fig. 2A). The rapid CORT condition on the other hand, did not show a significant difference in activity in this region from placebo (Fig. 2B).
Table I.
Peak voxel and corresponding F/T value of significantly activated clusters in the main effects of hydrocortisone (CORT)
| Main effect of drug | MNI coordinates | ||||||
|---|---|---|---|---|---|---|---|
| Brodmann area | x | y | z | Peak T‐score | Cluster size | P | |
| F‐contrast | |||||||
| Placebo vs. rapid CORT vs. slow CORT | |||||||
| Middle frontal gyrus, R | 45, 46, 48 | 32 | 28 | 26 | 13.16 | 456 | P < 0.001a |
| Placebo > rapid and slow CORT | |||||||
| Angular gyrus, L | 39 | −46 | −62 | 40 | 3.96 | 211 | P = 0.026a |
| Middle frontal gyrus, R | 9, 45, 46, 48 | 34 | 26 | 26 | 3.93 | 133 | P = 0.050b |
| Placebo > slow CORT | |||||||
| Mid occipital gyrus/angular gyrus, L | 39 | −38 | −66 | 36 | 4.34 | 489 | P < 0.001a |
| Middle cingulate gyrus, R | 23 | 8 | −30 | 40 | 4.32 | 622 | P < 0.001a |
| Middle frontal gyrus, R | 9, 32, 45, 48 | 34 | 26 | 26 | 5.09 | 2571 | P < 0.001a |
| L | −24 | 34 | 28 | 4.63 | |||
| Suporbitofrontal lobule, L | 10, 11 | −24 | 52 | −2 | 4.37 | 399 | P = 0.001a |
The peak x, y, and z coordinates are given in MNI152 standard space coordinates. All effects were analyzed using family wise error (FWE) correction for multiple comparisons at the cluster‐level (P fwe < 0.05), after using a height threshold of P < 0.001.
FWE‐corrected for whole brain volume.
FWE‐corrected for region of interest.
MNI, Montreal Neurological Institute; R, right; L, left.
Figure 2.
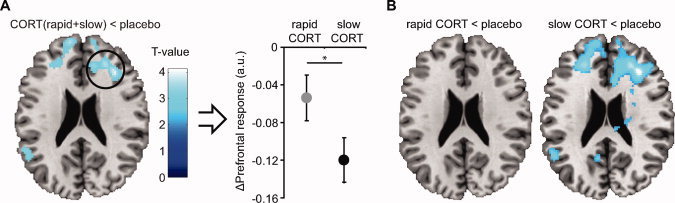
Effects of hydrocortisone (CORT) administration on brain activity during picture encoding. A, Negative main effect of CORT administration regardless of timing (z = 26); activity in prefrontal cortex was decreased due to CORT administration. Comparison of parameter estimates from this activation cluster (local maximum at [34, 26, 26]) to placebo revealed that PFC activity was significantly down regulated in the slow CORT condition. B, Simple effect contrasts of brain regions that were more active during picture processing under placebo conditions than under CORT (z = 26). The slow effects of corticosteroids clearly down regulated prefrontal cortex activity, whereas the rapid effects of corticosteroids did not. Baseline represents activity under placebo conditions. *P = 0.050. See Table I for formal statistical tests. Error bars represent SEM.
To correct for any potential effects of the remaining small but significant CORT increase during encoding in the slow CORT condition, a new general linear model was created using the normalized difference in hormone concentration between slow CORT and placebo conditions as a covariate. This correction did not change the pattern of results (Supporting Information, Table S3 and Fig. S4), indicating that the observed effects cannot easily be explained by the acute effects of the remaining small elevation in CORT levels, but are rather caused by the slow actions of CORT.
Second, we tested whether CORT had any effects on the hippocampus specifically. The initial analysis in SPM did not reveal a general main effect of CORT in this region, but since voxel‐wise analyses are most efficient in detecting focal effects, any effect may remain below the detection threshold if it is widely distributed across the entire hippocampus. Therefore, we averaged data from the anatomically defined hippocampi and tested for time‐specific CORT effects. As hypothesized, this analysis revealed reduced hippocampal responses compared with placebo due to the slow hydrocortisone effects (F (1,17) = 6.21; P = 0.023, Fig. 3). The rapid actions of corticosteroids did not seem to affect hippocampal activity, as activity observed in the rapid CORT condition was not significantly different from placebo (F (1,17) < 1). However, the difference in activity between both drug conditions (rapid vs. slow CORT) failed to reach significance (F (1,17) = 3.12; P = 0.095). Therefore, the effect of rapid corticosteroid actions on hippocampal activation remains to be resolved.
