Abstract
Chemotherapy is associated with cognitive impairment in a subgroup of breast cancer survivors, but the neural circuitry underlying this side effect is largely unknown. Moreover, long‐term impairment has not been studied well. In the present study, functional magnetic resonance imaging (fMRI) and neuropsychological testing were performed in breast cancer survivors almost 10 years after high‐dose adjuvant chemotherapy (chemo group, n = 19) and in breast cancer survivors for whom chemotherapy had not been indicated (control group, n = 15). BOLD activation and performance were measured during an executive function task involving planning abilities (Tower of London) and a paired associates task for assessment of episodic memory. For the chemo group versus the control group, we found hyporesponsiveness of dorsolateral prefrontal cortex in the Tower of London, and of parahippocampal gyrus in the paired associates task. Also, the chemo group showed significantly impaired planning performance and borderline significantly impaired recognition memory as compared to findings in the control group. Whole‐brain analyses demonstrated hyporesponsiveness of the chemo versus the control group in very similar regions of bilateral posterior parietal cortex during both the Tower of London and the paired associates task. Neuropsychological testing showed a relatively stable pattern of cognitive impairment in the chemo group over time. These results indicate that high‐dose adjuvant chemotherapy is associated with long‐term cognitive impairments. These impairments are underpinned by (a) task‐specific hyporesponsiveness of dorsolateral prefrontal cortex and parahippocampal gyrus, and (b) a generalized hyporesponsiveness of lateral posterior parietal cortex encompassing attentional processing. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: chemotherapy, breast cancer, functional magnetic resonance imaging, cognitive function, late effects
INTRODUCTION
Breast cancer survivors who have received adjuvant chemotherapy frequently complain of cognitive problems that have a negative impact on daily life function. This phenomenon is sometimes referred to as “chemobrain.” Even 5 years after treatment, up to 50% of disease‐free cancer survivors report complaints related to memory and concentration [Ahles and Saykin,2001; Schagen et al.,2001; van Dam et al.,1998; Vardy et al.,2008; Weis et al.,2009]. Neuropsychological studies have demonstrated the occurrence of cognitive deficits, and objectively impaired performance can be detected in about 20–40% of cancer survivors that have received chemotherapy [or recent reviews, see Correa and Ahles,2008; Wefel et al.,2008].
Breast cancer is the most common type of cancer in women. In the United States and Western Europe, approximately one in nine women will develop breast cancer [Ries et al.,2008]. Adjuvant chemotherapy increases the cure rate in patients with high‐risk primary breast cancer and is nowadays administered in as much as 60% of patients below the age of 60. The term “adjuvant” refers to chemotherapy given after surgery during which all detectable disease has been removed, but where there remains a risk of relapse due to occult disease. The high number of breast cancer patients receiving adjuvant chemotherapy and their high long‐term survival rates necessitate a greater understanding of chemotherapy‐induced cognitive dysfunction and its underlying neural circuitry. Therefore, in the present study, functional imaging of the brain was performed in breast cancer survivors that had received high‐dose adjuvant chemotherapy. In addition, neuropsychological data were obtained to assess cognitive performance on widely used standardized tests.
The pattern of chemotherapy‐related cognitive dysfunction as demonstrated by standardized cognitive tests is typically diffuse and spans the domains of processing speed, attention, memory, and executive function. Based on these findings, it has been suggested that chemotherapy‐induced cognitive deficits reflect a frontal, subcortical neurotoxicity profile [Meyers,2008; Schagen and Vardy,2007]. Cytotoxic regimens may differentially affect cognitive functions, although specific neurotoxicity profiles associated with various regimens have not yet been documented. The fact that cognitive deficits are found in a subset of cancer survivors many years after administration of chemotherapy indicates that chemotherapy‐related cognitive dysfunction may not be a transient phenomenon.
In contrast to an accumulating body of neuropsychological literature, functional imaging studies in this field are scarce. In a positron‐emission tomography (PET) study, higher radiolabeled water (H2 15O) PET regional perfusion of left inferior prefrontal cortex during a verbal, cued memory recall task was found in 16 breast cancer survivors 5–10 years after treatment with standard‐dose chemotherapy as compared to findings in a group composed of five survivors not treated with chemotherapy and three healthy controls [Silverman et al.,2007]. No performance measures of the recall task were reported. Moreover, 18fluorodeooxyglucose (18FDG)‐PET resting state metabolic activity was positively correlated with performance (outside the scanner) on the Rey‐Osterreith Complex Figure Delayed Recall task in patients after chemotherapy, whereas such a relation was not found for patients that did not receive chemotherapy. Increased regional perfusion as measured using H2 15O‐PET was interpreted as a compensatory response to decreased resting state metabolism in the patients after chemotherapy (although resting state metabolism did not differ significantly between groups). In an fMRI case study investigating monozygotic twins, more brain areas were activated during a working memory task in the twin that received chemotherapy than in the healthy, untreated twin, although performance differences were absent [Ferguson et al.,2007].
In the present study, breast cancer survivors that had undergone four courses of adjuvant standard‐dose chemotherapy, followed by one course of high‐dose chemotherapy almost 10 years before study entry, were compared with survivors with low‐risk breast cancer not requiring chemotherapy. All patients were recruited from a previously conducted neuropsychological study by our group [Schagen et al.,2006] and were comparable to those reported in an earlier cross‐sectional study [van Dam et al.,1998]. Some of these patients also participated in an event‐related brain potential (ERP) study [Kreukels et al.,2006; Kreukels et al.,2008], that was carried out several years after the neuropsychological study.
Our previous neuropsychological studies were conducted 6 months and 2 years after chemotherapy. Patients treated with adjuvant chemotherapy including one high‐dose cycle showed a significantly higher level of cognitive impairment compared to patients without chemotherapy [van Dam et al.,1998] and healthy controls [Schagen et al.,2006]. No pretreatment baseline differences in cognitive performance were observed [Schagen et al.,2006]. In the ERP study, we found significant reductions in the amplitude of the parietal P3 component in these cancer survivors, 4 years after chemotherapy as compared to survivors without chemotherapy. This reduction was found during a simple auditory [Kreukels et al.,2008] and a more complex visual attention task [Kreukels et al.,2006].
Based on findings from the literature and our previous studies, two cognitive functions were assessed while functional Magnetic Resonance Imaging (fMRI) scans were acquired: executive function and episodic memory. Executive function is a comprehensive term for a set of higher‐order cognitive functions that regulates an individual's ability to organize thoughts and activities, prioritize tasks, manage time efficiently, and make decisions. One major aspect of executive function is therefore planning, which is defined as the ability to achieve a goal through a series of intermediate steps. To investigate planning abilities, an fMRI‐adapted version of the Tower of London [Shallice,1982] was used [van den Heuvel et al.,2003]. To assess episodic memory, a paired associates memory encoding task was used with a recognition phase outside the scanner. Neuropsychological test data (current and past) were obtained to evaluate cognitive function on standardized tests. Also, self‐report data were collected to assess subjective cognitive complaints and to obtain a general impression of the mental and physical health of the participants. We expected that chemotherapy would have a negative effect on cognitive performance. Also, we expected altered brain activity in regions related to executive function and memory encoding, in particular dorsolateral prefrontal cortex (DLPFC) and (para)hippocampal regions, respectively.
