Abstract
Recognition of objects and their relations is necessary for orienting in real life. We examined cognitive processes related to recognition of objects, their relations, and the patterns they form by using the game of chess. Chess enables us to compare experts with novices and thus gain insight in the nature of development of recognition skills. Eye movement recordings showed that experts were generally faster than novices on a task that required enumeration of relations between chess objects because their extensive knowledge enabled them to immediately focus on the objects of interest. The advantage was less pronounced on random positions where the location of chess objects, and thus typical relations between them, was randomized. Neuroimaging data related experts' superior performance to the areas along the dorsal stream—bilateral posterior temporal areas and left inferior parietal lobe were related to recognition of object and their functions. The bilateral collateral sulci, together with bilateral retrosplenial cortex, were also more sensitive to normal than random positions among experts indicating their involvement in pattern recognition. The pattern of activations suggests experts engage the same regions as novices, but also that they employ novel additional regions. Expert processing, as the final stage of development, is qualitatively different than novice processing, which can be viewed as the starting stage. Since we are all experts in real life and dealing with meaningful stimuli in typical contexts, our results underline the importance of expert‐like cognitive processing on generalization of laboratory results to everyday life. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: expertise, object and pattern recognition, functional relations, eye movements, fMRI, chess
INTRODUCTION
Successful orientation in the environment requires fast recognition of objects and their relations. Although we rarely think about our everyday cognition as skilled cognition because it comes naturally and all of us possess it, we are all experts in mastering our everyday environment. The game of chess has been often used as a domain of research in cognitive science also because of its resemblance to real life [Charness, 1992; Simon and Chase, 1973; for generalization to other expertise domains, see reviews, Gobet et al. 2004; Reingold and Sheridan, in press]. Chess features various objects that form complex spatial and functional relations. To truly master the game, one needs to perfect numerous cognitive processes such as object and pattern recognition. Therein lies one of the most attractive features of chess—besides experts, it is also possible to find people who are not that good at the activity. Here, we use the expertise approach [Bilalić et al., 2010; Bukach et al., 2006; Charness, 1992; Gobet et al., 2004], and a combination of behavioral and neuroimaging techniques, to uncover cognitive and neural mechanisms behind everyday cognitive processes such as object and pattern recognition.
It is a human tendency to pick up regularities, explicitly or implicitly, in our environment [De Groot, 1978/1946]. These regularities are stored in long‐term memory as knowledge structures [called chunks, templates, schemas, or scripts—Biederman et al. 1982; Gobet et al., 2001; Shank and Abelson, 1977]. Once we face familiar situations, knowledge structures become activated [Chase and Simon, 1973; Ericsson and Kintsch, 1995; Gobet and Simon, 1996b;] and enable quick recognition and fast orientation in environment through a top‐down influence on perception [Chase and Simon, 1973; Ericsson and Kintsch, 1995; Gobet and Simon, 1996b]. The long‐term memory structures behind efficient orienting are based on objects and, in particular, their relations [Gobet and Simon, 1996a, 1998; Kiesel et al. 2009; McGregor and Howes, 2002]. A common finding is that simply placing randomly the same objects, and thus ruining the common relations between them, is enough to drastically impede experts' performance [for reviews, see Ericsson and Lehmann, 1996; Vicente and Wang, 1998].