Figure 3.
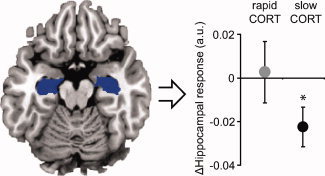
Effects of hydrocortisone (CORT) administration on hippocampal activity. The slow effects of corticosteroids reduced activity in the hippocampus bilaterally (anatomically defined), whereas corticosteroids' rapid effects did not have such an effect. Baseline represents activity under placebo conditions. *P < 0.05. Error bars represent SEM.
To investigate whether CORT also affected neural processes underlying memory formation we tested for interaction effects between drug and subsequent memory. No such interaction effects were found. Also, the observed arousal effects did not interact with drug administration.
DISCUSSION
In this study, we targeted time‐specific effects of corticosteroids on human memory formation by administering 20 mg hydrocortisone orally at two different time points before a memory encoding task executed during fMRI scanning. In line with previous animal studies, we found that corticosteroids affect neural processing in brain regions involved in memory formation in a dynamically changing manner. Specifically, corticosteroids' slow effects inhibited hippocampal and prefrontal processing, whereas corticosteroids' rapid actions did not show such an effect.
Previous work in animals has indicated that corticosteroids exert both rapid, nongenomic, and slow, genomic effects [Karst et al.,2005; Pavlides et al.,1995; Wiegert et al.,2005]. Here, we aimed to dissociate these two effects experimentally by administrating 20 mg of hydrocortisone at either 30 or 180 min before the memory task. The timing of the rapid corticosteroid condition was based on previous studies revealing (a) elevated salivary cortisol levels in humans within 15 min after hydrocortisone intake [van Stegeren et al.,2010], (b) highly significant covariation between salivary and free plasma cortisol levels following administration [Tunn et al.,1992], (c) a time‐delay between rodent peak plasma and brain levels of approximately 20 min [Droste et al.,2008], and (d) most prominent rapid, effects with corticosteroids administered directly to hippocampal slices [Karst et al.,2005]. The slow effects of corticosteroids are not expected to start earlier than approximately 90 min after corticosteroid administration, and last for hours [Joels and de Kloet,1992,1994; Joels et al.,2003]. We based the timing for targeting these effects on previous work showing suppressed LTP [Pavlides et al.,1995; Wiegert et al.,2005], and strongest corticosteroid effects on hippocampal gene expression at this time‐delay [Morsink et al.,2006]. Thus, administration of hydrocortisone at either 30 or 180 min before scanning allowed us to disentangle most optimally the rapid and slow corticosteroid effects, respectively.
Previous animal work on the genomic effects of corticosteroids showed that corticosteroid exposure suppresses hippocampal firing and LTP [Pavlides et al.,1995; Wiegert et al.,2005], presumably by modulating expression of over 200 genes [Datson et al.,2001] involved in many different cellular processes. Here we show that, in line with this animal work, the slow corticosteroid effects result in inhibition of human hippocampal processing. Most imaging studies on corticosteroid effects have found similar MTL down regulation by corticosteroid administration [de Quervain et al.,2003; Oei et al.,2007; van Stegeren et al.,2010], but lack time‐specificity of these corticosteroid effects. However, one very recent study [Lovallo et al.,2010] reports on rapidly decreased hippocampal and amygdala activity due to the immediate (thus presumably nongenomic) effects of corticosteroids, using i.v. administration of hydrocortisone immediately followed by fMRI scanning. The apparent discrepancy with our own findings (i.e., no effects at 30–75 min posthydrocortisone intake, but a decrease at 180–195 min postintake) could possibly be explained by differences in experimental setup; whereas Lovallo et al. [2010] investigated the effects of cortisol on resting BOLD signal in the brain (i.e., assessing a tonic state), we asked participants to memorize 160 complex pictures, and measured the brain responses to these stimuli (i.e., assessing phasic responses). Tonic and phasic brain responses may be altered differentially by corticosteroids, as is seen for other stress hormones [Valentino and Van, Bockstaele2008; Vijayraghavan et al.,2007], and might depend on the participants behavioral state [Makara and Haller,2001; Roozendaal,2002]. Moreover, our results add to these findings in showing that corticosteroids' influence on hippocampal activity remains discernable even when they are out of circulation.
