Abstract
Obesity and overweight are often defined by the body mass index (BMI), which associates with metabolic and cardiovascular disease, and possibly with dementia as well as variations in brain volume. However, body fat distribution and abdominal obesity (as measured by waist circumference) is more strongly correlated with cardiovascular and metabolic risk than is BMI. While prior studies have revealed negative associations between gray matter tissue volumes and BMI, the relationship with respect to waist circumference remains largely unexplored. We therefore investigated the effects of both BMI and waist circumference on local gray matter volumes in a group of 115 healthy subjects screened to exclude physical or mental disorders that might affect the central nervous system. Results revealed significant negative correlations for both BMI and waist circumference where regional gray matter effects were largest within the hypothalamus and further encompassed prefrontal, anterior temporal and inferior parietal cortices, and the cerebellum. However, associations were more widespread and pronounced for waist circumference than BMI. Follow‐up analyses showed that these relationships differed significantly across gender. While associations were similar for both BMI and waist circumference for males, females showed more extensive correlations for waist circumference. Our observations suggest that waist circumference is a more sensitive indicator than BMI, particularly in females, for potentially determining the adverse effects of obesity and overweight on the brain and associated risks to health. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: obesity, BMI, VBM, hypothalamus, morphometry, brain, sex differences
INTRODUCTION
Overweight and obesity represent a major public health concern; ∼ 1.6 billion and 400 million people, respectively meet criteria for these conditions worldwide [Abelson and Kennedy,2004; WHO,2006]. Body mass index (BMI) and clearly defined BMI cut‐offs [WHO,2000] are commonly used as the standard for measuring and diagnosing overweight and obesity. This highly reproducible measure is shown to correlate with metabolic or cardiovascular disease and other health risks [Field et al.,2001; Wang and Beydoun,2007; Wilson et al.,2002]. However, BMI does not reflect fat distribution within the body and a shift in body fat distribution toward abdominal obesity appears to be more closely correlated with secondary adverse effects than BMI [Dalton et al.,2003; de Koning et al.,2007; Zhu et al.,2004]. Moreover, initial evidence suggests that waist circumference, which estimates abdominal fat more directly, is increasing at greater rate than can be attributable to increases in BMI in US populations [Walls et al.,2010]. This is particularly salient given the apparent higher predictive value of waist circumference for determining secondary adverse effects of obesity.
The condition of overweight and obesity appears to affect most organ systems, including the central nervous system [Gustafson et al.,2003]. While a growing body of literature indicates that a higher BMI is associated with lower global or local brain volumes [Gustafson et al.,2004; Pannacciulli et al.,2006; Raji et al.,2010; Taki et al.,2008; Walther et al.,2010; Ward et al.,2005], evidence concerning the effects of fat distribution—e.g., as measured by waist circumference—on brain volume is extremely sparse. Notwithstanding, one recent study showed that whole brain volume reductions are more closely associated with visceral adiposity than overall body weight [Debette et al.,2010]. In addition, this prior study measured temporal horn ventricular volume and reported a trend to indicate relationships with higher BMI/waist circumference though no further local measures of gray matter volume were assessed. Thus the effects of body fat distribution on local brain volume and potential differences with respect to BMI remain largely unknown.
In sum, the reported higher predictive value of waist circumference for adverse health effects, proportionally larger population increases in waist circumference relative to BMI, and the findings by Debette et al. for overall brain volume suggest that waist circumference may constitute a medically more meaningful variable than BMI alone. Furthermore, in spite of the lack of prior data concerning the relationships between waist circumference and variations in local brain tissue volume, the above observations provide leverage to hypothesize that waist circumference may be a more relevant predictor of the effects of overweight and obesity on local brain volume. To determine and clarify the effects of BMI and waist circumference on regional gray matter volume we thus analyzed the high‐resolution whole brain images of 115 carefully screened healthy subjects using partial volume tissue segmentation and high dimensional warping for optimized voxel‐based morphometry. Further, since differential effects have been reported for males and females as well as with respect to age, at least with respect to BMI‐related influences on brain structure [Luchsinger and Gustafson,2009; Taki et al.,2008], follow‐up analyses were additionally performed to examine the modulating effects of these variables in the current study.
