Abstract
Patients suffering from schizophrenia have been characterized by an apparent lack of theta (around 6 Hz) and gamma (>40 Hz) brain oscillatory activity during task execution. The neurocognitive reasons for these abnormal synchronization patterns, however, remain elusive. Recording the electroencephalogramm (EEG) during a selective visual attention task, the current study investigates whether abnormal brain oscillatory resting‐state activity in the theta band might account for a lack of task‐related brain oscillatory activity in schizophrenia. EEGs were recorded from 26 patients with schizophrenia and 26 healthy matched controls during rest and during the execution of a selective visual attention task, in which an unexpected object (monkey) appeared on the screen. On a behavioral level, patients were less likely to report perceiving the unexpected event than controls. Controls showed a stronger increase in task‐related theta power than patients in prefrontal, parietal, and occipital brain regions. Task‐related theta power change differed between patients who perceived, and patients who did not perceive the unexpected event. Moreover, patients showed higher levels of theta power during rest than controls, whereas the absolute theta power values during the selective attention task did not differ between groups. These results suggest that the failure to increase oscillatory activity during a cognitive task can be accounted for by abnormally high oscillatory activity in a resting state. This finding has important implications for future studies examining abnormal brain oscillatory activity in schizophrenia, which usually treat resting‐state activity as a baseline for task‐related activity. Hum Brain Mapp 34:2266–2275, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: synchronization, schizophrenia, theta, oscillations, resting state, inattentional blindness
INTRODUCTION
Brain oscillations are rhythmic fluctuations of the local field potential and mediate complex cognitive tasks such as working memory and perception by establishing neural communication between cell assemblies (Fries, 2005). In particular, theta oscillations (around 6 Hz) play a crucial role for top–down executive control processes and working memory (see Sauseng et al., 2010, for a review). Working memory deficits are considered as one of the core cognitive deficits in schizophrenia typically manifested in markedly reduced processing capacities compared with healthy controls (Silver et al., 2003). Several studies demonstrate that patients show less increase in theta oscillatory activity during working memory than healthy subjects (Schmiedt et al., 2005; Haenschel et al., 2009). Furthermore, patients diagnosed with schizophrenia show deficient synchronization of task relevant oscillatory responses (Uhlhaas et al., 2008). Abnormal prefrontal neuronal synchrony in task‐related theta oscillations is associated with working memory performance in schizophrenia (Winterer et al., 2000) and is even considered an illness specific neurophysiological biomarker (Javitt et al., 2008).
It is recognized that the brain oscillatory activity evoked by an external stimulus or cognitive task strongly depends on resting‐state oscillatory dynamics (Hanslmayr et al., 2011). Although this has been predominantly shown for the alpha frequency band (8–12 Hz), resting‐state theta activity may also be relevant for cognitive processes. This is especially relevant for the current study, as patients with schizophrenia typically show abnormal high resting‐state theta activity (Boutros et al., 2008). Consistently, illness specific functional and structural alterations in synaptic signaling have been identified, which in turn are necessary for the generation of oscillatory activity required for information processing (Gonzalez‐Burgos and Lewis, 2008). Taken together, this body of evidence suggests that a lack of neural synchronization contributes to the cognitive deficits in schizophrenia. However, the neurophysiological dynamics potentially associated with the failure to increase brain oscillatory activity during cognitive tasks are not yet fully understood.
Theta oscillations are assumed to be mainly generated in prefrontal brain regions (Gevins et al., 1997; Hanslmayr et al., 2008), which are known to be of substantial relevance for working memory. In addition, it has been demonstrated that whether or not a visual stimulus is perceived depends on the current state of the brain, reflected by ongoing brain oscillations (Hanslmayr et al., 2011). Several studies demonstrated that the likelihood of a participant to perceive a briefly presented visual stimulus can be predicted by brain oscillatory resting‐state activity (Hanslmayr et al., 2007; van Dijk et al., 2008). Therefore, resting‐state activity may also correlate with the likelihood of whether a subject perceives an unexpected visual event. Thus, it is conceivable that the cognitive problems in schizophrenia and the task‐induced neurophysiological abnormalities are associated with abnormal resting‐state activity.
