Abstract
Neuroimaging studies provide evidence for organized intrinsic activity under task‐free conditions. This activity serves functionally relevant brain systems supporting cognition. Here, we analyze changes in resting‐state functional connectivity after videogame practice applying a test–retest design. Twenty young females were selected from a group of 100 participants tested on four standardized cognitive ability tests. The practice and control groups were carefully matched on their ability scores. The practice group played during two sessions per week across 4 weeks (16 h total) under strict supervision in the laboratory, showing systematic performance improvements in the game. A group independent component analysis (GICA) applying multisession temporal concatenation on test–retest resting‐state fMRI, jointly with a dual‐regression approach, was computed. Supporting the main hypothesis, the key finding reveals an increased correlated activity during rest in certain predefined resting state networks (albeit using uncorrected statistics) attributable to practice with the cognitively demanding tasks of the videogame. Observed changes were mainly concentrated on parietofrontal networks involved in heterogeneous cognitive functions. Hum Brain Mapp 34:3143–3157, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: resting‐state fMRI, functional connectivity, intelligence, cognitive practice, ICA‐dual regression
INTRODUCTION
Neuroimaging studies have shown structural and functional changes in the brain associated with varied motor, visuomotor, perceptual, and cognitive practice or training methods. These changes in cortical activity or structure (e.g. gray/white matter) are usually interpreted as reflecting the experience‐dependent plasticity of neural systems [Aydin et al., 2007; Olsen et al., 2003]. Thus, practice on specific tasks may improve performance and modify the underlying neural activation patterns and/or the macroscopic structure [Jolles et al., 2010].
Nevertheless, findings are heterogeneous because of factors such as the nature of practice or training demands (i.e. task domain), and the specific features of the methodological design (i.e. length of the practice period, comparing participants already trained who show specific talents and skills or analyzing changes induced by a particular practice program, or neuroimaging methods) [Kelly and Garavan, 2005]. Generally speaking, practice evokes changes in brain areas supporting the cognitive requirements of the practiced task, even when the specific pattern of changes varies across trained‐tasks or procedures.
For instance, task‐related functional magnetic resonance imaging (fMRI) studies have been shown that patterns of changes in activation associated with practice in sensory/motor and higher‐order cognitive tasks are different. Practice‐related changes in activation in sensory‐motor tasks may increase connectivity within the primary cortex [e.g. primary motor cortex, Karni et al., 1995], whereas activation changes related to practice on higher‐order cognitive tasks (e.g. working memory, visual attention, or planning) might be associated with connectivity changes in a parietofrontal distributed network [Dahlin et al., 2008; Hempel et al., 2004; Olesen et al., 2003]. For example, Wan et al. 2011 compared brain activity of shogi's professional and amateur players. Shogi is a board game with high‐level cognitive requirements. Activations typically associated with professional playing were located in the posterior precuneus (related to visuospatial imagery, episodic memory retrieval, and working memory) during perception of board patterns, and in the caudate nucleus (involved in goal‐directed behavior) during quick generation of the best next. Based on these findings, Kelly and Garavan 2005 have suggested that the predominant pattern of change involves reduced activation across the network of brain areas (association cortex) underlying task performance. Practice in complex cognitive tasks increases connectivity between hubs within this distributed network, perhaps increasing neural efficiency.
Also, it has been shown that functional connectivity at rest may change after perceptual [Lewis et al., 2009], motor [Ma et al., 2011], and complex cognitive training [e.g. working memory, Jolles et al., in press]. Analyses of resting‐state fMRI imaging data have demonstrated temporal correlations in low frequency BOLD signals of widely separated brain regions. These correlations quantify intrinsic functional connectivity [Biswal et al., 1995; De Luca et al., 2005; Fox and Raichle, 2007; Fox et al., 2005; Greicius et al., 2003; Raichle et al., 2001; Smith et al., 2009]. Synchronized blood flow fluctuations, or functionally connected networks, are spatially consistent across time (test–retest reliability), subjects, resting‐state conditions, conscious states, and analysis techniques [Beckmann et al., 2005; Biswal et al., 1995; Boly et al, 2009; Damoiseaux et al., 2006; De Luca et al., 2006; Fox and Raichle, 2007;Fox et al., 2005; Shehzad et al., 2009; Smith et al., 2004; Toro et al., 2008; Zuo et al., 2010] and they are significantly related to structural connections [Buckner et al., 2008; Hagmann et al., 2008, Honey et al., 2009, van den Heuvel et al., 2009]. Spatial patterns of coherent spontaneous fluctuations overlap functional brain systems activated by specific task‐evoked paradigms, including sensoriomotor, visual and auditory processing, language, working memory, or attention systems [Beckmann et al., 2005; Biswal et al., 1995; De Luca et al., 2005; Corbetta and Shulman, 2002; Cordes et al., 2000; Fox et al., 2006; Hampson et al., 2002, 2006a, 2006b; Vincent et al., 2006; Zuo et al., 2010]. Indeed, correlation patterns of spontaneous activity may predict the topography and/or variability of both brain responses and behavioral performance to a range of perceptual, motor, and cognitive tasks [Andrew–Hanna et al., 2007; Fox and Raichle, 2007; Fox et al., 2006; Hampson et al., 2006a; Kelly et al., 2008; Seeley et al., 2007]. The common architecture of these correlation patterns under rest conditions shows changes in the context of task performance. Synchrony among areas involved in the task increases, whereas it decreases for other networks, probably due to compensatory mechanisms. Thus, intrinsic activity might be understood as a dynamic prediction system: coactivations under resting conditions are possible because these regions have frequently been activated together for goal‐directed brain function; and these coactivations remain active as a prediction about future usage, mediating task preparation processes. In short, intrinsic activity might coordinate neuronal activity across several temporal and spatial ranges. So, coherence of oscillatory spontaneous fluctuations within functional systems increases for a better large‐distance neuronal communication, facilitating information processing and behavior [Buzsaki and Draguhn, 2004; Fox et al., 2006; Fox and Raichle, 2007; Salinas and Sejnowski, 2001; Varela et al., 2001].
Functional connectivity is thought to play “a dynamic role in brain functions, supporting the consolidation of previous experience… i.e., the history of network activation” [Lewis et al., 2009]. Consistently, the covariance structure of spontaneous brain networks engaged by varied practice or training methods may be modified and these changes might be related to performance improvements. For example, an intense training on a shape‐identification task modified the resting‐state functional connectivity and directed mutual interaction between trained visual cortex and frontal–parietal areas (they were independent before training and became negatively correlated after practice) [Lewis et al., 2009]. These changes were associated with the degree of perceptual learning (i.e. more negative correlation with improved perceptual learning). Jolles et al. 2011 found that functional connectivity within the parietofrontal network increased after practice on a verbal working memory task and these changes were associated to individual differences in after‐practice performance improvements.
