Abstract
The cerebral serotonin (5‐HT) system is involved in cognitive functions such as memory and learning and animal studies have repeatedly shown that stimulation of the 5‐HT type 4 receptor (5‐HT4R) facilitates memory and learning and further that the 5‐HT4R modulates cellular memory processes in hippocampus. However, any associations between memory functions and the expression of the 5‐HT4R in the human hippocampus have not been investigated. Using positron emission tomography with the tracer [11C]SB207145 and Reys Auditory Verbal Learning Test we aimed to examine the individual variation of the 5‐HT4R binding in hippocampus in relation to memory acquisition and consolidation in healthy young volunteers. We found significant, negative associations between the immediate recall scores and left and right hippocampal BPND, (p = 0.009 and p = 0.010 respectively) and between the right hippocampal BPND and delayed recall (p = 0.014). These findings provide evidence that the 5‐HT4R is associated with memory functions in the human hippocampus and potentially pharmacological stimulation of the receptor may improve episodic memory. Hum Brain Mapp 34:3066–3074, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: cerebral 5‐HT4 receptor, memory, hippocampus, Positron Emission Tomography, Reys Auditory Verbal Learning Test, human experimentation
INTRODUCTION
Learning and memory is essential for normal human behavior and disabilities in these cognitive functions are characteristic for a variety of psychiatric and neurologic diseases such as addiction, anxiety, depression, schizophrenia, and neurodegenerative diseases. Current treatments of memory and learning deficits are far from optimal and substantial effort is undertaken to find therapeutic approaches for example for preventing Alzheimer's Dementia [Frautschy and Cole, 2010]. Because of its implications in memory and learning [Schmitt et al. 2006] one of the potential therapeutic targets is the serotonin (5‐HT) system. Pharmacological intervention influencing the serotonin receptors can potentially improve memory and learning disabilities [Buhot et al., 2000; Cifariello et al., 2008; Perez‐Garcia and Meneses, 2008].
Numerous animal studies have repeatedly shown that stimulation of the 5‐HT type 4 receptor (5‐HT4R) facilitates memory and learning, and accordingly the receptor is an interesting pharmacologic target for treatment of memory deficits [Bockaert et al., 2004]. Pre‐task systemic injections in animals of the relatively nonselective 5‐HT4R agonists BIMU‐1 and BIMU‐8 or the more selective partial agonists such as RS17017 and RS6733 improved performance in a variety of memory tasks such as olfactory associative learning [Marchetti et al., 2004, 2000; Marchetti‐Gauthier et al., 1997], social memory [Letty et al., 1997], autoshaping [Meneses and Hong, 1997], place and object recognition [Lamirault and Simon, 2001], the Morris water maze [Lelong et al., 2001], and matching‐to‐sample [Terry et al., 1998]. Furthermore, the new highly selective and potent partial 5‐HT4R agonist, VRX‐03011, also enhanced memory in the delayed spontaneous alternation task [Mohler et al., 2007]. In all these studies the agonists were injected immediately before the memory task and therefore 5‐HT4R agonism seems to improve memory acquisition. The evidence for the 5‐HT4R's involvement in memory consolidation is less clear: For example, post‐task injection of a 5‐HT4R agonist impaired performance in an autoshaping task [Meneses and Hong, 1997], but improved performance in aged rats in an object recognition task [Lamirault and Simon, 2001]. In addition, administration of the 5‐HT4R antagonists SDZ205557 and GR125487 immediately after termination of the training session weakened passive avoidance memory [Galeotti et al., 1998]. With the radioligand [11C]SB207145 and positron emission tomography (PET) it has recently become possible to visualize and quantify the human cerebral 5‐HT4R in vivo [Marner et al., 2009].
Episodic memory is the memory for events and experiences and involves three cognitive processes; encoding, consolidation, and retrieval [Lezak et al., 2004]. During encoding, the new information presented is acquired and learned. Consolidation is the processing of encoded information into long‐term storage for later retrieval. The hippocampus is a crucial structure for both memory encoding and consolidation [Cipolotti and Bird, 2006; Desgranges et al., 1998]. In functional neuroimaging studies of humans, hippocampal activations are consistently observed during both memory encoding and retrieval [Nyberg et al., 2000]. The 5‐HT4R has a relatively high hippocampal density [Marner et al., 2010; Waeber et al., 1996] and plays a key role as a modulator of cellular memory processes in the hippocampus: 5‐HT4R agonists modulate long‐term potentiation and long‐term depression [Kemp and Manahan‐Vaughan, 2002, 2004; Marchetti et al., 2004] and further facilitates the neuronal excitability of pyramidal cells through cAMP mediated closure of potassium channels and regulation of calcium release [Andrade and Chaput, 1991; Fagni et al., 1992; Mlinar et al., 2006; Torres et al., 1996].
Episodic memory functions in humans can be measured with verbal or visual memory tasks. In this study we used Reys Auditory Verbal Learning Test and Rey‐Osterrieth's Complex Figure Test, to examine episodic memory function in relation to the individual variation of the in vivo 5‐HT4R binding in hippocampus in healthy young volunteers. On the basis of the experimental studies mentioned above, we hypothesized that the level of 5‐HT4Rs would be positively correlated with the immediate and delayed recall in the memory tasks.