METHODS
Participants
Participants were disease‐free breast cancer survivors from the Antoni van Leeuwenhoek Hospital/Netherlands Cancer Institute and the VU University medical center that were recruited from a database of a prospective neuropsychological study performed in the Antoni van Leeuwenhoek Hospital/Netherlands Cancer Institute [Schagen et al.,2006]. The experimental group (chemo group) consisted of survivors that had received standard‐dose chemotherapy (four cycles of FEC [5‐fluorouracil, 500 mg m−2, epirubicin, 90 mg m−2, cyclophosphamide, 500 mg m−2] followed by one cycle of high‐dose CTC [cyclophosphamide, 6 g m−2, thiotepa, 480 mg m−2, carboplatin, 1,600 mg m−2]) and autologous peripheral blood hematopoietic progenitor‐cell transplantation. At the time of treatment they were high‐risk breast cancer patients with at least four tumor‐positive axillary lymph nodes but no distant metastasis. These patients participated in a multicenter randomized trial comparing the efficacy of adjuvant high‐dose chemotherapy with optimal standard‐dose chemotherapy [Rodenhuis et al.,2003]. Patients from this group were subsequently treated with tamoxifen (40 mg daily) for 3.8 ± 1.7 years. The control group consisted of cancer survivors previously diagnosed with Stage I breast cancer that had not received any systemic therapy, except for one patient who was treated with tamoxifen for 5 years. All participants had undergone local surgery and locoregional radiation therapy.
The study was approved by the institutional review board of both institutes. Participants had to fulfill the following inclusion criteria: previous participation in our neuropsychological study, no presence of metastatic disease or relapse, no history of neurological or psychiatric signs that might lead to deviant test results, no use of medication that might lead to deviant test results, no alcohol or drug abuse, sufficient command of the Dutch language and eligibility to undergo the MRI scanning session. Written informed consent was obtained from all participants according to institutional guidelines and the declaration of Helsinki. The experiment was conducted at the Academic Medical Center of the University of Amsterdam.
The database yielded 40 potential candidates for the chemo group and 46 potential candidates for the control group. Of the 40 potential participants for the chemo group, 13 (32.5%) had died and 4 (10%) were excluded due to relapse or metastatic disease, leaving 23 (57.5%) eligible cancer survivors for the chemo group. Four (17.4%) out of 23 survivors refused to participate, of which 3 (75%) were interviewed by telephone to assess demographic variables and the occurrence of cognitive complaints. Thus, 19 out of 23 (82.6%) eligible cancer survivors constituted the chemo group in the present study. All were right‐handed. Of the 46 potential participants for the control group, 3 (6.5%) had died, 8 (17.4%) were excluded due to relapse or metastatic disease, 6 (13%) were excluded for other reasons [Parkinson's disease (2×), alcohol abuse, use of methotrexate for rheumatoid arthritis, stroke, recent hemithyreoidectomy], and 1 (2.2%) could not be traced, leaving 28 (60.9%) eligible cancer survivors for the control group. Thirteen out of 28 survivors (46.4%) refused to participate, of which 12 (92.3%) could be interviewed by telephone. Thus, 15 out of 28 eligible cancer survivors (53.6%) constituted the control group. Thirteen were right‐handed.
To obtain a general measure with regard to health status of the participants and to assess the influence of anxiety and depressive symptoms on cognitive function, health‐related quality of life was assessed with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire‐C30 [Aaronson et al.,1993] and anxiety and depressive symptoms were assessed with the Hopkins Symptoms Checklist‐25 [Hesbacher et al.,1980]. To assess patient eligibility, demographic variables and cognitive problems, a 30‐min structured telephone interview was held before including subjects in the study [Schagen et al.,1999]. Participants were asked to indicate on a five‐point Likert scale the extent to which problems in each of four domains (memory, attention, thinking, and language) occurred in their daily lives (never, occasionally, regularly, often, or always). For each domain, subjective cognitive impairment was considered present if participants reported cognitive complaints at least regularly.
Neuropsychological Tests
We carried out a subset of neuropsychological tests previously proven to be most sensitive for detecting cognitive impairment in our population. Seven tests were administered, yielding 16 test indices: Trail Making Test, reaction time (RT) to card A and B [Reitan,1958], Digit Symbol‐Coding Test of the Wechsler Adult Intelligence Scale (WAIS)‐III, number of correctly completed items [Wechsler,2000], Stroop Color‐Word Test, RT to cards 1, 2, 3, and 4 [Hammes,1978], Dutch version of the California Verbal Learning Test (CVLT), immediate recall, delayed recall and recognition [Mulder et al.,1996], Visual Reproduction Test of the Wechsler Memory Scale‐Revised (WMS‐R), immediate and delayed recall [Wechsler,1987], Word Fluency Test, number of animals and number of professions [Lezak,2004] and Fepsy Finger Tapping Test, number of taps for dominant and nondominant hand [Alpherts and Aldenkamp,1994]. The 16 test indices were grouped into 6 cognitive domains: focused and sustained attention (Trail Making Test RT to card A, Digit Symbol of the WAIS‐III, number of correctly completed items, Stroop Color‐Word Test, RT to cards 1 and 2), verbal memory (CVLT immediate recall, delayed recall and recognition), visual memory (Visual Reproduction Test of the WMS‐R, immediate and delayed recall), mental flexibility (Trail making Test, RT to card B, Stroop Color‐Word Test, RT to cards 3 and 4), verbal functioning (Word Fluency Test, number of animals and number of professions) and motor speed (Fepsy Finger Tapping Test, number of taps for dominant and nondominant hand). The Dutch Adult Reading Test was administered to obtain a measure of premorbid IQ [Schmand et al.,1992]. Data for the Fepsy Finger Tapping Test were missing for one participant from the chemo group (n = 18 instead of 19).
Experimental Procedures
The experimental procedure lasted ∼2.5 h. First, informed consent was signed and neuropsychological tests and questionnaires were completed (1 h and 15 min). After a 30‐min break, the fMRI tasks were practiced (15 min). Then, the MRI scanning session took place (1 h). Stimuli were presented on a screen positioned in front of the scanner. Participants lay supine in the scanner and viewed the stimuli through a mirror attached to the head coil. They wore ear pads and a headphone to reduce scanner noise. The headphone was attached to a microphone, enabling communication with the experimenter in‐between scan acquisitions. Subjects performed three fMRI tasks. The first was a Flanker task, the second the ToL and the last the paired associates task. Structural and MR spectroscopy scans were acquired in between the tasks. Results from these latter scans as well as the Flanker task will be reported elsewhere. After leaving the scanner room, participants immediately performed the retrieval part of the paired associates task.
Paradigms
The ToL paradigm used in the present study was an abbreviated version of van den Heuvel et al. [2003], a task that reliably activates brain regions associated with executive function, in particular bilateral DLPFC and parietal cortex. It consisted of two conditions (see Fig. 1). In the planning condition, a starting configuration and a target configuration were displayed on a single screen. In both configurations, three colored beads were placed on three vertical rods, which could accommodate one, two, and three beads, respectively. Participants were instructed to determine the minimum number of steps required to go from the starting to the target configuration by mentally moving beads one at a time. Two response options were displayed on the left and right side of the screen corresponding to either response button. The correct answer ranged from one to five steps. In the baseline condition, participants had to count the total number of yellow and blue beads. Directly after a button press, the next trial was presented. Maximum response time was 1 min. Trials were presented in a pseudorandom design. No feedback was given during the task, which lasted 8 min. Participants were instructed to focus on accuracy rather than on speed. Performance was calculated for the planning condition. The ToL was practiced outside the scanner.