Although previous animal studies indicated that corticosteroids' rapid actions enhance hippocampal excitability [Karst et al.,2005] and LTP [Wiegert et al.,2006], we did not observe any rapid corticosteroid effects on hippocampal processing. One possible explanation for this null finding is that corticosteroids' rapid effects manifest themselves by interacting with concurrent noradrenergic activation [Roozendaal et al.,2006]. Although corticosteroids' rapid effects were capable of increasing LTP after mild tetanization in the CA1 region of the hippocampus [Wiegert et al.,2006], concurrent noradrenergic stimulation was necessary to establish this effect in hippocampus' dentate gyrus [Pu et al.,2007]. For their augmenting effects on memory consolidation, corticosteroids also critically depend on noradrenergic activation [Roozendaal et al.,2006]. We tried to induce this activation by showing highly aversive pictures, but this effect might have been too subtle compared with a truly stressful event. Nevertheless, we show that corticosteroids' rapid, putatively nongenomic actions by themselves are not sufficient to amplify human hippocampal processing.
Besides affecting hippocampal processing, the slow effects of corticosteroids clearly down regulated activity of the prefrontal cortex (PFC) in a time‐specific manner. Time‐specific effects on rodent prefrontal cortex function have been reported before for stress [Jackson and Moghaddam,2006]; with acute stress producing immediate inhibition of PFC functioning, followed by subsequent recovery. Our findings in humans suggest that corticosteroids' rapid effects on their own are not able to induce such inhibition. Instead, other stress‐related neuromodulators, such as norepinephrine and dopamine [Arnsten,2009], might cause this stress‐induced impairment of PFC function, and their effects might potentially be amplified by corticosteroids' rapid actions [van Stegeren et al.,2010]. Corticosteroids slow, putatively genomic effects did down regulate the PFC. This novel finding is in line with previous studies on chronic stress, in which continuous (genomic) corticosteroid actions can be inferred, inducing both structural abnormalities [Liston et al.,2006] and functional disruption in the prefrontal cortex [Liston et al.,2006,2009].
The PFC has traditionally been associated with cognitive control processes, but its role in memory and interaction with the MTL is just as crucial [Fernandez and Tendolkar,2001]. The PFC and MTL contribute in different ways to the process of memory encoding, and their interaction is vital for successful memory in order to provide discrete and elaborated representations that fit long‐term storage [Fernandez and Tendolkar,2001]. Specifically, the region affected by corticosteroids in this study comprises parts of BA9, 45, 46, and 48 (Table I). Whereas the exact function of BA48—the retrosubicular area, located on the medial surface of the temporal lobe—remains unclear, all other regions have been implicated in memory processing. BA9 and 46 roughly correspond with the dorsolateral prefrontal cortex (DLPFC), which has traditionally been associated with its role in sustaining attention and working memory processing (WM). More recently, DLPFC has also been shown to promote long‐term memory formation through its role in WM organization [Blumenfeld and Ranganath,2006], memory maintenance [Leung et al.,2002], and associative memory processing [Murray and Ranganath,2007]. BA45 on the other hand has been typically associated with verbal processing, and has been implicated especially in intentional encoding paradigms [Braver et al.,2001] in which verbal elaboration has been shown to be an effective encoding strategy, predicting individual differences in memory performance [Kirchhoff and Buckner,2006]. Also in this study we find greater activity in the inferior‐middle frontal gyrus (BA9, 45, and 48) during the processing of items that are subsequently remembered compared with those later forgotten, implicating this region in memory formation (Supporting Information, Table S2). Therefore, the observed down regulation of both the hippocampus and the PFC indicates reduced processing due to the slow effects of corticosteroids.
Although such suppression of memory related areas by the slow effects of corticosteroids does not seem to be beneficial at first sight since it could be related to impaired memory for events following a stressful experience, one could speculate that it might actually aid memory for the stressful experience by reducing retroactive interference into the initial memory trace. Retroactive interference is assumed to be a major cause of forgetting. Forgetting can be induced by any subsequent task [Dewar et al.,2007], and has been shown to be reduced by preventing new learning [Sangha et al.,2005]. Therefore, the suppression of memory related areas might actually protect against the forgetting of the stressful event by reducing retroactive interference.
