Abstract
Some previous functional magnetic resonance imaging (fMRI) studies have revealed increased activation in amyotrophic lateral sclerosis (ALS) patients but longitudinal data on such activation changes are lacking. To assess the time course of changes in fMRI patterns and their potential contribution to the understanding of ALS pathophysiology, we, therefore, investigated a total of 22 patients with ALS and matched control participants while they performed a blocked motor task. Patients were assigned to three groups according to whether they had no (MRC grade 5), mild (MRC 4), or marked (MRC 3) weakness of the examined right hand. Significant activations were seen in primary motor and premotor cortex, somatosensory cortex, supplementary motor area and subcortical areas in all groups. The size of the activated area in the contralateral sensorimotor cortex was increased to a similar degree in all three ALS groups compared to control participants irrespective of weakness on clinical examination. Whereas movement related signal change and beta weights extracted from the activated cluster were unchanged relative to controls in ALS patients with no weakness, a marked decrease of these parameters was seen in patients with weakness. Two distinct stages of neuroplastic changes could be identified in ALS (first: increase of the activated area in contralateral sensorimotor cortex; second: reduction of signal change and beta weights with increasing weakness). We interpret the increase of the activated area as a result of decreased intracortical inhibition and the reduction of movement related signal change and beta weights as a consequence of loss of upper motor neurons. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: ALS, fMRI, neuroplasticity, disease progression, ipsilateral activation
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder involving upper and lower motor neurons (UMNs and LMNs) with a progressive limb and/or bulbar muscular weakness and wasting. The median survival from first symptom ranges from 2 to 4 years, but the survival time varies widely and has been found to be considerably shorter in patients with bulbar involvement [Haverkamp et al.,1995; Norris et al.,1993]. A suitable method to gain insight to the dynamics of neurodegeneration and possible compensatory processes is functional imaging. Indeed, a number of studies have assessed brain activations to movements of the upper extremities and have demonstrated increased activations in cortical and subcortical regions in ALS patients relative to healthy control subjects [Kew et al.,1993,1994; Konrad et al.,2002,2006; Schoenfeld et al.,2005; Stanton et al.,2007b], whereas motor imagery does not lead to activation increases in ALS [Stanton et al.,2007a]. While not undisputed, such increases of the activated areas have generally been interpreted in terms of cortical adaptation as a result of corticospinal tract damage. The neurodegenerative process is already advanced before muscle weakness and wasting occurs [Aggarwal and Nicholson,2002; de Carvalho and Swash,2006; Swash and Ingram,1988]. This implies that such increases should be present also for movements of unaffected limbs, if the aforementioned interpretation is correct. In this study, we therefore investigated motor activations using fMRI for unaffected limbs. At present, it is unknown whether compensatory processes go on throughout the disease or whether compensation is limited. In the former case one would expect further increases of activation with progressing weakness, while in the latter case a decrease of activity would be expected as the patient gets weaker. We therefore also assessed patients with different degrees of weakness. A subset of our ALS patients was scanned on multiple occasions allowing for a longitudinal assessment of the relationship of muscle strength and brain activation. Finally, to gain a more comprehensive picture of activation changes, we assessed different parameters of the activation (activated area, % signal change, beta weights).
METHODS
Two groups of participants were investigated using Blood‐oxygen‐level dependent (BOLD)‐fMRI. The first group comprised 22 ALS patients (nine women) who fulfilled during the course of the disease the diagnostic criteria for probable or definite ALS according to the revised El Escorial criteria of the World Federation of Neurology. The mean age at disease onset was 57 years (SD 12, range 37 to 71 years). According to the initial site of symptoms ALS patients were divided into bulbar‐onset (n = 6) and limb‐onset (n = 16) groups. The mean Amyotrophic Lateral Sclerosis Functional Rating Scale, Revised (ALSFRS)‐R score was 39.5 (SD 4.1, range 34 to 45). The mean interval between first symptom and the diagnosis was 8 months (SD 3, range 2 to 47). The mean time between the first symptom and the study was 13 months (SD 5, range 3 to 54). The interval between the diagnosis and the study (first scan) was 6 months (SD 3, range 1 to 15). None of the patients had other neurological diseases. None of the ALS patients needed noninvasive ventilation or percutaneous endoscopic gastrostomy. The mean time between two studies on the same patient was 4 months (SD 2, range 2 to 7).
The Second group comprised 22 healthy volunteers (12 women) aged from 42 to 67 years (mean age 61 years).
Limb reflexes, spread of limb reflexes, and muscle tone as signs of Upper Motor Neuron (UMN) involvement were scored and entered in a data base. Here, we consider the UMN signs of the right hand. LMN signs evaluated were atrophy and weakness. In this study, we used these data considering the muscles of the right hand; abductor digiti minimi (ADM) and first dorsal interosseous (FDI). The atrophy was scaled with 0 = no involvement, 1 = definite but trace involvement, 2 = moderate involvement, and 3 = severe involvement.
