Abstract
The familiarity to the subject of any potential stimuli presents one of the major difficulties for the investigation of the self; the separation of effects resulting from familiarity from self‐effects being extremely problematic. The aim of this study was thus to investigate the neural distinction between self and familiarity by combining two sets of fMRI data with a meta‐analysis. In the first fMRI experiment, regions responding to self/familiarity were investigated using the subject's own name and names of familiar others. These effects were confirmed and extended in a second fMRI experiment using the subject's own name and a stranger's name, as spoken by familiar and unfamiliar voices. Finally, a meta‐analysis of self‐ and familiarity‐related studies was conducted. Neural activity in the anterior brain regions, such as the anterior cingulate (ACC) and anterior insula (AI), was found to be specific for self‐specific stimuli. In contrast, posterior brain regions, such as the posterior cingulate, were activated by familiar stimuli. Finally, the distinction between anterior and posterior regions for self and familiarity was confirmed by meta‐analytic data. This study demonstrates a clear anterior–posterior cortical partition between self‐specificity and familiarity. Hum Brain Mapp, 2012. © 2010 Wiley Periodicals, Inc.
Keywords: self, familiarity, anterior cingulate, posterior cingulate, insula
INTRODUCTION
The neural basis of the self has been one of the most prominent problems in neuroscience [Gillihan and Farah,2005; Legrand and Ruby,2009; Northoff and Bermpohl,2004; Northoff et al.,2006]. Recent brain imaging studies have indicated that anterior and posterior cortical midline regions, such as the anterior cingulate cortex (ACC), medial prefrontal cortex (MPFC), and the posterior cingulate cortex (PCC), may display specific neural activity changes during the perception of self‐specific stimuli [Feinberg,2009; Feinberg et al.,2010; Kelley et al.,2002; Mitchell et al.,2005; Northoff and Bermpohl,2004; Northoff et al.,2006; Platek et al.,2006; Uddin et al.,2007; Yaoi et al.,2009; Zhu et al.,2007]. In this context, self‐specificity is an operational term that describes a specific experimental variable, a particular kind of stimuli used in experimental designs testing for the self. In addition, other anterior regions, including the anterior insula, have also been associated with the processing of self‐specific stimuli [Enzi et al.,2009; Modinos et al.,2009].
However, recent reviews have raised questions as to whether neural activity in these regions is really self‐specific [Gillihan and Farah,2005; Legrand and Ruby,2009]. Confounding factors, such as the degree of familiarity of the stimuli, or the composition of the task itself—for example, the cognitive processes involved in the evaluation of the stimuli (i.e., trait judgment task), separate from any self‐specific cognitive component—may by themselves lead to neural activity changes in the midline regions.
Although a recent study [Seger et al.,2004] has demonstrated a strong regional overlap between self‐specific and familiar stimuli in both the anterior and posterior midline regions, other studies have indicated a predominantly anterior cortical involvement, for instance in the ACC, during the processing of self‐specific stimuli [D'Argembeau et al.,2005,2007; Feinberg,2009; Feinberg et al.,2010; Gusnard and Raichle,2001; Han et al.,2009; Modinos et al.,2009; Ochsner et al.,2005; Zhu et al.,2007]. In contrast, studies of personal familiarity have generally indicated the involvement of posterior regions like the PCC in the processing of stimuli of this type [Nakamura et al.,2001; Shah et al.,2001; Sugiura et al.,2006; von Kriegstein et al.,2005]. As described above, personal familiarity is always a major confounding factor in studies of the self, from behavioral studies [Bower and Gilligan,1979] to brain imaging studies [Gillihan and Farah,2005; Seger et al.,2004; Sugiura et al.,2005]. Personal familiarity may be distinguished from familiarity more generally (such as the familiarity of a public figure whom one has, however, never met in person) through the involvement in it of autobiographical memory and self‐related emotional responses, as opposed to the recruitment of semantic memory in the case of public figures [Gobbini et al.,2004; Leibenluft et al.,2004; Sugiura et al.,2006,2008; Zhu et al.,2007]. Unfortunately, the neural distinction between self‐specificity and familiarity remains unclear, and no studies have yet investigated the relationship between self‐specific and personally familiar stimuli whereas at the same time controlling for possible confounding effects from the composition of the experimental task involved. Without such a distinction, the potential of studies into the neural underpinnings of the self must necessarily be limited.
The overall aim of our study was to investigate the relationship between self‐specific and personal familiar stimuli to clarify the regional specificity, if any, involved in the processing of each stimuli type. To do this, we first investigated those regions involved in the processing of self‐specific stimuli, hypothesizing, based on the studies described above, that the anterior cortical regions would be recruited under this condition. At the same time, possible familiarity‐specific regions were investigated, with the posterior cortical midline structures being hypothesized to be involved in the processing of stimuli of this type. According to the neural difference between personal familiar people and famous people [Gillihan and Farah,2005], and personal familiarity may involve more autographical memory and emotion response of the own person, that is, self whereas the familiarity of famous or public people may rather recruit semantic memory [Northoff et al.,2006], the stimuli about the personal familiar people were adopted.