Some limitations should be considered. First of all, we cannot claim that the peak cortisol levels in the rapid and slow CORT condition are the same. Figure 1 shows the salivary cortisol curves with cortisol levels peaking either during (rapid CORT condition) or 120 min before the scanning session. However, peak salivary cortisol levels in the rapid CORT condition seem lower than those induced in the slow CORT condition, although the dose of hydrocortisone administration was exactly the same. Possibly, cortisol‐binding globulin (CBG) levels were higher in the rapid than in the slow CORT condition. Approximately 95% of total cortisol is bound to carrier proteins, of which 80–90% to CBG [Lewis et al.,2005]. The measured levels in saliva represent the remaining cortisol that is unbound and free to diffuse across cell membranes and bind to intracellular glucocorticoid and mineralocorticoid receptors, and is thus highly dependent on the level of carrier proteins present. Reports on the circadian variations in CBG level are somewhat conflicting [Droste et al.,2009; Hsu and Kuhn,1988; Lewis et al.,2006]. Given that CBG binding affinity is temperature dependent [Henley and Lightman,2011], one would actually expect lower binding in the later afternoon (in the rapid CORT condition), i.e. the opposite of what we observed. Alternatively, free cortisol levels might have been influenced by circadian variations in 11β‐steroid dehydrogenase 1 efficacy [Veniant et al.,2009], which could indeed lead to lower peak levels. However, the most likely explanation for the difference in peak levels is that we might have missed the peak in salivary cortisol levels in the rapid CORT condition that is supposedly occurring 1 h postadministration (as seen for the slow CORT condition). Practical reasons restrained us from taking a saliva sample at that exact same time point, which is half way the encoding session, when subjects are in the scanner; chewing on the cotton swap might induce head movement and require new realignment for the second half of the session (and thereby require more time) and disturb the encoding process. This is supported by the fact that when the saliva samples taken in both drug conditions were time‐locked to the time of drug intake, they seemed to be comparable. IV injection of hydrocortisone combined with regular blood sampling might have resolved this issue and also have increased the time‐specificity of corticosteroid exposure to the brain. However, injections in general are known to induce stress in participants; a factor we would like to circumvent since we were specifically interested in the effects of corticosteroids.
Second, although we clearly found time‐dependent effects of corticosteroid application on neural responses in brain regions associated with memory encoding, we did not find a modulation of the subsequent memory effect in these regions (i.e., the difference in brain activation during the processing of subsequently remembered and forgotten items), nor a main effect of corticosteroids on memory performance. Significant effects on memory performance have been reported previously [Abercrombie et al.,2003; Buchanan and Lovallo,2001; Kuhlmann and Wolf,2006; Maheu et al.,2004; van Stegeren et al.,2010] and were also targeted in this study. One could speculate about the reason why we did not observe any of these effects. Most likely, specific properties of the study design have contributed. First, the intentional learning instruction might have led to an elaborate processing strategy for all items overriding or reducing some basal differential neuromodulatory effects that could have affected the difference between later remembered and later forgotten items [Kensinger et al.,2005; Talmi et al.,2008]. This might explain the absence of a corticosteroid (main) effect on memory performance that has been observed previously in incidental encoding paradigms [Abercrombie et al.,2003; Buchanan and Lovallo,2001; Kuhlmann and Wolf,2006; Maheu et al.,2004; van Stegeren et al.,2010]. Moreover, despite the counter balancing, the repeated testing could result in session order effects interacting with those of corticosteroids, as was seen in a recent study [Wirth et al., in press] However, a crossover design with repeated testing requires an intentional instruction as the participants would expect a memory test after the initial session. The only alternative design to circumvent this intentional encoding instruction would have been the use of a between‐subjects design, but this has other disadvantages, such as decreased power by introducing