Figure 1.

Tower of London (ToL). In the planning condition (A), subjects saw a starting configuration together with a target configuration with the instruction to “count the number of steps” required to achieve the target configuration. One bead could be moved at a time and only when there was no other bead on top. Two possible answers were shown. Subjects had to press the button corresponding to the side (left or right) of the screen where the correct answer was presented. In the baseline condition (B), subjects had to count the total number of yellow and blue beads. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We used a paired associates memory encoding task based on a task paradigm by Jager et al. [2007]. This task has been demonstrated to reliably activate the medial temporal lobe (parahippocampal gyrus [PHG] and hippocampus proper), amongst other brain areas. This paradigm consisted of three conditions (see Fig. 2). First, a low‐level baseline condition was presented in which participants were cued to press the left or right button according to the direction three arrowheads were pointing to (“<<<” or “>>>”). These were superimposed on blurred portrait and interior design photos to match the visual input of the associative learning condition. In the associative learning condition, participants were asked to indicate whether they thought a person depicted on a portrait photo was likely to live in the home interior depicted on a simultaneously presented photo. This was done by indicating “resident” or “visitor,” using a button press. In the high‐level baseline condition, two identical portrait photos or interior design photos were presented and participants had to indicate whether they saw a portrait photo or an interior design. The high level baseline was included to isolate memory encoding processes specifically related to associative learning (i.e., establishing a meaningful connection between the two simultaneously presented stimuli). The low level baseline was included to isolate not only associative learning but additional memory encoding processes driven by e.g., novelty detection [e.g., Kumaran and Maguire,2009]. Photos were projected on a white background. Each trial lasted 3 s (baseline conditions) or 7 s (memory encoding) and was followed by a white screen for 1 s. Six trials were presented per block, resulting in 24 (baseline conditions) or 48 (memory encoding) second epochs. The sequence of three task blocks was repeated twice. Before each stimulus sequence, an instruction screen was presented for 6 s. Consequently, the task lasted 5 min and 42 s. Outside the scanner, subject performed a recognition task during which all photos from the memory encoding task were presented again (50% as old pairs and 50% as new pairs). Participants were instructed to indicate with a button press whether they had seen the same combination in the scanner or not. The retrieval task was self‐paced and participants were encouraged to be accurate rather than fast. Both encoding and retrieval task were practiced outside the scanner.
Figure 2.
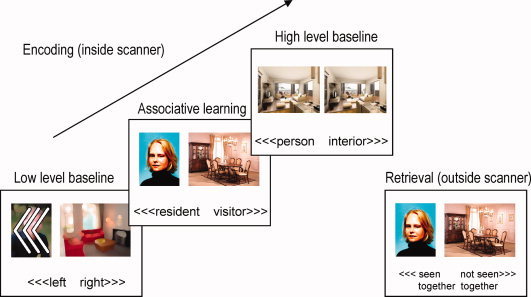
Paired Associates task. During associative learning, subjects indicated whether the depicted person matched the interior design by indicating “resident” or “visitor.” In the low level baseline, subjects made a left or right button press according to the direction of the arrows. In the high level baseline, subjects indicated whether they were looking at two identical pictures of a person or an interior design. Outside the scanner, subjects indicated whether they had seen the stimulus pair during scanning or not (recognition task). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Imaging Acquisition and Preprocessing
Imaging data were obtained using a 3.0 Tesla Intera full‐body fMRI scanner (Philips Medical Systems, Best, The Netherlands) with a phased array SENSE 6‐channel receiver head coil. Thirty‐five axial slices (voxel size 2.3 × 2.3 × 3 mm3, interslice gap 0 mm, matrix size 96 × 96 mm2, TR = 2 s, TE = 25 ms) of T2*‐weighted echo planar images (EPIs), sensitive to blood oxygenation level‐dependent (BOLD) contrast, were obtained. Also, a spoiled gradient echo structural T1‐weighed scan of 170 sagittal slices was made for coregistration with the fMRI data (voxel size 1 × 1 × 1 mm3). Imaging analysis was done using SPM5 (Statistical Parametric Mapping; Wellcome Department of Cognitive Neurology, London, UK). Images were slice‐timed, reoriented, and realigned to the first volume. Next, T1‐coregistered volumes were normalized to an SPM T1‐ template and spatially smoothed using an 8‐mm full width at half maximum (FWHM) Gaussian kernel.
Statistical Analysis
Demographic, self‐reported and neuropsychological data were analyzed with SPSS 15.0 (SPSS, Chicago, IL). Raw neuropsychological data were converted to standardized scores, based on the data of a control group of healthy women of similar age from our previous neuropsychological studies. Demographic and clinical data were analyzed by two‐tailed independent‐samples t‐tests (continuous variables) and χ2‐tests (categorical variables). Neuropsychological and fMRI performance data were analyzed with univariate analysis of variance (ANOVA), including age and estimated IQ as covariates (although the groups did not significantly differ on both variables, we still decided to include them, because small differences can have a substantial impact on test performance [Schilder et al., 2010]. Reaction time data analysis was performed on correct answers. We considered a participant to be cognitively impaired on a test index, if she scored 2 standard deviations (SDs) below the mean of a healthy control group on that test index [Schagen et al.,2006].
FMRI data were analyzed in the context of the general linear model, using epoch models (box‐car regressors) convolved with a canonical hemodynamic response function to model responses to each type of stimulus. Contrast images containing parameter estimates were entered into a second‐level (random effects) analysis. Main task effects for groups and group interactions were analyzed with a two‐sample t‐test implemented in SPM5 with age and estimated IQ as covariates. Main task effects for both groups are reported at a P < 0.05 corrected for multiple comparisons according to the False Discovery Rate (FDR) method [Genovese et al.,2002]. Whole‐brain group interactions are reported at P < 0.001, masked with the appropriate main effect at P < 0.05. A cluster size restriction of 10 voxels was maintained for these analyses. In addition, group interactions for hypothesis‐driven region‐of‐interest (ROI) analyses are reported at P < 0.005 with a five voxel cluster restriction. For the ToL, an ROI mask was used [Maldjian et al.,2003,2004] covering the DLPFC. This mask was constructed by combining Brodmann areas 9 and 46 bilaterally [Lancaster et al.,2000] and applying a 3D dilation mask (one iteration) because the Brodmann atlas areas define a relatively thin cortical strip. For the paired associates task, an ROI mask was used covering the PHG and hippocampus proper bilaterally [Tzourio‐Mazoyer et al.,2002]. The associations between performance and BOLD activation were examined for the two groups separately using the same statistical thresholds as for the group interaction analyses.
Three out of 19 cancer survivors from the chemo group were excluded from fMRI analysis. For one participant, no MRI scans were acquired due to claustrophobia. Another participant was not able to follow task instructions for the ToL and paired associates task due to severe visual impairment. For a third participant, fMRI scans for the ToL and the paired associates task were corrupt. Performance data of the latter participant were used for behavioral analysis.