The investigation of the processing of self‐specific and familiar stimuli was undertaken through two separate fMRI experiments and a meta‐analysis of imaging studies of self‐specificity and familiarity. The first fMRI experiment compared the neural response to the aural perception of the subject's own name with the response to hearing the names of people with whom the subject was familiar—such as a friend—and the names of people with whom they had no familiarity at all. In this experiment the names—self, familiar, and unknown—were presented using an event‐related study design. The second fMRI experiment then aimed to further typify the interplay between, and regional specificity of, self‐specificity and familiarity by investigating the neural response to the subject's own name when spoken by either an individual with whom they were familiar or by a stranger. Finally, to validate our results, and to go some way to ameliorating the issue of task‐effects on the results in this particular context, as described above, we carried out a meta‐analysis of self and familiarity and compared the results from this with those obtained in the fMRI experiments.
MATERIALS AND METHODS
Subjects
Seventeen healthy volunteers participated in the study (10 female; age range 20–27 years; 16 right handed). None had a history of neurological or psychiatric disorders or were taking medication. All the participants completed both the event‐related experiment and the block‐design experiments, as described below. The study was approved by the local Ethics Committees of the Institute of Psychology at Chinese Academy of Sciences.
Stimuli
Five auditory stimuli were used: the subjects own name (SON) spoken by a familiar voice (SON‐FV); SON spoken by an unknown voice (SON‐UV); a friend's name (Chinese) spoken by an unknown voice (FN‐UV); an unknown person's name (Chinese) spoken by an unknown voice (UN‐UV); and an English name spoken by an unknown voice (EN‐UV). The FN‐UV stimuli were one name of selected classmates of the subjects, of the same gender. The familiar voice used was specific to each participant, being a single classmate with whom they were friends, and was the same for each of the two experiments for each subject. Unknown voice stimuli were always said by the same person. Note also that subjects would have been in close contact with their classmates for a minimum of 2 years. The unknown names were disyllabic names in common usage in China. These were selected from a list of 200 names (100 for male and 100 for female) based on 100 undergraduate students' familiarity evaluation (5 = extremely common, 4 = very common, 3 = common, 2 = not common, 1 = very rare, those scored 4–5 were used). All stimuli were presented at 80 dB with a mean duration of 741 ± 84 ms (S.E.). During scanning, all participants confirmed that they could hear the names clearly.
Experimental Design
Experiment 1 (event‐related design): This experiment was used to determine those regions that were specific to the self, and not to familiarity. Sixty each of SON‐UV, UN‐UV, FN‐UV stimuli, and 24 EN‐UV stimuli, were presented in four runs, with stimulus onsets randomized between 4 and 12 s. Stimuli were presented in a pseudo‐random order. To ensure that subjects were attending to the stimuli during the experiment, subjects were asked to respond to EN‐UV stimuli by pressing one key using right thumb. The participants listened passively to all other name (with eyes open) without giving any response in order to eliminate the effect from tasks.
Experiment 2 (block design): This experiment was used to verify the result of the first experiment in different domain for familiarity. The subject was presented with either their own name spoken by a familiar voice (SON‐FV), their own name spoken by an unfamiliar voice (SON‐UV), or an unfamiliar name spoken by an unknown voice (UN‐UV). A condition in which the subject was presented with their own name spoken by themselves was not used, as people usually perceive their recorded voice as different from that which they hear when they hear themselves speak. Five blocks of each of the three stimuli were presented (15 blocks in total). Each 12 s block consisted of six stimulus presentations, with an inter‐trial interval of 16 s. During the experiment, participants listened passively to the stimuli.
MRI Scanning
MR images were acquired on a GE 3.0T Sigma Excite scanner. Functional images were acquired using a T2*‐weighted echo‐planar imaging (EPI) sequence (TR/TE/θ = 2,000 ms/30 ms/90°, FOV = 240 × 240 mm, matrix = 64 × 64, slice‐thickness = 4 mm, gap = 0 mm). Each volume had 31 axial slices, covering the whole brain. Following the functional scans, a fast SPoiled GRass (SPGR) sequence was used to obtain the 3D whole brain images (TR = 8.5 ms, TE = 3.4 ms, flip angle = 12°, FOV = 280 × 280 mm, Matrix = 256 × 256, slice‐thickness = 1 mm) for functional image registration and localization.
Data Analysis
Functional images were processed using the AFNI software package [Cox, 1996]. The data from the two experiments underwent a pre‐processing procedure that included two‐ and three‐dimensional head‐motion corrections, masking for the removal of the skull, and spatial smoothing using a kernel of 4 mm full‐width at half‐maximum. Scan data from Experiment 1 and 2 were converted to Talairach space and resampled to 1 mm isotropic voxels.
Event‐related design experiment: Following the above pre‐processing procedures, the data were submitted to deconvolution analysis to obtain a map of the estimated coefficients for the three stimuli (SON‐UV, UN‐UV, and FN‐UV). Group analyses were performed to produce the following three contrasts using paired t tests: SON‐UV versus UN‐UV, SON‐UV versus FN‐UV, and FN‐UV versus UN‐UV. To protect against Type I‐error, a P < 0.005 and a cluster volume exceeding 280 mm3 were considered to show a positive response (α level of 0.01). These thresholds were calculated with the AlphaSim program in AFNI with multiple testing, FWE corrections.