MATERIAL AND METHODS
Subjects
Participants included 115 healthy Caucasian adults (54 M/61 F, see Table I) initially recruited for inclusion in the international consortium for brain mapping (ICBM) database [Mazziotta et al.,1995]. All subjects received extensive medical and neurological examination to minimize the inclusion of subjects with any medical disorder that could possibly affect brain structure or function [Mazziotta et al.,2009]. That is, any history of medical, neurological, and psychiatric illnesses, any trauma leading to loss of consciousness, concussion, spinal cord or peripheral nerve injury, and with very few exceptions (e.g., history of antibiotics) any medication (prescription or over the counter) led to exclusion from the study. Furthermore, a clinical exam ensured that study participants had a blood pressure of <140/90 mm Hg, a pulse of <100 and >50 (without irregularities), and no signs of chronic or acute systemic disorder, as well as no abnormal neurological signs and a Mini Mental score of ≥ 28. Overweight and obesity as defined by BMI cutoffs were not exclusion criteria. Of all subjects, 11 qualified as obese with a BMI ≥ 30 and 31 qualified as overweight with a BMI between 25 and 30. The extensive screening procedure resulted in an extremely healthy sample without neurological or psychiatric disorders, no high blood pressure or evidence for chronic or acute diseases as assessed by a physical examination, known history of diabetes mellitus or lipid disorders, and no use of prescription, over the counter or illicit drugs with the exception of occasional use for disease prevention [for details see Mazziotta et al.,2009]. However, with respect to obesity it is important to note that the screening did not include specific laboratory testing for occult diabetes mellitus or lipid disorder. Thus, even if no evidence emerged from the medical history and the physical examination, it is possible that an undiagnosed metabolic syndrome in a given subject might have remained unrecognized. As part of the study procedures, weight, height, and waist circumference were measured and recorded. The BMI was calculated for each subject by dividing the weight in kilogram by the squared height in meters.
Table I.
Demographic data
| Mean ± std | Range | |
|---|---|---|
| Age (years) | 45.17 ± 15.45 | 18–80 |
| Height (m) | 1.71 ± 0.1 | 1.47–1.96 |
| Weight (kg) | 73.06 ± 14.76 | 44–137 |
| BMI (kg m−2) | 25.02 ± 4.13 | 18.18–42.37 |
| Waist circumference (m) | 0.87 ± 0.12 | 0.67–1.32 |
Image Acquisition
For each subject, five T1‐weighted images were sequentially acquired on a Siemens Sonata 1.5T Scanner at UCLA Brain Mapping Center using a high‐resolution T1‐weighted MP‐RAGE (magnetization‐prepared rapid‐acquisition gradient echo) sequence with the following parameters: repetition time = 1,900 ms; echo time = 4.38 ms; flip angle = 15°; 160 contiguous 1‐mm sagittal slices; field of view = 256 × 256 mm2; matrix size = 256 × 256; voxel size = 1.0 × 1.0 × 1.0 mm3. All five images per subject were realigned and averaged to gain a higher signal to noise ratio.
Image Preprocessing
Individual T1‐weighted structural images were processed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html—described in detail in http://dbm.neuro.uni-jena.de/vbm8/VBM8-Manual.pdf). In short, all images were bias‐field corrected and segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). The segmentation algorithm accounted for partial volume effects [Tohka et al.,2004] using adaptive maximum a posteriori estimations [Rajapakse et al.,1997] and a hidden Markov random field model [Cuadra et al.,2005]. Using the diffeomorphic registration algorithm described by Ashburner [Ashburner,2007], the individual GM and WM segments in native‐space were then nonlinearly normalized to the DARTEL‐Template supplied with the VBM8 toolbox (see http://dbm.neuro.uni-jena.de). The voxel values of the normalized tissue segments were then multiplied (“modulated”) with the nonlinear component of the Jacobian determinant, which was derived from the aforementioned normalization step. The resulting gray matter segments thus preserve the local gray matter volume as identified in native space corrected for total brain size [Buckner et al.,2004]. A quality check was performed using tools from the SPM Toolbox and individual visual assessment, which yielded no artifacts or failed segmentation and normalization of the data. Prior to statistical analysis the segments were smoothed with an 8 × 8 × 8 mm3 FWHM kernel.
Statistical Analysis
Effects of BMI and waist circumference on local gray matter volume were tested using multiple regression analyses in a general linear model framework. Because BMI and waist circumference are highly correlated (Pearson's r = 0.7, P = 3.6 × 10−18), they were tested in two different models, one for BMI and one for waist circumference [Poline,2007]. Age and gender were included as covariates. In addition, we tested for the possible modulating effects of age and gender by examining interactions between males (n = 54) and females (n = 61) and age. In the presence of a significant interaction, correlations were examined within each group separately. All results were corrected for multiple comparisons by controlling the false discovery rate (FDR) at q = 0.05. Additionally, a cluster extent threshold was applied to account only for clusters that exceeded the expected number of voxels per cluster as calculated according to random field theory [Worsley et al.,1996].