Here, changes in resting‐state functional connectivity, presumably induced by practice (16 h spread over 4 weeks) on a cognitively challenging videogame, are analyzed in a sample of young females using a test–retest design. A broad spectrum videogame was chosen, “Professor Layton and the Pandora's Box,” because of its demonstrated varied high level cognitive requirements (e.g. intellectual performance and working memory capacity) [Quiroga et al., 2009, 2011]. The practice group solved varied puzzles demanding reasoning, along with mental manipulation of verbal, spatial, and numerical information. These puzzles increased their complexity across sessions. The control group (carefully matched with the practice group across several relevant variables) remained in a “passive condition.” Therefore, we contrast gaming versus not gaming.
Structural and functional neuroimaging studies provide evidence for a distributed parietofrontal network relevant for high level cognition, such as reasoning, working memory, short‐term memory, and attention [Colom et al., 2006, 2007, 2009, 2010a, 2011; Corbetta and Shulman, 2002; Gläscher et al., 2010; Hampson et al., 2006a, 2006b; Jung and Haier, 2007; Karama et al., 2009, 2011, Song et al., 2008, 2009; van den Heuvel et al., 2009; Vincent et al., 2006; Wang et al., 2011]. Because of the nature of the selected videogame, changes in brain networks topographically overlapping these parietofrontal regions are predicted. Probable effects due to both the passage of time on functional connectivity and baseline intellectual performance are controlled by comparing the practice and control groups. Reliable networks are selected for studying changes in resting state connectivity presumably induced by practice.
METHOD
Participants
The sample comprised 20 young females (mean age for the complete group = 18.95; SD = 2.65, mean age practice group = 19.60; SD = 3.69, mean age control group = 18.30; SD = 0.48) from “Colegio Universitario Cardenal Cisneros” (Madrid). Volunteers were selected from a N = 100 database taking into account the following criteria: (1) no history of psychiatric or neurological illness, (2) little previous experience with videogames and no experience with any version of 'Professor Layton' game (by Nintendo), (3) right‐handed, and (4) varied in their level of cognitive abilities and skills assessed with a set of standardized tests. The practice and control groups were carefully matched by general cognitive ability [differences between groups were not significant (p < 0.01)]. Table 2 shows the descriptive statistics for each group and the complete sample on the cognitive ability tests. Written informed consent was obtained in accordance with regulations of Fundación CIEN‐Fundación Reina Sofia (Madrid). The local ethical committee approved the study.
Table 2.
Descriptive statistics and correlation matrix for the intelligence measures (P < 0.05*; P < 0.01**) across sessions (test and retest)
| Intelligence measures | RAPM test | DAT‐AR test | DAT‐SR test | DAT‐VR test | RAPM retest | DAT‐AR retestT | DAT‐SR retest | DAT‐VR retest | Total test | Total retest |
|---|---|---|---|---|---|---|---|---|---|---|
| RAPM test | 0.30 | 0.34 | 0.15 | 0.32 | 0.27 | 0.31 | 0.24 | 0.51* | 0.35 | |
| DAT‐AR test | 0.62** | 0.44 | 0.60** | 0.58** | 0.65** | 0.53* | 0.87** | 0.61** | ||
| DAT‐SR test | 0.30 | 0.35 | 0.59** | 0.72** | 0.40 | 0.84** | 0.65** | |||
| DAT‐VR test | 0.21 | 0.24 | 0.23 | 0.34 | 0.62** | 0.30 | ||||
| RAPM retest | 0.57** | 0.55* | 0.43 | 0.52* | 0.76** | |||||
| DAT‐AR retest | 0.71** | 0.67** | 0.62** | 0.89** | ||||||
| DAT‐SR retest | 0.57** | 0.71** | 0.87** | |||||||
| DAT‐VR retest | 0.53* | 0.77** | ||||||||
| Total test | 0.73** | |||||||||
| Total retest | ||||||||||
| Mean (total sample) (SD) | 10.60 (2.0) | 12.95 (4.4) | 12.30 (4.5) | 11.05 (2.9) | 11.70 (3.5) | 11.20 (3.9) | 14.05 (4.8) | 12.00 (2.9) | 46.90 (10.4) | 48.95 (12.6) |
| Mean (practice group) (SD) | 10.60 (1.7) | 12.00 (4.24) | 12.70 (4.06) | 10.80 (3.4) | 11.10 (4.4) | 11.20 (4.1) | 13.60 (5.0) | 11.70 (3.5) | 46.10 (9.9) | 47.60 (14.4) |
| Mean (control group) (SD) | 13.40 (9.0) | 13.90 (4.53) | 11.90 (5.0) | 11.30 (2.5) | 12.30 (2.3) | 11.20 (3.9) | 14.50 (4.9) | 12.30 (2.2) | 47.70 (11.4) | 50.30 (11.0) |
Advanced Progressive Matrices test (RAPM), abstract reasoning (DAT‐AR), verbal reasoning (DAT‐VR), and spatial relations (DAT‐SR) subtests from the Differential Aptitude test (DAT‐5) Battery. Correlations were computed from accuracy scores. For general cognitive ability a summary score was obtained (RAPM + DAT‐AR + DAT‐VR + DAT‐SR = TOTAL).
Procedure
Participants completed a battery of four reasoning tests and they were submitted to a 6‐min resting‐state fMRI scanner (rest quietly with eyes closed) in two blocks (both intelligence measures and rsfMRI scan) separated by 4 weeks (test–retest). Between these blocks, 10 females (practice group) were trained during a maximum of 16 h (2 h by session, two sessions by week) on a pack of reasoning puzzles (Professor Layton and The Pandora's Box, by Nintendo) under strict supervision in the laboratory. Retest (Session 2) replicated the first, just changing the items (even‐test vs. odd‐retest numbered items). In order to control for retest effects, resting brain activity of the practice group was compared with the remaining females (control group). General cognitive ability scores were also considered for control purposes. This is unusual in previous research, but we reasoned that individual differences in intelligence might mediate both videogame performance [Colom et al., 2010b, Quiroga et al., 2009, 2011] and intersubject variability in the analyzed resting state networks [Song et al., 2008, 2009; van den Heuvel et al., 2009; Wang et al., 2011].
Behavioral Measures
Intelligence
Screening versions of four well‐known reasoning tests were used: (1) the Raven Advanced Progressive Matrices test (RAPM), along with the (2) abstract reasoning (AR), (3) verbal reasoning (VR), and (4) spatial relations (SR) subtests from the Differential Aptitude Test (DAT‐5) Battery. Accuracy scores were calculated for each measure. For general intelligence (g) an omnibus measure was obtained (RAPM + DAT‐AR + DAT‐VR + DAT‐SR = 83 items).
