Abstract
Despite advances in the treatment of patients with human immunodeficiency virus (HIV), HIV‐associated neurocognitive disorder occurs in 15–50% of HIV‐infected individuals, and may become more apparent as ageing advances. In the present study we investigated regional cerebral blood flow (rCBF) and regional cerebral metabolic rate of glucose uptake (rCMRglc) in medically and psychiatrically stable HIV‐1‐infected participants in two age‐groups. Positron emission tomography (PET) and magnetic resonance imaging (MRI)‐based arterial spin labeling (ASL) were used to measure rCMRglc and rCBF, respectively, in 35 HIV‐infected participants and 37 HIV‐negative matched controls. All participants were currently asymptomatic with undetectable HIV‐1 viral loads, without medical or psychiatric comorbidity, alcohol or substance misuse, stable on medication for at least 6 months before enrolment in the study. We found significant age effects on both ASL and PET with reduced rCBF and rCMRglc in related frontal brain regions, and consistent, although small, reductions in rCBF and rCMRglc in the anterior cingulate cortex (ACC) in HIV, a finding of potential clinical significance. There was no significant interaction between HIV status and the ageing process, and no significant HIV‐related changes elsewhere in the brain on PET or ASL. This is the first paper to combine evidence from ASL and PET method in HIV participants. These finding provide evidence of crossvalidity between the two techniques, both in ageing and a clinical condition (HIV). Hum Brain Mapp 34:2484–2493, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: PET, ASL, rCBF, glucose uptake, ageing, HIV
INTRODUCTION
Since its introduction in 1996, highly active antiretroviral therapy (HAART) for human immunodeficiency virus (HIV) has led to dramatic decreases in HIV‐associated mortality and morbidity for these individuals [Detels et al., 2001; Dore et al., 2003; Hammer et al., 1997; Palella et al., 1998]. However, rates of cognitive impairment remain high: for example, the CHARTER study reported 33% for asymptomatic neurocognitive impairment, 12% for mild neurocognitive impairment, and 2% HIV associated dementia [Heaton et al., 2010, 2011]. Robertson et al. 2007 found 39% neurocognitive impairment, and Simoni et al. 2010 69%. In a recent review, Schouten et al. 2011 described abnormal scores on neuropsychological tests in 15–50% of HIV‐infected individuals in studies from different parts of the world.
HIV‐1 is known to enter the CNS early in the course of infection, even before antibodies are detectable in the blood [Thurnher and Donovan‐Post, 2008]. As a result, the brain may be affected by a variety of HIV‐1 associated abnormalities, with knowledge of these abnormalities being important for detection and diagnosis and for initiation of treatment [Thurnher and Donovan‐Post, 2008]. Neuroimaging has already offered significant insight into the nature and extent of underlying HIV‐1 CNS changes [Paul et al., 2008] and the availability of newer techniques offers the opportunity to expand upon this further [Tucker et al., 2004]. In our own previous study, we found a region of reduced gray matter volume in the superior and medial frontal gyri [Towgood et al., 2011]. Of particular interest is the potential to identify surrogate markers of disease burden, particularly early in the disease process. These markers could help identify those at risk of developing neurocognitive disorders and/or dementia [Ances et al., 2009] and may in turn be beneficial for drug trials in early CNS infection [Thompson et al., 2006].
Positron emission tomography (PET) has been used to explore regional cerebral metabolic rate of glucose (rCMRglc) in a limited number of studies in HIV‐infected participants and has provided some important insights into the nature of HIV‐infection. A consistent finding from these investigations in symptomatic HIV‐1 infection has been of increased basal ganglia uptake [Hinkin et al., 1995; Rottenberg et al., 1996; van Gorp et al., 1992]. Two hypotheses have been put forward to explain this result. The first is that globally reduced uptake in the cortex, with relative sparing of subcortical uptake, produces the appearance of elevated uptake in the latter [van Gorp et al., 1992]. The second hypothesis attributes this finding to an absolute increase in uptake in subcortical structures. In all of these studies, [18F]2‐fluoro‐2‐deoxy‐d‐glucose (FDG) PET was used to investigate rCMRglc in participants with acquired immunodeficiency syndrome (AIDS). Whilst these studies all reported on participants in the pre‐HAART era, the single study conducted to date conducted with participants from the post‐HAART era confirms these findings [von Giesen et al., 2000]. Very few asymptomatic HIV‐infected participants have been included in existing studies.
A recent advance in neuroimaging which offers the potential to further explore the physiological changes associated with HIV‐infection is arterial spin labeling (ASL). ASL is a magnetic resonance imaging (MRI) technique which can be used to generate reliable maps of regional cerebral blood flow (rCBF) in a noninvasive manner, and has shown promise for evaluation of the central nervous system disorders [Detre and Alsop, 1999; Detre et al., 2009]. In addition, ASL appears to be a reliable correlate of regional tissue metabolism. It is also a sensitive marker of tissue pathology as shown recently in the case of Alzheimer's Disease [Chao et al.2010].
