Abstract
A meta‐analysis of 140 neuroimaging studies was performed using the activation‐likelihood‐estimate (ALE) method to explore the location and extent of activation in the brain in response to noxious stimuli in healthy volunteers. The first analysis involved the creation of a likelihood map illustrating brain activation common across studies using noxious stimuli. The left thalamus, right anterior cingulate cortex (ACC), bilateral anterior insulae, and left dorsal posterior insula had the highest likelihood of being activated. The second analysis contrasted noxious cold with noxious heat stimulation and revealed higher likelihood of activation to noxious cold in the subgenual ACC and the amygdala. The third analysis assessed the implications of using either a warm stimulus or a resting baseline as the control condition to reveal activation attributed to noxious heat. Comparing noxious heat to warm stimulation led to peak ALE values that were restricted to cortical regions with known nociceptive input. The fourth analysis tested for a hemispheric dominance in pain processing and showed the importance of the right hemisphere, with the strongest ALE peaks and clusters found in the right insula and ACC. The fifth analysis compared noxious muscle with cutaneous stimuli and the former type was more likely to evoke activation in the posterior and anterior cingulate cortices, precuneus, dorsolateral prefrontal cortex, and cerebellum. In general, results indicate that some brain regions such as the thalamus, insula and ACC have a significant likelihood of activation regardless of the type of noxious stimuli, while other brain regions show a stimulus‐specific likelihood of being activated. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: pain, fMRI, PET, brain, human
INTRODUCTION
Advances in brain imaging techniques, including functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), have permitted a detailed view of nociceptive processing in the human brain. Reviews of neuroimaging studies examining “pain‐evoked” activation in the brain have reported an extensive network of cortical regions involved in nociceptive processing, including the primary (SI) and secondary (SII) somatosensory cortices, the anterior cingulate cortex (ACC), the insula, the prefrontal cortex, and the thalamus [Apkarian et al.,2005; Iadarola and Coghill,1999; Peyron et al.,1999]. While these reviews have been important for collating information, they only report common regions of “pain‐evoked” activation. For example, Apkarian et al. [2005] in their review of the pain neuroimaging literature reported that the most commonly activated region in response to noxious stimuli was the anterior insular cortex. While this information is useful in the general sense, such qualitative approaches do not permit a quantitative appreciation of the probabilistic spatial extent of “pain‐related” activation nor do they allow a more detailed assessment of the relative influence of experimental variables on the likelihood of observing this activation within the broad network of regions implicated in pain processing. Recent advances in meta‐analytic methods of assessing brain activation allow some of these limitations to be addressed. Meta‐analysis is a statistical technique whereby data are collected, analyzed, and compared from multiple independent studies to examine a particular research question. This approach is especially relevant to the study of the cortical and subcortical responses to noxious stimuli. By its nature, pain is a multidimensional sensory experience that leads to numerous candidate areas of brain activation; meta‐analysis can be a tool to help decipher the functionality of these varied regions of activation. The quantitative approach of this method yields a brain volume in which the probability of observing activation in response to noxious stimuli is computed at each voxel based on a large number of neuroimaging studies.
This review applies meta‐analytic techniques to examine journal articles published between 1991 and 2011, which report peak activation coordinates in response to noxious stimuli (Study 1). Additionally, as pain can be evoked by different types of peripheral stimuli (e.g., heat, cold, impact, and capsaicin injection) and under different experimental conditions, the large number of studies included in this review facilitated the exploration of three additional fundamental questions related to the study of brain activation in response to noxious stimuli. The second analysis, presented in Study 2, addresses the specificity of activation across different stimulus modalities by comparing activation sites associated with noxious cold stimulation with those evoked by noxious heat. The third analysis (Study 3) examines the influence of one particular aspect of experimental design (the use of a resting baseline or an innocuous warm stimulus condition) on the apparent activation evoked by noxious heat stimuli. The fourth analysis (Study 4) tests for possible evidence of hemispheric dominance for activation in response to noxious stimuli. Finally, the fifth analysis (Study 5) compared activation in response to noxious muscle stimuli with noxious cutaneous stimuli.
Study 1: Brain Activation in Response to All Noxious Stimuli
To provide improved information on the localization and spatial extent of pain‐related activation in the brain, the first meta‐analysis explores brain activation in response to all forms of noxious stimuli applied to the skin, muscle or viscera.
Study 2: Differential Brain Activation in Response to Noxious Cold and Heat Stimuli
The second section of this review examines differences in brain regions that process experimental noxious cold stimuli in comparison to those that process noxious heat stimuli. Three previous studies have suggested that cold pain evokes a similar pattern of brain activation as that seen in response to heat pain [Casey et al.,1996; Craig et al.,1996; Tracey et al.,2000]; however, cold pain is typically induced using the cold‐pressor task, which is considered a significant autonomic stressor with a high degree of unpleasantness. Kwan et al. reported large inter‐individual differences in brain activation evoked by cold‐pain stimulation [Kwan et al.,2000], which could be explained by the potential cultural and situational influences on pain affect. However, it is difficult to draw conclusions based on the results of these previous studies as they used relatively small numbers of subjects (N = 6 − 13) and did not perform any direct subtractions on the data to determine which brain areas were preferentially associated with processing noxious cold or noxious heat stimuli.
Study 3: Control Conditions for Noxious Heat
During pain‐imaging experiments, noxious heat stimuli are commonly generated using contact thermodes. The probe is placed on the skin and kept at a baseline temperature (30–32°C) between stimulus presentations. During the stimulation period, the temperature is increased to reach a level that is rated as painful by the subject. The gradual rise in temperature will inherently activate fibers that transmit warmth information [Raja et al.,1999] and may trigger orienting responses towards the stimulus. Therefore, when using a resting baseline as a control condition for noxious heat stimuli, the resulting statistical maps may reflect a contamination of the “pain‐related” brain activation with that associated with the warming of the skin and orienting responses that preceded the perception of pain.
Only a few imaging studies have specifically examined brain activation in response to warmth. Two of these studies reported that warm stimuli activate brain areas similar to those that process pain, with somewhat less robust activation [Becerra et al.,1999; Craig et al.,1996]; however, one group reported both similar regions and similar activation levels in the brain in response to noxious heat and innocuous warm stimuli [Moulton et al.,2005]. If innocuous warm‐ and noxious heat‐responsive cortical neurons are distinct and coexist within spatially defined regions of the brain, then warm stimuli may be an inappropriate control for a noxious‐heat condition, since a statistical comparison between the two may result in an underestimation of activation associated with noxious stimuli.
However, another potential confound may result if warm stimuli evoke activation in brain regions that do not process pain. For example, Sung et al. [2007] reported activation in several regions outside of the commonly described “pain matrix” (as well as in regions frequently associated with pain perception) evoked by warm stimuli that were perceived as pleasant and comfortable. Although Sung et al. [2007] did not present a noxious heat condition, their results underscore the potential problems that would arise in a statistical comparison for “pain‐evoked” responses across regions that are more activated during a warm “control” condition (i.e., apparent inhibition by noxious heat, which may or may not be an appropriate interpretation). Furthermore, as indicated by the perceptual ratings of the warm stimuli used by Sung et al. [2007], statistical contrasts between innocuous and noxious heat stimuli may not be appropriate, as the perception of warmth is not merely a lower intensity of thermal pain or unpleasantness, but may be considered a separate sensory modality with distinctly different (positive) affective qualities. In turn, this may render the subsequent subtractions difficult to interpret.
To date, no study has compared the effects of using either a resting baseline or innocuous warm stimuli on the apparent activation in the brain in response to noxious heat stimuli. We examined the advantages and disadvantages of the two subtraction strategies by performing a meta‐analysis on a similar number of studies that used one or the other contrast.
Study 4: Hemispheric Dominance for Activation in Response to Noxious Stimuli
It is generally believed that somatosensory stimuli are processed primarily or preferentially by the hemisphere that is contralateral to the point of stimulation. However, evidence from clinical studies in patients with brain lesions and from brain imaging studies of normal pain processing has called this theory into question.
Results suggesting the possibility of a bilateral pain‐processing network come from psychophysical data obtained from patients. For example, hemispherectomized patients can perceive painful stimuli that are either contralateral or ipsilateral to their only functioning hemisphere, albeit with poor localization [Olausson et al.,2001]. Additionally, recent evidence from an fMRI study with callosotomized patients demonstrated that ipsilateral brain regions responsible for processing pain (SI, SII, insula, and cingulate cortex) could be activated in response to noxious heat stimuli [Duquette et al.,2008].
Neuroimaging studies examining the BOLD nociceptive signal associated with stimuli applied exclusively to one side of the body have often reported bilateral activation in a number of brain regions involved in sensory‐discriminative and affective‐motivational pain processing. Common regions of bilateral activation include ACC, prefrontal cortex, SII, insula, thalamus, inferior parietal lobule [for example see Bingel et al.,2004b,2007a,b; Boly et al.,2007; Bornhovd et al.,2002; Buchel et al.,2002], and in some instances, SI [for example see Bingel et al.,2004b; Cole et al., 2008; Staud et al.,2007; Straube et al.,2008]. A previous activation‐likelihood‐estimate (ALE) meta‐analysis examined concordant brain activation sites evoked by noxious stimuli from 22 original studies that applied stimuli to the upper arms [Farrell et al.,2005]. These authors reported that the likelihood of activation was generally bilateral, except in left prefrontal cortex and right SI. However, the finding of a significant likelihood of activation in right SI, instead of in bilateral SI (as would be predicted given that the stimuli were applied to both sides of the body) was likely due to the inclusion of a greater number of foci from studies that had presented stimuli to the left arms (Left: 249 vs. Right: 140). For this reason, it is difficult to draw conclusions about lateralization of nociceptive processing from this previous meta‐analysis, as they did not perform their comparisons on a similar number of activation sites.
Additional evidence that is inconsistent with a strictly ontralateral processing of nociceptive information comes from psychophysical studies on healthy subjects suggesting a possible right‐hemisphere dominance for pain processing. For example, individuals exhibit lower pain thresholds and rate pain as more intense when noxious stimuli are applied to the left side of the body (processed by the contralateral right hemisphere) [Haslam,1970; Jensen et al.,1992; Lugo et al.,2002; Pauli et al.,1999b; Sarlani et al.,2003]. In a study of chronic pain patients, Hsieh et al. [1995] found activation lateralized to the right ACC regardless of the limb in which pain was experienced. However, other regions, such as the anterior insula, posterior parietal, lateral inferior prefrontal, and posterior cingulate cortices, were activated bilaterally.
Two imaging studies, which specifically tested for hemispheric differences in pain processing in healthy subjects, have provided additional evidence that some brain regions in the right hemisphere preferentially process pain. Coghill et al. [2001] reported right‐lateralized activation in thalamus, inferior parietal lobule, dorsolateral, and dorsal prefrontal cortex in response to noxious and innocuous heat stimuli applied to either forearm. More recently, Symonds et al. [2006] described an fMRI study in which noxious electrical stimuli applied to the right and left fingertips evoked a predominant right hemispheric activation of the ACC (BA 32), the middle frontal gyrus (BA 9/46/10), the medial and superior frontal gyri (BA 6/8), ventrolateral prefrontal cortex, and the inferior parietal lobule. Both studies, however, used relatively small samples (N = 9), making generalizability of results rather uncertain.
To better distinguish brain regions that may participate in a lateralized dominance of pain processing, we conducted a meta‐analysis on a similar number of imaging studies that applied noxious stimuli to the left or to the right side of the body.
Study 5: Neural Processing of Noxious Muscle and Cutaneous Stimuli
The majority of functional neuroimaging studies have primarily focused on exploring the cerebral mechanisms that process cutaneous pain; however, the majority of chronic pain syndromes originate in muscles (i.e., myositides, fibromyalgia, muscle ischemia, and some forms of low back pain), joints (i.e., rheumatoid arthritis), and viscera (i.e., irritable bowel syndrome). Moreover, the sensations associated with even a minor deep tissue injury can result in prolonged allodynia near and adjacent to the site, while a minor skin injury can cause a more spatially localized sensation. Psychophysical studies have documented that painful sensations due to muscle or skin damage are perceived differently. Muscle injury is referred to as diffuse, aching, or cramping, and is often poorly localized as the sensation of muscle pain can be referred to distant sites [Arendt‐Nielsen and Svensson,2001; Graven‐Nielsen and Mense,2001; Graven‐Nielsen et al.,1997; Mense,1993]. Conversely, noxious cutaneous stimuli are described as sharp, burning and localized [Mense,1993]. Due to the prolonged and radiating pain associated with muscle damage, it has been suggested that these types of pain are mediated by central mechanisms [Wall and Woolf,1984]. Therefore, potentially different cerebral mechanisms are responsible for the processing of noxious muscle and cutaneous stimuli that could account for these perceptual differences. Thus, we assessed with a fifth meta‐analysis whether noxious muscle stimuli evoked activation in specific or overlapping brain structures that also process noxious cutaneous stimuli.
METHODS
Article Selection
The Study 1 database (all noxious stimuli) was created from a compilation of journal articles retrieved from several sources and using noxious stimuli applied to the skin, muscle, or viscera. Articles reporting brain activation coordinates in response to noxious stimuli were retrieved initially using reference lists from the more recent reviews of “pain‐evoked” activation brain imaging studies [Apkarian et al.,2005; Farrell et al.,2005]. A subsequent Medline search was initiated using the keywords: pain, noxious, PET, fMRI, experimental, and healthy. Articles were also retrieved from the references in the original research articles collected. The database variables included (1) Author names; (2) Year of publication; (3) Size of the Gaussian smoothing filter; (4) Number of subjects; (5) Stimulus modality (laser, electrical, impact, etc.); (6) System targeted by noxious stimuli (cutaneous, muscle, visceral, etc.); (7) Side of the body; (8) Body part; (9) Type of standardized space; (10) Brain activation coordinates.
We initially conducted a search of the neuroimaging literature published between 1980 and 2011 to retrieve articles that used noxious stimuli. Articles selected for inclusion in the database satisfied the following criteria: (a) data were acquired in healthy subjects; (b) the activation sites were the result of a contrast that compared a noxious stimulus condition to a resting baseline, or to a control condition, or to a noxious stimulus condition that was rated by participants as less painful, or to a no‐stimulus condition conducted in a control group of participants. Likewise, articles were included in which the activation sites were determined by correlating brain activity with participants' perceptual levels of pain intensity or unpleasantness. Excluded from the analysis were studies that reported coordinates that combined painful and nonpainful stimuli.
In total 140 studies were included in the Study 1 analysis, 8 of which were based on further analysis of data from previous publications, leading to a total of 132 original articles (Table I). The majority of studies (104) used cutaneously administered stimuli (contact thermodes, laser, impact, pressure, electric shock, pinprick, topical capsaicin, or incision). However, some of these studies used more than one type of noxious stimulus within the same experimental protocol. Eleven studies used painful visceral stimuli (esophageal, rectal, stomach, and vascular distension), while four used intracutaneous stimuli (ethanol injection, capsaicin injection, electric shock, or infusion of a phosphate buffer), seven used transcutaneous stimuli (electric shock), seven used subcutaneous injections (ascorbic acid, capsaicin, and hypertonic saline), seven were intramuscular (electric shock, hypertonic saline injection, and infusion of a phosphate buffer), three were muscular, four were intracutaneous, one used intranasal gaseous CO2, and one applied noxious stimuli to the tooth pulp. In most instances, stimuli were applied to the upper limbs (104 studies). Of the remaining studies, 22 used noxious stimuli applied to the lower limbs, 11 to the face, 3 to the trunk, and 10 were applied internally.
Table I.
List of studies included in study 1 (all noxious stimuli)
| Study # | Author | Year | Imaging | N | Stimuli | Side | Body Part | Notes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Modality | System | ||||||||
| 1 | Adler et al. | 1997 | PET | 9 | Heat | Thermal | Cutaneous | Left | Forearm | |
| 2 | Aharon et al. | 2006 | fMRI 1.5 | 6 | Heat | Thermal | Cutaneous | Left | Hand | |
| 3 | Albanese et al. | 2007 | fMRI 1.5 | 8 | Heat | Thermal | Cutaneous | Right | Hand | |
| 4 | Andersson et al. | 1997 | PET | 6 | Capsaicin injection | Chemical | Intracutaneous | Right | Hand and foot | |
| 5 | Apkarian et al. | 2000 | fMRI 1.5 | 7 | Heat | Thermal | Cutaneous | Right | Fingers | |
| 6 | Aziz et al. | 1997 | PET | 8 | Esophageal distention | Mechanical | Visceral | Bilateral | Esophagus | |
| 7 | Becerra et al. | 1999 | fMRI 1.5 | 6 | Heat | Thermal | Cutaneous | Left | Hand | |
| 8 | Becerra et al. | 2001 | fMRI 1.5 | 8 | Heat | Thermal | Cutaneous | Left | Hand | |
| 9 | Bingel et al. | 2002 | fMRI 1.5 | 14 | Laser | Thermal | Cutaneous | Left and Right | Hand | |
| 10 | Bingel et al. | 2003 | fMRI 1.5 | 14 | Laser | Thermal | Cutaneous | Left and Right | Hand | Data are shared with Bingel et al., 2002 |
| 11 | Bingel et al. | 2006 | fMRI 1.5 | 19 | Laser | Thermal | Cutaneous | Left and Right | Hand | |
| 12 | Bingel et al. | 2004a | fMRI 1.5 | 20 | Laser | Thermal | Cutaneous | Left and Right | Hand and foot | |
| 13 | Bingel et al. | 2004b | fMRI 1.5 | 18 | Laser | Thermal | Cutaneous | Left | Hand and foot | |
| 14 | Bingel et al. | 2007a | fMRI 3 | 16 | Laser | Thermal | Cutaneous | Left | Hand | |
| 15 | Bingel et al. | 2007b | fMRI 3 | 20 | Heat | Thermal | Cutaneous | Left | Forearm | |
| 16 | Binkofski et al. | 1998 | fMRI 1.5 | 5 | Esophageal distention | Mechanical | Visceral | Bilateral | Esophagus | |
| 17 | Boly et al. | 2007 | fMRI 3 | 24 | Laser | Thermal | Cutaneous | Left | Hand | |
| 18 | Bornhovd et al. | 2002 | fMRI 1.5 | 9 | Laser | Thermal | Cutaneous | Left | Hand | Data are shared with Buchel et al., 2002 |
| 19 | Borsook et al. | 2003 | fMRI 1.5 | 9 | Heat | Thermal | Cutaneous | Right | Face | Data are shared with Dasilva et al., 2002 |
| 20 | Botvinick et al. | 2005 | fMRI 1.5 | 12 | Heat | Thermal | Cutaneous | Left | Thenar Eminence | |
| 21 | Brooks et al. | 2005 | fMRI 3 | 14 | Heat | Thermal | Cutaneous | Right | Face, foot, and hand | |
| 22 | Buchel et al. | 2002 | fMRI 1.5 | 9 | Laser | Thermal | Cutaneous | Left | Hand | |
| 23 | Carlsson et al. | 2006 | fMRI 1.5 | 9 | Electrical shock | Electrical | Cutaneous | Right | Wrist | |
| 24 | Casey et al. | 1994 | PET | 18 | Heat | Thermal | Cutaneous | Left | Arm | |
| 25 | Casey et al. | 1996 | PET | 27 | Cold | Thermal | Cutaneous | Left | Hand | |
| 26 | Casey et al. | 2000 | PET | 11 | Cold | Thermal | Cutaneous | Left | Hand | |
| 27 | Casey et al. | 2001 | PET | 14 | Heat | Thermal | Cutaneous | Left | Forearm | |
| 28 | Chen et al. | 2002 | fMRI 1.5 | 4 | Heat | Thermal | Cutaneous | Left | Inner calf | |
| 29 | Christmann et al. | 2007 | fMRI 1.5 | 6 | Electrical Shock | Electrical | Transcutaneous | Right | Thumb | |
| 30 | Coen et al. | 2007 | fMRI 1.5 | 7 | Esophageal distention | Mechanical | Visceral | Bilateral | Esophagus | |
| 31 | Coghill et al. | 1994 | PET | 9 | Heat | Thermal | Cutaneous | Left | Forearm | |
| 32 | Coghill et al. | 1999 | PET | 16 | Heat | Thermal | Cutaneous | Right | Upper arm | |
| 33 | Coghill et al. | 2001 | PET | 9 | Heat | Thermal | Cutaneous | Left and Right | Forearm | |
| 34 | Coghill and Eisenach; Coghill et al. | 2003 | fMRI 1.5 | 17 | Heat | Thermal | Cutaneous | Right | Leg | |
| 35 | Cole et al. | 2008 | fMRI 1.5 | 30 | Pressure | Mechanical | Cutaneous | Right | Thumb | |
| 36 | Craig et al. | 1996 | PET | 11 | Cold | Thermal | Cutaneous | Right | Hand | |
| 37 | Craig et al. | 1996 | Heat | Thermal | Cutaneous | Right | Hand | |||
| 38 | DaSilva et al. | 2002 | fMRI 1.5 | 9 | Heat | Thermal | Cutaneous | Right | Opthalmic, Maxillary, Mandibular, thumb | |
| 39 | Davis and Pope | 2002 | fMRI 1.5 | NR | Cold Prickle | Thermal and mechanical | Cutaneous | Right | Thenar eminence | |
| 40 | de Leeuw et al. | 2006 | fMRI 1.5 | 9 | Heat | Thermal | Cutaneous | Left | Masseter muscle | |
| 41 | Derbyshire and Jones; Derbyshire et al. | 1998 | PET | 7 | Heat | Thermal | Cutaneous | Left | Hand | Data are shared with Vogt et al., 1996 |
| 42 | Derbyshire et al. | 1997 | PET | 12 | Laser | Thermal | Cutaneous | Right | Hand | |
| 43 | Derbyshire and Jones; Derbyshire et al. | 1998 | PET | 12 | Heat | Thermal | Cutaneous | Right | Hand | |
| 44 | Derbyshire et al. | 2002a,b | PET | 21 | Laser | Thermal | Cutaneous | Right | Hand | |
| 45 | Derbyshire et al. | 2002a,b | PET | 16 | Heat | Thermal | Cutaneous | Right | Hand | |
| 46 | Derbyshire | 2004 | fMRI 3 | 8 | Heat | Thermal | Cutaneous | Right | Hand | |
| 47 | Downar et al. | 2003 | fMRI 1.5 | 10 | Electrical Shock | Electrical | Transcutaneous | Right | Median nerve | |
| 48 | Dunckley et al. | 2005 | fMRI 3 | 10 | Heat and rectal distention | Thermal and mechanical | Cutaneous and visceral | Bilateral/ Left | Back and rectum/Foot | |
| 49 | Fairhurst et al. | 2007 | fMRI 3 | 12 | Heat | Thermal | Cutaneous | Left | Hand | |