The RAPM comprises a matrix figure with three rows and three columns with the lower right hand entry missing. Participants choose, among eight alternatives, the one completing the 3 × 3 matrix figure. DAT‐AR is a series test based on abstract figures. Each item includes four figures following a given rule, and participants choose one of five possible alternatives. DAT‐SR is a mental folding test. Each item is composed by an unfolded figure and four folded alternatives. The unfolded figure is shown at the left, whereas figures at the right depict folded versions. Participants choose one folded figure corresponds to the unfolded figure at the left. DAT‐VR is verbal reasoning test. A given sentence stated like an analogy must be completed. The first and last words from the sentence are missing, so a pair of words must be selected to complete the sentence from five possible alternative pairs of words. For instance: “… is to water like eating is to … (A) Travelling‐Driving, (B) Foot‐Enemy, (C) Drinking‐Bread, (D) Girl‐Industry, (E) Drinking‐Enemy”. Only one alternative is correct.
Cognitive practice
“Professor Layton and The Pandora's Box” is a “solving history” (mystery) videogame. To advance on the story, the player must solve puzzles built with different characters. The game includes 138 puzzles (plus 15 extra puzzles) demanding reasoning and mental manipulation of spatial, numerical, and verbal information (examples are depicted in Fig. 1). Solved puzzles per hour of practice were systematically registered.
Figure 1.
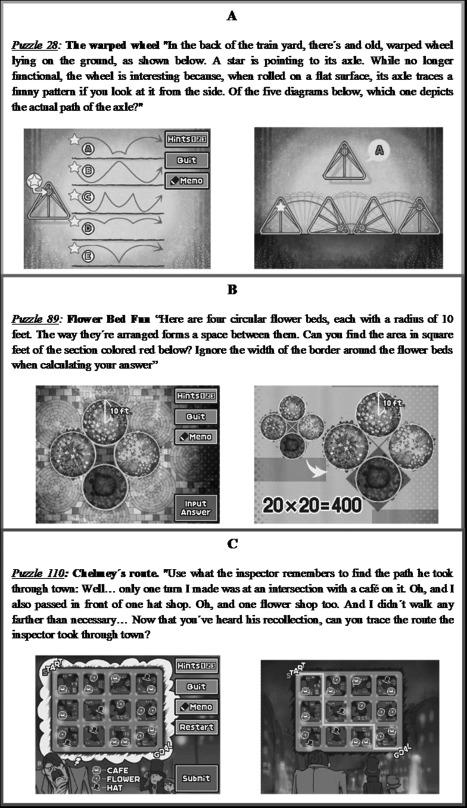
Professor Layton and the Pandora's Box. Examples of (A) “spatial” puzzle, (B) “general” puzzle, and (C) “verbal” puzzles. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Imaging Methods
Data acquisition
Images were acquired on a General Electric Signa 3T MR Scanner (General ElectricHealthcare, Farfield, CT) using a whole‐body radiofrequency (RF) coil for signal excitation and quadrature 8‐channel coil for reception. For the functional scan, 6 min Gradient‐Echo EPI were acquired with the following sequence parameters: repetition time (TR) = 3,000 ms, echo time (TE) = 28.1, flip angle = 90°, 36 oblique ACPC‐oriented slices, FoV = 24 cm, slice thickness = 2.4 mm, spacing between slices = 0.3 mm, acquisition matrix = 96 × 96. One hundred twenty volumes were acquired during the resting‐state scan.
For the structural image, a high‐resolution three‐dimensional T1‐weighted Gradient Echo‐SPGR with parameters: 1‐mm slice thickness, 260 × 260 matrix, Preparation Time = 500 ms, TE = 4.2 ms, TR = 9.2 ms, flip angle 8°, complete volume with 158 sagittal slices.
Data preprocessing
RsfMRI images were analyzed using FSL (fMRIB Software Library; available at: http://www.fmrib.ox.ac.uk/fsl) [Smith et al., 2004; Woolrich et al., 2009]. The preprocessing method applied to the images was divided into different stages. First, a slice timing correction for interleaved bottom‐up acquisition using a sinc interpolation was applied; then head motion correction was performed using the algorithm MCFLIRT [Jenkinson et al., 2002] to obtain spatially realigned images across volumes. Furthermore, a spatial smoothing of 6 mm FWHM and a high‐pass temporal filter (100 s) were used. In addition, functional images were coregistered to its three‐dimensional high‐resolution anatomical images and normalized to MNI152 phantom using the FLIRT algorithm as found in FSL.
ICA analysis and dual regression
To obtain group independent spatial maps, the multivariate exploratory linear optimized decomposition into independent components (MELODIC) [Beckmann and Smith, 2004] toolbox in FSL (FMRIB Software Library; available at: http://www.fmrib.ox.ac.uk/fsl) was used. Temporal Concatenation Group Independent Component Analysis (TC‐GICA) was conducted to generate group‐level components across all participants and sessions [Beckmann et al., 2005]. This approach consists of three fundamental steps: (1) estimation of a mean covariance matrix: all 40 preprocessed and normalized to MNI152 standard space individual fMRI datasets (20 test and 20 retest; 120 volumes each) were temporally concatenated and used to estimate the mean covariance matrix; (2) PCA reduction of individual datasets. The number of components was restricted, as suggested by Smith et al. 2004, to study large‐scale spatial networks. Here it was fixed to 25 components as reported by Damoiseaux et al. 2006. Before PCA, data must be demeaned; also, as part of the pre‐ICA processing, all voxels' time series were variance normalized [Smith et al., 2009]; and (3) probabilistic ICA was conducted on temporally concatenated data. This procedure produced 25 group independent spatial maps.
A spatial mixture model was then applied to each component map to infer whether the voxels were significantly modulated by the associated estimated independent component (IC) timeseries (p < 0.5). All components were standardized into Z‐scores maps by dividing the relevant component weight by the standard deviation of the background noise. These maps were a measurement of the signal‐to‐noise ratio (SNR). The 25 ICs were sorted into two broad classes: biologically plausible/functionally relevant components or RSNs, and scanner/physiological artifactual components (cerebrospinal fluid, white matter, head motion, and large vessels artifacts). The inspection was made visually based, primarily, on each component's spatial profile following criteria purposed by Kelly et al. 2005: a component was classified as “noise” (NIC) when 90% or more of it “activations” or “deactivations” were localized “in peripheral areas or in a spotty or speckled pattern… without regard for functional–anatomical boundaries” (p. 234). In contrast, they were identified as “functionally relevant network” (RSN) when at least 10% of the activations or deactivations were found in small to larger grey matter biologically plausible brain areas. The second step examined the time series‐based power spectrum profile. Components were considered “noise” when (1) more than 50% of the power in the Fourier Frequency spectrum of the component's time course is over 0.1 Hz; or/and (2) one or more large (greater than five standard deviations), abrupt (over 6 s or less) changes appear in the normalized time course; or/and (3) there is a sharply and regularly alternating up‐and‐down time course; (4) sinus coactivation: roughly 10 or more thresholded voxels is presented in the superior sagittal sinus (p. 234–235). Nine RSNs previously related to functionally relevant brain functions were chosen [Beckman et al., 2005; Damoiseaux, 2006, 2008; Filippini et al., 2009; Perlbarg and Marrelec, 2008; Smith et al., 2009; Shehzad et al., 2009; Toro et al., 2008; Zuo et al., 2010] and labeled accordingly (see Fig. 2).