To identify those brain regions specifically responsive to self‐specific stimuli and at the same time to minimize the impact of non‐specific familiarity in SON‐UN versus FN‐UV, we identified those areas that overlapped between the two contrasts (SON‐UV vs. FN‐UV and SON‐UV vs. UN‐UV). A comparison of overlapping regions between the contrasts (FN‐UV vs. SON‐UV and FN‐UV vs. UN‐UV) was then carried out to identify those regions that responded specifically to familiarity as distinguished from both self‐ and non‐self‐specificity. Finally, to identify those regions that overlap between self‐specificity and familiarity as distinguished from non‐self‐specificity and non‐familiarity, a third comparison, between the contrasts (SON‐UV vs. UN‐UV and FN‐UV vs. UN‐UV) was carried out.
Block design experiment: The pre‐processed data were submitted to deconvolution analysis to obtain a map of the estimated coefficients for the SON‐FV, SON‐UV, and UN‐UV conditions. Because of severe head movement (>2 mm), data from two subjects were discarded. The brain regions identified in the overlap comparisons (self‐specificity, familiarity, and regions overlapping for both) for the first experiment were used as regions of interest (ROI) in the analysis of the block design experiment. These ROIs were applied to the data from the block design experiment, and the mean estimated coefficients in these regions for SON‐FV, SON‐UV, and UN‐UV were calculated. Comparisons between these mean coefficients were then carried out (SON‐FV vs. SON‐UV, SON‐FV vs. UN‐UV, and SON‐UV vs. UN‐UV) in all ROIs (paired t tests, two‐tailed) using SPSS version 13.0 (SPSS, Chicago, IL).
Meta‐Analysis
Literature search and coordinates selection
In addition to the two fMRI experiments described above, we undertook a meta‐analysis of the literature available on imaging studies of the self and familiarity. This allowed us to investigate the consistency between the results of the present study and those produced by previous studies, providing an independent validation of the ROIs used to undertake our second level analysis.
All the studies were selected from the search result in Pubmed from 1999 to August of 2009. Both the fMRI and PET results were included in the current meta‐analysis. For all the four conditions, the following inclusion criteria were applied:
-
1
Only data (brain activity coordinates) from the adult healthy subjects were included whereas those from neurological or psychiatric patients were excluded.
-
2
Only studies measuring brain activity in the whole brain were included whereas the studies based on regions of interest (ROI) analysis were excluded.
-
3
All the brain activity reporting significant activity changes in the single studies were included; for the meta‐analysis, only the coordinates of the peak voxel maxima were included whereas we did not consider the volume of the activated clusters.
-
4
The significant activity coordinates within the whole brain from each single study were included as distinguished from merely considering coordinates in predefined special brain regions, see for instance Northoff et al. [2006], who focused only on the midline regions.
-
5
The data about the brain activity revealed by task comparison [image subtraction method, parametric designs and brain imaging (fMRI, PET)‐other signal (behavior, ERP) correlations] were included. In contrast, data about functional connectivity were not considered.
-
6
The coordinates reported in the space of the MNI template or the atlas of Talairach and Tournoux were included.
See the following information for each condition.
Self Condition
We included 57 recent articles about self‐specific processing (see Supporting Information Table 4). We used a rather broad and unspecific definition of self‐related tasks describing all tasks where some material or content had to be related to the persons themselves, that is, their own selves. This broader definition was used rather than just “self‐specific” to obtain a wider range of potential studies, as well as because other terms, such as “self‐related,” have frequently been used in the literature when studying self‐specific stimuli. Compared with self‐specificity, the concept of self‐related processing does not describe an operational term and thus an experimental variable but rather a specific neuronal process assumed to be tapped in when applying self‐specific stimuli. We used the following keywords to find the studies for the self condition: “fMRI” or “PET” with “self,” “self‐related,” “self‐relevant,” “own name,” “own face,” “autobiographical,” “first person perspective,” and “agency” in the title or abstract of the studies. In addition, some studies from a previous corresponding meta‐analysis [Vanderwal et al.,2008] were included. The tasks used in these articles included trait adjective judgment, retrieval of personality traits, face recognition, body recognition, personal thinking, name perception, autobiographical memory, own feeling, self‐administered pain, person perspective tasks, and agency tasks (see Supporting Information Table 4 for more information). The following contrasts were used in the single studies: self versus personal familiarity, self versus control/baseline, self versus public people, first‐person perspective versus third‐person perspective, and self versus other (agency task). The coordinates of the brain regions involved in correlation between self evaluation and BOLD signal were also included. The coordinates that showed significantly stronger brain activity comparing the self condition with other conditions (even all the condition showed deactivation compared with the baseline) in the single studies were included.
Familiarity Condition
Intending to keep consistency with the above experiments, our familiarity condition included 23 recent articles that investigated the neural effects of personally familiar people, for example, participants' family, friends, classmates, and relatives (Supporting Information Table 5). We used the following keywords to find the studies for the familiarity condition: “fMRI” or “PET” with “familiarity,” “familiar name,” “familiar face,” and “familiar voice” in the title or abstract of the studies. The tasks adopted in the single studies on familiarity included face recognition, body recognition, voice recognition, trait adjective judgment, and name recognition. The following contrasts were used in the single studies: personal familiarity versus self, personal familiarity versus stranger/baseline, and personal familiarity versus public people. The coordinates that showed significantly stronger brain activity in personal familiarity condition when compared with the other condition in single studies were included. Finally, coordinates that showed common brain activity for self and familiarity [Wager et al.,2009] were included in the familiarity condition rather than the self condition as it was assumed that the common regions for self and familiarity represented the personal familiarity of the stimuli.