To date only two studies have explored the use of ASL in HIV‐infection [Ances et al., 2006, 2009]. These researchers found reduced caudate rCBF in HIV‐1‐infected patients with cognitive impairment [Ances et al., 2006] and reduced lenticular nucleus and caudate rCBF in individuals who had only recently seroconverted (become HIV positive), and who were not yet expressing any signs of neuropsychological impairment [Ances et al., 2009].
The main objective of the current study was to describe the rCBF and rCMRglc in the brains of HIV‐1 positive individuals who were medically and psychiatrically asymptomatic and stable on HAART. We selected a study population which was not confounded by other risk factors, such as history of alcohol or substance use/dependence or by significant previous neurological or psychiatric disorder. This offered the significant advantage of potentially detecting HIV‐related cerebral changes, independent of confounding factors. Detailed cognitive and structural MRI findings in our samples are reported elsewhere [Towgood et al., 2011].
MATERIALS AND METHODS
Participant Population
A total of 95 participants who self‐reported as “men who have sex with men” were recruited between October 2005 and December 2008. They were divided into four groups: HIV‐1‐infected patients aged 20–40; those aged 50–75, and age‐ and education‐matched younger (20–40) and older (50–75) HIV negative healthy controls.
To participate in the study, HIV‐1‐infected participants were required to have been stable on HAART with undetectable viral loads of <50 copies ml−1 and CD4 cell count >200 cells μl−1 for at least 6 months. HAART regimens had been prescribed as clinically judged by the primary HIV physician and, with the exception of two cases, all HIV‐1‐infected participants were being treated with at least one CNS penetrating antiretroviral drug at the time of the study. All participants were of white/Caucasian ethnicity and proficient in the English language. Research Ethics Committee approval for the project was granted by the Joint South London and Maudsley and Institute of Psychiatry Research Ethics Committee (Approval Number 05/Q0706/267). Consent was obtained according to the Declaration of Helsinki.
Participants underwent a brief medical and neurological examination performed by an HIV physician. Blood samples were obtained to verify HIV status, CD4 cell count and plasma levels of HIV‐RNA burden and to check for the presence of syphilis and hepatitis B and C infection as well as full blood count, renal and liver function, bone and lipid profile, vitamin B12, folate and glucose levels, thyroid stimulating hormone (TSH), and C‐reactive protein. All participants were also evaluated for psychiatric disorder or a history of alcohol and substance usage using a structured clinical interview administered by a consultant psychiatrist to establish a diagnosis according to DSM‐IV criteria [American Psychiatric Association, 1994]. In addition, depression and anxiety symptoms were assessed using the Beck Depression Inventory (BDI) and the Beck Anxiety Inventory (BAI) [Beck, 1987; Beck and Steer, 1990].
Participants were excluded if they met criteria for any previous or current CNS‐AIDS‐defining‐conditions as defined by the CDC classification of confounding neurological disorder; history of head trauma with loss of consciousness for more than 10 min or had a history of harmful alcohol or substance usage. Harmful alcohol usage was defined as more than 25 U of alcohol per week. Harmful substance usage was defined as any use of cannabis within the 2 weeks prior to screening or use more frequently than once a month; use of other substances such as MDMA or cocaine more than three times a year, or any use of heroin. Other exclusion criteria were chronic medical illness that might affect cognition such as cardiac, hepatic, or renal dysfunction, moderate to severe psychiatric disorder, and/or current use of psychotropic medication. Patients with contraindications for MRI scans were also excluded.
Details of patient recruitment are presented in Figure 1.
Figure 1.
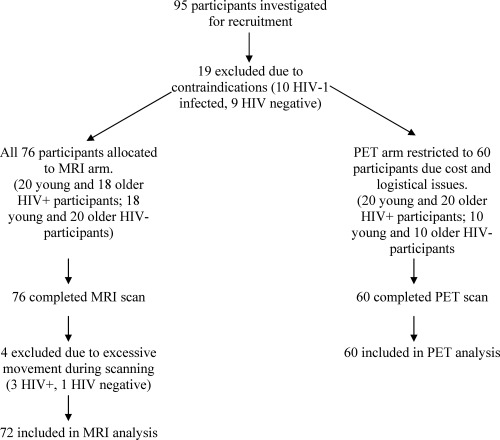
Study recruitment.
Procedures
Neuropsychological examination
A range of neuropsychological tests of known reliability and validity were administered. These were broadly based on the Heaton et al., 1995 HIV battery, although equivalent tests developed and standardized in the United Kingdom were substituted where available. The tests were administered by trained psychologists according to standardized procedures. The order of testing was the same for all participants. The neuropsychological battery took ∼ 2 h to administer.