| 50 | Farrell et al. | 2006 | PET | 10 | Pressure | Mechanical | Cutaneous | Left | Thumb | |
| 51 | Ferretti et al. | 2003 | fMRI 1.5 | 8 | Electrical Shock | Electrical | Cutaneous | Right | Median nerve | |
| 52 | Frankenstein et al. | 2001 | fMRI 1.5 | 12 | Cold | Thermal | Cutaneous | Right | Foot | |
| 53 | Gelnar et al. | 1999 | fMRI 1.5 | 9 | Heat | Thermal | Cutaneous | Right | Finger | |
| 54 | Gyulai et al. | 1997 | PET | 5 | Heat | Thermal | Cutaneous | Left | Forearm | |
| 55 | Helmchen et al. | 2003 | fMRI 1.5 | 18 | Heat | Thermal | Cutaneous | Right | Hand | |
| 56 | Helmchen et al. | 2006 | fMRI 1.5 | 18 | Heat | Thermal | Cutaneous | Right | Hand | Data are shared with Helmchen et al., 2003 |
| 57 | Henderson et al. | 2007 | fMRI 3 | 23 | Hypertonic Saline Injection | Mechanical | Intramuscular and subcutaneous | Right | Leg and forearm | |
| 58 | Hofbauer et al. | 2001 | PET | 10 | Heat | Thermal | Cutaneous | Left | Hand | |
| 59 | Hofbauer et al. | 2004 | PET | 15 | Heat | Thermal | Cutaneous | Left | Forearm | |
| 60 | Hsieh et al. | 1996 | PET | 4 | Ethanol Injection | Chemical | Intracutaneous | Right | Upper arm | |
| 61 | Iadarola et al. | 1998 | PET | 13 | Capsaicin injection | Chemical | Subcutaneous | Left | Forearm | |
| 62 | Iannilli | 2008 | fMRI 1.5 | 18 | Electric shock, Gaseous CO2 | Electrical and chemical | Cutaneous and intranasal | Right | Forehead and trigeminal branch | |
| 63 | Ibinson et al. | 2004 | fMRI 1.5 | 6 | Electrical shock | Electrical | Cutaneous | Right | Median nerve | |
| 64 | Jantsch et al. | 2005 | fMRI 1.5 | 8 | Electrical Shock | Electrical | Cutaneous | Left | Upper incisor | |
| 65 | Keltner et al. | 2006 | fMRI 4.0 | 16 | Heat | Thermal | Cutaneous | Left | Hand | |
| 66 | Kong et al. | 2006 | fMRI 3 | 16 | Heat | Thermal | Cutaneous | Right | Forearm | |
| 67 | Korotkov et al. | 2002 | PET | 16 | Hypertonic Saline Injection | Mechanical | Intramuscular | Left | Tricep | |
| 68 | Koyama et al. | 2003 | fMRI 1.5 | 9 | Heat | Thermal | Cutaneous | Right | Leg | |
| 69 | Koyama et al. | 2005 | fMRI 1.5 | 10 | Heat | Thermal | Cutaneous | Right | Leg | |
| 70 | Kupers et al. | 2004 | PET | 10 | Hypertonic Saline Injection | Mechanical | Intramuscular | Right | Masseter muscle | |
| 71 | Kurata et al. | 2002 | fMRI 3 | 5 | Heat | Thermal | Cutaneous | Left and Right | Forearm | |
| 72 | Kurata et al. | 2005 | fMRI 3 | 6 | Heat | Thermal | Cutaneous | Right | Forearm | |
| 73 | Ladabaum et al. | 2001 | PET | 15 | Gastric Distention | Mechanical | Visceral | Bilateral | Stomach | |
| 74 | Ladabaum et al. | 2001 | fMRI 1.5 | 10 | Gastric Distention | Mechanical | Visceral | Bilateral | Stomach | |
| 75 | Lorenz et al. | 2002 | PET | 14 | Heat | Thermal | Cutaneous | Left | Forearm | |
| 76 | Lorenz et al. | 2008 | fMRI 1.5 | 11 | Pressure | Mechanical | Cutaneous | Right | Tibia | |
| 77 | Lu et al. | 2004 | fMRI 3 | 10 | Gastric Distention | Mecahnical | Visceral | Bilateral | Gastric fundus | |
| 78 | Lui et al. | 2008 | fMRI 1.5 | 14 | Pin Prick | Mechanical | Cutaneous | Right | Hand | |
| 79 | Maihofner et al. | 2004 | fMRI 1.5 | 11 | Heat and topical capsaicin | Thermal and chemical | Cutaneous | Left | Forearm | |
| 80 | Maihofner and Handwerker | 2005 | fMRI 1.5 | 12 | Pin prick and topical capsaicin | Mechanical and thermal | Cutaneous | Left | Forearm | |
| 81 | Maihofner et al. | 2006 | fMRI 1.5 | 14 | Heat and impact | Thermal and mechanical | Cutaneous | Right | Forearm | |
| 82 | Maihofner et al. | 2011 | fMRI 1.5 | 12 | Impact | Mechanical | Cutaneous | Left | Hand | |
| 83 | Mainero | 2007 | fMRI 3 | 11 | Heat/Capsaicin | Thermal/ Chemical | Cutaneous | Right | Ophthalmic | |
| 84 | May et al. | 1998 | PET | 7 | Capsaicin injection | Thermal | Subcutaneous | Right | Forehead | |
| 85 | Mochizuki et al. | 2007 | fMRI 3 | 14 | Cold | Thermal | Cutaneous | Left and Right | Wrist and hand | |
| 86 | Mohr et al. | 2008 | fMRI 1.5 | 17 | Heat | Thermal | Cutaneous | Right | Thigh | |
| 87 | Mobascher et al. | 2010 | fMRI 3 | 12 | Laser | Heat | Cutaneous | Left | Hand | |
| 88 | Moulton et al. | 2011 | fMRI 3 | 11 | Heat | Thermal | Cutaneous | Left | Hand | |
| 89 | Nash et al. | 2010 | fMRI 3 | 28 | Hypertonic saline injection | Mechanical | Muscular and subcutaneous | Right | Masseter muscle and Jaw | |
| 90 | Nemoto et al. | 2003 | PET | 12 | Laser | Thermal | Cutaneous | Right | Forearm | |
| 91 | Niddam et al. | 2002 | fMRI 3 | 10 | Electrical Shock | Electrical | Intramuscular | Left | Hand | |
| 92 | Ochsner et al. | 2006 | fMRI 3 | 13 | Heat | Thermal | Cutaneous | Right | Forearm | |
| 93 | Oshiro et al. | 2007 | fMRI 1.5 | 12 | Heat | Thermal | Cutaneous | Left | Leg | |
| 94 | Owen et al. | 2008 | fMRI 3 | 14 | Heat | Thermal | Cutaneous | Left | Hand | |
| 95 | Owen et al. | 2010 | fMRI 3 | 13 | Hypertonic saline injection | Mechanical | Muscular | Left | Forearm | |
| 96 | Paulson et al. | 1998 | PET | 20 | Heat | Thermal | Cutaneous | Left | Forearm | |
| 97 | Petrovic et al. | 2002 | PET | 7 | Cold | Thermal | Cutaneous | Left | Hand | |
| 98 | Petrovic et al. | 2004a | PET | 7 | Cold | Thermal | Cutaneous | Left | Hand | Data are shared with Petrovic et al., 2002 |
| 99 | Petrovic et al. | 2004b | PET | 10 | Cold | Thermal | Cutaneous | Left | Hand | |
| 100 | Peyron et al. | 1999 | PET | 7 | Heat | Thermal | Cutaneous | Left and Right | Hand | |
| 101 | Pogatzki‐Zahn et al. | 2010 | fMRI 3 | 30 | Incision | Tissue damage | Cutaneous | Right | Forearm | |
| 102 | Porro et al. | 1998 | fMRI 1.5 | 24 | Ascorbic acid injection | Chemical | Subcutaneous | Left and Right | Foot | |
| 103 | Porro et al. | 2002 | fMRI 1.5 | 26 | Ascorbic Acid Injection | Chemical | Subcutaneous | Left and Right | Foot | |
| 104 | Qiu et al. | 2006 | fMRI 3 | 13 | Laser | Thermal | Cutaneous | Right | Hand | |
| 105 | Raij et al. | 2005 | fMRI 3 | 14 | Laser | Thermal | Cutaneous | Left | Hand | |
| 106 | Rainville et al. | 1997 | PET | 8 | Heat | Thermal | Cutaneous | Left | Hand | |
| 107 | Remy et al. | 2003 | fMRI 3 | 12 | Heat | Thermal | Cutaneous | Left | Hand | |
| 108 | Rolls et al. | 2003 | fMRI 3 | 8 | Pressure | Mechanical | Cutaneous | Left | Hand | |
| 109 | Ruehle et al. | 2006 | fMRI 1.5 | 11 | Electrical Shock | Electrical | Transcutaneous and Intracutaneous | Right | Foot | |
| 110 | Sawamoto et al. | 2000 | fMRI 1.5 | 10 | Laser | Thermal | Cutaneous | Right | Hand | |
| 111 | Schneider et al. | 2001 | fMRI 1.5 | 6 | Vascular Distention | Mechanical | Vascular | Left | Foot | |
| 112 | Schoedel et al. | 2008 | fMRI 1.5 | 11 | Impact | Mechanical | Cutaneous | Left | Middle finger | |
| 113 | Schreckenberger et al. | 2005 | PET | 10 | Infusion of phosphate buffer | Mechanical | Intracutaneous and intramuscular | Left | Hand | |
| 114 | Seifert and Maihofner | 2007 | fMRI 1.5 | 12 | Cold | Thermal | Cutaneous | Right | Forearm | |
| 115 | Seifert et al. | 2010 | fMRI 3 | 10 | Pin Prick | Mechanical | Cutaneous | Right | Forearm | |
| 116 | Seminowicz et al. | 2004 | fMRI 1.5 | 16 | Electrical Shock | Electrical | Transcutaneous | Left | Median nerve | |
| 117 | Seminowicz and Davis | 2006 | fMRI 1.5 | 22 | Electrical Shock | Electrical | Transcutaneous | Left | Median nerve | |
| 118 | Seminowicz and Davis | 2007 | fMRI 1.5 | 23 | Electrical Shock | Electrical | Transcutaneous | Left | Median nerve | |
| 119 | Song et al. | 2006 | fMRI 3 | 12 | Cold and distention | Thermal and Mechanical | Cutaneous and visceral | Left/ bilateral | Foot/rectum | |
| 120 | Sprenger et al. | 2006 | PET | 8 | Heat | Thermal | Cutaneous | Right | Forearm | |
| 121 | Stammler et al. | 2008 | fMRI 1.5 | 12 | Pin prick | Mechanical hyperalgesia | Cutaneous | Right | Forearm | |
| 122 | Staud et al. | 2007 | fMRI 3 | 11 | Heat | Thermal | Cutaneous | Right | Foot | |
| 123 | Straube et al. | 2008 | fMRI 1.5 | 24 | Electrical Shock | Electrical | Cutaneous | Left | Finger | |
| 124 | Strigo et al. | 2003 | fMRI 1.5 | 7 | Esophageal Distention and Heat | Mechanical and thermal | Visceral and Cutaneous | Bilateral | Esophagus and Chest | |
| 125 | Strigo et al. | 2005 | fMRI 1.5 | 7 | Esophageal Distention and Heat | Mechanical and thermal | Visceral and Cutaneous | Bilateral | Esophagus | |
| 126 | Svensson et al. | 1997 | PET | 10 | Electrical Shock and laser | Electrical and thermal | Intramuscular and Cutaneous | Left | Forearm and Elbow | |
| 127 | Svensson et al. | 1998 | PET | 10 | Heat | Thermal | Cutaneous | Right | Forearm | |
| 128 | Symonds et al. | 2006 | fMRI 3 | 9 | Electrical shock | Electrical | Transcutaneous | Left and Right | Index finger | |
| 129 | Talbot et al. | 1991 | PET | 8 | Heat | Thermal | Cutaneous | Right | Forearm | |
| 130 | Terekhin and Forster | 2006 | fMRI 1.5 | 14 | Impact | Mechanical | Cutaneous | Right | Index Finger | |
| 131 | Thunberg et al. | 2005 | PET | 19 | Hypertonic Saline Injection | Mechanical | Intramuscular | Right | Erector Spinae muscle | |
| 132 | Tolle | 1999 | PET | 12 | Heat | Thermal | Cutaneous | Right | Forearm | |
| 133 | Tracey et al. | 2000 | fMRI 1.5 | 6 | Cold and heat | Thermal | Cutaneous | Left | Hand | |
| 134 | Uematsu et al. | 2011 | fMRI 1.5 | 17 | Pressure | Mechanical | Muscular | right | Calf | |
| 135 | Vandenbergh et al. | 2005 | PET | 11 | Gastric Distention | Mechanical | Visceral | Bilateral | Stomach | |
| 136 | Vogt et al. | 1996 | PET | 7 | Heat | Thermal | Cutaneous | Left | Hand | |
| 137 | Wagner et al. | 2007 | PET | 7 | Heat | Thermal | Cutaneous | Right | Forearm | |
| 138 | Weigelt et al. | 2010 | fMRI 1.5 | 13 | Electric shock | Electrical | Cutaneous | Bilateral | Upper and lower canines | |
| 139 | Xu et al. | 1997 | PET | 6 | Laser | Thermal | Cutaneous | Left | Foot and hand | |
| 140 | Yilmaz et al. | 2010 | fMRI 1.5 | 21 | Pressure | Mechanical | Cunateous | Left | Index finger | |
Study 1 (all noxious stimuli): List of studies reporting brain activation coordinates evoked by externally and internally applied noxious stimuli. Abbreviations: fMRI, functional magnetic resonance imaging; PET, positron emission tomography; n, sample size; NR, not reported.
Study 2 consists of two meta‐analyses conducted on reports selected from the database described in Study 1. The first meta‐analysis was performed on 112 coordinates obtained from 9 studies that applied noxious cold stimuli to the upper limbs (Table II). The stimulus conditions included water baths, contact thermodes, and ice packs. For purposes of comparison, the second meta‐analysis was conducted on 122 activation foci from 9 studies employing noxious contact heat stimuli applied to the upper limbs (Table III). Studies for the noxious heat meta‐analysis were selected if they employed stimuli that were similar to those included in the noxious cold analysis in terms of stimulation site, imaging modality, and year of publication. Additionally, the stimuli included in the two meta‐analyses were matched for intensity (P = 0.57). As the ALE method does not take into consideration the number of studies but rather the number of coordinates, the studies were also selected so that they would be matched in terms of the number of reported coordinates. A Mann‐Whitney U test was performed to assess the number of coordinates reported in the studies selected for these two meta‐analyses and results indicated no difference between them (P = 0.5).
Table II.
List of studies included in study 2 (noxious cold)
| Author | Year | Imaging | Subject (N) | Stimuli | NRS | |||
|---|---|---|---|---|---|---|---|---|
| Modality | System | Side | Body Part | |||||
| Casey et al. | 2000 | PET | 11 | Thermal | Cutaneous | Left | Hand | NR |
| Casey et al. | 1996 | PET | 27 | Thermal | Cutaneous | Left | Hand | 7.89 |
| Craig | 1996 | PET | 11 | Thermal | Cutaneous | Right | Hand | NR |
| Davis and Pope | 2002 | fMRI 1.5 | NR | Thermal/mechanical | Cutaneous | Right | Palm | NR |
| Mochizuki et al. | 2007 | fMRI 3 | 14 | Thermal | Cutaneous | Left | Wrist | 7 |
| Petrovic et al. | 2002 | PET | 7 | Thermal | Cutaneous | Left | Hand | 5.3 |
| Petrovic et al. | 2004b | PET | 10 | Thermal | Cutaneous | Left | Hand | 5.9 |
| Seifert and Maihofner | 2007 | fMRI 1.5 | 12 | Thermal | Cutaneous | Right | Forearm | 4.08 |
| Tracey et al. | 2000 | fMRI 1.5 | 6 | Thermal | Cutaneous | Left | Hand | 7.9 |
Study 2 (noxious cold): List of studies reporting brain activation coordinates evoked by noxious cold stimuli. Abbreviations: fMRI, functional magnetic resonance imaging; PET, positron emission tomography; NR, not reported; NRS, Numerical rating scale.
Table III.
List of studies included study 2 (noxious heat)
| Author | Year | Imaging | Subject (N) | Type | Stimuli | Body Part | NRS | ||
|---|---|---|---|---|---|---|---|---|---|
| Modality | System | Side | |||||||
| Botvinick et al. | 2005 | fMRI 1.5 | 12 | Heat | Thermal | Cutaneous | Left | Forearm | 7 |
| Brooks et al. | 2005 | fMRI 3 | 14 | Heat | Thermal | Cutaneous | Right | Hand | 5.5 |
| Casey et al. | 2001 | PET | 14 | Heat | Thermal | Cutaneous | Left | Forearm | 8.93 |
| Coghill et al. | 1994 | PET | 9 | Heat | Thermal | Cutaneous | Left | Forearm | 8 |
| Lorenz et al. | 2002 | PET | 14 | Heat | Thermal | Cutaneous | Left | Forearm | 6 |
| Maihofner et al. | 2006 | fMRI 1.5 | 14 | Heat | Thermal | Cutaneous | Right | Forearm | 4 |
| Nemoto et al. | 2003 | PET | 12 | Laser | Thermal | Cutaneous | Right | Forearm | 7.6 |
| Tracey et al. | 2000 | fMRI 1.5 | 6 | Heat | Thermal | Cutaneous | Left | Hand | 7.7 |
| Xu et al. | 1997 | PET | 6 | Laser | Thermal | Cutaneous | Left | Hand | NR |
Study 2 (noxious heat): List of studies reporting brain activation coordinates evoked by noxious heat stimuli. Abbreviations: fMRI, functional magnetic resonance imaging; PET, positron emission tomography; NR, not reported; NRS, Numerical rating scale.
Study 3 was created based on a search of the general meta‐analysis of Study 1 for articles that used either innocuous warm stimuli or a resting baseline as a control condition for evaluating brain activation associated with noxious heat stimuli (applied to any part of the body). Nine studies that reported 131 activation foci described in the Study 1 database matched the inclusion criterion for examining noxious heat in comparison to a warm control condition (Table IV). Nine studies that reported a total of 149 coordinates from Study 1 met our inclusion criterion of comparing noxious heat stimuli with a resting baseline (Table V). These nine studies were matched to those included in the first analysis according to the following criteria: imaging modality, number and extent of activation sites, year of publication, and site of stimulation (Table IX). The pain intensity ratings reported in the two sets of studies were not significantly different from one another (P = 0.9). A Mann‐Whitney U test applied to the data indicated no significant differences between the numbers of activation foci included in the two meta‐analyses (P = 0.5).
Table IV.
List of studies included in study 3 (noxious heat vs. warm)
| Author | Year | Imaging | Subject (N) | Type | Stimuli | Body Part | NRS | ||
|---|---|---|---|---|---|---|---|---|---|
| Modality | System | Side | |||||||
| Adler et al. | 1997 | PET | 9 | Heat | Thermal | Cutaneous | Left | Forearm | 6.7 |
| Botvinick et al. | 2005 | fMRI 1.5 | 12 | Heat | Thermal | Cutaneous | Left | Hand | 7 |
| Casey et al. | 2001 | PET | 14 | Heat | Thermal | Cutaneous | Left | Forearm | 8.93 |
| Vogt et al. | 1996 | PET | 7 | Heat | Thermal | Cutaneous | Left | Hand | 6.2 |
| Derbyshire et al. | 1997 | PET | 12 | Laser | Thermal | Cutaneous | Right | Hand | 7 |
| Derbyshire and Jones; Derbyshire et al. | 1998 | PET | 12 | Heat | Thermal | Cutaneous | Right | Hand | 5.85 |
| Ochsner et al. | 2006 | fMRI 3 | 13 | Heat | Thermal | Cutaneous | Right | Forearm | 7 |
| Svensson et al. | 1998 | PET | 10 | Heat | Thermal | Cutaneous | Right | Forearm | 8 |
| Wagner et al. | 2007 | PET | 7 | Heat | Thermal | Cutaneous | Right | Forearm | 6.8 |
Study 3 (noxious heat vs. warm): List of studies reporting brain activation coordinates evoked by noxious heat stimuli in comparison to a warm control condition. Abbreviations: fMRI, functional magnetic resonance imaging; PET, positron emission tomography; NR, not reported; NRS, Numerical rating scale.
Table V.
List of studies included in study 3 (noxious heat vs. resting baseline)
| Author | Year | Imaging | Subject (N) | Type | Stimuli | Body Part | NRS | ||
|---|---|---|---|---|---|---|---|---|---|
| Modality | System | Side | |||||||
| Albanese et al. | 2007 | fMRI 1.5 | 8 | Heat | Thermal | Cutaneous | Right | Hand | 7 |
| Coghill et al. | 1994 | PET | 9 | Heat | Thermal | Cutaneous | Left | Forearm | 8 |
| Coghill et al. | 2001 | PET | 9 | Heat | Thermal | Cutaneous | Left | Forearm | 7.6 |
| Kurata et al. | 2005 | fMRI 3 | 6 | Heat | Thermal | Cutaneous | Right | Hand | 6.8 |
| Kurata et al. | 2002 | fMRI 3 | 5 | Heat | Thermal | Cutaneous | Right | Forearm | 7 |
| Maihofner et al. | 2006 | fMRI 1.5 | 14 | Heat | Thermal | Cutaneous | Right | Forearm | 4.3 |
| Nemoto et al. | 2003 | PET | 12 | Laser | Thermal | Cutaneous | Right | Forearm | 7.4 |
| Tracey et al. | 2000 | fMRI 1.5 | 6 | Heat | Thermal | Cutaneous | Left | Hand | 7.7 |
| Xu et al. | 1997 | PET | 6 | Laser | Thermal | Cutaneous | Left | Hand | NR |
Study 3 noxious heat vs. resting baseline: List of studies reporting brain activation coordinates evoked by noxious heat stimuli in comparison to a resting baseline. Abbreviations: fMRI, functional magnetic resonance imaging; PET, positron emission tomography; NR, not reported; NRS, Numerical rating scale.
Study 4 examined a possible hemispheric dominance for processing noxious stimuli. The database for Study 1 was searched to select different sets of studies that applied noxious stimuli either exclusively to the left side or to the right side of the body. For both meta‐analyses, studies were selected if they applied stimuli to the arms, legs, or sides of the face. However, to simplify the comparison, the meta‐analysis included studies that used stimuli generated using contact thermodes or laser stimuli, since other modalities of noxious stimulation may evoke activation that is unequally weighted in terms of the intensity or emotional valence, which might lead to a nonuniform comparison among studies and brain activation coordinates. The data from the studies included in both meta‐analyses were from contrasts that resulted from a noxious stimulus (heat or cold) compared to either a resting baseline or a control condition (innocuous warm or cool). Coordinates that were reported based on correlations of pain ratings with percent blood‐oxygen‐level‐dependent (BOLD) signal change were also included in the analyses. Studies were excluded if they applied stimuli to the midline (back or chest), simultaneously to both sides of the body, or if they reported data combined from scans in which stimuli were applied to either side of the body.
The left‐sided meta‐analysis included 43 studies and a total of 694 coordinates (Table VI). Studies chosen for the right‐sided meta‐analysis were matched to those included in the left‐sided meta‐analysis based on the year of publication, the imaging modality, and the site of stimulation. Additionally, to have an equal number of coordinates to compare across the two sets of studies, we selected 40 studies for the right‐sided meta‐analysis (Table VII). The studies were matched for stimulus intensity as determined by comparing subjects' ratings using an unpaired t‐test (P = 0.08). A Mann‐Whitney U test was performed to assess the mean and the distribution of coordinates reported in the studies included in the two comparison groups, which indicated that no single study unduly influenced the calculations of the meta‐analyses (P = 0.190).
Table VI.
List of studies included in study 4 (noxious stimuli applied to the left side of the body)
| Author | Year | Imaging | FWHM | Subject (N) | Stimuli | Side | Body Part | NRS | Notes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Modality | System | |||||||||
| Adler et al. | 1997 | PET | 6 | 9 | Heat | Thermal | Cutaneous | Left | Forearm | 6.7 | |
| Aharon et al. | 2006 | fMRI 1.5 | 5 | 6 | Heat | Thermal | Cutaneous | Left | Hand | 8.2 | |
| Becerra et al. | 1999 | fMRI 1.5 | NR | 6 | Heat | Thermal | Cutaneous | Left | Hand | 7.24 | |
| Becerra et al. | 2001 | fMRI 1.5 | 6 | 8 | Heat | Thermal | Cutaneous | Left | Hand (dorsum) | 7.8 | |
| Bingel et al. | 2003 | fMRI 1.5 | 6 | 14 | Laser | Thermal | Cutaneous | Left | Hand | NR | |
| Bingel et al. | 2004a | fMRI 1.5 | 6 | 20 | Laser | Thermal | Cutaneous | Left | Hand and foot | NR | |
| Bingel et al. | 2004b | fMRI 1.5 | 6 | 18 | Laser | Thermal | Cutaneous | Left | Hand | NR | |
| Bingel et al. | 2007a | fMRI 3 | 8 | 16 | Laser | Thermal | Cutaneous | Left | Hand | 4 | |
| Bingel et al. | 2007b | fMRI 3 | 8 | 20 | Heat | Thermal | Cutaneous | Left | Forearm | 6.7 | |
| Boly et al. | 2007 | fMRI 3 | 6 | 24 | Laser | Thermal | Cutaneous | Left | Hand | 8 | |
| Bornhovd et al. | 2002 | fMRI 1.5 | 6 | 9 | Laser | Thermal | Cutaneous | Left | Hand | 8 | Data are shared with Buchel et al. [2002] |
| Botvinick et al. | 2005 | fMRI 1.5 | 12 | 12 | Heat | Thermal | Cutaneous | Left | Thenar Eminence | 7 | |
| Buchel et al. | 2002 | fMRI 1.5 | 6 | 9 | Laser | Thermal | Cutaneous | Left | Hand | 8 | |
| Casey et al. | 1994 | PET | NR | 18 | Heat | Thermal | Cutaneous | Left | Arm | 8.9 | |
| Casey et al. | 1996 | PET | 9 | 27 | Cold | Thermal | Cutaneous | Left | Hand | 8.9 | |
| Casey et al. | 2000 | PET | 9 | 11 | Cold | Thermal | Cutaneous | Left | Hand | NR | |
| Casey et al. | 2001 | PET | 9 | 14 | Heat | Thermal | Cutaneous | Left | Forearm | 8.93 | |
| Chen et al. | 2002 | fMRI 1.5 | 6 | 4 | Heat | Thermal | Cutaneous | Left | Inner calf | 8.2 | |
| Coghill et al. | 1994 | PET | 20 | 9 | Heat | Thermal | Cutaneous | Left | Forearm | 7.8 | |
| Coghill et al. | 2001 | PET | 13.3 | 9 | Heat | Thermal | Cutaneous | Left | Forearm | 7.8 | |
| de Leeuw et al. | 2006 | fMRI 1.5 | 3 | 9 | Heat | Thermal | Cutaneous | Left | Face | 4.4 | |
| Derbyshire and Jones; Derbyshire et al. | 1998 | PET | 20 | 7 | Heat | Thermal | Cutaneous | Left | Hand | 6.7 | Data are shared with Vogt et al. [1996] |
| Dunckley et al. | 2005 | fMRI 3 | 5 | 10 | Heat | Thermal | Cutaneous | Left | Foot | 6.5 | |
| Fairhurst et al. | 2007 | fMRI 3 | 5 | 12 | Heat | Thermal | Cutaneous | Left | Hand | 5.7 | |
| Gyulai et al. | 1997 | PET | 20 | 5 | Heat | Thermal | Cutaneous | Left | Forearm | 6.7 | |
| Hofbauer et al. | 2001 | PET | 14 | 10 | Heat | Thermal | Cutaneous | Left | Hand | 6 | |
| Hofbauer et al. | 2004 | PET | 14 | 15 | Heat | Thermal | Cutaneous | Left | Forearm | 6 | |
| Keltner et al. | 2006 | fMRI 4 | 8 | 16 | Heat | Thermal | Cutaneous | Left | Hand | 8.2 | |
| Kurata et al. | 2002 | fMRI 3 | 0.5 | 5 | Heat | Thermal | Cutaneous | Left | Forearm | 7.6 | |
| Lorenz et al. | 2002 | PET | 9 | 14 | Heat | Thermal | Cutaneous | Left | Forearm | 4.6 | |
| Oshiro et al. | 2007 | fMRI 1.5 | 5 | 12 | Heat | Thermal | Cutaneous | Left | Leg | 6.4 | |
| Owen et al. | 2008 | fMRI 3 | 8 | 14 | Heat | Thermal | Cutaneous | Left | Hand | 8 | |
| Paulson et al. | 1998 | PET | 9 | 20 | Heat | Thermal | Cutaneous | Left | Forearm | 8.75 | |
| Petrovic et al. | 2002 | PET | 16 | 7 | Cold | Thermal | Cutaneous | Left | Hand | 5.3 | |
| Petrovic et al. | 2004a | PET | 16 | 7 | Cold | Thermal | Cutaneous | Left | Hand | 5.3 | Data are shared with Petrovic et al., [2002] |
| Petrovic et al. | 2004b | PET | 10 | 10 | Cold | Thermal | Cutaneous | Left | Hand | 5.9 | |
| Raij et al. | 2005 | fMRI 3 | 8 | 14 | Laser | Thermal | Cutaneous | Left | Hand | 6.5 | |
| Rainville | 1997 | PET | NR | 8 | Heat | Thermal | Cutaneous | Left | Hand | 7.5 | |
| Remy et al. | 2003 | fMRI 3 | 8 | 12 | Heat | Thermal | Cutaneous | Left | Hand | 6.3 | |
| Svensson et al. | 1997 | PET | 15 | 11 | Laser | Thermal | Cutaneous | Left | Elbow | 7.9 | |
| Tracey et al. | 2000 | fMRI 1.5 | 1.5 | 6 | Heat and cold | Thermal | Cutaneous | Left | Hand | 7.8 | |
| Vogt et al. | 1996 | PET | 20 | 7 | Heat | Thermal | Cutaneous | Left | Hand | NR | |
| Xu et al. | 1997 | PET | NR | 6 | Laser | Thermal | Cutaneous | Left | Hand and foot | NR | |
Study 4 (noxious stimuli applied to the left side of the body): List of studies reporting brain activation coordinates evoked by noxious stimuli applied to the left side of the body. Abbreviations: fMRI, functional magnetic resonance imaging; PET, positron emission tomography; NR, not reported; NRS, Numerical rating scale.