The Medical Research Council (MRC) scale was used for the evaluation of muscle strength [Paternostro‐Sluga et al.,2008]. The measurement involved adductor policis brevis muscle and grip flexion. Based on the MRC, score of the right‐hand patients were classified into three groups (ALS‐MRC‐5, ALS‐MRC‐4, and ALS‐MRC‐3).
Grip strength is considered to be a good indicator of upper limb strength. We therefore used the Martin‐Type Squeeze Dynamometer (Vigorimeter, Martin, Tuttlingen, Germany) to assess grip strength of the right hand to supplement our clinical assessment. The medium‐sized bulb was positioned in the palm of the participant's hand with the air tube extending out between the individual's thumb and index finger and with the fingers wrapped around the bulb such that the fingers touched the surface of the bulb as much as possible. Three measurements with maximum voluntary force were taken and averaged to obtain the grip strength score. Pauses of 30 s were taken in between trials. This method is known to be precise and reliable [Merkies et al.,2000,2003].
Experimental Design
Each functional session comprised one run of the motor paradigm (flexion and extension of fingers in the right hand) with four blocks of alternating task and rest conditions each lasting 20 s. The frequency of the hand movements was practiced outside of the scanner to assure a highly similar frequency across patients and disease stages.
Image Acquisition
Magnetic‐resonance images were acquired on a 3‐T Siemens Magnetom Scanner (Erlangen, Germany) equipped with a standard head coil. A total of 83 T2*‐weighted volumes of the whole brain (EPI‐sequence; TR 2000 ms, TE 30 ms, flip angle 80°, Field of View (FOV) 192 mm, matrix 642, 34 slices, slice thickness 3 mm, interslice gap 0.75 mm) parallel to the AC‐PC line were recorded for functional imaging. A T1‐weighted high resolution data set was acquired using a 3D‐MPRAGE sequence for anatomical information (matrix 192 × 256, 1 mm isovoxel). The subject's head was immobilized by foam padding to avoid head movements. Furthermore, participants were asked to avoid any movements with head and body other than those required during the scan.
fMRI Data Analysis
Analysis and visualization of the data were performed using Brain Voyager QX (Brain Innovation BV, Maastricht, The Netherlands) software. Slice scan time correction, 3D motion correction, and correction of linear trend were applied to all functional data using the preprocessing procedures implemented in Brain Voyager QX. Structural and functional data were spatially transformed into the Talairach standard space [Lancaster et al.,2000] using a 12‐parameter affine transformation. Functional echo‐planar imaging (EPI) volumes were spatially smoothed with an 8 mm full‐width half‐maximum isotropic Gaussian kernel to accommodate residual anatomical differences across volunteers. The first three volumes of the functional measurement were discarded to allow for steady state magnetization.
For the statistical model, a design matrix including all conditions was specified using a hemodynamic response function. This function was created by convolving the rectangle function with the model of Boynton [Boynton et al.,1996] using delta = 2.5, tau = 1.25, and n = 3, i.e., standard values. Thereafter, a multi‐subject random effects (RFX) analysis of variance model (ANOVA) with stimulation (task vs. rest) as the first main within‐subject factor was used to identify t‐maps for each group. In addition, further analyses contrasting the task effects for different groups were also conducted. The false discovery rate threshold of q(FDR) < 0.01 [Genovese et al.,2002] was chosen for identification of the activated voxels. Furthermore, a cluster volume threshold of eight contiguous voxels (i.e. a volume of 216 mm3) was chosen to exclude small spurious activations. All voxels fulfilling these criteria are reported. The centers of mass of suprathreshold regions were localized using Talairach coordinates and the Talairach Daemon tool [Lancaster et al.,2000].
Activated clusters involving supplementary motor area (SMA) and contralateral sensorimotor cortex were identified for each subject, using Talairach coordinates and the Talairach Daemon tool [Lancaster et al.,2000], and in addition to cluster size, the beta values and mean signal change (%) were extracted and used for further analysis. The size of an activated cluster reflects the extent of the neuronal populations recruited by a task, whereas both, signal change and beta values, provide measures for the effect size and, indirectly [Logothetis and Wandell,2004; Logothetis et al.,2001], of the degree of neural activity within a region of interest.
RESULTS
Patient Classification
ALS‐MRC‐5
In 11 patients, the right hand was clinically unaffected at the time of study. Eight patients showed signs of UMN involvement for the right upper extremity including increased and spread of limb reflexes but all patients had normal muscle tone. None of the patients showed muscle atrophy of the right hand. Six patients showed only bulbar signs, two mild atrophy of the left hand, and the rest showed weakness and atrophy of the lower limbs.