MATERIALS AND METHODS
Participants
Thirty healthy adults (6 females, 24 males) were recruited through newspaper advertisements. Since the memory test scores are reported to decrease with age we only included participants below 45 years of age (mean age 27.2 ± 6.3 (s.d.) years; range, 20.0–44.7 years), to avoid confounding effects of aging. Written informed consent was obtained according to the Declaration of Helsinki II, and the Copenhagen Region Ethics Committee approved the study. Thirteen of the participants had previously been included in other studies of the 5‐HT4R in humans [Madsen et al., 2010a; Marner et al., 2009, 2010].
Exclusion criteria included significant medical history (including obesity), drug or alcohol abuse, psychiatric disorders or head trauma. All participants had a normal neurological examination and an unremarkable magnetic resonance imaging (MRI) scan of the brain. All participants had completed some education after high‐school with a mean of 15.7 ± 2.1 (s.d.) years of education in total. IQ in the cohort was investigated with two subscales (the number series and verbal analogies) of the Intelligenz‐Struktur‐Test 2000 R [Amthauer 2001; Neubauer et al., 2005] and found to be a little over average for the general population (mean 104.4 ± 10.5 (s.d.); range, 90.5–123.5).
Memory Testing
Two experienced neuropsychologists conducted memory testing on a day separate from the day of the PET scan (mean time‐interval: 45.1 ± 37.6 (s.d.) days).
Two memory tests were used: Rey Auditory Verbal Learning Test (RAVLT) to assess episodic verbal memory and Rey‐Osterrieth's Complex Figure Test (ROCFT) to evaluate visual nonverbal memory [Lezak et al., 2004]. In RAVLT, a list of 15 emotionally neutral words was read aloud by the neuropsychologist with a 2‐s interval in the same sequence five times. After each presentation of the list, the participant delivered a free immediate, verbal recall with a time limit of 60‐s. These five presentations were followed by presentation and recall of an interference list and after a 30‐min delay the participant was asked to recall the first list again. To reflect the cognitive process of memory encoding we used the total number of words the individuals acquired during the five first trials (immediate recall, maximum score 75) and to reflect memory consolidation we used the number of recalled words after 30‐min (delayed recall, maximum score 15) [Vakil et al., 2010]. In our set‐up it was not possible to separate the cognitive process of retrieval from encoding and consolidation, since we did not have a recognition trial at the end of the test.
In ROCFT, participants copied a complex geometric figure and then reproduced it from memory after 3‐min and after a delay of 30‐min. A 36‐point scoring system was used to evaluate the reproductions. To measure memory encoding the scores of 3‐min were used (immediate recall) and to measure memory consolidation the delayed scores at 30‐min were chosen (delayed recall). Again, in this test it was not possible to eliminate memory retrieval from memory encoding and consolidation.
PET and MR Imaging
[11C]SB207145 was synthesized using a fully automated radio‐synthesis system as previously described [Gee et al., 2008; Gillings and Larsen, 2005]. Immediately after an intravenous bolus injection of [11C]SB207145, (mean 490.3 ± 143.0 (s.d.) MBq; range, 206–617 MBq) a 120 min dynamic 3D PET scan (6 × 5 s, 10 × 15 s, 4 × 30 s, 5 × 120 s, 5 × 300 s, and 8 × 600 s) was initiated using either an eighteen ring GE‐Advance scanner (GE, Milwaukee, WI) with an approximate in‐plane resolution of 6 mm (n = 13) or a High Resolution Research Tomograph (HRRT) with an approximate in plane resolution of 1.5 mm (n = 17) [Olesen et al., 2009]. The images from the Advance scanner were reconstructed with filtered back projection and corrected for attenuation, dead time and scatter. The HRRT scans were reconstructed using the iterative PSF reconstruction with attenuation map improvements [Comtat 2008; Sureau et al., 2008].
MRI was conducted on a 3T Siemens Magnetom Trio scanner (Erlangen, Germany). High‐resolution 3D T1‐weighted (matrix 256 × 256; 1 × 1 × 1 mm3 voxels) and 2D T2‐weighted sequences were acquired and corrected for spatial distortions and nonuniformity [Jovicich et al., 2006; Sled et al., 1998]. The T1‐weighted brain MRIs were segmented into gray matter, white matter, and cerebrospinal fluid using SPM5 (Wellcome Department of Cognitive Neurology, London, UK) and each voxel was assigned to the tissue class with the highest probability and this segmentation was subsequently used for delineation of the region of interest. The T2 weighted images served for brain masking purposes.
Quantification of Hippocampal 5HT4Rs
The frames of the dynamic PET scan were aligned to correct for head‐motion artifacts of more than 3 mm using the AIR routines (version 5.2.5). A flow‐weighted mean emission image was automatically aligned to the same individuals MRI using either AIR routines (GE Advance scans) or SPM5 (HRRT scans).
The quantitative analysis to obtain the binding potential (BP) of the 5‐HT4R was performed with the simplified reference tissue model (SRTM) [Lammertsma and Hume, 1996] using PMOD (PMOD Inc, Zürich, Switzerland) since it was found to be a reliable and reproducible method for quantification of [11C]SB207145 receptor binding in humans [Marner et al., 2009]. The cerebellum was used as a reference region since blocking with a selective 5‐HT4R compound prior to radiotracer administration did not alter the cerebellar binding [Marner et al., 2009]. The model estimates the nondisplaceable BP (BPND), which is defined as:
where f ND is the free fraction of tracer in nondisplaceable tissue compartment, B max is the receptor concentration, and K d is the equilibrium dissociation constant for the tracer.
Regional Analysis
The regions of interest in the present study were left and right hippocampus. The gray matter tissue concentration of radioactivity in the regions of interest was obtained by automatic delineation on each participants MRI in a user‐independent fashion with the Pvelab software package [Svarer et al., 2005].