Table VII.
List of studies included in study 4 (noxious stimuli applied to the right side of the body)
| Author | Year | Imaging | FWHM | Subject (N) | Stimuli | Side | Body Part | NRS | Notes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Modality | System | |||||||||
| Albanese et al. | 2007 | fMRI 1.5 | 6 | 8 | Heat | Thermal | Cutaneous | Right | Hand | 7 | |
| Apkarian | 2000 | fMRI 1.5 | 5 | 7 | Heat | Thermal | Cutaneous | Right | Fingers | 5.95 | |
| Bingel et al. | 2003 | fMRI 1.5 | 6 | 14 | Laser | Thermal | Cutaneous | Right | Hand | NR | |
| Bingel et al. | 2004a | fMRI 1.5 | 6 | 20 | Laser | Thermal | Cutaneous | Right | Hand and foot | NR | |
| Borsook et al. | 2003 | fMRI 1.5 | 6 | 9 | Heat | Thermal | Cutaneous | Right | Face | 6.1 | Data are shared with DaSilva et al., [2002] |
| Brooks et al. | 2005 | fMRI 3.0 | 2 | 14 | Heat | Thermal | Cutaneous | Right | Face, hand, and foot | 5.5 | |
| Coghill et al. | 1999 | PET | 13.3 | 16 | Heat | Thermal | Cutaneous | Right | Upper Arm | 5.1 | |
| Coghill et al. | 2001 | PET | 13.3 | 9 | Heat | Thermal | Cutaneous | Right | Ventral Forearm | 7.8 | |
| Coghill and Eisenach; Coghill et al. | 2003 | fMRI 1.5 | 7.5 | 17 | Heat | Thermal | Cutaneous | Right | Leg | 7.43 | |
| Craig | 1996 | PET | 18 | 11 | Cold and heat | Thermal | Cutaneous | Right | Hand | NR | |
| DaSilva et al. | 2002 | fMRI 1.5 | 6 | 9 | Heat | Thermal | Cutaneous | Right | Face and thumb | 6.1 | |
| Davis and Pope | 2002 | fMRI 1.5 | 6 | 0 | Cold | Thermal/mechanical | Cutaneous | Right | Thernar eminence | NR | |
| Derbyshire et al. | 1997 | PET | 10 | 12 | Laser | Thermal | Cutaneous | Right | Hand | 7 | |
| Derbyshire and Jones; Derbyshire et al. | 1998 | PET | 8 | 12 | Heat | Thermal | Cutaneous | Right | Hand | 5.85 | |
| Derbyshire et al. | 2002a,b | PET | 12 | 21 | Laser | Thermal | Cutaneous | Right | Hand | 6.6 | |
| Derbyshire et al. | 2002a,b | PET | 10 | 16 | Heat | Thermal | Cutaneous | Right | Hand | 6 | |
| Derbyshire | 2004 | fMRI 3.0 | 10 | 8 | Heat | Thermal | Cutaneous | Right | Hand | 5.6 | |
| Frankenstein et al. | 2001 | fMRI 1.5 | 8 | 12 | Cold | Thermal | Cutaneous | Right | Foot | 5.8 | |
| Gelnar et al. | 1999 | fMRI 1.5 | 5 | 9 | Heat | Thermal | Cutaneous | Right | Finger | 3.4 | |
| Helmchen et al. | 2003 | fMRI 1.5 | 5 | 18 | Heat | Thermal | Cutaneous | Right | Hand | 6.3 | |
| Helmchen et al. | 2006 | fMRI 1.5 | 5 | 18 | Heat | Thermal | Cutaneous | Right | Hand | 6.3 | Data are shared with Helmchen et al., 2003 |
| Kong et al. | 2006 | fMRI 3.0 | 8 | 16 | Heat | Thermal | Cutaneous | Right | Forearm | 7.4 | |
| Koyama et al. | 2003 | fMRI 1.5 | 5 | 9 | Heat | Thermal | Cutaneous | Right | Leg | 6.8 | |
| Koyama et al. | 2005 | fMRI 1.5 | 5 | 10 | Heat | Thermal | Cutaneous | Right | Leg | 3.8 | |
| Kurata et al. | 2002 | fMRI 3.0 | 0.5 | 5 | Heat | Thermal | Cutaneous | Left | Forearm | 7 | |
| Kurata et al. | 2005 | fMRI 3.0 | 4 | 6 | Heat | Thermal | Cutaneous | Right | Forearm | 6.8 | |
| Maihofner et al. | 2006 | fMRI 1.5 | 4 | 14 | Heat | Thermal | Cutaneous | Right | Forearm | 4.3 | |
| Mohr et al. | 2005 | fMRI 1.5 | 5 | 16 | Heat | Thermal | Cutaneous | Right | Hand | 6.3 | Data are shared with Helmchen et al., 2003 |
| Mohr et al. | 2008 | fMRI 1.5 | 8 | 17 | Heat | Thermal | Cutaneous | Right | Thigh | 5.1 | |
| Nemoto et al. | 2003 | PET | 16 | 12 | Laser | Thermal | Cutaneous | Right | Forearm | 7.6 | |
| Ochsner et al. | 2006 | fMRI 3.0 | 6 | 13 | Heat | Thermal | Cutaneous | Right | Forearm | 7 | |
| Qiu et al. | 2006 | fMRI 3.0 | 8 | 13 | Laser | Thermal | Cutaneous | Right | Hand | NR | |
| Sawamoto et al. | 2000 | fMRI 1.5 | 5.16 | 10 | Laser | Thermal | Cutaneous | Right | Hand | 9.2 | |
| Seifert and Maihofner | 2007 | fMRI 1.5 | 4 | 12 | Cold | Thermal | Cutaneous | Right | Forearm | 4.08 | |
| Sprenger et al. | 2006 | PET | 6 | 8 | Heat | Thermal | Cutaneous | Right | Forearm | 7.4 | |
| Staud et al. | 2007 | fMRI 3.0 | 4 | 11 | Heat | Thermal | Cutaneous | Right | Foot | 4.5 | |
| Svensson et al. | 1998 | PET | 12 | 10 | Heat | Thermal | Cutaneous | Right | Forearm | 8 | |
| Talbot et al. | 1991 | PET | 7 | 8 | Heat | Thermal | Cutaneous | Right | Forearm | 8 | |
| Tolle | 1999 | PET | 8 | 12 | Heat | Thermal | Cutaneous | Right | Forearm | 5.7 | |
| Wagner et al. | 2007 | PET | 12 | 7 | Heat | Thermal | Cutaneous | Right | Forearm | 6.8 | |
Study 4 (noxious stimuli applied to the right side of the body): List of studies reporting brain activation coordinates evoked by noxious stimuli applied to the right side of the body. Abbreviations: fMRI, functional magnetic resonance imaging; PET, positron emission tomography; NR, not reported; NRS, Numerical rating scale.
Study 5 examined potential regional specificity for processing noxious muscle stimuli in comparison to noxious cutaneous stimuli. The Study 1 database was searched for articles that had reported activation in response to noxious stimuli applied to muscles and resulted in a total of 10 studies (Table VIII). An equal number of studies that applied noxious stimuli to the skin were selected for purposes of comparison and were matched to the noxious muscle stimuli studies based on the year of publication, the imaging modality, and the site of stimulation (Table IX). An unpaired t‐test revealed no significant differences in pain intensity ratings obtained for the sets of studies included in either meta‐analysis (P = 0.9). The noxious muscle stimuli studies reported a total of 172 coordinates and the noxious cutaneous studies reported 133 activation foci. A Mann‐Whitney U test showed no significant differences between the two sets of coordinates reported for the two meta‐analyses (P = 0.5).
Table VIII.
List of studies included in study 5 (noxious muscle stimuli)
| Author | Year | Imaging | Subject (N) | Stimuli | Side | Body Part | NRS | Notes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Modality | System | ||||||||
| Henderson et al. | 2007 | fMRI 3 | 23 | Hypertonic Saline Injection | Mechanical | Intramuscular | Right | Leg | 6.5 | |
| Henderson et al. | 2007 | fMRI 3 | 23 | Hypertonic Saline Injection | Mechanical | Intramuscular | Right | Forearm | 7 | Data are shared with Henderson et al., 2007 |
| Korotkov et al. | 2002 | PET | 16 | Hypertonic Saline Injection | Mechanical | Intramuscular | Left | Tricep | 3.2 | |
| Kupers et al. | 2004 | PET | 10 | Hypertonic Saline Injection | Mechanical | Intramuscular | Right | Masseter muscle | 7.5 | |
| Nash et al. | 2010 | fMRI 3 | 28 | Hypertonic saline injection | Mechanical | Muscular | Right | Masseter muscle | 4.73 | |
| Niddam et al. | 2002 | fMRI 3 | 10 | Electrical Shock | Electrical | Intramuscular | Left | Hand | 2.22 | |
| Owen et al. | 2010 | fMRI 3 | 13 | Hypertonic saline injection | Mechanical | Muscular | Left | Forearm | 6 | |
| Schreckenberger et al. | 2005 | PET | 10 | Infusion of phosphate buffer | Mechanical | Intramuscular | Left | Hand | 4 | |
| Svensson et al. | 1997 | PET | 10 | Electrical Shock | Electrical | Intramuscular | Left | Forearm | 7.5 | |
| Thunberg et al. | 2005 | PET | 19 | Hypertonic Saline Injection | Mechanical | Intramuscular | Right | Erector spinae muscle | 4.6 | |
| Uematsu et al. | 2011 | fMRI 1.5 | 17 | Pressure | Mechanical | Muscular | Right | Calf | 4.7 | |
Study 5 (Noxious muscle stimuli): List of studies reporting brain activation coordinates in response to noxious stimuli applied to muscles. Abbreviations: fMRI, functional magnetic resonance imaging; PET, positron emission tomography; NRS, Numerical rating scale.
Table IX.
List of studies included in study 5 (Noxious cutaneous stimuli)
| Author | Year | Imaging | Subject (N) | Stimuli | Side | Body Part | NRS | ||
|---|---|---|---|---|---|---|---|---|---|
| Type | Modality | System | |||||||
| Koyama et al. | 2005 | fMRI 1.5 | 10 | Heat | Thermal | Cutaneous | Right | Leg | 3.7 |
| Raij et al. | 2005 | fMRI 3 | 14 | Laser | Thermal | Cutaneous | Left | Hand | 6.2 |
| DaSilva et al. | 2002 | fMRI 1.5 | 9 | Heat | Thermal | Cutaneous | Right | Mandibular (V3) | 6 |
| Brooks et al. | 2005 | fMRI 3 | 14 | Heat | Thermal | Cutaneous | Right | Face | 5.5 |
| Symonds et al. | 2006 | fMRI 3 | 9 | Electrical shock | Electrical | Transcutaneous | Left | Index finger | 4.5 |
| Maihofner et al. | 2004 | fMRI 1.5 | 11 | Heat | Thermal | Cutaneous | Left | Forearm | 3.9 |
| Fairhurst et al. | 2007 | fMRI 3 | 12 | Heat | Thermal | Cutaneous | Left | Hand | 5.71 |
| Seminowicz et al. | 2004 | fMRI 1.5 | 16 | Electrical shock | Electrical | Transcutaneous | Left | Median nerve | 5.5 |
| Dunckley et al. | 2005 | fMRI 3 | 10 | Heat | Thermal | Cutaneous | Bilateral | Back | 5.8 |
| Lorenz et al. | 2008 | fMRI 1.5 | 11 | Pressure | Mechanical | Cutaneous | Right | Tibia | 4.6 |
Study 5 (Noxious cutaneous stimuli): List of studies reporting brain activation coordinates in response to noxious stimuli applied to the skin for purposes of comparison with noxious stimuli applied to muscles. Abbreviations: fMRI, functional magnetic resonance imaging; PET, positron emission tomography; NRS, Numerical rating scale.
Quantitative Analyses
Probabilistic maps of activation evoked by all noxious stimuli (Study 1), noxious cold and heat stimuli (Study 2), noxious stimuli in comparison to a resting baseline or innocuous warm stimuli (Study 3), noxious stimuli applied to the left and right sides of the body (Study 4), and noxious stimuli applied to muscle and skin (Study 5), were generated using the ALE analytic strategy as described by Laird et al. [2005]. Briefly, the ALE statistic is calculated for each voxel in the template MRI signifying the probability of evoking activation in response to noxious stimuli. Reported coordinates were recorded in their original space and then transformed into Talairach space [Talairach and Tournoux,1988] using a conversion provided in the GingerALE (v.1.0) software [Lancaster et al.,2007]. The ALE maps were created by smoothing the activation foci using a full‐width half maximum (FWHM) of 8 mm, which was the average size of the Gaussian smoothing filter among the studies included in the Study 1 database. This latter step ensures that the data are a realistic reflection of the peak activation sites as all data included in the analysis were smoothed by an average Gaussian smoothing filter of this size. The statistical significance of the ALE maps was determined by performing a permutation test (N = 5,000) and the data were thresholded using a false discovery rate (FDR) correction of q = 0.05 [Genovese et al.,2002]. The ALE method calculates the likelihood that one peak (out of the total number of peaks) actually occurred within a given voxel in the template MRI and tests this against the null hypothesis that the points are randomly distributed across the brain. The resulting ALE values indicate that the likelihood that any single peak of the total peaks actually occurred in a single voxel located in the template MRI. These ALE values range from ∼ 0.003 to a theoretical maximum of 1.0.
Subtraction Analyses
To test for brain regions preferentially associated with the processing of noxious cold or heat stimuli (Study 2), noxious heat compared to innocuous warm or a resting baseline (Study 3), right or left sided noxious stimuli (Study 4), and muscle or cutaneous pain (Study 5), we performed a voxel‐by‐voxel subtraction of the two ALE maps included in each of the four meta‐analyses.
The analysis involved the subtraction of the ALE values in condition 2 from the ALE values in condition 1 at each voxel (Step 1). Two sets of random peak coordinates are then generated using the same number of peaks observed in conditions 2 and 1 and the random ALE maps undergo a pair‐wise subtraction (Step 2). Subsequently, this method of random peak generation and subtraction is repeated 5,000 times (Step 3). This process results in a single statistical map representing a null distribution of activation peaks (Step 4). At each voxel, the observed ALE statistic in the original subtraction map (Step 1) is compared to the random ALE statistic subtraction map (Step 4) and a P value is generated to denote the statistical significance of the test. The ALE map is then thresholded at P < 0.05 using the FDR method.
RESULTS
Study 1: All Noxious Stimuli
An ALE analysis was performed on 2,873 coordinate points associated with activation in response to all noxious stimuli. The greatest likelihood of evoking activation in the cortex in response to all types of noxious stimuli was in the right anterior insula (ALE = 0.238) and ACC (BA 24, ALE = 0.245; Fig. 1). The resulting ALE values reflect the likelihood of activation in a single voxel, which is a very small region of gray matter within the insula and ACC. The likelihood of activation occurring in the full brain regions is of course much larger. Note that these ALE values are large compared with the likelihood (0.003) of the highest value in the background noise being interpreted as an activated voxel during the permutation testing.
Figure 1.
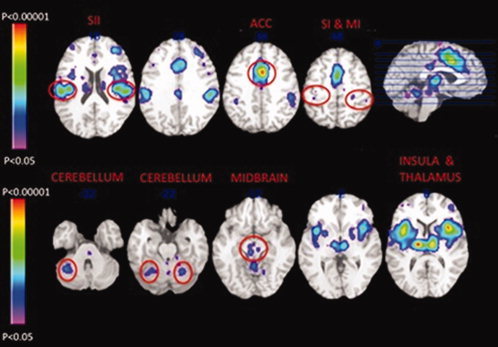
Study 1: ALE map describing the likelihood of evoking activation in the brain in response to noxious stimuli applied to the skin, muscle, or viscera. Brain regions having a significant likelihood of being activated by noxious stimuli included the secondary somatosensory cortex (SII), the anterior cingulate cortex (ACC), the primary somatosensory and motor (SI/MI) cortices, the cerebellum, the midbrain, and the insula (anterior, middle, and dorsal posterior regions), and the thalamus. The z‐values for the horizontal images are in Talairach space [Talairach and Tournoux1988].
Additional cortical regions with significant likelihoods of activation were observed in left insula (ALE = 0.219), bilateral SII (right: ALE = 0.186; left: ALE = 0.182), the prefrontal cortex (right BA 44, ALE = 0.144; left BA 10, ALE = 0.099), and SI/PPC (right: ALE = 0.064; left: ALE = 0.07). A complete list of brain regions with significant likelihoods of being activated is detailed in Table X.
Table X.
Spatial location and extent of ALE values for study 1 (all noxious stimuli)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Left | Thalamus | −14 | −16 | 8 | 0.272 | <0.000001 | 1 | 96168 | |
| Right | Anterior insula | 36 | 12 | 8 | 0.238 | <0.000001 | |||
| Right | Thalamus | 10 | −18 | 6 | 0.227 | <0.000001 | |||
| Left | Anterior insula | −36 | 4 | 6 | 0.219 | <0.000001 | |||
| Left | Posterior insula | −40 | −20 | 16 | 0.191 | <0.000001 | |||
| Right | SII | 40 | 52 | −26 | 22 | 0.186 | <0.000001 | ||
| Left | SII | 40 | −52 | −24 | 20 | 0.182 | <0.000001 | ||
| Right | Anterior insula | 36 | −20 | 16 | 0.175 | <0.000001 | |||
| Right | Inferior frontal gyrus | 44 | 50 | 2 | 10 | 0.144 | <0.000001 | ||
| Left | Putamen | −24 | −2 | 6 | 0.097 | <0.000001 | |||
| Right | Putamen | 20 | 8 | 4 | 0.085 | <0.000001 | |||
| Left | Inferior frontal gyrus | 6 | −54 | −2 | 8 | 0.081 | <0.000001 | ||
| Left | Inferior frontal gyrus | 43 | −54 | −6 | 12 | 0.079 | <0.000001 | ||
| Left | Posterior insula | −38 | −18 | −4 | 0.072 | <0.000001 | |||
| Right | IPL | 40 | 46 | −38 | 42 | 0.072 | <0.000001 | ||
| Left | Lentiform nucleus | −24 | 0 | −2 | 0.068 | <0.000001 | |||
| Left | IPL | 40 | −58 | −38 | 28 | 0.064 | <0.000001 | ||
| Right | Lentiform nucleus | 20 | −4 | 0 | 0.064 | <0.000001 | |||
| Right | IPL | 40 | 52 | −44 | 38 | 0.059 | <0.000001 | ||
| Right | IPL | 40 | 46 | −54 | 44 | 0.046 | 0.0008 | ||
| Right | Cingulate gyrus | 32/24 | 2 | 8 | 38 | 0.245 | <0.000001 | 2 | 2420 |
| Right | Cingulate gyrus | 32 | 6 | 22 | 28 | 0.127 | <0.000001 | ||
| Left | Anterior cingulate gyrus | 32 | −2 | 32 | 22 | 0.057 | <0.000001 | ||
| Right | Medial frontal gyrus | 6 | 2 | −10 | 64 | 0.047 | 0.0004 | ||
| Right | Middle frontal gyrus | 10 | 34 | 42 | 20 | 0.099 | <0.000001 | 3 | 5872 |
| Right | Superior frontal gyrus | 9 | 28 | 40 | 30 | 0.079 | <0.000001 | ||
| Right | Middle frontal gyrus | 10 | 42 | 46 | 12 | 0.078 | <0.000001 | ||
| Right | Middle frontal gyrus | 47 | 38 | 38 | −6 | 0.046 | 0.0006 | ||
| Left | Cerebellum | −34 | −56 | −32 | 0.071 | <0.000001 | 4 | 3224 | |
| Left | Cerebellum | −30 | −58 | −30 | 0.071 | <0.000001 | |||
| Left | Cerebellum | −22 | −60 | −24 | 0.056 | <0.000001 | |||
| Right | Cerebellum | 0 | −48 | −16 | 0.085 | <0.000001 | 5 | 2384 | |
| Right | Cerebellum | 4 | −62 | −16 | 0.063 | <0.000001 | |||
| Right | Cerebellum | 24 | −60 | −22 | 0.067 | <0.000001 | 6 | 2024 | |
| Right | Cerebellum | 18 | −62 | −14 | 0.054 | <0.000001 | |||
| Right | Cerebellum | 18 | −48 | −22 | 0.047 | <0.000001 | |||
| Left | Cingulate gyrus | 23 | 0 | −28 | 28 | 0.082 | <0.000001 | 7 | 1440 |
| Left | Cingulate gyrus | 24 | 0 | −20 | 36 | 0.051 | <0.000001 | ||
| Left | SI | 2 | −32 | −36 | 60 | 0.065 | <0.000001 | 8 | 1432 |
| Left | MI | 4 | −32 | −24 | 52 | 0.060 | <0.000001 | ||
| Left | MI | 4 | −38 | −26 | 62 | 0.057 | <0.000001 | ||
| Right | MI | 4 | 32 | −28 | 56 | 0.073 | <0.000001 | 9 | 1176 |
| Left | IPL | 40 | −40 | −40 | 40 | 0.066 | <0.000001 | 10 | 816 |
| Right | SI/PPC | 5 | 20 | −44 | 64 | 0.064 | <0.000001 | 11 | 560 |
| Left | Superior frontal gyrus | 10 | −34 | 48 | 18 | 0.052 | 0.0002 | 12 | 496 |
| Left | Middle frontal gyrus | 9 | −32 | 38 | 26 | 0.043 | 0.0001 | ||
| Right | MI | 6 | 26 | −16 | 52 | 0.055 | 0.0002 | 14 | 264 |
| Right | Inferior frontal gyrus | 9 | 48 | 6 | 26 | 0.045 | 0.0001 | 15 | 104 |
ALE values for Study 1. ALE values refer to the likelihood of obtaining activation evoked by noxious stimuli in a given voxel of the standard template MRI. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster : The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; IPL, inferior parietal lobule; SI, primary somatosensory cortex; PPC, posterior parietal cortex; SII, secondary somatosensory cortex; MI, primary motor cortex.
Study 2: Noxious Cold Versus Noxious Heat
An ALE analysis was performed on 112 coordinate sites compiled from the nine studies that used noxious cold stimuli applied to the upper limbs. For the noxious cold stimuli meta‐analysis, the likelihood of activation was significant in several brain regions involved in affective pain processing such as bilateral insula/claustrum (right: ALE = 0.03; left: ALE = 0.028), right subgenual ACC (ALE = 0.023) and the amygdala (ALE = 0.012; Table XI).
Table XI.