ALS‐MRC‐4
Seventeen fMRI scans were obtained in patients who had mild weakness of the right hand. Out of these, 10 patients had belonged to ALS‐MRC‐5 but had developed weakness of the right hand on follow‐up. All of these patients showed mild atrophy of the right hand. The mean scores (± SD) for ADM and FDI were 1.1 ± 0.6 and 1.2 ± 0.4, respectively. Twelve patients showed UMN involvement of the right upper extremity, i.e., increased and spread of limb reflexes with normal muscle tone.
ALS‐MRC‐3
Nineteen fMRI scans were obtained in patients with significant weakness of the performing right hand. Out of these, 10 had belonged to ALS‐MRC‐5 and 4 had belonged to ALS‐MRC‐4 but had developed weakness on further follow up. All of these patients showed significant atrophy in right hand. The mean scores (± SD) for ADM and FDI were 2.5 ± 0.4, and 2.4 ± 0.5, respectively. Eleven patients showed unequivocal signs of UMN involvement of the right hand.
To summarize, 10 patients were examined thrice, once as MRC5, once as MRC4, and once as MRC3. Data from all patients were analysed for each MRC‐group to increase the power, in particular for correlational analyses. In addition data from the 10 patients who were examined thrice were analyzed using a within subject design.
Grip Strength
The mean (± SD) grip strength of the right hand was 109 ± 11 Kpa in the group of healthy volunteers, 108 ± 12 Kpa in ALS‐MRC‐5 group, 87 ± 8 Kpa in ALS‐MRC‐4 group and 66 ± 9 Kpa in ALS‐MRC‐3 group. Grip strength of the ALS‐MRC‐5 group was thus virtually identical to that of the healthy volunteers. However, patients with ALS‐MRC‐4 were significantly different from controls (P < 0.001, t = 4.22), ALS‐MRC‐5 (P < 0.001, t = 3.83) and ALS‐MRC‐3 (P < 0.001, t = 4.21). Also, patients with ALS‐MRC‐3 showed a reduced grip strength compared to controls (P < 0.001, t = 7.89) and ALS‐MRC‐5 (P < 0.001, t = 6.59).
Imaging Results
Movement led to significant activations in contralateral primary motor and premotor cortex (precentral gyrus; BA 4, 6), somatosensory cortex (postcentral gyrus; BA 2, 3), the supplementary motor area (SMA, BA 6) and subcortical areas in all groups (see Fig. 1). Table I summarizes specific data for each area in healthy controls and ALS‐MRC‐5.
Figure 1.
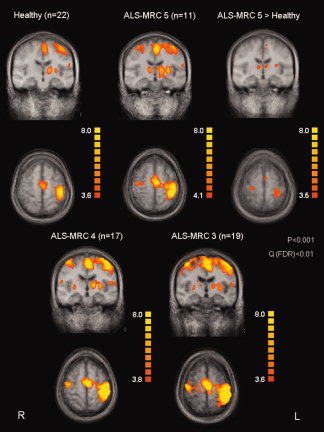
Activation patterns in the healthy group and ALS patients in different disease stages. The contrast “ALS‐MRC‐5 > controls” demonstrates significantly increased activity in ALS patients during the motor task performed with the clinically unaffected hand.
Table I.
Regions of activation in different groups as indicated. P < 0.05 Bonferroni‐corrected
| Talairach coordinates | ||||||
|---|---|---|---|---|---|---|
| Regions of activation | X | Y | Z | Volume (mm3) | T value | P uncorrected |
| Healthy volunteers | ||||||
| Contralateral primary motor, somatosensory, and posterior premotor cortex (BA 1, 2, 3, 4, 6) | −37 | −25 | 50 | 8,279 | 6.3 | 0.00004 |
| SMA | −2 | −8 | 51 | 7,093 | 6.2 | 0.00003 |
| Contralateral parietal cortex (BA 40) | −52 | −23 | 25 | 3,258 | 7.7 | 0.00004 |
| Ipsilateral parietal cortex (BA 40) | 54 | −25 | 24 | 1,390 | 7.1 | 0.00009 |
| Contralateral putamen | −26 | −14 | 7 | 509 | 5.2 | 0.00007 |
| Contralateral thalamus | −14 | −15 | 7 | 1,196 | 4.2 | 0.00005 |
| ALS‐MRC 5 | ||||||
| Contralateral primary motor, somatosensory, and posterior premotor cortex (BA 1, 2, 3, 4, 6) | −35 | −28 | 45 | 14,318 | 7.2 | 0.00005 |
| Ipsilateral primary motor, somatosensory, and posterior premotor cortex (BA 1, 2, 3, 4, 6) | 32 | −14 | 56 | 556 | 7.6 | 0.00003 |