In our previous study [Bilalić et al., 2010], we started investigating the cognitive processes important in everyday life such as object and pattern recognition using the game of chess. Although there were other studies that used chess for their neuroimaging investigations [Amidzic et al. 2001; Atherton et al. 2003; Campitelli et al. 2005, 2007, 2008; Nichelli et al., 1994; Onofrj et al., 1995; Righi et al., in press; Saariluoma et al., 2004], this was the first study to use the expertise approach of comparing experts and novices in conjunction with a clear‐cut task aimed at studying pattern and object recognition processes. We employed a version of a visual search task where participants had to enumerate the number of certain objects in chess positions. Here, we expand on the research using a similar visual search task adding another cognitive process—explicit recognition of relations among objects in an environment. In the current study, participants had to indicate the number of certain relations among pieces on a board (so called threats, where one object attacks the other by the opponent—see Fig. 1A and Method for detailed explanation). Henceforth, we will refer to this task as the Threats task. In contrast to the task used in our previous study that needed only recognition of objects, the current task requires repeatedly retrieval of object functions and investigation of relations between objects. Common to both tasks is that recognition processes of objects and their relations were not captured in isolation. As it is the case with everyday objects [e.g., Bar, 2004], chess objects are also found jointly with other chess objects on a chess board. The placement of chess pieces and their relations form together a meaningful unit, called position, to chess experts. It is not unlike in everyday life where the distribution of furniture and other objects in a room makes a distinct kind of room. Just like we can very quickly recognize the kind of room in question based on our previous experience, chess experts also need little time to grasp the essence of a chess position. The Threats task thus also captures complex pattern recognition processes. Knowing where to expect certain objects and their relations to other objects will certainly improve performance. To directly test this assumption, we devised two types of chess stimuli—normal positions, where the objects are on their common places and form common relations, and random positions, where the objects are randomly distributed (and thus form unusual relations). In general, we expect expert chess players to solve the task faster than novices. However, this randomization should particularly affect experts, who possess memory structures necessary for efficiently processing common relations. Since novices possess very few of those memory structures, they should be largely unaffected.
Figure 1.
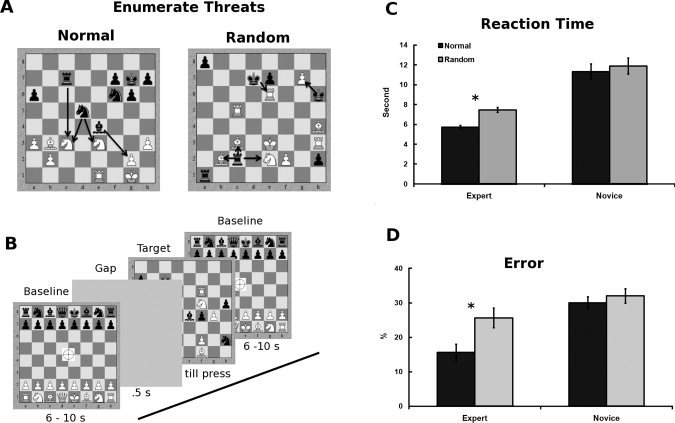
Design and reaction time data. A: The stimuli used in the task. Participants had to indicate whether the number of Black threats (how many times Black can take White) was four. Left side presents normal positions taken from masters games unknown to participants; right side depicts random positions obtained by distributing pieces randomly on the board. The relations are highlighted by lines between the encircled objects—not seen by participants. B: Trial structure. Baseline stimulus was an initial chess board configuration with a fixation cross; its duration was jittered. A gap in stimulus presentation was used as a warning about the upcoming stimulus. The actual chess stimulus (normal and random positions) was then presented. After the players indicated their answers by pressing one of the response buttons, the baseline stimulus of the next trial was presented. C: Time (in seconds) experts and novices took to complete the task depending on the type of position. D: Errors (in percentage) experts and novices make while completing the chess and control tasks depending on the type of position. Blue color represents experts; red color novices. Error bars indicate the standard error of the mean (SEM). *P < 0.01 in a t‐test for dependent samples.
If the Threats task indeed captures expert processes the time necessary to complete the task (reaction time) would provide useful information. However, this measurement cannot provide insight into the nature of the mechanisms behind the differences among experts and novices. That is why we also recorded participants' eye movements and their brain activity using functional magnetic resonance imaging (fMRI). Eye movements will provide insight into the strategies that experts and novices employ [Rayner, 1998; Underwood, 2005]. More specifically, we will be able to check the assumption that experts draw their advantage from previous knowledge by comparing their search patterns in normal and random positions, in addition to the comparison to the search patterns among novices. Finally, fMRI will identify the necessary brain structures for the successful cognitive processing [Henson, 2005; Shallice, 2003; Wilkinson and Halligan, 2004].