Spatial location and extent of ALE values for study 2 (noxious cold)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Right | Anterior insula/Claustrum | 28 | 6 | 12 | 0.030 | <0.00001 | 1 | 1656 | |
| Right | Anterior insula | 40 | 8 | 0 | 0.013 | 0.0006 | |||
| Left | Anterior insula | −38 | 6 | 4 | 0.028 | 0.0002 | 2 | 1520 | |
| Left | Claustrum | −36 | −8 | 4 | 0.013 | 0.001 | |||
| Left | Anterior insula | −38 | 4 | 14 | 0.013 | 0.0006 | |||
| Left | Cingulate gyrus | 32 | −10 | 6 | 40 | 0.021 | <0.00001 | 3 | 1496 |
| Right | Cingulate gyrus | 24 | 2 | 2 | 36 | 0.019 | <0.00001 | ||
| Left | Cingulate gyrus | 32 | 0 | 10 | 38 | 0.017 | 0.0002 | ||
| Left | Thalamus | 0 | −20 | 6 | 0.023 | <0.00001 | 4 | 1232 | |
| Right | Thalamus | 6 | −22 | 14 | 0.014 | <0.00001 | |||
| Right | Thalamus | 16 | −22 | 12 | 0.014 | 0.001 | |||
| Right | Thalamus | 4 | −12 | 12 | 0.013 | 0.001 | |||
| Left | Claustrum | −30 | 10 | 14 | 0.015 | 0.0002 | 5 | 712 | |
| Left | Claustrum | −30 | 12 | 10 | 0.014 | 0.0006 | |||
| Left | Putamen | −26 | 6 | 12 | 0.014 | 0.0002 | |||
| Left | Putamen | −18 | 4 | 8 | 0.014 | 0.0002 | |||
| Left | Caudate | −12 | 8 | 10 | 0.013 | 0.001 | |||
| Right | Cingulate gyrus | 24 | 12 | 14 | 30 | 0.023 | <0.00001 | 6 | 656 |
| Right | Thalamus | 12 | −4 | 8 | 0.021 | <0.00001 | 7 | 552 | |
| Right | Middle frontal gyrus | 10 | 42 | 46 | 12 | 0.020 | <0.00001 | 8 | 544 |
| Left | SI/PPC | 43 | −54 | −6 | 14 | 0.019 | <0.00001 | 9 | 520 |
| Right | Subgenual ACC | 47/25 | 18 | 18 | −10 | 0.023 | <0.00001 | 10 | 496 |
| Right | Medial frontal gyrus | 25 | 10 | 16 | −14 | 0.013 | 0.0004 | ||
| Right | Claustrum | 38 | −14 | 8 | 0.020 | <0.00001 | 11 | 400 | |
| Left | Putamen | −22 | 12 | −8 | 0.024 | <0.00001 | 12 | 384 | |
| Right | Claustrum | 36 | −4 | 0 | 0.020 | <0.00001 | 13 | 352 | |
| Right | MI | 4 | 32 | −26 | 56 | 0.017 | <0.00001 | 14 | 328 |
| Right | SII | 46 | −24 | 16 | 0.016 | <0.00001 | 15 | 256 | |
| Left | SII | −40 | −46 | 46 | 0.016 | 0.0006 | 16 | 248 | |
| Right | Midbrain | 8 | −20 | −2 | 0.014 | 0.005 | 17 | 240 | |
| Left | Medial frontal gyrus | 6 | −4 | −10 | 56 | 0.014 | 0.001 | 18 | 240 |
| Right | Inferior frontal gyrus | 9 | 50 | 4 | 24 | 0.014 | <0.00001 | 19 | 232 |
| Right | Inferior frontal gyrus | 9 | 52 | 10 | 26 | 0.014 | <0.00001 | ||
| Left | Superior frontal gyrus | 10 | −26 | 44 | 18 | 0.013 | 0.0006 | 20 | 184 |
| Left | Middle frontal gyrus | 10 | −30 | 38 | 14 | 0.013 | 0.001 | ||
| Right | Posterior insula | 50 | −40 | 18 | 0.014 | 0.001 | 21 | 152 | |
| Right | Posterior insula | 44 | −36 | 20 | 0.013 | 0.0006 | |||
| Right | Cerebellum | 2 | −58 | −20 | 0.013 | 0.001 | 22 | 72 | |
| Right | Middle frontal gyrus | 9 | 38 | 28 | 32 | 0.012 | <0.00001 | 23 | 72 |
| Right | Middle frontal gyrus | 9 | 38 | 28 | 34 | 0.012 | <0.00001 | ||
| Right | Lingual gyrus | 19 | 30 | −68 | −2 | 0.011 | 0.002 | 24 | 64 |
| Right | Superior frontal gyrus | 10 | 28 | 54 | 4 | 0.012 | 0.001 | 25 | 64 |
| Right | Premotor | 6 | 50 | −2 | 10 | 0.011 | 0.004 | 26 | 64 |
| Right | Posterior insula | 36 | −16 | 20 | 0.012 | 0.001 | 27 | 64 | |
| Right | Paracentral lobule | 31 | 6 | −10 | 46 | 0.013 | 0.001 | 28 | 64 |
| Left | Cingulate gyrus | 24 | 0 | 0 | 46 | 0.013 | 0.0006 | 29 | 64 |
| Right | MI | 4 | 24 | −22 | 50 | 0.013 | 0.0008 | 30 | 64 |
| Right | Cerebellum | 26 | −64 | −22 | 0.013 | 0.0008 | 31 | 56 | |
| Right | Thalamus | 12 | −30 | 6 | 0.013 | 0.0004 | 32 | 56 | |
| Right | Anterior insula | 38 | 18 | 8 | 0.013 | 0.0004 | 33 | 56 | |
| Left | Thalamus | −10 | −16 | 8 | 0.012 | 0.003 | 34 | 56 | |
| Right | Posterior insula | 46 | −12 | 12 | 0.013 | 0.001 | 35 | 56 | |
| Left | Cingulate gyrus | 32 | −10 | 18 | 26 | 0.013 | 0.0004 | 36 | 56 |
| Right | Cingulate gyrus | 24 | 6 | −10 | 32 | 0.013 | 0.0002 | 37 | 56 |
| Right | SI | 3 | 44 | −24 | 52 | 0.013 | 0.0004 | 38 | 56 |
| Left | Fusiform gyrus | 19 | −22 | −66 | −6 | 0.013 | 0.001 | 39 | 48 |
| Right | Superior frontal gyrus | 6 | 12 | −6 | 64 | 0.013 | 0.0002 | 40 | 48 |
| Left | Midbrain | −4 | −18 | −10 | 0.013 | 0.0008 | 41 | 40 | |
| Left | Putamen | −20 | 16 | 2 | 0.012 | 0.002 | 42 | 40 | |
| Left | Cerebellum | −36 | −56 | −32 | 0.012 | 0.002 | 43 | 32 | |
| Right | Parahippocampal gyrus | 35 | 22 | −8 | −22 | 0.012 | 0.002 | 44 | 32 |
| Right | Amygdala | 24 | −8 | −22 | 0.012 | 0.002 | |||
| Right | Amygdala | 22 | −8 | −20 | 0.012 | 0.002 | |||
| Right | Amygdala | 24 | −8 | −20 | 0.012 | 0.002 |
ALE values for Study 2 (noxious cold). ALE values refer to the likelihood of obtaining activation evoked by noxious cold stimuli in a given voxel of the standard template. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; IPL, inferior parietal lobule; SI, primary somatosensory cortex; PPC, posterior parietal cortex; SII, secondary somatosensory cortex; MI, primary motor cortex.
For the comparative noxious heat meta‐analysis, the ALE analysis was conducted on 122 coordinates that were published in the nine selected studies. Areas with the most significant likelihood of activation associated with noxious heat stimulation were observed in bilateral insula/claustrum (right: ALE = 0.033; left: ALE = 0.025), the left ACC (ALE = 0.024), the right thalamus [ALE = 0.029, and SII (ALE = 0.021; Table XII)].
Table XII.
Spatial location and extent of ALE values for study 2 (noxious heat)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Left | Anterior insula | −40 | 18 | 6 | 0.025 | <0.00001 | 1 | 4432 | |
| Left | Posterior insula | −44 | −24 | 16 | 0.024 | <0.00001 | |||
| Left | Lentiform Nucleus | −22 | 0 | −2 | 0.024 | <0.00001 | |||
| Left | Lentiform Nucleus | −22 | −10 | 8 | 0.018 | 0.0004 | |||
| Left | Mid‐insula/claustrum | −34 | 4 | 6 | 0.017 | 0.0002 | |||
| Left | Anterior insula/claustrum | −34 | 10 | 6 | 0.017 | 0.0002 | |||
| Left | Anterior insula | −30 | 18 | 8 | 0.017 | 0.0004 | |||
| Left | Lentiform Nucleus | −24 | −4 | 6 | 0.016 | <0.00001 | |||
| Left | Posterior insula/claustrum | −34 | −16 | 10 | 0.014 | 0.0006 | |||
| Right | Mid‐insula/claustrum | 34 | 4 | 10 | 0.033 | <0.00001 | 2 | 2432 | |
| Right | Anterior insula/claustrum | 34 | 12 | 6 | 0.030 | <0.00001 | |||
| Right | Thalamus | 12 | −20 | 4 | 0.029 | <0.00001 | 3 | 1544 | |
| Right | Thalamus | 10 | −10 | 6 | 0.013 | 0.001 | |||
| Left | Cingulate Gyrus | 32 | −4 | 10 | 40 | 0.024 | 0.0002 | 4 | 1288 |
| Left | Cingulate Gyrus | 24 | −4 | 12 | 32 | 0.016 | 0.0002 | ||
| Right | SII | 40 | 52 | −30 | 22 | 0.021 | 0.0002 | 5 | 1160 |
| Right | Posterior insula | 40 | 52 | −22 | 14 | 0.014 | 0.0006 | ||
| Right | Lentiform Nucleus | 30 | −14 | 8 | 0.018 | <0.00001 | 6 | 688 | |
| Right | Posterior insula | 32 | −10 | 18 | 0.018 | 0.0002 | |||
| Right | Cingulate Gyrus | 24 | 2 | −4 | 44 | 0.020 | <0.00001 | 7 | 576 |
| Left | Thalamus | −12 | −24 | 12 | 0.022 | <0.00001 | 8 | 552 | |
| Right | Inferior Frontal Gyrus | 38 | 46 | 2 | 0.021 | <0.00001 | 9 | 464 | |
| Right | Caudate | 16 | 8 | 12 | 0.021 | <0.00001 | 10 | 368 | |
| Left | MI | 4 | −32 | −22 | 50 | 0.020 | <0.00001 | 11 | 304 |
| Right | Cingulate Gyrus | 32 | 4 | 22 | 26 | 0.018 | <0.00001 | 12 | 256 |
| Left | Anterior insula | −46 | 6 | 16 | 0.013 | 0.0006 | 13 | 168 | |
| Left | Inferior Frontal Gyrus | 44 | −48 | 0 | 12 | 0.013 | 0.0008 | ||
| Right | Middle Frontal Gyrus | 46 | 42 | 36 | 24 | 0.014 | <0.00001 | 14 | 152 |
| Right | Superior Frontal Gyrus | 9 | 40 | 34 | 28 | 0.013 | 0.0004 | ||
| Right | Anterior insula | 46 | 6 | 16 | 0.014 | 0.0002 | 15 | 144 | |
| Right | Inferior Frontal Gyrus | 44 | 52 | 6 | 12 | 0.014 | 0.0006 | ||
| Left | Posterior insula | −40 | −4 | 10 | 0.013 | 0.0006 | 16 | 80 | |
| Left | Superior Temporal Gyrus | 42 | −54 | −30 | 14 | 0.013 | 0.001 | 17 | 80 |
| Right | Thalamus | 6 | −18 | 14 | 0.013 | 0.0008 | 18 | 72 | |
| Left | Posterior insula | −52 | −34 | 20 | 0.013 | 0.0006 | 19 | 64 | |
| Left | Cingulate Gyrus | 24 | −10 | 4 | 30 | 0.013 | 0.001 | 20 | 64 |
| Right | Lentiform Nucleus | 24 | 4 | 16 | 0.014 | 0.0006 | 21 | 56 | |
| Right | IPL | 39 | 48 | −62 | 38 | 0.013 | 0.0008 | 22 | 56 |
| Left | Medial Frontal Gyrus | 6 | 0 | −10 | 52 | 0.013 | 0.0008 | 23 | 56 |
| Right | Lentiform Nucleus | 22 | −4 | 12 | 0.012 | 0.001 | 24 | 48 | |
| Right | Posterior insula | 36 | −18 | 20 | 0.012 | 0.001 | 25 | 48 | |
| Left | Superior Frontal Gyrus | 6 | −4 | 8 | 60 | 0.013 | 0.0008 | 26 | 48 |
| Right | Medial Frontal Gyrus | 6 | 2 | −12 | 62 | 0.011 | 0.003 | 27 | 48 |
| Right | Cerebellum | 30 | −76 | −28 | 0.013 | 0.001 | 28 | 40 | |
| Right | Cerebellum | 8 | −60 | −12 | 0.012 | 0.001 | 29 | 40 | |
| Right | Precentral Gyrus | 6 | 50 | −4 | 38 | 0.012 | 0.001 | 30 | 40 |
| Right | SI | 20 | −36 | 52 | 0.013 | 0.001 | 31 | 40 | |
| Right | Middle Frontal Gyrus | 6 | 18 | −10 | 58 | 0.013 | 0.001 | 32 | 40 |
| Left | Cerebellum | −28 | −40 | −42 | 0.012 | 0.001 | 33 | 32 | |
| Right | Cerebellum | 18 | −72 | −30 | 0.012 | 0.001 | 34 | 32 | |
| Left | Cerebellum | −20 | −60 | −20 | 0.013 | 0.001 | 35 | 32 | |
| Right | Cerebellum | 0 | −52 | −16 | 0.012 | 0.001 | 36 | 32 | |
| Right | Inferior Frontal Gyrus | 47 | 42 | 20 | −4 | 0.013 | 0.001 | 37 | 32 |
| Right | Posterior insula/claustrum | 36 | −6 | 0 | 0.013 | 0.001 | 38 | 32 | |
| Right | Lentiform Nucleus | 20 | 10 | 0 | 0.012 | 0.001 | 39 | 32 | |
| Right | SII | 42 | 56 | −12 | 12 | 0.013 | 0.001 | 40 | 32 |
| Left | Posterior insula | −48 | −20 | 24 | 0.012 | 0.001 | 41 | 32 | |
| Left | IPL | 40 | −62 | −40 | 28 | 0.012 | 0.001 | 42 | 32 |
| Right | Cingulate Gyrus | 23 | 4 | −22 | 28 | 0.012 | 0.001 | 43 | 32 |
| Right | Medial Frontal Gyrus | 8 | 14 | 30 | 38 | 0.012 | 0.001 | 44 | 32 |
| Left | Paracentral Lobule | 5 | −10 | −34 | 46 | 0.012 | 0.001 | 45 | 32 |
ALE values for Study 2 (noxious heat). ALE values refer to the likelihood of obtaining activation evoked by noxious heat stimuli in a given voxel of the standard template. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann's Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; SI, primary somatosensory cortex; IPL, inferior parietal lobule; SII, secondary somatosensory cortex; MI, primary motor cortex.
Both types of stimuli were found to significantly activate the ACC [Brodmann Area (BA) 24], and insula (Supporting Information Fig. 1). Statistical subtractions of the noxious cold and noxious heat maps revealed that the likelihood of noxious cold‐related activation was significantly greater in the amygdala and the subgenual ACC (BA 25/47; Table XIII) while the likelihood of noxious heat‐related activation was significantly greater in bilateral SII (Table XIV).
Table XIII.
Spatial location and extent of ALE values for study 2 (noxious cold minus noxious heat)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Right | Cingulate Gyrus | 24 | 12 | 14 | 30 | 0.023 | <0.00001 | 1 | 408 |
| Left | Anterior insula | −40 | 6 | 2 | 0.023 | <0.00001 | 2 | 384 | |
| Right | Lentiform Nucleus | 26 | 6 | 12 | 0.022 | <0.00001 | 3 | 360 | |
| Right | Middle Frontal Gyrus | 10 | 42 | 46 | 12 | 0.020 | 0.0002 | 4 | 360 |
| Right | Subgenual ACC | 25/47 | 18 | 18 | −10 | 0.023 | <0.00001 | 5 | 344 |
| Left | SII | 43 | −54 | −6 | 14 | 0.018 | 0.0002 | 6 | 296 |
| Right | Thalamus | 12 | −4 | 8 | 0.019 | 0.0002 | 7 | 280 | |
| Left | Lentiform Nucleus | −22 | 12 | −8 | 0.022 | <0.00001 | 8 | 264 | |
| Left | Thalamus | 0 | −20 | 6 | 0.021 | <0.00001 | 9 | 248 | |
| Left | IPL | 40 | −40 | −46 | 46 | 0.016 | <0.00001 | 10 | 232 |
| Left | Cingulate Gyrus | 32 | −10 | 6 | 40 | 0.019 | <0.00001 | 11 | 224 |
| Right | MI | 4 | 32 | −26 | 56 | 0.017 | 0.0002 | 12 | 208 |
| Right | Posterior insula/claustrum | 38 | −14 | 8 | 0.016 | 0.0004 | 13 | 88 | |
| Right | Inferior Frontal Gyrus | 9 | 52 | 10 | 26 | 0.014 | 0.002 | 14 | 88 |
| Left | Cingulate Gyrus | 24 | 0 | 2 | 36 | 0.015 | 0.002 | 15 | 64 |
| Right | Lingual Gyrus | 19 | 32 | −68 | −2 | 0.011 | 0.002 | 16 | 48 |
| Right | Mid‐insula/claustrum | 36 | −2 | −2 | 0.013 | 0.002 | 17 | 48 | |
| Right | Posterior insula | 46 | −24 | 16 | 0.013 | 0.001 | 18 | 40 | |
| Left | Superior Frontal Gyrus | 10 | −26 | 44 | 18 | 0.013 | 0.0008 | 19 | 40 |
| Left | Caudate | −12 | 8 | 10 | 0.013 | 0.002 | 20 | 32 | |
| Left | Cerebellum | −36 | −54 | −32 | 0.012 | 0.003 | 21 | 24 | |
| Right | Parahippocampal Gyrus | 35 | 22 | −8 | −22 | 0.012 | 0.003 | 22 | 24 |
| Right | Amygdala | 24 | −8 | −22 | 0.012 | 0.003 | |||
| Right | Amygdala | 24 | −8 | −20 | 0.012 | 0.004 |
ALE values for Study 2. ALE maps of noxious heat were subtracted from noxious cold. ALE values refer to the likelihood of obtaining activation in response to noxious cold stimuli in a given voxel of the standard template. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; IPL, inferior parietal lobule; SII, secondary somatosensory cortex; MI, primary motor cortex.
Table XIV.
Spatial location and extent of ALE values for study 2 (noxious heat minus noxious cold)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Left | Putaman | −22 | 0 | −2 | 0.024 | 0.005 | 1 | 1072 | |
| Right | Anterior insula/claustrum | 34 | 12 | 6 | 0.023 | <0.00001 | 2 | 960 | |
| Right | Mid‐insula/claustrum | 34 | 2 | 10 | 0.023 | <0.00001 | |||
| Right | Anterior insula | 34 | 22 | 8 | 0.014 | 0.002 | |||
| Left | Anterior insula | −40 | 18 | 6 | 0.025 | <0.00001 | 3 | 616 | |
| Left | Anterior insula | −30 | 20 | 8 | 0.014 | 0.001 | |||
| Right | SII/IPL | 52 | −32 | 22 | 0.020 | 0.0002 | 4 | 584 | |
| Left | Posterior insula | −44 | −24 | 16 | 0.024 | <0.00001 | 5 | 576 | |
| Right | Thalamus | 14 | −20 | 4 | 0.021 | <0.00001 | 6 | 504 | |
| Right | Posterior insula | 32 | −8 | 18 | 0.017 | <0.00001 | 7 | 360 | |
| Right | Lentiform Nucleus | 30 | −14 | 8 | 0.016 | 0.0004 | |||
| Left | Thalamus | −12 | −24 | 12 | 0.022 | <0.00001 | 8 | 360 | |
| Right | Inferior Frontal Gyrus | 38 | 46 | 2 | 0.020 | <0.00001 | 9 | 352 | |
| Left | MI | 4 | −32 | −22 | 50 | 0.020 | <0.00001 | 10 | 296 |
| Right | Cingulate Gyrus | 32 | 4 | 22 | 26 | 0.018 | <0.00001 | 11 | 200 |
| Right | Caudate | 14 | 8 | 12 | 0.020 | 0.0002 | 12 | 168 | |
| Left | Cingulate Gyrus | 24 | −6 | 12 | 32 | 0.014 | 0.001 | 13 | 112 |
| Left | Cingulate Gyrus | 24 | 0 | −6 | 42 | 0.015 | 0.001 | 14 | 72 |
| Left | Superior Temporal Gyrus | 42 | −54 | −30 | 14 | 0.013 | 0.003 | 15 | 64 |
| Right | Anterior insula | 46 | 6 | 16 | 0.013 | 0.0004 | 16 | 64 | |
| Right | Inferior frontal gyrus | 44 | 52 | 6 | 12 | 0.013 | 0.002 | ||
| Right | Middle Frontal Gyrus | 46 | 42 | 36 | 24 | 0.014 | 0.0008 | 17 | 40 |
| Right | Cingulate Gyrus | 23 | 2 | −22 | 28 | 0.012 | 0.003 | 18 | 32 |
| Right | SII/IPL | 39 | 48 | −62 | 38 | 0.013 | 0.0008 | 19 | 32 |
| Left | SI/PPC | 5 | −10 | −34 | 46 | 0.012 | 0.003 | 20 | 32 |
| Left | SI/PPC | 5 | −10 | −32 | 46 | 0.012 | 0.003 | ||
| Right | SI/PPC | 5 | 30 | −42 | 58 | 0.012 | 0.004 | 21 | 32 |
| Left | Posterior insula | −52 | −34 | 20 | 0.012 | 0.002 | 21 | 24 | |
| Left | Posterior insula | −48 | −20 | 24 | 0.012 | 0.003 | 21 | 24 |
ALE values for Study 2. ALE maps of noxious cold were subtracted from noxious heat pain. ALE values refer to the likelihood of obtaining activation in response to noxious heat vs. noxious cold in a given voxel of the standard template. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; IPL, inferior parietal lobule; SI/PPC, primary somatosensory cortex/posterior parietal cortex; MI, primary motor cortex.
Study 3: Noxious Heat Minus Warm Versus Noxious Heat Minus Resting Baseline
An initial ALE analysis was conducted on 131 coordinate sites compiled from the nine studies that used a warm‐stimulus control in comparison to noxious heat stimulation. Results of this ALE analysis yielded 31 regions with a significant likelihood (ranging from 0.013 to 0.048) of showing “pain‐related” brain activation. The greatest likelihood that activation will be evoked in the cortex in response to noxious heat stimuli in comparison to warm was in the anterior and posterior cingulate gyrus (BA 24, ALE = 0.048 and BA 23, ALE = 0.029), the insula (ALE = 0.028), followed by SI and SII (both ALEs = 0.014; Table XV). Additionally, the likelihood of evoking activation in response to noxious heat stimuli was significant within the cerebellum, thalamus, and basal ganglia.
Table XV.
Spatial location and extent of ALE values for study 3 (noxious heat minus warm)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Left | SII | −50 | −4 | 6 | 0.027 | <0.00001 | 1 | 3432 | |
| Left | Anterior insula | −48 | 6 | 4 | 0.015 | 0.0002 | |||
| Left | Anterior insula | −44 | 6 | 2 | 0.015 | 0.0008 | |||
| Left | Inferior frontal gyrus | 44 | −46 | 8 | 12 | 0.014 | 0.0004 | ||
| Right | Anterior insula | 38 | 8 | −4 | 0.028 | <0.00001 | 2 | 1448 | |
| Right | Mid‐insula | 38 | 0 | 12 | 0.020 | <0.00001 | |||
| Right | Cingulate gyrus | 24 | 4 | 2 | 38 | 0.027 | <0.00001 | 3 | 1440 |
| Left | Cingulate gyrus | 24 | −6 | −4 | 40 | 0.022 | <0.00001 | ||
| Right | Medial frontal gyrus | 2 | 0 | 52 | 0.014 | 0.001 | |||
| Right | Cingulate gyrus | 24 | 6 | 20 | 24 | 0.048 | <0.00001 | 4 | 1096 |
| Right | Thalamus | 6 | −20 | 0 | 0.026 | <0.00001 | 5 | 824 | |
| Right | Thalamus | 12 | −22 | 8 | 0.016 | 0.0004 | |||
| Left | Cingulate gyrus | 23 | −2 | −22 | 32 | 0.029 | <0.00001 | 6 | 800 |
| Right | Cerebellum | 16 | −58 | −12 | 0.027 | <0.00001 | 7 | 752 | |
| Right | Putamen | 30 | −14 | 8 | 0.018 | 0.0002 | 8 | 712 | |
| Right | Posterior insula | 36 | −12 | 16 | 0.015 | 0.0004 | |||
| Right | Posterior insula | 34 | −22 | 14 | 0.014 | 0.0002 | |||
| Right | Posterior insula | 36 | −18 | 20 | 0.014 | 0.0006 | |||
| Left | Posterior insula | −40 | −20 | 16 | 0.029 | <0.00001 | 9 | 648 | |
| Left | Thalamus | −8 | −16 | 8 | 0.017 | <0.00001 | 10 | 480 | |
| Right | Thalamus | 30 | 44 | 20 | 0.026 | <0.00001 | 11 | 360 | |
| Left | Thalamus | −22 | −16 | 10 | 0.014 | 0.0002 | 12 | 208 | |
| Left | Putamen | −26 | −20 | 12 | 0.014 | 0.0004 | |||
| Right | SII | 48 | −38 | 30 | 0.014 | 0.0008 | 13 | 184 | |
| Right | SII | 52 | −34 | 24 | 0.013 | 0.0008 | |||
| Right | Cerebellum | 22 | −58 | −28 | 0.014 | 0.0008 | 14 | 168 | |
| Right | Cerebellum | 22 | −60 | −32 | 0.014 | 0.001 | |||
| Left | SII | −50 | −26 | 28 | 0.014 | 0.0004 | 15 | 152 | |
| Left | SI | 2 | −48 | −20 | 26 | 0.014 | 0.002 | ||
| Right | Cingulate gyrus | 32 | 4 | 42 | 12 | 0.013 | 0.0006 | 16 | 120 |
| Left | Cingulate gyrus | 24 | 0 | 38 | 6 | 0.013 | 0.002 |
ALE values for Study 3. ALE values refer to the likelihood of obtaining activation in response to noxious heat stimuli contrasted with innocuous warm stimuli in a given voxel of the standard template. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; SI, primary somatosensory cortex; PPC, posterior parietal cortex; SII, secondary somatosensory cortex.
The nine studies that used a resting baseline in comparison to noxious heat stimulation had a total of 149 coordinate sites that were then subjected to an ALE analysis. As expected, the noxious heat versus baseline condition yielded a substantially greater number of activation loci than had been observed in the more restrictive comparison of noxious heat to warm stimulation (40 vs. 31 regions). Brain regions of interest that had a significant likelihood of exhibiting stimulus‐related activation in comparison to a resting baseline were observed throughout the cortex and included the ACC (BA32, 0.042), the inferior frontal gyrus (BA 44, 0.026), the insula (0.024), SI (0.019), and SII (left and right: 0.014), and the superior frontal gyrus BA 6, 0.021); see Table XVI for a complete list.
Table XVI.
