Abstract
TRACK‐HD is a multicentre longitudinal observational study investigating the use of clinical assessments and 3‐Tesla magnetic resonance imaging as potential biomarkers for future therapeutic trials in Huntington's disease (HD). The cross‐sectional data from this large well‐characterized dataset provide the opportunity to improve our knowledge of how the underlying neuropathology of HD may contribute to the clinical manifestations of the disease across the spectrum of premanifest (PreHD) and early HD. Two hundred and thirty nine gene‐positive subjects (120 PreHD and 119 early HD) from the TRACK‐HD study were included. Using voxel‐based morphometry (VBM), grey and white matter volumes were correlated with performance in four domains: quantitative motor (tongue force, metronome tapping, and gait); oculomotor [anti‐saccade error rate (ASE)]; cognition (negative emotion recognition, spot the change and the University of Pennsylvania smell identification test) and neuropsychiatric measures (apathy, affect and irritability). After adjusting for estimated disease severity, regionally specific associations between structural loss and task performance were found (familywise error corrected, P < 0.05); impairment in tongue force, metronome tapping and ASE were all associated with striatal loss. Additionally, tongue force deficits and ASE were associated with volume reduction in the occipital lobe. Impaired recognition of negative emotions was associated with volumetric reductions in the precuneus and cuneus. Our study reveals specific associations between atrophy and decline in a range of clinical modalities, demonstrating the utility of VBM correlation analysis for investigating these relationships in HD. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: TRACK‐HD, magnetic resonance imaging, voxel‐based morphometry
INTRODUCTION
Huntington's disease (HD) is an autosomal dominant neurodegenerative disorder caused by a CAG repeat expansion on chromosome 4 [The Huntington's Disease Collaborative Research Group, 1993] and consequently can be identified before symptom onset by predictive genetic testing. The clinical characteristics are progressive motor dysfunction, cognitive decline and psychiatric disturbance. Formal diagnosis is based on the presence of unequivocal and otherwise unexplained movement disorder [Huntington's Disease Study Group, 1996].
There is now a wealth of evidence showing subtle functional deficits occur many years prior to clinical diagnosis. Recent studies describe impairment in motor and cognitive function up to 15 years before predicted disease onset [Biglan et al., 2009; Duff et al., 2010; Paulsen et al., 2008; Stout et al., 2011; Tabrizi et al., 2009, 2011]. Impaired oculomotor function has been reported in premanifest gene carriers (PreHD) [Blekher et al., 2006; Golding et al., 2006; Hicks et al., 2008; Lasker and Zee, 1997; Tabrizi et al., 2009] as have deficits in emotion recognition [Johnson et al., 2007; Tabrizi et al., 2009] and neuropsychiatric disturbances [Berrios et al., 2002; Julien et al., 2007; Tabrizi et al., 2009]. Structural neuroimaging studies reveal macroscopic effects of the underlying neuropathology with striatal volume loss [Aylward et al., 1997, 2004, 2010; Hobbs et al., 2009; Tabrizi et al., 2009, 2011] and global brain atrophy [Henley et al., 2009; Squitieri et al., 2009; Tabrizi et al., 2009, 2011; Thieben et al., 2002] as well as cortical thinning [Nopoulos et al., 2010; Rosas et al., 2002, 2005; Tabrizi et al., 2009] evident in both manifest and premanifest cohorts. Recent work has demonstrated a close association between structural loss and clinical phenotype [Henley et al., 2008; Rosas et al., 2005, 2008].
Studying incipient functional deficits and the distribution of structural changes in individuals destined to develop HD and in the early manifest stages increases our understanding of how the disease progresses and how underlying neuropathology may contribute to the clinical manifestations of HD. At this early stage deficits are specific and isolated, unlike in the advanced stages where structural loss and functional impairment become increasingly global.
TRACK‐HD is a large prospective multicentre longitudinal observational study, with the aim of identifying potential biomarkers for future use in disease‐modifying clinical trials, which has been described in detail previously [Tabrizi et al., 2009, 2011]. We sought to examine the relationship between these clinical deficits and regional atrophy across the spectrum of premanifest to early Huntington's disease.
METHODS
Subjects
All subjects were recruited as part of the TRACK‐HD study [Tabrizi et al., 2009] which included 120 PreHD and 123 early HD subjects. Assessments were performed at four centres: the Department of Neurology at Leiden University Medical Centre, the National Hospital for Neurology and Neurosurgery, London, the Department of Medical Genetics at the University of British Columbia, Vancouver, and the Department of Genetics and Cytogenetics at the Hôpital de la Salpêtrière‐Université Pierre and Marie Curie, Paris. Clinical assessment was standardized across sites and performed using the Unified Huntington's Disease Rating Scale UHDRS‐99, medical and psychiatric history, current medications, HD history, clinical motor scores, portions of the cognitive component and functional capacity. PreHD subjects required a disease‐burden score of >250 [Penney et al., 1997] and a total motor score of ≤5 in the UHDRS motor assessment indicating lack of significant motor signs. Early HD subjects included HD Stage I and HD Stage II, based on a total functional capacity score of >7 [Shoulson and Fahn, 1979]. The study was approved by the local ethical committees and written informed consent was obtained from each subject according to the Declaration of Helsinki.
