Abstract
It was recently observed that dehydration causes shrinkage of brain tissue and an associated increase in ventricular volume. Negative effects of dehydration on cognitive performance have been shown in some but not all studies, and it has also been reported that an increased perceived effort may be required following dehydration. However, the effects of dehydration on brain function are unknown. We investigated this question using functional magnetic resonance imaging (fMRI) in 10 healthy adolescents (mean age = 16.8, five females). Each subject completed a thermal exercise protocol and nonthermal exercise control condition in a cross‐over repeated measures design. Subjects lost more weight via perspiration in the thermal exercise versus the control condition (P < 0.0001), and lateral ventricle enlargement correlated with the reduction in body mass (r = 0.77, P = 0.01). Dehydration following the thermal exercise protocol led to a significantly stronger increase in fronto‐parietal blood‐oxygen‐level‐dependent (BOLD) response during an executive function task (Tower of London) than the control condition, whereas cerebral perfusion during rest was not affected. The increase in BOLD response after dehydration was not paralleled by a change in cognitive performance, suggesting an inefficient use of brain metabolic activity following dehydration. This pattern indicates that participants exerted a higher level of neuronal activity in order to achieve the same performance level. Given the limited availability of brain metabolic resources, these findings suggest that prolonged states of reduced water intake may adversely impact executive functions such as planning and visuo‐spatial processing. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: adolescent, dehydration, executive function, fMRI, lateral ventricle
INTRODUCTION
Adequate levels of fluid intake are crucial to maintain vital physiological functions. Adverse effects of reduced fluid intake on physical performance have long been known [Grandjean and Grandjean, 2007; Lieberman, 2007]. In addition there is accumulating evidence that acute states of dehydration may also impair cognitive function [Baker et al., 2007; Cian et al., 2001; Gopinathan et al., 1988; McMorris et al., 2006]. These effects can lead to accidents at work [Kenefick and Sawka, 2007] and are likely to impact on vulnerable populations, such as children and adolescents whose brains are still developing [Bar‐David et al., 2005; D'Anci et al., 2006], and elderly individuals, whose ability to self‐regulate appropriate fluid levels is often impaired [Suhr et al., 2004]. Accordingly, there has been considerable scientific and public interest in the recommended amount of water to be consumed daily and there has been a lively debate around appropriate policy implementations in the workplace and school [Valtin, 2002].
The issue is complicated by studies failing to find negative effects of dehydration on cognitive performance [Ackland et al., 2008; Grego et al., 2005; Patel et al., 2007; Szinnai et al., 2005; Tomporowski et al., 2007] and by recent evidence that public policy attempts to increase the amount of water drunk by the general public may not be associated with the expected improvements in health [Negoianu and Goldfarb, 2008; Valtin, 2002]. However, previous studies have not examined the effects of dehydration on measures of brain function in humans. Functional magnetic resonance imaging (fMRI) allows the indirect measurement of neural activity during performance of cognitive tasks. Structural magnetic resonance imaging (sMRI) methods have been employed previously in studies of dehydration and have shown that acute [Dickson et al., 2005; Kempton et al., 2009] or prolonged [Duning et al., 2005] dehydration through physical exercise or restricted fluid intake causes reversible brain changes. These changes consist of reduced brain volume [Duning et al., 2005] and associated increases in ventricular volume [Kempton et al., 2009].
We used fMRI, sMRI, and arterial spin labelling (ASL) to study the objective effects of acute dehydration on brain function, structure, and blood flow, respectively. Specifically we investigated the effects of dehydration on executive function as it has been theorized that dehydration may adversely affect this cognitive domain [Wilson and Morley, 2003] and measured the volume of the lateral ventricles as our previous research [Kempton et al., 2009] suggested these structures were the most sensitive to the effects of dehydration. To examine whether any observed changes in blood‐oxygen‐level‐dependent (BOLD) signal may be due to changes in blood flow, we also measured resting regional cerebral blood flow using ASL. We studied young people as we were interested in the effects of acute dehydration on the developing brain.
MATERIALS AND METHODS
Ten adolescent subjects participated in a randomized, cross‐over, and repeated‐measures study. Each subject attended the laboratory on two occasions and underwent sMRI, fMRI, and ASL before and after a thermal‐exercise dehydration or exercise control condition.
Participants
Ten healthy adolescents (five females) were recruited from schools and colleges in Chichester, UK. Physical characteristics for the group (mean ± SD) were 16.81 ± 0.49 years of age and 66.1 ± 9 kg body mass. Participants undertook regular physical activity but were naïve to dehydration methods. Participants and their parents gave written informed consent. Ethical approval was obtained from King's College London Research Ethics Committee.
Procedure
Participants first underwent a medical assessment, comprising initial clinical history, including details of any current or previous medical or surgical morbidity, medication treatment, recent substance use, or allergies. This was followed by a full physical examination, including basic observations (pulse, supine blood pressure, respiratory rate), cardiovascular, respiratory, gastrointestinal and neurological assessment, including fundoscopy. All participants were noted to be fit and healthy following assessment.