The revised National Adult Reading test [Nelson and Willison, 1991] was administered as a test of general premorbid ability. General intelligence was assessed based on the vocabulary, digit‐symbol coding, similarities, block design, matrix reasoning, digit span, digit symbol, and letter‐number sequencing subtests from the Wechsler Adult Intelligence Scale (Third Edition) [Wechsler, 1997].
Image acquisition
ASL and conventional MRI scans were acquired on a General Electric SIGNA HDx 3.0T MR scanner (General Electric, Milwaukee WI) at the Centre for Neuroimaging Sciences, Institute of Psychiatry, King's College London. For radiological purposes, we acquired a T 2‐weighted fast spin echo (FSE) sequence (T R = 4,380 ms, T E = 65 ms, slice thickness = 4 mm, 36 slices, image matrix = 320 × 320, reconstructed as 512 × 512, FOV = 24 × 24 cm2) and a fluid attenuated inversion recovery (FLAIR) sequence (T R = 8,000 ms, T I = 2,000 ms, T E = 127 ms, with the same slice prescription and resolution).
Resting state rCBF measurements were made using a pseudo‐continuous ASL technique developed by Dai et al. 2009. In this technique, arterial blood is labeled using a 1.5‐s train of Hanning shaped RF pulses of 1,500 μs duration and 500 μs spacing. After a postlabeling delay of 1.5 s, the image is acquired with a 3D fast spin echo (FSE) spiral multishot readout (TE 32 ms; TR = 5,500 ms; ETL = 64). Two factors make this an ideal sequence for resting‐state perfusion studies: first, the long adiabatic RF pulse labels a substantial amount of arterial blood and therefore increases the amplitude of the perfusion contrast (contrast to noise ratio). Second, the FSE signal readout minimizes signal losses due to magnetic susceptibility induced gradients, an artifact commonly observed when using gradient recalled, echo planar imaging techniques, particularly in the basal ganglia, orbito‐frontal areas and inferior temporal regions. Images are reconstructed to a 2562 matrix, such that the resulting rCBF maps have a voxel size of 1 × 1 × 3 mm3. Three pairs of tagged–untagged images were collected, together with two calibration volumes for flow quantification, for a total acquisition time of 6:08 min.
PET scans were performed in the PET Imaging Centre at St Thomas's Hospital, King's College London, using a GE Discovery ST PET/CT scanner (GE Medical System, Milwaukee, WI) with a 15.4‐cm axial field of view. Participants were instructed to refrain from eating or drinking anything except water for 3 h prior to the scan. On arrival each participant was cannulated in the antecubital fossa and injected with 250 MBq (18F)‐FDG. After a 30‐min uptake period in which they rested in a dimly lit quiet room the participants were put on the PET scanner, with their head secured by a head rest. A planar CT scout was acquired to localize the participant's brain in the PET field of view. A single low dose CT was also acquired for attenuation correction of the PET scan.
Each PET scan was acquired in 3D mode over 15 min and reconstructed using the 3D FORE algorithm and 2D filtered back projection with scatter correction, geometric correction and CT‐based attenuation correction. The resulting images had voxel dimensions 128 × 128 × 47 with voxel sizes 2 × 2 × 3.27 mm3.
Image processing
For the ASL images, the preprocessing steps were conducted in the following manner. First, extracerebral signal was removed from the T 2‐weighted FSE scan using the “brain extraction tool” (BET; the Functional Software Library, Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, University of Oxford, UK). The resulting “skull stripped” T 2 volume and its corresponding binary mask were then coregistered to the rCBF map. Next, the coregistered, brain‐only mask was multiplied by the rCBF map to remove extracerebral signal from this scan. The skull stripped T 2 and rCBF maps were coregistered back to the space of the original T 2‐weighted scan. Finally, the original T 2‐weighted scan was normalized to the MNI (Montreal Neurological Institute) T 2 template provided in the SPM suite (SPM5, Wellcome Department of Imaging Neurosciences, University College London, UK) and the transformation matrix was applied to both the rCBF map and the T 2‐weighted scan to transform them into standard anatomical space. All normalized rCBF maps were manually inspected to assess the quality of the normalization using major anatomical landmarks as well as reference points on the surface of the brain. Transformed images were then smoothed with a Gaussian kernel with a full width half maximum of 6 mm.
PET images were also spatially normalized into standard anatomical space (again conforming to the standard MNI template used in SPM5) to allow interparticipant averaging and comparison. Transformed images were then smoothed with a Gaussian kernel with a full width half maximum of 6 mm. Although FDG rCMRglc images do provide a sensitive measure of rCMRglc, they are not fully quantitative. Regional differences in rCMRglc were therefore explored by normalizing the whole brain uptake for each scan.