RESULTS
Subject Attrition
Decliners in either group did not differ from participants with respect to age or the presence of cognitive complaints as assessed during the telephone interview (all Ps > 0.2). Nor did they differ in estimated IQ or the number of test indices they were impaired on, based on an earlier assessment (all Ps > 0.1)
Demographic and Clinical Data
Table I summarizes demographic and clinical characteristics for the chemo group and the control group. The groups did not differ with regard to age and estimated IQ, nor were significant differences found with regard to scores on measures for quality of life, anxiety, and depression. Cancer survivors that had received chemotherapy reported significantly more memory complaints than cancer survivors without chemotherapy (χ2‐test).
Table I.
Demographic and clinical characteristics of the study population
| Chemo group (n = 19) | Control group (n = 15) | |
|---|---|---|
| Age | 56.3 (5.5) | 58.2 (5.8) |
| Estimated IQ (NART) | 101.1 (17.9) | 100.7 (17.3) |
| Years since surgery | 9.9 (0.5)* | 9.2 (0.5) |
| Years since chemotherapy | 9.5 (0.8) | NA |
| EORTC QLQ‐C30 | ||
| Global quality of life | 82.0 (12.1) | 81.1 (16.2) |
| Physical Functioning | 83.5 (12.2) | 88.4 (11.7) |
| Fatigue | 25.7 (14.4) | 23.0 (19.4) |
| HSCL‐25 total score | 11.30 (6.4) | 14.84 (16.3) |
| Cognitive complaints | ||
| Concentration, n (%) | 8 (42.1%) | 6 (40%) |
| Memory, n (%) | 11 (57.9%)* | 3 (20%) |
| Thinking, n (%) | 0 | 1 (6.7%) |
| Language, n (%) | 3 (15.8%) | 6 (40%) |
| Cognitive impairment, number of tests (%) | 1.71 (10.7%)* | 0.83 (5.2%) |
Abbreviations: NART = Dutch version of the National Adult Reading Test; EORTC QLQ‐C30 = European Organization for Research and Treatment of Cancer health‐related Quality‐of‐Life Questionnaire: scores range from 0 to 100; HSCL‐25 = Hopkins Symptom Checklist‐25: scores range from 0 to 100, higher score indicates higher levels of anxiety and depression; Cognitive complaints = number (and percentage) of subjects reporting cognitive complaints in telephone interview; Cognitive impairment = number (and percentage) of tests that subjects were impaired on (see text for details); NA = not applicable.
P < 0.05.
Neuropsychological Tests
The chemo group performed significantly worse on the Word Fluency profession test (verbal functioning domain, chemo group 17.26 ± 3.74 items, control group 19.87 ± 4.32 items, F(1, 30) = 5.86, P < 0.05) and showed a trend for worse performance on the Fepsy Finger Tapping Test, dominant hand (motor speed domain, chemo group 59.03 ± 6.14 items, control group 60.93 ± 5.79 items, F(1, 29) = 2.94, P = 0.097). On 13 out of 14 remaining test indices, the chemo group performed numerically, albeit not significantly, worse than the control group.
Comparing earlier and current neuropsychological performance (less than 2 years and more than 9 years after chemotherapy, or yoked intervals for the control group) on the individual level, a significant main effect of Group (F(1, 30) = 5.07, P < 0.05) indicated that subjects from the chemo group were cognitively impaired on more tests than those from the control group [chemo group 1.71 ± 1.97 out of 16 test indices (10.7%), control group 0.83 ± 0.72 (5.2%), Table I]. No Group × Time interaction was found (Fs < 1, NS), so no statistical support was found for a change over time of this effect. Separate ANOVAs for the two measurements revealed that within 2 years after chemotherapy, the chemo group was impaired on 2.05 ± 3.17 test indices (12.8%) and the control group on 0.87 ± 0.92 test indices (5.4%, F(1, 30) = 2.39, NS), and 9.5 years after chemotherapy the chemo group was impaired on 1.37 ± 1.86 test indices (8.6%) and the control group on 0.80 ± 1.15 test indices (5.0%), F(1, 30) = 3.27, P = 0.081). To examine the consistency of impairment over time with respect to deviant test performance, we compared test scores of subjects that showed impairment on at least one test index on both measurements. In the chemo group, six subjects fulfilled this criterion. All six subjects showed overlap in the cognitive domain(s) affected (encompassing the domains of focused and sustained attention, mental flexibility, verbal memory, and visual memory). In the control group, five subjects showed impairment on at least one test index on both assessments. None of the subjects showed overlap in a cognitive test across assessments.
Relation Between Cognitive Test Performance and Self‐Reported Measures
No significant associations were found between the number of tests scored in the impaired range and self‐reported cognitive complaints or HSCL total score. Cognitive complaints about concentration, memory, and language were positively correlated with HSCL total score (r = 0.35, 0.35, 0.52, respectively).
ToL
The chemo group performed significantly worse on the ToL than the control group (F(1, 28) = 4.66, P < 0.05; chemo group 73% ± 14% correct responses, control group 83% ± 12% correct responses). The chemo group responded significantly faster than the control group (F(1, 28) = 5.55, P < 0.05; RT for chemo group 8.93 ± 3.06 s, for control group 11.93 ± 3.78 s). To investigate whether this was a general effect, independent of response accuracy, we also compared RT for incorrect responses. Indeed, the chemo group also responded faster than the control group on error trials (RT for chemo group 9.88 ± 4.59 s, for control group 11.78 ± 4.20 s), but this effect did not reach statistical significance (F(1, 28) = 1.72, NS).
Imaging results showed significant BOLD activation in dorsolateral and ventrolateral prefrontal cortex, precuneus and lateral posterior parietal cortex (PPC), premotor cortex and dorsal striatum for the planning vs. baseline contrast. Additionally, significant activation was found in the occipital cortex and inferior temporal gyrus. These effects were all found bilaterally for both the chemo group and the control group, demonstrating the robustness of the task in eliciting BOLD activation in relevant brain areas (Fig. 3A and Table II). ROI analysis demonstrated hypoactivation of left DLPFC in the chemo group relative to the control group. In addition, whole brain analysis demonstrated hypoactivation of bilateral PPC in the chemo group relative to the control group (Fig. 3B,C, Table II).
Figure 3.