Meta‐Analysis Methods
We used Multilevel Kernel Density Analysis (MKDA) [Kober et al.,2008; Wager et al.,2009] to process our meta‐analysis, In MKDA, the coordinates are treated as the location of the activation, and the coordinates from one contrast in one study make up a particular statistical contrast map (SCM), there could be some different SCM from one study. The main aim of the MKDA is to reconstruct a map of significant regions for each statistical contrast map within study, and analyze the consistency and specificity across all the studies in the neighborhood of each voxel.
In the following, the detailed method used in the present study will be described. The coordinates (peak activation) in each single study were transferred in MKDA to a standard brain from the Montreal Neurologic Institute as distributed with SPM2 software (Wellcome Department of Imaging Neuroscience, London, UK). The coordinates from each study contrast were treated as an individual SCM. To then integrate the coordinates in space, the coordinates in each SCM were considered as one spherical kernel with a radius of 10 mm. This means that the voxels in a 10 mm radius around each contrast's coordinates were regarded as activated, the maximum value of these voxels being restricted to 1. This contributed to construct one indicator map for each SCM where the value 1 in the voxel represented a coordinate (reported in the single study) in the neighborhood. These indicator maps were weighted by the number of the subjects in the particular study and the kind of data analysis (random or fixed effects). The current version of MKDA weights each SCM by the square root of the number of the subjects, whereas the SCM from those studies using fixed effects analysis are down weighted by a factor of 0.75. We did not consider the z scores of the single studies because first they are not provided by all studies and second their inclusion has been shown to confound with the replicability of activation across studies, hence making interpretation more difficult [Wager et al.,2009].
The weighted average of the indicator maps was compared with the maximum proportion of activated comparison maps expected under the null hypothesis that there is no coherent spatial consistence across the SCMs. During this calculation, a random effects analysis was used. For the thresholding of the results, MKDA uses a threshold derived from Monte Carlo Simulation of the global null hypothesis. The contiguous activated clusters of each SCM were identified, and were selected at random within a gray matter mask (smoothed to include an 8 mm border, derived from segmentation of the avg152T1.img template using SPM2). In the present study, we used 5,000 Monte Carlo iterations (note that results should be stabilize after 2,000 iterations) [Enzi et al.,2009; Modinos et al.,2009]. The significance threshold was set at to P < 0.05, corrected for multiple comparisons (FWE‐correction), with a cluster extent threshold of 10 voxels.
Comparison of fMRI and Meta‐Analysis Results
To verify the consistency between our experiments and previous studies, and thus give preliminary evidence that the self and familiarity effects shown in the fMRI component of the study were not stimulus‐specific, we identified the overlap between the regions identified in the experiments and those identified in the meta‐analysis. We converted the image results of the meta‐analysis to AFNI format and then identified the overlapping regions between the self‐specific regions identified in the fMRI component (i.e., the overlap between SON‐UV vs. FN‐UV and SON‐UV vs. UN‐UV) and the self meta‐analysis; as well as the overlapping regions between the familiarity‐specific regions (i.e., the overlap between FN‐UV vs. SON‐UV and FN‐UV vs. UN‐UV) from our experiment and the familiarity meta‐analysis. This was done to ensure that the contrasts (i.e., overlaps) were the same in both event/block design studies and meta‐analysis.
RESULTS
Signal Changes Associated with Self‐Specific Stimuli
To identify those signal changes that are specifically related to self‐specific stimuli (i.e., the subject's own name), as distinguished from both familiar stimuli and general stimulus/task‐related effects, we identified the regions that overlapped between the two contrasts (SON‐UV vs. FN‐UV and SON‐UV vs. UN‐UV), from the data obtained in Experiment 1. This analysis yielded significant signal changes in the caudal ACC (cACC), anterior ACC (aACC), supplementary motor area (SMA), bilateral anterior insula, and right middle insula (Fig. 1A,B and Supporting Information Table 1). We then took these regions as regions of interest (ROI), and used these in our analysis of the data obtained in Experiment 2, extracting the mean signal change during each condition from each ROI.
Figure 1.
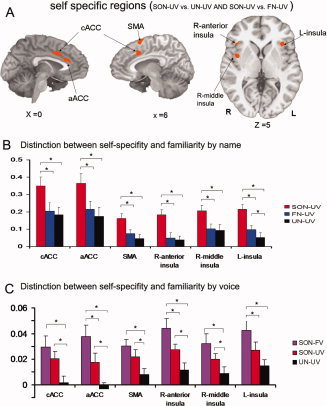
A: Brain activations for self‐specific stimuli from overlap of contrasts (SON‐UV vs. FN‐UV and SON‐UV vs. UN‐UV; P = 0.01, FWE corrected). B: Parameter estimates (Mean ± SE) for SON‐UV, FN‐UV, and UN‐UV in the cACC, SMA, aACC, and insula in the event‐related experiment (Experiment 1). C: Parameter estimates (Mean ± SE) for SON‐FV, SON‐UV, and UN‐UV in the same ROIs for the block‐design experiment (Experiment 2). *Denotes P < 0.05; cACC, caudal anterior cingulate cortex; aACC, anterior ACC; SMA, supplementary motor area. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The subject's own name (SON‐FV and SON‐UV) induced significantly stronger signal changes in the cACC, the SMA and the right middle insula, when compared to the signal changes induced by the hearing of an unknown name (UN‐UV). These same regions did not show any significant difference in mean signal change between the responses to the subject's own name when spoken by a familiar voice compared to the response to the subject's own name spoken by an unknown voice. In contrast, signal changes in the aACC and bilateral anterior insula did show significant differences between the response to the subject's own name, as spoken by familiar voice (SON‐FV), and the subject's own name as spoken by an unknown voice (SON‐UV) and both SON‐UV and SON‐FV show significant difference compared with unknown name (Fig. 1C).