| SMA | −1 | −5 | 49 | 9,590 | 5.9 | 0.00008 |
| Contralateral parietal cortex (BA 40) | −50 | −25 | 24 | 6,600 | 6.7 | 0.00005 |
| Ipsilateral parietal cortex (BA 40) | 53 | −25 | 26 | 3,624 | 6.1 | 0.0001 |
| Contralateral putamen | −27 | −11 | 6 | 869 | 5.2 | 0.00009 |
| Contralateral thalamus | −13 | −12 | 6 | 2,291 | 5.1 | 0.00002 |
| Ipsilateral thalamus | 13 | −11 | 11 | 257 | 4.9 | 0.0004 |
| ALS‐MRC 5 > healthy group | ||||||
| Contralateral primary motor, somatosensory, and posterior premotor cortex (BA 1, 2, 3, 4, 6) | −26 | −31 | 56 | 3,733 | 6.7 | 0.00002 |
| Ipsilateral primary motor, somatosensory, and posterior premotor cortex (BA 6) | 23 | −20 | 62 | 353 | 6.3 | 0.00001 |
| SMA | 0 | −3 | 52 | 2,230 | 5.5 | 0.00005 |
| Contralateral putamen | −28 | −13 | 8 | 340 | 5.6 | 0.00007 |
| Contralateral thalamus | −5 | −13 | 11 | 762 | 5.1 | 0.00006 |
| Ipsilateral thalamus | 12 | −17 | 10 | 119 | 4.8 | 0.00009 |
Statistical comparison between ALS‐MRC‐5 and control participants revealed a significant increase of the activated area in the patients (Fig. 1; Table I). This increase was also evident at the single subject level as illustrated by Figure 2 showing data of five healthy volunteers (left column) and five ALS‐MRC‐5 patients (right column) who performed the motor task with the unaffected hand.
Figure 2.
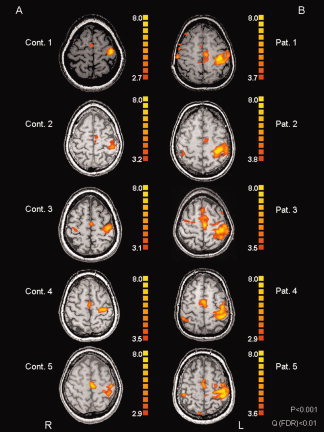
Data from five typical healthy volunteers (A: Cont. 1 to Cont. 5) and five typical ALS patients (B) who performed the motor task with unaffected hand.
Activated cluster volume in the contralateral sensorimotor cortex was compared for ALS‐patients in the different stages of disease and controls participants (Fig. 3; one‐way ANOVA: F(3,65) = 36.79, P < 0.001). Post hoc tests (Tukey) showed that all ALS‐groups were different from the control group (all P < 0.001) but not different from each other. When the analysis was restricted to those patients (n = 17) who were followed over different stages of the disease analogous results were obtained (see Fig. 3).
Figure 3.
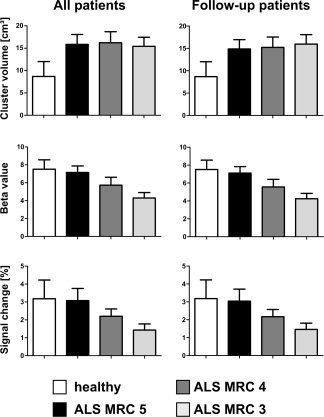
Cluster volume, beta value, and signal change of the contralateral sensorimotor cortex as a function of strength of the tested limb. The left column shows data for all patients, the right column illustrates data for those 11 patients who were tested three times during the progression of the disease. Cluster volume is markedly increased even in patients who not yet show muscle weakness but does not show further increase in later disease stages. By contrast, beta value and signal change show a gradual decrease with increasing weakness.
A very different picture emerged when the beta values within the activated cluster in the contralateral sensorimotor cortex were considered (main effect of group: F(3,65) = 53.18, P < 0.001). Here, no difference between controls and ALS‐MRC‐5 was observed (Tukey test: n.s.), but patients with ALS‐MRC‐4 were significantly different from controls, ALS‐MRC‐5 and ALS‐MRC‐3 on post‐hoc testing (all P < 0.001, Fig. 3). Also, ALS‐MRC‐3 patients were different from controls and ALS‐MRC‐5 (both P < 0.001). Again, analogous results were obtained, if only data of those patients were considered that were followed up over different stages (see Fig. 3). Beta weights showed a positive correlation to the ALSFRS score (Pearson correlation, r 2 = 0.19, P < 0.01; Fig. 4).
Figure 4.
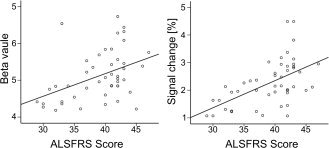
Correlation between ALSFRS‐Score and the Beta value of the contralateral sensorimotor cortex and the corresponding Signal change.