Statistical methods
Voxel wise analysis
An independent samples t test was computed to assess global CBF variability across the two groups, HIV‐1 infected and HIV negative. Differences in rCBF between the HIV‐1‐infected participants and HIV negative controls were then evaluated using SPM5. A two‐way (HIV‐1 status by Age group) analysis of covariance model was fitted at each intracerebral voxel in standard space, covarying for mean whole brain global perfusion. Because of the well known insensitivity of ASL in white matter, an absolute threshold of 20 ml/100 g/min was applied to the CBF maps to eliminate the majority of white matter tissue from the statistical comparison.
Differences in FDG uptake between the HIV‐1‐infected participants and HIV negative controls were also evaluated using SPM5. Again, a two‐way (HIV‐1 status by Age group) analysis of covariance model was fitted at each intracerebral voxel in standard space, with images normalized to the whole brain mean rCMRglc. For all analysis an uncorrected voxelwise P value of 0.001 and a cluster P value of 0.05 (corrected for multiple comparisons) were set as our threshold for significant values.
Region of interest analysis
To aid comparison with previous research that found significant rCBF changes in HIV [Ances et al., 2009] we conducted a region of interest analysis within the caudate and lenticular nuclei. rCBF and rCMRglc values from these regions were extracted using the WFU Pick Atlas facility within SPM5. Mean rCBF values within each of these regions were calculated for each participant. An independent t test analysis was conducted to explore the difference between HIV‐infected participants and healthy controls. An alpha level of 0.05 uncorrected was set as our threshold for significant values.
RESULTS
Demographic Data
Tables 1 and 2 describe the basic demographic, HIV‐1 history, and immune status data for the four participant groups. Full details of neuropsychological outcome in this population have been reported in a previous paper [Towgood et al., 2011]. No significant differences were observed in terms of age, IQ, or mood variables between HIV‐1‐infected older participants and their matched HIV‐negative older controls, or between HIV‐1‐infected younger participants and their matched HIV‐1‐negative controls. In addition, no significant differences were noted on current CD4 and nadir CD4 cell counts between the HIV‐1‐infected younger group (20‐ to 40‐years old) and HIV‐1‐infected older group (50‐ to 75‐years old). A significant difference was however noted between these two HIV‐1‐infected groups for years since diagnosis (Mann–Whitney U = 102.5, P = 0.023) and years since commencing HIV treatment (Mann–Whitney U = 87.5, P = 0.006).
Table 1.
Means (SD) for baseline characteristics of study participants for ASL analysis
| HIV infected | HIV negative | |||
|---|---|---|---|---|
| Older group (N = 15) | Younger group (N = 20) | Older group (N = 19) | Younger group (N = 18) | |
| Age | 56.67 (5.12) | 34.25 (4.39) | 56.16 (6.52) | 31.39 (6.13) |
| IQ | ||||
| WAIS FSIQa | 118.67 (11.88) | 115.55 (11.33) | 118.63 (10.78) | 120.22 (13.14) |
| WAIS VIQb | 116.73 (12.50) | 112.95 (11.67) | 117.42 (13.37) | 118.00 (12.84) |
| WAIS PIQc | 116.79 (13.19) | 115.05 (15.26) | 116.05 (9.41) | 118.44 (14.42) |
| Mood | ||||
| Depression | 5.64 (4.52) | 4.50 (5.07) | 3.32 (2.98) | 3.82 (5.89) |
| Anxiety | 7.71 (7.15) | 3.42 (4.32) | 4.21 (5.89) | 2.82 (3.38) |
| HIV variables | ||||
| CD4 cells mm−1 | 700.87 (261.8) | 596.45 (191.4) | ||
| Nadir CD4 cells mm−1 | 166.93 (98.0) | 175.4 (101.72) | ||
| Yrs since diagnosisd | 12.0 (8.0) | 8.10 (4.40) | ||
| Yrs since treatmentd | 9.2 (4.49) | 5.0 (2.32) | ||
Wechsler adult intelligence scale third edition full scale intelligence quotient, standardized scores.
Wechsler adult intelligence scale third edition verbal intelligence quotient, standardized scores.
Wechsler adult intelligence scale third edition performance intelligence quotient, standardized scores.
Significant differences found between groups.
Table 2.