Spatial location and extent of ALE values for study 3 (noxious heat vs. baseline)
| Side | Region | BA | X | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Left | Cingulate gyrus | 32 | −2 | 10 | 40 | 0.042 | <0.00001 | 1 | 3768 |
| Left | Cingulate gyrus | 24 | −4 | 12 | 30 | 0.029 | <0.00001 | ||
| Left | Cingulate gyrus | 24 | 0 | −2 | 44 | 0.024 | <0.00001 | ||
| Left | Cingulate gyrus | 32 | −8 | 24 | 30 | 0.014 | 0.0002 | ||
| Left | Supplementary motor area | 6 | 0 | −10 | 52 | 0.013 | 0.004 | ||
| Right | SII | 43 | 50 | −18 | 16 | 0.020 | <0.00001 | 2 | 2408 |
| Right | Posterior insula | 36 | −20 | 16 | 0.020 | <0.00001 | |||
| Right | IPL | 40 | 48 | −34 | 28 | 0.018 | 0.0004 | ||
| Right | IPL | 40 | 56 | −30 | 24 | 0.016 | 0.0002 | ||
| Right | IPL | 40 | 60 | −30 | 26 | 0.016 | 0.0002 | ||
| Right | SII | 40 | 50 | −32 | 34 | 0.014 | 0.0008 | ||
| Right | SII | 56 | −12 | 12 | 0.014 | 0.0006 | |||
| Right | Putaman | 30 | 2 | 8 | 0.032 | <0.00001 | 3 | 1952 | |
| Right | Mid‐insula/claustrum | 30 | 4 | 12 | 0.031 | <0.00001 | |||
| Left | Mid‐insula | −40 | 2 | 8 | 0.024 | 0.0002 | 4 | 1360 | |
| Left | Anterior insula | −46 | 6 | 16 | 0.013 | 0.003 | |||
| Left | Posterior insula | −42 | −10 | 12 | 0.013 | 0.002 | |||
| Left | Mid−insula/claustrum | −30 | 4 | 8 | 0.013 | 0.001 | |||
| Left | Thalamus | −16 | −20 | 12 | 0.037 | <0.00001 | 5 | 1032 | |
| Right | Inferior frontal gyrus | 44 | 52 | 6 | 10 | 0.026 | <0.00001 | 6 | 824 |
| Right | Cerebellum | 0 | −66 | −16 | 0.023 | <0.00001 | 7 | 784 | |
| Right | Cerebellum | 2 | −62 | −14 | 0.021 | 0.0002 | |||
| Right | Thalamus | 12 | −20 | 4 | 0.027 | <0.00001 | 8 | 760 | |
| Right | Cerebellum | 20 | −66 | −24 | 0.020 | <0.00001 | 9 | 656 | |
| Right | Cerebellum | 30 | −76 | −28 | 0.014 | 0.0004 | |||
| Left | Posterior insula | −44 | −24 | 16 | 0.024 | <0.00001 | 10 | 576 | |
| Right | Medial frontal gyrus | 6 | 6 | −6 | 62 | 0.019 | 0.0002 | 11 | 408 |
| Left | Putaman | −22 | 0 | −2 | 0.022 | <0.00001 | 12 | 312 | |
| Right | PPC | 5 | 22 | −42 | 66 | 0.021 | <0.00001 | 13 | 280 |
| Left | MI | 4 | −32 | −22 | 50 | 0.020 | 0.0002 | 14 | 264 |
| Right | SI | 3 | 30 | −30 | 62 | 0.019 | <0.00001 | 15 | 264 |
| Left | Putaman | −28 | −14 | 10 | 0.014 | 0.0004 | 16 | 256 | |
| Left | Putaman | −22 | −10 | 8 | 0.014 | 0.001 | |||
| Left | Putaman | −30 | −12 | 2 | 0.013 | 0.002 | |||
| Right | Posterior insula | 32 | −10 | 18 | 0.017 | 0.0002 | 17 | 248 | |
| Left | Cerebellum | −22 | −54 | −28 | 0.016 | 0.0006 | 18 | 240 | |
| Right | Premotor cortex | 6 | 46 | 0 | 30 | 0.018 | <0.00001 | 19 | 232 |
| Right | Cerebellum | 38 | −54 | −36 | 0.017 | 0.0004 | 20 | 216 | |
| Right | Anterior insula | 36 | 18 | 8 | 0.015 | 0.0004 | 21 | 160 | |
| Left | Superior frontal gyrus | −10 | −8 | 72 | 0.021 | <0.00001 | 22 | 152 |
ALE values for Study 3. ALE values refer to the likelihood of obtaining activation evoked by noxious heat stimuli in comparison to resting baseline. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; IPL, inferior parietal lobule; SI, primary somatosensory cortex; PPC, posterior parietal cortex; SII, secondary somatosensory cortex; MI, primary motor cortex.
The two sets of peak ALE values (noxious heat vs. warm and noxious heat vs. resting baseline) were examined for common brain regions demonstrating a significant likelihood of being activated using either type of contrast. It was evident that for both types of contrasts, the likelihood of activation was significant within the ACC (BA 24), supplementary motor area (SMA), insula, SII, and thalamus (Supporting Information Fig. 2).
We performed a direct subtraction of the two maps to assess significant differences in the patterns of activation that resulted from the two analysis strategies. Studies using a no‐stimulation baseline control as a comparison for noxious heat stimuli were more likely to reveal stimulus‐related activation in the anterior portion of the ACC (BA 32; ALE = 0.039; Table XVII), and SI/PPC (ALE = 0.019); while those using a warm‐control condition as a comparison were significantly more likely to observe noxious‐heat‐related activation in the middle regions of the ACC (BA 24; ALE = 0.048), and the posterior cingulate cortex (ALE = 0.029; Table XVIII).
Table XVII.
Spatial location and extent of ALE values for study 3 (noxious heat vs. baseline minus noxious heat vs. warm)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Left | Cingulate gyrus | 32 | −2 | 10 | 40 | 0.039 | <0.00001 | 1 | 2168 |
| Left | Cingulate gyrus | 24 | −6 | 14 | 30 | 0.029 | <0.00001 | ||
| Left | Cingulate gyrus | 32 | −8 | 24 | 30 | 0.013 | 0.002 | ||
| Right | SII | 43 | 50 | −18 | 16 | 0.020 | <0.00001 | 2 | 1040 |
| Right | SII | 40 | 60 | −30 | 26 | 0.015 | 0.0004 | ||
| Right | SII | 42 | 56 | −12 | 12 | 0.014 | 0.0008 | ||
| Right | Putamen | 30 | 2 | 6 | 0.027 | <0.00001 | 3 | 944 | |
| Left | Thalamus | −16 | −20 | 12 | 0.035 | <0.00001 | 4 | 688 | |
| Right | Inferior frontal gyrus | 44 | 54 | 6 | 10 | 0.026 | <0.00001 | 5 | 624 |
| Right | Cerebellum | 0 | −66 | −16 | 0.023 | <0.00001 | 6 | 536 | |
| Right | Cerebellum | 20 | −66 | −24 | 0.020 | 0.0004 | 7 | 488 | |
| Right | Cerebellum | 30 | −76 | −28 | 0.014 | 0.0008 | |||
| Left | Mid‐insula | −40 | 0 | 8 | 0.019 | <0.00001 | 8 | 448 | |
| Left | Anterior insula | −38 | 12 | 12 | 0.014 | 0.002 | |||
| Right | Medial frontal gyrus | 6 | 6 | −6 | 62 | 0.019 | 0.0006 | 9 | 320 |
| Right | SI/PPC | 22 | −42 | 66 | 0.021 | 0.0002 | 10 | 240 | |
| Left | MI | 4 | −32 | −22 | 50 | 0.020 | 0.0002 | 11 | 224 |
| Right | SI | 3 | 30 | −30 | 62 | 0.019 | 0.0004 | 12 | 216 |
| Left | Putamen | −22 | 0 | −4 | 0.021 | 0.0002 | 13 | 192 | |
| Left | Posterior insula | −44 | −26 | 16 | 0.018 | <0.00001 | 14 | 168 | |
| Right | Thalamus | 14 | −20 | 4 | 0.018 | 0.0002 | 15 | 160 | |
| Right | Inferior frontal gyrus | 6 | 46 | 0 | 30 | 0.017 | 0.0004 | 16 | 152 |
| Right | Cerebellum | 38 | −54 | −36 | 0.016 | 0.0002 | 17 | 144 | |
| Left | Superior frontal gyrus | 6 | −10 | −8 | 72 | 0.021 | 0.0002 | 18 | 120 |
| Right | Posterior insula/claustrum | 30 | −8 | 18 | 0.016 | 0.0002 | 19 | 112 |
Study 3: ALE values refer to the likelihood of obtaining activation evoked by noxious heat stimuli in comparison to resting baseline minus ALE values obtained for noxious heat in comparison to innocuous warm stimuli. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; SI, primary somatosensory cortex; PPC, posterior parietal cortex; SII, secondary somatosensory cortex; MI, primary motor cortex.
Table XVIII.
Spatial location and extent of ALE values for study 3 (noxious heat vs. warm minus noxious heat vs. baseline)
| Side | Region | BA | x | y | z | ALE value | P values | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Right | Cingulate Gyrus | 24 | 6 | 20 | 24 | 0.048 | <0.00001 | 1 | 976 |
| Left | Cingulate Gyrus | 23 | −2 | −22 | 32 | 0.029 | <0.00001 | 2 | 600 |
| Right | Anterior insula | 13 | 38 | 8 | −4 | 0.028 | <0.00001 | 3 | 464 |
| Right | Cerebellum | 16 | −58 | −12 | 0.021 | <0.00001 | 4 | 256 | |
| Right | Lentiform Nucleus | 30 | −14 | 8 | 0.018 | 0.0002 | 5 | 192 | |
| Left | Superior Temporal Gyrus | 22 | −50 | −4 | 6 | 0.021 | <0.00001 | 6 | 184 |
| Right | Cingulate Gyrus | 24 | 6 | 0 | 38 | 0.019 | <0.00001 | 7 | 184 |
| Left | Posterior insula | 13 | −38 | −20 | 14 | 0.018 | 0.0004 | 8 | 152 |
| Right | Mid‐insula | 13 | 38 | −2 | 12 | 0.016 | 0.001 | 9 | 136 |
| Left | Cingulate Gyrus | 24 | −6 | −6 | 40 | 0.018 | 0.0004 | 10 | 112 |
| Right | Thalamus | 4 | −20 | 0 | 0.017 | <0.00001 | 11 | 104 | |
| Left | Thalamus | −4 | −14 | 8 | 0.015 | 0.001 | 12 | 104 |
Study 3: ALE values refer to the likelihood of obtaining activation evoked by noxious heat stimuli in comparison to innocuous warm stimuli subtracting ALE values obtained for noxious heat minus baseline. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior.
Study 4: Left‐ Versus Right‐Sided Stimuli
ALE maps of noxious stimuli applied to the left side of the body were created using the 694 coordinates extracted from the publications included in the left‐sided meta‐analysis. According to predictions, analysis of studies using left‐sided stimulation showed a substantially larger number of sites with significant activation‐likelihood values in the contralateral right hemisphere, compared to those observed in the left hemisphere (31 vs. 18). The most statistically significant ALE sites were located in the right insula (ALE = 0.11) and right ACC (ALE = 0.095). Significant ALE values were also found in bilateral thalamus (right: ALE = 0.089; left: ALE = 0.082). Other brain regions that also had a significant likelihood of being activated are listed in Table XIX.
Table XIX.
Spatial location and extent of ALE values for Study 4 (noxious stimuli applied to the left side of the body)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Right | Thalamus | 10 | −20 | 6 | 0.089 | <0.00001 | 1 | 18,008 | |
| Left | Thalamus | −12 | −16 | 10 | 0.082 | <0.00001 | |||
| Left | Mid‐insula/claustrum | −34 | 2 | 8 | 0.069 | <0.00001 | |||
| Right | Thalamus | 16 | −18 | 14 | 0.055 | <0.00001 | |||
| Left | Anterior insula | −32 | 12 | 10 | 0.044 | <0.00001 | |||
| Right | Thalamus | 10 | −6 | 6 | 0.042 | 0.0002 | |||
| Left | Midbrain | −2 | −16 | −8 | 0.036 | <0.00001 | |||
| Right | Lentiform Nucleus | 18 | −6 | 0 | 0.032 | <0.00001 | |||
| Right | Putamen | 2 | −28 | −6 | 0.029 | <0.00001 | |||
| Left | Lentiform Nucleus | −28 | −10 | 4 | 0.028 | <0.00001 | |||
| Left | Anterior insula | −40 | 6 | −4 | 0.027 | 0.0004 | |||
| Right | Posterior insula | 36 | −20 | 18 | 0.108 | <0.00001 | 2 | 17,912 | |
| Right | Mid‐insula/claustrum | 32 | 4 | 12 | 0.098 | <0.00001 | |||
| Right | IPL | 40 | 52 | −30 | 26 | 0.073 | <0.00001 | ||
| Right | Superior Temporal Gyrus | 22 | 52 | 4 | 8 | 0.050 | <0.00001 | ||
| Right | IPL | 40 | 46 | −34 | 40 | 0.031 | 0.0001 | ||
| Right | Anterior insula | 46 | 10 | 0 | 0.027 | 0.0002 | |||
| Right | Posterior insula/claustrum | 36 | −12 | −4 | 0.022 | 0.002 | |||
| Right | Cingulate Gyrus | 24 | 2 | 4 | 38 | 0.095 | <0.00001 | 3 | 12,552 |
| Right | Medial Frontal Gyrus | 6 | 2 | 2 | 52 | 0.079 | <0.00001 | ||
| Right | Medial Frontal Gyrus | 6 | 10 | 8 | 50 | 0.049 | <0.00001 | ||
| Left | Cingulate Gyrus | 24 | −8 | 16 | 28 | 0.048 | <0.00001 | ||
| Left | Medial Frontal Gyrus | 6 | −2 | −10 | 52 | 0.037 | <0.00001 | ||
| Right | Cingulate Gyrus | 24 | 8 | −12 | 40 | 0.037 | <0.00001 | ||
| Right | Medial Frontal Gyrus | 6 | 8 | −10 | 52 | 0.027 | <0.00001 | ||
| Right | Superior Frontal Gyrus | 6 | 14 | −6 | 62 | 0.026 | 0.0004 | ||
| Left | IPL | 40 | −52 | −32 | 28 | 0.032 | <0.00001 | 4 | 1384 |
| Left | IPL | −50 | −36 | 22 | 0.032 | <0.00001 | |||
| Left | SII | −56 | −22 | 20 | 0.030 | 0.0002 | |||
| Left | Cerebellum | −34 | −56 | −30 | 0.033 | <0.00001 | 5 | 1352 | |
| Left | Cerebellum | −28 | −54 | −30 | 0.031 | <0.00001 | |||
| Left | Cerebellum | −24 | −56 | −18 | 0.031 | <0.00001 | |||
| Right | SI/PPC | 5 | 22 | −42 | 64 | 0.040 | <0.00001 | 6 | 1048 |
| Right | SI | 3 | 30 | −30 | 62 | 0.033 | <0.00001 | ||
| Right | Cerebellum | 4 | −58 | −14 | 0.032 | <0.00001 | 7 | 864 | |
| Right | Cerebellum | 0 | −50 | −16 | 0.026 | 0.001 | |||
| Right | Precentral Gyrus | 6 | 26 | −16 | 54 | 0.042 | <0.00001 | 8 | 784 |
| Right | MI | 4 | 34 | −18 | 58 | 0.026 | 0.001 | ||
| Right | Middle Frontal Gyrus | 10 | 32 | 40 | 22 | 0.029 | <0.00001 | 9 | 720 |
| Right | Middle Frontal Gyrus | 10 | 40 | 38 | 22 | 0.029 | 0.0004 | ||
| Right | Superior Frontal Gyrus | 9 | 28 | 40 | 30 | 0.026 | 0.0004 | ||
| Left | SII | −40 | −24 | 14 | 0.029 | <0.00001 | 11 | 456 | |
| Right | Inferior Frontal Gyrus | 38 | 22 | 6 | 0.027 | 0.0006 | 12 | 184 | |
| Right | Cerebellum | 24 | −66 | −24 | 0.025 | 0.001 | 13 | 160 | |
| Right | Paracentral Lobule | 5 | 8 | −40 | 60 | 0.030 | 0.0004 | 14 | 152 |
| Left | Posterior insula/claustrum | −34 | −18 | 4 | 0.025 | 0.0004 | 15 | 128 | |
| Right | Cingulate Gyrus | 32 | 4 | 22 | 26 | 0.027 | 0.001 | 16 | 128 |
| Left | Cingulate Gyrus | 32 | −6 | 32 | −4 | 0.028 | 0.0006 | 17 | 104 |
ALE values for Study 4. ALE values refer to the likelihood of obtaining activation evoked by noxious stimuli applied to the left side of the body. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; IPL, inferior parietal lobule; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; MI, primary motor cortex.
The ALE analysis for right‐sided stimuli was calculated on 699 coordinates taken from the studies. Surprisingly, the number of statistically significant activation sites was equivalent in both hemispheres (24 vs. 24), rather than being concentrated in the left hemisphere, as suggested by the traditional view of preferential cutaneous processing through the contralateral sensory pathways. The highest likelihood of evoking activation in response to noxious stimuli applied to the right side of the body was found in right anterior insula (ALE = 0.11). Other regions showing high likelihood values were the left insula (ALE = 0.1), bilateral SII (right = 0.084; left = 0.091), left thalamus (ALE = 0.082), and the right ACC (BA 24, ALE = 0.081). These results provide strong support for a right hemispheric dominance for pain processing. A complete list of the ALE values for right‐sided noxious stimulation is in Table XX.
Table XX.
Spatial location and extent of ALE values for (noxious heat applied to the right side of the body)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Right | Anterior insula | 34 | 12 | 8 | 0.105 | <0.000001 | 1 | 41,464 | |
| Left | Mid‐insula | −38 | 4 | 4 | 0.100 | <0.000001 | |||
| Left | SII | −54 | −26 | 22 | 0.091 | <0.000001 | |||
| Right | SII | 56 | −22 | 20 | 0.084 | <0.000001 | |||
| Left | Posterior insula | −38 | −20 | 14 | 0.082 | <0.000001 | |||
| Left | Thalamus | −16 | −16 | 10 | 0.082 | <0.000001 | |||
| Right | Thalamus | 4 | −18 | 4 | 0.069 | <0.000001 | |||
| Right | Thalamus | 12 | −12 | 8 | 0.054 | <0.000001 | |||
| Right | Lentiform Nucleus | 24 | −2 | 8 | 0.049 | <0.000001 | |||
| Left | Precentral Gyrus | 43 | −54 | −8 | 12 | 0.043 | <0.000001 | ||
| Right | Precentral Gyrus | 44 | 50 | 6 | 12 | 0.040 | <0.000001 | ||
| Right | Mid‐insula | 36 | −2 | 14 | 0.040 | <0.000001 | |||
| Right | Inferior Frontal Gyrus | 47 | 42 | 18 | −2 | 0.039 | <0.000001 | ||
| Left | Lentiform Nucleus | −20 | 4 | 10 | 0.038 | <0.000001 | |||
| Left | Precentral Gyrus | 6 | −52 | −4 | 6 | 0.035 | <0.000001 | ||
| Left | Thalamus | −4 | −26 | 0 | 0.035 | <0.000001 | |||
| Right | Posterior insula | 38 | −14 | 16 | 0.034 | <0.000001 | |||
| Right | Posterior insula | 44 | −14 | 16 | 0.033 | <0.000001 | |||
| Right | Inferior Frontal Gyrus | 42 | 26 | 4 | 0.026 | <0.000001 | |||
| Left | Lentiform Nucleus | −20 | 12 | 0 | 0.025 | 0.0008 | |||
| Left | Supramarginal Gyrus | 40 | −54 | −38 | 32 | 0.022 | 0.002 | ||
| Right | Cingulate Gyrus | 24 | 4 | 8 | 36 | 0.081 | <0.000001 | 2 | 14,016 |
| Right | Cingulate Gyrus | 32 | 6 | 22 | 26 | 0.067 | <0.000001 | ||
| Left | Cingulate Gyrus | 24 | −4 | −4 | 42 | 0.062 | <0.000001 | ||
| Left | Cingulate Gyrus | 32 | −2 | 24 | 38 | 0.046 | <0.000001 | ||
| Left | Anterior Cingulate | 24 | −4 | 20 | 24 | 0.045 | <0.000001 | ||
| Left | Medial Frontal Gyrus | 8 | −10 | 26 | 42 | 0.025 | 0.001 | ||
| Left | SII | 3 | −32 | −34 | 60 | 0.049 | <0.000001 | 3 | 1664 |
| Left | MI | 4 | −38 | −24 | 62 | 0.034 | <0.000001 | ||
| Right | Middle Frontal Gyrus | 10 | 30 | 44 | 20 | 0.036 | <0.000001 | 4 | 1424 |
| Right | Middle Frontal Gyrus | 10 | 42 | 46 | 14 | 0.030 | 0.0002 | ||
| Right | Superior Frontal Gyrus | 9 | 38 | 36 | 26 | 0.028 | <0.000001 | ||
| Right | Middle Frontal Gyrus | 9 | 38 | 30 | 30 | 0.024 | 0.001 | ||
| Left | Cerebellum | −34 | −54 | −36 | 0.034 | <0.000001 | 5 | 968 | |
| Left | Cerebellum | −20 | −62 | −24 | 0.033 | <0.000001 | |||
| Left | Cerebellum | −30 | −58 | −30 | 0.029 | <0.000001 | |||
| Right | Cerebellum | 22 | −58 | −24 | 0.040 | <0.000001 | 6 | 720 | |
| Right | Cerebellum | 2 | −46 | −16 | 0.034 | <0.000001 | 7 | 616 | |
| Right | IPL | 40 | 50 | −32 | 34 | 0.038 | <0.000001 | 8 | 480 |
| Left | Supramarginal Gyrus | 40 | −40 | −40 | 36 | 0.042 | <0.000001 | 9 | 480 |
| Left | Cerebellum | −4 | −56 | −28 | 0.028 | 0.0002 | 10 | 328 | |
| Right | Cerebellum | 4 | −62 | −16 | 0.034 | <0.000001 | 11 | 288 | |
| Right | IPL | 40 | 50 | −46 | 38 | 0.031 | <0.000001 | 12 | 280 |
| Left | Middle Frontal Gyrus | 10 | −30 | 46 | 4 | 0.033 | <0.000001 | 13 | 216 |
| Left | Angular Gyrus | 39 | −40 | −58 | 34 | 0.030 | 0.0002 | 14 | 160 |
| Left | Medial Frontal Gyrus | 6 | −4 | −20 | 66 | 0.027 | <0.000001 | 15 | 128 |
| Right | Uncus | 36 | 20 | −4 | −34 | 0.025 | 0.001 | 16 | 104 |
| Right | Medial Frontal Gyrus | 6 | 6 | 2 | 62 | 0.025 | 0.0008 | 17 | 104 |
Study 4: ALE values refer to the likelihood of obtaining activation evoked by noxious heat stimuli applied to the right side of the body. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; IPL, inferior parietal lobule; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; MI, primary motor cortex.
When directly comparing noxious stimuli applied to the right or left side of the body, the greatest likelihood of evoking activation in the cortex in response to noxious stimuli presented to either side of the body was in the right anterior insula. Additionally, in both meta‐analyses large clusters of likelihood estimate values were significant within the right ACC (Supporting Information Fig. 3).
Upon performing the subtractions, (left‐sided stimuli minus right‐sided stimuli) the results showed preferential likelihood values that were significant within contralateral (right) SI, MI, PPC, and the superior frontal gyrus, and the ipsilateral (left) midbrain (Table XXI). The likelihood of activation evoked by right‐sided stimuli was significant (exclusively) within contralateral (left) SI, ACC (BA32), MI, inferior parietal lobule, and the medial frontal gyrus. However, some regions in the right hemisphere were also found to have distinctive activation likelihood values in response to right‐sided stimuli such as ACC (BA 32), the inferior parietal lobule, and the middle frontal gyrus (Table XXII).
Table XXI.
Spatial location and extent of ALE values for study 4 (left‐sided stimuli minus right‐sided stimuli)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Right | Posterior insula | 36 | −20 | 18 | 0.093 | <0.000001 | 1 | 3024 | |
| Right | Posterior insula/claustrum | 38 | −14 | 8 | 0.040 | <0.000001 | |||
| Right | Cingulate Gyrus | 24 | 4 | 2 | 38 | 0.047 | <0.000001 | 2 | 2576 |
| Right | Medial Frontal Gyrus | 6 | 2 | 2 | 54 | 0.046 | <0.000001 | ||
| Right | Medial Frontal Gyrus | 6 | 12 | 8 | 50 | 0.043 | <0.000001 | ||
| Right | Cingulate Gyrus | 24 | 8 | −12 | 42 | 0.029 | <0.000001 | ||
| Right | Medial Frontal Gyrus | 6 | 8 | −10 | 52 | 0.024 | 0.0008 | ||
| Right | Mid‐insula/claustrum | 32 | 4 | 12 | 0.055 | <0.000001 | 3 | 1976 | |
| Right | Thalamus | 16 | −18 | 14 | 0.049 | <0.000001 | 4 | 1488 | |
| Right | Thalamus | 10 | −20 | 4 | 0.041 | <0.000001 | |||
| Right | SII | 52 | −30 | 26 | 0.058 | <0.000001 | 5 | 1272 | |
| Right | SI/PPC | 5 | 22 | −42 | 64 | 0.039 | <0.000001 | 6 | 880 |
| Right | SI | 3 | 30 | −30 | 62 | 0.031 | 0.0002 | ||
| Right | Precentral Gyrus | 6 | 26 | −16 | 54 | 0.041 | <0.000001 | 7 | 712 |
| Right | MI | 4 | 34 | −18 | 58 | 0.025 | 0.0008 | ||
| Left | Thalamus | −10 | −16 | 10 | 0.035 | <0.000001 | 8 | 416 | |
| Left | Thalamus | −6 | −20 | 16 | 0.027 | 0.0002 | |||
| Left | Midbrain | −2 | −16 | −10 | 0.031 | <0.000001 | 9 | 304 | |
| Left | Cingulate Gyrus | 24 | −10 | 16 | 28 | 0.035 | <0.000001 | 10 | 264 |
| Right | Inferior frontal gyrus | 44 | 52 | 2 | 4 | 0.031 | <0.000001 | 11 | 240 |
| Left | Cerebellum | −24 | −56 | −18 | 0.029 | 0.0002 | 12 | 232 | |
| Right | SI/PPC | 5 | 8 | −40 | 60 | 0.030 | <0.000001 | 13 | 168 |
| Right | Superior Frontal Gyrus | 6 | 14 | −6 | 62 | 0.025 | 0.001 | 14 | 160 |
| Left | Medial Frontal Gyrus | 6 | −4 | −12 | 56 | 0.026 | 0.0004 | 15 | 152 |
| Right | Lentiform Nucleus | 18 | −6 | 0 | 0.025 | 0.0004 | 16 | 144 | |
| Right | Thalamus | 10 | −4 | 2 | 0.022 | 0.002 | |||
| Left | Mid‐insula | −46 | 2 | 14 | 0.027 | 0.0006 | 17 | 136 | |
| Left | Cingulate gyrus | 32 | −6 | 32 | −4 | 0.028 | <0.000001 | 18 | 128 |
| Left | Posterior insula | −50 | −36 | 22 | 0.027 | 0.0002 | 19 | 128 |
ALE values for Study 4. ALE values for applying noxious stimuli to the left side of the body subtracting the ALE maps for applying stimuli to the right side of the body. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; SI, primary somatosensory cortex; PPC, posterior parietal cortex; SII, secondary somatosensory cortex; MI, primary motor cortex.