RESULTS
For both BMI and waist circumference, widespread negative correlations with gray matter were observed throughout the brain. These negative correlations were located in the vicinity of the hypothalamus, the frontal and temporal lobes as well as the cerebellum. Significant correlations for waist circumference were more spatially expansive than those observed for BMI (Fig. 1). In the frontal lobe, the effects of both measures comprised the orbitofrontal cortex, frontal pole, the anterior parts of inferior and superior frontal gyri, as well as dorsomesial prefrontal cortices. Within the temporal lobe, reductions in the hippocampi and the middle and superior temporal gyri were observed. In the cerebellum both the vermis and widespread parts of the cerebellar hemispheres were affected. Regardless of the measure of interest (BMI or waist circumference), the global maximum (i.e., the smallest P‐value) was located in the hypothalamus (see Figs. 2 and 3, and Tables II and III). Additional less significant regions were also observed in the insula, the globus pallidus, and the inferior parietal lobe including the angular and supramarginal gyri (for waist circumference only). Throughout the brain, no positive correlations between BMI or waist circumference and gray matter volume were observed.
Figure 1.
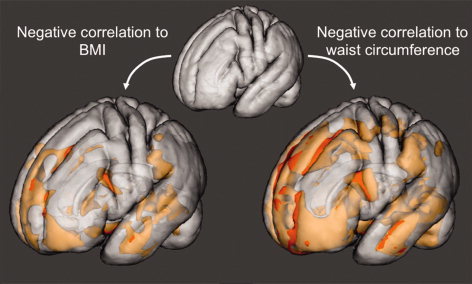
Negative correlations with BMI (left) and waist circumference (right) overlaid onto the semitransparent mean template created from the whole study population (P < 0.05, FDR corrected for multiple comparisons). Negative correlations with waist circumference are more widespread but comprise of similar areas as negative correlations with BMI. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 2.
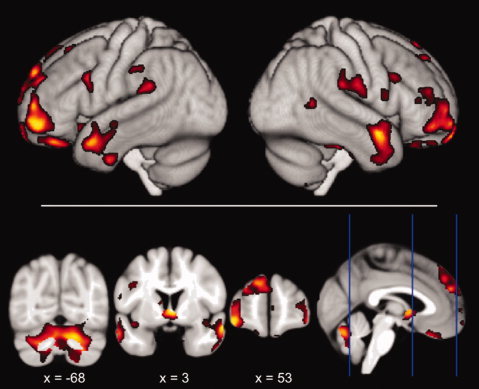
Negative correlations with BMI overlaid onto the mean template created from the whole study population (FDR corrected for multiple comparisons), depicted in neurological convention. In particular, prefrontal, hypothalamic, cerebellar, and temporal regions show a decrease of gray matter with increasing BMI. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
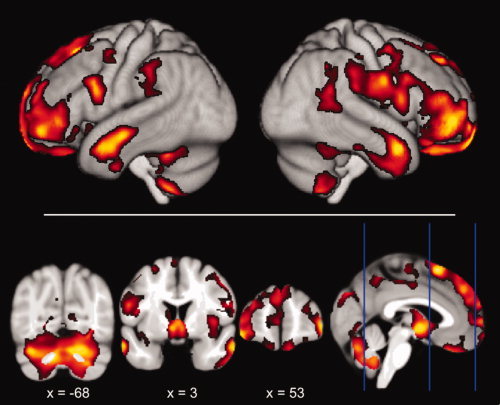
Negative correlations with waist circumference overlaid onto the mean template created from the whole study population (FDR corrected for multiple comparisons) in neurological convention. The decrease of gray matter with increasing waist circumference is more extended than with BMI, but also most pronounced in prefrontal, hypothalamic, cerebellar, and temporopolar regions. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Maxima of significant clusters negatively correlating with BMI, listed by cluster