Participants were instructed not to participate in strenuous activity, to maintain a normal diet and to avoid alcohol consumption during the 24 h before the study. Participants consumed 500 ml of water during the evening before reporting to the laboratory the following morning. On arrival at the laboratory [temperature 19 (±1)°C, relative humidity (40% ± 5%)] participants provided a urine sample for the determination of urine osmolality (mOsm/kg) (Model 3300 Osmometer, Vitech Scientific Ltd, West Sussex, UK). Participants consumed a further 500 ml of water 1 h before physical exercise. Participants were weighed to determine nude body mass (accurate to ±50 g, Avery scales, Birmingham, England), practiced on the fMRI task (see below for description) and received a baseline brain scan before undertaking the exercise protocol.
Exercise Protocol
The design was randomized, cross‐over, and repeated‐measures contrasting thermal‐exercise dehydration with an exercise control condition. Both conditions comprised an identical 90‐min exercise protocol during which core temperature and heart rate were measured. The thermal‐exercise dehydration protocol was devised to decrease body mass by 1–2% in 90 min [Smith 1998]. The thermal component required participants to wear three layers of clothing: Layer 1 consisted of a modified black bin liner (Sainsbury's, UK) fitted next to the skin; Layer 2 was a chemical warfare protective suit that comprised a hooded impermeable plastic top and bottoms (Royal Navy, Ministry of Defence, UK); Layer 3 was a sports tracksuit comprising a cotton top and bottoms (Adidas, Germany) and trainers. These clothes were worn throughout the thermal‐exercise protocol. In the exercise control condition participants wore light sports clothing (t‐shirt, shorts, socks, and trainers).
The exercise protocol was identical for both conditions and was divided into four phases. Phase 1 involved cycling on a Monark‐E824E ergometer (Monark, Sweden) at 130W for 50 min. Phase 2 was a 10‐min seated recovery. Phase 3 required 20 min cycling at 130W. Phase 4 was a further 10‐min seated recovery followed by reweighing.
After reweighing participants were given a 15‐min supine recovery period, practiced the fMRI tasks again, and then completed the post‐dehydration fMRI scan.
Physiological and Mood State Measures
Body core temperature was measured at 10‐min intervals during the 90‐min exercise protocol using a rectal probe inserted 8 cm past the anal sphincter (Edale Instruments, Cambridge, UK). Heart rate was monitored at the same intervals (Polar S810i, Kempele, Finland).
Mood state was measured before and after exercise using the Physical Sedation (PS) and Mental Sedation (MS) factors of the visual analogue rating scales (VARS) as described by Norris (1971). PS consisted of the items strong—feeble, well coordinated—clumsy, lethargic—energetic (inversely scored), incompetent—proficient (inversely scored). MS consisted of the items alert—drowsy, muzzy—clear headed (inversely scored), mentally slow—quick witted (inversely scored), attentive—dreamy. Each pair of items was presented on paper and was linked by a horizontal line measuring 11.5 cm. Participants were asked to tick the line to indicate their current mood state. Ratings were averaged across items in each factor for statistical analysis.
Structural MRI
MRI scanning was performed on a 1.5 Tesla SIGNA HDx scanner (General Electric, Milwaukee, WI). High resolution structural images were acquired axially using a three‐dimensional, T1‐weighted, inversion‐recovery prepared, steady‐state, spoiled gradient‐echo sequence (TR = 11.1 ms, TE = 4.8 ms, TI = 300 ms, flip angle = 18°, slice thickness = 1.1 mm, matrix size = 256 × 256, voxel dimensions 1.09 × 1.09 × 1.10 mm3, NEX = 1).
We developed an automated outlining procedure to measure the volume of the lateral ventricles using existing neuroimaging analysis software. Normalized and modulated CSF segmented images were produced from each structural image using unified segmentation [Ashburner and Friston, 2005] in SPM5 (http://www.fil.ion.ucl.ac.uk/spm). A mask of the lateral ventricles was created in normalized space to extract this structure from the segmented images. This was achieved by outlining the lateral ventricles on the mean CSF image calculated from all 40 segmented images. Outlining was carried out using MRIcro (version 1.40) [Rorden and Brett, 2000]. As the segmented images already demarcate the main boundaries of the lateral ventricles, the purpose of the mask was to exclude CSF outside the lateral ventricles, such as the third ventricle, superior cistern and sulcal CSF. The mask was applied to each normalized segmented image. As the segmented images were modulated, the total volume of the lateral ventricles was calculated by integrating the intensity over each masked segmented image. We have validated the above methodology in an independent sample of seven subjects; there was excellent agreement in lateral ventricle volume between a trained rater using established stereological techniques [Kempton et al., 2009] and the automated outlining procedure, ICC = 0.99.