Table XXII.
Spatial location and extent of ALE values for study 4 (right‐sided stimuli minus left‐sided stimuli)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Left | SI | −54 | −26 | 22 | 0.067 | <0.000001 | 1 | 8128 | |
| Left | Anterior insula | −38 | 6 | 4 | 0.066 | <0.000001 | |||
| Left | Posterior insula | −38 | −18 | 12 | 0.061 | <0.000001 | |||
| Left | SII | −56 | −10 | 12 | 0.039 | <0.000001 | |||
| Left | Precentral Gyrus | 6 | −52 | −4 | 6 | 0.030 | 0.0004 | ||
| Left | Cingulate Gyrus | 32 | −2 | 24 | 40 | 0.042 | <0.000001 | 2 | 2256 |
| Right | Cingulate Gyrus | 32 | 6 | 20 | 26 | 0.042 | <0.000001 | ||
| Right | Cingulate Gyrus | 32 | 2 | 24 | 32 | 0.040 | <0.000001 | ||
| Left | Cingulate Gyrus | 24 | −2 | 20 | 24 | 0.033 | <0.000001 | ||
| Left | Medial Frontal Gyrus | 8 | −10 | 26 | 42 | 0.025 | 0.0008 | ||
| Right | Anterior insula | 34 | 14 | 8 | 0.079 | <0.000001 | 3 | 1648 | |
| Left | Cingulate Gyrus | 32 | −8 | 8 | 38 | 0.041 | <0.000001 | 4 | 1568 |
| Left | Superior Frontal Gyrus | 6 | 0 | 12 | 48 | 0.034 | <0.000001 | ||
| Right | Cingulate Gyrus | 32 | 8 | 12 | 40 | 0.033 | <0.000001 | ||
| Right | Cingulate Gyrus | 32 | 12 | 14 | 38 | 0.033 | <0.000001 | ||
| Left | SI | 3 | −32 | −34 | 60 | 0.049 | <0.000001 | 5 | 1528 |
| Left | MI | 4 | −38 | −24 | 62 | 0.033 | <0.000001 | ||
| Right | SII | 40 | 56 | −22 | 20 | 0.051 | <0.000001 | 6 | 656 |
| Left | Cingulate Gyrus | 24 | −6 | −4 | 42 | 0.049 | <0.000001 | 7 | 528 |
| Right | Lentiform Nucleus | 24 | −4 | 8 | 0.040 | <0.000001 | 8 | 480 | |
| Left | Supramarginal Gyrus | 40 | −40 | −40 | 36 | 0.042 | <0.000001 | 9 | 432 |
| Right | Cerebellum | 22 | −56 | −26 | 0.032 | <0.000001 | 10 | 296 | |
| Left | Cerebellum | −4 | −54 | −28 | 0.027 | 0.0006 | 11 | 240 | |
| Right | IPL | 40 | 50 | −46 | 38 | 0.031 | 0.0002 | 12 | 232 |
| Left | Middle Frontal Gyrus | 10 | −30 | 46 | 4 | 0.033 | <0.000001 | 13 | 224 |
| Left | Lentiform Nucleus | −20 | 4 | 10 | 0.032 | <0.000001 | 14 | 224 | |
| Right | Inferior Frontal Gyrus | 47 | 42 | 20 | −4 | 0.032 | <0.000001 | 15 | 208 |
| Left | Anterior insula | −32 | 20 | 4 | 0.029 | 0.0006 | 16 | 176 | |
| Right | Thalamus | 2 | −16 | 4 | 0.032 | <0.000001 | 17 | 168 | |
| Left | Angular Gyrus | 39 | −40 | −58 | 34 | 0.030 | 0.0002 | 18 | 160 |
| Right | Middle Frontal Gyrus | 46 | 42 | 46 | 16 | 0.024 | 0.001 | 19 | 144 |
| Left | Thalamus | −4 | −26 | 0 | 0.029 | <0.000001 | 20 | 120 | |
| Left | Cerebellum | −34 | −52 | −38 | 0.027 | <0.000001 | 21 | 104 |
ALE values for Study 4. ALE values refer to the likelihood of obtaining activation evoked by noxious stimuli applied to the right side of the body subtracting the ALE maps for applying noxious stimuli to the left side of the body. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; MI, primary motor cortex.
Study 5: Noxious Muscle Versus Cutaneous Stimuli
An ALE analysis was applied to the 172 activation foci reported for the 10 studies that applied noxious stimuli to muscles. Some brain regions demonstrating significant ALE values included the anterior insula (ALE = 0.037), thalamus (P = 0.04), and the anterior (BA 24:ALE = 0.025 and 32:ALE = 0.023) and posterior cingulate (ALE = 0.021; Table XXIII).
Table XXIII.
Spatial location and extent of ALE values for study 5 (noxious muscle stimuli)
| Side | Region | BA | x | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Right | Anterior insula | 36 | 14 | 10 | 0.037 | <0.000001 | 1 | 6168 | |
| Left | Thalamus | −12 | −16 | 8 | 0.04 | <0.000001 | 2 | 4984 | |
| Left | Cingulate gyrus | 24 | 0 | 16 | 24 | 0.025 | <0.000001 | 3 | 1904 |
| Left | Mid‐insula | −34 | 2 | 20 | 0.026 | <0.000001 | 4 | 1888 | |
| Left | Posterior insula | −36 | −22 | 12 | 0.034 | <0.000001 | 5 | 1616 | |
| Right | PPC | 40 | 64 | −22 | 22 | 0.026 | <0.000001 | 6 | 896 |
| Right | Middle frontal gyrus | 10 | 32 | 42 | 16 | 0.034 | <0.000001 | 7 | 848 |
| Left | Precentral | 6 | −56 | −2 | 8 | 0.027 | <0.000001 | 8 | 704 |
| Left | IPL | −58 | −38 | 28 | 0.023 | <0.000001 | 9 | 672 | |
| Left | SII | 41 | −58 | −18 | 14 | 0.023 | <0.000001 | 10 | 488 |
| Left | Posterior cingulate gyrus | 23 | −4 | −26 | 28 | 0.021 | 0.0002 | 11 | 440 |
| Left | Cingulate gyrus | 32 | −8 | 30 | 24 | 0.023 | <0.000001 | 12 | 352 |
| Left | Posterior insula | −38 | −18 | −6 | 0.024 | <0.000001 | 13 | 344 | |
| Right | Cingulate gyrus | 32 | 8 | 10 | 38 | 0.019 | 0.0002 | 14 | 344 |
| Left | Superior temporal gyrus | 22 | −50 | 6 | −6 | 0.024 | <0.000001 | 15 | 328 |
| Left | Precuneus | 7 | −8 | −70 | 36 | 0.021 | 0.0001 | 16 | 320 |
| Left | Cerebellum | −2 | −26 | −14 | 0.022 | <0.000001 | 17 | 312 | |
| Right | Cerebellum | 24 | −62 | −18 | 0.022 | <0.000001 | 18 | 280 | |
| Left | Cerebellum | −38 | −54 | −36 | 0.021 | <0.000001 | 19 | 272 | |
| Left | Middle frontal gyrus | 9 | −30 | 40 | 28 | 0.02 | <0.000001 | 20 | 272 |
| Left | Anterior insula | −28 | 16 | 2 | 0.015 | 0.001 | 21 | 152 |
ALE values for Study 5. ALE values refer to the likelihood of obtaining activation evoked by noxious stimuli applied to the skin. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; SII, secondary somatosensory cortex; MI, primary motor cortex; PPC, posterior parietal cortex.
A comparable number of studies were included in a comparative meta‐analysis that used 133 activation foci obtained from studies that had applied noxious stimuli to the skin. Significant ALE values were found in SII (ALE = 0.035), thalamus (ALE = 0.027), mid‐insula (ALE = 0.032), and the ACC (BA 42:ALE = 0.027; Table XXIV).
Table XXIV.
Study 5 (noxious cutaneous stimuli)
| Side | Region | BA | X | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Right | Thalamus | 14 | −18 | 4 | 0.027 | <0.00001 | 1 | 4328 | |
| Left | Mid‐insula | −38 | −2 | 4 | 0.032 | <0.00001 | 2 | 4064 | |
| Right | Dorsal posterior insula | 42 | −18 | 14 | 0.035 | <0.00001 | 3 | 1696 | |
| Left | Cingulate gyrus | 24 | −2 | −4 | 44 | 0.027 | <0.00001 | 4 | 1424 |
| Right | Anterior insula | 36 | 10 | 0 | 0.02 | <0.00001 | 5 | 960 | |
| Right | IPL | 40 | 48 | −28 | 26 | 0.017 | 0.0002 | 6 | 648 |
| Left | SII | −58 | −20 | 22 | 0.021 | <0.00001 | 7 | 472 | |
| Right | Mid‐insula/Mid‐Clastrum | 36 | −4 | 6 | 0.017 | <0.00001 | 8 | 408 | |
| Left | Cingulate gyrus | 24 | 0 | 16 | 26 | 0.017 | <0.00001 | 10 | 384 |
| Left | SII | −50 | −30 | 16 | 0.024 | <0.00001 | 11 | 368 | |
| Right | IPL | 40 | 26 | −36 | 54 | 0.022 | <0.00001 | 12 | 368 |
| Right | Precentral gyrus | 44 | 48 | 2 | 8 | 0.017 | 0.004 | 13 | 320 |
| Right | Posterior insula | 44 | −10 | −4 | 0.02 | <0.00001 | 14 | 304 | |
| Right | SI/PPC | 7 | 28 | −44 | 46 | 0.017 | <0.00001 | 15 | 296 |
| Right | IPL | 52 | −44 | 28 | 0.016 | <0.00001 | 16 | 280 | |
| Right | Inferior frontal gyrus | 47 | 40 | 28 | 2 | 0.016 | 0.0002 | 17 | 272 |
| Left | Middle frontal gyrus | 9 | −24 | 32 | 30 | 0.013 | 0.0004 | 18 | 200 |
ALE values for Study 5. ALE values refer to the likelihood of obtaining activation evoked by noxious stimuli applied to muscles. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; IPL, inferior parietal lobule; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; MI, primary motor cortex; PPC, posterior parietal cortex.
Upon examination of the ALE values for each meta‐analysis, both types of stimuli were found to significantly activate SII, ACC, dorsal posterior insula, and the thalamus (Supporting Information Fig. 4).
Subsequent subtraction analyses (noxious muscle stimuli minus noxious cutaneous stimuli) revealed a significant likelihood of spatially specific activation in response to noxious muscle stimuli in the precuneus (ALE = 0.021; Table XXV), the posterior cingulate (BA 32; ALE = 0.021), the dorsolateral prefrontal cortex (DLPFC BA 9; ALE = 0.017), and the cerebellum (ALE = 0.019). The reverse subtraction (noxious cutaneous stimuli—noxious muscle stimuli) showed a preferential likelihood of activation in SI (ALE = 0.022) and the ventrolateral prefrontal cortex (VLPFC BA 47; ALE = 0.016; Table XXVI).
Table XXV.
Spatial location and extent of ALE values for study 5 (noxious muscle stimuli minus noxious cutaneous stimuli)
| Side | Region | BA | X | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Right | Anterior insula | 36 | 16 | 10 | 0.029 | <0.000001 | 1 | 3288 | |
| Left | Anterior insula | −34 | 2 | 20 | 0.026 | <0.000001 | 2 | 784 | |
| Left | Thalamus | −12 | −16 | 10 | 0.027 | <0.000001 | 3 | 720 | |
| Right | Middle frontal gyrus | 10 | 32 | 42 | 14 | 0.034 | <0.000001 | 4 | 704 |
| Left | Thalamus | −16 | −26 | 14 | 0.022 | <0.000001 | 5 | 664 | |
| Left | Cingulate gyrus | 32 | −4 | 20 | 38 | 0.023 | <0.000001 | 6 | 664 |
| Left | Precentral gyrus | 6 | −56 | −2 | 8 | 0.027 | <0.000001 | 7 | 656 |
| Right | SII | 64 | −24 | 22 | 0.023 | <0.000001 | 8 | 584 | |
| Left | Posterior insula | −36 | −24 | 10 | 0.03 | <0.000001 | 9 | 560 | |
| Left | Posterior insula | −40 | −18 | 20 | 0.028 | <0.000001 | 10 | 416 | |
| Left | Superior temporal gyrus | −50 | 6 | −6 | 0.024 | <0.000001 | 11 | 296 | |
| Right | Cerebellum | 24 | −62 | −18 | 0.022 | <0.000001 | 12 | 288 | |
| Left | Cingulate gyrus | 32 | −8 | 30 | 24 | 0.023 | <0.000001 | 13 | 288 |
| Left | Precuneus | 7 | −8 | −70 | 36 | 0.021 | <0.000001 | 14 | 272 |
| Left | Posterior cingulate gyrus | 23 | −4 | −26 | 28 | 0.021 | <0.000001 | 15 | 256 |
| Right | Thalamus | 6 | −22 | 8 | 0.022 | <0.000001 | 16 | 248 | |
| Left | IPL | 40 | −60 | −38 | 26 | 0.021 | <0.000001 | 17 | 232 |
| Left | Transverse temporal gyrus | −58 | −16 | 12 | 0.019 | <0.000001 | 18 | 208 | |
| Right | Thalamus | 14 | −10 | 8 | 0.016 | 0.0004 | 19 | 184 | |
| Left | Cerebellum | −40 | −54 | −36 | 0.019 | <0.000001 | 20 | 176 | |
| Left | IPL | 40 | −56 | −28 | 32 | 0.016 | 0.0008 | 21 | 168 |
| Left | Midbrain | −2 | −26 | −14 | 0.021 | <0.000001 | 22 | 160 | |
| Left | Middle frontal gyrus | 9 | −30 | 40 | 28 | 0.017 | 0.0002 | 23 | 152 |
ALE values for Study 5. ALE values refer to the likelihood of obtaining activation evoked by noxious muscle stimuli in comparison to noxious cutaneous stimuli. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; IPL, inferior parietal lobule; SII, secondary somatosensory cortex; MI, primary motor cortex; PPC, posterior parietal cortex.
Table XXVI.
Spatial location and extent of ALE values for study 5 (noxious cutaneous stimuli minus noxious muscle stimuli)
| Side | Region | BA | X | y | z | ALE value | P value | Cluster # | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| Left | Mid‐insula | −38 | −4 | 6 | 0.022 | <0.000001 | 1 | 1048 | |
| Right | Posterior insula | 42 | −20 | 14 | 0.031 | <0.000001 | 2 | 968 | |
| Left | Cingulate gyrus | 24 | −2 | −4 | 44 | 0.024 | <0.000001 | 3 | 592 |
| Right | Midbrain | 4 | −16 | −8 | 0.019 | 0.0002 | 4 | 384 | |
| Left | Thalamus | −6 | −10 | 12 | 0.018 | 0.0004 | 5 | 344 | |
| Right | SII | 48 | −28 | 26 | 0.017 | 0.0006 | 6 | 344 | |
| Left | SII | −50 | −30 | 16 | 0.024 | <0.000001 | 7 | 312 | |
| Right | Anterior insula | 36 | 10 | 0 | 0.018 | 0.0006 | 8 | 256 | |
| Right | SI | 26 | −36 | 54 | 0.022 | <0.000001 | 9 | 240 | |
| Left | SI | −58 | −20 | 22 | 0.020 | 0.0002 | 10 | 192 | |
| Left | Putamen | −26 | 6 | −6 | 0.016 | 0.0002 | 11 | 144 | |
| Right | Inferior frontal gyrus | 47 | 40 | 28 | 2 | 0.016 | 0.0002 | 12 | 144 |
| Right | Precuneus | 7 | 28 | −44 | 46 | 0.017 | 0.0008 | 13 | 144 |
| Right | SII | 52 | −44 | 28 | 0.016 | 0.0004 | 14 | 128 |
ALE values for Study 5. ALE values refer to the likelihood of obtaining activation evoked by noxious cutaneous stimuli in comparison to noxious to the skin stimuli. Coordinates are in Talairach space [Talairach and Tournoux 1988]. Cluster #: The clusters are ranked according to their size in millimeters cubed (mm3). Abbreviations: BA, Brodmann Area; x, medial‐lateral; y, anterior posterior; z, superior‐inferior; SII, secondary somatosensory cortex; PPC, posterior parietal cortex.
DISCUSSION
Study 1: Meta‐Analysis of Activation Evoked by All Types of Noxious Stimuli
We explored common brain regions activated by noxious stimuli by performing a meta‐analysis on the activation sites reported by 140 fMRI and PET studies published between 1991–2011. In contrast to previous reviews, our approach provides a quantitative assessment of activation in the brain in response to noxious stimuli through the creation of likelihood estimate maps, which permit precise localization of cortical regions involved in processing pain. The maps can be particularly useful for targeting subregions of a brain area such as SII, which has no anatomically distinct boundaries to delineate the extent and location of where to predict activation evoked by noxious stimuli.
Our results are consistent with previous qualitative reviews of the literature that have described a “pain network” comprised of SI, SII, ACC, insula, prefrontal cortex, and the thalamus [Apkarian et al.,2005; Iadarola and Coghill,1999; Peyron et al.,1999]. The results are somewhat consistent with one of these previous reviews [Apkarian et al.,2005] that found the insula to be the most commonly reported activation site evoked by noxious stimuli. However, our quantitative results expand upon these previous reviews by providing the precise spatial location and extent of the likelihood of activation in response to noxious stimuli, thus providing more detailed and accurate information that is based on previous data. Using this data driven method, the left thalamus, the right ACC, bilateral anterior insulae, and left dorsal posterior insula had the highest likelihood of activation in response to noxious stimuli, providing a new quantitative 3D matrix in which to predict pain‐evoked activation. In addition, although largely confirming findings from qualitative reviews, the results of this study point to the inclusion of the posterior cingulate cortex and the basal ganglia as key brain regions involved in processing nociception.
An important finding was that in the cortex, the right ACC (BA 32/24) had the highest likelihood of being activated in response to noxious stimuli. The ACC has been implicated in processing the emotional salience or unpleasantness of painful stimuli as suggested by research in animals and humans. This region receives nociceptive input from dorsal horn neurons via the medial‐dorsal (MD) and intralaminar thalamic nuclei [Giguere and Goldman‐Rakic 1988; Goldman‐Rakic and Porrino1985; Krettek and Price 1977; Wang and Shyu2004]. Cingulotomy for alleviation of chronic pain reduces affective responses with no concomitant disruption of the ability to appreciate somatosensory aspects of painful stimuli [Ballantine et al.1967; Foltz and White 1962]. Additionally, a number of functional neuroimaging studies have also implicated the ACC in processing affective aspects of pain [Kulkarni et al., 2005; Rainville et al.,1997]. For example, hypnotic modulation of pain unpleasantness was correlated with ACC activation, with no concurrent changes in regions involved in sensory‐discriminative processing [Rainville et al.,1997]. However, the ACC may also subserve some sensory‐discriminative aspects of pain processing. Electrophysiological studies, in both humans and animals, have reported neuronal firing frequencies in the ACC that were correlated with stimulus intensity [Hutchison et al.,1999; Yamamura et al. 1996]. Additionally, a crude somatotopic organization of nociceptive stimuli has been reported in this region, thus implicating the ACC in stimulus localization [Arienzo et al.,2006]. An important note is that these findings have been questioned as several electrophysiological studies have reported that the ACC contains large, bilateral receptive fields [Hutchison et al.,1999; Kuo and Yen,2005; Sikes and Vogt1992]. Based on these previous findings, the significant likelihood of evoking activation in the ACC in response to noxious stimuli may reflect both the processing of the affective component of pain and potentially the localization of stimuli applied to the body.
Bilateral anterior insulae and the left dorsal posterior insula were other cortical regions that had a significant likelihood of being activated by noxious stimuli. The insula is a complex, multisensory integration area that is involved in processing many aspects involved with the conscious experience of pain such as affect [Berthier et al.,1988; Schon et al.,2008], autonomic activity [Cameron and Minoshima,2002; Cechetto and Saper,1987; Critchley et al.,2000; Gianaros et al.,2007; Yasui et al.,1991; Zhang et al.,1999], interoception [Critchley et al.,2004], and temperature [Craig et al.,2000]. The anterior insula receives input from peripheral autonomic receptors, and therefore it may become activated during affective tasks or during the perception of pain due to increases in heart rate, changes in blood pressure, etc. [Cechetto and Saper,1987; Yasui et al.,1991; Zhang et al.,1999]. A number of neuroimaging studies have reported activation in the insula during tasks that involve heightened autonomic activity [Cameron and Minoshima,2002; Critchley et al.,2000; Gianaros et al.,2007]. Furthermore, the right anterior insula also has a key role in interoception, or monitoring the internal state of the body [Critchley et al.,2004]. The dorsal posterior insula has been shown to receive nociceptive input from the posterior portion of the ventromedial nucleus in the thalamus [Blomqvist et al.,2000; Craig and Dostrovsky2001; Craig et al.,1994]. Brain activation in this region, as revealed by fMRI, is directly related to changes in temperature [Craig et al.,2000], indicating that it is a primary locus for processing thermosensory information. In turn, a significant likelihood of obtaining activation in response to noxious stimuli in the insula may reflect an increased awareness of physiological functions during exposure to noxious stimuli.
While the anterior insula is a major site for emotional processing, it also processes sensory‐discriminative aspects of pain perception. For example, direct electrophysiological stimulation of the anterior insula produces painful and nonpainful somesthetic responses [Ostrowsky et al.,2002; Penfield and Faulk,1955]. Furthermore, an imprecise somatotopic organization was reported in the insula based on electrophysiological stimulation and functional neuroimaging of this region [Henderson et al.,2007; Ostrowsky et al.,2000].
Surprisingly, the likelihood of activation in SI was significant even though the values reported in this region are from the global analysis and included studies that stimulated different parts of the body. As this region has a detailed somatotopic organization, the activation peaks were in different locations of the postcentral gyrus. Another important factor is that large individual differences in the location of the central sulcus may reduce the ability to detect spatially restricted activation in SI based on multiple‐subject averaging [Geyer et al.,2000]. Therefore, the probabilistic values in SI produced by this meta‐analysis may not accurately reflect the likelihood of activation in this region in individual studies.
The role of SI in the perception of pain has been disputed since some of the first published experiments in the early 20th century and continues to this day. This began with an early report by Head and Holmes [1911] that pain perception remained intact after damage to SI. Subsequent electrophysiological studies demonstrated contradictory findings, whereby neuronal responses to noxious stimuli were recorded in SI [Chudler et al.1990; Kenshalo et al.,2000; Kenshalo and Isensee1983]. Later brain imaging studies reported mixed findings with some studies reporting activation in SI [e.g., Talbot et al.,1991] and others finding an absence of SI activation [e.g., Jones et al., 1991]. These results could be due to many factors; however, these meta‐analyses of all existing brain imaging studies of nociception have provided evidence that SI is involved in the processing of some aspects of nociception, although with a relatively small likelihood of being activated in response to noxious stimuli.
The meta‐analysis has also identified cortical regions that are not typically associated with nociceptive processing, such as the posterior cingulate gyrus. Activation in the posterior cingulate cortex is often reported in pain neuroimaging studies as a finding being unrelated to processing noxious stimuli, as its role in pain processing has not been thoroughly explored. However, studies in animals have indicated that this region receives a direct projection from the main pain and temperature transmitting pathway in the spinal cord, the spinothalamic tract, [Apkarian and Shi,1998] and contains nociceptive neurons [Sikes et al.,2008] thus suggesting it processes sensory‐discriminative aspects of pain. Additionally, the meta‐analysis identified motor regions that invariably become activated during a pain imaging experiment. As of late, pain neuroimaging studies often discount activation in motor regions because of preparatory motor responses. However, Melzack and Wall [1965] noted in their seminal work on peripheral and central processing of pain that motor responses are an integral part of the exposure to a noxious stimulus. They note that many actions can occur after a noxious stimulus is applied to the body such as a startle response and orienting of the head and eyes. Therefore, the significant likelihood of activation in motor areas found in the current experiment reinforces this long held view of pain processing. Additionally, several motor areas, such as the nuclei in the basal ganglia, are directly responsive to noxious stimuli [Chudler and Dong,1995] with some regions showing a nociceptive somatotopic organization [Bingel et al.,2004a] consistent with an involvement in stimulus localization.
To conclude, this meta‐analysis represents a comprehensive quantitative review identifying the specific location and spatial extent of activation evoked by noxious stimuli in the brain. Given the all‐inclusive nature of the types of stimuli included in the analysis, the specific role of these structures in processing noxious stimuli cannot be addressed within the limits of the current study. More detailed information can be obtained by contrasting activation likelihood estimates associated with distinct noxious stimuli as discussed in the following sections.
Study 2: Noxious Cold Compared with Noxious Heat
This is the first meta‐analysis of brain imaging data to directly compare noxious cold with noxious heat. The most important finding from the noxious cold meta‐analysis was that these stimuli were associated with the activation of a number of sensory and affective pain processing cortical regions, including bilateral insular cortices, the right ACC, subcallosal gyrus, SII, and the right amygdala. In comparison, the highest likelihood of obtaining activation in response to noxious heat was localized in bilateral insulae and thalamus. Based on the subtraction analysis (noxious heat minus noxious cold), noxious‐heat related activation was more likely to occur in somatosensory cortices, which perhaps reflects the substantially lesser autonomic reaction and unpleasantness associated with these stimuli [Rainville et al.,1992].
To date, very few imaging studies have explored the neural representations of noxious cold and noxious heat pain within the same experimental protocol [Casey et al.,1996; Craig et al.,1996; Tracey et al.,2000]. In one study, Tracey et al. [2000] reported that cold and heat pain activated similar brain areas. However, these authors applied cold stimuli using relatively short (30 s) stimuli delivered via a computer‐controlled thermode that were potentially not as aversive as the stimuli used in the other cold‐pain studies included in the meta‐analysis.