The current study explores the neurocognitive deficits in patients with schizophrenia during an “inattentional blindness” paradigm. Inattentional blindness (Mack and Rock, 1998; Simons and Chabris, 1999) refers to the inability to detect a salient but unexpected stimulus during the execution of a demanding selective attention task. In their classic experiment, Simons and Chabris (1999) instructed subjects to watch a basketball playing movie and to count the passes of one of two teams. During the movie, a man wearing a gorilla costume crossed the scene. Although the gorilla was well‐visible for several seconds, half of the subjects failed to notice anything unusual. The likelihood of perceiving the unexpected event strongly depends on the resources demanded by the selective attention task. For instance, less subjects perceived the gorilla if the counting of the passes was made more difficult (Simons and Chabris, 1999). Similarly, subjects with high working‐memory capacity, or subjects who are well practiced in the selective attention task, are more likely to perceive the unexpected event (Richards et al., 2010).
The current study used a selective visual attention task in which a salient but unexpected visual event (monkey) occurred (Fig. 1; see Supporting Information for animations). It was hypothesized that schizophrenic patients are less likely to perceive the unexpected event than healthy controls, as patients have fewer cognitive resources available to detect the unexpected event (Silver et al., 2003). It was further expected to replicate previous findings in showing that patients with schizophrenia show a less pronounced increase in task‐related oscillatory theta power. It was of particular interest whether the changes in task‐related theta power, and the likelihood of perceiving the unexpected visual stimulus, would be related to increased brain oscillatory resting‐state power. Such a finding would provide a first link between abnormal resting‐state activity and the failure to increase task‐related brain oscillatory activity in schizophrenia.
Figure 1.
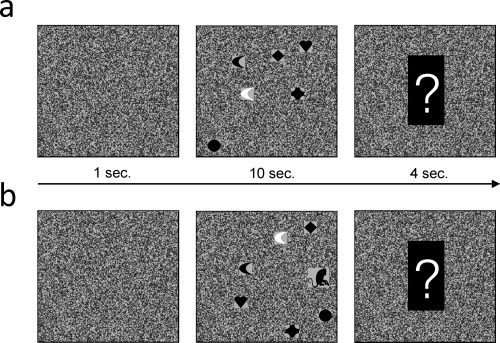
The selective visual attention task. (a) Each trial started with a square depicting static noise for 1 s. Thereafter, six objects were presented which moved around randomly for 10 s. The subject's had to count how often the white object bounced against one of the edges of the square. At the end of each trial a question mark was shown, prompting the subjects to report the number of edge bounces. (b) The last, “critical” trial is shown. Note the monkey (unexpected event) which crossed the square from right to left (see Supporting Information, for animations).
METHODS AND MATERIALS
Subjects
Patients
Twenty‐six patients (see Table 1, for demographic data) were recruited from the local psychiatric clinic (Regensburg, Germany). All patients suffered from schizophrenia of either the paranoid‐hallucinatory (n = 19; ICD‐10, F20.0) or hebephrenic subtype (n = 7; ICD‐10, F20.1). The patients had a mean duration of illness of 48.7 months (SD, 50.3). One patient received a combination of typical and atypical neuroleptic medication, 22 patients received atypical neuroleptics only, and three patients received no medication at all. Mean chlorpromazine equivalent dosage (CPZ) was 466 mg/d (SD, 519; Woods, 2003). Four patients received antidepressant medication (selective serotonin reuptake inhibitors, or tetracyclic antidepressants). Exclusion criteria were neurological diseases, additional psychiatric diseases, vision impairment, or medication with tranquillizers. Current clinical symptoms were assessed with the positive and negative syndrome scale (PANSS; mean score, 73.2; SD, 16.2, Kay et al., 1987).
Table 1.
Demographic data of patients and controls
| Gender (m/f) | Age | Education | Handedness RH/LH | Cigarettes per day | Verbal IQ | |
|---|---|---|---|---|---|---|
| Controls (n = 26) | 21/5 | 24.65 (4.35) | 3 | 25 / 1 | 8.08 (7.45) | 103 (8.8) |
| Patients (n = 26) | 21/5 | 27.35 (5.66) | 3 | 23 / 3 | 11.27 (11.31) | 102 (13.4) |
| Stat. difference | X 2 = 0.0; P > 0.5 | t 50 = 1.92; P > 0.05 | Z = 1.53; P > 0.1 | X 2 = 1.08; P > 0.25 | t 50 = 1.2; P > 0.2 | t 50 = 0.6; P > 0.5 |
Mean values and standard deviation (in brackets) are reported for age, cigarettes per day and verbal IQ. Median values are reported for education level.
Controls
Twenty‐six healthy volunteers (Table 1) served as control subjects, matched according to gender, age, educational level (0: no graduation–4: university entrance diploma), handedness, amount of cigarettes smoked per day, and premorbid IQ (measured with the “Mehrfachwahl–Wortschatz–Intelligenztest”, Lehrl, 2005). The demographic, and IQ data are plotted in Table 1. All participants gave written informed consent. The protocol was approved by the local ethical review board.