Considering the mean signal change within the activated contralateral sensorimotor area (F(3,65) = 23.50, P < 0.001) again there was no difference between controls and ALS‐MRC‐5. As was the case with the beta values, patients with ALS‐MRC‐4 were significantly different from controls, ALS‐MRC‐5 and ALS‐MRC‐3 on post hoc testing (all P < 0.001, Fig. 3). In addition, ALS‐MRC‐3 patients were different from controls and ALS‐MRC‐5 (both P < 0.001). The follow‐up data showed a gradual reduction of signal change with increasing weakness (see Fig. 3). Signal change showed a positive correlation to the ALSFRS score (Pearson correlation, r 2 = 0.13, P < 0.01; Fig. 4).
The cluster volumes in the SMA were highly similar for ALS patients in the different stages of the disease but were different from the control participants (F(3,65) = 26.86, P < 0.001; post hoc tests: all ALS groups were different from the controls, all P < 0.001, but not from each other, Fig. 5). Cluster volumes in the SMA showed no correlation to muscle atrophy or weakness in the performing right hand. Again, beta values in this area decreased from ALS‐MRC‐5 to ALS‐MRC‐3 without significant difference between control and ALS‐MRC‐5 (F(3,65) = 51.81, P < 0.001, post hoc tests: ALS‐MRC‐4 different from controls, ALS‐MRC‐5 and ALS‐MRC‐3, ALS‐MRC‐3 different from controls and ALS‐MRC‐5, all P < 0.001, Fig. 5). The mean signal change within the SMA (F(3,65) = 24.92, P < 0.001) was not different between control and ALS‐MRC‐5 but decreased significantly in ALS‐MRC‐4 and ALS‐MRC‐3 (see Fig. 5).
Figure 5.
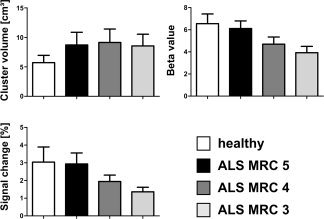
Cluster volume, Beta value, and Signal change of the SMA in all subjects.
Only 2 of the 22 control subjects showed ipsilateral activation of motor cortex, whereas 5 of 11 patients in the ALS‐MRC‐5 group, 11 of 17 patients in the ALS‐MRC‐4 group and 14 of 19 patients in the ALS‐MRC‐3 group showed ipsilateral activation.
The cluster volume of an activated area in the contralateral thalamus was increased in ALS patients compared to controls (Table I) but did not change over different stages of the disease. The beta values from the thalamus showed no differences between disease stages. In none of control subjects activation of the ipsilateral thalamus was seen. By contrast, ipsilateral thalamus showed activation in 33% of patients independent of disease stage.
Finally, grip strength showed no significant correlation with cluster volumes of the different activated clusters or the beta weights obtained from those clusters. However, we observed a significant correlation (Pearson, r 2 = 0.29, P < 0.01) between grip strength and the signal change within the activated contralateral sensorimotor area.
DISCUSSION
The present investigation examined changes in activation patterns in ALS as a function of disease stage with fMRI. Whereas previous fMRI investigations have focused on statistical comparisons of the activation maps of patients and controls, we compared size of activated clusters nor % signal change beta values derived from regions of interest between patients and controls and between patients in different stages of the disease. This approach revealed evidence for two distinct phases of neuroplastic changes.
In the first phase, during which there was no weakness of the tested limb (ALS‐MRC‐5 in the current study), the size of the activated cluster in the contralateral sensorimotor region was increased but the mean signal change and the beta weights characterizing activation strength were not changed compared to healthy controls. In the second phase, decreasing muscle strength (ALS‐MRC‐4 → ALS‐MRC‐3) was accompanied by gradual decreases in mean signal change and beta weights, whereas the size of the activation of the sensorimotor cortex remained unchanged. A similar pattern of results was seen in the SMA. Moreover, in the second phase, an increasing proportion of patients was showing ipsilateral activation of the sensorimotor cortex.
First Phase of Neuroplastic Changes: ALS MRC 5
An increase of activated areas has been found in a number of previous studies [Konrad et al.,2002,2006; Schoenfeld et al.,2005] but has been the subject of intense discussion. Schoenfeld et al. [2005] found an increased size of the activation in the contralateral sensorimotor cortex as well as ipsilateral activation in ALS patients relative to healthy controls. These authors argued that the increased activation might have been due to the fact that the task was simply more difficult for the patients. Indeed when the level of difficulty was increased for control participants in a second experiment, ipsilateral recruitment was seen as well as activity in the cerebellar hemisphere contralateral to the hand that executed the movement. The authors concluded that this pattern does not reflect cortical reorganization in the true sense of the term. Rather, the pattern was thought to be due to a difficulty effect.
As our ALS‐MRC‐5 patients had virtually identical grip strength compared with the control participants (109 Kpa vs. 108 Kpa, see methods) and the used hand movement was very easy to perform, it seems that a difficulty explanation can not be put forward for this group of patients. Indeed, Konrad et al. [2006] also found differences between ALS patients and controls when a similar relative force output (10% of maximum) was required, thus effectively equating difficulty between patients and controls.