Tower of London (ToL). A: BOLD activations for the Active > Baseline contrast for breast cancer survivors after chemotherapy (Chemo) and breast cancer survivors without chemotherapy (Control), FDR corrected at P < 0.05; B: group interactions showing hyporesponsiveness of the chemo group versus the control group. Left: bilateral DLPFC (ROI analysis shown at P < 0.05), right: bilateral PPC (whole brain analysis shown at P < 0.01). Vertical bars show T‐values. C: Contrast estimates with 90% confidence intervals for group interactions at left DLPFC and right PPC (MNI coordinates −48, 6, 39 and 39, −60, 54, respectively). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Tower of London
| R/L | Chemo group | Control group | Chemo < control group | Voxels | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Z value | x | y | z | Z value | x | y | z | Z value | |||
| Dorsolateral PFC | R | 48 | 27 | 36 | 4.17 | 30 | 36 | 42 | 5.62 | |||||
| L | −48 | 18 | 45 | 4.36 | −48 | 27 | 33 | 5.06 | −48 | 6 | 39 | 3.25 | 16a | |
| Ventrolateral PFC | R | 36 | 57 | 3 | 3.73 | 48 | 42 | −9 | 3.36 | |||||
| L | −48 | 42 | −6 | 3.69 | −45 | 48 | −3 | 3.94 | ||||||
| Premotor cortex | R | 30 | 21 | 45 | 4.13 | 27 | 21 | 45 | 5.01 | |||||
| L | −27 | 21 | 48 | 4.02 | −27 | 21 | 48 | 5.30 | ||||||
| L | −6 | 27 | 54 | 5.11 | −6 | 27 | 45 | 5.40 | ||||||
| Precuneus | R | 9 | −72 | 54 | 5.71 | 9 | −72 | 54 | 6.73 | |||||
| L | −9 | −60 | 57 | 4.92 | −9 | −60 | 57 | 5.72 | ||||||
| PPC | R | 51 | −45 | 51 | 4.73 | 51 | −45 | 51 | 5.90 | 39 | −60 | 54 | 3.94 | 17 |
| 48 | −33 | 48 | 3.83 | 16 | ||||||||||
| L | −45 | −51 | 45 | 4.70 | −33 | −75 | 48 | 5.63 | −30 | −60 | 57 | 3.43 | 10 | |
| Dorsal striatum | R | 15 | −3 | 18 | 3.27 | 12 | −6 | 18 | 4.08 | |||||
| L | −9 | −3 | 15 | 3.70 | −9 | 0 | 12 | 4.15 | ||||||
| Inferior temporal G | R | 54 | −63 | −12 | 3.40 | 54 | −63 | −9 | 4.90 | |||||
| L | −57 | −60 | −3 | 5.00 | −57 | −57 | −3 | 5.38 | ||||||
| Occipital cortex | R | 15 | −54 | 21 | 4.02 | 15 | −60 | 21 | 4.11 | |||||
| L | −15 | −63 | 24 | 3.31 | −15 | −60 | 21 | 3.45 | ||||||
BOLD activations (MNI coordinates) for the Active > Baseline contrast for breast cancer survivors after chemotherapy (Chemo Group), breast cancer survivors without chemotherapy (Control Group) and group interactions.
Abbreviations: R, right; L, left; PFC, prefrontal cortex; PPC, lateral posterior parietal cortex; G, gyrus. Main task effects for each group are reported at P < 0.05, FDR corrected. Group interactions are reported at P < 0.001 unless indicated otherwise.
ROI analysis at P < 0.005 uncorrected.
Paired Associates
Recognition memory performance (assessed after the scanning session) for the paired associates task was marginally worse for the chemo group than for the control group (F(1, 28) = 3.27, P = 0.081; chemo group 26% ± 15% correct, control group 36% ± 23% correct). The chemo group also responded somewhat faster during the memory recognition task but this effect was not significant (F< 1, NS; RT for chemo group 3.63 ± 1.14 s, for control group 4.21 ± 1.99 s). During memory encoding, the chemo group responded marginally faster than the control group (F(1, 28) = 3.74, P = 0.063; RT for chemo group 3.29 ± 0.28 s, for control group 3.57 ± 0.49 s). No other behavioral group differences were found for the paired associates task.
Memory encoding vs. low‐level baseline was associated with significant bilateral activation of inferior occipital cortex, fusiform gyrus, PHG and hippocampus proper in both groups, demonstrating reliable activation of the ventral stream. Bilateral middle occipital cortex and precuneus were also activated in both groups. In addition, (pre)frontal and middle temporal regions were activated in both groups although less extensively in the chemo group than in the control group. Lateral PPC was solely and bilaterally activated in the control group, as well as dorsal striatum and anterior cingulate cortex. Right amygdala was only significantly activated in the chemo group (Fig. 4A, Table III). ROI‐based group comparisons demonstrated significant hypoactivation of right PHG for the chemo group compared to the control group. An additional whole‐brain group interaction analysis revealed significant bilateral hypoactivation of lateral PPC for the chemo group relative to the control group (Fig. 4B,C, Table III). In addition, significant hypoactivation for the chemo group compared to the control group was found for left precuneus, right dorsal striatum, right inferior parietal cortex and left middle temporal gyrus (Table III). The only region that showed more activation in the chemo group than the control group was the left parietal operculum. The memory encoding versus high‐level baseline contrast did not reveal significant group differences.
Figure 4.

Paired associates task. A: BOLD activations for the Associative learning > Low‐level baseline contrast for breast cancer survivors after chemotherapy (Chemo) and breast cancer survivors without chemotherapy (Control), FDR corrected at P < 0.05. B: group interactions showing hyporesponsiveness of the chemo group versus the control group. Left: bilateral PHG (ROI analysis shown at P < 0.05), right: bilateral PPC (whole brain analysis shown at P < 0.01). Vertical bars show T‐values. C: Contrast estimates with 90% confidence intervals for group interactions at right PHC and right PPC (MNI coordinates 21, −39, −6 and 42, −60, 54, respectively). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table III.
Paired associates task
| R/L | Chemo group | Control group | Chemo < control group | Voxels | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Z‐value | x | y | z | Z value | x | y | z | Z value | |||
| Dorsolateral PFC | R | 51 | 33 | 24 | 4.12 | 45 | 27 | 21 | 4.92 | |||||
| L | −54 | 24 | 27 | 4.73 | ||||||||||
| Ventrolateral PFC | R | 33 | 30 | −18 | 4.41 | 42 | 33 | −15 | 4.26 | |||||
| L | −45 | 27 | −12 | 3.89 | ||||||||||
| Premotor | R | 3 | 24 | 45 | 3.54 | 6 | 27 | 54 | 4.67 | |||||
| L | −3 | 18 | 48 | 3.60 | −6 | 39 | 42 | 4.99 | −6 | 39 | 42 | 4.04 | 10 | |
| Precuneus | R | 15 | −54 | 15 | 4.13 | 6 | −51 | 12 | 4.45 | |||||
| L | −3 | −57 | 12 | 4.77 | −3 | −51 | 15 | 4.44 | ||||||
| PPC | R | 39 | −57 | 54 | 4.74 | 42 | −60 | 54 | 4.26 | 161 | ||||
| L | −33 | −63 | 51 | 5.26 | −30 | −66 | 51 | 3.74 | 36 | |||||
| L | −42 | −69 | 45 | 4.85 | −42 | −69 | 45 | 3.71 | 17 | |||||
| Dorsal striatum | R | 12 | 0 | 9 | 5.03 | 15 | 0 | 15 | 3.53 | 15 | ||||
| L | −9 | 3 | 0 | 4.14 | ||||||||||
| Inferior occipital C | R | 33 | −90 | −6 | 6.19 | 33 | −90 | −6 | 7.34 | 27 | −87 | −9 | 3.89 | 20 |
| L | −36 | −90 | −6 | 4.03 | −36 | −87 | −6 | 5.87 | ||||||
| L | −24 | −96 | 0 | 5.48 | −18 | −90 | −12 | 5.51 | ||||||
| Middle occipital C | R | 39 | −75 | 21 | 3.85 | −45 | −81 | 3 | 4.79 | |||||
| L | −45 | −78 | 15 | 3.84 | 36 | −78 | 21 | 5.40 | ||||||
| Hippocampus | R | 21 | −18 | −12 | 3.69 | 21 | −27 | −6 | 3.46 | |||||
| L | 21 | −27 | −18 | 3.81 | −24 | −18 | −21 | 3.65 | ||||||
| Parahippocampal G | R | 18 | −42 | −9 | 3.44 | 21 | −39 | −9 | 4.68 | 21 | −39 | −6 | 3.03 | 5a |
| L | −33 | −45 | −9 | 3.07 | −27 | −42 | −9 | 4.92 | ||||||
| Amygdala | R | 21 | 0 | −15 | 4.32 | |||||||||
| L | −18 | −6 | −15 | 3.04 | ||||||||||
| Fusiform | R | 27 | −48 | −12 | 5.24 | 42 | −51 | −18 | 5.86 | |||||
| L | −39 | −36 | −21 | 4.57 | −36 | −51 | −15 | 5.45 | ||||||
| Middle temporal G | R | 51 | −9 | −21 | 3.41 | |||||||||
| R | 63 | −39 | 0 | 3.59 | ||||||||||
| L | −57 | −6 | −12 | 3.72 | −51 | 6 | −30 | 3.55 | ||||||
| L | −57 | −6 | −12 | 4.51 | ||||||||||
| L | −63 | −39 | −6 | 4.82 | −63 | −48 | 3 | 3.56 | 12 | |||||
| Anterior cingulate C | L | −3 | 21 | 24 | 3.26 | |||||||||
| Chemo > control group | ||||||||||||||
| Parietal operculum | L | −48 | −9 | 15 | 3.56 | 11 | ||||||||
BOLD activations (MNI coordinates) for the Encoding – Low level baseline contrast for breast cancer survivors after chemotherapy (Chemo Group), breast cancer survivors without chemotherapy (Control Group) and group interactions.