Statistics
We modeled the association between the 5‐HT4R binding and memory functions using regression analyses. The four memory test scores used in the models were: (1) RAVLT immediate recall (sum of the five initial trials), (2) the RAVLT delayed recall, (3) the immediate recall in ROCFT, and (4) delayed recall score in ROCFT. These outcomes were one by one compared to the 5‐HT4R BPND in left and right hippocampus. Ceiling effects were found for both RAVLT scores (immediate and delayed recall), which are often observed in young and well‐educated cohorts [Uttl, 2005]. The ceiling effect causes bias in regression parameters in a standard linear regression (under‐estimation of the association between memory score and 5‐HT4R BPND), and to limit this effect we conducted the analyses of RAVLT scores using Tobit regression [Tobin, 1958]. The Tobit model was designed to estimate linear relationships between censored variables. In this study we have a censoring, where individuals with a RAVLT memory score at or above the threshold of 15 words, take on the value of that threshold, so that the true memory score might be equal to the threshold of 15 words, but it might also be higher. In every regression analyses we adjusted for the influence of the type of PET scanner used by including the scanner type as a class variable. Age, gender and IQ were furthermore included in the regression models and eliminated if nonsignificant. Additionally, we used linear regressions to evaluate whether BPND correlated to the nonspecific binding in the brain (computed as the area‐under‐the curve in the reference region) or injected amount of cold dose/kg. Further, we evaluated with linear regressions if BPND or memory scores correlated with gender corrected grey matter volumes. A significance level of P = 0.05 was adopted throughout the analyses, and all tests were two‐tailed. All analyses were carried out in SAS (v. 9.1 SAS Institute Inc.) or R (v. 2.1.1, The R Foundation for Statistical Computing).
Voxel‐Based Analysis
In addition to the regional analyses we performed voxel‐based analyses to reveal effects of interest within the structure of hippocampus. The parametric time‐activity curves from the HRRT scanner and the GE Advance scanner were smoothed with a 6 mm and a 4 mm full‐width‐half‐maximum (FWHM) Gaussian kernel respectively to obtain the same level of noise in the images. To generate parametric images of the BPND in each voxel, we used the basis function implementation of SRTM [Gunn et al., 1997]. Then the single‐subject BPND parametric maps were warped to MNI space within SPM8 (http://www.fil.ion.ucl.ac.uk/spm) using the single‐subject GM segmented MRI and the SPM8 apriori grey.nii image as the template. Final voxel‐size was 2 × 2 × 2 mm2. The parametric images from the GE Advance scanner were additionally smoothed with a 12 mm FWHM Gaussian kernel after normalization. We defined hippocampus using the Wake Forest University Pickatlas (v3.0) [Maldjian et al., 2004, 2003]. A voxel‐wise Tobit regression analysis was conducted with each of RAVLT immediate and delayed recall as dependent variable and 5‐HT4R BPND and scanner type as covariates. We corrected for multiple comparisons using Monte Carlo simulation in 3dClustSim within AFNI (http://afni.nimh.nih/gov/afni). In the hippocampal volume the method yielded a cluster extent significance threshold of k ≥ 30 voxels (P < 0.05).
RESULTS
Our regional Tobit regression analyses of left and right hippocampus revealed significant, negative associations between the RAVLT immediate recall scores and left and right hippocampal BPND, (P = 0.009 and P = 0.010, respectively) (Table 1 and Fig. 1). Correspondingly, the right hippocampal BPND and RAVLT delayed recall showed a significant, negative association (P = 0.014), whereas this relationship was not found for the left hippocampus (P = 0.25) (Table 1 and Fig. 1). The model assumptions of linearity and distribution were found reasonable when assessed by inclusion of polynomial terms of the predictor and by graphical comparison of the Kaplan‐Meier estimator and the model‐specific distribution of the residuals. Linear regression analyses between ROCFT immediate and delayed recall scores and binding potentials in left and right hippocampus were not significant (P > 0.29). Age, gender, and IQ were included in the regression models, but eliminated from the analyses, as they did not contribute significantly to the model (All P‐values > 0.23). The voxel‐level Tobit analyses of associations between left hippocampal BPND and RAVLT immediate recall revealed two inversely associated clusters, which were significant after corrections for multiple comparisons (MNI coordinates −30, −16, −16, z = 3.23, 51 voxels, P < 0.05; MNI coordinates −30, −32, −8, z = 3.72, 31 voxels, P < 0.05) (Fig. 2). We also identified two clusters within the right hippocampus where RAVLT immediate recall was inversely associated with 5‐HT4R binding, however these did not survive correction for multiple comparisons (MNI coordinates 32, −16, −18, z = 3.58, 24 voxels, P > 0.05; MNI coordinates 30, −26, −12, z = 3.36, 14 voxels, P > 0.05). No clusters in left or right hippocampus were associated with RAVLT delayed recall after corrections for multiple comparisons. We found zero positively associated voxels between RAVLT immediate or delayed recall and 5‐HT4R binding.
Table 1.