Thus, the current data as well as the Konrad et al. [2006] study suggest that the increase in the activated area of the contralateral sensorimotor cortex is a true effect rather than the result of differential task difficulty. What mechanism could underlie this increase in activation then? Studies employing transcranial magnetic stimulation have found significantly reduced short‐interval intracortical inhibition (SICI) in ALS [Sommer,1999; Stefan et al.,2001; Vucic and Kiernan,2006; Zanette et al.,2002; Ziemann et al.,1997]. Another sign of increased excitability is the decrease of cortical silent periods [Vucic et al.,2006]. It has been proposed that the reduction of SICI in ALS is due to the loss parvalbumin‐positive inhibitory cortical interneurons, which is evident in neuropathological studies [Nihei et al.,1993]. Importantly, neuropathological studies have shown that loss of parvalbumin‐positive interneurons is seen for all levels of severity in ALS, i.e., irrespective of the degree of loss of Betz‐cells in the motor cortex [Nihei et al.,1993]. Other neuropathological observations [Maekawa et al.,2004] also found degeneration of GABAergic interneurons in the primary motor cortex and beyond (dorsolateral prefrontal cortex and anterior cingulate cortex) in ALS. Using flumazenil C11 positron emission tomography Turner et al. [2005a] noted reduced binding in patients with sporadic ALS (but not in patients with homozygous for the D90A SOD1 mutation) which is indicative of reduced intracortical inhibition in ALS. This is accompanied by increased cortical excitability in the former but not in the latter group of patients [Turner et al.,2005b]. The same mechanism that leads to reduced SICI and enhanced excitability in ALS in TMS‐studies might also underlie the increase of the activated area in ALS patients that do not yet suffer from weakness.
Second Phase of Neuroplastic Changes: ALS‐MRC‐4 and ‐3
ALS patients with increasing weakness in the studied hand exhibited a decrease of the maximum signal change of the BOLD signal as well as a decrease of β‐weights, both determined in the contralateral sensorimotor cortex, whereas the size of the activated area remained unchanged compared to the ALS‐MRC‐5 patients. Obviously, mean signal change of the BOLD response is a rather indirect measure of the changes in neuronal firing patterns that occur over the course of ALS. Studies directly comparing the BOLD signal with neuronal electrical activity are therefore important in interpreting the current results. Logothetis and colleagues [Logothetis and Wandell,2004; Logothetis et al.,2001] simultaneously measured neuronal activity and the hemodynamic response and found that the BOLD response in primates directly reflects an increase in neural activity. In particular, the BOLD response correlated with local field potentials which represent synchronized synaptic inputs of a given neuronal population. Other recent simultaneous fMRI and electrophysiological measurements in rats suggest that the BOLD response from the cerebral cortex is closely linked to neurotransmitter release and energetic demand of glutamatergic neurons [Kida and Hyder,2006].
We therefore suggest that the decrease of % signal change and beta values for ALS‐MRC‐4 and ALS‐MRC‐3 patients reflects less neuronal activity, which in turn may correspond to the loss of neurons in primary motor cortex in these patients. The fact that the size of the activated cluster did not change between the ALS groups suggests that the spread of activation due to loss of intracortical inhibition reaches a ceiling early in the disease.
Ipsilateral Activations
Whereas only 2 of 22 control participants exhibited activation of the ipsilateral sensorimotor cortex, this was seen in most ALS patients, in particular those with more severe weakness (14 of 19 patients with ALS‐MRC‐3). Ipsilateral activation is also often observed after strokes involving the motor system [Calautti et al.,2001; Johansen‐Berg et al.,2002; Marshall et al.,2000; Nair et al.,2007]. In stroke, such increased activation in the healthy hemisphere is most likely due to loss of inhibition that occurs from the lesional hemisphere to the healthy hemisphere [Liepert et al.,2000]. In healthy persons, inhibitory transcallosal conduction occurs between the contralateral and ipsilateral motor cortex during unimanual motor tasks [Allison et al.,2000; Kobayashi et al.,2004] which is compromised after stroke. A similar mechanism of reduced transcallosal inhibition might underlie the ipsilateral activation in ALS. In support of this notion, TMS studies studying transcallosal inhibition in ALS have found this to be reduced [Karandreas et al.,2007; Wittstock et al.,2007].