Task and Procedure
The selective visual attention task was programmed using Presentation (©Neurobehavioral Systems). The task was designed to be comparable to inattentional blindness tasks used in previous studies (Simons and Chabris, 1999; Most et al., 2001). The structure of one trial is depicted in Figure 1A (see Supporting Information for animations). Each trial started with the presentation of a square depicting static noise (visual angle 8.6°). The square was shown for 1 s. Then, a cartoon‐like animation was presented in which six little geometric objects moved around randomly within the square. The geometric objects were little hearts, diamonds, circles, half‐moons, and crosses with a visual angle of 0.8°. One of the six objects was white, the other five were black. The shape of the white object (heart, diamond, etc.) was selected randomly from trial to trial. During the animation, the objects occasionally bounced against the edges of the square. The subjects' task was to focus attention on the white object and to ignore the black objects. Specifically, the subjects were instructed to quietly count how often the white object bounced against the edges of the square. The duration of the movie was 10 s. Thereafter, a question mark appeared which told the subjects to indicate the number of edge bounces. The question mark was shown for 4 s, before the next trial started. The whole experiment consisted of 25 trials, and the EEG signal was collapsed across these 25 trials.
The last, 25th trial, was the critical trial. This trial started like all other previous trials, but after 3.5 s. a monkey crossed the screen horizontally from right to left (Fig. 1b). The monkey was slightly bigger than the other objects (visual angle 1.3°) and was fully visible for 2.8 s. Before the experiment, five practice trials were performed to familiarize the subjects with the task, and participants also had to rate on a scale from 1 to 10, how wakeful they were.
To assess whether the subject reported perceiving the monkey, three questions were asked immediately after the critical, last trial. (i) Did you recognize anything unusual during the last trial, which was different from the previous trials? (ii) Did you recognize anything unusual in the background, other than the objects, during the last trial? (iii) Did you recognize that a monkey appeared on the screen in the last trial? If at any point the subjects mentioned the unexpected event, the remaining questions were skipped. If the subject answered all questions with no, the last trial was shown again. Only subjects, who answered all questions with no were counted as being “inattentionally blind”. All inattentionally blind subjects were clearly surprised to not having perceived the monkey. At the end of the experiment, the subjects additionally had to rate task difficulty on a scale from 1 to 10.
Resting‐States
Two resting‐state EEGs were recorded. One was recorded prior and one after the experiment, each lasting 2 min. During the resting‐states the subjects were instructed to keep their eyes open and to fixate the black monitor in front of them. An eyes‐open resting state was used instead of an eyes‐closed resting‐state, because it represents a more appropriate baseline for task‐related activity. Subjects were told to relax as much as possible and not to think of anything specific. For EEG analysis, the data of both resting‐state recordings were collapsed together.
EEG Recording
EEGs were recorded at a sampling rate of 500 Hz from 61 equidistant passive electrodes mounted in an elastic cap (Montage 10, EasyCap, Herrsching‐Breitbrunn, Germany). The EEGs were amplified using BrainAmp DC (©Brain Products, GmbH, Gilchingen, Germany). Impedances were kept below 10 kOhm. The signals were amplified from 0.1 to 250 Hz, with a notch filter at 50 Hz. The data were recorded referenced to electrode Cz and re‐referenced to average reference after raw‐data preprocessing (see below).
EEG Preprocessing and Analysis
Frequency and source localization analysis of the EEG data were carried out using fieldtrip (http://fieldtrip.fcdonders.nl/, Oostenveld et al., 2011) running under Matlab (© The Mathworks, Munich, Germany). Before frequency analysis, the EEG data during the selective visual attention task (onset of the movie to the onset of the question mark; see Fig. 1), and during the resting‐states, were segmented into epochs of 2 s. The epochs were visually inspected for artifacts, and epochs containing large artifacts were discarded from further analysis. These data were then subjected to an independent component analysis, as implemented in EEGLAB (http://sccn.ucsd.edu/eeglab). Components corresponding to vertical or horizontal eye‐movements, blinks or muscle artifacts were rejected. Thereafter, the EEG data were re‐referenced to average reference. For the patients, an average of 115.9 (range, 95–125) and 113.2 epochs (range, 63–30) remained for analysis of the selective visual attention task and the resting‐state, respectively. For the control subjects, an average of 111.8 (range, 50–125) and 113.4 (range, 72–135) epochs remained for the selective visual attention task and the resting‐state, respectively.