Regions Specifically Implicated in Familiarity
To identify those regions that are specifically responsive to familiar stimuli (i.e., familiar name), as distinguished from both those regions that are responsive to self‐specific stimuli, and general stimulus/task‐related effects, we identified those regions that overlap between the two contrasts (FN‐UV vs. SON‐UV and FN‐UV vs. UN‐UV), from the data obtained in Experiment 1. This analysis identified significant signal changes in the bilateral PCC that are specific for familiarity, as distinguished from the response to self‐specific stimuli (Fig. 2A,B and Supporting Information Table 2).
Figure 2.
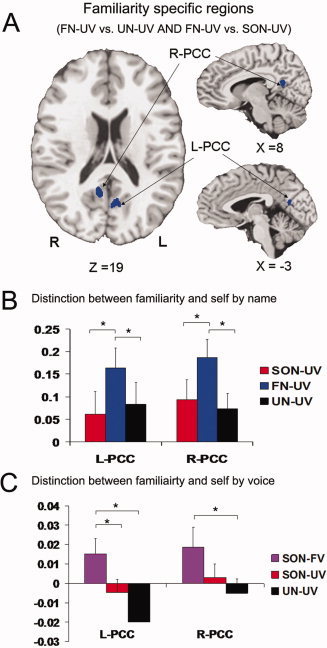
A: Brain activations for familiarity from overlap of contrasts (FN‐UV vs. SON‐UV and FN‐UV vs. UN‐UV; P = 0.01, FWE corrected). B: Parameter estimates (Mean ± SE) for SON‐UV, FN‐UV, and UN‐UV in the R‐PCC and L‐PCC in the event‐related experiment (Experiment 1). C: Parameter estimates (Mean ± SE) for SON‐FV, SON‐UV, and UN‐UV in the same three ROIs for the block‐design experiment (Experiment 2). *Denotes P < 0.05; R‐PCC, right posterior cingulate cortex; L‐PCC, left posterior cingulate cortex. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Applying the left and right PCC as regions of interest to the data from Experiment 2, signal changes in the left PCC during the own name called by a familiar voice (SON‐FV) condition were found to be significantly greater than those produced in this region during both the own name called by unknown voice (SON‐UV), and unknown name called by unknown voice (UN‐UV) conditions. In contrast, there was no difference between the signal changes in this region during the SON‐UV and UN‐UV conditions. Similar to the left PCC, signal changes in the right PCC did differ in SON‐FV when compared to UN‐UV, with no difference between SON‐FV and SON‐UV, and SON‐UV and UN‐UV (Fig. 2C).
Common Regions for Self‐Specific and Familiar Stimuli
To identify those regions responsive to both self‐specific and familiar stimuli, we further identified the overlapping brain regions between the two contrasts (SON‐UV vs. UN‐UV and FN‐UV vs. UN‐UV), from the data obtained in Experiment 1. The medial prefrontal cortex (MPFC), a distinct subregion of the PCC, and the bilateral temporoparietal junction (TPJ) were identified as showing overlapping areas of activation to both self‐specific and familiar stimuli (Fig. 1A,B and Supporting Information Table 3). Using these regions as ROIs in the analysis of Experiment 2, it was found that the PCC and left TPJ showed significant stronger signal changes in SON‐FV than UN‐UV; whereas no difference was found between the responses to the SON‐FV and SON‐UV conditions, nor between the responses to the SON‐UV and UN‐UV conditions, in these regions. There was no difference between different stimuli in the MPFC and right TPJ (Fig. 3C).
Figure 3.
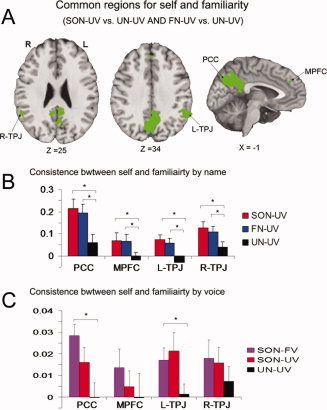
A: Brain activation for common regions between the self and familiarity from overlap of contrasts (SON‐UV vs. UN‐UV and FN‐UV vs. UN‐UV; P = 0.01, FWE corrected). B: Parameter estimates (Mean ± SE) for SON‐UV, FN‐UV, and UN‐UV in the MPFC, PCC, R‐TPJ, and L‐TPJ in the event‐related experiment (Experiment 1). C: Parameter estimates (Mean ± SE) for SON‐FV, SON‐UV, and UN‐UV in the same three ROIs for the block‐design experiment (Experiment 2). *Denotes P < 0.05; MPFC, medial prefrontal cortex; R‐TPJ, right temporoparietal junction; L‐TPJ, left temporoparietal junction. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Comparison Between fMRI and Meta‐Analytic Data
To provide an independent validation of the results obtained from the present study, we compared the regions identified in Experiment 1 with the results of an MKDA‐based meta‐analysis of imaging studies investigating the neural effects of self‐specific and familiar stimuli. This comparison revealed a strong overlap between the regions identified in Experiment 1 as being responsive to self‐specific stimuli, specifically the aACC and left anterior insula, and those regions identified as such in the meta‐analysis (Fig. 4A). Similarly, those regions identified as being responsive to familiar stimuli in Experiments 1, specifically the bilateral PCC, showed an overlap with the regions identified as such in the meta‐analysis (Fig. 4B).