CONCLUSIONS
Our study revealed distinct stages of fMRI activation changes in ALS. In light of the pertinent neuropathological and electrophysiological literature, these changes may reflect a decreased intracortical inhibition early in the disease that is reflected in the increase of the activated brain areas. Over the course of the disease and with increasing weakness, the signal change in the activated areas is reduced which might be related to loss of pyramidal cells. In addition, increased activation of ipsilateral sensorimotor cortex likely reflects reduced transcallosal inhibition. This interpretation of the observed two stage pattern needs to be substantiated by further studies. In particular, the relationship between neurophysiological markers of decreased intracortical inhibition (SICI, silent period) and transcallosal inhibition and the contra‐ and ipsilateral activation changes needs to be explored. Moreover, changes in fMRI pattern should be studied in relation to structural changes of the central motor system as assessed, e.g., by the determination of cortical thickness [Agosta et al.,2007; Roccatagliata et al.,2009] or diffusion tensor imaging of the pyramidal tract [Sage et al.,2007; Wong et al.,2007]. Finally, on the basis of careful clinical and neuropathological observations Ravits and colleagues [Ravits and La Spada,2009; Ravits et al.,2007] have recently suggested that motor neuron degeneration in ALS is a focal process at both upper and LMN levels which actively propagates in an orderly fashion. Longitudinal studies of patients using fMRI similar to the one presented here will be important to further substantiate these findings.
REFERENCES
- Aggarwal A, Nicholson G ( 2002): Detection of preclinical motor neurone loss in SOD1 mutation carriers using motor unit number estimation. J Neurol Neurosurg Psychiatry 73: 199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Pagani E, Rocca MA, Caputo D, Perini M, Salvi F, Prelle A, Filippi M ( 2007): Voxel‐based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability. Hum Brain Mapp 28: 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC ( 2000): Functional MRI cerebral activation and deactivation during finger movement. Neurology 54: 135–142. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ ( 1996). Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16: 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti C, Leroy F, Guincestre JY, Baron JC ( 2001): Dynamics of motor network overactivation after striatocapsular stroke: A longitudinal PET study using a fixed‐performance paradigm. Stroke 32: 2534–2542. [DOI] [PubMed] [Google Scholar]
- de Carvalho M, Swash M ( 2006): The onset of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 77: 388–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Haverkamp LJ, Appel V, Appel SH ( 1995). Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 118 ( Part 3): 707–719. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM ( 2002): Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 125: 2731–2742. [DOI] [PubMed] [Google Scholar]
- Karandreas N, Papadopoulou M, Kokotis P, Papapostolou A, Tsivgoulis G, Zambelis T ( 2007): Impaired interhemispheric inhibition in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 8: 112–118. [DOI] [PubMed] [Google Scholar]
- Kew JJ, Leigh PN, Playford ED, Passingham RE, Goldstein LH, Frackowiak RS, Brooks DJ ( 1993): Cortical function in amyotrophic lateral sclerosis. A positron emission tomography study. Brain 116 ( Part 3): 655–680. [DOI] [PubMed] [Google Scholar]
- Kew JJ, Brooks DJ, Passingham RE, Rothwell JC, Frackowiak RS, Leigh PN ( 1994). Cortical function in progressive lower motor neuron disorders and amyotrophic lateral sclerosis: a comparative PET study. Neurology 44: 1101–1110. [DOI] [PubMed] [Google Scholar]
- Kida I, Hyder F ( 2006): Physiology of functional magnetic resonance imaging: Energetics and function. Methods Mol Med 124: 175–195. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual‐Leone A ( 2004): Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology 62: 91–98. [DOI] [PubMed] [Google Scholar]
- Konrad C, Henningsen H, Bremer J, Mock B, Deppe M, Buchinger C, Turski P, Knecht S, Brooks B ( 2002): Pattern of cortical reorganization in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Exp Brain Res 143: 51–56. [DOI] [PubMed] [Google Scholar]
- Konrad C, Jansen A, Henningsen H, Sommer J, Turski PA, Brooks BR, Knecht S ( 2006): Subcortical reorganization in amyotrophic lateral sclerosis. Exp Brain Res 172: 361–369. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT ( 2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C ( 2000): Treatment‐induced cortical reorganization after stroke in humans. Stroke 31: 1210–1216. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA ( 2004): Interpreting the BOLD signal. Annu Rev Physiol 66: 735–769. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- Maekawa S, Al‐Sarraj S, Kibble M, Landau S, Parnavelas J, Cotter D, Everall I, Leigh PN ( 2004): Cortical selective vulnerability in motor neuron disease: A morphometric study. Brain 127: 1237–1251. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL ( 2000): Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke 31: 656–661. [DOI] [PubMed] [Google Scholar]