Results of Tobit regressions (RAVLT) and linear regressions (ROCFT) analyses of left and right hippocampus with the memory scores as outcome variable and 5‐HT4R binding and scanner type as explanatory variables
| Memory score | Region | Estimate (β) ± SE | R 2 | 95% CI | P‐value |
|---|---|---|---|---|---|
| RAVLT immediate recall | Left hippocampus | −37.9 ± 14.5 /BPND | 0.23a | −66.4 to −9.4 | 0.009 |
| Right hippocampus | −37.3 ± 14.5 /BPND | 0.21a | −65.8 to −8.8 | 0.010 | |
| RAVLT delayed recall | Left hippocampus | −4.6 ± 4.0 /BPND | 0.05a | −12.5 to 3.3 | 0.25 |
| Right hippocampus | −9.6 ± 3.9 /BPND | 0.17a | −17.2 to −1.9 | 0.014 | |
| ROCFT immediate recall | Left hippocampus | −1.9 ± 5.4 /BPND | 0.02 | −13.1 to 9.2 | 0.71 |
| Right hippocampus | −3.7 ± 5.7 /BPND | 0.03 | −15.4 to 8.0 | 0.52 | |
| ROCFT delayed recall | Left hippocamp−us | −5.1 ± 5.1 /BPND | 0.05 | −16.0 to 4.9 | 0.29 |
| Right hippocampus | −4.7 ± 5.4 /BPND | 0.04 | −15.8 to 6.3 | 0.39 |
McKelvey‐Zavoina Pseudo‐R 2 defined as ratio of variance of prediction and the latent response [Veall and Zimmermann, 1994].
RAVLT, Reys Auditory Verbal Learning Test; ROCFT, Rey‐Osterrieth's Complex Figure Test; SE, standard error; CI, confidence interval.
Figure 1.
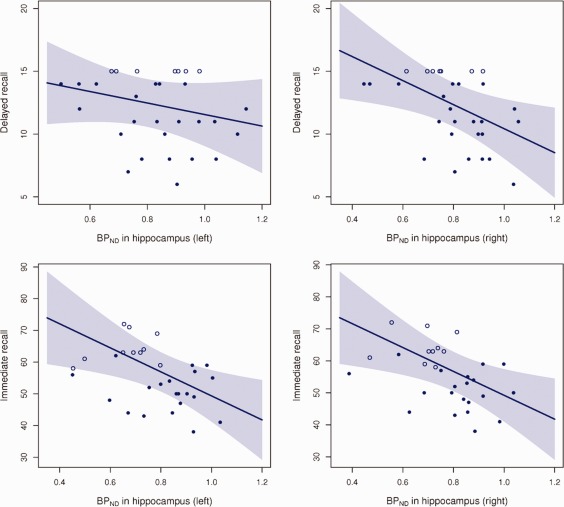
Four plots showing estimated linear associations between RAVLT immediate recall and delayed recall and the left and right regional BPND in hippocampus. BPND values are adjusted for the estimated scanner effect. The shaded region is the pointwise 95% confidence limits. The censored observations—the unfilled circles—all scored 15 in the delayed recall or in one or more of the five immediate recall sessions.
Figure 2.
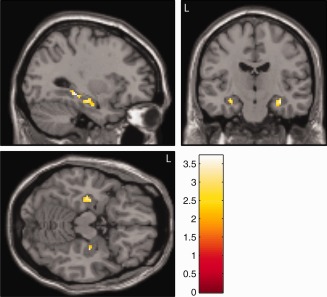
Statistical parametric map overlaid on a T1‐weighted MR image showing the negative associations between 5HT4R BPND and RAVLT immediate recall in hippocampus (P < 0.05). Two clusters in the left hippocampus were significant after corrections for multiple comparisons while two clusters within the right hippocampus were below the cluster extent threshold. The color bar depicts the T values. MNI coordinates for the sagittal image is X = −30, the coronal image is Y = −16 and the axial image is Z = −16.
Since the BPND value of the hippocampus was associated with verbal but not nonverbal memory performance, we examined the interrelationships between these two kinds of memory test scores. In a univariate linear regression immediate and delayed recall scores were strongly correlated in both RAVLT (P < 0.001) and ROCFT (P < 0.001). Further, we observed strong correlations between verbal and nonverbal immediate recall scores (P < 0.001) as well as verbal and nonverbal delayed recall scores (P < 0.001).
We did not see any significant correlation between (1) gray matter volumes and BPND or memory scores or (2) BPND and nonspecific binding or cold dose/kg injected (All P‐values > 0.26).
DISCUSSION
This is the first human in vivo study to examine relationships between 5‐HT4R‐binding and memory functions. In 30 young and healthy individuals we found a significant negative correlation of the 5‐HT4R expression in left and right hippocampus and immediate recall and further a significant negative correlation between the right hippocampal 5‐HT4R expression and delayed recall. Our results suggest that the 5‐HT4R in humans is involved in memory encoding and consolidation and that fewer hippocampal 5‐HT4Rs are representative of a better episodic memory function. As receptor stimulation according to the animal literature generally has a facilitatory effect on memory acquisition and as receptor expression is augmented when rats undergo memory training [Manuel‐Apolinar et al., 2005], the result does not call for a straightforward interpretation. However, most of the animal studies examined functional effects of agonism or antagonism of the receptor, which could potentially explain the difference between the present clinical study and the experimental studies.