Figure 4.
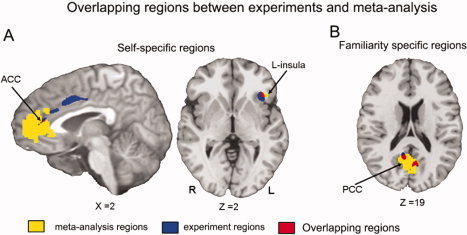
A: The brain overlapping between the self specific regions from overlap of contrasts (SON‐UV vs. FN‐UV and SON‐UV vs. UN‐UV) and meta‐analysis for the self condition. B: The brain overlapping between the familiarity regions from overlap of contrasts (FN‐UV vs. SON‐UV and FN‐UV vs. UN‐UV) and meta‐analysis for the self condition. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Our first main finding consists in differential recruitment of anterior and posterior cortical regions during the processing of self‐specific and familiar stimuli. In Experiment 1, self‐specific stimuli were shown to recruit predominantly anterior cortical regions (ACC, AI and SMA), whereas familiar stimuli were associated instead with responses in the posterior cortical regions (PCC). Although controlling for possible task‐related effects, these findings were confirmed by both our second fMRI study and by the observed overlap between the regions identified in the fMRI experiments and the regions obtained in the meta‐analytic study. Taken together, our data demonstrate a neural divide between self‐specificity and familiarity in anterior and posterior cortical regions, suggesting that both concepts, self and familiarity, may need to be distinguished from each other.
We observed that self‐specific stimuli recruited neural activity changes in anterior cortical regions including the ACC and bilateral insula. This is well in line with previous studies showing the specific involvement of these regions in self‐specificity [Craig,2009; Critchley et al.,2004; Devinsky et al.,1995]. Our study extends these results by controlling for familiarity by using three sets of brain region overlaps that allow, as much as possible, for the exclusion of the confounding effects of the latter on the former. In addition, this result was further validated by the second fMRI experiment. Finally, our results were further validated by the observed overlap between the regions identified in the fMRI component of the study and those identified via the meta‐analysis.
The AI is well known to be involved in the processing of interoceptive stimuli [Craig, 2002, 2003, 2004]. At the same time, the AI is closely connected with the ACC, which has been assumed to be crucial in generating consciousness [Northoff et al.,2006] and a sense of self [Augustine,1996; Mesulam and Mufson,1982; Vogt and Pandya,1987]. What have remained unclear so far, however, are the exact neural mechanisms that enable the ACC to potentially constitute consciousness and self.
On the basis of our findings, one may hypothesize that the close anatomical linkage between AI and ACC may allow the incoming exteroceptive stimulus (such as the person's name, as in our study) to be functionally linked to the interoceptive stimuli originating from the body [See also Enzi et al.,2009; Feinberg,2009]. It may thus be such interoceptive–exteroceptive interaction via AI‐ACC connectivity that enables the transformation of a non‐self‐specific stimulus into a self‐specific one [2009]. This hypothesis is further supported by the results from a previous study by Taylor et al. [Nakamura et al.,2001; Shah et al.,2001; Sugiura et al.,2006; von Kriegstein et al.,2005], in which it was demonstrated, using an analysis of resting‐state functional connectivity, that the bilateral insula and ACC display closely correlated activity patterns. However, to further support this hypothesis, future studies are needed that specifically focus on the interoceptive–exteroceptive interaction and how this affects the subsequent perception and conscious experience of incoming exteroceptive stimulus.
In contrast to self‐specific stimuli, familiar stimuli recruited neural activity changes in posterior cortical regions, in particular the bilateral PCC. The involvement of these regions is very much in accordance with previous studies on familiarity [Frith and Frith,1999,2003,2006; Uddin et al.,2007; Van Overwalle,2009]; as well as with studies involving the perception of other people, such as in mind‐reading tasks, which also show the involvement of these regions [Gillihan and Farah,2005; Legrand and Ruby,2009]. By using the above described analysis, our results extend previous studies by controlling for self‐specificity, which in this context must be considered a confounding factor. Furthermore, we were able to confirm and validate these results by both our second fMRI experiment and the meta‐analysis. Taken together with the above discussed results on self‐specificity, our findings indicate an anterior–posterior cortical divide between self‐specificity and familiarity.