Means (SD) for baseline characteristics of study participants for PET analysis
| HIV infected | HIV negative | |||
|---|---|---|---|---|
| Older group (N = 20) | Younger group (N = 20) | Older group (N = 10) | Younger group (N = 10) | |
| Age | 58.70 (6.62) | 34.25 (4.39) | 58.90 (8.52) | 31.90 (4.20) |
| IQ | ||||
| WAIS FSIQa | 117.35 (12.41) | 115.55 (11.33) | 122.20 (11.15) | 118.90 (15.46) |
| WAIS VIQb | 117.05 (12.76) | 112.95 (11.67) | 121.80 (16.57) | 117.90 (16.48) |
| WAIS PIQc | 113.95 (13.92) | 115.05 (15.26) | 121.80 (16.57) | 116.10 (14.78) |
| Mood | ||||
| Depression | 5.17 (4.20) | 4.50 (5.07) | 3.10 (3.73) | 3.22 (2.44) |
| Anxiety | 6.37 (6.57) | 3.42 (4.32) | 5.70 (7.85) | 2.11 (1.96) |
| HIV variables | ||||
| CD4 cells mm−1 | 735.5 (343.53) | 596.45 (191.4) | ||
| Nadir CD4 cells mm−1 | 170.7 (91.99) | 175.4 (101.72) | ||
| Yrs since diagnosisd | 13.00 (7.00) | 8.10 (4.40) | ||
| Yrs since treatmentd | 8.85 (4.26) | 5.0 (2.32) | ||
Wechsler adult intelligence scale third edition full scale intelligence quotient, standardized scores.
Wechsler adult intelligence scale third edition verbal intelligence quotient, standardized scores.
Wechsler adult intelligence scale third edition performance intelligence quotient, standardized scores.
Significant differences found between groups.
Voxelwise Analysis
rCBF
Global CBF
The ASL data showed no significant difference in whole brain global rCBF between the groups (see Table 3).
Table 3.
Means (SD) for global CBF
| HIV infected (N = 35 ) | HIV negative (N = 37) | P value (t test) | |
|---|---|---|---|
| Mean global rCBF | 394.70 (72.48) | 416.49 (86.20) | 0.251 |
Main effect of aging
Significant effects of aging were detected with the older individuals having significantly decreased rCBF (relative to whole brain mean) in three clusters in the frontal lobes in the midline and left dorso‐lateral regions (cluster level corrected P < 0.05, see Fig. 2). In the absence of absolute threshold masking, there was also significantly increased rCBF (relative to the whole brain mean) in four clusters situated in subcortical white matter, bilateral putamen (lenticular nucleus) and bilateral pulvinar (posterior thalamus), (cluster level corrected P < 0.05, see Fig. 3).
Figure 2.
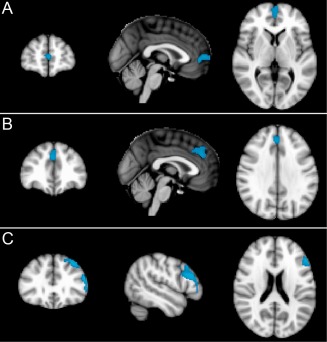
ASL: Areas of significantly (cluster level corrected P < 0.05) reduced regional cerebral blood flow (rCBF) in older relative to younger participants in three clusters in the frontal lobes in the midline and left dorso‐lateral frontal regions. Slides in radiological convention with left hemisphere to the right of the image.
Figure 3.
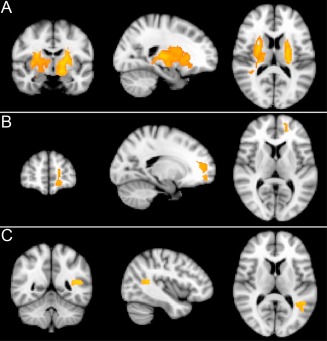
ASL: Areas of significantly (cluster level corrected P < 0.05) increased regional cerebral blood flow (rCBF) in older relative to younger participants in four clusters situated in sub cortical white matter bilateral putamen (lenticular nucleus) and bilateral pulvinar (posterior thalamus). Slides in radiological convention.
Main effect of HIV status
There were no significant effects of HIV status detected.
Interaction between HIV status and age
No significant interactions were detected between HIV‐1 status and aging on measures of rCBF.
Regional cerebral metabolic rate of glucose (rCMRglc)
Main effect of aging
Significant effects of aging were detected with the older individuals having significantly decreased rCMRglc (relative to whole brain mean) in left dorso‐lateral frontal and bilateral midline frontal/anterior cingulate regions (cluster level corrected P < 0.05, see Fig. 4). Significantly increased rCMRglc (relative to whole brain mean) was found in bilateral pulvinar and white matter, upper brain stem, and cerebellar regions (cluster level corrected P < 0.05, see Fig. 5).
Figure 4.
PET: Area of significantly (cluster level corrected P < 0.05) reduced regional cerebral metabolic rate of glucose (rCMRglc) in older relative to younger participants in left dorsolateral and bilateral midline frontal/anterior cingulate regions. Slides in radiological convention.
Figure 5.