Image Acquisition and Processing
The 3T MRI data were acquired according to a standardized T1‐weighted protocol developed for this study [Tabrizi et al., 2009]. Details are provided in the Supporting Information. Four early HD subjects had scans which were unsuitable for VBM analysis due to poor scan quality resulting in 239 gene‐positive subjects (120 PreHD and 119 early HD) being included in the study. Total intracranial volume was calculated using the MIDAS software [Freeborough et al., 1997] following a semiautomated protocol previously described [Whitwell et al., 2001]. VBM was performed using Statistical Parametric Mapping version 5 (http://www.fil.ion.ucl.ac.uk/spm). Initial tissue classification was performed using the unified segmentation process with rigid alignment. DARTEL (diffeomorphic anatomical registration using exponentiated lie algebra) [Ashburner, 2007] was used to create a study‐specific template. The initial tissue segmentations were spatially normalized using high dimensional warping onto the template then modulated to account for volume change during the normalisation process. Because of the improved registration accuracy of DARTEL and to improve anatomical localisation, we smoothed with a relatively small kernel of 4‐mm full width at half maximum. All images were visually inspected blind to diagnosis to ensure segmentation accuracy.
Assessments
A large number of assessments were performed on the TRACK‐HD cohort; here we report on a subset from each of the modalities that showed the most significant crosssectional differences at baseline [Tabrizi et al., 2009]. Additional details on these assessments are provided in the Supporting Information.
Quantitative motor
Isometric force during sustained tongue protrusion was recorded using a force transducer [Reilmann et al., 2010]. The logarithm of the coefficient of variation in tongue force was used in our assessment. The same force transducer system was used for the metronome tapping task, which involved subjects first tapping their index finger in response to tones produced by a metronome and then continuing to tap at this rhythm without further cues [Bechtel et al., 2010]. We measured the precision of the taps produced without cues by the nondominant hand, which was defined as 1/SD of the deviation of taps from the training tap rate (reported in [Tabrizi et al., 2009] and referred to as Delta‐MTI in [Bechtel et al., 2010]). For gait, stride length variability was assessed during normal speed walking using a GAITrite system (CIR Systems, Havertown, PA) [Rao et al., 2008]. The log of the coefficient of variation in stride length was calculated.
Oculomotor
Horizontal eye position was recorded using the Saccadometer Advanced (Ober Consulting, Poznan, Poland) during a random, centrally cued mixed pro/antisaccade task [Hicks et al., 2008] and the rate of errors in antisaccade component was calculated.
Cognitive
Recognition of negative emotions (sadness, anger, fear, or disgust) was assessed using a subset of the Ekman and Friesen facial stimuli [Ekman and Friesen, 1976]. Spot the change was designed to assess visual working memory and involved participants identifying changes in colored squares presented on a computer screen and the number correct was adjusted for guessing. An abbreviated (20‐item) version of the University of Pennsylvania smell identification test (UPSIT; Sensonics, Haddon Heights, NJ), was used to assess recognition of common odors.
Neuropsychiatric
A brief semistructured interview that was a shortened form of the problem behaviors assessment (PBA‐s) [Craufurd et al., 2001] was administered by raters trained to meet reliability thresholds. Apathy, irritability, and affect scores were calculated as the frequency × severity of each symptom over the past month and the square root of the scores was entered into the model.
Statistical Analysis
All gene‐positive carriers (PreHD and early HD) were included in the correlation analysis. For the metronome tapping task, only right‐handed subjects were included. See Supporting Information Table I for details of nonmissing values for each assessment.
Table I.
Subject demographics
| PreHD (n = 120) | Early HD (n = 119) | |
|---|---|---|
| Age (years) | 40.8a (8.9) | 48.5a (9.9) |
| Gender (M:F) | 66:54 | 64:55 |
| CAG length | 43.1 (2.4) | 43.7 (3.0) |
| UHDRS total motor score | 2.5a (1.6) | 23.6a (10.8) |
| Tongue force (log coefficient of variation) | 3.6a (0.5) | 4.4a (0.6) |
| Metronome tapping precision (1/SD deviation from taps) | 32.9a (11.9) | 16.2a (10.2) |
| Gait (log coefficient of variation) | 1.0a (0.3) | 1.3a (0.4) |
| Anti‐saccade error rate (%) | 33.3a (19.6) | 58.0a (25.3) |
| Recognition of negative emotions (no correct/50) | 23.4a (5.7) | 16.7a (6.2) |
| Spot the change 5 K score (no correct, corrected for guessing) | 2.8a (1.3) | 1.6a (1.3) |
| UPSIT (no correct/20) | 16.5a (2.6) | 13.6a (3.2) |
| PBA Apathy Score (frequency × severity) | 0.5a (0.9) | 1.1a (1.2) |
| PBA irritability score (frequency × severity) | 1.5b (1.3) | 2.0b (1.3) |
| PBA affect score (frequency × severity) | 2.0 (1.4) | 2.1 (1.3) |
Mean (standard deviation).
UPSIT = University of Pennsylvania Smell Identification Test; PBA = Problem Behaviours Assessment.
PreHD significantly different from early HD P < 0.0001.
PreHD significantly different from early HD P < 0.001.