As shown in the methods section the binding potential of the 5‐HT4R is a composite measure of receptor expression and affinity [Lammertsma and Hume, 1996]. Since [11C]SB207145 has not been found sensitive to acute endogenous serotonin release [Marner et al., 2010] and since the affinity is assumed to be equivalent in hippocampus and the reference region, the BPND in this study is proportional to the receptor density in hippocampus. The 5‐HT4R is expressed in hippocampus in central locations, where both intrinsic hippocampal and extrinsic cortical and subcortical circuits are modified such as the CA1, the dentate gyrus and the subiculum [King et al., 2008]. The precise hippocampal neuronal localization has not yet been specified, but lesion studies indicate that the receptor is present on glutamatergic neurons [Vilaro et al., 2005]. Furthermore, stimulation of the 5‐HT4R leads to modulation of the acetylcholine and GABA release in hippocampus, so the receptor is likely to be localized on these neurons, even though indirect effects cannot be excluded [Bianchi et al., 2002; Bockaert et al., 2006; Matsumoto et al., 2001]. As the in vivo binding of [11C]SB207145 cannot differentiate the type of neurons or the specific location of the 5‐HT4R within hippocampus, a low receptor density in hippocampus cannot simply be linked to low neuronal activity and functions. Further, stimulation of the receptor is also not in a simple way linked to better memory. For example, in healthy volunteers a low dose of selective serotonin reuptake inhibitors (SSRI) improved short‐term memory, while a high dose impaired it [Dumont et al., 2005] and, as mentioned in the introduction, 5‐HT4R agonists impaired memory function in young rats while it improved memory functions in old rats [Lamirault and Simon, 2001]. Therefore, various explanations of the results in this study can be proposed. Acute tryptophan depletion impairs episodic memory [Mendelsohn et al., 2009; Sambeth et al., 2007], and therefore efficient serotonergic innervation and adequate 5‐HT interstitial levels in hippocampus seem important for memory functions. Since 5‐HT4R binding was found to be down‐regulated after chronic SSRI treatment [Licht et al., 2009; Vidal et al., 2009], high serotonin levels in hippocampus may reduce the levels of the 5‐HT4R. This might explain the paradoxical finding that the participants in this study with a high memory performance have lower levels of the receptor. On the other hand, since subchronic serotonin depletion was found to cause an up‐regulation of the 5‐HT4R [Licht et al., 2009] subjects with an inefficient memory function may improve the functioning of the serotonin system by up‐regulating the 5‐HT4R, that both may improve memory directly and indirectly by stimulating 5‐HT release in hippocampus [Ge and Barnes 1996; Licht et al., 2010]. However, hypothetically this up‐regulation is insufficient to boost memory function to the level of the better performing subjects. This is in line with a recent study of Alzheimer's disease from our group, where accumulation of β‐amyloid and thereby possible decline of interstitial serotonin levels was associated with an up‐regulation of the 5‐HT4Rs [Madsen et al., 2011]. In this context we also speculate that the relation between the serotonin system and memory may be inversely u‐shaped as it is assumed for dopaminergic modulation of cognitive functions [Cools and D'Esposito, 2011]: both low and high 5‐HT4R binding may reflect levels of serotonin outside the optimal range for memory function. However, this study lacks variation in memory scores, with few low scoring subjects, to find evidence for this hypothesis.
We previously investigated the relation between the RAVLT memory scores and the serotonin transporter (SERT) and found no correlation [Madsen et al., 2011]. The reason for these differing results may be that SERT is considered to be a marker of general serotonergic innervation with complex postsynaptic actions, while the 5‐HT4R is likely to be directly involved in memory function as previously show in the introduction.
It was clear from the voxel‐based analysis within hippocampus that the association between memory functions and receptor expression was found both in ventral and dorsal hippocampus, which have been assigned to dissimilar functional roles: Dorsal hippocampus is mainly participating in cognitive functions while ventral hippocampus is mainly involved in emotions, stress and affect [Fanselow and Dong, 2010]. This is in line with the roles of the 5‐HT4R in the brain. As shown both from the previous animal literature and this study the receptor is likely to play a role in memory and learning processes, however, the receptor is also likely to be involved in emotional processes as studies has linked the receptor to regulation of the hedonic part of food intake [Compan et al., 2010; Francis et al., 2011; Jean et al., 2007] and emotional processes such as stress [Compan et al., 2004].
It was evident from the overlapping confidence intervals (Table 1) that the difference between left and right binding potentials would not statistically reach a 5% significance level. However, the RAVLT delayed recall scores were only significant in the right hippocampus. This finding could be due to differences in cognitive processes in the left and right hippocampus as some studies suggest that encoding processes may be left‐lateralized and recall processes right‐lateralized [Desgranges et al., 1998]. Another explanation may be that the 5‐HT4Rs differ in functionality with regard to memory encoding and consolidation as the previously mentioned animal literature suggest. This may result in differential regulation of the receptor dependent on the cognitive process.
In contrast to the verbal memory performance we did not find significant correlations between the nonverbal memory task and hippocampal 5‐HT4Rs even though the memory tests correlated strongly. These results could be explained by the specific functions of the 5‐HT4R. It has been shown that neuronal networks of encoding and retrieval can be distinguished by their reaction to specific stimuli (e.g., verbal vs. visual) even though interactions between the systems are evident [Nyberg et al., 2000]. Our results suggest that the 5‐HT4R primarily affects the verbal memory network and not the visual. It is not possible to confirm this result in the existing literature, as 5‐HT4R agonism seems to improve all the various animal memory tests, at least in the acquisition phase. However, in animals it is difficult to precisely imitate the visual and verbal memory tasks used in humans, as species differences may be considerable. Thus, rather than drawing any specific conclusions, we recommend that future human studies seek to clarify these associations between serotonergic tone, 5‐HT4Rs and verbal and visual memory.