Our findings address a recent debate revolving around whether there are regions in the brain whose activity is specific for self‐specificity or not [Gillihan and Farah,2005]. Gillihan and Farah [2005] make the argument that many of the regional activity changes observed during self‐specific stimuli may be traced back to the high degree of familiarity of these stimuli [Cavanna and Trimble,2006; Gusnard et al.,2001; Wagner et al.,2005]. By controlling for self‐specificity in our analysis of familiarity and, conversely, for familiarity in our data on self‐specificity, the here observed anterior–posterior divide does not lend empirical support to this argument. Instead, the clear divide between self‐specificity and familiarity in anterior and posterior cortical regions suggests that both must be considered distinct. This, however, raises the question as to what accounts for such a distinction. As described above, what makes an exteroceptive stimulus self‐specific, rather than merely familiar, may be its linkage to interoceptive stimuli, with such a linkage seemingly being mediated by anterior cortical regions. An exteroceptive stimulus may thus be processed as being familiar, rather than self‐specific, when there is no such linkage between it and concurrent interoceptive stimuli via the anterior insula. Instead, such non‐interoceptively linked exteroceptive stimuli may rather recruit neural activity changes in posterior cortical regions, such as the PCC/precuneus. Interestingly, these posterior regions have been seen to be closely involved in the retrieval of episodic memories [Gillihan and Farah,2005; Northoff et al.,2006]. This hypothesis remains rather speculative at this point, however, and as such requires further experimental testing; for instance through the use of experimental paradigms that focus upon the interaction between interoceptive and exteroceptive, as well as self‐specific and familiar, stimuli.
The observed anterior–posterior divide between self‐specificity and familiarity is, however, not a mutually exclusive distinction. We observed a regional overlap between self‐specificity and familiarity in the MPFC, PCC, and TPJ, thus implicating in some way those posterior regions that were associated with familiarity in the processing of self‐specific stimuli. This can be interpreted in two different ways: First, this regional overlap may reflect the high degree of familiarity of self‐specific stimuli, with this interpretation being consistent with the often observed involvement of these regions in the many imaging studies of self‐specificity that have not controlled for familiarity effects [2003]. Alternatively, one could argue that even self‐specific stimuli are processed in the context of the wider environment, both physical and social, and thus in relation to non‐self‐specific stimuli. If so, one would hypothesize that it is the neural balance between anterior and posterior regions rather than the exclusive recruitment (or non‐involvement) of anterior regions that predisposes the stimulus to become either self‐specific or familiar. This however remains to be tested in the future; for instance, by investigating the potential differences in functional connectivity between anterior and posterior regions during both self‐specific and familiar stimuli.
In a similar context, the overlapping region identified in the MPFC is similar to those identified by Kampe et al. [Holeckova et al.,2006,2008] in a study that also utilized the hearing of one's own name. They interpret the activity in this region as being associated with “theory of mind.” The current data would also broadly fit with this interpretation, with the hearing of one's own name, as well as the name of a familiar person, potentially both requiring this putative theory of mind in the interpretation of the intent of the individual calling the name. Studies targeted at this particular issue would be required to expand on this possibility, however.
In addition to a neural overlap between self‐specificity and familiarity in posterior regions, we also observed some possible interaction effects between both in anterior regions, specifically the bilateral AI and anterior ACC. Although we did not find any effect of familiarity alone in these regions, familiar stimuli were able to enhance neural activity in these regions during the processing of self‐specific stimuli (for analogous results in EEG, although note that these results do not treat the same regions as studied here). This suggests that neural activity in these anterior regions may be not exclusively sensitive to self‐specific stimuli, but rather that they may also be modulated by familiarity when processing self‐specificity.
It should be noted, however, that the interaction between self‐specificity and familiarity was not tested in a properly controlled 2 × 2 factorial design. These results must therefore be treated with some caution. Furthermore, neither fMRI experiment included a control for interoceptive stimuli. Our assumption that insula activity reflects interoceptive processing should thus be regarded as a preliminary treatment of this issue, requiring further future investigation. A final potential limitation of the study is that with stimuli being presented repeatedly in the block‐design experiment there is the potential for repetition effects confounding the findings detailed here (the randomization and long gaps between stimuli in the event‐related experiment should minimize this possibility in it). An analysis of the BOLD responses in our regions of interest does suggest that this is not the case (see Supporting Information Figs. 1 and 2), but further investigation of potential differential repetition effects between self‐specific and familiar stimuli may be warranted.
CONCLUSION
By using two independent fMRI experiments, combined with a meta‐analytic study, we were here able to demonstrate an anterior–posterior divide in the neural processing of self‐specific and familiar stimuli. Although self‐specific stimuli were associated with anterior cortical regions, such as the ACC and the AI, familiar stimuli recruited neural activity changes rather in posterior cortical regions, such as the PCC and TPJ. Taken together, our results provide an empirical argument against previous assumptions of self‐specificity being merely a high degree of familiarity. Finally, it is suggested that the involvement of the identified anterior cortical regions may be crucial for neurally distinguishing between self‐specific and familiar stimuli, with this involvement possibly reflecting an interoceptive–exteroceptive interaction.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting information
Contributor Information
Xuchu Weng, Email: wengxc@psych.ac.cn.
Georg Northoff, Email: georg.northoff@rohcg.on.ca.