Figure 2.
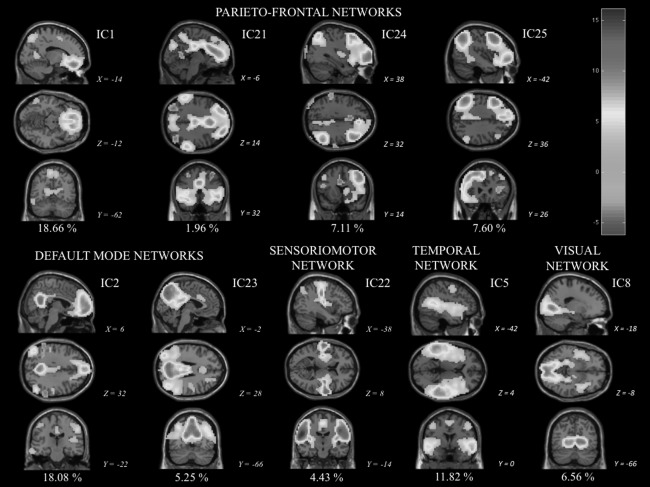
Visually identified group‐level components obtained by temporal concatenation group independent component analysis (TC‐GICA): fourth parietofrontal networks (IC1, IC21, IC24, and IC25), two default mode networks (IC2; IC23 = posterior cingulate/ precuneus subsytem), one sensoriomotor (IC22) network, one mainly temporal network (IC5), and one visual network (IC8). Sagittal, coronal, and axial views (showed as x, y, z according to MNI152 standard space) of these nine functionally relevant group‐level independent components (ICs) are displayed according to neurological convention (right on right). The percentage of variance explained by each IC is displayed below each IC. The z‐statistic map legend for all networks is shown in the up right corner. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
It is important to note that TC‐GICA analysis was applied using the entire dataset from the 20 participants across the two scans (a total of 40 scans: test and retest). This was done for providing a setup equivalent to studies based on control‐patient populations making their group level analyses over the large dataset rather than over a single subset [Damoiseaux et al., 2006; Filippini et al., 2009; Rombouts et al., 2009; Zuo et al 2010]. Further, this allows obtaining the best possible ICA estimation multiplying sample size by a factor of 2.
To perform intersubject analysis, each subject‐specific temporal dynamics and spatial map related to each group IC was computed using a dual regression procedure. This was done in two steps: (1) each group IC spatial map was used as a mask in a spatial regression obtaining a subject‐specific time course associated to that group IC, and (2) the obtained time courses in the first regression were used in a temporal regression to estimate a subject‐specific spatial map per group component. The result of this dual regression approach provided 40 × 25 subject‐specific spatial maps related to the 25 groups ICs per subject and session.
Statistical Analyses
Test–retest reliability
Intraclass correlation coefficients (ICC) [Shrout and Fleiss, 1979] were used for assessing intersession reliability in the RsfMRI per group level IC. The specific form used here is [Caceres et al., 2009]:
where, BMS = sum of mean squares between subjects, EMS = error mean squares, k = number of repeated sessions
Test–retest reliability was assessed using the dedicated MATLAB reliability toolbox [Caceres et al., 2009] in conjunction with SPM8. One ICC per relevant group‐level component was computed using as input the individual dual regression outputs per subject and session (20 pairs of contrast images). The mode [the first most frequent ICC value, like in Zuo et al., 2010] of the ICC distributions per group‐level component were obtained for three different brain areas: (1) the whole brain, where the nonbrain structures were masked, (2) the activation network, defined by voxels in each relevant spatial group IC map, where Z‐scores exceeded 2.3 (p < 0.01), and (3) the deactivation network, defined by voxels in each relevant spatial group IC map, where the Z‐scores were lower than −2.3 (p < 0.01).
Voxel‐wise analyses
For studying practice effects on functional connectivity, IC maps for the practice and control groups at both sessions were compared using permutation‐based inference (5,000 permutations) [Nichols and Holmes, 2002] with a threshold‐free cluster enhancement (TFCE) method as it is found in FSL (randomize) [Smith and Nichols, 2009]. The multiple comparisons nonparametric permutation approach relies on minimal assumptions, deals with the multiple comparisons issue, and can be applied when the assumptions of a parametric approach are untenable for obtaining an empirical null distribution. The rationale for choosing non‐parametric permutation is directly related to the nature of the analyzed sample and to the employed experimental design. It is better for multisubject fMRI involving small numbers of subjects, where analysis must be conducted at the subject level to account for intersubject variability [Nichols and Holmes, 2002]. Also, the TFCE method is more sensitive than voxel‐wise thresholding in finding the “true signal” making possible cluster‐based inference without specifying an arbitrary cluster‐forming threshold. TFCE allows enhancing individual voxels proportionally to their level of local cluster support at a wide range of different thresholds, while still facilitating a fundamentally voxel‐wise comparison.
Two t‐tests were computed, adding Z‐normalized intelligence scores as a confound regressor: (a) paired t‐test for related measures (two statistical contrasts obtained: retest > test and retest < test) for the practice group to inspect significant increases or decreases on regional functional connectivity after practice, (b) unpaired t‐test for related measures for both sessions in the two groups [two statistical contrasts obtained: practice group (retest–test) > control group (retest–test) and control group (retest–test) > practice group (retest–test)] to study specific effects of practice on functional connectivity. Results were considered significant at P < 0.005 uncorrected and cluster size = 10, because statistical maps did not survived FWE correction. We assume these as non‐“false‐positive” results noting that (1) we applied an accurate and sensitive methodology for detecting variations across regions in a small sample, and (2) significant results are coherent with theoretical expectations based on previous research (regions that exhibited changes were indeed located in the parietofrontal network). Moreover, chosen RSN were obtained by ICA (which distinguishes artifactual noise and functionally relevant signal) and selected from a set of networks according to their intersession reliability (ICC) in our sample (which secures the analysis of practice‐related changes on stable networks). Nevertheless, because uncorrected statistics were considered results should be interpreted with caution.
We also directly link changes on functional connectivity (individual IC maps) and increases in cognitive performance after practice sessions. A statistical permutation test was computed per component of interest using randomize from FSL. We defined as regressor the mean of difficulty solved by each subject in the training group (10 females). Z‐normalized intelligence scores in Session 1 were added as confound covariable. Each statistical analysis used as input the difference between sessions (Retest minus Test) in individual beta maps obtained after the dual‐regression procedure. Results were considered significant for P < 0.005 uncorrected using a threshold‐free cluster enhancement (TFCE) [Smith and Nichols, 2009].