The segmented epochs (2 s) were then multiplied with a Hanning taper, and subjected to a fast fourier transformation as implemented in fieldtrip. This results in a power spectrum with a frequency resolution of 0.5 Hz, for each epoch, without the time information. The powerspectra were then averaged across the epochs to obtain mean power levels for the selective visual attention task and the resting‐state for each subject. The power values for the selective visual attention task were transformed to relative power changes in percent from baseline power (see formula below). The power values were collapsed to obtain five frequency bands; delta (1–3 Hz), theta (4–7.5 Hz), alpha (8–1.5), beta 1 (12–19.5), beta 2 (20–29.5), and gamma (30–44.5).
Statistical Analysis
To investigate the difference in the occurrence of inattentional blindness between patients and control subjects, nonparametric Chi‐square tests were used. For between subjects, comparisons of the number of errors that were conducted in the selective attention task (counting the edge bounces), t tests were used (the data fulfilled the requirements for parametric statistical tests). For comparisons of the rating scales between the two groups, nonparametric Mann–Whitney tests were carried out.
For statistical comparisons of the EEG data between control subjects and patients, nonparametric Mann–Whitney tests were calculated for each electrode and frequency band, because the EEG data did not fulfill the requirements for parametric statistical tests (e.g., difference in variance homogeneities). To control for multiple comparisons, a nonparametric randomization approach as implemented in self‐written Matlab codes was used (Hanslmayr et al., 2009). This procedure uses a two‐step randomization approach, to estimate the likelihood of finding a given number of significant electrodes by chance. In a first step, Mann–Whitney tests were conducted between the control subjects and patients to investigate, how many electrodes show a significant difference between the two groups (P < 0.05). In a second step, 1000 randomization runs were carried out in which the subjects were randomly assigned to one of two groups. This randomization procedure returns a corrected P‐level (P corr), which is an estimate of the likelihood to find a given number of significant electrodes by chance. This method effectively controls for type‐I errors that arise due to multiple testing across electrode positions. The statistical comparisons were focused on theta power, for which specific hypothesis were formulated. Exploratory analyses were also performed for the other five frequency bands. Post hoc analyses were conducted for clusters of significant electrodes.
Source Analysis
For source reconstruction, a standardized boundary element model was used, which was derived from an averaged T1‐weighted MRI dataset (MNI, http://www.mni.mcgill.ca). A previous study demonstrated that similar results are obtained from such a standard head model and individual head models (Fuchs et al., 2002). To estimate the sources of the activity induced by the visual attention task, a frequency‐domain adaptive spatial filtering algorithm, which enables the dynamic imaging of coherent sources (DICS; Gross et al., 2001), was applied as implemented in the fieldtrip toolbox. DICS constructs a spatial filter optimized for a specific location (voxel), based on the cross‐spectral density matrices obtained from the data. Source analysis was carried out for those frequency bands, which revealed significant results on the scalp level. Statistics on the source level were performed using nonparametric Mann–Whitney tests.
RESULTS
Behavioral Results
The results of perceiving the unexpected event (monkey) are reported in Table 2. Control subjects were much more likely to report perceiving the monkey than patients (χ2=7.59; P < 0.01; Table 2). Although most of the control subjects (88%) reported perceiving the unexpected event, only half of the patients (54%) reported perceiving the monkey. There was no difference between the groups in the number of errors conducted in counting, how often the to‐be‐attended object bounced against the edges (see Table 3). There were also no significant differences between controls and patients in the rated difficulty of the task and the wakefulness rating (Table 3). Furthermore, the patients who reported perceiving the monkey did not differ from patients who reported not perceiving the monkey in terms of medication (CPZ equivalent), diagnose, illness severity (PANSS score), duration, and IQ (Table S1).
Table 2.
Within group proportion of subjects who did, or did not report perceiving the unexpected event (monkey), listed separately for patients and controls
| Monkey | ||
|---|---|---|
| Perceived | Not perceived | |
| Patients | 54% (n = 14) | 46% (n = 12) |
| Controls | 88% (n = 23) | 12% (n = 3) |
| Stat. difference | X 2 = 7.59; P < 0.01 | |
Table 3.