One should consider the limitations of this study. We found ceiling effects in RAVLT, which in young and well‐educated cohorts is a prevalent finding with the applied testing procedures [Uttl, 2005; Van der Elst et al., 2005]. We corrected for this bias by using a censored regression model to avoid inconsistent estimates present in an ordinary linear regression in these kinds of data. However, if the data was analyzed using an ordinary linear regression model, we still found significant correlations between the immediate recall scores and the 5‐HT4R levels in left hippocampus (P = 0.04) and right hippocampus (P = 0.02) and between RAVLT delayed scored and the receptor level in right hippocampus (P = 0.03). Another issue was that the subjects were scanned on two different PET scanners. We compensated for this by including scanner type in the regressions models, but it is not possible to exclude that some scanner effects were still present. However, we would point out that scanner type was insignificant in the regression models in all analyses and we did not observe any tendencies toward main effects of scanner type on immediate or delayed memory scores.
In conclusion, this is the first study in humans to examine the association between the 5‐HT4R in hippocampus and memory functions. The study provides evidence that the 5‐HT4R is associated with memory functions in hippocampus and supports an association between 5‐HT4R and the previously reported asymmetry of hippocampal function in memory. Since 5‐HT4R stimulation generally enhances memory performance across various domains in animals, it was an unexpected finding that the 5HT4R level correlated negatively with measures of memory function. This finding may be explained by the complex interactions between the intrinsic serotonergic tonus and receptor functions in the hippocampus. Further studies in humans are needed to elucidate the functional significance of this study; however, we speculate that stimulation of the human 5‐HT4R could improve memory functions.
REFERENCES
- Amthauer R (2001): Intelligenz‐Struktur‐Test 2000. R. Hogrefe. [Google Scholar]
- Andrade R, Chaput Y (1991): 5‐Hydroxytryptamine4‐like receptors mediate the slow excitatory response to serotonin in the rat hippocampus. J Pharmacol Exp Ther 257:930–937. [PubMed] [Google Scholar]
- Bianchi C, Rodi D, Marino S, Beani L, Siniscalchi A (2002): Dual effects of 5‐HT4 receptor activation on GABA release from guinea pig hippocampal slices. Neuroreport 13:2177–2180. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P (2006): Neuronal 5‐HT metabotropic receptors: Fine‐tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res 326:553–572. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Compan V, Dumuis A (2004): 5‐HT4 receptors. Curr Drug Targets CNS Neurol Disord 3:39–51. [DOI] [PubMed] [Google Scholar]
- Buhot MC, Martin S, Segu L (2000): Role of serotonin in memory impairment. Ann Med 32:210–221. [DOI] [PubMed] [Google Scholar]
- Cifariello A, Pompili A, Gasbarri A (2008): 5‐HT(7) receptors in the modulation of cognitive processes. Behav Brain Res 195:171–179. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Bird CM (2006): Amnesia and the hippocampus. Curr Opin Neurol 19:593–598. [DOI] [PubMed] [Google Scholar]
- Compan V, Charnay Y, Dusticier N, Daszuta A, Hen R, Bockaert J (2004): [Feeding disorders in 5‐HT4 receptor knockout mice]. J Soc Biol 198:37–49. [PubMed] [Google Scholar]
- Compan V, Jean A, Laurent L, Aribo O, Malapris C, Dantec C, Barrot M, Neve RL, Dusticier N, Nieoullon A, Hen R, Bockaert J (2010): Anorexia coexists with psychoactive and rewarding effects when meidated by serotonin 4 receptors in the nucleus accembens (abstract). Society for Neuroscience, Annual Meeting, San Diego, California Online:299.2/JJJ32.
- Comtat C (2008): Image based resolution modeling for the HRRT OSEM reconstructions software. IEEE MIC Conf. Rec, Dresden. [Google Scholar]
- Cools R, D'Esposito M (2011): Inverted‐U‐shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69:e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, Eustache F (1998): The functional neuroanatomy of episodic memory: The role of the frontal lobes, the hippocampal formation, and other areas. Neuroimage 8:198–213. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, de Visser SJ, Cohen AF, van Gerven JM (2005): Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. Br J Clin Pharmacol 59:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L, Dumuis A, Sebben M, Bockaert J (1992): The 5‐HT4 receptor subtype inhibits K+ current in colliculi neurones via activation of a cyclic AMP‐dependent protein kinase. Br J Pharmacol 105:973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW (2010): Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis HM, Kraushaar NJ, Hunt LR, Cornish JL (2011): Serotonin 5‐HT4 receptors in the nucleus accumbens are specifically involved in the appetite suppressant and not locomotor stimulant effects of MDMA (‘ecstasy’). Psychopharmacology (Berl) 213:355–363. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Cole GM (2010): Why pleiotropic interventions are needed for Alzheimer's disease. Mol Neurobiol 41( 2–3):392–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C, Bartolini A (1998): Role of 5‐HT4 receptors in the mouse passive avoidance test. J Pharmacol Exp Ther 286:1115–1121. [PubMed] [Google Scholar]