Correlations between grey‐matter (GM) or white‐matter (WM) volumes and performance on the various assessments were examined using linear regression models within SPM5. Age, gender, study site, education, total intracranial volume, CAG repeat length and disease‐burden score [Penney et al., 1997] were included as covariates in the model. CAG repeat length and disease‐burden score were included to adjust for overall disease severity to identify more specific relationships between task performance and tissue loss. Age and CAG repeat length are known to influence the onset and trajectory of the disease and disease‐burden score was included to represent an interaction between these two terms.
An explicit binary mask was used for analysis, generated using the optimal thresholding technique described by Ridgway et al. [2009]. Results were corrected for multiple comparisons using familywise error at the P < 0.05 level. Reverse contrasts were examined to investigate whether there was any evidence of greater GM or WM volume associated with task impairment. Anatomical localisation was performed in consultation with a trained neuroradiologist (HH) using xjview (http://www.alivelearn.net/xjview/).
RESULTS
Subject Demographics
The characteristics of the PreHD and early HD cohort are shown in Table I. Details of medication usage are provided in Supporting Information Table II. As expected due to the progressive nature of the disease, the mean ages of these two groups were significantly different (P < 0.001). There were no significant differences in mean CAG repeat length (P = 0.09). With the exception of the affect score (P = 0.38), all assessments showed significant impairment in the early HD compared with the PreHD group (P < 0.001).
Table II.
Anatomical regions of correlations across the whole cohort
| Anatomical location | Cluster size | T score | P value (FWE corrected) | ||
|---|---|---|---|---|---|
| Tongue force | GM | R caudate | 1007 | 7.27 | <0.001 |
| R precentral gyrus | 385 | 6.93 | <0.001 | ||
| L caudate | 738 | 6.72 | <0.001 | ||
| R cuneus | 115 | 6.46 | <0.001 | ||
| R precuneus | 295 | 6.43 | <0.001 | ||
| L precuneus | 90 | 6.09 | <0.001 | ||
| WM | R occipital lobe | 27 | 5.46 | 0.003 | |
| L occipital lobe | 43 | 5.38 | 0.004 | ||
| R internal capsule | 36 | 5.38 | 0.004 | ||
| Splenium corpus callosum | 31 | 5.05 | 0.017 | ||
| L frontal lobe | 3 | 5.03 | 0.018 | ||
| Metronome tapping precision | GM | L putamen | 754 | 6.65 | <0.001 |
| R putamen | 905 | 6.59 | <0.001 | ||
| WM | R internal capsule | 272 | 6.11 | <0.001 | |
| L internal capsule | 435 | 5.92 | <0.001 | ||
| R external capsule | 15 | 5.37 | 0.005 | ||
| Antisaccade error rate | GM | L caudate | 1179 | 7.02 | <0.001 |
| R caudate | 870 | 6.99 | <0.001 | ||
| L lateral occipital gyrus | 12 | 5.26 | 0.017 | ||
| R superior temporal gyrus | 4 | 5.18 | 0.023 | ||
| L putamen | 3 | 5.08 | 0.036 | ||
| R inferior frontal gyrus | 2 | 5.03 | 0.043 | ||
| WM | L external capsule | 78 | 5.98 | <0.001 | |
| R internal capsule | 387 | 5.68 | 0.001 | ||
| L internal capsule | 234 | 5.67 | 0.001 | ||
| R occipital lobe | 71 | 5.54 | 0.002 | ||
| L parietal lobe | 68 | 5.34 | 0.005 | ||
| R frontal lobe | 23 | 5.15 | 0.011 | ||
| Recognition of negative emotions | GM | L precentral gyrus | 16 | 5.26 | 0.018 |
| R precuneus | 8 | 5.26 | 0.018 | ||
| R cuneus | 23 | 5.25 | 0.019 | ||
| L lingual gyrus | 4 | 5.24 | 0.019 | ||
| R lingual gyrus | 5 | 5.19 | 0.024 | ||
| WM | L external capsule | 61 | 5.52 | 0.002 | |
| R parietal lobe | 42 | 5.17 | 0.011 | ||
| R superior frontal lobe | 17 | 5.15 | 0.011 | ||
| R inferior parietal lobe | 40 | 5.10 | 0.013 |
Results shown are the most significant local maxima more than 8‐mm apart with a familywise error corrected P value of <0.05.
Quantitative Motor
Tongue Force
There was a strong correlation between tongue force variability and GM reduction in the striatum bilaterally and in the right precentral gyrus which included sensory and motor regions (Fig. 1, Supporting Information Fig. 1 and Table II). There was also involvement of the right cuneus and the precuneus bilaterally. Increased tongue force variability was also associated with WM reductions in the left and right occipital lobes as well as the splenium of the corpus callosum and the internal capsule.
Figure 1.
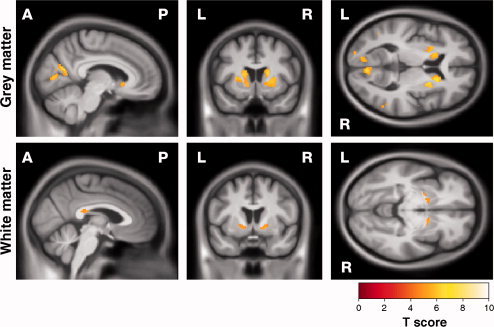
Statistical parametric map (SPM) showing correlations between increased variability in tongue force and reduction in (top) GM and (bottom) WM. Results are shown overlaid on a mean customized template at P < 0.05, corrected for multiple comparisons using familywise error. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Finger tapping
Reduced precision on metronome tapping was associated with bilateral reduction of GM throughout the striatum and WM bilaterally in the internal capsule and in the right external capsule (Fig. 2, Supporting Information Fig. 2 and Table II).