Statistical analyses
Statistical analyses for the behavioral measures were conducted using SPSS 15 (Statistical Package for the Social Sciences).
RESULTS
Group‐Level Components: Test–Retest Reliability Across Sessions and Subjects
Twenty‐five group‐level components resulting from TC‐GICA analysis across sessions and subjects (approximately 70% of explained variance) were visually inspected. Based on biological relevance and plausibility of their spatial maps plus time series power spectrum profiles, nine ICNs (Fig. 2) previously related to relevant and high‐order brain functions or systems [Beckman et al., 2005; Demoiseaux et al., 2006, 2008; Perlbarg and Marrelec, 2008; Shehzard et al., 2009; Toro et al., 2008; Zuo et al., 2010] were chosen: four parietofrontal networks (IC1, IC21, IC24, and IC25), two default mode networks (IC2; IC23 = posterior cingulate/precuneus subsytem), one sensoriomotor (IC22) network, one mainly temporal network (IC5), and one visual network (IC8).
ICCs were obtained for all relevant visually selected ICs. As Table 1 shows, activations within networks were more reliable than deactivations (except for IC1 and IC21), and both were more reliable than the whole brain. Components showed moderate to high test–retest reliability (modal ICC: 0.50 to 0.65). Only two components, IC5 and IC8, exhibited low reliability (modal ICC: 0.378 and 0.046, respectively) so they were excluded for subsequent analyses.
Table 1.
Mode for the intraclass correlation coefficients (ICC) distributions per group‐level component obtained for three different brain areas: (1) brain: the whole brain, where the nonbrain structures were masked; (2) network: the activation network, defined by the voxels in each relevant spatial group IC map, where z‐scores exceeded 2.3 (P < 0.01) (activations); and (3) deactivations: the deactivation network, defined by the voxels in each relevant spatial Group IC map, where the z‐scores were lower than −2.3 (P < 0.01)
| IC | Brain | Network | Deactivations |
|---|---|---|---|
| 1 | 0.431 | 0.435 | 0.439 |
| 2 | 0.343 | 0.624 | 0.440 |
| 5 | 0.154 | 0.378 | 0.198 |
| 8 | 0.068 | 0.046 | 0.199 |
| 21 | 0.275 | 0.416 | 0.617 |
| 22 | 0.108 | 0.424 | 0.382 |
| 23 | 0.286 | 0.547 | 0.395 |
| 24 | 0.329 | 0.687 | 0.294 |
| 25 | 0.302 | 0.587 | 0.309 |
Intelligence Differences and Performance in Practice Sessions
A mean of solved difficulty per hour score was computed taking into account the difficulty rating by puzzle (provided by Nintendo). Difficulty scores during practice sessions revealed strong improvements over time (Fig. 3). Solved puzzles were mainly grouped and labeled “verbal,” “spatial,” “abstract,” and “general,” according to their mental processing requirements.
Figure 3.
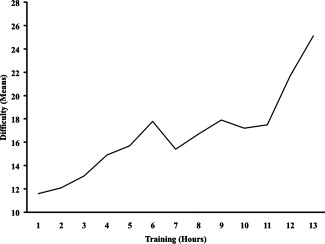
Practice improvements in “Professor Layton and The Pandora's Box” measured by difficulty levels of the solved puzzles. The graph only extends to 13 h on the x‐axis because from that point several participants systematically finish the standard game: three in 13 h, one in 14 h, one in 15 h, and one in 16 h. These six participants keep playing but using problems that cannot be compared with those organized for the standard game.
The descriptive statistics and correlation matrix for the intelligence measures in both sessions are shown in Table 2. The administered intelligence tests showed distinguishable loadings on a composite factor representing general intelligence (g). Further, these measures were also distinguishable by performance variability. Changes on intelligence scores between sessions were also studied computing a t‐test for related measures. Results showed nonsignificant differences (P < 0.01) between Session 1 and Session 2 in both groups (practice and control) for all the intelligence measures.
Stepwise linear regression was carried out for predicting intelligence measures. General intelligence (g) and DAT‐VR scores in Session 1, were predicted by verbal puzzles [(β = 0.78; R 2 corrected = 0.56, P < 0.01) and (β = 0.79; R 2 corrected = 0.58, P < 0.01) respectively]; DAT‐AR scores were predicted by verbal and spatial puzzles (β = 0.82 and β = −0.45, respectively; R 2 corrected = 0.70, P < 0.01); RAPM scores were predicted by abstract puzzles (β = 0.71; R 2 corrected = 0.44, P < 0.05); DAT‐SR scores were predicted by general puzzles (β = 0.71; R 2 corrected = 0.44. P < 0.05). Therefore, solving puzzles during practice sessions required reasoning, verbal, and spatial skills.
Intelligence differences at Session 1 predict variability on behavioral performance improvements during practice sessions: mean of difficulty solved (β = 0.77; R 2 corrected = 0.54, P < 0.01) and number of hours needed to finish the videogame (β = 0.71; R 2 corrected = 0.43, P < 0.05). Females who finished the videogame (t‐test for independent samples, P < 0.01) were on average more intelligent at Session 1 (mean = 51.67) than those that failed to finish (mean = 37.75) (t(8) = 3.557; d = 2.52).
Regional Changes on Functional Connectivity After Videogame Practice
Voxel‐wise analyses using permutation methods (TFCE; p < 0.005; cluster size = 10) were used to study significant test–retest changes on functional connectivity in the practice group compared with the control group, controlling for intelligence differences (unpaired t‐test for related measures).
Figure 4 shows several areas in which changes could be attributed to practice (i.e. where beta values at Session 2 were greater than at Session 1 in the practice group as compared with the control group). Importantly, no significant changes were found in the control group as compared with the practice group.
Figure 4.
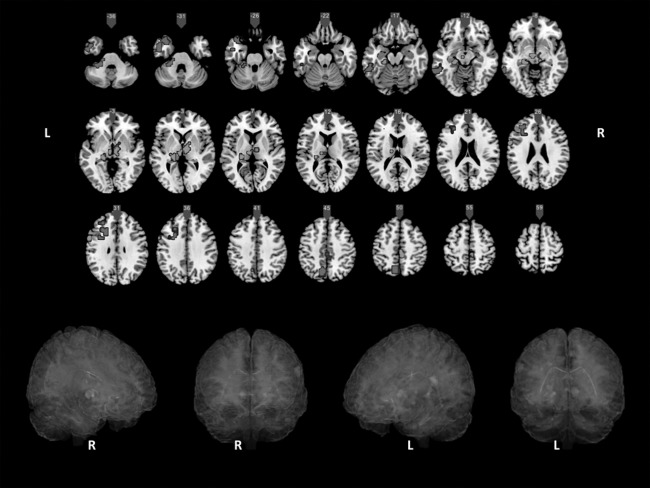
Voxel‐wise analyses using permutation methods (TFCE p < 0.005; cluster size = 10). Areas in which changes in functional connectivity after practice were significantly higher for the practice group compared with the control group are displayed (IC1 = red; IC2 = green; IC23 = violet; IC22 = blue; L =left; R = right). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Almost all relevant changes were localized at the left hemisphere, mainly on parietal, prefrontal, and temporal regions: lateral temporal (posterior inferior and middle, fusiform and temporal pole), medial temporal (hippocampus, parahippocampal gyrus), anterior and central precuneus, dorsolateral prefrontal, and ventrolateral prefrontal. Significant changes on bilateral thalamus, right precuneus, bilateral posterior cingulate (PCC), and left anterior cerebellum were also found.