Abbreviations: R, right; L, left; PFC, prefrontal cortex; PPC, lateral posterior parietal cortex; C, cortex; G, gyrus. Main task effects for each group are reported at P < 0.05, FDR corrected. Group interactions are reported at P < 0.001 unless indicated otherwise.
ROI analysis at P < 0.005 uncorrected.
Group Interactions Across ToL and Paired Associates
Whole‐brain group interaction analysis revealed significant bilateral hypoactivation of lateral PPC for the chemo group relative to the control group across the ToL and paired associates task (MNI coordinates right PPC 42, −57, 54; Z‐value 5.59, left PPC, −33, −72, 48, Z‐value 3.87, significant at P < 0.05 corrected for multiple comparisons). The opposite contrast revealed no significant effects.
Regression Analysis of BOLD With Performance
For the ToL, ROI regression analysis in the chemo group with performance showed two foci in right DLPFC (MNI coordinates 48, 24, 30, Z‐value = 3.52, P < 0.001, r = 0.77, 40 voxels (Fig. 5A) and MNI coordinates 27, 30, 42, Z‐value = 3.48, P < 0.001, r = 0.77, 14 voxels, bordering on ventral DLPFC) and one focus in left DLPFC (MNI coordinates −42, 15, 36, Z‐value = 3.52, P < 0.001, r = 0.77, 27 voxels). Whole‐brain analysis showed additional activation in right PPC (MNI coordinates 39, −75, 39, Z‐value = 4.04, P < 0.001, r = 0.84, 35 voxels) and right precuneus (MNI coordinates 6, −66, 54, Z‐value = 3.67, P < 0.001, r = 0.79, 20 voxels). For the control group, only when omitting the five voxel cluster size restriction, a correlation of BOLD activation in right DLPFC was found (MNI coordinates 54, 15, 42, Z‐value = 3.30, P < 0.001, r = 0.76, 1 voxel). Whole‐brain analysis did not reveal additional activation. For the paired associates task, ROI regression analysis in the chemo group of BOLD activation during memory encoding in hippocampus and PHG with recognition performance outside the scanner showed activation in left PHG (MNI coordinates −27, −18, −27, Z‐value = 3.61, P < 0.001, r = 0.79, 18 voxels, Fig. 5B). Whole‐brain analysis did not reveal additional activation. For the control group, only when omitting the five voxel cluster size restriction, a correlation of recognition memory with BOLD activation in left hippocampus was found (MNI coordinates −18, −6, −24, Z‐value = 2.90, P < 0.005, r = 0.70, 2 voxels). Whole‐brain analysis did not reveal additional activation.
Figure 5.

Regression analyses of performance with BOLD activation for the chemo group. A. Tower of London. Regression of performance with BOLD activation in right DLPFC (MNI coordinates 48, 24, 30, Z‐value = 3.52, P < 0.001, r = 0.77); B. Memory encoding/retrieval. Regression of memory recognition performance with BOLD activation during associative learning in left PHG (MNI coordinates −27, −18, −27, Z‐value = 3.61, P < 0.001, r = 0.79.
DISCUSSION
To our knowledge, this is the first study to show decreased responsiveness of brain regions related to executive function and memory encoding, almost 10 years after adjuvant chemotherapy for breast cancer. During the ToL, an executive function task measuring planning abilities, an ROI analysis showed decreased activation of DLPFC. In addition, whole‐brain analysis showed decreased activation of bilateral PPC. These hypoactivations were accompanied by significantly worse performance of the chemo group compared with the control group. In addition to performing worse, the chemo group also responded faster than the control group. Participants were explicitly instructed to emphasize accuracy rather than speed during the ToL. Possibly, the chemo group responded more impulsively than the control group due to impaired attentional abilities. Within the chemo group, activation of DLPFC was significantly correlated with task performance. For the control group, a significant correlation was found only when applying a less stringent cluster threshold. These findings support the notion that planning behavior was impaired in the chemo group and that this behavioral impairment was related to hypoactivation of DLPFC. Both DLPFC and PPC are consistently activated in studies employing the ToL, as well as in other executive function tasks [for reviews, see Cabeza and Nyberg,2000; Wager and Smith,2003]. The DLPFC is most closely linked to executive function, in particular active manipulation in working memory [Fletcher and Henson,2001; Levy and Goldman‐Rakic,2000], whereas the function of PPC is most consistently related to attention, in particular visuospatial attention [Cabeza and Nyberg,2000; Levy and Goldman‐Rakic,2000; Ungerleider and Mishkin,1982; Wager and Smith,2003]. Thus, impaired visuospatial planning as observed in the chemo group may result when both cognitive functions have been compromised.
When performing the paired associates task, an ROI analysis showed reduced activation of the PHG in cancer survivors after chemotherapy as compared to cancer survivors in the control group. Moreover, whole‐brain analysis demonstrated strong and large bilateral hypoactivation of PPC. Also, the chemo group showed a trend toward impaired recognition memory as compared to the control group. It should be noted that overall memory performance on the paired associates task was low (31%), so floor effects may have partly concealed memory impairment in the chemo group. Within the chemo group, activation of PHG during the memory encoding task was significantly correlated with recognition memory performance outside the scanner, indicating that higher activation of the PHG during encoding was related to higher subsequent recognition memory performance. For the control group, an effect was only found when omitting our minimum cluster size criterion (similar to the regression analysis of BOLD activation with ToL performance).
The encoding vs. high‐level baseline comparison served to specifically isolate memory encoding processes related to associative learning, but did not reveal any significant group interactions. This may indicate that the reduced activation of PHG of the chemo group was related to other processes such as novelty detection [Kumaran and Maguire,2009]. Regression analysis indicated that recognition memory in the chemo group was solely associated with activation in PHG. The parietal hypoactivations found in the chemo group may therefore not be specifically associated with aberrant memory encoding, but with a more general cognitive (dys)function like visual attention.