REFERENCES
- Augustine JR ( 1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Bower GH, Gilligan SG ( 1979): Remembering information related to one's self. J Res Personality 13: 420–432. [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2009): How do you feel‐‐now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ ( 2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E ( 2005): Self‐referential reflective activity and its relationship with rest: A PET study. NeuroImage 25: 616–624. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E ( 2007): Distinct regions of the medial prefrontal cortex are associated with self‐referential processing and perspective taking. J Cogn Neurosci 19: 935–944. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA ( 1995): Contributions of anterior cingulate cortex to behavior. Brain 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Enzi B, de Greck M, Prosch U, Tempelmann C, Northoff G ( 2009): Is our self nothing but reward? Neuronal overlap and distinction between reward and personal relevance and its relation to human personality. PLoS One 4: e8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg TE, Venneri A, Simone AM, Fan Y, Northoff G ( 2010): The neuroanatomy of asomatognosia and somatoparaphrenia. J Neurol Neurosurg Psychiatry 81: 276–281. [DOI] [PubMed] [Google Scholar]
- Feinberg TT ( 2009): From Axons to Identity: Neurological Explorations of the Nature of the Self. New York: W. W. Norton. [Google Scholar]
- Frith CD, Frith U ( 1999): Interacting minds—A biological basis. Science 286: 1692–1695. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U ( 2006): The neural basis of mentalizing. Neuron 50: 531–534. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD ( 2003): Development and neurophysiology of mentalizing. Philos Trans R Soc London Ser B: Biol Sci 358: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ ( 2005): Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychol Bull 131: 76–97. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Leibenluft E, Santiago N, Haxby JV ( 2004): Social and emotional attachment in the neural representation of faces. NeuroImage 22: 1628–1635. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME ( 2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Han S, Gu X, Mao L, Ge J, Wang G, Ma Y ( 2009): Neural substrates of self‐referential processing in Chinese Buddhists. Soc Cogn Affect Neurosci 5: 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holeckova I, Fischer C, Giard MH, Delpuech C, Morlet D ( 2006): Brain responses to a subject's own name uttered by a familiar voice. Brain Res 1082: 142–152. [DOI] [PubMed] [Google Scholar]
- Holeckova I, Fischer C, Morlet D, Delpuech C, Costes N, Mauguiere F ( 2008): Subject's own name as a novel in a MMN design: A combined ERP and PET study. Brain Res 1189: 152–165. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Frith U ( 2003): “Hey John”: Signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. J Neurosci 23: 5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss‐Moreau E, Lindquist K, Wager TD ( 2008): Functional grouping and cortical‐subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. NeuroImage 42: 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand D, Ruby P ( 2009): What is self‐specific? Theoretical investigation and critical review of neuroimaging results. Psychol Rev 116: 252–282. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV ( 2004): Mothers' neural activation in response to pictures of their children and other children. Biol Psychiatry 56: 225–232. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ ( 1982): Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol 212: 38–52. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN ( 2005): The link between social cognition and self‐referential thought in the medial prefrontal cortex. J Cogn Neurosci 17: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A ( 2009): Activation of anterior insula during self‐reflection. PLoS One 4: e4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Sugiura M, Kato T, Nakamura A, Hatano K, Nagumo S, Kubota K, Fukuda H, Ito K, Kojima S ( 2001): Neural substrates for recognition of familiar voices: A PET study. Neuropsychologia 39: 1047–1054. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 3: 102–107. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. NeuroImage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D'Esposito M ( 2005): The neural correlates of direct and reflected self‐knowledge. NeuroImage 28: 797–814. [DOI] [PubMed] [Google Scholar]
- Platek SM, Loughead JW, Gur RC, Busch S, Ruparel K, Phend N, Panyavin IS, Langleben DD ( 2006): Neural substrates for functionally discriminating self‐face from personally familiar faces. Hum Brain Map 27: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP ( 2004): Cortical activations during judgments about the self and an other person. Neuropsychologia 42: 1168–1177. [DOI] [PubMed] [Google Scholar]
- Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR ( 2001): The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain 124 ( Part 4): 804–815. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R ( 2005): Cortical mechanisms of visual self‐recognition. NeuroImage 24: 143–149. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Watanabe J, Akitsuki Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R ( 2006): Cortical mechanisms of person representation: Recognition of famous and personally familiar names. NeuroImage 31: 853–860. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Watanabe J, Akitsuki Y, Maeda Y, Matsue Y, Kawashima R ( 2009): Anatomical segregation of representations of personally familiar and famous people in the temporal and parietal cortices. J Cogn Neurosci 21: 1855–1868. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD ( 2009): Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Map 30: 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP ( 2007): The self and social cognition: The role of cortical midline structures and mirror neurons. Trends Cogn Sci 11: 153–157. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F ( 2009): Social cognition and the brain: A meta‐analysis. Hum Brain Map 30: 829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T, Hunyadi E, Grupe DW, Connors CM, Schultz RT ( 2008): Self, mother and abstract other: An fMRI study of reflective social processing. NeuroImage 41: 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN ( 1987): Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol 262: 271–289. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Kleinschmidt A, Sterzer P, Giraud AL ( 2005): Interaction of face and voice areas during speaker recognition. J Cogn Neurosci 17: 367–376. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX ( 2009): Evaluating the consistency and specificity of neuroimaging data using meta‐analysis. NeuroImage 45 ( 1 Suppl): S210–S221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL ( 2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9: 445–453. [DOI] [PubMed] [Google Scholar]
- Yaoi K, Osaka N, Osaka M ( 2009): Is the self special in the dorsomedial prefrontal cortex? An fMRI study. Social Neurosci 1–9. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S ( 2007): Neural basis of cultural influence on self‐representation. NeuroImage 34: 1310–1316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting information