Voxel‐wise analyses using permutation methods and controlling for intelligence differences (p < 0.005 uncorrected; cluster size = 5) showed several areas in which mean of solved difficulty score were positively related to changes on connectivity. As it can be seen in Figure 5, improvements on videogame basically showed positive correlations with large‐scale synchronization between several parietal, frontal, and temporal regions involved in high‐order cognitive processing across components. Main regional findings are concentrated on bilateral superior parietal (precuneus), posterior cingulated, retrosplenial cortex, inferior parietal/supramarginal (BA40), temporoparietal and occipitotemporal junctions and posterior temporal (BA21/22 Wernicke's area), left temporal pole, bilateral bilateral parahippocampal gyrus, left inferior frontal gyrus, bilateral dorsolateral and ventromedial prefrontal (BA10, 11), left middle frontal gyrus (BA9), anterior cingulate (BA24, 32) and bilateral cuneus (BA18, 19), bilateral cerebellum, and thalamus. No negative correlations were found. Thus, improvements on videogame were also related to connectivity changes on several key regions for high order cognition.
Figure 5.
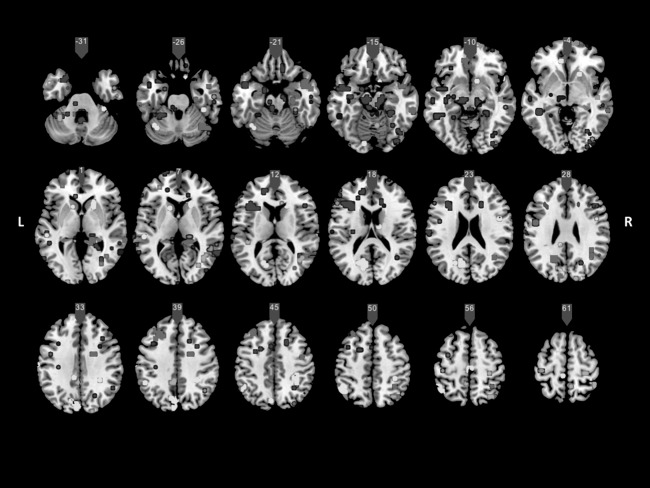
Voxel‐wise analyses using permutation methods (TFCE p < 0.005 uncorrected; cluster size = 5). Areas in which mean of solved difficulty per hour score (practice group) were positively related to changes on connectivity (retest–test) across relevant independent components (IC) are shown (IC1 = red; IC2 = dark blue; IC21 = green; IC22 = yellow; IC23 = pink; IC24 = violet; IC25 = light blue; L =left; R = right). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Statistical maps for the practice group before and after videogame practice (paired t‐tests), suggest that the observed changes (in both above‐mentioned contrasts) mainly denote connectivity improvements (increases in functional connectivity measured as z‐value in dual regression individual maps).
DISCUSSION
Here we have shown that practice on a videogame with high‐level cognitive requirements can be tentatively associated with changes in the synchrony levels of low frequency spontaneous fluctuations in some, but not all, resting state networks. Changes occurred mainly on left temporal, parietal, and frontal resting networks, most probably because they were especially required during practice. Behavioral improvements in the videogame across practice sessions were related to more efficient large‐scale connected key regions presumably involved in varied memory and executive functions. Admittedly, a small sample was considered, but participants within the practice and control groups were carefully selected for representing a wide range in general cognitive ability, which is unusual. Nevertheless, because uncorrected statistics were used, results should be interpreted with caution, even when they support the main prediction. Further, they cannot be immediately generalized to other videogames or samples.
Considering the proposed role of functionally organized spontaneous BOLD activity in brain function [Buzsaki and Draguhn, 2004; Fox and Raichle, 2007; Fox et al., 2005; Salinas and Sejnowski, 2001; Varela et al., 2001] changes mainly in temporal‐parietofrontal functional connectivity might be understood from the frequent coactivation of these regions during videogame practice.
Changes were prominent on parietofrontal network especially devoted to a wide variety of complex cognitive processing (such as attention, working memory, and episodic memory retrieval). Comparing control and practice groups, functional connectivity changes on parietal cortex were commonly detected in central precuneus, which plays a multimodal integrative role [Margulies et al., 2009]. Consistently, long‐distance central precuneus and left dorsolateral prefrontal brain regions were simultaneously modified in the same component. This central precuneus region has been previously related to the parietofrontal control system, because of its strong resting state functional connectivity with the multisensory angular gyrus and dorsal prefrontal cortex, which is consistent with our results. Thus, considering the role of this network on integration and control of information processing, we suggest that practice on this type of videogame increases the functional connectivity within brain networks underlying higher‐order executive processing, such as monitoring information in working memory and action planning.
Moreover, functional connectivity in the posterior cingulate cortex was modified along with two functionally relevant pathways, namely (1) the parietofrontal control system, including multimodal integration temporal areas, and (2) the hippocampal memory system, including the left prefrontal cortex, hippocampus, and parahippocampus. Interestingly, functional connectivity on bilateral thalamus was also modified. This structure plays an important role in sensory and motor integration. In addition, thalamic regions interact with the hippocampus for supporting learning and memory [Aggleton et al., 2010]. These interactions seem relevant for episodic memory.
Taken together, these results suggest that the cognitive practice employed here involve prefrontal regions interacting with posterior memory‐related areas for cognitive control of encoding and retrieval processes when the maintained information is monitored and manipulated in the working memory system [Takahashi et al., 2007]. This key finding is consistent with previous studies exploring how resting‐state functional connectivity is modified by practice, specifically functional systems presumably involved in the practiced cognitive activity. Several studies have found that parietofrontal regions are especially responsive to practice on working memory [Jolles et al., in press], visuomotor [Albert et al., 2009], or visuospatial tasks [Lewis et al., 2009].