Some studies in the meta‐analysis administered noxious cold stimuli using the cold pressor task, which involves the immersion of a limb into freezing water for several minutes. In general, subjects report cold‐pain sensations to be “aching” and “deep,” in comparison to heat pain, which has been described as “stinging” and “superficial” [Davis et al.,1998]. Additionally, subjects rate cold pain as more unpleasant than heat pain [Greenspan et al.,2003; Rainville et al.,1992]. In turn, the findings from the noxious cold meta‐analysis are in line with results showing high probabilistic values in regions associated with emotional processing and negative affect such as the amygdala, insula, and the ACC [Mayberg et al.,1999; Neugebauer et al.,2004; Wiech and Tracey,2009]. The subgeniculate area of the ACC projects to the amygdala, hypothalamus, and the periaqueductal gray, brain regions known to process emotional motivational stimuli, and also autonomic processing, indicating that the increased likelihood of activation in these regions during noxious cold stimuli may reflect both affect‐related physiological and behavioral reactions that occur during unpleasant stimuli. In line with these findings is that activation has been reported in the ACC and the amygdala for noxious muscle stimuli, but not for noxious cutaneous stimuli when presented within the same experimental design [Takahashi et al.,2011]. These findings were also related to the enhanced emotional response elicited by muscle stimuli.
Study 3: Localizing Activation in Response to Noxious Heat Stimuli
In this systematic study, we examined the effects of using either innocuous warm stimuli or a resting baseline as the control condition on the apparent brain activation evoked by noxious heat stimuli. As expected, our findings indicate that contrasts with a resting baseline suggest a more widespread network of brain regions activated by the noxious stimuli. This was demonstrated by the greater number of ALE peaks, the larger clusters of significant ALE values, and the detection of activation peaks outside of the classical spino‐thalamo‐cortical system (e.g., in the superior frontal gyrus). Of particular interest, the contrast with a resting baseline has the advantage of increasing the likelihood of detecting stimulus‐evoked activation in SI, an area that is often missed because of a variety of factors difficult to control in brain imaging studies, as discussed above.
A major finding from the meta‐analysis in which innocuous warm stimuli was used as a control condition for noxious heat was the localized peak ALE values in BA 24 of the ACC. This important result suggests that the pain versus warm contrast may not simply reveal a subset of activation peaks detected in pain versus baseline. Electrophysiological studies have recorded neurons in the ACC responding to noxious stimuli, with or without attentional modulation, or solely during attentive tasks [Davis et al.,2000; Hutchison et al.,1999]. An fMRI study examined BOLD activity either during the presentation of a painful stimulus or an attention‐demanding task [Davis et al.,1997]. Activation evoked by pain was reported in BA 24, while the attention‐demanding task activated BA 32. In these results, the significant probabilistic value in BA 32 for the pain vs. baseline condition might reflect attentional resources directed towards the stimuli. Notably, this cluster largely overlapped with another cluster that had a significant likelihood of being activated in the pain versus warm contrast, consistent with increased attention‐related responses to pain. However, the more ventral peaks found in the pain versus warm contrast are consistent with a spino‐thalamo‐cortical input to BA 24 [Sikes and Vogt,1992], which might be more closely related to the processing of noxious signals and to the experience of pain. Potentially, the likelihood of activation in BA 24 area could also be a result of weaker signal change in this region in response to innocuous warm stimuli or even “deactivation,” or negative signal change. Certainly, activation only in response to innocuous warm stimuli has been found to produce activation in this region [e.g., Becerra et al.,1999]. Therefore, the findings suggest that BA 24 does process innocuous warm stimuli, but not more so than noxious stimuli. Deactivation or negative signal in response to warm stimuli may also have produced a better contrast to localize pain‐specific activation in the brain. The neurophysiological mechanisms underlying negative BOLD‐signal change in functional neuroimaging data are unclear, and have been a focus of interest in the brain imaging literature [Kastrup et al.,2008; Menon et al.,1995; Shmuel et al.,2002,2006]. It has been theorized that negative BOLD‐signal change in the somatosensory system may be a result of inhibitory surround receptive fields [Apkarian et al.,2000]. Whether similar mechanisms may underlie the processing of innocuous warm stimuli in the ACC remains uncertain; however, this may explain the stronger signal change in response to noxious stimuli when using an innocuous warm control. In any case, whether the signal change in BA 24 in response to innocuous warm stimuli is absent, weak, or negative, an important incentive for using warmth as a control for pain is that it may help to discriminate activation associated with nociceptive processes and pain experiences from cognitive processes involved in the encoding and attention to both noxious and innocuous stimuli.
Study 4: Hemispheric Lateralization of Nociceptive Processing
This fourth meta‐analysis examined the hemispheric lateralization of nociceptive processing by comparing two groups of independent studies that reported brain activation coordinates evoked by noxious stimuli applied either to the left or the right sides of the body. Regardless of whether the left or the right sides of the body received noxious stimulation, the meta‐analysis revealed that the most significant probabilistic values were in the right insular cortex. Additionally, the other region to show large clusters and significant ALE values for both analyses was the right ACC (BA 24).
The likelihood of activation in the contralateral hemisphere was significant within right SI, MI, PPC, and the superior frontal gyrus, for the left‐sided stimuli. For the right‐sided meta‐analysis, the likelihood of contralateral activation was significant within left SI, ACC (BA32), MI, inferior parietal lobule, and the medial frontal gyrus.
In the ipsilateral hemisphere, the likelihood of activation was significant within the midbrain for the left‐sided stimuli. The likelihood of activation in the ipsilateral hemisphere for right‐sided stimuli was significant within the ACC (BA 32), inferior parietal lobule, and the middle frontal gyrus.
Findings from this meta‐analysis provide credence to the previously proposed right hemispheric dominance for pain processing [Craig,2005]. This is likely due to the role of the right hemisphere in mediating affective processing, which has been seen across a number of sensory modalities [Borod et al.,1998; Coen et al.,2009; Killgore and Yurgelun‐Todd,2007]. Pain in itself is recognized as an emotional state, and in turn is highly modifiable by emotions and mood [Meagher et al.,2001; Villemure and Bushnell,2002], an effect recently shown to involve the right anterior insula [Craig,2005; Roy et al.,2009]. An additional consideration is that unlike sensory aspects of pain, emotional responses to pain do not depend on localization, and therefore may not rely on precise spatial topographically organized maps. This is consistent with our findings of significant activation likelihood within contralateral SI.
It should be noted that the majority of studies included in the meta‐analysis tested only right‐handed individuals, and therefore the results may not be applicable to the population as a whole. In turn, the results may reflect differential pain processing by right‐handed people. For example, pain is more tolerable when presented to the dominant (right) side of the body [Pauli et al.,1999a]. In contrast, pain sensitivity measures in left‐handed people are essentially equivalent for stimuli presented to either side of the body [Pauli et al.,1999b]. Therefore, left‐handed individuals may process pain either in additional brain regions or in a more distributed fashion in comparison to right‐handed people.
Study 5: Differential Processing Associated with Noxious Muscle and Cutaneous Stimuli
The fifth meta‐analysis examined preferential neural processing associated with noxious muscle or cutaneous stimuli. Common areas that had a similar likelihood of being activated by both types of stimuli included the thalamus, anterior cingulate cortices, the anterior, mid‐ and posterior insula cortices, SII, and the posterior parietal cortices. These findings are consistent with evidence suggesting that convergence of afferent inputs in the periphery terminate on projection neurons in the spino‐thalamic tract [Mense,1993]. Additionally, electrophysiological studies have recorded responses in neurons in the dorsal horn [Amano et al.,1986; Asato and Yokota,1989] and the thalamus [Kawakita et al.,1993] to noxious cutaneous, muscle, and visceral stimuli.
The likelihood of activation specifically associated with noxious muscle stimuli was significant in the precuneus, the mid/posterior cingulate gyrus, the DLPFC and the cerebellum. The likelihood of activation in some of these regions may be more associated with cognitive aspects of processing muscle pain as this type of pain is generally unavoidable and often evokes uncontrollability [Graven‐Nielsen and Mense2001], and freezing behavior [Fanselow 1986; Rhudy and Meagher 2000]. Therefore, the likelihood of activation in the posterior cingulate cortex may be associated with the aversive nature of the stimuli as activation in this region has been associated with the fear of a noxious stimulus [Ochsner et al.,2006].
Noxious cutaneous stimuli were associated with a significant likelihood of activation in SI and the VLPFC. Cutaneous receptive fields are located in SI with a discrete somatotopic organization [Geyer et al.,1999; Kenshalo et al.,2000]. The significant likelihood of activation in this region likely reflects the fact that cutaneous stimuli are often well localized on the body.
CONCLUSIONS AND FUTURE WORK
Substantial information from functional brain imaging research can be gained through our ability to combine results across multiple studies that used a large variety of experimental conditions. Meta‐analytic techniques permit the extraction of common patterns of brain responses thought to reflect the processes that are common across studies. This meta‐analysis provides a detailed assessment of brain responses to different types of noxious stimuli. This technique allowed for an objective, quantitative determination of findings across imaging studies, and produced a spatial likelihood map of activation evoked by noxious stimuli. Meta‐analyses can be used to expand upon the findings of single studies in that they permit the collation of data across studies to examine robustness and heterogeneity of findings. An important consideration is that single studies are often limited by time and financial ability to test large numbers of subjects. The average number of subjects that were tested in the Study 1 database was 12.5. Therefore, meta‐analyses can be used to identify brain regions that are consistently activated in response to noxious stimuli across studies, despite the limitations pertaining to single studies. In addition to providing very strong confirmatory evidence for the activation of brain areas typically associated with pain, and supporting a right‐hemisphere dominance in the processing of noxious stimuli, the detailed analyses further demonstrated significant differences associated with the type of noxious stimulus employed, as well as the control condition used to reveal noxious‐related responses.
While this work provides confirmatory evidence for the involvement of SI, SII, ACC, insula, prefrontal cortex, thalamus, and basal ganglia in processing nociceptive stimuli, it is not possible to determine based on the results of this study if any of these brain regions have pain‐specific responses or work in a network to produce the perception of pain. Several recent studies have called into question the specificity of brain responses to pain [Baliki et al.,2009; Iannetti et al.,2008; Mouraux and Iannetti,2009; Mouraux et al.,2011]. Some brain regions such as the ACC, insula and SII have been shown to respond to multimodal stimuli with no evidence of a nociceptive specific response [Mouraux et al.,2011]. In that study, neural responses were correlated with the magnitude of the stimulus indicating that the pattern of brain activation seen in response to pain may only be a reflection of estimating pain intensity. In these analyses, data were obtained from several studies that required participants to rate the intensity of the stimuli. A future meta‐analysis could compare and contrast data from studies that required rating the stimuli during the experiment with those that simply presented noxious stimuli to subjects in the absence of qualitative estimation of the stimuli.
Future research lies in comparing data from this work with brain activation associated with spontaneously induced pain in chronic pain patients. Few studies have directly compared brain activation evoked by chronic and acute pain; however, a review article indicated that chronic pain patients were more likely to have activation in the prefrontal cortex [Apkarian et al.,2005]. A whole brain meta‐analysis would offer a more expansive comparison with patient data to explore additional areas of the brain demonstrating differential activation in response to chronic versus acute pain.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Figure S1, S2, and S3
Acknowledgements
The authors would like to thank Dr. Gary Duncan (University of Montreal) for aiding in the development of the experimental methodology and providing comments on an earlier version of the manuscript. Additionally, the authors are indebted to Joyce Fu and Jen‐I Chen for aiding in data entry.
REFERENCES
- Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL ( 1997): Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg 84: 120–126. [DOI] [PubMed] [Google Scholar]
- Aharon I, Becerra L, Chabris CF, Borsook D ( 2006): Noxious heat induces fMRI activation in two anatomically distinct clusters within the nucleus accumbens. Neurosci Lett 392: 159–164. [DOI] [PubMed] [Google Scholar]
- Albanese MC, Duerden EG, Rainville P, Duncan GH ( 2007): Memory traces of pain in human cortex. J Neurosci 27: 4612–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano N, Hu JW, Sessle BJ ( 1986): Responses of neurons in feline trigeminal subnucleus caudalis (medullary dorsal horn): To cutaneous, intraoral, and muscle afferent stimuli. J Neurophysiol 55: 227–243. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Lilja A, Hartvig P, Langstrom B, Gordh T, Handwerker H, Torebjork E ( 1997): Somatotopic organization along the central sulcus, for pain localization in humans, as revealed by positron emission tomography. Exp Brain Res 117: 192–199. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Shi T ( 1998): Thalamocortical connections of the cingulate and insula in relation to nociceptive inputs to the cortex In: Ayrapeytyan SN, Apkarian AV, editors. Pain mechanisms and management. Amsterdam, Berlin, Oxford, Tokyo, Washington DC: TOS Press; pp 212–220. [Google Scholar]
- Apkarian AV, Gelnar PA, Krauss BR, Szeverenyi NM ( 2000): Cortical responses to thermal pain depend on stimulus size: A functional MRI study. J Neurophysiol 83: 3113–3122. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK ( 2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen L, Svensson P ( 2001): Referred muscle pain: Basic and clinical findings. Clin J Pain 17: 11–19. [DOI] [PubMed] [Google Scholar]
- Arienzo D, Babiloni C, Ferretti A, Caulo M, Del Gratta C, Tartaro A, Rossini PM, Romani GL ( 2006): Somatotopy of anterior cingulate cortex (ACC) and supplementary motor area (SMA) for electric stimulation of the median and tibial nerves: An fMRI study. Neuroimage 33: 700–705. [DOI] [PubMed] [Google Scholar]
- Asato F, Yokota T ( 1989): Responses of neurons in nucleus ventralis posterolateralis of the cat thalamus' to hypogastric inputs. Brain Res 488: 135–142. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Andersson JL, Valind S, Sundin A, Hamdy S, Jones AK, Foster ER, Langstrom B, Thompson DG ( 1997): Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology 113: 50–59. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV ( 2009): Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol 101: 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantine HT Jr., Cassidy WL, Flanagan NB, Marino R Jr. ( 1967): Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg 26: 488–495. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D ( 2001): Reward circuitry activation by noxious thermal stimuli. Neuron 32: 927–946. [DOI] [PubMed] [Google Scholar]
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D ( 1999): Human brain activation under controlled thermal stimulation and habituation to noxious heat: An fMRI study. Magn Reson Med 41: 1044–1057. [DOI] [PubMed] [Google Scholar]
- Berthier M, Starkstein S, Leiguarda R ( 1988): Asymbolia for pain: A sensory‐limbic disconnection syndrome. Ann Neurol 24: 41–49. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C ( 2002): Subcortical structures involved in pain processing: Evidence from single‐trial fMRI. Pain 99: 313–321. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C ( 2003): Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. Neuroimage 18: 740–748. [DOI] [PubMed] [Google Scholar]
- Bingel U, Glascher J, Weiller C, Buchel C ( 2004a): Somatotopic representation of nociceptive information in the putamen: An event‐related fMRI study. Cereb Cortex 14: 1340–1345. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Glauche V, Knab R, Glascher J, Weiller C, Buchel C ( 2004b): Somatotopic organization of human somatosensory cortices for pain: A single trial fMRI study. Neuroimage 23: 224–232. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C ( 2006): Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120: 8–15. [DOI] [PubMed] [Google Scholar]
- Bingel U, Rose M, Glascher J, Buchel C ( 2007a): fMRI reveals how pain modulates visual object processing in the ventral visual stream. Neuron 55: 157–167. [DOI] [PubMed] [Google Scholar]
- Bingel U, Schoell E, Herken W, Buchel C, May A ( 2007b): Habituation to painful stimulation involves the antinociceptive system. Pain 131: 21–30. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Schnitzler A, Enck P, Frieling T, Posse S, Seitz RJ, Freund HJ ( 1998): Somatic and limbic cortex activation in esophageal distention: A functional magnetic resonance imaging study. Ann Neurol 44: 811–815. [DOI] [PubMed] [Google Scholar]
- Blomqvist A, Zhang E‐T, Craig AD ( 2000): Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the posterior portion of the ventral medial nucleus, in the human thalamus. Brain 123: 601–619. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S ( 2007): Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci U S A 104: 12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C ( 2002): Painful stimuli evoke different stimulus‐response functions in the amygdala, prefrontal, insula and somatosensory cortex: A single‐trial fMRI study. Brain 125: 1326–1336. [DOI] [PubMed] [Google Scholar]
- Borod JC, Cicero BA, Obler LK, Welkowitz J, Erhan HM, Santschi C, Grunwald IS, Agosti RM, Whalen JR ( 1998): Right hemisphere emotional perception: Evidence across multiple channels. Neuropsychology 12: 446–458. [DOI] [PubMed] [Google Scholar]
- Borsook D, DaSilva AF, Ploghaus A, Becerra L ( 2003): Specific and somatotopic functional magnetic resonance imaging activation in the trigeminal ganglion by brush and noxious heat. J Neurosci 23: 7897–7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM ( 2005): Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage 25: 312–319. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I ( 2005): Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage 27: 201–209. [DOI] [PubMed] [Google Scholar]
- Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C ( 2002): Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: A parametric single‐trial laser functional magnetic resonance imaging study. J Neurosci 22: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B ( 1999): Pain perception: Is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A 96: 7705–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron OG, Minoshima S ( 2002): Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosom Med 64: 851–861. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M ( 2006): Predictability modulates the affective and sensory‐discriminative neural processing of pain. Neuroimage 32: 1804–1814. [DOI] [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Morrow TJ, Koeppe RA ( 1996): Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol 76: 571–581. [DOI] [PubMed] [Google Scholar]
- Casey KL, Svensson P, Morrow TJ, Raz J, Jone C, Minoshima S ( 2000): Selective opiate modulation of nociceptive processing in the human brain. J Neurophysiol 84: 525–533. [DOI] [PubMed] [Google Scholar]
- Casey KL, Morrow TJ, Lorenz J, Minoshima S ( 2001): Temporal and spatial dynamics of human forebrain activity during heat pain: Analysis by positron emission tomography. J Neurophysiol 85: 951–959. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB ( 1987): Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol 262: 27–45. [DOI] [PubMed] [Google Scholar]
- Chen JI, Ha B, Bushnell MC, Pike B, Duncan GH ( 2002): Differentiating noxious‐ and innocuous‐related activation of human somatosensory cortices using temporal analysis of fMRI. J Neurophysiol 88: 464–474. [DOI] [PubMed] [Google Scholar]
- Christmann C, Koeppe C, Braus DF, Ruf M, Flor H ( 2007): A simultaneous EEG‐fMRI study of painful electric stimulation. Neuroimage 34: 1428–1437. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Dong WK ( 1995): The role of the basal ganglia in nociception and pain. Pain 60: 3–38. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Anton F, Dubner R, Kenshalo DR Jr. ( 1990): Responses of nociceptive SI neurons in monkeys and pain sensation in humans elicited by noxious thermal stimulation: Effect of interstimulus interval. J Neurophysiol 63: 559–569. [DOI] [PubMed] [Google Scholar]
- Coen SJ, Gregory LJ, Yaguez L, Amaro E Jr., Brammer M, Williams SC, Aziz Q ( 2007): Reproducibility of human brain activity evoked by esophageal stimulation using functional magnetic resonance imaging. Am J Physiol Gastrointest Liver Physiol 293: G188–G197. [DOI] [PubMed] [Google Scholar]
- Coen SJ, Yaguez L, Aziz Q, Mitterschiffthaler MT, Brammer M, Williams SC, Gregory LJ ( 2009): Negative mood affects brain processing of visceral sensation. Gastroenterology 137: 253–261, e251–252. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Eisenach J ( 2003): Individual differences in pain sensitivity: Implications for treatment decisions. Anesthesiology 98: 1312–1314. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH ( 1994): Distributed processing of pain and vibration by the human brain. J Neurosci 14: 4095–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ ( 1999): Pain intensity processing within the human brain: A bilateral, distributed mechanism. J Neurophysiol 82: 1934–1943. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Gilron I, Iadarola MJ ( 2001): Hemispheric lateralization of somatosensory processing. J Neurophysiol 85: 2602–2612. [DOI] [PubMed] [Google Scholar]
- Coghill RC, McHaffie JG, Yen YF ( 2003): Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A 100: 8538–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LJ, Farrell MJ, Gibson SJ, Egan GF ( 2010): Age‐related differences in pain sensitivity and regional brain activity evoked by noxious pressure. Neurobiol Aging 31: 494–503. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2005): Forebrain emotional asymmetry: A neuroanatomical basis? Trends Cogn Sci 9: 566–571. [DOI] [PubMed] [Google Scholar]
- Craig AD, Dostrovsky JO ( 2001): Differential Projections of thermoreceptive and nociceptive lamina i trigeminothalamic and spinothalamic neurons in the cat. J Neurophys 86: 856–870. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC, Zhang ET, Blomqvist A ( 1994): A thalamic nucleus specific for pain and temperature sensation. Nature 372: 770–773. [DOI] [PubMed] [Google Scholar]
- Craig AD, Reiman EM, Evans A, Bushnell MC ( 1996): Functional imaging of an illusion of pain. Nature 384: 258–260. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM ( 2000): Thermosensory activation of insular cortex. Nat Neurosci 3: 184–190. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ ( 2000): Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. J Physiol 523( Pt 1): 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ ( 2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D ( 2002): Somatotopic activation in the human trigeminal pain pathway. J Neurosci 22: 8183–8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Pope GE ( 2002): Noxious cold evokes multiple sensations with distinct time courses. Pain 98: 179–185. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ ( 1997): Functional MRI of pain‐ and attention‐related activations in the human cingulate cortex. J Neurophysiol 77: 3370–3380. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ ( 1998): Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol 80: 1533–1546. [DOI] [PubMed] [Google Scholar]
- Davis KD, Hutchison WD, Lozano AM, Tasker RR, Dostrovsky JO ( 2000): Human anterior cingulate cortex neurons modulated by attention‐demanding tasks. J Neurophysiol 83: 3575–3577. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Davis CE, Albuquerque R, Carlson CR, Andersen AH ( 2006): Brain activity during stimulation of the trigeminal nerve with noxious heat. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102: 750–757. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK ( 1998): Cerebral responses to a continual tonic pain stimulus measured using positron emission tomography. Pain 76: 127–135. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL ( 1997): Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 73: 431–445. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Vogt BA, Jones AK ( 1998): Pain and Stroop interference tasks activate separate processing modules in anterior cingulate cortex. Exp Brain Res 118: 52–60. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Nichols TE, Firestone L, Townsend DW, Jones AK ( 2002a): Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain 3: 401–411. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Creed F, Starz T, Meltzer CC, Townsend DW, Peterson AM, Firestone L ( 2002b): Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage 16: 158–168. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Whalley MG, Stenger VA, Oakley DA (2004): Cerebral activation during hypnotically induced and imagined pain. Neuroimage 23:392–401 [DOI] [PubMed] [Google Scholar]
- Downar J, Mikulis DJ, Davis KD ( 2003): Neural correlates of the prolonged salience of painful stimulation. Neuroimage 20: 1540–1551. [DOI] [PubMed] [Google Scholar]
- Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I ( 2005): A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci 25: 7333–7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette M, Rainville P, Alary F, Lassonde M, Lepore F ( 2008): Ipsilateral cortical representation of tactile and painful information in acallosal and callosotomized subjects. Neuropsychologia 46: 2274–2279. [DOI] [PubMed] [Google Scholar]
- Fairhurst M, Wiech K, Dunckley P, Tracey I ( 2007): Anticipatory brainstem activity predicts neural processing of pain in humans. Pain 128: 101–110. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Laird AR, Egan GF ( 2005): Brain activity associated with painfully hot stimuli applied to the upper limb: A meta‐analysis. Hum Brain Mapp 25: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Egan GF, Zamarripa F, Shade R, Blair‐West J, Fox P, Denton DA ( 2006): Unique, common, and interacting cortical correlates of thirst and pain. Proc Natl Acad Sci U S A 103: 2416–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti A, Babiloni C, Gratta CD, Caulo M, Tartaro A, Bonomo L, Rossini PM, Romani GL ( 2003): Functional topography of the secondary somatosensory cortex for nonpainful and painful stimuli: An fMRI study. Neuroimage 20: 1625–1638. [DOI] [PubMed] [Google Scholar]
- Foltz EL, White LE ( 1968): The role of rostral cingulumotomy in “pain” relief. Int J Neurol 6: 353–373. [PubMed] [Google Scholar]
- Frankenstein UN, Richter W, McIntyre MC, Remy F ( 2001): Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage 14: 827–836. [DOI] [PubMed] [Google Scholar]