| Maximum (anatomic location) | Extent (mm3) | Significance | MNI | |||
|---|---|---|---|---|---|---|
| P (FDR) | T | X | Y | Z | ||
| Hypothalamus | 1,333 | 0.0174 | 5.1453 | 3 | 3 | 1.5 |
| 0.0225 | 3.5118 | 6 | −3 | −9 | ||
| Left caudate head | 0.0218 | 3.5793 | −7.5 | 13.5 | 0 | |
| Left superior frontal gyrus | 9,400 | 0.0174 | 4.8610 | −24 | 24 | 51 |
| Left mid frontal gyrus | 0.0174 | 4.7594 | −39 | 52.5 | −10.5 | |
| Right frontal pole | 0.0174 | 4.5808 | 9 | 63 | −21 | |
| Right superior temporal sulcus | 1,356 | 0.0174 | 4.7986 | 60 | 3 | −18 |
| Right mid temporal gyrus | 0.0242 | 3.3667 | 54 | 10.5 | −34.5 | |
| Right superior temporal gyrus | 0.0249 | 3.3229 | 57 | 9 | −12 | |
| Right cerebellar hemisphere | 10,540 | 0.0174 | 4.5589 | 18 | −66 | −30 |
| Cerebellar vermis | 0.0174 | 4.1783 | −1.5 | −72 | −24 | |
| Left cerebellar hemisphere | 0.0174 | 4.1176 | 25.5 | −64.5 | −36 | |
| Left superior temporal sulcus | 982 | 0.0174 | 4.3029 | −54 | 9 | −27 |
| Left mid temporal gyrus | 0.0192 | 3.8222 | −54 | −7.5 | −40.5 | |
| 0.0286 | 3.1445 | −63 | −6 | −21 | ||
| Left inferior frontal gyrus | 620 | 0.0187 | 3.8795 | −49.5 | 10.5 | 27 |
| Right mid temporal gyrus | 234 | 0.0192 | 3.8209 | 55.5 | −52.5 | 6 |
| 0.0355 | 2.8813 | 51 | −57 | 15 | ||
| Left supramarginal gyrus | 1,221 | 0.0198 | 3.7747 | −54 | −34.5 | 25.5 |
| 0.0212 | 3.6709 | −45 | −36 | 24 | ||
| Left superior temporal gyrus | 0.0200 | 3.7564 | −48 | −40.5 | 16.5 | |
| Left fusiform gyrus | 298 | 0.0211 | 3.6894 | −37.5 | −43.5 | −16.5 |
| 0.0389 | 2.7812 | −37.5 | −33 | −24 | ||
| Left intraparietal sulcus | 208 | 0.0212 | 3.6565 | −33 | −52.5 | 36 |
| Right supramarginal gyrus | 528 | 0.0215 | 3.6067 | 63 | −27 | 22.5 |
| 0.0276 | 3.1864 | 57 | −25.5 | 30 | ||
| Right postcentral gyrus | 0.0222 | 3.5254 | 64.5 | −15 | 22.5 | |
| Left hippocampus | 250 | 0.0224 | 3.5190 | −21 | −33 | −12 |
| Left inferior frontal gyrus | 827 | 0.0245 | 3.3443 | −28.5 | 24 | −15 |
| Left anterior basal insula | 0.0257 | 3.2829 | −33 | 16.5 | −13.5 | |
| Left basal insula | 0.0274 | 3.2014 | −39 | 3 | −15 | |
| Right mid cingulate gyrus | 198 | 0.0360 | 2.8654 | 9 | −4.5 | 40.5 |
| 0.0372 | 2.8293 | 12 | −9 | 46.5 | ||
| 0.0445 | 2.6334 | 9 | −19.5 | 40.5 | ||
P = corrected P‐value; T = T‐value; X,Y,Z = stereotactic coordinates in MNI space.
Table III.