The identification of brain structures related to superior performance of experts is important for several reasons. First, it provides a neural basis behind the processes necessary for mastering one of the most complex and intellectual activities [Newell et al., 1963]. Second, it corroborates the findings on the cognitive processes in everyday life as many of them are similar, if not the same, to those found in the game of chess [Charness, 1992; Gobet et al., 2004]. Finally, it also provides insights into the developmental trajectory of expertise. It is possible that experts' processing of objects and their relations and complex patterns is based on the same but more efficient processes found in novices. In that case expertise would probably require the same brain structures that would engage in more or less processing to enable superior performance. It is, however, possible that expertise requires qualitatively different processing that would need to be accommodated by engaging additional brain structures [Bilalić et al., 2010; Henson, 2005; Palmeri and Gauthier, 2004].
In this particular task, we can investigate the neural basis behind object recognition. Chess objects, usually called pieces, have characteristic visual features that distinguish them from other objects but also among each other. We know the ventral stream [Goodale and Milner, 1992; Ungerleider and Mishkin, 1982] is responsible for object recognition: from the lateral occipital complex (LOC) associated with perception of shape and form [Grill‐Spector and Malach, 2004], to lateral and medial parts of the fusiform gyrus (FG) connected to the recognition of full objects and parts of human body [Downing et al., 2001; Mahon et al., 2007; Martin et al., 1995; Miceli et al., 2001; Weisberg et al., 2007]. Chess pieces also have clearly defined functions reinforced through chess rules (e.g., bishop moves diagonally). It is known that objects with clearly defined functions also engage left lateral areas, in particular the left posterior middle temporal gyrus (pMTG) close to the left posterior parietal‐occipito‐temporal junction [POTJ; Johnson‐Frey, 2004; Lewis, 2006; Mahon and Caramazza, 2009]. Expert chess players are faster than novices in recognizing chess pieces, but this advantage is comparably small to the advantage experts have in recognition of chess pieces' functions [Bilalić et al., 2011; Kiesel et al., 2009; Saariluoma, 1990, 1995]. We expect then the differences between experts and novices to be more visible in the lateral temporal areas connected to recognition of functions than in the ventral temporal areas connected with the recognition of shapes and forms of objects. Indeed, our previous study [Bilalić et al., 2010] employed a task where the recognition of chess objects was necessary and found expertise related differences in lateral brain areas around occipito‐parietal junction. However, unlike in most studies using other objects where the activation is left lateralized [e.g., Johnson‐Frey, 2004; Lewis, 2006; Mahon and Caramazza, 2009], both hemispheres displayed higher activations in experts. The present study, which uses a task that encompass the cognitive processes necessary for the previous task but also features additional processes, will further help to disentangle the neural basis of skilled object recognition.
Another brain area related to recognition of objects is the supramarginal gyrus (SMG) in the left inferior parietal lobe (IPL). SMG is important not only for the actual execution of an action with an object [Boronat et al., 2005; Canessa et al., 2008) but also for the retrieval of actions of an object [Kellenbach et al., 2003; Martin et al., 1996]. This particular area showed no different activation patterns among experts and novices in our previous study, but our task did not explicitly require retrieval of relations between chess objects. Our current task, however, requires identifying relations between numerous objects for which it is necessary to extract a function of the object and relate it to the other. This process is likely to activate SMG and given the behavioral differences in retrieval of function, we may expect that SMG is related to expertise.
Finally, recognition of complex objects with multiple elements such as scenes or places involves the activation around the collateral sulcus (CoS) in the inferior temporal lobe [IT; Bar, 2004; Epstein, 2008; Epstein and Kanwisher, 1998]. A part of the medial temporal lobe, the medial part of CoS, was related to pattern recognition differences in our previous study [Bilalić et al., 2010]. If this area were indeed related to pattern recognition processes, we would expect the same structure to be differently active among experts and novices on normal and random positions. More specifically, CoSs should show different sensitivity to normal and random positions among experts, while the position type should not matter among novices who do not possess knowledge to utilize it in normal positions. The current task, however, also involves recognizing relations between pieces and it is not clear whether the CoS, or some other additional brain areas, will be sensitive to pattern recognition in this context.