We relied upon a cognitive practice program based on the commercial videogame “Professor Layton and The Pandora's Box” (by Nintendo). To successfully play this game, involvement of several complex cognitive processes and skills is strongly required [Quiroga et al., 2009, 2011]. These processes and abilities are related to variability on standardized intelligence measures, which is consistent with our findings showing that intelligence differences predicted variability in practice performance (achieved mean difficulty and number of hours needed to finish the videogame). These shared requirements of high‐level cognition might explain why changes on functional connectivity related to improvements on the videogame (mean of difficulty solved) overlap discrete brain regions supporting intelligent performance as summarized by the Parieto‐frontal Integration Theory (P‐FIT) [Colom and Thompson, 2011; Colom et al., 2010a; Jung and Haier, 2007]. This model nominates discrete brain regions supporting distinguishable information processing stages: (1) occipital and temporal areas for processing sensory information, (2) integration and abstraction of sensory information by parietal regions, (3) interaction between parietal areas and the frontal lobes for supporting problem solving, evaluation, and hypothesis testing, and (4) response selection and inhibition of alternative responses supported by the anterior cingulate. All these regions are relevant, but Jung and Haier 2007 underscore the dorsolateral prefrontal cortex (BAs 9, 10, 45, 46, and 47) and the parietal cortex (BAs 7 and 40) for intelligence and working memory capacity. Consistently, here we reported improvements in functional connectivity between dorsolateral prefrontal and parietal (precuneus and supramarginal) regions related to highest videogame performance, even when individual intelligence differences were controlled.
Finally, it has been suggested that cognitive training or practice programs affecting higher association cortices, and, specifically, neural changes in the parietofrontal network, should improve performance in nontrained similar tasks [see Klingberg, 2010]. Here we reported regional improvements in functional connectivity after playing a commercial game, suggesting that this practice requires higher‐order abilities. But even when these changes overlap key regions for intellectual performance and reflects improvements solving the videogame challenges, intelligence scores did not increase after practice. Actually, it is not rare. The long history of research on cognitive training have been showing that although performance on trained tasks can increase dramatically after practice, transfer, or generalizability to other tasks or domains usually fails [Colom et al., 2010]. Therefore, it is unclear whether or not these functional connectivity improvements are stronger enough for influencing behavioral performance in related areas like intelligence tests. However, although highly interesting, this point is far beyond our main research goal.
CONCLUSION
The present study suggests that practice on a cognitively demanding commercial videogame increases correlated activity in certain predefined theoretically relevant resting state networks (albeit using uncorrected statistics). As demonstrated by Quiroga et al. [2009, 2011] cognitive practice programs demanding high‐level cognition involve (a) novelty, (b) moderate complexity, and (c) working memory capacity. These cognitive requirements are consistent with connectivity changes in spontaneous long‐distance brain networks after practice revealed by the present study. Specifically, functional connectivity among discrete regions presumably involved in (1) early stages of new learning (thalamus‐hippocampus‐prefrontal) and (2) processing within the working memory system of previously integrated incoming information (temporal‐parietal‐prefrontal) was modified.
ACKNOWLEDGMENTS
The authors thank “Colegio Universitario Cardenal Cisneros” (Madrid, Spain) for providing assistance and facilities for participants' selection and behavioral data acquisition.
REFERENCES
- Aggleton JP, O'Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT (2010): Hippocampal‐anterior thalamic pathways for memory: Uncovering a network of direct and indirect actions. Eur J Neurosci 31:2292–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert NB, Robertson EM, Miall RC (2009): The resting human brain and motor learning. Curr Biol 19:1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL (2007): Disruption of large‐scale brain systems in advanced aging. Neuron 56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin K, Ucar A, Oguz KK, Okur OO, Agayev A, Unal Z, Yilmaz S, Ozturk C (2007): Increased gray matter density in the parietal cortex of mathematicians: A voxel‐based morphometry study. Am J Neuroradiol 28:1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM (2004): Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med. Imaging 23:137–152. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Mackay CE, Filippini N, Smith SM (2009): Group comparison of resting‐state FMRI data using multi‐subject ICA and dual regression. NeuroImage 47(Suppl 1):S39–S41.
- Biswal B, Yetkin F, Haughton V, Hyde J (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP (2010): Toward discovery science of human brain function. Proc Natl Acad. Sci USA 102:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang‐Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S (2008): Intrinsic brain activity in altered states of consciousness: How conscious is the default mode of brain function. Ann NY Acad Sci 1129:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A (2004): Neuronal oscillations in cortical networks. Science 304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Caceres A, Hall DL, Zelaya FO, Williams ACR, Mehta M (2009): Measuring fMRI reliability with the intra‐class correlation coefficient. Neuroimage 45:758–768. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T (2009): A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage 45:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Jonides J, Smith EE, Noll DC (1997): Temporal dynamics of brain activity during a working memory task. Nature 386:604–608. [DOI] [PubMed] [Google Scholar]
- Colom R, Jung R, Haier RJ (2006): Distributed brain sites for the g‐factor of intelligence. Neuroimage 31:1359–1365. [DOI] [PubMed] [Google Scholar]
- Colom R, Jung RE, Haier RJ (2007): General intelligence and memory span: Evidence for a common neuro‐anatomic framework. Cognit Neuropsychol 24:867–878. [DOI] [PubMed] [Google Scholar]
- Colom R, Haier RJ, Head K, Alvarez‐Linera J, Quiroga MA, Shih PC, Jung RE (2009): Gray matter correlates of fluid, crystallized, and spatial intelligence: Testing the P‐FIT model. Intelligence 37:124–135. [Google Scholar]
- Colom R, Karama S, Jung RE, Haier RJ (2010a)Human intelligence and brain networks. Dialogue Clin Neurosci 12:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Quiroga MA, Shih PC, Martínez K, Burgaleta M, Martínez‐Molina A, Román FJ, Requena L, Ramírez I (2010b)Improvement in working memory is not related to increased intelligence scores. Intelligence 38:497–505. [Google Scholar]
- Colom R, Thompson PM (2011): Understanding human intelligence by imaging the brain In: Chamorro‐Premuzic T, von Stumm S, Furnham A, editors.The Wiley‐Blackwell Handbook of Individual Differences.London:Wiley‐Blackwell; pp330–352. [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:215–229. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2000): Mapping functionally related regions of brain with functional connectivity MR imaging. Am J Neuroradiol 21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Dahlin E, Stigsdotter Neely A, Larsson A, Bäckman L, Nyberg L (2008): Transfer of learning after updating training mediated by the striatum. Science 320:1510–1512. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA (2008): Reduced resting‐state brain activity in the default network in normal aging. Cereb Cortex 18:1856–1864. [DOI] [PubMed] [Google Scholar]
- De Luca M, Smith SM, De Stefano N, Federico A, Matthews PM (2005): Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp Brain Res 167:587–594. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM (2006): fMRI resting state networks define distinct modes of long‐distance interactions in the human brain. Neuroimage 29:1359–1367. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004): Changes in grey matter induced by training. Nature 427:311–312. [DOI] [PubMed] [Google Scholar]