Figure 2.
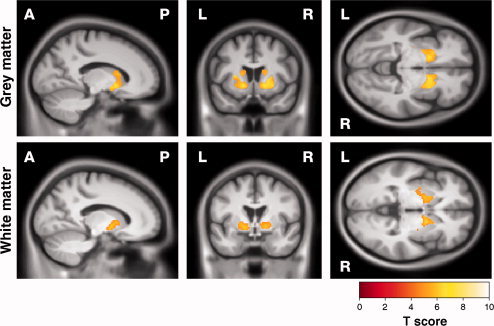
SPM showing correlations between decreased precision in metronome tapping and reduction in (top) GM and (bottom) WM. Results are shown overlaid on a mean customised template at P < 0.05, corrected for multiple comparisons using familywise error. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Gait
Significant associations between increased variability in stride and structural loss were localised to a small area of GM in the left hippocampus (Supporting Information Fig. 3 and Supporting Information Table III).
Figure 3.
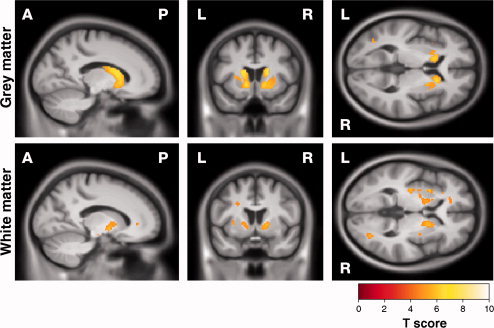
SPM showing correlations between increased anti‐saccade error rate and reduction in (top) GM and (bottom) WM. Results are shown overlaid on a mean customised template at P < 0.05, corrected for multiple comparisons using familywise error. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Oculomotor
Increased antisaccade error rate was associated with bilateral GM reduction in the striatum as well as localised regions of the left lateral occipital lobe, right superior temporal and right inferior frontal gyri (Fig. 3, Supporting Information Fig. 4 and Table II). Increased error rates were also associated with WM reduction in the internal capsules bilaterally and the left external capsule as well as the parieto‐occipital and frontostriatal regions.
Figure 4.
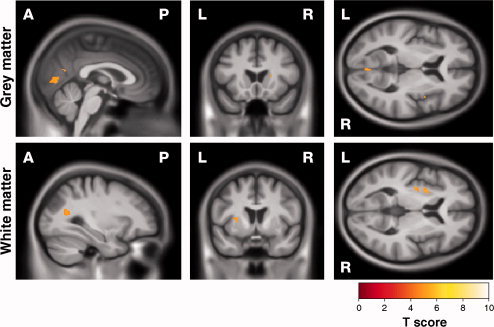
SPM showing correlations between decrease in recognition of negative emotion and reduction in (top) GM and (bottom) WM. Results are shown overlaid on a mean customised template at P < 0.05, corrected for multiple comparisons using familywise error. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Cognitive
Emotion
Poor performance on the negative emotion recognition task correlated with GM reduction in the right cuneus and precuneus, the left precentral gyrus close to the sensorimotor area and bilaterally in the lingual gyri (Fig. 4, Supporting Information Fig. 5 and Table II). Impairment of negative emotion recognition correlated with WM reduction in the left external capsule and the right parietal area.
Spot the Change
Increased error on the spot the change task was related to an isolated GM region in the left middle frontal gyrus as well as single voxels in the right inferior parietal lobe and the right fusiform. Associations with WM reduction were seen in the genu of the corpus callosum (Supporting Information Fig. 6 and Supporting Information Table III).
UPSIT
There was a correlation between impairment in smell identification and reduced GM volume of the superior right hippocampus and the left insula (Supporting Information Fig. 7 and Supporting Information Table III) and reduced WM volume bilaterally in the temporal stems and the right parietal and frontal regions.
Neuropsychiatric
There was little evidence of associations between the neuropsychiatric variables examined here and volume reduction with the exception of a single voxel in the WM of the right lingual gyrus (Supporting Information Fig. 8 and Supporting Information Table III).
Reverse contrasts
There were no regions of significantly larger GM or WM volumes associated with impairment on any of the assessments.
DISCUSSION
We present new findings showing how clinical impairment in premanifest and early HD associates with regionally specific GM and WM atrophy across three assessment modalities, namely quantitative motor, oculomotor, and emotion recognition. In contrast, there was little evidence relating neuropsychiatric abnormalities to structural brain deficits.
Deficits in tongue protrusion force coordination were associated with bilateral loss in the striatal areas as would be expected from a motor‐dependent task. In addition, there was involvement superiorly of GM including both the sensory and motor areas. It is possible that sensory feedback via the tongue itself is also impaired in HD, which is supported by evidence from a recent diffusion tensor imaging (DTI) study reporting an association between reduced WM integrity in this region and deficits in tongue force coordination [Dumas et al., 2011]. This task is also heavily reliant on visual feedback as it allows subjects to monitor their performance and the association with the occipital lobe supports the suggestion that visual functions are also impaired in HD. This is consistent with a recent study demonstrating an association between visual motor impairment and cortical thinning in the occipital lobe [Rosas et al., 2008].