Performance data during the selective attention task, and ratings of wakefulness and task difficulty
| Commissions | Omissions | Errors total | Wakefulness | Difficulty | |
|---|---|---|---|---|---|
| Patients | 3.57 (3.02) | 5.26 (4.08) | 8.83 (3.67) | 6.5 (1.77) | 4.7 (1.85) |
| Controls | 4.54 (4.77) | 3.59 (2.33) | 8.13 (4.05) | 6.5 (1.82) | 5.5 (1.68) |
| Stat. difference | t 50 = 0.88; P > 0.35 | t 50 = 1.81; P > 0.05 | t 50 = 0.65; P > 0.5 | Z = 0.1; P > 0.5 | Z = 1.46; P > 0.1 |
Commissions and omissions refer to errors due to counting too many (false alarms) or too few (misses) edge bounces, respectively. Digits in brackets refer to standard deviation.
EEG Results
Task‐related power change
Comparing the relative power changes during the selective attention task between patients and controls a significant effect emerged in the theta band (Fig. 2a). This effect was due to a pronounced increase in task‐related compared with resting‐state theta power in control subjects, which did not occur in patients (P corr < 0.001). The effect was evident over fronto‐central and parietal electrode sites (Fig. 2a). No differences were observed in any of the other frequency bands (P corr's > 0.05; see also supporting Information, Fig. S2). To exclude the possibility that the effect in theta band is due to residual eye‐movement artifacts, a control analysis on EEG data during voluntary eye movements was performed. The results of this analysis are plotted in Supporting Information, Fig. S1, and demonstrate that voluntary eye‐movements elicit very strong artifacts in the delta band, and much weaker artifacts in the theta frequency band. Therefore, it is very unlikely that the difference in task‐related theta power was driven by residual eye‐movement artifacts.
Figure 2.
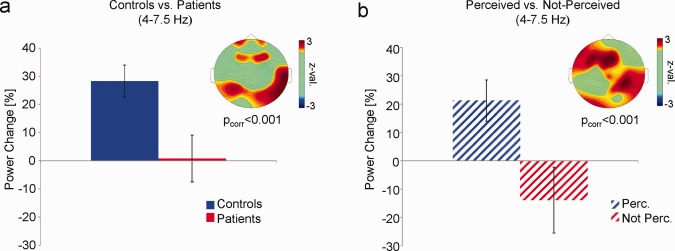
Differences in task‐related theta power. (a) Task‐related theta power values (% power change) averaged over electrodes showing a significant difference between controls and patients are shown. The topography depicts z values obtained from nonparametric Mann–Whitney tests. (b) Task‐related theta power values are shown for patients who perceived and patients who did not perceive the unexpected event. Error bars indicate mean standard error. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To investigate whether the effect in the theta frequency band varied with reporting perception of the unexpected event, we contrasted task‐related theta power between patients who reported perceiving, and patients who did not report perceiving the unexpected event (Fig. 2b). Patients who reported perceiving the unexpected event showed a stronger increase in task‐related theta power than patients who did not report perceiving the unexpected stimulus (P corr < 0.001). Moreover, patients who reported perceiving the unexpected event resembled the level of task‐related theta power in control subjects (Z = −0.62; P > 0.5). In contrast, patients who failed to perceive the monkey even showed a decrease in theta power during task execution, compared with resting‐state theta power. Note, that only three of the healthy control subjects did not report perceiving the unexpected event, which precluded a similar analysis for healthy controls.
Source analysis
The results of the source analysis of differences in task‐related theta power between patients and controls are illustrated in Figure 3. Controls showed a higher level of relative theta power than patients in prefrontal regions: the left superior frontal gyrus (∼BA 6, ∼BA 9) and the right inferior frontal gyrus (∼BA 46); parietal brain regions: precuneus (∼BA 7), and right temporo‐parietal junction (∼BA 22); and occipital regions: cuneus and middle occipital gyrus (∼BA 18).
Figure 3.
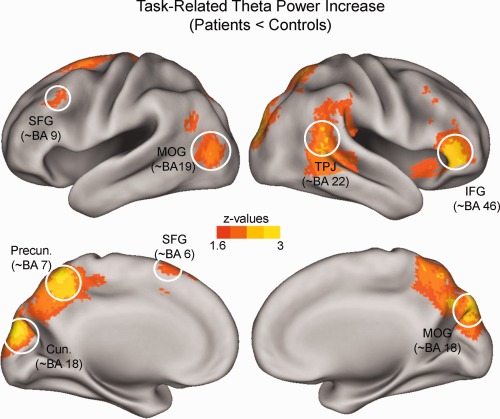
Source‐localization results. Differences in task‐related theta power between controls and patients are projected onto an inflated cortical surface. Z values were obtained from nonparametric Mann–Whitney tests. Controls show a stronger increase in theta power in prefrontal, parietal, and occipital brain regions (SFG: superior‐frontal‐gyrus; IFG: inferior‐frontal‐gyrus; Precun: Precuneus; Cun: Cuneus; TPJ: temporo‐parietal‐junction; MOG: middle‐occipital‐gyrus). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Resting‐state
Contrasting EEG resting‐state activity between controls and patients confirmed group differences in the theta band (P corr < 0.001). This effect was due to higher theta power over fronto‐central and right parietal electrodes in patients compared with controls (Fig. 4a, see also Supporting Information Fig. S3) with a similar topography as the group difference in task‐related theta power (Fig. 2a). Contrasting theta resting power between patients who reported perceiving and patients who did not report perceiving the unexpected event a statistical trend was observed (Z = ‐1.44; P = 0.075), suggesting that patients who failed to perceive the unexpected event showed higher levels of theta resting power than patients who perceived the monkey (Fig. 4b).