- Ge J, Barnes NM (1996): 5‐HT4 receptor‐mediated modulation of 5‐HT release in the rat hippocampus in vivo. Br J Pharmacol 117:1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee AD, Martarello L, Passchier M (2008): Synthesis and evaluation of [11C]SB207145 as the first in vivo serotonin 5‐HT4 receptor radioligand for PET imaging in man. Curr Radiopharma 110–114. [Google Scholar]
- Gillings N, Larsen P (2005): A highly flexible modular radiochemistry system [abstract]. J Label Comp Radiopharm 48 (Suppl):338. [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ (1997): Parametric imaging of ligand‐receptor binding in PET using a simplified reference region model. Neuroimage 6:279–287. [DOI] [PubMed] [Google Scholar]
- Jean A, Conductier G, Manrique C, Bouras C, Berta P, Hen R, Charnay Y, Bockaert J, Compan V (2007): Anorexia induced by activation of serotonin 5‐HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci USA 104:16335–16340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A (2006): Reliability in multi‐site structural MRI studies: Effects of gradient non‐linearity correction on phantom and human data. Neuroimage 30:436–443. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan‐Vaughan D (2004): Hippocampal long‐term depression and long‐term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci USA 101:8192–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MV, Marsden CA, Fone KC (2008): A role for the 5‐HT(1A), 5‐HT4 and 5‐HT6 receptors in learning and memory. Trends Pharmacol Sci 29:482–492. [DOI] [PubMed] [Google Scholar]
- Kulla A, Manahan‐Vaughan D (2002): Modulation by serotonin 5‐HT(4) receptors of long‐term potentiation and depotentiation in the dentate gyrus of freely moving rats. Cereb Cortex 12:150–162. [DOI] [PubMed] [Google Scholar]
- Lamirault L, Simon H (2001): Enhancement of place and object recognition memory in young adult and old rats by RS 67333, a partial agonist of 5‐HT4 receptors. Neuropharmacology 41:844–853. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP (1996): Simplified reference tissue model for PET receptor studies. Neuroimage 4( Part 1):153–158. [DOI] [PubMed] [Google Scholar]
- Lelong V, Dauphin F, Boulouard M (2001): RS 67333 and D‐cycloserine accelerate learning acquisition in the rat. Neuropharmacology 41:517–522. [DOI] [PubMed] [Google Scholar]
- Letty S, Child R, Dumuis A, Pantaloni A, Bockaert J, Rondouin G (1997): 5‐HT4 receptors improve social olfactory memory in the rat. Neuropharmacology 36( 4–5):681–687. [DOI] [PubMed] [Google Scholar]
- Lezak M, Howieson DB, Loring DW, Hannay HJ, Fischer JS (2004): Neuropsychological Assessment. Oxford: Oxford University Press. [Google Scholar]
- Licht CL, Knudsen GM, Sharp T (2010): Effects of the 5‐HT(4) receptor agonist RS67333 and paroxetine on hippocampal extracellular 5‐HT levels. Neurosci Lett 476:58–61. [DOI] [PubMed] [Google Scholar]
- Licht CL, Marcussen AB, Wegener G, Overstreet DH, Aznar S, Knudsen GM (2009): The brain 5‐HT4 receptor binding is down‐regulated in the Flinders Sensitive Line depression model and in response to paroxetine administration. J Neurochem 109:1363–1374. [DOI] [PubMed] [Google Scholar]
- Madsen K, Marner L, Haahr M, Gillings N, Knudsen GM (2010a): Age and sex effects on 5‐HT4 receptors in the human brain—A [11C]SB207145 PET study. J Cerebral Blood Flow Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen K, Neumann WJ, Holst K, Marner L, Haahr MT, Lehel S, Knudsen GM, Hasselbalch SG (2011): Cerebral serotonin 4 receptors and amyloid‐beta in early Alzheimer's disease. J Alzheimers Dis 26:457–466. [DOI] [PubMed] [Google Scholar]
- Madsen K, Erritzoe D, Mortensen EL, Gade A, Madsen J, Baare W, Knudsen GM, Hasselbalch SG (2011): Cognitive function is related to fronto‐striatal serotonin transporter levels—A brain PET study in young healthy subjects. Psychopharmacology (Berl) 213( 2–3):573–581. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH (2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21:450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Manuel‐Apolinar L, Rocha L, Pascoe D, Castillo E, Castillo C, Meneses A (2005): Modifications of 5‐HT4 receptor expression in rat brain during memory consolidation. Brain Res 1042:73–81. [DOI] [PubMed] [Google Scholar]
- Marchetti E, Chaillan FA, Dumuis A, Bockaert J, Soumireu‐Mourat B, Roman FS (2004): Modulation of memory processes and cellular excitability in the dentate gyrus of freely moving rats by a 5‐HT4 receptors partial agonist, and an antagonist. Neuropharmacology 47:1021–1035. [DOI] [PubMed] [Google Scholar]
- Marchetti E, Dumuis A, Bockaert J, Soumireu‐Mourat B, Roman FS (2000): Differential modulation of the 5‐HT(4) receptor agonists and antagonist on rat learning and memory. Neuropharmacology 39:2017–2027. [DOI] [PubMed] [Google Scholar]
- Marchetti‐Gauthier E, Roman FS, Dumuis A, Bockaert J, Soumireu‐Mourat B (1997): BIMU1 increases associative memory in rats by activating 5‐HT4 receptors. Neuropharmacology 36( 4–5):697–706. [DOI] [PubMed] [Google Scholar]
- Marner L, Gillings N, Comley RA, Baare WF, Rabiner EA, Wilson AA, Houle S, Hasselbalch SG, Svarer C, Gunn RN, Laruelle M, Knudsen GM (2009): Kinetic modeling of 11C‐SB207145 binding to 5‐HT4 receptors in the human brain in vivo. J Nucl Med 50:900–908.2009. [DOI] [PubMed] [Google Scholar]