A reduction in precision of metronome tapping was strongly associated with bilateral caudate and putaminal volume reduction as well as the associated striatal WM, consistent with previous work using this dataset [Bechtel et al., 2010]. This is in contrast to the findings of Dumas et al. who report no association between tapping and DTI‐derived measures of WM degradation in this region, also using the TRACK dataset [Dumas et al., 2011]. However, Dumas et al. used a smaller subset of the TRACK cohort so may not have had sufficient power to detect this effect. Methodological differences such as the different properties examined by DTI compared with structural MRI, plus the fact that the study did not adjust for overall disease severity whereas our study did, may also explain this apparent discrepancy. In addition to motor demands, metronome tapping is heavily reliant on time perception. Previous work has suggested that the basal ganglia play an important role in time perception at the sub‐second level [Wiener et al., 2010] and the striatal beat frequency model suggests that the striatum contributes to an “internal clock” which is able to monitor the similarity between the current time interval and that previously encountered [Matell and Meck, 2004]. Our findings are supportive of these studies implicating the striatum in the perception of time.
We found a strong association between increased anti‐saccade error rates and bilateral loss of the striatum accompanied by WM reduction in the external and internal capsules. There was also a correlation with reduction of GM and WM in the occipital and frontal lobes. This task involves both inhibition of a reflexive response towards a stimulus and a voluntary saccade away from it and the importance of the connection between the frontal eye fields and the caudate in voluntary saccades has been described previously [Ettinger et al., 2008; Kloppel et al., 2008]. The association with the occipital lobe suggests that deficits in more fundamental basic visual processing skills may also underlie impairment on this task.
Impaired recognition of negative facial emotions correlated with atrophy of the cuneus and the lingual region of the occipital lobe, which is unsurprising in a task requiring processing of visual stimuli. In contrast to a previous study by Henley et al. [2008], but in agreement with another report [Johnson et al., 2007], we found no significant association between negative emotion recognition and striatal volume. Our study differed in design from that of Henley et al as we used composite scores of negative emotions. Furthermore, adjusting for disease severity may have compensated for general disease‐related striatal change, improving the specificity of the identified associations. Further examination of separate negative emotions may provide more information about specific associations between brain anatomy and identification of emotional stimuli.
Our findings are in agreement with other studies identifying correlations between structural change and functional impairment [Bechtel et al., 2010; Biglan et al., 2009; Bohanna et al., 2011; Dumas et al., 2011]. Despite the robust associations discussed above, our VBM analysis did not detect associations between atrophy and impairment in other domains known to be affected in premanifest and early HD, namely gait, spot the change, UPSIT, or any of the neuropsychiatric measures. It is possible that there are certain characteristics of these assessments which make it more difficult to detect such associations. For example, performance can fluctuate greatly and may be more impacted by external factors than other assessments in this study. Many of the Pre‐HD subjects showed floor effects in the neuropsychiatric assessments; in addition the range of values in these measures was small, hence there may have been insufficient power to detect a correlation. Furthermore, a large proportion of the subjects were being treated for neuropsychiatric disorders such as depression so it may be that the true underlying psychopathology is being masked by medication and is not reflected in the variables examined here. Our neuropsychiatric results are supported by two other studies who report no association between the Beck depression inventory and DTI‐derived measures of structural integrity [Bohanna et al., 2011; Dumas et al., 2011] and a study by Starkstein et al. who found no association between atrophy and apathy in Alzheimer's disease but showed a correlation with WM hyperintensities [Starkstein et al., 2009]. It is also possible that deficits in these domains are the result of more subtle early neuronal dysfunction prior to the widespread loss of tissue and hence would not have been detectable with VBM. Further work with other imaging modalities in HD such as DTI, functional MRI or FDG‐PET may shed light on the underlying mechanism behind loss of function; these techniques have already proved sensitive at detecting early functional deficits prior to clinical onset [Kloppel et al., 2009; Bohanna et al., 2011].
A strength of our study is the fact that we have taken into account the estimated pathological burden for each subject. This may in part explain discrepancies with previous work [Bohanna et al., 2011; Henley et al., 2008; Rosas et al., 2008]. Clearly, all subjects with manifest disease display atrophy and impairment on a variety of tasks and this needs to be taken into account when looking for specific associations, especially with a cross‐sectional analysis. We included not only age and CAG repeat length, but disease burden [Penney et al., 1997] which acts as an interaction term between these two factors. The differential associations we describe for each task suggest that we are not merely detecting global disease effects but specific links between clinical impairment and neuroanatomy. Another strength is that we combine the whole gene‐positive group, negating the need to apply an arbitrary cutoff that defines disease onset. This allows us to examine a range of disease severities from the earliest possible signs of impairment. Also, by using an automated whole‐brain technique such as VBM we did not need to make any a priori assumptions about where associations were expected.