BOLD fMRI
During fMRI subjects completed the Tower of London (ToL) task, a measure of executive function chosen to activate the functionally relevant cognitive demand network. The task requires subjects to mentally rearrange objects and plan a complex visuospatial response. Each array in the ToL [Williams‐Gray et al., 2007] consisted of three pockets, which each contained up to three circles colored in red, blue, or green. The task consisted of a “planning” condition tapping executive functions and a “subtracting” control condition, matched for visuomotor responses. In the “planning” condition, participants indicated the number of moves required to rearrange one array so that it matches another array. For the “subtracting” condition subjects were required to calculate the difference in the number of objects between arrays. Only trials requiring responses of 2, 3, or 4 were used. The paradigm took 10 min, during which ToL and control trials were presented alternating with an intervening rest interval (5–15 s duration). Trials began with an instruction (“plan” or “subtract”) and participants received feedback (“correct” or “incorrect”) after responding. The difficulty level of the problems displayed varied according to a randomized sequence. Trial durations were response‐driven; therefore, the number of trials varied between participants.
fMRI was performed on a 1.5 Tesla SIGNA HDx scanner (GE, Milwaukee, WI). In each session, T2*‐weighted echo planar images of the whole head depicting the blood‐oxygen‐level‐dependent (BOLD) response were acquired axially from bottom to top using an interleaved sequence yielding 252 volumes, each with 36 slices of 3 mm thickness and 0.3 mm gap. An 8‐channel headcoil was used for radiofrequency transmission and reception. Images were aligned parallel to the intercommissural plane. fMRI parameters were: TR = 2,500 ms, TE = 40 ms, flip angle = 90°, FOV = 24 cm, NEX = 1, voxel dimensions 3.75 × 3.75 × 3.3 mm3. Four dummy‐data‐acquisition scans were performed before each run.
Analysis of fMRI data was carried out using SPM5. Pre‐processing involved motion correction along the first image in the time series, normalization into standard space (MNI template) using affine registration and nonlinear transformations, and spatial smoothing with an 8 mm full‐width‐at‐half‐maximum Gaussian kernel. The fMRI BOLD response was modeled to the onsets and durations of planning and subtracting trials. Onsets were defined as stimulus appearance; durations were defined as the time from onset to response (button press). Two contrasts were calculated for each participant: “planning” minus baseline (rest) and “planning” minus “subtracting”. We used a region‐of‐interest approach as we wished to determine how dehydration specifically affects task‐related regions of activation. Analyses of dehydration effects were thus restricted to the network of brain areas showing selective activation during ToL performance, subtracting nonspecific activations due to visual input and motor responses (“planning” minus “subtracting”). These areas were identified using a random effects F test of all sessions (dehydration/control, pre/post) and all participants (P = 0.05, family‐wise error corrected, extent threshold = 10 voxels). MNI coordinates were transformed into Talairach coordinates (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) and brain regions were identified using the Talairach atlas [Talairach and Tournoux, 1988]. Mean activation levels of these areas were then extracted for the contrast “planning” minus “baseline”, which was expected to generate maximal signal change, for each participant, session (dehydration/control, pre/post) and region using the Marsbar toolbox in SPM5 [Brett et al., 2002]; these data were then entered into statistical analysis of dehydration effects as described below.
ToL performance measures were reaction time (RT) from stimulus appearance to button press (ms), frequency of errors (%), and number of trials achieved.
ASL MRI
ASL data were collected at rest on the same scanner using a pseudo‐continuous ASL tagging scheme with a 3D interleaved spiral FSE readout [Garcia et al., 2005; Wang et al., 2006]. Each subject's images were realigned to their first session and normalized using the SPM5 PET template. A brain mask was applied to exclude extra‐cerebral voxels and mean perfusion rates were extracted from the regions of interest produced from the fMRI analysis using the Marsbar toolbox [Brett et al., 2002].
Statistical Analysis
Urine osmolality values were compared across Conditions (dehydration, control) using paired‐samples t‐test in SPSS 15.0 (SPSS Inc, Chicago, IL). Analysis of heart rate and body temperature focussed on Phase 1 (six measurements before rest period) and Phase 3 (two measurements after rest period). To assess effects of dehydration in Phase 1, 6 × 2 repeated‐measures analyses of variance (ANOVA) were carried out with Time (Sections 1–6) and Condition (dehydration, control) as within‐subject variables and, in separate analyses, heart rate and body temperature as dependent variables. To assess effects of dehydration in Phase 3, 2 × 2 ANOVA were carried out with Time (Sections 1–2) and Condition (dehydration, control) as independent variables and, in separate analyses, heart rate, and body temperature as dependent variables.
Data of body mass, structural, behavioral, VARS, BOLD and ASL measures were entered into 2‐by‐2 repeated‐measures ANOVAs in SPSS 15.0 with Condition (dehydration, control) and Time (pre‐exercise, post‐exercise) as within‐subjects factor. Correlations between measures were assessed with Pearson's r implemented in SPSS 15.0.
RESULTS
We first measured urine osmolality to confirm normal hydration status prior to the experiment. Urine osmolality scores ranged from 347–675 mOsm/kg, indicating normal levels of hydration. There was no difference (P = 0.39) in osmolality before dehydration (mean = 479.50, SD = 71.53) and control (mean = 498.60, SD = 114.01) sessions.
Following exercise, body mass decreased significantly (F[1,9] = 298.80, P < 0.001), an effect that was modulated by a Time x Condition interaction (F[1,9] = 115.69, P < 0.001, see Table I). This interaction indicated that the decrease in body mass was more pronounced following the thermal‐exercise dehydration condition (mean = 1.64%, SD = 0.26) than the exercise control condition (mean = 0.53%, SD = 0.15). There was no main effect of Condition (P = 0.23).