Maxima of significant clusters negatively correlating with waist circumference listed by cluster
| Maximum (anatomic location) | Extent (mm3) | Significance | MNI | |||
|---|---|---|---|---|---|---|
| P (FDR) | T | X | Y | Z | ||
| Hypothalamus | 76,992 | 0.0013 | 5.6049 | 6 | −3 | −6 |
| Left mid frontal gyrus | 0.0013 | 5.4619 | −24 | 22.5 | 52.5 | |
| Hypothalamus | 0.0013 | 5.3409 | −4.5 | −1.5 | −6 | |
| This cluster extends over frontal pole, orbitofrontal cortex, inferior frontal cortex, superior frontal cortex, insulae, right inferior parietal cortex, pallidum, hippocampi, fusiform gyri, and cerebellum | ||||||
| Right superior temporal sulcus | 1,697 | 0.0013 | 5.1171 | 58.5 | 7.5 | −25.5 |
| Right mid temporal gyrus | 0.0046 | 3.6726 | 55.5 | 3 | −39 | |
| 0.0108 | 3.1697 | 61.5 | −13.5 | −33 | ||
| Left superior temporal sulcus | 2,800 | 0.0013 | 4.8653 | −60 | −13.5 | −15 |
| Left mid temporal gyrus | 0.0015 | 4.6759 | −63 | −4.5 | −24 | |
| 0.0015 | 4.6315 | −58.5 | 3 | −24 | ||
| Left inferior frontal gyrus | 4,207 | 0.0016 | 4.5379 | −52.5 | 9 | 24 |
| Left supramarginal gyrus | 0.0017 | 4.4851 | −49.5 | −34.5 | 31.5 | |
| 0.0024 | 4.2287 | −43.5 | 0 | 15 | ||
| Right cuneus | 1,394 | 0.0017 | 4.5245 | 6 | −78 | 36 |
| 0.0127 | 3.0715 | 6 | −90 | 19.5 | ||
| Left cuneus | 0.0048 | 3.6539 | −4.5 | −81 | 33 | |
| Left angular gyrus | 474 | 0.0034 | 3.8998 | −39 | −58.5 | 36 |
| Left mid occipital gyrus | 430 | 0.0129 | 3.0649 | −34.5 | −79.5 | 27 |
| 0.0325 | 2.4737 | −24 | −81 | 18 | ||
| Right lingual gyrus | 451 | 0.0205 | 2.7723 | 15 | −58.5 | 7.5 |
| Right calcarine gyrus | 0.0219 | 2.7279 | 10.5 | −66 | 6 | |
P = corrected P‐value; T = T‐value; X,Y,Z = stereotactic coordinates in MNI space. Note that the first cluster encompasses large parts of the brain that do not show their own maxima despite being identified as significant (descriptively listed below).
In addition, we tested for possible age and gender interactions to determine if these variables may influence the observed correlations with BMI and waist circumference in this healthy sample. While significant interactions for age were not detected in any brain region, significant interactions for gender for both BMI and waist circumferences were observed (maps not shown). Correlations were thus subsequently examined within males and females separately. For BMI, follow‐up analyses (Fig. 4) within females revealed significant negative correlations with local gray matter within one cluster in the cerebellum only. In males BMI was negatively correlated with local gray matter across more extensive and distributed brain regions. These regions comprised of prefrontal, mesial frontal and orbitofrontal, insular and temporal cortices, and also included more spatially localized hypothalamic, hippocampal, parietal, occipital, and cerebellar regions. The most pronounced effect (i.e., the region exhibiting the smallest P‐value) was located in the right mesial frontal cortex.
Figure 4.
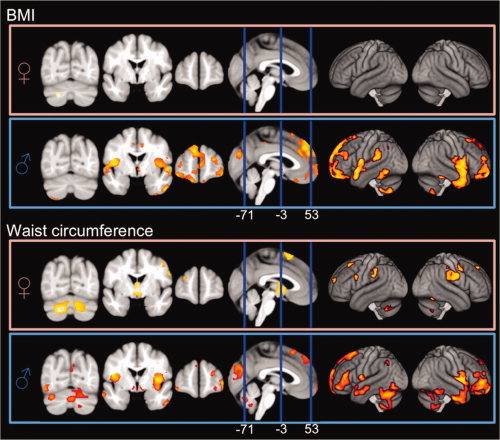
Negative correlations between BMI/waist circumference and local gray matter volume for male and female groups (FDR corrected for multiple comparisons). In males, correlations with respect to BMI/waist circumference are relatively similar. In females, BMI is negatively correlated in the left cerebellum only, while waist circumference is negatively correlated with numerous additional regions. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In contrast to BMI, for waist circumference negative correlations were much more expansive when examined in females separately. Significance was observed in the hypothalamus, widespread cerebellar regions, and smaller parietal, frontal, right parahippocampal, and occipital regions, with the most pronounced effects observed in the left cerebellum (smallest P‐value). For males, negative correlations with waist circumference were relatively similar to those seen for BMI, although associations became less pronounced within frontal regions and more pronounced within the cerebellum and hypothalamus. The overall smallest P‐value was located in left mid‐insula cortex. Positive correlations between local gray matter volume and BMI/waist circumference were absent in both males and females.