Figure 4.
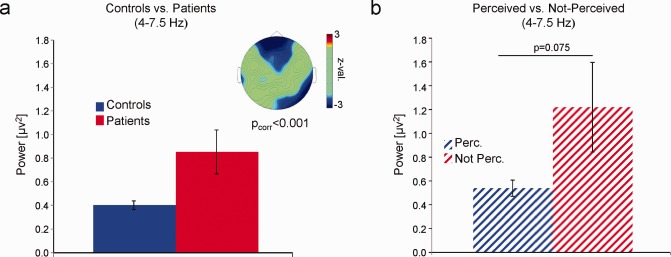
Differences in resting‐state power. (a) Mean resting‐state theta power (y axis) is shown averaged over the electrodes showing a significant difference between patients and controls. The topography plots z values obtained by nonparametric statistical tests. (b) Mean resting‐state theta power for patients who reported perceiving and patients who did not report to perceive the unexpected event is plotted. Theta power values were averaged over those electrodes showing a significant difference in resting‐state power between patients and controls. Error bars indicate mean standard error. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Absolute power during task
In a last step, the absolute theta power values during the selective attention task, without transforming the power values to percent change from rest, were compared between patients and controls. This analysis revealed no significant differences between the two groups (P corr > 0.5). Similarly, patients who reported perceiving the monkey did not differ from patients who did not report perceiving the monkey in absolute theta power during the task (P corr > 0.5). These null‐results can be explained by the fact that controls showed a strong increase from rest to task in theta power, whereas the patients failed to increase theta power (Fig. 2a), or even showed a decrease in theta power (Fig. 2b), thus diminishing any differences in absolute theta power during task execution.
DISCUSSION
The present results confirm that patients with schizophrenia show a reduced increase of task‐relevant theta power, which was specifically related to the failure to perceive a salient, but unexpected stimulus during a demanding selective visual attention task. Central to the aim of the current study, the lack of task‐related theta power in patients was paired with abnormally high resting‐state theta power. These findings suggest that abnormal task‐related oscillatory activity in schizophrenia, and the illness‐associated neurocognitive deficits, could arise from abnormal high oscillatory resting‐state activity.
Abnormal resting‐state activity in schizophrenia has been traditionally recognized in the EEG (Boutros et al., 2008) and recently also in functional magnetic resonance imaging (fMRI) (Bluhm et al., 2007; Greicius, 2008). For instance, enhanced theta oscillatory activity in schizophrenia has been reported in previous EEG studies (Boutros et al., 2008). Recent fMRI studies (Bluhm et al., 2007; Greicius, 2008) demonstrated reduced activity in patients with schizophrenia during rest in brain regions corresponding to the default‐mode network (Gusnard and Raichle, 2001). A combined EEG–fMRI study reported a close relationship between theta power and BOLD signal during rest and demonstrated that frontal theta power was negatively correlated with the BOLD signal in the default‐mode network (Scheeringa et al., 2008). The current findings of increased theta power during rest in schizophrenia are, thus, in line with these prior observations. These results are unlikely to be driven by increased drowsiness in patients (due to medication), as wakefulness ratings did not differ between groups and vigilance related increases in theta power are typically also paralleled by changes in delta and alpha activity (Olbrich et al., 2009). Thus, the current study demonstrates that abnormal resting‐state theta oscillatory activity can account for reduced task‐related oscillatory activity, which is increasingly recognized as a central mechanism associated with the neurocognitive deficits in schizophrenia (Kissler et al., 2000; Uhlhaas et al., 2008).