- Filippini N, Macintosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE (2009): Distinct patterns of brain activity in young carriers of the APOE epsilon 4 allele. Proc Natl Acad Sci USA 106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anti‐correlated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME (2006): Coherent spontaneous activity accounts for trial‐to–trial variability in human evoked brain responses. Nat Neurosci 9:23–25. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G (2003): Brain structures differ between musicians and non‐musicians. J Neurosci 23:9240–9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, Adolphs R (2010): The distributed neural system for general intelligence revealed by lesion mapping. Proc Natl Acad Sci USA 107:4705–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Karama S, Leyba L, Jung RE (2009): MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual‐spatial task. BMC Res Notes 2:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC (2002): Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp 15:247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT (2006a)Brain connectivity related to working memory performance. J Neurosci 26:13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT (2006b)Connectivity‐behavior analysis reveals that functional connectivity between left BA39 and Broca's area varies with reading ability. Neuroimage 31:513–519. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yücell M (2008): Modulation of brain resting‐state networks by sad mood induction. PLoS One 3:e1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel A, Giesel FL, Garcia Caraballo NM, Amann M, Meyer H, Wüstenberg T, Essig M, Schröder J (2004): Plasticity of cortical activation related to working memory during training. The Am J Psychiatry 161:745–747. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P (2009): Predicting human resting‐state functional connectivity from structural connectivity. Proc Natl Acad Sci USA 106:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ (2008): Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA 105:6829–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady J, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jolles DD, Grol MJ, Van Buchem MA, Rombouts Serge ARB, Crone EA (2010): Practice effects in the brain: Changes in cerebral activation after working memory practice depend on task demands. Neuroimage 52:658–668. [DOI] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, Crone EA, Rombouts SA (2011): Functional brain connectivity at rest changes after working memory training. Hum Brain Mapp. doi: 10.1002/hbm.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ (2007): The parieto‐frontal integration theory (PFIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci 30:135–187. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995): Functional MRI evidence for adultmotor cortex plasticity duringmotor skill learning. Nature 377:155–158. [DOI] [PubMed] [Google Scholar]
- Karama S, Ad‐dab'bagh Y, Haier RJ, Deary IJ, Lyttelton OC, Lepage C, Evans AC, The Brain Development Cooperative Group (2009): Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year‐olds. Intelligence 37:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier RJ, Waber DP, Lepage C, Ganjavi H, Jung R, Evans AC, The Brain Development Cooperative Group (2011): Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. Neuroimage 55:1443–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Garavan H (2005): Human functional neuroimaging of brain changes associated with practice. Cereb Cortex 15:1089–1102. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2008): Competition between functional brain networks mediates behavioral variability. Neuroimage 39:527–537. [DOI] [PubMed] [Google Scholar]
- Kelly RE Jr, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ (2010): Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. J Neurosci Methods 189:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T (2010): Training and plasticity of working memory. Trends Cognit Sci 14:317–324. [DOI] [PubMed] [Google Scholar]
- Lee B, Park J, Jung WH, Kim HS, Oh JS, Choi C, Jang JH, Kang D, Kwon JS (2010): White matter neuroplastic changes in long‐term trained players of the game of “Baduk” (GO): A voxel‐based diffusion‐tensor imaging study. Neuroimage 52:9–19. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M (2009): Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci USA 106:17558–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Narayana SH, Robin DA, Fox PT, Xiong J (2011): Changes occur in resting state network of motor system during 4 weeks of motor skill learning. Neuroimage 58:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M (2009): Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA 106:20069–20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T (2003): Increased prefrontal and parietal activity after training of working memory. Nat Neurosci 14:75–79. [DOI] [PubMed] [Google Scholar]
- Perlbarg V, Marrelec G (2008): Contribution of exploratory methods to the investigation of extended large‐scale brain networks in functional MRI: Methodologies, results, and challenges. Int J Biomed Imaging 2008:218519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyka M, Beckmann CF, Schoning S, Hauke S, Heider D, Kugel H, Arolt V, Konrad C (2009): Impact of working memory load on FMRI resting state pattern in subsequent resting phases. PLoS One 4:e7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga MA, Herranz M, Gómez‐Abad M, Kebir M, Ruiz J, Colom R (2009): Video‐games: Do they require general intelligence? Comput Educ 53:414–418. [Google Scholar]
- Quiroga MA, Román FJ, Catalán A, Rodríguez H, Ruiz J, Herranz M, Gómez‐Abad M, Colom R (2011): Videogame performance (not always) requires intelligence. Int J Online Pedagogy Course Des 1:18–32. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Damoiseaux JS, Goekoop R, Barkhof F, Scheltens P, Smith SM, Beckmann CF (2009): Model‐free group analysis shows altered BOLD FMRI networks in dementia. Hum Brain Mapp 30:256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ (2001): Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP (2009): The resting brain: Unconstrained yet reliable. Cereb Cortex 19:2209–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout P, Fleiss J (1979): Intraclass correlations: Uses in assessing rater reliability. Psychol Bull 86:420–428. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister P, De Luca CJ, Drobnjak I, Flitney DE, Nianzy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Mathews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:208–219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localization in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, Jiang T (2008): Brain spontaneous functional connectivity and intelligence. Neuroimage 41:1168–1176. [DOI] [PubMed] [Google Scholar]
- Song M, Liu Y, Zhou Y, Wang K, Yu C, Jiang T (2009): Default network and intelligence difference. Conf Proc IEEE Eng Med Biol Soc 2009:2212–2215. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Ohki K, Kim DS (2007): Diffusion tensor studies dissociated two fronto‐temporal pathways in the human memory system. Neuroimage 34:827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Marrett S, Saad ZS, Ruff DA, Martin A, Bandettini PA (2009): Functional but not structural changes associated with learning: An exploration of longitudinal voxel‐based morphometry (VBM). Neuroimage 48:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox P, Paus T (2008): Functional coactivation map of the human brain. Cereb Cortex 18:2553–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS, Pol H (2009): Efficiency of functional brain networks and intellectual performance. J Neurosci 29:7619–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE (2009): Functionally linked resting‐state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 30:3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J (2001): The brainweb: Phase synchronization and large‐scale integration. Nat Rev Neurosci 2:229–239. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL (2006): Coherent spontaneous activity identifies a hippocampal‐parietal mnemonic network. J Neurophysiol 96:3517–3531. [DOI] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD (2005): Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp 24:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Nakatani H, Ueno K, Asamizuya T, Cheng K, Tanaka K (2011): The neural basis of intuitive best next‐move generation in board game experts. Science 331:341–346. [DOI] [PubMed] [Google Scholar]
- Wang L, Song M, Jiang T, Zhanga Y, Yua C (2011): Regional homogeneity of the resting‐state brain activity correlates with individual intelligence. Neurosci Lett 488:275–278. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren HR, Preusse F, Kramer J, van der Meer E (2009): Cerebral correlates of analogical processing and their modulation by training. Neuroimage 48:291–302. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM (2009): Bayesian analysis of neuroimaging data in FSL. Neuroimage 45:173–186. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, and Milham MP (2010): Reliable intrinsic connectivity networks: Test–retest evaluation using ICA and dual regression approach. Neuroimage 49:2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]