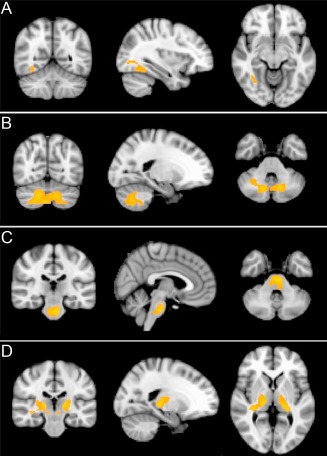
PET: Areas of significantly (cluster level corrected p < 0.05) increased regional cerebral metabolic rate of glucose (rCMRglc) in older relative to younger participants in bilateral pulvinar and white matter, upper brain stem, and cerebellar regions. Slides in radiological convention.
Main effect of HIV status
There were no significant effects of HIV status detected.
Interaction between HIV status and age
No significant interactions were detected between HIV‐1 status and aging on measures FDG uptake.
Region of Interest Analysis
We did not find evidence of significantly reduced rCBF in the lenticular nucleus or the caudate for HIV‐1‐infected participants versus controls for rCBF (see Table 4) or for rCMRglc (see Table 5), although we did find a trend toward significance for rCBF in the lenticular nucleus (P < 0.06).
Table 4.
rCBF (ASL) region of interest values (ml of blood per 100 g)
| HIV infected (N = 35) | HIV negative (N = 37) | t | P | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Caudate N | 34.21 | 6.14 | 36.82 | 7.99 | 1.55 | 0.126 |
| Lenticular N | 38.98 | 4.60 | 41.84 | 7.45 | 2.70 | 0.056 |
Table 5.
RCMRglc (PET) region of interest values (% of whole brain uptake)
| HIV infected (N = 40) | HIV negative (N = 20) | t | P | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Caudate N | 113.28 | 14.12 | 114.45 | 16.72 | 0.285 | 0.777 |
| Lenticular N | 158.06 | 7.06 | 159.13 | 8.85 | 0.504 | 0.616 |
Further analysis
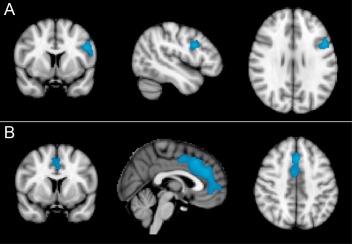
As we considered it important to verify the negative results in relationship to HIV status we performed a secondary analysis in which we deliberately traded specificity for additional sensitivity (i.e., accepted the likelihood of a higher number of Type I errors, to still further reduce the likelihood of Type II errors). By relaxing the voxel‐wise threshold value at P = 0.01 and the cluster P value to an uncorrected value of P < 0.05, a single significant cluster appeared for the main effect of HIV status in the PET data set and the ASL data set (see Fig. 6). Interestingly, these clusters were located in near‐identical anatomical regions, in the right anterior and inferior portion of the anterior cingulate cortex (ACC), in each independent data set.
Figure 6.
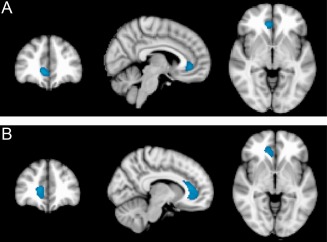
Area of reduced (A) regional cerebral blood flow (rCBF) and (B) regional cerebral metabolic rate of glucose (rCMRglc) in HIV‐infected participants relative to HIV‐negative controls in the right anterior and inferior region of anterior cingulate cortex (cluster level uncorrected P < 0.05). Slides in radiological convention.
DISCUSSION
PET and ASL imaging in HIV‐1 infection offer the potential to reveal regional, metabolic and physiological changes in the brain during the early stages of HIV‐1 infection [Ances et al., 2009; Chang et al., 2003]. In PET investigations, symptomatic HIV‐infection has shown to be associated with increased basal ganglia uptake [Hinkin et al., 1995; Rottenberg et al., 1996; van Gorp et al., 1992]. In addition, Ances et al. [2006, 2009] have similarly found significant reductions in caudate and lenticular nucleus blood flow and volume in HIV‐1‐infected patients using ASL.
To date there is limited evidence with regard to rCBF and FDG uptake in asymptomatic HIV‐infection, and no studies which have used both together. This study did not replicate the findings of Ances et al. 2009 of reduced rCBF in the lenticular nucleus in HIV‐1‐infected participants in the asymptomatic stage, although we did find a trend (P < 0.06) toward a significant effect in the lenticular nucleus. However, as Ances et al. 2009 note, many of their participants had detectable plasma viral loads, whereas our sample all had undetectable plasma viral loads. Other potentially important differences between the studies are treatment rates. In the current study 100% of participants were treated with HAART medication. In the Ances et al. 2009 study this rate was 82%. These differences in treatment, and in particular differences in the CNS penetration effectiveness, may also partially explain the differences in our findings.