Previous studies demonstrated that the likelihood of perceiving unexpected stimuli in inattentional blindness tasks strongly depends on the resources that are consumed by the task. For instance, subjects are more likely to miss the unexpected event if the selective attention task is more demanding (Simons and Chabris, 1999), or if they have low working‐memory capacity (Richards et al., 2010). The present finding that patients suffering from schizophrenia have problems with perceiving the unexpected event fits with these observations as impaired processing resources are one of the major cognitive deficits in schizophrenia (Silver et al., 2003). Crucially, there were no differences in medication, IQ, or duration of illness between patients who reported to perceive and patients who did not report to perceive the unexpected event. Thus, the group differences in inattentional blindness cannot be attributed to unspecific effects (e.g. medication) alone. One could argue that the behavioral measure of inattentional blindness is based on a single trial, which might be too “noisy” for experimental investigation. However, the inattentional blindness phenomenon is considered a robust and highly replicable finding (Simons and Chabris, 1999; Most et al., 2001; Richards et al., 2010), despite being measured on a single trial basis supporting its applicability in experimental neurophysiological studies.
Group differences in task‐related brain oscillatory activity arose specifically in the theta band, which replicates findings demonstrating that patients with schizophrenia show less task‐related theta activity, especially during working memory (Schmiedt et al., 2005; Haenschel et al., 2009). Importantly, differences in task‐related theta power were also found between patients who reported to perceive and patients who did not report perceiving the unexpected event, which demonstrates that the differences in task‐related theta power cannot be attributed to medication. Interestingly, the patients who reported perceiving the unexpected event showed similar resting‐state and task‐related theta activity as healthy controls. These results are in line with a previous study showing residual cognitive functions in patients with schizophrenia (Haenschel et al., 2010), and suggest resting‐state theta oscillations to be an index of such preserved cognitive functions.
Source localization demonstrated a lack of task‐related theta power in left superior prefrontal and parietal regions (superior‐frontal‐gyrus and precuneus) and right inferior prefrontal and parietal regions (inferior‐frontal‐gyrus and temporo‐parietal‐junction). These regions correspond to the dorsal and ventral attention networks which control goal‐directed (top‐down) and stimulus‐driven (bottom‐up) direction of attention (Corbetta and Shulman, 2002). According to this framework, the right ventral attentional system controls attention which is captured by an unexpected event. The finding that patients diagnosed with schizophrenia show less task‐related theta activity in these brain regions, therefore, fits the behavioral observation that patients were more likely to miss the unexpected event. Group differences in task‐related theta power emerged also in the dorsal attention network, presumably reflecting top–down control of attention, consistent with prior findings indicating that patients with schizophrenia have difficulties in goal directed attention (Gur et al., 2007). Reduced theta power in patients was also present in visual processing areas, which corroborates a previous fMRI study showing reduced activity in visual processing regions in patients with schizophrenia (Haenschel et al., 2007), and is consistent with numerous behavioral observations of impaired visual perception in schizophrenia (Cadenhead et al., 1998; Butler et al., 2003).
The inattentional blindness task that was used in the current study likely triggers two cognitive processes: (i) working memory (counting the edge‐bounces), and (ii) selective attention (focusing attention on the white object). Prior studies suggest that inattentional blindness is modulated by both working memory (Richards et al., 2010), and selective attention processes (Most et al., 2001). Thus, it is unclear whether the present group difference in inattentional blindness was driven by a deficit in working memory or attention or both. As theta oscillations have been linked to working memory processes (Sauseng et al., 2010), the current results suggest a working memory rather than a selective attention deficit in schizophrenia. Whether this holds, however, has to be examined by future studies.
CONCLUSIONS
To conclude, the current study demonstrates that abnormally high resting‐state theta power can account for the lack of task‐related oscillatory activity in schizophrenia, and the inability to perceive a salient stimulus during the execution of a demanding cognitive task. These results highlight the role of resting‐state brain oscillations for task‐related oscillatory activity and behavior (Hanslmayr et al., 2011) and provide important implications for future studies. Investigations of abnormal brain oscillatory activity induced by sensory stimulation or cognitive tasks in psychiatric disorders should also examine differences in resting‐state activity. Moreover, the current data suggest that theta activity during rest might represent a potential candidate for neurocognitive remediation in schizophrenia (e.g., via EEG‐Biofeedback, pharmacotherapy or psychotherapy).
Supporting information
Supporting Information Movie 1
Supporting Information Movie 2
Supporting Information
Acknowledgments
The authors thank Brigitte Rockstroh and Corinna Haenschel for their insightful comments on previous versions of the manuscript.