The only brain region that was more active for the chemo group than the control group during the paired associates task was the anterior portion of the left parietal operculum (the inferior part of the postcentral gyrus). Together with the inferior part of the precentral gyrus this region constitutes the rolandic operculum. As this area is involved in speech production [e.g., Ghosh et al.2008; Indefrey et al.,2001; Tonkonogy and Goodglass,1981] it may be the case that the chemo group relied more on (subvocal) verbal strategies for task performance, possibly as a compensatory mechanism for reduced visual attention abilities.
During the ToL, the chemo group showed planning‐related hypoactivity in very similar regions of PPC as during the paired associates task, as was confirmed by between‐group analysis incorporating both tasks. The fact that parietal hypoactivation occurred across two very different task paradigms supports the idea that chemotherapy may be related to long‐term effects on attention. Both tasks involved focusing attention on stimuli presented in different locations, supporting the interpretation that in the present study, particularly visuospatial attention was compromised. The findings in our previous ERP study of a chemotherapy‐related reduction in parietal P3 amplitude suggest that compromise of attentional abilities might not be limited to the visuospatial domain. In that study [Kreukels et al.,2006,2008], a P3 reduction was seen in two different experimental tasks (visual flanker and auditory oddball) that did not involve visuospatial attention. This finding was interpreted as a loss of information transmission because of impaired focusing of attention toward important stimuli [Kreukels et al.,2006]. A specific deficit in attention was not confirmed by the current neuropsychological test battery which may have been due to the fact that impairments are particularly found for more complex tests than those used in the present study [Wefel et al.,2008].
Cancer survivors that had received chemotherapy reported significantly more memory complaints than cancer survivors without chemotherapy. Frequency of complaints about concentration, language, and thinking did not differ between groups. Moreover, we found that participants that had received chemotherapy were cognitively impaired on a significantly higher number of tests than cancer survivors without chemotherapy, irrespective of time of assessment (less than 2 years, as well as more than 9 years after administration of chemotherapy), which implies a relatively stable pattern of chemotherapy‐induced cognitive impairment over time. Comparisons of raw neuropsychological test scores were suggestive of subtle impairments across all six cognitive domains, although mostly not significantly different. This was possibly the case because the study was underpowered for this type of examination. Assessments at the subjective and neuropsychological level thus corroborate our fMRI results in the general sense that chemotherapy was associated with adverse effects on subjective memory and neuropsychological test performance, although both types of measures were not significantly correlated. Subjective cognitive impairment, however, was correlated with levels of anxiety and depression. This pattern of findings is very common in cancer patients that received chemotherapy, and also in other populations characterized by mild cognitive impairment, e.g., HIV‐patients [Woods et al.,2009]. Intact cognitive functioning is clearly critical for successful daily coping. The fact that the magnitude of associations between neuropsychological tests and measures of “real world” cognitive skills is often in the moderate range [Chaytor and Schmitter‐Edgecombe,2003] likely reflects the idiographic nature of the impact of a given degree of cognitive dysfunction on a specific activity for each individual.
Few imaging studies have been published that we can compare our findings with. A PET study by Silverman et al. [2007] employing a verbal memory recall task has reported increased activation in left inferior frontal gyrus in breast cancer survivors that received chemotherapy, while performance differences were absent. The authors suggested that the higher activation of left inferior prefrontal cortex indicated a compensatory response to lower resting metabolism in this brain region associated with chemotherapy. Combining these results with our present data, it may be hypothesized that deficient memory encoding (associated with regional cerebral hypoactivation) may necessitate increased task effort with associated hyperactivation during memory retrieval. Therefore, in future studies it would be interesting to examine encoding as well as retrieval memory with fMRI. In a second study, employing fMRI in a prospective design, breast cancer patients receiving chemotherapy (n = 18) were compared with patients receiving only radiotherapy (n = 12) and healthy controls (n = 17) on an auditory verbal working memory task (poster presented at Human Brain Mapping, 2007 by Andrew J. Saykin and colleagues). Preliminary results showed a trend for impaired performance for the patients on chemotherapy 1 month after completion. Also, relative to healthy controls cancer patients demonstrated decreased activation in bilateral prefrontal regions, in particular the chemotherapy group. These preliminary findings thus corroborate our pattern of results with regard to prefrontal hypoactivation and impaired performance, although these authors did not report parietal hypoactivation as observed in the present study.
Several direct and indirect mechanisms have been proposed to underlie chemotherapy‐induced cognitive dysfunction [Ahles and Saykin,2007]. Cytotoxic agents that are able to cross the blood‐brain barrier might have direct neurotoxic effects, while indirect effects may also occur, such as cytokine‐mediated inflammatory processes. The breast cancer survivors in our group had been treated with five different cytotoxic agents, some of which cross the blood‐brain barrier more easily than others. From our data, it cannot be inferred which agents and mechanisms were responsible for the observed cognitive impairment and cerebral hypoactivations.
It should be noted that a healthy control group was not included, which leaves the question unanswered whether cancer survivors that only received radiotherapy also showed cognitive impairment and aberrant brain activation patterns. Also, it cannot be ruled out that the use of tamoxifen influenced our results [Schilder et al.,2009]. This selective estrogen receptor modulator was prescribed to all breast cancer survivors from the chemo group, but to only one cancer survivor from the control group. However, cancer survivors for the present study were recruited from a previous study in which patients were randomly assigned to high‐dose or standard‐dose chemotherapy [Schagen et al.,2006; van Dam et al.,1998]. All patients were treated with tamoxifen. It was shown that high‐dose chemotherapy was associated with a significantly higher risk of cognitive impairment than standard‐dose chemotherapy. It is thus unlikely that tamoxifen treatment is solely or exclusively responsible for the current findings. Because we did not compare different chemotherapeutic regimens, we cannot infer whether our results are specific for high‐dose chemotherapy or whether they also pertain to standard‐dose regimens.
Concluding, results from the present study indicate long‐term negative effects on cognitive function and associated regional brain activity in breast cancer survivors, 10 years after adjuvant systemic therapy consisting of standard‐dose and high‐dose chemotherapy, followed by tamoxifen. Dysfunction of PPC may be related to impaired attentional capacities and may partly underlie the diffuse pattern of cognitive dysfunction observed in these patients. The results of this cross‐sectional study stress the importance for continuing research into the cognitive effects of cytotoxic treatments and the need for larger, prospective neuroimaging studies investigating other chemotherapeutic regimens.
Acknowledgements
The authors thank Sabine Linn for her valuable advice on study design and Emiel Rutgers and Marie‐Jeanne Vrancken Peeters for their help in patient recruitment.