DISCUSSION
In the present analysis we observed widespread reductions of gray matter volume in association with BMI and waist circumference in bilateral prefrontal cortex, anterior temporal cortex, inferior parietal cortex and cerebellar and midbrain hypothalamic regions. Waist circumference, which estimates abdominal fat, was associated with more spatially extensive reductions in gray matter volume than BMI. Together with prior findings indicating that a fat distribution biased toward abdominal adiposity more accurately predicts the risk of cardiovascular and metabolic diseases [Dalton et al.,2003; Zhu et al.,2004], our observations suggest that abdominal obesity is more closely linked with regional gray matter variations than is BMI and that associations with waist circumference may be of greater medical relevance.
BMI has been used as the standard to measure and define obesity given that it is considered relatively accurate, reproducible, accessible, and inexpensive. These characteristics have thus led to the more frequent use of BMI rather than of other measures of body composition, such as hydrostatic weighing, bioelectrical impedance, or devices such as the BOD POD, even though they may estimate the percentage of body fat more precisely. However, while BMI allows adjustment of body weight to height, it cannot distinguish between tissue content and distribution. This can lead to bias, as muscles have a higher specific weight than fat. A relative increase in muscular mass can therefore lead to an overestimation of BMI or vice versa. Still, while the aforementioned measures of body composition can overcome this drawback, none gives an estimate of the fat distribution toward abdominal obesity, which appears to be more closely correlated with secondary adverse consequences to health than BMI [Dalton et al.,2003; de Koning et al.,2007; Zhu et al.,2004].
In contrast to other measures, waist circumference yields an estimate of abdominal adiposity and is less sensitive to changes in muscle mass. Our observations of more widespread negative correlations for gray matter volume with waist circumference than with BMI may thus indicate two different but probably complementary conclusions: On the one hand, a shift in body fat distribution toward the abdomen may be a critical part of the already observed association between BMI and gray matter volume. Abdominal adiposity would then likely be a more sensitive measure for indicating possible neurological risks such as dementia or other neurodegenerative diseases associated with obesity [Doherty et al.,2008; Gustafson et al.,2003]. On the other hand the measured waist circumference might be less biased by variations in tissue content, which would at least partly explain the more extensive and pronounced correlations with regional gray matter reductions. In either case, overall BMI appears a less sensitive measure with which to investigate the effects of overweight and obesity on the brain. However, it remains the defining measure to differentiate weight status into “normal,” “overweight,” and “obese” categories.
Prior studies commonly report a widespread negative correlation between BMI and regional brain [Ho et al.,2010; Raji et al.,2010] and gray matter volumes [Pannacciulli et al.,2006; Taki et al.,2008; Walther et al.,2010]. This replication of results between different studies certainly provides some confidence in their validity. The present findings of associations between BMI and diminished local gray matter volumes match well with the observations frequently reported in literature. To our knowledge, very few studies have investigated the relation between waist circumference and brain volume. However, one report confirms stronger associations between waist circumference and diminished whole brain volume than BMI and diminished whole brain volume [Debette et al.,2010]. Furthermore, a trend for larger temporal horn volumes was observed, which were interpreted to reflect potentially linked reductions of hippocampal volume. In the present study, we show that there are stronger associations between local gray matter volumes and waist circumference than between BMI and local gray matter volumes—which is consistent with the findings of Debette et al. [2010]. In addition, the present study demonstrates more directly the negative association between waist circumference and hippocampal volume (see Table III), which was implicated in the prior study.
In agreement with prior studies showing differential effects in men and women with respect to BMI–gray matter relationships [Taki et al.,2008], the present study similarly found associations to be modulated by gender. Specifically, our results showed relatively prominent BMI effects within males, which were remained similar for waist circumference. In contrast, effects of BMI in females were subtle though more pronounced and regionally expansive for waist circumference. These findings match well the results of Taki et al. [2008], who observed effects of BMI in males, but not in females. Further, results are in line with observations focused on such relationships with respect to cardiovascular risk. That is, in females waist circumference alone explained the observed cardiovascular risk; in males a combination of both waist circumference and BMI was optimal for risk prediction [Zhu et al.,2004]. These results might suggest that BMI/waist circumference effects on the brain may also indicate cardiovascular risk (at least in healthy subjects). This assumption, however, warrants further investigation.