Table I.
Changes in body mass by condition
| Dehydration | Control | |
|---|---|---|
| Pre‐exercise; Body mass, kg | 66.14 (8.65) | 66.11 (8.99) |
| Post‐exercise; Body mass, kg | 65.05 (8.51) | 65.76 (8.96) |
The table shows pre and post exercise body mass by Condition (dehydration, control). Data are given as means (standard deviation). N = 10.
In Phase 1 of the exercise period (before rest), dehydration caused a stronger increase in body temperature (Condition × Time interaction: F[5,45] = 2.78, P = 0.03) and heart rate (Condition × Time interaction: F[5,45] = 10.02, P < 0.001) than the control condition (see Table II). Main effects of Time indicated a general increase in temperature (F[5,45] = 34.51, P < 0.001) and heart rate (F[5,45] = 125.82, P < 0.001). Dehydration led to higher heart rates (main effect of Condition: F[1,9] = 15.08, P < 0.001) but not temperature (P = 0.13) overall.
Table II.
Physiological data by condition
| Dehydration | Control | |||
|---|---|---|---|---|
| Heart rate, bpm | Temperature, °C | Heart rate, bpm | Temperature, °C | |
| Phase 1 | ||||
| 1 | 74.10 (9.66) | 37.24 (0.38) | 75.60 (7.20) | 37.12 (0.25) |
| 2 | 142.10 (19.27) | 37.44 (0.36) | 129.10 (17.80) | 37.30 (0.24) |
| 3 | 151.30 (21.32) | 37.66 (0.38) | 132.30 (15.96) | 37.48 (0.24) |
| 4 | 150.10 (16.49) | 37.80 (0.32) | 131.40 (14.12) | 37.59 (0.29) |
| 5 | 153.00 (20.46) | 37.92 (0.34) | 132.00 (14.63) | 37.67 (0.30) |
| 6 | 155.30 (19.41) | 38.01 (0.37) | 135.20 (14.99) | 37.70 (0.33) |
| Rest | 105.80 (15.85) | 37.83 (0.44) | 86.10 (14.95) | 37.38 (0.32) |
| Phase 3 | ||||
| 1 | 149.90 (22.03) | 37.90 (0.40) | 131.30 (17.80) | 37.45 (0.30) |
| 2 | 152.00 (17.35) | 37.98 (0.46) | 132.70 (17.88) | 37.53 (0.28) |
| Rest | 101.10 (19.37) | 37.66 (0.23) | 79.00 (9.30) | 37.42 (0.17) |
The table shows heart rate and body core temperature by Condition (dehydration, control) for each segment in Phase 1 (numbered 1‐6) and Phase 3 (numbered 1‐2). Measurements were taken every 10 min, for details see method section. Data are given as means (standard deviation). N = 10, bpm = beats per minute.
In Phase 3 (after rest), temperature and heart rate were higher with dehydration than control (main effect of Condition on temperature: F[1,9] = 7.98, P = 0.02; main effect of Condition on heart rate: F[1,9] = 13.62, P = 0.005) and there was a further increase in temperature (main effect of Time: F[1,9] = 16.00, P = 0.003) but not heart rate (P = 0.35). There were no Condition × Time interactions for either variable in this phase (P > 0.69).
VARS ratings showed that exercise had effects on both measures of sedation: there were main effects of Time on PS (F[1,9] = 38.33, P < 0.001) and MS (F[1,9] = 20.62, P < 0.001), but no Condition × Time interactions (both P > 0.17). For PS (F[1,9] = 5.83, P = 0.04) but not MS (P = 0.14) there was a main effect of Condition, indicating higher sedation ratings on dehydration days irrespective of Time.
Using sMRI, we observed that lateral ventricular volume increased after exercise (main effect of Time F[1,9] = 9.59, P = 0.01). There was no main effect of Condition and no Time × Condition interaction (P > 0.19). One sample t‐tests on percentage volume change in the two conditions revealed significant ventricular expansion in the dehydration condition (+3.3%, P = 0.01) but not in the control condition (P = 0.12).
In the dehydration condition we observed a significant correlation between percentage change in body mass and percentage change in ventricular volume (r = −0.77, P = 0.01), indicating that greater reductions in body mass were associated with greater increases in the volume of the lateral ventricles (see Fig. 1).
Figure 1.
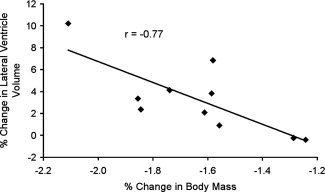
Relationship between change in lateral ventricle volume and body mass. The graph shows data of the association between change in ventricular volume and change in body mass. Data are from the dehydration condition only.