REFERENCES
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RWJ, Théberge J, Schaefer B, Williamson P (2007): Spontaneous low‐frequency fluctuations in the BOLD signal in schizophrenic patients: Anomalies in the default network. Schizophr Bull 33:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W (2008): The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res 99:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, DeSanti LA, Maddox J, Harkavy‐Friedman JM, Amador XF, Goetz RR, Javitt DC, Gorman JM (2003): Visual backward‐masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res 59:199–209. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Serper Y, Braff DL (1998): Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol. Psychiatry 43:132–138. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Fries P (2005): A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cognit Sci 9:474–480. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Kastner J, Wagner M, Hawes S, Ebersole JS (2002): A standardized boundary element method volume conductor model. Clin Neurophysiol 113:702–712. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D (1997): High‐resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cerebral Cortex 7:374–385. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Burgos G, Lewis DA (2008): GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull 34:944–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M (2008): Resting‐state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 21:424–430. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R (2001): Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA 98:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Loughead J, Snyder W, Kohler C, Elliott M, Pratiwadi R, Ragland JD, Bilker WB, Siegel SJ, Kanes SJ, Arnold SE, Gur RC (2007): Visual attention circuitry in schizophrenia investigated with oddball event‐related functional magnetic resonance imaging. Am J Psychiatry 164:442–449. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001): Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska‐Jagiela A, Maurer K, Singer W, Linden DEJ (2007): Contribution of impaired early‐stage visual processing to working memory dysfunction in adolescents with schizophrenia: A study with event‐related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry 64:1229–1240. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, Linden DEJ, Rodriguez E (2009): Cortical oscillatory activity is critical for working memory as revealed by deficits in early‐onset schizophrenia. J Neurosci 29:9481–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenschel C, Linden DE, Bittner RA, Singer W, Hanslmayr S (2010): Alpha phase locking predicts residual working memory performance in schizophrenia. Biol Psychiatry 68:595–598. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bäuml, K‐H (2007): Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage 37:1465–1473. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Gross J, Klimesch W, Shapiro KL (2011): The role of alpha oscillations in temporal attention. Brain Res Rev 67:331–343. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Pastötter B, Bäuml K‐H, Gruber S, Wimber M, Klimesch W (2008): The electrophysiological dynamics of interference during the Stroop task. J Cognit Neurosci 20:215–225. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Spitzer B, Bäuml K‐H (2009): Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cerebral Cortex 19:1631–1640. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajós M (2008): Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov 7:68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987): The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Kissler J, Müller MM, Fehr T, Rockstroh B, Elbert T (2000): MEG gamma band activity in schizophrenia patients and healthy subjects in a mental arithmetic task and at rest. Clin Neurophysiol 111:2079–2087. [DOI] [PubMed] [Google Scholar]
- Lehrl S (2005): Mehrfachwahl‐Wortschatz‐Intelligenztest MWT‐B. Bahlingen: Spitta Verlag. [Google Scholar]
- Mack A, Rock I (1998): Inattentional Blindness. Cambridge, MA: MIT Press. [Google Scholar]
- Most SB, Simons DJ, Scholl BJ, Jimenez R, Clifford E, Chabris CF (2001): How not to be seen: The contribution of similarity and selective ignoring to sustained inattentional blindness. Psychol Sci 12:9–17. [DOI] [PubMed] [Google Scholar]
- Olbrich S, Mulert C, Karch S, Trenner M, Leicht G, Pogarell O, Hegerl U (2009): EEG‐vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage 45:319–332. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J‐M (2011): FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A, Hannon EM, Derakshan N (2010): Predicting and manipulating the incidence of inattentional blindness. Psychol Res 74:513–523. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, Klimesch W (2010): Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev 34:1015–1022. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MCM, Petersson KM, Oostenveld R, Norris DG, Hagoort P (2008): Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int J Psychophysiol 67:242–251. [DOI] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Basar‐Eroglu C (2005): Event‐related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cognit Brain Res 25:936–947. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC (2003): Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry 160:1809–1816. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Chabris CF (1999): Gorillas in our midst: sustained inattentional blindness for dynamic events. Perception 28:1059–1074. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolić D, Singer W (2008): The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull 34:927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen J‐M, Oostenveld R, Jensen O (2008): Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci 28:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, Herrmann WM, Coppola R (2000): Schizophrenia: reduced signal‐to‐noise ratio and impaired phase‐locking during information processing. Clin Neurophysiol 111:837–849. [DOI] [PubMed] [Google Scholar]
- Woods SW (2003): Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64:663–667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Movie 1
Supporting Information Movie 2
Supporting Information