In examining for age effects, we found significantly reduced rCBF and reduced FDG uptake (P < 0.05, cluster level corrected in both cases) in bilateral midline frontal and left dorso‐lateral frontal regions; and increased rCBF and rCMRglc in various regions including the pulvinar (posterior thalamus) bilaterally. These findings match the results of studies of normal ageing which have found reduced rCBF in a number of limbic and association cortex areas including cingulate, parahippocampal, superior temporal, medial frontal, and posterior parietal cortices bilaterally, and in the left insular and left posterior prefrontal cortices [e.g., Martin et al., 1991]. Interestingly, in further analyses, we found that by relaxing our significance levels (P < 0.05, uncorrected) we were able to detect areas of reduced rCBF and rCMRglc in near identical anatomical regions involving the ACC. Whilst this should be regarded as preliminary, very few studies in any disorder to date have included both PET and ASL, and the two findings together provide convergent evidence.
The ACC is an area involved in higher cortical functions and has been hypothesized to play a role in initiation, motivation and goal‐directed behaviors [Devinsky et al., 1995]. The ACC has also been hypothesized to play a role in the regulation of affect, and lesions of the region have been found to produce such changes as disinhibition, and impulsivity [Devinsky et al., 1995]. It has been suggested that oxidative stress‐mediated neuronal apoptosis associated with HIV‐infection may damage the attention network, including the ACC [Ernst et al., 2009]. Alternatively, it could be argued that impaired ACC function might predispose HIV‐infected individuals to engaging in behavior which increases their risk of secondary infection and cognitive impairment. A third possibility is that changes observed were because of HAART medication, rather than any premorbid or disease‐related factors.
In this study of medically and psychiatrically stable HIV‐1‐infected participants, there was no significant interaction between HIV status and the ageing process. This finding is consistent with our findings in a parallel study investigating gray and white matter integrity [Towgood et al., 2011], although we did find a region of HIV‐related loss of gray matter volume in the superior and medial frontal lobes in that investigation. However, in the present study, we have found related age reductions in rCBF and rCMRglc in bilateral midline frontal and left dorso‐lateral frontal regions on ASL and PET, respectively. We have also found converging evidence of reduced rCBF and rCMRglc in the ACC in HIV which, although minor in degree in these asymptomatic HIV participants, may be a precursor of future behavioral change and have clinical significance. In addition, it is important to note that this is the first paper to combine evidence from ASL and PET methods, and it is encouraging that we have obtained similar results using both techniques. Further examination of the correlation between these two imaging techniques would be valuable. Our study has also indicated that there are no significant HIV‐related changes elsewhere in the brain if HIV has been well controlled and confounding disorders, including substance or alcohol misuse, have been avoided.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of all the participants who took part in the study. They also thank the staff at the Lawson Unit at Brighton and Sussex University Hospitals NHS Trust and the HIV teams at St Mary's Hospital, Paddington, the Royal Free Hospital, and King's College Hospital for their much appreciated help and support in recruiting participants. They are indebted to David Alsop for making the ASL pulse sequence available. They also thank Professor Steve Williams and the radiography staff at the Centre for Neuroimaging Sciences, nstitute of Psychiatry, King's College London (KCL) and the staff at the PET Imaging Centre, St Thomas's Hospital for their assistance.
REFERENCES
- American Psychiatric Association (1994):Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM‐IV).Washington, DC:American Psychiatric Association. [Google Scholar]
- Ances BM, Roc AC, Wang J, Korczykowski M, Okawa J, Stern J, Kim J, Wolf R, Lawler K, Kolson DL, Detre JA (2006): Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology 66:862–866. [DOI] [PubMed] [Google Scholar]
- Ances BM, Sisti D, Vaida F, Liang CL, Leontiev O, Perthen JE, Buxton RB, Benson D, Smith DM, Little SJ, Richman DD, Moore DJ, Ellis RJ; HNRC group (2009)Resting cerebral blood flow: A potential biomarker of the effects of HIV in the brain. Neurology 73:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA (1987): Beck Depression Inventory Manual, The Psychological Corporation, Harcourt Brace Jovanovich Inc., San Antonio. [Google Scholar]
- Beck AT, Steer RA (1990):Beck Anxiety Inventory.San Antonio, TX:The Psychological Corporation. [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Walot I, Jovicich J, DeSilva M, Trivedi N, Speck O, Miller EN (2003): Persistent brain abnormalities in antiretroviral‐naive HIV patients 3 months after HAART. Antivir Ther 8:17–26. [PubMed] [Google Scholar]
- Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, Weiner MW (2010): ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord 2010;24:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Garcia D, de BC, Alsop DC (2009): Continuous flow‐driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008;60:1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detels R, Tarwater P, Phair JP, Margolick J, Riddler SA, Munoz A (2001): Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis. AIDS 15:347–355. [DOI] [PubMed] [Google Scholar]