One limitation of our study is that we infer functional associations on the basis of structural data; an association between impairment on a particular task and structural loss does not prove causality. Other techniques such as functional imaging may offer more direct evidence of a link between task performance and neuronal functional impairment. However, acquiring functional data on a large cohort is problematic due to difficulty in ensuring consistency across sites and it is nevertheless likely that neuronal death and structural loss underlie much of the clinical impairment apparent in premanifest and early stage HD. Finally, whilst we assume that VBM is detecting disease‐related volume loss, it is possible that these changes may also reflect pathological changes in signal intensity. Nevertheless, previous volumetry work published on this cohort has demonstrated significant volume loss in both the premanifest and early HD subjects, particularly in the striatal region [Tabrizi et al., 2009], suggesting that VBM is indeed detecting this prominent atrophy, supportive of our conclusions here.
We have demonstrated that clinical impairment in a number of domains is associated with atrophy in both GM and WM, including extra‐striatal regions. The impairments seen in HD are often explained as consequences of atrophy in the striatum, and associated frontostriatal connections. It is clear from our work that atrophy in these areas is indeed associated with a range of impairments. However there is increasing evidence that other regions such as the occipital lobe are undergoing early structural change [Lange et al., 1976; Rosas et al., 2005; Tabrizi et al., 2009]. Our findings suggest that low‐level visuospatial processing deficits may also contribute to the pattern of performance in HD. This has implications both for our conceptualization of the tasks used (e.g., visuospatial skills are often required in tasks that are purported to measure other cognitive domains), and to further our understanding of the difficulties experienced by those with HD. This may be an important area for further detailed research. The TRACK‐HD cohort are being followed longitudinally allowing detailed definitions of the patterns of progressive atrophy and associated clinical disease progression in PreHD and early HD.
In summary, our study has revealed new and specific associations between atrophy and clinical impairment across the spectrum of premanifest and early stage HD. Such detailed studies are important for a number of reasons: (a) they yield insights into anatomical regions underlying some of the earliest functional deficits that can be measured and may help us to understand some of the heterogeneity inherent in the manifestation of HD; (b) if imaging is to be useful as a biomarker of disease progression for future clinical trials, we need to better understand the functional correlates of brain atrophy and (c) disease‐modifying treatments aimed at restoring function (such as neural transplantation and mutant‐huntingtin‐lowering therapies) in specific brain regions in HD rely on a detailed understanding of the regional functionality of these areas in the progression of the disease. This study demonstrates the utility of VBM correlation analysis for investigating associations between structural loss and clinical manifestation in Huntington's disease.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.
Supporting Information Figure 4.
Supporting Information Figure 5.
Supporting Information Figure 6.
Supporting Information Figure 7.
Supporting Information Figure 8.
Acknowledgements
The authors thank all those at the CHDI/High Q Foundation responsible for the TRACK‐HD study, in particular Beth Borowsky, Allan Tobin, Daniel van Kammen, Ethan Signer, and Sherry Lifer. They also extend their gratitude to the TRACK‐HD study participants, to Saiqah Munir for her contribution to the coordination of the TRACK‐HD project, to Clare Gibbard for reviewing the manuscript, and to Ray Young for his help with the figures.
The TRACK‐HD Investigators: Natalie Arran, Manchester; Eric Axelson, Iowa; Eric Bardinet, Paris; Simon J.A. van Bogaard, Leiden; Jenny Callaghan, Manchester; Colin Campbell and Melissa Campbell, Monash; Allison Coleman, Rachelle Dar Santos and Joji Decolongon, Vancouver; Eve Dumas, Leiden; Nick Fox, UCL; Ellen Frajman, Monash; Jeroen van der Grond and Ellen t'Hart, Leiden; Arndt Hoffman, Bochum; Céline Jauffret, Paris; Damian Justo, Paris; Siobhan Keenan, Imperial College London; Nayana Lahiri, UCL; Bernhard Landwehmeyer, Ulm; Stephanie Lee, MGH; Cecilia Marelli, Paris; Cassie Milchman, Monash; Jim Mills, Iowa; William Monaco, MGH; Kevin Nigaud, Paris; Alison O'Regan, Monash; Roger Ordidge, UCL; Gail Owen, UCL; Tracey Pepple, UCL; Sarah Queller, Indiana; Joy Read, UCL; H Diana Rosas, MGH; Aaron Sturrock, Vancouver; Romain Valabrègue, Paris; Chiachi Wang, Iowa; Kathryn Whitlock, Indiana.