- Marner L, Gillings N, Madsen K, Erritzoe D, Baare WF, Svarer C, Hasselbalch SG, Knudsen GM (2010): Brain imaging of serotonin 4 receptors in humans with [11C]SB207145‐PET. Neuroimage 50:855–861. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Togashi H, Mori K, Ueno K, Ohashi S, Kojima T, Yoshioka M (2001): Evidence for involvement of central 5‐HT(4) receptors in cholinergic function associated with cognitive processes: Behavioral, electrophysiological, and neurochemical studies. J Pharmacol Exp Ther 296:676–682. [PubMed] [Google Scholar]
- Mendelsohn D, Riedel WJ, Sambeth A (2009): Effects of acute tryptophan depletion on memory, attention and executive functions: A systematic review. Neurosci Biobehav Rev 33:926–952. [DOI] [PubMed] [Google Scholar]
- Meneses A, Hong E (1997): Effects of 5‐HT4 receptor agonists and antagonists in learning. Pharmacol Biochem Behav 56:347–351. [DOI] [PubMed] [Google Scholar]
- Mlinar B, Mascalchi S, Mannaioni G, Morini R, Corradetti R (2006): 5‐HT4 receptor activation induces long‐lasting EPSP‐spike potentiation in CA1 pyramidal neurons. Eur J Neurosci 24:719–731. [DOI] [PubMed] [Google Scholar]
- Mohler EG, Shacham S, Noiman S, Lezoualc'h F, Robert S, Gastineau M, Rutkowski J, Marantz Y, Dumuis A, Bockaert J, Gold PE, Ragozzino ME (2007): VRX‐03011, a novel 5‐HT4 agonist, enhances memory and hippocampal acetylcholine efflux. Neuropharmacology 53:563–573. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Grabner RH, Fink A, Neuper C (2005): Intelligence and neural efficiency: Further evidence of the influence of task content and sex on the brain‐IQ relationship. Brain Res Cogn Brain Res 25:217–225. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Persson J, Habib R, Tulving E, McIntosh AR, Cabeza R, Houle S (2000): Large scale neurocognitive networks underlying episodic memory. J Cogn Neurosci 12:163–173. [DOI] [PubMed] [Google Scholar]
- Olesen O, Sibomana M, Keller S, Andersen F, Jensen J, Holm S, Svarer C, H⊘jgaard L (2009): Spatial resolution of the HRRT PET scanner using 3D‐OSEM PSF reconstruction. IEEE MIC Record, Orlando. [Google Scholar]
- Perez‐Garcia G, Meneses A (2008): Memory formation, amnesia, improved memory and reversed amnesia: 5‐HT role. Behav Brain Res 195:17–29. [DOI] [PubMed] [Google Scholar]
- Sambeth A, Blokland A, Harmer CJ, Kilkens TO, Nathan PJ, Porter RJ, Schmitt JA, Scholtissen B, Sobczak S, Young AH, Riedel WJ (2007): Sex differences in the effect of acute tryptophan depletion on declarative episodic memory: A pooled analysis of nine studies. Neurosci Biobehav Rev 31:516–529. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Wingen M, Ramaekers JG, Evers EA, Riedel WJ (2006): Serotonin and human cognitive performance. Curr Pharm Des 12:2473–2486. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97. [DOI] [PubMed] [Google Scholar]
- Sureau FC, Reader AJ, Comtat C, Leroy C, Ribeiro MJ, Buvat I, Trebossen R (2008): Impact of image‐space resolution modeling for studies with the high‐resolution research tomograph. J Nucl Med 49:1000–1008. [DOI] [PubMed] [Google Scholar]
- Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbol S, Frokjaer VG, Holm S, Paulson OB, Knudsen GM (2005): MR‐based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage 24:969–979. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Buccafusco JJ, Jackson WJ, Prendergast MA, Fontana DJ, Wong EH, Bonhaus DW, Weller P, Eglen RM (1998): Enhanced delayed matching performance in younger and older macaques administered the 5‐HT4 receptor agonist, RS 17017. Psychopharmacology (Berl) 135:407–415. [DOI] [PubMed] [Google Scholar]
- Tobin J (1958): Estimation of relationships for limited dependent variables. Econometrica 26:24–36. [Google Scholar]
- Torres GE, Arfken CL, Andrade R (1996): 5‐Hydroxytryptamine4 receptors reduce afterhyperpolarization in hippocampus by inhibiting calcium‐induced calcium release. Mol Pharmacol 50:1316–1322. [PubMed] [Google Scholar]
- Uttl B (2005): Measurement of individual differences: Lessons from memory assessment in research and clinical practice. Psychol Sci 16:460–467. [DOI] [PubMed] [Google Scholar]
- Vakil E, Greenstein Y, Blachstein H (2010): Normative data for composite scores for children and adults derived from the Rey Auditory Verbal Learning Test. Clin Neuropsychol 24:662–677. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J (2005): Rey's verbal learning test: Normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc 11:290–302. [DOI] [PubMed] [Google Scholar]
- Veall MT, Zimmermann KF (1994): Goodness of fit measures in the Tobit Model. Oxford Bull Econ Stat 56:485–499. [Google Scholar]
- Vidal R, Valdizan EM, Mostany R, Pazos A, Castro E (2009): Long‐term treatment with fluoxetine induces desensitization of 5‐HT4 receptor‐dependent signalling and functionality in rat brain. J Neurochem 110:1120–1127. [DOI] [PubMed] [Google Scholar]
- Vilaro MT, Cortes R, Mengod G (2005): Serotonin 5‐HT4 receptors and their mRNAs in rat and guinea pig brain: Distribution and effects of neurotoxic lesions. J Comp Neurol 484:418–439. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Bockaert J, Dumuis A (1996): Regional distribution and ontogeny of 5‐HT4 binding sites in rat brain. Behav Brain Res 73( 1–2):259–262. [DOI] [PubMed] [Google Scholar]