between‐subject variance. Moreover, incidental encoding would most likely have resulted in decreased memory performance because intentional encoding ensures deeper encoding by e.g. conscious semantic encoding strategies [Braver et al.,2001; Kirchhoff and Buckner,2006], increased motivation or elevated attention to the exact details of the encoded information. For fMRI analysis proper performance was necessary since a sufficient number of remembered neutral and aversive pictures were required. Using a recognition memory paradigm could have been an alternative approach, but recall measures provide a cleaner measure of episodic memory retrieval than recognition memory, which can be confounded by familiarity judgments, and seem to be more sensitive to corticosteroid modulation [Buchanan and Lovallo,2001]. A second explanation might be a lack of power of our neuroimaging study in comparison with behavioral studies, which have tested larger groups of subjects [Abercrombie et al.,2003; Buchanan and Lovallo,2001; Kuhlmann and Wolf,2006; Maheu et al.,2004]. Since brain activity is a more sensitive measure than behavioral output, which is the consequence of many parallel neural operations, regional differences in brain activity are more easily detected with smaller samples. However, these samples offer little power to observe behavioral effects. A third explanation for the absence of a behavioral effect might be corticosteroids' dependence on noradrenergic activation, which naturally joins corticosteroid release during exposure to stress. Since corticosteroids' rapid effects on hippocampal activity might depend on noradrenergic activation [Pu et al.,2007], the same might be true to corticosteroids' facilitating effects on memory formation under conditions of stress [Abercrombie et al.,2006]. Moreover, previous animal work has shown that corticosteroids critically depend on noradrenergic activation for their augmenting effects on memory consolidation as well [Roozendaal et al.,2006]. Therefore, corticosteroids' delayed genomic effects might also depend on noradrenergic activation in preserving (by reducing retroactive interference) what was earlier encoded under stressful conditions. The fact that also corticosteroid's slow effects are modulated by noradrenergic activation is supported by a recent study that shows that gene binding of the GR is targeted to preexisting foci of accessible chromatin [John et al.,2011]. Because of this dependence of GR‐binding on preexisting chromatin architecture, stress or arousal induced alterations in chromatin structure might modulate these effects. Previous research in rodents has indicated that stressful challenges (e.g. forced swimming [Bilang‐Bleuel et al.,2005], novelty [Chandramohan et al.,2007], and fear conditioning [Chwang et al.,2006; Gupta et al.,2010] evoke such post‐translational changes in dentate gyrus neurons. The rapid effects of corticosteroids were also shown to play a role in establishing the observed epigenetic modifications (histone modifications and DNA (de‐)methylation) and conformational changes in the chromatin. These effects were mediated by GRs interacting with the NMDA‐receptor activated ERK MAPK pathway in a rapid, non‐genomic fashion [Trollope et al., in press]. This suggests that corticosteroids' slow genomic effects might be modulated by earlier rapidly induced changes by corticosteroid signaling and concurrent noradrenergic activation. Thus, both the rapid and slow effects of corticosteroids by themselves may not be sufficient to result in clear mnemonic effects. Although we tried to induce sufficient noradrenergic activation by showing highly aversive pictures to the participants, this effect might have been too subtle to generate the necessary interactions with corticosteroids.
A final limitation to this study is that it investigated men only, thus the obtained results cannot be readily generalized to women. Hydrocortisone administration has been shown to result in differential effects between women and men, both in behavior [Andreano and Cahill,2006; Bohnke et al.,2010] and brain activation [Merz et al.,2010; Stark et al.,2006]. Moreover, the hippocampus of women displays a more distinct affinity for corticosteroids than that of men [Madeira and Lieberman,1995], which might contribute to different effects on exposure to corticosteroids during memory formation. Although important, sex differences were beyond the scope of this initial study, which is why we opted to recruit male subjects only, allowing easier comparison with an earlier study in stressed individuals [Henckens et al.,2009].