Several different mechanisms may account for the observed association between diminished gray matter and high BMI/waist circumference. For example, growing evidence points to associations between overweight and obesity with genetic factors [Maes et al.,1997; Meyre et al.,2009; Willer et al.,2009], while other data supports the influence of the signaling properties of the hormone leptin [Moraes et al.,2009; Scarpace and Zhang,2009] and/or points to behavior as a possible mediator [Benoit et al.,2010; Cohen,2008; Stoeckel et al.,2008]. However, none of these factors alone can be shown as causal. Rather, they seem linked and to interact leading to the outcome of overweight and obesity [Chisholm et al.,1998].
In the present study we included well‐screened healthy adults with a focus on Caucasian subjects to exclude the possibility that variations in body composition or brain structure amongst different racial or ethnic groups could influence findings [Camhi et al.,2010]. Although we cannot completely rule out a covert diabetes mellitus or lipid disorder that may be linked to obesity, we can exclude the possibility that these diseases were known, medicated, or had already led to clinical sequella that would have been evident during history taking and physical examination. It is therefore rather unlikely that possible confounds such as the presence of a metabolic syndrome can explain the observed effects.
It is important to note that the rigorous screening, although facilitating investigation of the effects of obesity on the brain independent of secondary disorders, may also be viewed a potential limitation. As reported previously [Mazziotta et al.,2009], only 10.7% of all subjects who considered themselves healthy and willing to participate, satisfied the inclusion criteria. Given that obesity is linked with several secondary disorders [Field et al.,2001; Wang and Beydoun,2007; Wilson et al.,2002], and patients with these related disorders have been excluded from this study, the current sample might not be strictly representative of the average obese population (especially in the upper age ranges, where links between obesity and disease risk become stronger).
CONCLUSION
Results from this study support that regional reductions in gray matter are associated with obesity and point to a stronger association between abdominal obesity and diminished gray matter than with BMI, particularly in females. This may suggest that abdominal obesity is a more relevant risk factor for cardiovascular or metabolic disease, and potentially also for neurodegenerative disorders. However, future studies may clarify the impact of fat distribution versus the impact of tissue content on the brain to establish a more predictive measure than BMI. Subsequent studies may also thus investigate the interactions between genes, hormones and behavior toward obesity‐related changes in brain structure more effectively. In view of the high prevalence of obesity [WHO,2006], defined to have reached epidemic proportions, a better understanding of the links with central nervous system structure and function could have major implications for healthcare and prevention.
REFERENCES
- Abelson P, Kennedy D ( 2004): The obesity epidemic. Science 304: 1413. [DOI] [PubMed] [Google Scholar]
- Anan F, Masaki T, Shimomura T, Fujiki M, Umeno Y, Eshima N, Saikawa T, Yoshimatsu H. ( 2010): Abdominal visceral fat accumulation is associated with hippocampus volume in nondementia patients with type 2 diabetes mellitus. Neuroimage 49: 57–62. [DOI] [PubMed] [Google Scholar]
- Ashburner J ( 2007): A fast diffeomorphic image registration algorithm. Neuroimage 38: 95–113. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Davis JF, Davidson TL ( 2010): Learned and cognitive controls of food intake. Brain Res 1350: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ ( 2004): A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas‐based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage 23: 724–738. [DOI] [PubMed] [Google Scholar]
- Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, Ravussin E, Ryan DH, Smith SR, Katzmarzyk PT ( 2011): The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: Sex and race differences. Obesity (Silver Spring) 19: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm DJ, Samaras K, Markovic T, Carey D, Lapsys N, Campbell LV ( 1998): Obesity: Genes, glands or gluttony? Reprod Fertil Dev 10: 49–53. [DOI] [PubMed] [Google Scholar]
- Cohen DA ( 2008): Neurophysiological pathways to obesity: Below awareness and beyond individual control. Diabetes 57: 1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP ( 2005): Comparison and validation of tissue modelization and statistical classification methods in T1‐weighted MR brain images. IEEE Trans Med Imaging 24: 1548–1565. [DOI] [PubMed] [Google Scholar]
- Dalton M, Cameron AJ, Zimmet PZ, Shaw JE, Jolley D, Dunstan DW, Welborn TA ( 2003): Waist circumference, waist‐hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med 254: 555–563. [DOI] [PubMed] [Google Scholar]
- de Koning L, Merchant AT, Pogue J, Anand SS ( 2007): Waist circumference and waist‐to‐hip ratio as predictors of cardiovascular events: Meta‐regression analysis of prospective studies. Eur Heart J 28: 850–856. [DOI] [PubMed] [Google Scholar]