For reaction time (RT) during the ToL task, there was a main effect of Time (F[1,9] = 87.68, P < 0.001) indicating faster RT after exercise. This effect was similar for both conditions (effects of Condition or Time × Condition interaction: both P > 0.78). There were no main or interaction effects for the percentage of errors or the number of trials achieved (all P > 0.13). In the dehydration condition the mean reaction time was 6.6 (SD = 1.4) and 6.2 (SD = 1.4) seconds pre‐ and post–exercise, respectively. In the control condition the values were 6.4 (SD = 1.7) seconds pre‐exercise, and 6.0 (SD = 1.4) post‐exercise.
Using fMRI, we found that the ToL task activated the previously shown [Williams‐Gray et al., 2007] bilateral network of rostral prefrontal cortex, middle frontal gyrus (MFG), intraparietal sulcus (IPS), and inferior parietal lobule (see Table III and Fig. 2). Analysis of dehydration effects within these areas showed the following pattern: Significant main effects of Time were observed for right (F[1,9] = 5.99, P = 0.04) and left (F[1,9] = 6.53, P = 0.03) IPS as well as right (F[1,9] = 7.23, P = 0.03) and left (F[1,9] = 11.17, P = 0.009) MFG, suggesting increased BOLD response post‐exercise compared with pre‐exercise. Significant Time‐by‐Condition interactions were observed for right (F[1,9] = 6.36, P = 0.03) and left (F[1,9] = 7.14, P = 0.03) IPS and left MFG (F[1,9] = 6.67, P = 0.03), indicating that the pre‐to‐post increase in BOLD was greater for dehydration than control (see Fig. 3). There were no other main or interaction effects for any area (all P > 0.06).
Table III.
BOLD response during the Tower‐of‐London task
| Macroanatomical Label | Brodmann Area | Stereotaxic coordinates | Z | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left intraparietal sulcus | 7 | −6 | −58 | 47 | 7.37 |
| Right intraparietal sulcus | 7 | 14 | −61 | 58 | 7.25 |
| Right middle frontal gyrus | 6 | 30 | 3 | 53 | 6.86 |
| Left middle frontal gyrus | 6 | −26 | 3 | 51 | 6.70 |
| Right rostral prefrontal cortex | 10 | 34 | 59 | 6 | 5.39 |
| Left rostral prefrontal cortex | 10 | −28 | 55 | 6 | 5.21 |
| Right inferior parietal lobule | 40 | 46 | −45 | 41 | 4.92 |
| Left inferior parietal lobule | 40 | −42 | −46 | 43 | 4.91 |
Stereotaxic coordinates are given in Talairach space which was also used to derive Brodmann areas and macroanatomical labels.
Figure 2.
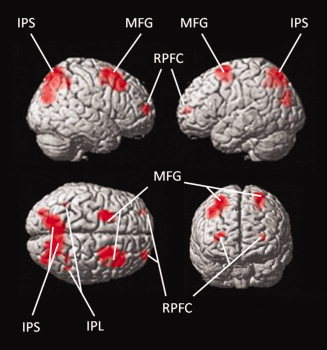
BOLD response during the Tower‐of‐London task. The images shows significant activations (family‐wise error corrected at P < 0.05) across both visits and all subjects during the Tower‐of‐London task. IPS, intraparietal sulcus; IPL, inferior parietal lobule; MFG, middle frontal gyrus; RPFC, rostral prefrontal cortex.
Figure 3.
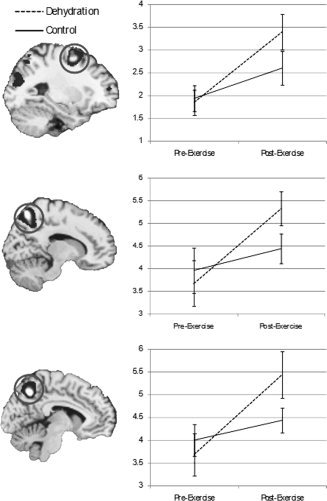
Effects of dehydration on BOLD response in middle frontal gyrus and intraparietal sulcus. The figure shows mean BOLD responses (weighted parameter estimates) in the left middle frontal gyrus (top), right intraparietal sulcus (middle), and left intraparietal sulcus (bottom) as a function of Time (pre‐exercise, post‐exercise) and Condition (dehydration, control). Error bars are standard errors.
We then carried out two tests to investigate whether the dehydration effects observed represent in fact an increase in neural effort in fronto‐parietal cortex, or a more general effect due to differences in the dynamics of blood flow change underlying the BOLD response. First, an analysis of BOLD response in visual cortex (the voxel showing maximal activation; Talairach coordinates x = −22, y = −80, z = −13) showed a main effect of Time (F[1,9] = 8.47, P = 0.02) but no main effect of Condition or Time‐by‐Condition interaction (P > 0.59), suggesting an anatomically specific effect of dehydration on neural effort in MFG and IPS.
Second, analysis of ASL data in the right and left IPS showed main effects of Time (both P < 0.03) indicating that cerebral blood flow within these regions increased following exercise. There was no main effect of Time in the left MFG (P = 0.96) and no main effect of Condition or Time‐by‐Condition interaction in any region (all P > 0.37), suggesting cerebral perfusion in fronto‐parietal cortex was not affected by dehydration.