METHOD
Participants
Eight experts (mean age ± standard deviation, 30 ± 5 years) and 15 novices (mean age 29 ± 4) participated in the study. All were male and right‐handed. Written informed consent was obtained from all participants, and the study was approved by the ethics committee of Tübingen University. Our expert sample corresponds size‐wise to the expert samples used in behavioral research on expertise [e.g., Bilalić et al., 2008a, b, 2009; Brockmole et al., 2008; Kiesel et al., 2009] and is larger than the few neuroimagining studies involving chess experts [e.g., Campitelli et al., 2005, 2007, 2008]. Our experts were exceptionally skilled practitioners. In competitive chess, players get rated based on their performance against other rated players. The international chess Elo scale [Elo, 1978] is an interval scale with a theoretical mean of 1,500 and standard deviation of 200. Beginners have a rating of around 500 while the best players, Grand Masters, have ratings over 2,500. Experts are players with a rating of 2,000 Elo points or more. Our experts were highly rated—on average 2,108 ± 148 points—and were better than 99 % of all chess players. Novice players were hobby players who played chess occasionally. They were, however, not beginners whom they would beat easily.
Task
In the task, players had to decide whether there were four threats by Black, that is, whether Black had four possibilities to take White in a given position (there were between 4 and 6 threats in each position). Participants had to indicate “yes” if there were exactly 4 threats and “no” if there were more than four or less than four threats. This task requires chess knowledge because it is necessary to discriminate between different pieces, retrieve their function, and relate that function to other pieces in order to check whether the threat is present. The task thus captures the recognizing of chess objects and their functions. Another factor manipulated (chess) relations by using normal and random positions. Chess positions involve a chess board and chess pieces on it. The location of pieces is extremely important because pieces form relations. These relations are reinforced by chess specific rules (e.g., bishops move diagonally but cannot jump over other pieces) and together with pieces they form the basis of complex chess knowledge [Chase and Simon, 1973; Gobet and Simon, 1996a]. Normal positions were taken from master games and thus involved typical relations between pieces which can be exploited by experts. Random positions involved the same pieces but these pieces were scattered randomly around the board. The randomization of pieces disturbs the common relations between pieces and makes the use of pattern recognition difficult. The position type factor thus captures the complex processes related to the processing of relations between objects, which is one of the major components of any expertise [Gobet et al., 2001].
Stimuli and Apparatus
There were 20 normal chess positions and 20 random chess positions in the task. The normal positions were taken from a large database of over 4 million games (ChessBase Mega Base 2007, ChessBase GmbH, Hamburg, Germany; http://www.chessbase.com). These were normal middle‐game positions by masters and it is highly unlikely that the games were known to the participants. The random positions were generated by distributing the pieces on the board randomly using the rule that any piece of either color can occur on any square [Gobet and Waters, 2003; Vicente and Wang, 1998]. All positions contained between 15 and 18 pieces. The dimension of the whole stimulus was 305 × 305 pixels, while the board with the pieces had a dimension of 276 × 276 pixels. The dimension of a single square was 34 × 34 pixels. The stimuli were projected onto a screen above the head of the participants via a video projector in the adjacent room. Participants saw the stimuli through a mirror mounted on the head coil. The physical dimensions of the stimulus were: 256 mm for the whole stimulus, 230 mm for the board, and 29 mm for the single square. The setup resulted in a visual field of 14.6° for the whole stimulus, 13.2° for the chess board, and 1.65° for a single square in the board.