- Gelnar PA, Krauss BR, Sheehe PR, Szeverenyi NM, Apkarian AV ( 1999): A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. Neuroimage 10: 460–482. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K ( 1999): Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage 10: 63–83. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K ( 2000): Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space Neuroimage 11: 684–696. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SWG, Matthews KA ( 2007): Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension 49: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere M, Goldman‐Rakic PS ( 1988): Mediodorsal nucleus: Areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol 277: 195–213. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS, Porrino LJ ( 1985): The primate mediodorsal (MD): Nucleus and its projection to the frontal lobe. J Comp Neurol 242: 535–560. [DOI] [PubMed] [Google Scholar]
- Graven‐Nielsen T, Mense S ( 2001): The peripheral apparatus of muscle pain: Evidence from animal and human studies. Clin J Pain 17: 2–10. [DOI] [PubMed] [Google Scholar]
- Graven‐Nielsen T, Arendt‐Nielsen L, Svensson P, Jensen TS ( 1997): Quantification of local and referred muscle pain in humans after sequential i.m. injections of hypertonic saline. Pain 69: 11–17. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Roy EA, Caldwell PA, Farooq NS ( 2003): Thermosensory intensity and affect throughout the perceptible range. Somatosens Mot Res 20: 19–26. [DOI] [PubMed] [Google Scholar]
- Gyulai FE, Firestone LL, Mintun MA, Winter PM ( 1997): In vivo imaging of nitrous oxide‐induced changes in cerebral activation during noxious heat stimuli. Anesthesiology 86: 538–548. [DOI] [PubMed] [Google Scholar]
- Haslam DR ( 1970): Lateral dominance in the perception of size and of pain. Q J Exp Psychol 22: 503–507. [DOI] [PubMed] [Google Scholar]
- Head H, Holmes G ( 1911): Sensory disturbances from cerebral lesions. Brain 34:102–254. [Google Scholar]
- Helmchen C, Mohr C, Erdmann C, Petersen D, Nitschke MF ( 2003): Differential cerebellar activation related to perceived pain intensity during noxious thermal stimulation in humans: A functional magnetic resonance imaging study. Neurosci Lett 335: 202–206. [DOI] [PubMed] [Google Scholar]
- Helmchen C, Mohr C, Erdmann C, Binkofski F, Buchel C ( 2006): Neural activity related to self‐ versus externally generated painful stimuli reveals distinct differences in the lateral pain system in a parametric fMRI study. Hum Brain Mapp 27: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Gandevia SC, Macefield VG ( 2007): Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: A single‐trial fMRI study. Pain 128: 20–30. [DOI] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC ( 2001): Cortical representation of the sensory dimension of pain. J Neurophysiol 86: 402–411. [DOI] [PubMed] [Google Scholar]
- Hofbauer RK, Fiset P, Plourde G, Backman SB, Bushnell MC ( 2004): Dose‐dependent effects of propofol on the central processing of thermal pain. Anesthesiology 100: 386–394. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Belfrage M, Stone‐Elander S, Hansson P, Ingvar M ( 1995): Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain 63: 225–236. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Hannerz J, Ingvar M ( 1996): Right‐lateralised central processing for pain of nitroglycerin‐induced cluster headache. Pain 67: 59–68. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO ( 1999): Pain‐related neurons in the human cingulate cortex. Nat Neurosci 2: 403–405. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Coghill RC ( 1999): Imaging of pain: Recent developments. Curr Opin Anaesthesiol 12: 583–589. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Berman KF, Zeffiro TA, Byas‐Smith MG, Gracely RH, Max MB, Bennett GJ ( 1998): Neural activation during acute capsaicin‐evoked pain and allodynia assessed with PET. Brain 121( Pt 5): 931–947. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Hughes NP, Lee MC, Mouraux A ( 2008): Determinants of laser‐evoked EEG responses: Pain perception or stimulus saliency? J Neurophysiol 100: 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannilli E, Gerber J, Frasnelli J, Hummel T ( 2007): Intranasal trigeminal function in subjects with and without an intact sense of smell. Brain Res 1139: 235–244. [DOI] [PubMed] [Google Scholar]
- Ibinson JW, Small RH, Algaze A, Roberts CJ, Clark DL, Schmalbrock P ( 2004): Functional magnetic resonance imaging studies of pain: An investigation of signal decay during and across sessions. Anesthesiology 101: 960–969. [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Tanaka M, Iriki A, Taoka M, Toda T ( 2002): Processing of tactile and kinesthetic signals from bilateral sides of the body in the postcentral gyrus of awake monkeys. Behav Brain Res 135: 185–190. [DOI] [PubMed] [Google Scholar]
- Jantsch HH, Kemppainen P, Ringler R, Handwerker HO, Forster C ( 2005): Cortical representation of experimental tooth pain in humans. Pain 118: 390–399. [DOI] [PubMed] [Google Scholar]
- Jensen R, Rasmussen BK, Pedersen B, Lous I, Olesen J ( 1992): Cephalic muscle tenderness and pressure pain threshold in a general population. Pain 48: 197–203. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Baudewig J, Schnaudigel S, Huonker R, Becker L, Sohns JM, Dechent P, Klingner C, Witte OW ( 2008): Behavioral correlates of negative BOLD signal changes in the primary somatosensory cortex. Neuroimage 41: 1364–1371. [DOI] [PubMed] [Google Scholar]
- Kawakita K, Dostrovsky JO, Tang JS, Chiang CY ( 1993): Responses of neurons in the rat thalamic nucleus submedius to cutaneous, muscle and visceral nociceptive stimuli. Pain 55: 327–338. [DOI] [PubMed] [Google Scholar]
- Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL ( 2006): Isolating the modulatory effect of expectation on pain transmission: A functional magnetic resonance imaging study. J Neurosci 26: 4437–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenshalo DR Jr., Isensee O ( 1983): Responses of primate SI cortical neurons to noxious stimuli. J Neurophysiol 50: 1479–1496. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Iwata K, Sholas M, Thomas DA ( 2000): Response properties and organization of nociceptive neurons in area 1 of monkey primary somatosensory cortex. J Neurophysiol 84: 719–729. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun‐Todd DA ( 2007): The right‐hemisphere and valence hypotheses: Could they both be right (and sometimes left)? Soc Cogn Affect Neurosci 2: 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL ( 2006): Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp 27: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkov A, Ljubisavljevic M, Thunberg J, Kataeva G, Roudas M, Pakhomov S, Radovanovic S, Lyskov E, Medvedev S, Johansson H ( 2002): Changes in human regional cerebral blood flow following hypertonic saline induced experimental muscle pain: A positron emission tomography study. Neurosci Lett 335: 119–123. [DOI] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC ( 2003): The single‐epoch fMRI design: Validation of a simplified paradigm for the collection of subjective ratings. Neuroimage 19: 976–987. [DOI] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC ( 2005): The subjective experience of pain: Where expectations become reality. Proc Natl Acad Sci U S A 102: 12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL ( 1977): The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171: 157–191. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Yen CT ( 2005): Comparison of anterior cingulate and primary somatosensory neuronal responses to noxious laser‐heat stimuli in conscious, behaving rats. J Neurophysiol 94: 1825–1836. [DOI] [PubMed] [Google Scholar]
- Kupers RC, Svensson P, Jensen TS ( 2004): Central representation of muscle pain and mechanical hyperesthesia in the orofacial region: A positron emission tomography study. Pain 108: 284–293. [DOI] [PubMed] [Google Scholar]
- Kurata J, Thulborn KR, Gyulai FE, Firestone LL ( 2002): Early decay of pain‐related cerebral activation in functional magnetic resonance imaging: Comparison with visual and motor tasks. Anesthesiology 96: 35–44. [DOI] [PubMed] [Google Scholar]
- Kurata J, Thulborn KR, Firestone LL ( 2005): The cross‐modal interaction between pain‐related and saccade‐related cerebral activation: A preliminary study by event‐related functional magnetic resonance imaging. Anesth Analg 101: 449–456, table of contents. [DOI] [PubMed] [Google Scholar]
- Kwan CL, Crawley AP, Mikulis DJ, Davis KD ( 2000): An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 85: 359–374. [DOI] [PubMed] [Google Scholar]
- Ladabaum U, Minoshima S, Hasler WL, Cross D, Chey WD, Owyang C ( 2001): Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology 120: 369–376. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT ( 2005): ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT ( 2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28: 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz IH, Egger K, Schubert H, Schnurer C, Tiefenthaler W, Hohlrieder M, Schocke MF, Kremser C, Esterhammer R, Ischebeck A, Moser PL, Kolbitsch C ( 2008): Lornoxicam characteristically modulates cerebral pain‐processing in human volunteers: A functional magnetic resonance imaging study. Br J Anaesth 100: 827–833. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE, Casey KL ( 2002): A unique representation of heat allodynia in the human brain. Neuron 35: 383–393. [DOI] [PubMed] [Google Scholar]
- Lu CL, Wu YT, Yeh TC, Chen LF, Chang FY, Lee SD, Ho LT, Hsieh JC ( 2004): Neuronal correlates of gastric pain induced by fundus distension: A 3T‐fMRI study. Neurogastroenterol Motil 16: 575–587. [DOI] [PubMed] [Google Scholar]
- Lugo M, Isturiz G, Lara C, Garcia N, Eblen‐Zaijur A ( 2002): Sensory lateralization in pain subjective perception for noxious heat stimulus. Somatosens Mot Res 19: 207–212. [DOI] [PubMed] [Google Scholar]
- Lui F, Duzzi D, Corradini M, Serafini M, Baraldi P, Porro CA ( 2008): Touch or pain? Spatio‐temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain 138: 362–374. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO ( 2005): Differential coding of hyperalgesia in the human brain: A functional MRI study. Neuroimage 28: 996–1006. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Schmelz M, Forster C, Neundorfer B, Handwerker HO ( 2004): Neural activation during experimental allodynia: A functional magnetic resonance imaging study. Eur J Neurosci 19: 3211–3218. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Herzner B, Otto Handwerker H ( 2006): Secondary somatosensory cortex is important for the sensory‐discriminative dimension of pain: A functional MRI study. Eur J Neurosci 23: 1377–1383. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Seifert F, Decol R ( 2011): Activation of central sympathetic networks during innocuous and noxious somatosensory stimulation. Neuroimage 55: 216–224. [DOI] [PubMed] [Google Scholar]
- Mainero C, Zhang WT, Kumar A, Rosen BR, Sorensen AG (2007): Mapping the spinal and supraspinal pathways of dynamic mechanical allodynia in the human trigeminal system using cardiac‐gated fMRI. Neuroimage 35:1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Kaube H, Buchel C, Eichten C, Rijntjes M, Juptner M, Weiller C, Diener HC ( 1998): Experimental cranial pain elicited by capsaicin: A PET study. Pain 74: 61–66. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT ( 1999): Reciprocal limbic‐cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry 156: 675–682. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Arnau RC, Rhudy JL ( 2001): Pain and emotion: Effects of affective picture modulation. Psychosom Med 63: 79–90. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD ( 1965): Pain mechanisms: A new theory. Science 150: 971–979. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Hu X, Strupp JP, Anderson P, Ugurbil K ( 1995): BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: Echo‐planar imaging correlates with previous optical imaging using intrinsic signals. Magn Reson Med 33: 453–459. [DOI] [PubMed] [Google Scholar]
- Mense, S ( 1993): Nociception from skeletal muscle in relation to clinical muscle pain. Pain 54: 241–289. [DOI] [PubMed] [Google Scholar]
- Mobascher A, Brinkmeyer J, Warbrick T, Musso F, Schlemper V, Wittsack HJ, Saleh A, Schnitzler A, Winterer G ( 2010): Brain activation patterns underlying fast habituation to painful laser stimuli. Int J Psychophysiol 75: 16–24. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Sadato N, Saito DN, Toyoda H, Tashiro M, Okamura N, Yanai K ( 2007): Neural correlates of perceptual difference between itching and pain: A human fMRI study. Neuroimage 36: 706–717. [DOI] [PubMed] [Google Scholar]
- Mohr C, Binkofski F, Erdmann C, Buchel C, Helmchen C ( 2005): The anterior cingulate cortex contains distinct areas dissociating external from self‐administered painful stimulation: A parametric fMRI study. Pain 114: 347–357. [DOI] [PubMed] [Google Scholar]
- Mohr C, Leyendecker S, Helmchen C ( 2008): Dissociable neural activity to self‐ vs. externally administered thermal hyperalgesia: A parametric fMRI study. Eur J Neurosci 27: 739–749. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD ( 2005): Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. J Neurophysiol 93: 2183–2193. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D ( 2011): Aversion‐related circuitry in the cerebellum: Responses to noxious heat and unpleasant images. J Neurosci 31: 3795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD ( 2009): Nociceptive laser‐evoked brain potentials do not reflect nociceptive‐specific neural activity. J Neurophysiol 101: 3258–3269. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD ( 2011): A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage 54: 2237–2249. [DOI] [PubMed] [Google Scholar]
- Nash PG, Macefield VG, Klineberg IJ, Gustin SM, Murray GM, Henderson LA ( 2010): Bilateral activation of the trigeminothalamic tract by acute orofacial cutaneous and muscle pain in humans. Pain 151: 384–393. [DOI] [PubMed] [Google Scholar]
- Nemoto H, Toda H, Nakajima T, Hosokawa S, Okada Y, Yamamoto K, Horiuchi R, Endo K, Ida I, Mikuni M, Goto F ( 2003): Fluvoxamine modulates pain sensation and affective processing of pain in human brain. Neuroreport 14: 791–797. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS ( 2004): The amygdala and persistent pain. Neuroscientist 10: 221–234. [DOI] [PubMed] [Google Scholar]
- Niddam DM, Yeh TC, Wu YT, Lee PL, Ho LT, Arendt‐Nielsen L, Chen AC, Hsieh JC ( 2002): Event‐related functional MRI study on central representation of acute muscle pain induced by electrical stimulation. Neuroimage 17: 1437–1450. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, Mackey SC ( 2006): Neural correlates of individual differences in pain‐related fear and anxiety. Pain 120: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Ha B, Duncan GH, Morin C, Ptito A, Ptito M, Marchand S, Bushnell MC ( 2001): Cortical activation by tactile and painful stimuli in hemispherectomized patients. Brain 124: 916–927. [DOI] [PubMed] [Google Scholar]
- Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC ( 2007): Brain mechanisms supporting spatial discrimination of pain. J Neurosci 27: 3388–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowsky K, Isnard J, Ryvlin P, Guenot M, Fischer C, Mauguiere F ( 2000): Functional mapping of the insular cortex: Clinical implication in temporal lobe epilepsy. Epilepsia 41: 681–686. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F ( 2002): Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cereb Cortex 12: 376–385. [DOI] [PubMed] [Google Scholar]
- Owen DG, Bureau Y, Thomas AW, Prato FS, St Lawrence KS ( 2008): Quantification of pain‐induced changes in cerebral blood flow by perfusion MRI. Pain 136: 85–96. [DOI] [PubMed] [Google Scholar]
- Owen DG, Clarke CF, Ganapathy S, Prato FS, St Lawrence KS ( 2010): Using perfusion MRI to measure the dynamic changes in neural activation associated with tonic muscular pain. Pain 148: 375–386. [DOI] [PubMed] [Google Scholar]
- Pauli P, Wiedemann G, Nickola M ( 1999a): Pain sensitivity, cerebral laterality, and negative affect. Pain 80: 359–364. [DOI] [PubMed] [Google Scholar]
- Pauli P, Wiedemann G, Nickola M ( 1999b): Pressure pain thresholds asymmetry in left‐ and right‐handers: Associations with behavioural measures of cerebral laterality. Eur J Pain 3: 151–156. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Minoshima S, Morrow TJ, Casey KL ( 1998): Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain 76: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Faulk MEJ ( 1955): The insula: Further observations on its function. Brain 78: 445–470. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Hansson P, Ingvar M ( 2002): A regression analysis study of the primary somatosensory cortex during pain. Neuroimage 16: 1142–1150. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Hansson P, Ingvar M ( 2004a): Brainstem involvement in the initial response to pain. Neuroimage 22: 995–1005. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M ( 2004b): Context‐dependent deactivation of the amygdala during pain. J Cogn Neurosci 16: 1289–1301. [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia‐Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B ( 1999): Haemodynamic brain responses to acute pain in humans: Sensory and attentional networks. Brain 122( Pt 9): 1765–1780. [DOI] [PubMed] [Google Scholar]
- Pogatzki‐Zahn EM, Wagner C, Meinhardt‐Renner A, Burgmer M, Beste C, Zahn PK, Pfleiderer B ( 2010): Coding of incisional pain in the brain: A functional magnetic resonance imaging study in human volunteers. Anesthesiology 112: 406–417. [DOI] [PubMed] [Google Scholar]
- Porro CA, Cettolo V, Francescato MP, Baraldi P ( 1998): Temporal and intensity coding of pain in human cortex. J Neurophysiol 80: 3312–3320. [DOI] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P ( 2002): Does anticipation of pain affect cortical nociceptive systems? J Neurosci 22: 3206–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Noguchi Y, Honda M, Nakata H, Tamura Y, Tanaka S, Sadato N, Wang X, Inui K, Kakigi R ( 2006): Brain processing of the signals ascending through unmyelinated C fibers in humans: An event‐related functional magnetic resonance imaging study. Cereb Cortex 16: 1289–1295. [DOI] [PubMed] [Google Scholar]
- Raij TT, Numminen J, Narvanen S, Hiltunen J, Hari R ( 2005): Brain correlates of subjective reality of physically and psychologically induced pain. Proc Natl Acad Sci U S A 102: 2147–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Feine JS, Bushnell MC, Duncan GH ( 1992): A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens Mot Res 9: 265–277. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC ( 1997): Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277: 968–971. [DOI] [PubMed] [Google Scholar]
- Raja S, Meyer R, Ringkamp M, Campbell J ( 1999): Peripheral neural mechanisms of nociception In: Wall PD, Melzack R, editors. Textbook of Pain. New York: Churchill Livingston; pp 11‐57. [Google Scholar]
- Remy F, Frankenstein UN, Mincic A, Tomanek B, Stroman PW ( 2003): Pain modulates cerebral activity during cognitive performance. Neuroimage 19: 655–664. [DOI] [PubMed] [Google Scholar]
- Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F ( 2003): Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex 13: 308–317. [DOI] [PubMed] [Google Scholar]
- Roy M, Piche M, Chen JI, Peretz I, Rainville P ( 2009): Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci USA 106:20090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehle BS, Handwerker HO, Lennerz JK, Ringler R, Forster C ( 2006): Brain activation during input from mechanoinsensitive versus polymodal C‐nociceptors. J Neurosci 26: 5492–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlani E, Farooq N, Greenspan JD ( 2003): Gender and laterality differences in thermosensation throughout the perceptible range. Pain 106: 9–18. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H ( 2000): Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: An event‐related functional magnetic resonance imaging study. J Neurosci 20: 7438–7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Holthusen H, Kessler C, Posse S, Muller‐Gartner HW, Arndt JO ( 2001): Subjective ratings of pain correlate with subcortical‐limbic blood flow: An fMRI study. Neuropsychobiology 43: 175–185. [DOI] [PubMed] [Google Scholar]
- Schoedel AL, Zimmermann K, Handwerker HO, Forster C ( 2008): The influence of simultaneous ratings on cortical BOLD effects during painful and non‐painful stimulation. Pain 135: 131–141. [DOI] [PubMed] [Google Scholar]
- Schon D, Rosenkranz M, Regelsberger J, Dahme B, Buchel C, von Leupoldt A ( 2008): Reduced perception of dyspnea and pain after right insular cortex lesions. Am J Respir Crit Care Med 178:1173–1179. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Siessmeier T, Viertmann A, Landvogt C, Buchholz HG, Rolke R, Treede RD, Bartenstein P, Birklein F ( 2005): The unpleasantness of tonic pain is encoded by the insular cortex. Neurology 64: 1175–1183. [DOI] [PubMed] [Google Scholar]
- Seifert F, Maihofner C ( 2007): Representation of cold allodynia in the human brain–a functional MRI study. Neuroimage 35: 1168–1180. [DOI] [PubMed] [Google Scholar]
- Seifert F, Fuchs O, Nickel FT, Garcia M, Dörfler A, Schaller G, Kornhuber J, Sperling W, Maihˆfner C ( 2010): A functional magnetic resonance imaging navigated repetitive transcranial magnetic stimulation study of the posterior parietal cortex in normal pain and hyperalgesia. Neuroscience 170: 670–677. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD ( 2006): Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 120: 297–306. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD ( 2007): Interactions of pain intensity and cognitive load: The brain stays on task. Cereb Cortex 17: 1412–1422. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mikulis DJ, Davis KD ( 2004): Cognitive modulation of pain‐related brain responses depends on behavioral strategy. Pain 112: 48–58. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K ( 2002): Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36: 1195–1210. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK ( 2006): Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9: 569–577. [DOI] [PubMed] [Google Scholar]
- Sikes RW, Vogt BA ( 1992): Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol 68: 1720–1732. [DOI] [PubMed] [Google Scholar]
- Sikes RW, Vogt LJ, Vogt BA ( 2008): Distribution and properties of visceral nociceptive neurons in rabbit cingulate cortex. Pain 135: 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder‐Smith CH ( 2006): Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain 126: 79–90. [DOI] [PubMed] [Google Scholar]
- Sprenger T, Valet M, Boecker H, Henriksen G, Spilker ME, Willoch F, Wagner KJ, Wester HJ, Tolle TR ( 2006): Opioidergic activation in the medial pain system after heat pain. Pain 122: 63–67. [DOI] [PubMed] [Google Scholar]
- Stammler T, De Col R, Seifert F, Maihofner C ( 2008): Functional imaging of sensory decline and gain induced by differential noxious stimulation. Neuroimage 42: 1151–1163. [DOI] [PubMed] [Google Scholar]
- Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD ( 2007): Brain activity related to temporal summation of C‐fiber evoked pain. Pain 129: 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH ( 2008): Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum Brain Mapp 30:689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Duncan GH, Boivin M, Bushnell MC ( 2003): Differentiation of visceral and cutaneous pain in the human brain. J Neurophysiol 89: 3294–3303. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Duncan GH, Bushnell MC, Boivin M, Wainer I, Rodriguez Rosas ME, Persson J ( 2005): The effects of racemic ketamine on painful stimulation of skin and viscera in human subjects. Pain 113: 255–264. [DOI] [PubMed] [Google Scholar]
- Sung EJ, Yoo SS, Yoon HW, Oh SS, Han Y, Park HW. (2007). Brain activation related to affective dimension during thermal stimulation in humans: a functional magnetic resonance imaging study. Int J Neurosci 117:1011–1027. [DOI] [PubMed] [Google Scholar]
- Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL ( 1997): Cerebral processing of acute skin and muscle pain in humans. J Neurophysiol 78: 450–460. [DOI] [PubMed] [Google Scholar]
- Svensson P, Johannsen P, Jensen TS, Arendt‐Nielsen L, Nielsen J, Stodkilde‐Jorgensen H, Gee AD, Baarsgaard Hansen S, Gjedde A ( 1998): Cerebral blood‐flow changes evoked by two levels of painful heat stimulation: A positron emission tomography study in humans. Eur J Pain 2: 95–107. [DOI] [PubMed] [Google Scholar]
- Symonds LL, Gordon NS, Bixby JC, Mande MM ( 2006): Right‐lateralized pain processing in the human cortex: An FMRI study. J Neurophysiol 95: 3823–3830. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Taguchi T, Tanaka S, Sadato N, Qiu Y, Kakigi R, Mizumura K. Painful muscle stimulation preferentially activates emotion‐related brain regions compared to painful skin stimulation. Neurosci Res (in press). [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH ( 1991): Multiple representations of pain in human cerebral cortex. Science 251: 1355–1358. [DOI] [PubMed] [Google Scholar]
- Terekhin P, Forster C ( 2006): Hypocapnia related changes in pain‐induced brain activation as measured by functional MRI. Neurosci Lett 400: 110–114. [DOI] [PubMed] [Google Scholar]
- Thunberg J, Lyskov E, Korotkov A, Ljubisavljevic M, Pakhomov S, Katayeva G, Radovanovic S, Medvedev S, Johansson H ( 2005): Brain processing of tonic muscle pain induced by infusion of hypertonic saline. Eur J Pain 9: 185–194. [DOI] [PubMed] [Google Scholar]
- Tölle TR, Kaufmann T, Siessmeier T, Lautenbacher S, Berthele A, Munz F, Zieglgänsberger W, Willoch F, Schwaiger M, Conrad B, Bartenstein P (1999): Region‐specific encoding of sensory and affective components of pain in the human brain: a positron emission tomography correlation analysis. Ann Neurol 45:40‐7. [DOI] [PubMed] [Google Scholar]
- Tracey I, Becerra L, Chang I, Breiter H, Jenkins L, Borsook D, Gonzalez RG ( 2000): Noxious hot and cold stimulation produce common patterns of brain activation in humans: A functional magnetic resonance imaging study. Neurosci Lett 288: 159–162. [DOI] [PubMed] [Google Scholar]
- Uematsu H, Shibata M, Miyauchi S, Mashimo T ( 2011): Brain imaging of mechanically induced muscle versus cutaneous pain. Neurosci Res 70:78–84. [DOI] [PubMed] [Google Scholar]
- Vandenbergh J, Dupont P, Fischler B, Bormans G, Persoons P, Janssens J, Tack J ( 2005): Regional brain activation during proximal stomach distention in humans: A positron emission tomography study. Gastroenterology 128: 564–573. [DOI] [PubMed] [Google Scholar]
- Villemure C, Bushnell MC ( 2002): Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain 95: 195–199. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Derbyshire S, Jones AK ( 1996): Pain processing in four regions of human cingulate cortex localized with co‐registered PET and MR imaging. Eur J Neurosci 8: 1461–1473. [DOI] [PubMed] [Google Scholar]
- Wagner KJ, Sprenger T, Kochs EF, Tolle TR, Valet M, Willoch F ( 2007): Imaging human cerebral pain modulation by dose‐dependent opioid analgesia: A positron emission tomography activation study using remifentanil. Anesthesiology 106: 548–556. [DOI] [PubMed] [Google Scholar]
- Wall PD, Woolf CJ ( 1984): Muscle but not cutaneous C‐afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol 356: 443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Shyu BC ( 2004): Differential projections from the mediodorsal and centrolateral thalamic nuclei to the frontal cortex in rats. Brain Res 995: 226–235. [DOI] [PubMed] [Google Scholar]
- Wiech K, Tracey I ( 2009): The influence of negative emotions on pain: Behavioral effects and neural mechanisms. Neuroimage 47: 987–994. [DOI] [PubMed] [Google Scholar]
- Weigelt A, Terekhin P, Kemppainen P, Dörfler A, Forster C ( 2010): The representation of experimental tooth pain from upper and lower jaws in the human trigeminal pathway. Pain 149: 529–538. [DOI] [PubMed] [Google Scholar]
- Xu X, Fukuyama H, Yazawa S, Mima T, Hanakawa T, Magata Y, Kanda M, Fujiwara N, Shindo K, Nagamine T, Shibasaki H ( 1997): Functional localization of pain perception in the human brain studied by PET. Neuroreport 8: 555–559. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DF ( 1991): Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 303: 355–374. [DOI] [PubMed] [Google Scholar]
- Yilmaz P, Diers M, Diener S, Rance M, Wessa M, Flor H ( 2010): Brain correlates of stress‐induced analgesia. Pain 151: 522–529. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Dougherty PM, Oppenheimer SM ( 1999): Monkey insular cortex neurons respond to baroreceptive and somatosensory convergent inputs. Neuroscience 94: 351–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Figure S1, S2, and S3