To ensure changes in brain activation were not due to differences in body core temperature between the conditions, we estimated body core temperature during the scan. Body core temperature decreased after subjects stopped exercising (see Table II) and the difference between the control and dehydration condition in the last rest period was 0.24°C. When the temperature change was extrapolated forward 10 min (using the same rate of change from Phase 3.2‐rest) there was essentially no difference in temperature (37.34°C during dehydration and 37.31°C in the control condition). As there was at least 10 min between the last temperature measurement and the start of the fMRI task we would not expect there to be a significant difference in core body temperature between the conditions during the fMRI scanning session. However, to verify that there was no relationship between the difference in temperature at the end of the exercise between conditions (ΔT) and difference in post‐exercise BOLD responses between conditions (ΔBOLD) we examined correlations between these variables. We examined ΔBOLD in brain regions where we had found an effect of dehydration, namely the right and left intraparietal sulcus and left middle frontal gyrus. There was no significant correlation between ΔT and ΔBOLD in any of these brain regions, suggesting that the results were not explained by differences in body core temperature.
Finally we investigated inter‐relationships amongst brain functional (BOLD and ASL), structural, performance, and weight changes following dehydration. We did not find any significant relationships between these variables, other than the association between greater weight loss and greater ventricular enlargement reported above (all P > 0.05).
DISCUSSION
The finding of ventricular enlargement following dehydration, which is proportional to bodyweight lost is consistent with previous research [Dickson et al., 2005; Duning et al., 2005; Kempton et al., 2009] and extends this finding to adolescents. Dehydration decreases blood volume (hypovolaemia) [Costill and Fink, 1974] which may be detected as reduced brain volume and associated increase in ventricular volume. Acute dehydration also increases serum osmolality, generating an osmotic gradient which results in water leaving intracellular stores. This causes shrinkage of cells, in particular astrocytes which play an important role in water transport [Gullans and Verbalis, 1993]. The relatively subtle reduction in overall brain volume observed in previous studies of dehydration may also be expressed in a secondary (or compensatory) increase in ventricular volume [Dickson et al., 2005; Duning et al., 2005; Kempton et al., 2009].
As expected both exercise conditions increased core body temperature and cardiovascular function. There was also an increase in mental and physical sedation following exercise, but no differential effect of dehydration. BOLD response in the dorsal fronto‐parietal network mediating executive functions increased after dehydration. While BOLD response in this network generally rose following exercise, the slope of this increase was significantly steeper for dehydration than the exercise control condition. Given there was no effect of dehydration on cognitive performance, this suggests that participants exerted a higher level of neuronal activity in order to achieve the same performance level.
Our findings may provide a neurobiological explanation for a previously observed phenomenon: Szinnai et al. [ 2005] showed no effects of dehydration in healthy subjects on performance of a number of cognitive tasks. However, they observed that dehydration caused changes in subjective ratings, such as increased tiredness, and higher levels of perceived effort and concentration. The present findings suggest the increase in fatigue is overcome by an increase in fronto‐parietal brain activation, which may be experienced by participants as increased effort. This pattern fits with previous observations of the distinction between effectiveness, i.e. the level of performance, and efficiency, i.e. the cost required to achieve this level of performance [Eysenck and Calvo, 1992]. Interestingly, the effects of dehydration on BOLD were not paralleled by selective increases in subjective ratings of sedation, which were similar for the two conditions.
Although the majority of studies examining the effect of dehydration on cognition have been performed in adults, a small number of studies have focused on children and adolescents. Edmonds and Burford [ 2009] reported that children who drank additional water performed better on visual attention tasks, while Benton and Burgess [ 2009] found that children who had consumed water were better at recalling objects which had previously been presented to them. Although we found no significant differences in task performance, our study also suggests that dehydration negatively impacts brain functions underlying important cognitive processes in young people. These findings may be relevant to school policies regarding the availability of water as it has been shown that school children may suffer from moderate to severe dehydration, especially in hot environments [Bar‐David et al., 2009] and that many teachers do not appreciate the importance of children being adequately hydrated [Molloy et al., 2008].
We were able to detect a significant difference in BOLD response despite only a 1.1% difference in weight loss due to dehydration. Although the percentage change between the groups was small the effect was highly significant (P < 0.0001) because the weight variance in each group was minimal (i.e. subjects consistently lost more weight in the dehydration condition). In addition the BOLD signal is an objective measure of neurophysiological processes [Logothetis et al., 2001] and as such may be more sensitive to dehydration than behavioral measures which show larger intersubject variability.
It is important to note that the increases in task‐related BOLD signal observed in this study were not accompanied by regional increases in blood flow during a rest condition. Instead, we observed a regional increase in the BOLD signal that is likely to be explained by a demand for a higher level of neuronal activity in the dehydrated state. The perfusion data suggest that this regional increase in the BOLD signal is supported by an increase in the rate of blood circulation. While this increase is not significantly different from the increase observed following the exercise control condition, its magnitude must be sufficiently large to support any regional increases in the rate of oxygen consumption as a result of increased neuronal output.