- Debette S, Beiser A, Hoffmann U, Decarli C, O'Donnell CJ, Massaro JM, Au R, Himali JJ, Wolf PA, Fox CS, Seshadri S ( 2010): Visceral fat is associated with lower brain volume in healthy middle‐aged adults. Ann Neurol 68: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GH, Oldreive C, Harvey J ( 2008): Neuroprotective actions of leptin on central and peripheral neurons in vitro. Neuroscience 154: 1297–1307. [DOI] [PubMed] [Google Scholar]
- Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA ( 2001): Impact of overweight on the risk of developing common chronic diseases during a 10‐year period. Arch Intern Med 161: 1581–1586. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I ( 2003): An 18‐year follow‐up of overweight and risk of Alzheimer disease. Arch Intern Med 163: 1524–1528. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I ( 2004): A 24‐year follow‐up of body mass index and cerebral atrophy. Neurology 63: 1876–1881. [DOI] [PubMed] [Google Scholar]
- Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, Dinov ID, Toga AW, Saykin AJ, Shen L, et al. ( 2010): A commonly carried allele of the obesity‐related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci USA 107: 8404–8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Gustafson DR ( 2009): Adiposity and Alzheimer's disease. Curr Opin Clin Nutr Metab Care 12: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Eaves LJ ( 1997): Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 27: 325–351. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J ( 1995): A probabilistic atlas of the human brain: Theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage 2: 89–101. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Woods R, Iacoboni M, Sicotte N, Yaden K, Tran M, Bean C, Kaplan J, Toga AW ( 2009): The myth of the normal, average human brain—The ICBM experience: (1) Subject screening and eligibility. Neuroimage 44: 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, Durand E, Vatin V, Degraeve F, Proenca C, et al. ( 2009): Genome‐wide association study for early‐onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet 41: 157–159. [DOI] [PubMed] [Google Scholar]
- Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, et al. ( 2009): High‐fat diet induces apoptosis of hypothalamic neurons. PLoS One 4: e5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA ( 2006): Brain abnormalities in human obesity: A voxel‐based morphometric study. Neuroimage 31: 1419–1425. [DOI] [PubMed] [Google Scholar]
- Poline JKF, Pallier C, Penny WD ( 2007): Contrasts and classical inference In: Friston KAJT, Kiebel SJ, Nichols TE, Penny WD, editors. Statistical Parametric Mapping. London: Elsevier; pp 126–139. [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL ( 1997): Statistical approach to segmentation of single‐channel cerebral MR images. IEEE Trans Med Imaging 16: 176–186. [DOI] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM ( 2010): Brain structure and obesity. Hum Brain Mapp 31: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpace PJ, Zhang Y ( 2009): Leptin resistance: A prediposing factor for diet‐induced obesity. Am J Physiol Regul Integr Comp Physiol 296: R493–R500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW III, Twieg DB, Knowlton RC, Cox JE ( 2008): Widespread reward‐system activation in obese women in response to pictures of high‐calorie foods. Neuroimage 41: 636–647. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H ( 2008): Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 16: 119–124. [DOI] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A ( 2004): Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage 23: 84–97. [DOI] [PubMed] [Google Scholar]
- Walls HL, Stevenson CE, Mannan HR, Abdullah A, Reid CM, McNeil JJ, Peeters A ( 2011): Comparing trends in BMI and waist circumference. Obesity (Silver Spring) 19: 216–219. [DOI] [PubMed] [Google Scholar]
- Walther K, Birdsill AC, Glisky EL, Ryan L ( 2010): Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp 31: 1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA ( 2007): The obesity epidemic in the United States—Gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta‐regression analysis. Epidemiol Rev 29: 6–28. [DOI] [PubMed] [Google Scholar]
- Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC ( 2005): The effect of body mass index on global brain volume in middle‐aged adults: A cross sectional study. BMC Neurol 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO ( 2000): Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894: i–xii, 1–253. [PubMed] [Google Scholar]
- WHO . 2006. Obesity and Overweight. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, et al. ( 2009): Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB ( 2002): Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch Intern Med 162: 1867–1872. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC ( 1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Zhu S, Heshka S, Wang Z, Shen W, Allison DB, Ross R, Heymsfield SB ( 2004): Combination of BMI and waist circumference for identifying cardiovascular risk factors in whites. Obes Res 12: 633–645. [DOI] [PubMed] [Google Scholar]