REFERENCES
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC ( 1993): The European organization for research and treatment of cancer QLQ‐C30: A quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365–376. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ ( 2001): Cognitive effects of standard‐dose chemotherapy in patients with cancer. Cancer Invest 19: 812–820. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ ( 2007): Candidate mechanisms for chemotherapy‐induced cognitive changes. Nat Rev Cancer 7: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpherts W, Aldenkamp AP. 1994. FePsy: The Iron Psyche. Heemstede: Instituut voor Epilepsiebestrijding. [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Chaytor N, Schmitter‐Edgecombe M ( 2003): The ecological validity of neuropsychological tests: A review of the literature on everyday cognitive skills. Neuropsychol Rev 13: 181–197. [DOI] [PubMed] [Google Scholar]
- Correa DD, Ahles TA ( 2008): Neurocognitive changes in cancer survivors. Cancer J 14: 396–400. [DOI] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA ( 2007): Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. J Clin Oncol 25: 3866–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN ( 2001): Frontal lobes and human memory: Insights from functional neuroimaging. Brain 124: 849–881. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH ( 2008): A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. J Speech Lang Hear Res 51: 1183–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes J ( 1978): De Stroop Kleur‐Woord Test. Handleiding, 2nd ed. Lisse: Swets & Zeitlinger. [Google Scholar]
- Hesbacher PT, Rickels K, Morris RJ, Newman H, Rosenfeld H ( 1980): Psychiatric illness in family practice. J Clin Psychiatry 41: 6–10. [PubMed] [Google Scholar]
- Indefrey P, Brown CM, Hellwig F, Amunts K, Herzog H, Seitz RJ, Hagoort P ( 2001): A neural correlate of syntactic encoding during speech production. Proc Natl Acad Sci USA 98: 5933–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MM, Kahn RS, Van den Brink W, Van Ree JM, Ramsey NF ( 2007): Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur. Neuropsychopharmacol 17: 289–297. [DOI] [PubMed] [Google Scholar]
- Kreukels BP, Schagen SB, Ridderinkhof KR, Boogerd W, Hamburger HL, Muller MJ, van Dam FS ( 2006): Effects of high‐dose and conventional‐dose adjuvant chemotherapy on long‐term cognitive sequelae in patients with breast cancer: An electrophysiologic study. Clin Breast Cancer 7: 67–78. [DOI] [PubMed] [Google Scholar]
- Kreukels BP, Hamburger HL, de Ruiter MB, van Dam FS, Ridderinkhof KR, Boogerd W, Schagen SB ( 2008): ERP amplitude and latency in breast cancer survivors treated with adjuvant chemotherapy. Clin Neurophysiol 119: 533–541. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA ( 2009): Novelty signals: A window into hippocampal information processing. Trends Cogn Sci 13: 47–54. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT ( 2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman‐Rakic PS ( 2000): Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res 133: 23–32. [DOI] [PubMed] [Google Scholar]
- Lezak M ( 2004): Neuropsychological Assessment. New York: Oxford University Press. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. NeuroImage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH ( 2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage 21: 50–455. [DOI] [PubMed] [Google Scholar]
- Meyers CA ( 2008): How chemotherapy damages the central nervous system. J Biol 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder JL, Dekker R, Dekker PH ( 1996): Verbale Leer en Geheugen Test. Lisse: Swets & Zeitlinger. [Google Scholar]
- Reitan R ( 1958): Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 8: 271–276. [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK ( 2008): SEER Cancer Statistics Review, 1975–2005. Bethesda: National Cancer Institute. [Google Scholar]
- Rodenhuis S, Bontenbal M, Beex LV, Wagstaff J, Richel DJ, Nooij MA, Voest EE, Hupperets P, van Tinteren H, Peterse HL, TenVergert EM, de Vries EG ( 2003): High‐dose chemotherapy with hematopoietic stem‐cell rescue for high‐risk breast cancer. N Engl J Med 349: 7–16. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Vardy J ( 2007): Cognitive dysfunction in people with cancer. Lancet Oncol 8: 852–853. [DOI] [PubMed] [Google Scholar]
- Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF ( 1999): Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer 85: 640–650. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Hamburger HL, Muller MJ, Boogerd W, van Dam FS ( 2001): Neurophysiological evaluation of late effects of adjuvant high‐dose chemotherapy on cognitive function. J Neurooncol 51: 159–165. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS ( 2006): Change in cognitive function after chemotherapy: A prospective longitudinal study in breast cancer patients. J Natl Cancer Inst 98: 1742–1745. [DOI] [PubMed] [Google Scholar]
- Schilder CM, Eggens PC, Seynaeve C, Linn SC, Boogerd W, Gundy CM, Beex LV, van Dam FS, Schagen SB ( 2009): Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC‐chemotherapy: Cross‐sectional findings from the neuropsychological TEAM‐side study. Acta Oncol 48: 76–85. [DOI] [PubMed] [Google Scholar]
- Schilder CM,Seynaeve C,Linn SC,Boogerd W,Gundy CM,Beex LV,van Dam FS,Schagen SB ( 2010): The impact of different definitions and reference groups on the prevalence of cognitive impairment: A study in postmenopausal breast cancer patients before the start of adjuvant systemic therapy. Psychooncology 19:415–422. [DOI] [PubMed] [Google Scholar]
- Schmand B, Lindeboom J, van Harskamp F ( 1992): De Nederlandse Leestest Voor Volwassenen. Lisse: Swets & Zeitlinger. [Google Scholar]
- Shallice T ( 1982): Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 298: 199–209. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA ( 2007): Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant‐treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat 103: 303–311. [DOI] [PubMed] [Google Scholar]
- Tonkonogy J, Goodglass H ( 1981): Language function, foot of the third frontal gyrus, and rolandic operculum. Arch Neurol 38: 486–490. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M ( 1982): Two cortical visual systems In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge: MIT Press; pp 549–586. [Google Scholar]
- van Dam, FS , Schagen SB, Muller MJ, Boogerd W, van der Wall E, Droogleever Fortuyn ME, Rodenhuis S ( 1998): Impairment of cognitive function in women receiving adjuvant treatment for high‐risk breast cancer: High‐dose versus standard‐dose chemotherapy. J Natl Cancer Inst 90: 210–218. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Groenewegen HJ, Barkhof F, Lazeron RH, van, Dyck R, Veltman DJ ( 2003): Frontostriatal system in planning complexity: A parametric functional magnetic resonance version of Tower of London task. NeuroImage 18: 367–374. [DOI] [PubMed] [Google Scholar]
- Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB ( 2008): Cancer and cancer‐therapy related cognitive dysfunction: An international perspective from the Venice cognitive workshop. Ann Oncol 19: 623–629. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE ( 2003): Neuroimaging studies of working memory: A meta‐analysis. Cogn Affect Behav Neurosci 3: 255–274. [DOI] [PubMed] [Google Scholar]
- Wechsler D ( 1987): Wechsler Memory Scale: Revised. New York: Psychological Corporation. [Google Scholar]
- Wechsler D ( 2000): WAIS‐III. Nederlandstalige Bewerking. Technische Handleiding. Lisse: Swets & Zeitlinger. [Google Scholar]
- Wefel JS, Witgert ME, Meyers CA ( 2008): Neuropsychological sequelae of non‐central nervous system cancer and cancer therapy. Neuropsychol Rev 18: 121–131. [DOI] [PubMed] [Google Scholar]
- Weis J, Poppelreuter M, Bartsch HH ( 2009): Cognitive deficits as long‐term side‐effects of adjuvant therapy in breast cancer patients: “Subjective” complaints and “objective” neuropsychological test results. Psychooncology 18: 775–782. [DOI] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I ( 2009): Cognitive neuropsychology of HIV‐associated neurocognitive disorders. Neuropsychol Rev 19: 152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]