It must be stressed that the measurements of cerebral perfusion were made a few minutes after the execution of the task and therefore, they reflect only the resting state perfusion characteristics of the subject population in the absence of cognitive demands. To fully understand the regional physiological changes that support any changes in neuronal activity, task specific functional imaging maps must be made employing techniques like pulsed ASL, that are able to reveal brain activation by means of blood flow driven contrast instead of BOLD.
Limitations
While it is possible that structural brain changes could affect the measurement of brain function these were subtle, and do not invalidate the interaction observed in brain activations as no volumetric interaction due to dehydration was observed. Including a control condition in this study ensured that the changes observed were not due to exercise alone. However core body temperature was higher during the last phase of the exercise period in the dehydration condition compared to the control condition. Although we suggest there was no difference in temperature during the fMRI task using extrapolation, this could have been accurately measured using an MRI compatible thermometer. Future studies using a restricted fluid intake design may be able to more effectively separate the effects of dehydration and raised body core temperature. The control condition was chosen to match the amount of exercise undertaken in the two conditions; however it may have been informative to include an additional experimental procedure of no exercise to examine the effect of physical exertion on brain structure and function.
Dehydration was quantified using changes in body mass; however, other measures such as plasma osmolality and protein levels would have given further information about the degree of dehydration.
Although our sample size was modest, we previously reported structural brain changes following dehydration in seven participants [Kempton et al., 2009] and indeed we detected significant changes in brain activation in this sample.
Finally, our findings concerning cognitive performance are limited to executive function and further research is needed to clarify if dehydration affects other cognitive domains such as sustained attention, learning, and memory.
Implications
In this study we have shown that dehydration led to a stronger increase in fronto‐parietal brain activations during a task of executive function, and the extent of dehydration correlated with an increase in lateral ventricular volume. The present findings may be of relevance not only to the developing brain, as argued above, but also in individuals with psychiatric or neurological conditions with poorly maintained fluid intake; cognitive impairments in these populations may be confounded further by effects of dehydration [Suhr et al., 2004]. Our study has implications for the distinction between acute and prolonged dehydration and its dissociable effects. Although BOLD response appears to be a more sensitive indicator of dehydration effects, impairments in cognitive performance may become more apparent after longer time intervals and with greater severity of dehydration. In other words, while the brain can compensate for water restriction in the short term, this increased effort may not be sustained in the longer term. Evidence for this hypothesis comes from a study by Bar‐David et al. [ 2005], who showed that dehydrated and normally hydrated school children did not differ in cognitive performance at the beginning of the day; however, after several hours of school work dehydrated children showed a significant drop in performance levels.
Acknowledgements
The authors thank the volunteers for taking part in this study and the radiographic team at the Centre for Neuroimaging Sciences for their support. Validation of the ventricular mask was based on regions outlined by Anne Schmechtig. They would like to thank GlaxoSmithKline for supplying the recovery solutions post experiment and David Alsop for providing the ASL sequence. Ulrich Ettinger was funded by a NIHR (National Institute for Health Research) Personal Award. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR or Department of Health. The authors thank the volunteers from Chichester High Schools Sixth Form and Chichester College for taking part in the study.
REFERENCES
- Ackland GL, Harrington J, Downie P, Holding JW, Singh‐Ranger D, Griva K, Mythen MG, Newman SP ( 2008): Dehydration induced by bowel preparation in older adults does not result in cognitive dysfunction. Anesth Analg 106: 924–929, table of contents. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2005): Unified segmentation. Neuroimage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- Baker LB, Conroy DE, Kenney WL ( 2007): Dehydration impairs vigilance‐related attention in male basketball players. Med Sci Sports Exerc 39: 976–983. [DOI] [PubMed] [Google Scholar]
- Bar‐David Y, Urkin J, Kozminsky E ( 2005): The effect of voluntary dehydration on cognitive functions of elementary school children. Acta Paediatr 94: 1667–1673. [DOI] [PubMed] [Google Scholar]
- Bar‐David Y, Urkin J, Landau D, Bar‐David Z, Pilpel D ( 2009): Voluntary dehydration among elementary school children residing in a hot arid environment. J Hum Nutr Diet 22: 455–460. [DOI] [PubMed] [Google Scholar]
- Benton D, Burgess N ( 2009): The effect of the consumption of water on the memory and attention of children. Appetite 53: 143–146. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline JB ( 2002): Region of interest analysis using an SPM toolbox, abstract 497 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan.