REFERENCES
- Ashburner J ( 2007): A fast diffeomorphic image registration algorithm. Neuroimage 38: 95–113. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Li Q, Stine OC, Ranen N, Sherr M, Barta PE, Bylsma FW, Pearlson GD, Ross CA ( 1997): Longitudinal change in basal ganglia volume in patients with Huntington's disease. Neurology 48: 394–399. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, Brandt J, Gourley LM, Liang K, Zhou H, Margolis RL, Ross CA ( 2004): Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology 63: 66–72. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Nopoulos PC, Ross CA, Langbehn DR, Pierson RK, Mills JA, Johnson HJ, Magnotta VA, Juhl AR, Paulsen JS ( 2010): Longitudinal change in regional brain volumes in prodromal Huntington disease. J Neurol Neurosurg Psychiatry 22: 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel N, Scahill RI, Rosas HD, Acharya T, van den Bogaard SJ, Jauffret C, Say MJ, Sturrock A, Johnson H, Onorato CE, Salat DH, Durr A, Leavitt BR, Roos RA, Landwehrmeyer GB, Langbehn DR, Stout JC, Tabrizi SJ, Reilmann R ( 2010): Tapping linked to function and structure in premanifest and symptomatic Huntington disease. Neurology 75: 2150–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrios GE, Wagle AC, Markova IS, Wagle SA, Rosser A, Hodges JR ( 2002): Psychiatric symptoms in neurologically asymptomatic Huntington's disease gene carriers: A comparison with gene negative at risk subjects. Acta Psychiatr Scand 105: 224–230. [DOI] [PubMed] [Google Scholar]
- Biglan KM, Ross CA, Langbehn DR, Aylward EH, Stout JC, Queller S, Carlozzi NE, Duff K, Beglinger LJ, Paulsen JS ( 2009): Motor abnormalities in premanifest persons with Huntington's disease: The PREDICT‐HD study. Mov Disord 24: 1763–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blekher T, Johnson SA, Marshall J, White K, Hui S, Weaver M, Gray J, Yee R, Stout JC, Beristain X, Wojcieszek J, Foroud T ( 2006): Saccades in presymptomatic and early stages of Huntington disease. Neurology 67: 394–399. [DOI] [PubMed] [Google Scholar]
- Bohanna I, Georgiou‐Karistianis N, Sritharan A, Asadi H, Johnston L, Churchyard A, Egan G ( 2011): Diffusion tensor imaging in Huntington's disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain Imaging Behav 5: 171–80. [DOI] [PubMed] [Google Scholar]
- Craufurd D, Thompson JC, Snowden JS ( 2001): Behavioral changes in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol 14: 219–226. [PubMed] [Google Scholar]
- Duff K, Paulsen J, Mills J, Beglinger LJ, Moser DJ, Smith MM, Langbehn D, Stout J, Queller S, Harrington DL ( 2010): Mild cognitive impairment in prediagnosed Huntington disease. Neurology 75: 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas EM, van den Bogaard SJ, Ruber ME, Reilman RR, Stout JC, Craufurd D, Hicks SL, Kennard C, Tabrizi SJ, van Buchem MA, van der Grond J, Roos RA ( 2011): Early changes in white matter pathways of the sensorimotor cortex in premanifest Huntington's disease. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV ( 1976): Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, Williams SC ( 2008): Decomposing the neural correlates of antisaccade eye movements using event‐related FMRI. Cereb Cortex 18: 1148–1159. [DOI] [PubMed] [Google Scholar]
- Freeborough PA, Fox NC, Kitney RI ( 1997): Interactive algorithms for the segmentation and quantitation of 3‐D MRI brain scans. Comput Methods Programs Biomed 53: 15–25. [DOI] [PubMed] [Google Scholar]
- Golding CV, Danchaivijitr C, Hodgson TL, Tabrizi SJ, Kennard C ( 2006): Identification of an oculomotor biomarker of preclinical Huntington disease. Neurology 67: 485–487. [DOI] [PubMed] [Google Scholar]
- Henley SM, Wild EJ, Hobbs NZ, Warren JD, Frost C, Scahill RI, Ridgway GR, Macmanus DG, Barker RA, Fox NC, Tabrizi SJ ( 2008): Defective emotion recognition in early HD is neuropsychologically and anatomically generic. Neuropsychologia 46: 2152–2160. [DOI] [PubMed] [Google Scholar]
- Henley SM, Wild EJ, Hobbs NZ, Frost C, MacManus DG, Barker RA, Fox NC, Tabrizi SJ ( 2009): Whole‐brain atrophy as a measure of progression in premanifest and early Huntington's disease. Mov Disord 24: 932–936. [DOI] [PubMed] [Google Scholar]
- Hicks SL, Robert MP, Golding CV, Tabrizi SJ, Kennard C ( 2008): Oculomotor deficits indicate the progression of Huntington's disease. Prog Brain Res 171: 555–558. [DOI] [PubMed] [Google Scholar]
- Hobbs NZ, Henley SM, Wild EJ, Leung KK, Frost C, Barker RA, Scahill RI, Barnes J, Tabrizi SJ, Fox NC ( 2009): Automated quantification of caudate atrophy by local registration of serial MRI: Evaluation and application in Huntington's disease. Neuroimage 47: 1659–1665. [DOI] [PubMed] [Google Scholar]
- Huntington's Disease Study Group ( 1996): Unified Huntington's disease rating scale: Reliability and consistency. Mov Disord 11: 136–142. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Stout JC, Solomon AC, Langbehn DR, Aylward EH, Cruce CB, Ross CA, Nance M, Kayson E, Julian‐Baros E, Hayden MR, Kieburtz K, Guttman M, Oakes D, Shoulson I, Beglinger L, Duff K, Penziner E, Paulsen JS ( 2007): Beyond disgust: Impaired recognition of negative emotions prior to diagnosis in Huntington's disease. Brain 130: 1732–1744. [DOI] [PubMed] [Google Scholar]