In conclusion, this study is first in showing that corticosteroids affect neural processing in brain regions involved in human memory formation in a time‐dependent manner. Specifically, corticosteroid's slow, putatively genomic effects reduced activity in hippocampus and prefrontal cortex, whereas no changes were observed due to corticosteroid's rapid actions. Down regulation of these memory‐related brain regions might minimize subsequent interference into the initial memory trace by poststress experiences, and therefore aid consolidation of the stressful event most optimally. Thus, we provide an initial mechanistic account of how corticosteroids affect memory in humans.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
REFERENCES
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ ( 2003): Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav Neurosci 117: 505–516. [DOI] [PubMed] [Google Scholar]
- Abercrombie HC, Speck NS, Monticelli RM ( 2006): Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology 31: 187–196. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L ( 2006): Glucocorticoid release and memory consolidation in men and women. Psychol Sci 17: 466–470. [DOI] [PubMed] [Google Scholar]
- Arnsten AF ( 2009): Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2000): Voxel‐based morphometry—The methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer R, Brown GK ( 2002): Beck Depression Inventory‐II‐NL. Handleiding. De Nederlandse versie van de Beck Depression Inventory. Lisse: Swets Test Publishers. [Google Scholar]
- Bilang‐Bleuel A, Ulbricht S, Chandramohan Y, De Carli S, Droste SK, Reul JM ( 2005): Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: Involvement in a glucocorticoid receptor‐dependent behavioural response. Eur J Neurosci 22: 1691–1700. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C ( 2006): Dorsolateral prefrontal cortex promotes long‐term memory formation through its role in working memory organization. J Neurosci 26: 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnke R, Bertsch K, Kruk MR, Richter S, Naumann E ( 2010): Exogenous cortisol enhances aggressive behavior in females, but not in males. Psychoneuroendocrinology 35: 1034–1044. [DOI] [PubMed] [Google Scholar]
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ ( 2009): Adolescents' cortisol responses to awakening and social stress: Effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology 34: 884–893. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ ( 1994): Measuring emotion: The self‐assessment Manikin and the semantic differential. J Behav Ther Exp Psychiatry 25: 49–59. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE ( 2001): Direct comparison of prefrontal cortex regions engaged by working and long‐term memory tasks. Neuroimage 14: 48–59. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD ( 1998): Making memories: Brain activity that predicts how well visual experience will be remembered. Science 281: 1185–1187. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR ( 2001): Enhanced memory for emotional material following stress‐level cortisol treatment in humans. Psychoneuroendocrinology 26: 307–317. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Reul JM ( 2007): Novelty stress induces phospho‐acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N‐methyl‐d‐aspartate receptor and the glucocorticoid receptor: relevance for c‐fos induction. J Neurochem 101: 815–828. [DOI] [PubMed] [Google Scholar]
- Chwang WB, O'Riordan KJ, Levenson JM, Sweatt JD ( 2006): ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem 13: 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT Jr, McCrae RR ( 1992). Revised NEO Personality Inventory (NEO‐PI‐R) and the Five Factor Inventory (NEO‐FFI): Professional Manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E ( 2001): Identification of corticosteroid‐responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci 14: 675–689. [DOI] [PubMed] [Google Scholar]
- de Kloet ER ( 1991): Brain corticosteroid receptor balance and homeostatic control. Front Neuroendocrinol 12: 95–164. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Karst H, Joels M (2008): Corticosteroid hormones in the central stress response: quick‐and‐slow. Front Neuroendocrinol 29: 268–272. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C ( 2003): Glucocorticoid‐induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci 17: 1296–1302. [DOI] [PubMed] [Google Scholar]
- Dewar MT, Cowan N, Sala SD ( 2007): Forgetting due to retroactive interference: A fusion of Muller and Pilzecker's (1900) early insights into everyday forgetting and recent research on anterograde amnesia. Cortex 43: 616–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DD, Campbell AM, Park CR, Halonen J, Zoladz PR ( 2007): The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress‐induced amnesia, flashbulb and traumatic memories, and the Yerkes‐Dodson law. Neural plast 2007: 608033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R ( 2004): Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron 42: 855–863. [DOI] [PubMed] [Google Scholar]
- Droste SK, Collins A, Lightman SL, Linthorst AC, Reul JM ( 2009): Distinct, time‐dependent effects of voluntary exercise on circadian and ultradian rhythms and stress responses of free corticosterone in the rat hippocampus. Endocrinology 150: 4170–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC ( 2008): Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology 149: 3244–3253. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Tendolkar I ( 2001): Integrated brain activity in medial temporal and prefrontal areas predicts subsequent memory performance: Human declarative memory formation at the system level. Brain Res Bull 55: 1–9. [DOI] [PubMed] [Google Scholar]
- Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD ( 2010): Histone methylation regulates memory formation. J Neurosci 30: 3589–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE ( 2004): Nonstationary cluster‐size inference with random field and permutation methods. Neuroimage 22: 676–687. [DOI] [PubMed] [Google Scholar]
- Henckens MJ, Hermans EJ, Pu Z, Joels M, Fernandez G ( 2009): Stressed memories: How acute stress affects memory formation in humans. J Neurosci 29: 10111–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley DE, Lightman SL ( 2011): New insights into corticosteroid‐binding globulin and glucocorticoid delivery. Neuroscience 180: 1–8. [DOI] [PubMed] [Google Scholar]
- Hsu BR, Kuhn RW ( 1988): The role of the adrenal in generating the diurnal variation in circulating levels of corticosteroid‐binding globulin in the rat. Endocrinology 122: 421–426. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B ( 2006): Distinct patterns of plasticity in prefrontal cortex neurons that encode slow and fast responses to stress. Eur J Neurosci 24: 1702–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, de Kloet ER ( 1989): Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science 245: 1502–1505. [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER ( 1992): Control of neuronal excitability by corticosteroid hormones. Trends Neurosci 15: 25–30. [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER ( 1994): Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol 43: 1–36. [DOI] [PubMed] [Google Scholar]
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ ( 2006): Learning under stress: How does it work? Trends Cogn Sci 10: 152–158. [DOI] [PubMed] [Google Scholar]
- Joels M, Velzing E, Nair S, Verkuyl JM, Karst H ( 2003): Acute stress increases calcium current amplitude in rat hippocampus: Temporal changes in physiology and gene expression. Eur J Neurosci 18: 1315–1324. [DOI] [PubMed] [Google Scholar]
- John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA ( 2011): Chromatin accessibility pre‐determines glucocorticoid receptor binding patterns. Nat Genet 43: 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI ( 2006): The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31: 151–178. [DOI] [PubMed] [Google Scholar]
- Karssen AM, Meijer OC, van der Sandt IC, Lucassen PJ, de Lange EC, de Boer AG, de Kloet ER ( 2001): Multidrug resistance P‐glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology 142: 2686–2694. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M ( 2005): Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102: 19204–19207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Piguet O, Krendl AC, Corkin S ( 2005): Memory for contextual details: Effects of emotion and aging. Psychol Aging 20: 241–250. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL ( 2006): Functional‐anatomic correlates of individual differences in memory. Neuron 51: 263–274. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH ( 1994): Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 19: 313–333. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH ( 1999): Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus‐pituitary‐adrenal axis. Psychosom Med 61: 154–162. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI ( 2009): Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci 12: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann S, Wolf OT ( 2006): Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behav Neurosci 120: 217–223. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN ( 1999): International Affective Picture System (IAPS): Technical manual and affective ratings. Gainesville, FL: University of Florida. [Google Scholar]
- Leung HC, Gore JC, Goldman‐Rakic PS ( 2002): Sustained mnemonic response in the human middle frontal gyrus during on‐line storage of spatial memoranda. J Cogn Neurosci 14: 659–671. [DOI] [PubMed] [Google Scholar]
- Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ ( 2005): Plasma free cortisol fraction reflects levels of functioning corticosteroid‐binding globulin. Clin Chim Acta 359: 189–194. [DOI] [PubMed] [Google Scholar]
- Lewis JG, Mopert B, Shand BI, Doogue MP, Soule SG, Frampton CM, Elder PA ( 2006): Plasma variation of corticosteroid‐binding globulin and sex hormone‐binding globulin. Horm Metab Res 38: 241–245. [DOI] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ ( 2009): Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA 106: 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS ( 2006): Stress‐induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set‐shifting. J Neurosci 26: 7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Robinson JL, Glahn DC, Fox PT ( 2010): Acute effects of hydrocortisone on the human brain: An fMRI study. Psychoneuroendocrinology 35: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MD, Lieberman AR ( 1995): Sexual dimorphism in the mammalian limbic system. Prog Neurobiol 45: 275–333. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Collicutt P, Kornik R, Moszkowski R, Lupien SJ ( 2005): The perfect time to be stressed: A differential modulation of human memory by stress applied in the morning or in the afternoon. Prog Neuropsychopharmacol Biol Psychiatry 29: 1281–1288. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Joober R, Beaulieu S, Lupien SJ ( 2004): Differential effects of adrenergic and corticosteroid hormonal systems on human short‐ and long‐term declarative memory for emotionally arousing material. Behav Neurosci 118: 420–428. [DOI] [PubMed] [Google Scholar]