- Cian C, Barraud PA, Melin B, Raphel C ( 2001): Effects of fluid ingestion on cognitive function after heat stress or exercise‐induced dehydration. Int J Psychophysiol 42: 243–251. [DOI] [PubMed] [Google Scholar]
- Costill DL, Fink WJ ( 1974): Plasma volume changes following exercise and thermal dehydration. J Appl Physiol 37: 521–525. [DOI] [PubMed] [Google Scholar]
- D'Anci KE, Constant F, Rosenberg IH ( 2006): Hydration and cognitive function in children. Nutr Rev 64( Part 1): 457–464. [DOI] [PubMed] [Google Scholar]
- Dickson JM, Weavers HM, Mitchell N, Winter EM, Wilkinson ID, Van Beek EJ, Wild JM, Griffiths PD ( 2005): The effects of dehydration on brain volume—Preliminary results. Int J Sports Med 26: 481–485. [DOI] [PubMed] [Google Scholar]
- Duning T, Kloska S, Steinstrater O, Kugel H, Heindel W, Knecht S ( 2005): Dehydration confounds the assessment of brain atrophy. Neurology 64: 548–550. [DOI] [PubMed] [Google Scholar]
- Edmonds CJ, Burford D ( 2009): Should children drink more water? The effects of drinking water on cognition in children. Appetite 52: 776–779. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG ( 1992): Anxiety and performance: The processing efficiency theory. Cogn Emotion 6: 409–434. [Google Scholar]
- Garcia DM, Bazelaire C, Alsop DC ( 2005): Pseudo‐continuous flow driven adiabatic inversion for arterial spin labeling. Proc ISMRM, Abstract 37. [Google Scholar]
- Gopinathan PM, Pichan G, Sharma VM ( 1988): Role of dehydration in heat stress‐induced variations in mental performance. Arch Environ Health 43: 15–17. [DOI] [PubMed] [Google Scholar]
- Grandjean AC, Grandjean NR ( 2007): Dehydration and cognitive performance. J Am Coll Nutr 26( 5 Suppl): 549S–554S. [DOI] [PubMed] [Google Scholar]
- Grego F, Vallier JM, Collardeau M, Rousseu C, Cremieux J, Brisswalter J ( 2005): Influence of exercise duration and hydration status on cognitive function during prolonged cycling exercise. Int J Sports Med 26: 27–33. [DOI] [PubMed] [Google Scholar]
- Gullans SR, Verbalis JG ( 1993): Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu Rev Med 44: 289–301. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Ettinger U, Schmechtig A, Winter EM, Smith L, McMorris T, Wilkinson ID, Williams SC, Smith MS ( 2009): Effects of acute dehydration on brain morphology in healthy humans. Hum Brain Mapp 30: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenefick RW, Sawka MN ( 2007): Hydration at the work site. J Am Coll Nutr 26( 5 Suppl): 597S–603S. [DOI] [PubMed] [Google Scholar]
- Lieberman HR ( 2007): Hydration and cognition: A critical review and recommendations for future research. J Am Coll Nutr 26( 5 Suppl): 555S–561S. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- McMorris T, Swain J, Smith M, Corbett J, Delves S, Sale C, Harris RC, Potter J ( 2006): Heat stress, plasma concentrations of adrenaline, noradrenaline, 5‐hydroxytryptamine and cortisol, mood state and cognitive performance. Int J Psychophysiol 61: 204–215. [DOI] [PubMed] [Google Scholar]
- Molloy CJ, Gandy J, Cunningham C, Slattery G ( 2008): An exploration of factors that influence the regular consumption of water by Irish primary school children. J Hum Nutr Diet 21: 512–515. [DOI] [PubMed] [Google Scholar]
- Negoianu D, Goldfarb S ( 2008): Just add water. J Am Soc Nephrol 19: 1041–1043. [DOI] [PubMed] [Google Scholar]
- Norris H ( 1971): The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology 10: 181–191. [DOI] [PubMed] [Google Scholar]
- Patel AV, Mihalik JP, Notebaert AJ, Guskiewicz KM, Prentice WE ( 2007): Neuropsychological performance, postural stability, and symptoms after dehydration. J Athl Train 42: 66–75. [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M ( 2000): Stereotaxic display of brain lesions. Behav Neurol 12: 191–200. [DOI] [PubMed] [Google Scholar]
- Smith MS ( 1998): Sport specific ergometry and the physiological demands of amateur boxing. PhD Thesis, Southampton University.
- Suhr JA, Hall J, Patterson SM, Niinisto RT ( 2004): The relation of hydration status to cognitive performance in healthy older adults. Int J Psychophysiol 53: 121–125. [DOI] [PubMed] [Google Scholar]
- Szinnai G, Schachinger H, Arnaud MJ, Linder L, Keller U ( 2005): Effect of water deprivation on cognitive‐motor performance in healthy men and women. Am J Physiol Regul Integr Comp Physiol 289: R275–R280. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): A Co‐planar Stereotactic Atlas of a Human Brain. Stuttgart: Thieme. [Google Scholar]
- Tomporowski PD, Beasman K, Ganio MS, Cureton K ( 2007): Effects of dehydration and fluid ingestion on cognition. Int J Sports Med 28: 891–896. [DOI] [PubMed] [Google Scholar]
- Valtin H ( 2002): “Drink at least eight glasses of water a day.” Really? Is there scientific evidence for “8 × 8”? Am J Physiol Regul Integr Comp Physiol 283: R993–R1004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fernandez‐Seara MA, Alsop DC, Liu WC, Benasich A, Wang J, Detre JA, CASL perfusion MRI in normal infant brain development. Proceedings of ISMRM . ( 2006): Abstract 3403.
- Williams‐Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA ( 2007): Catechol O‐methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci 27: 4832–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MM, Morley JE ( 2003): Impaired cognitive function and mental performance in mild dehydration. Eur J Clin Nutr 57( Suppl 2): S24–S29. [DOI] [PubMed] [Google Scholar]