- Merkies IS, Schmitz PI, Samijn JP, Meche FG, Toyka KV, Van Doorn PA ( 2000): Assessing grip strength in healthy individuals and patients with immune‐mediated polyneuropathies. Muscle Nerve 23: 1393–1401. [DOI] [PubMed] [Google Scholar]
- Merkies IS, Schmitz PI, Van Der Meche FG, Van Doorn PA ( 2003): Comparison between impairment and disability scales in immune‐mediated polyneuropathies. Muscle Nerve 28: 93–100. [DOI] [PubMed] [Google Scholar]
- Nair DG, Hutchinson S, Fregni F, Alexander M, Pascual‐Leone A, Schlaug G ( 2007): Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well‐recovered patients. Neuroimage 34: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei K, McKee AC, Kowall NW ( 1993): Patterns of neuronal degeneration in the motor cortex of amyotrophic lateral sclerosis patients. Acta Neuropathol 86: 55–64. [DOI] [PubMed] [Google Scholar]
- Norris F, Shepherd R, Denys E, U K, Mukai E, Elias L, Holden D, Norris H ( 1993): Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci 118: 48–55. [DOI] [PubMed] [Google Scholar]
- Paternostro‐Sluga T, Grim‐Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, Bittner C, Fialka‐Moser V ( 2008): Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med 40: 665–671. [DOI] [PubMed] [Google Scholar]
- Ravits J, Paul P, Jorg C ( 2007): Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology 68: 1571–1575. [DOI] [PubMed] [Google Scholar]
- Ravits JM, La Spada AR ( 2009): ALS motor phenotype heterogeneity, focality, and spread: Deconstructing motor neuron degeneration. Neurology 73: 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccatagliata L, Bonzano L, Mancardi G, Canepa C, Caponnetto C ( 2009): Detection of motor cortex thinning and corticospinal tract involvement by quantitative MRI in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 10: 47–52. [DOI] [PubMed] [Google Scholar]
- Sage CA, Peeters RR, Gorner A, Robberecht W, Sunaert S ( 2007): Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage 34: 486–499. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Tempelmann C, Gaul C, Kuhnel GR, Duzel E, Hopf JM, Feistner H, Zierz S, Heinze HJ, Vielhaber S ( 2005): Functional motor compensation in amyotrophic lateral sclerosis. J Neurol 252: 944–952. [DOI] [PubMed] [Google Scholar]
- Sommer M ( 1999): Riluzole does not have an acute effect on motor thresholds and the intracortical excitability in amyotrophic lateral sclerosis. J Neurol Suppl 246: 22–26. [DOI] [PubMed] [Google Scholar]
- Stanton BR, Williams VC, Leigh PN, Williams SC, Blain CR, Giampietro VP, Simmons A ( 2007a): Cortical activation during motor imagery is reduced in Amyotrophic Lateral Sclerosis. Brain Res 1172: 145–151. [DOI] [PubMed] [Google Scholar]
- Stanton BR, Williams VC, Leigh PN, Williams SC, Blain CR, Jarosz JM, Simmons A ( 2007b): Altered cortical activation during a motor task in ALS. Evidence for involvement of central pathways. J Neurol 254: 1260–1267. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Classen J ( 2001): Effects of riluzole on cortical excitability in patients with amyotrophic lateral sclerosis. Ann Neurol 49: 537–540. [PubMed] [Google Scholar]
- Swash M, Ingram D ( 1988): Preclinical and subclinical events in motor neuron disease. J Neurol Neurosurg Psychiatry 51: 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MR, Hammers A, Al‐Chalabi A, Shaw CE, Andersen PM, Brooks DJ, Leigh PN ( 2005a): Distinct cerebral lesions in sporadic and ‘D90A’ SOD1 ALS: Studies with [11C]flumazenil PET. Brain 128: 1323–1329. [DOI] [PubMed] [Google Scholar]
- Turner MR, Osei‐Lah AD, Hammers A, Al‐Chalabi A, Shaw CE, Andersen PM, Brooks DJ, Leigh PN, Mills KR ( 2005b): Abnormal cortical excitability in sporadic but not homozygous D90A SOD1 ALS. J Neurol Neurosurg Psychiatry 76: 1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Kiernan MC ( 2006): Novel threshold tracking techniques suggest that cortical hyperexcitability is an early feature of motor neuron disease. Brain 129: 2436–2446. [DOI] [PubMed] [Google Scholar]
- Vucic S, Howells J, Trevillion L, Kiernan MC ( 2006): Assessment of cortical excitability using threshold tracking techniques. Muscle Nerve 33: 477–486. [DOI] [PubMed] [Google Scholar]
- Wittstock M, Wolters A, Benecke R ( 2007): Transcallosal inhibition in amyotrophic lateral sclerosis. Clin Neurophysiol 118: 301–307. [DOI] [PubMed] [Google Scholar]
- Wong JC, Concha L, Beaulieu C, Johnston W, Allen PS, Kalra S ( 2007): Spatial profiling of the corticospinal tract in amyotrophic lateral sclerosis using diffusion tensor imaging. J Neuroimaging 17: 234–240. [DOI] [PubMed] [Google Scholar]
- Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N ( 2002): Different mechanisms contribute to motor cortex hyperexcitability in amyotrophic lateral sclerosis. Clin Neurophysiol 113: 1688–1697. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Winter M, Reimers CD, Reimers K, Tergau F, Paulus W ( 1997): Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis: Evidence from paired transcranial magnetic stimulation. Neurology 49: 1292–1298. [DOI] [PubMed] [Google Scholar]