- Makara GB, Haller J ( 2001): Non‐genomic effects of glucocorticoids in the neural system. Evidence, mechanisms and implications. Prog Neurobiol 65: 367–390. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Morris RG ( 2002): New life in an old idea: The synaptic plasticity and memory hypothesis revisited. Hippocampus 12: 609–636. [DOI] [PubMed] [Google Scholar]
- McEwen BS ( 1994): Corticosteroids and hippocampal plasticity. Ann NY Acad Sci 746: 134–142; discussion 142–144, 178–179. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT ( 2010): Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 35: 33–46. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd , Wang S, Mason J, Southwick SM, Fox P, Hazlett G, Charney DS, Greenfield G ( 2000): Hormone profiles in humans experiencing military survival training. Biol Psychiatry 47: 891–901. [DOI] [PubMed] [Google Scholar]
- Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joels M, De Kloet ER, Datson NA ( 2006): Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol 18: 239–252. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C ( 2007): The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci 27: 5515–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei NY, Elzinga BM, Wolf OT, De Ruiter MB, Damoiseaux JS, Kuijer JPA, Veltman DJ, Scheltens P, Rombouts SA ( 2007): Glucocorticoids decrease hippocampal and prefrontal activation during declarative memory retrieval in young men. Brain Imaging Behav 1: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Backstrom T, Fernandez G ( 2010): Neural mechanisms underlying changes in stress‐sensitivity across the menstrual cycle. Psychoneuroendocrinology 35: 47–55. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Kimura A, Magarinos AM, McEwen BS ( 1995): Hippocampal homosynaptic long‐term depression/depotentiation induced by adrenal steroids. Neuroscience 68: 379–385. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I ( 2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Pu Z, Krugers HJ, Joels M ( 2007): Corticosterone time‐dependently modulates beta‐adrenergic effects on long‐term potentiation in the hippocampal dentate gyrus. Learn Mem 14: 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B ( 2002): Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem 78: 578–595. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL ( 2006): Glucocorticoids interact with emotion‐induced noradrenergic activation in influencing different memory functions. Neuroscience 138: 901–910. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo‐Nava MT ( 2007): Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast 2007: 78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Scheibenstock A, Martens K, Varshney N, Cooke R, Lukowiak K ( 2005): Impairing forgetting by preventing new learning and memory. Behav Neurosci 119: 787–796. [DOI] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Schienle A, Vaitl D ( 2006): Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. Neuroimage 32: 1290–1298. [DOI] [PubMed] [Google Scholar]
- Talmi D, Anderson AK, Riggs L, Caplan JB, Moscovitch M ( 2008): Immediate memory consequences of the effect of emotion on attention to pictures. Learn Mem 15: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollope AF, Gutierrez‐Mecinas M, Mifsud KR, Collins A, Saunderson EA, Reul JM: Stress, epigenetic control of gene expression and memory formation. Exp Neurol [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Tunn S, Mollmann H, Barth J, Derendorf H, Krieg M ( 1992): Simultaneous measurement of cortisol in serum and saliva after different forms of cortisol administration. Clin Chem 38: 1491–1494. [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E ( 2008): Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 583: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joels M ( 2010): Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem 93: 56–65. [DOI] [PubMed] [Google Scholar]
- Veniant MM, Hale C, Komorowski R, Chen MM, St Jean DJ, Fotsch C, Wang M ( 2009): Time of the day for 11beta‐HSD1 inhibition plays a role in improving glucose homeostasis in DIO mice. Diabetes Obes Metab 11: 109–117. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF ( 2007): Inverted‐U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10: 376–384. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL ( 1998): Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science 281: 1188–1191. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A ( 1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wiegert O, Joels M, Krugers H ( 2006): Timing is essential for rapid effects of corticosterone on synaptic potentiation in the mouse hippocampus. Learn Mem 13: 110–113. [DOI] [PubMed] [Google Scholar]
- Wiegert O, Pu Z, Shor S, Joels M, Krugers H ( 2005): Glucocorticoid receptor activation selectively hampers N‐methyl‐d‐aspartate receptor dependent hippocampal synaptic plasticity in vitro. Neuroscience 135: 403–411. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Scherer SM, Hoks RM, Abercrombie HC: The effect of cortisol on emotional responses depends on order of cortisol and placebo administration in a within‐subject design. Psychoneuroendocrinology [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