- Detre JA, Alsop DC (1999): Perfusion magnetic resonance imaging with continuous arterial spin labeling: Methods and clinical applications in the central nervous system. Eur J Radiol 30:115–124. [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J, Wang Z, Rao H (2009): Arterial spin‐labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol 22:348–355. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behavior. Brain 118( Part 1):279–306. [DOI] [PubMed] [Google Scholar]
- Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ (2003): Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS 17:1539–1545. [DOI] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo‐Dukelow ML, Chang L (2009): Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol 65:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ Jr, Feinberg JE, Balfour HH Jr, Deyton LR, Chodakewitz JA, Fischl MA (1997): A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 337:725–733. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, Wolfson T, Velin R, Marcotte TD, Hesselink JR, Jernigan TL, Chandler J, Wallace M, Abramson I; HNRC group (1995): The HNRC 500—Neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc 1:231–251. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera‐Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema‐Notestine C, Jernigan TL, Wong J, Grant I; Charter Group (2010): HIV‐associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema‐Notestine C, Jernigan TL, Wong J, Grant I; Charter Group; HNRC Group (2011): HIV‐associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J Neurovirol 17( Part 1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, van Gorp WG, Mandelkern MA, Gee M, Satz P, Hoiston S, Marcotte TD, Evans G, Paz DH, Ropchan JR, Quinones N, Khonsary A, Blahd WH (1995): Cerebral metabolic change in patients with AIDS: Report of a six‐month follow‐up using positron‐emission tomography. J Neuropsychiatry Clin Neurosci 7:180–187. [DOI] [PubMed] [Google Scholar]
- Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS (1991): Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 11:684–689. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Willison J (1991):National Adult Reading Test (NART): Test Manual, 2nd edWindsor, UK:NFER‐Nelson Publishing Company. [Google Scholar]
- Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Furher J, Satten GA, Aschman DJ, Holmberg SD (1998): Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860. [DOI] [PubMed] [Google Scholar]
- Paul RH, Ernst T, Brickman AM, Yiannoutsos CT, Tate DF, Cohen RA, Navia BA; ACTG 301 team; ACTG 700 team (2008): Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. J Int Neuropsychol Soc 14:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ (2007): The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 21:1915–1921. [DOI] [PubMed] [Google Scholar]
- Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, Price RW (1996): Abnormal cerebral glucose metabolism in HIV‐1 seropositive subjects with and without dementia. J Nucl Med 37:1133–1141. [PubMed] [Google Scholar]
- Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P (2011): HIV‐1 infection and cognitive impairment in the cART‐era: A review. AIDS 25:561–575. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Amico KR, Smith L, Nelson K (2010): Antiretroviral adherence interventions: Translating research findings to the real world. Curr HIV/AIDS Rep 7:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez S, Baril L, Stankoff B, Khellaf M, Dubois B, Lubetzki C, Bricaire F, Hauw JJ (2001): Outcome of patients with HIV‐1‐related cognitive impairment on highly active antiretroviral therapy. AIDS 15:195–200. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, Lopez OL, Aizensthein HJ, Toga AW, Becker JT (2006): 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage 31:12–23. [DOI] [PubMed] [Google Scholar]
- Thurnher MM, Donovan‐Post MJ (2008): Neuroimaging in the brain in HIV‐1‐infected patients. Neuroimaging Clin N Am 18:93–117. [DOI] [PubMed] [Google Scholar]
- Towgood KJ, Pitkanen M, Kulasegaram R, Fradera A, Kumar A, Soni S, Sibtain NA, Reed L, Bradbeer C, Barker GJ, Kopelman MD (2011): Mapping the brain in younger and older asymptomatic HIV‐1 men: Frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex 48:230–241. [DOI] [PubMed] [Google Scholar]
- Tucker KA, Robertson KR, Lin W, Smith K, An H, Chen Y, Aylward SR, Hall CD (2004): Neuroimaging in human immunodeficiency virus infection. J Neuroimmunol 157:153–162. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Mandelkern MA, Gee M, Hinkin CH, Stern CE, Paz DK, Dixon W, Evans G, Flynn F, Frederick CJ (1992): Cerebral metabolic dysfunction in AIDS: Findings in a sample with and without dementia. J Neuropsychiatry Clin Neurosci 4:280–287. [DOI] [PubMed] [Google Scholar]
- von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ, Arendt G. (2000): Potential time course of human immunodeficiency virus type 1‐associated minor motor deficits: Electrophysiologic and positron emission tomography findings. Arch Neurol 57:1601–1607. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997):Wechsler Adult Intelligence Scale—Administration and Scoring Manual, 3rd edLondon, UK:The Psychological Corporation. [Google Scholar]