- Julien CL, Thompson JC, Wild S, Yardumian P, Snowden JS, Turner G, Craufurd D ( 2007): Psychiatric disorders in preclinical Huntington's disease. J Neurol Neurosurg Psychiatry 78: 939–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppel S, Draganski B, Golding CV, Chu C, Nagy Z, Cook PA, Hicks SL, Kennard C, Alexander DC, Parker GJ, Tabrizi SJ, Frackowiak RS ( 2008): White matter connections reflect changes in voluntary‐guided saccades in pre‐symptomatic Huntington's disease. Brain 131: 196–204. [DOI] [PubMed] [Google Scholar]
- Kloppel S, Draganski B, Siebner HR, Tabrizi SJ, Weiller C, Frackowiak RS ( 2009): Functional compensation of motor function in pre‐symptomatic Huntington's disease. Brain 132: 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange H, Thorner G, Hopf A, Schroder KF ( 1976): Morphometric studies of the neuropathological changes in choreatic diseases. J Neurol Sci 28: 401–425. [DOI] [PubMed] [Google Scholar]
- Lasker AG, Zee DS ( 1997): Ocular motor abnormalities in Huntington's disease. Vision Res 37: 3639–3645. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH ( 2004): Cortico‐striatal circuits and interval timing: Coincidence detection of oscillatory processes. Brain Res Cogn Brain Res 21: 139–170. [DOI] [PubMed] [Google Scholar]
- Nopoulos PC, Aylward EH, Ross CA, Johnson HJ, Magnotta VA, Juhl AR, Pierson RK, Mills J, Langbehn DR, Paulsen JS ( 2010): Cerebral cortex structure in prodromal Huntington disease. Neurobiol Dis 40: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M ( 2008): Detection of Huntington's disease decades before diagnosis: The Predict‐HD study. J Neurol Neurosurg Psychiatry 79: 874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney JB Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH ( 1997): CAG repeat number governs the development rate of pathology in Huntington's disease. Ann Neurol 41: 689–692. [DOI] [PubMed] [Google Scholar]
- Rao AK, Muratori L, Louis ED, Moskowitz CB, Marder KS ( 2008): Spectrum of gait impairments in presymptomatic and symptomatic Huntington's disease. Mov Disord 23: 1100–1107. [DOI] [PubMed] [Google Scholar]
- Reilmann R, Bohlen S, Klopstock T, Bender A, Weindl A, Saemann P, Auer DP, Ringelstein EB, Lange HW ( 2010): Tongue force analysis assesses motor phenotype in premanifest and symptomatic Huntington's disease. Mov Disord 25: 2195–2202. [DOI] [PubMed] [Google Scholar]
- Ridgway GR, Omar R, Ourselin S, Hill DL, Warren JD, Fox NC ( 2009): Issues with threshold masking in voxel‐based morphometry of atrophied brains. Neuroimage 44: 99–111. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B ( 2002): Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58: 695–701. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B ( 2005): Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology 65: 745–747. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, Greve D, Hevelone N, Hersch SM ( 2008): Cerebral cortex and the clinical expression of Huntington's disease: Complexity and heterogeneity. Brain 131: 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulson I, Fahn S ( 1979): Huntington disease: Clinical care and evaluation. Neurology 29: 1–3. [DOI] [PubMed] [Google Scholar]
- Squitieri F, Cannella M, Simonelli M, Sassone J, Martino T, Venditti E, Ciammola A, Colonnese C, Frati L, Ciarmiello A ( 2009): Distinct brain volume changes correlating with clinical stage, disease progression rate, mutation size, and age at onset prediction as early biomarkers of brain atrophy in Huntington's disease. CNS Neurosci Ther 15: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mizrahi R, Capizzano AA, Acion L, Brockman S, Power BD ( 2009): Neuroimaging correlates of apathy and depression in Alzheimer's disease. J Neuropsychiatry Clin Neurosci 21: 259–265. [DOI] [PubMed] [Google Scholar]
- Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, Carlozzi N, Duff K, Beglinger LJ, Langbehn DR, Johnson SA, Biglan KM, Aylward EH ( 2011): Neurocognitive signs in prodromal Huntington disease. Neuropsychology 25: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, Borowsky B, Tobin AJ, Rosas HD, Johnson H, Reilmann R, Landwehrmeyer B, Stout JC ( 2009): Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK‐HD study: Cross‐sectional analysis of baseline data. Lancet Neurol 8: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR, Jones R, Landwehrmeyer GB, Fox NC, Johnson H, Hicks SL, Kennard C, Craufurd D, Frost C, Langbehn DR, Reilmann R, Stout JC ( 2011): Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK‐HD study: The 12‐month longitudinal analysis. Lancet Neurol 10: 31–42. [DOI] [PubMed] [Google Scholar]
- The Huntington's Disease Collaborative Research Group ( 1993): A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72: 971–983. [DOI] [PubMed] [Google Scholar]
- Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, McCusker E, Frackowiak RS ( 2002): The distribution of structural neuropathology in pre‐clinical Huntington's disease. Brain 125: 1815–1828. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Crum WR, Watt HC, Fox NC ( 2001): Normalization of cerebral volumes by use of intracranial volume: Implications for longitudinal quantitative MR imaging. AJNR 22: 1483–1489. [PMC free article] [PubMed] [Google Scholar]
- Wiener M, Turkeltaub P, Coslett HB ( 2010): The image of time: A voxel‐wise meta‐analysis. Neuroimage 49: 1728–1740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.
Supporting Information Figure 4.
Supporting Information Figure 5.
Supporting Information Figure 6.
Supporting Information Figure 7.
Supporting Information Figure 8.