Design and Procedure
Participants indicated their decision by pressing one of two buttons of an MRI‐compatible response device held in the right hand (left button was for YES and right button for NO). The number of correct YES and NO responses was equal in both conditions. Before the actual sessions, participants were given two practice trials for each task. The structure of the trial is presented diagrammatically in Figure 1B. We first presented a starting board (all pieces at their initial location) with a fixation cross. This stimulus was used as the baseline and its duration was jittered (6–10 s). After a short gap (0.5 s), the target stimulus was presented. The stimulus disappeared after participants indicated their response, and the baseline of the next trial was then presented. The described sequence was repeated 20 times during each run. There were two runs. In one run, 10 meaningful and 10 meaningless stimuli were presented randomly. Both runs were a part of a larger project [Bilalic et al., 2008c] and were carried out during a single session with participants. Reaction time (or the time to complete the task) was the time from stimulus appearance until response. Although there were errors, the eye‐movement protocols showed that participants carried out the task with best intentions (error happened when participants overlooked a threat, which is understandable given the number of objects and relations in a position). Excluding the error trials produced similar reaction times patterns and there were also no differences in brain activations between correct and incorrect trials. Given that all participants executed the same task and the errors did not influence the behavioral or fMRI results, we did not deem it necessary to separately analyze the correct and erroneous trials.
Apparatus
Participants' eye movements were recorded by an infra‐red remote long‐range eye‐tracking device (iView X MEyeTrack Long Range, SensoMotoric Instruments, Berlin, Germany; http://www.smi.de) sampling at 50 Hz while they were executing tasks in a 3‐T scanner (Siemens Trio) with a 12‐channel head coil at the fMRI center in Tübingen, Germany. The whole brain was covered using a standard echo‐planar‐imaging sequence with the following parameters: repetition time [TR] = 2.5 s; field of view = 192 × 192; echo time [TE] = 35 ms; matrix size = 64 × 64, 36 slices with a thickness of 3.2 mm ± 0.8 mm gap resulting in voxels with the resolution of 3 × 3 × 4 mm3. Anatomical images covering the whole brain with 176 sagittal slices were obtained after the functional runs using an MP‐RAGE sequence with a voxel resolution of 1 × 1 × 1 mm3 (TR = 2.3 s, TI = 1.1 s, TE = 2.92 ms).
The eye tracking system had an error of 0.5–1°, corresponding to 8.6–17.1 mm (or less than half a square) on the board. All devices were MRI‐compatible and did not interfere with participants' performance. Participants saw the stimuli through a mirror mounted on the head coil. The stimuli were projected onto a screen above the head of the participants via a video projector in the adjacent room.
Behavioral Analysis
We analyzed the behavioral data using a 2 × 2 (expertise [experts/novices] × type of position [normal/random]) analysis of variance (ANOVA) for the task. Finally, t‐tests for dependent samples were used to check differences between normal and random positions among experts and novices, separately.
Eye‐Movement Analysis
We used a nine‐point calibration with bi‐quadratic functions before each run. We created a program in MatLab 7.1 (The MathWorks Inc., Natic, MA; http://www.mathworks.com) to analyze the eye‐movement data of five experts and six novices (technical problems prevented eye movement measurement in the other participants). First, we defined a fixation as an event where participants kept their eyes within a diameter of 34 pixels for 80 ms or more. The diameter of 34 pixels is roughly the size of a square on the chess board. We then extracted the fixations for each participant on each position in each task. Supporting Information presents the number of fixations and average duration of fixations among expert and novice chess players on the normal and random positions.
To differentiate between relevant and irrelevant objects in the stimuli, we identified the areas of interest for each position. These were the chess objects forming threat relations—both Black pieces, which can take, and White pieces, which can be taken (see circles and lines in Fig. 1A). We then calculated the percentage of fixations falling on these objects of interest (Fig. 2A) as well as the percentage of fixations falling on any object in the position, irrespective of its relevance (Fig. 2B). Given that we were also interested in the beginning of trials because the fMRI analysis featured the first 3 s of a trial, the same analyses of the number and duration of fixation as well as the percentage of fixations on objects of interest and any object were done for the first 3 s of the trial (see Supporting information). The averaged values were then used in a 2 × 2 (expertise [experts/novices] × type of position [normal/random]) analysis of variance (ANOVA). As with reaction times, t‐tests for dependent samples were used to check the differences between normal and random positions among experts and novices, separately.
Figure 2.
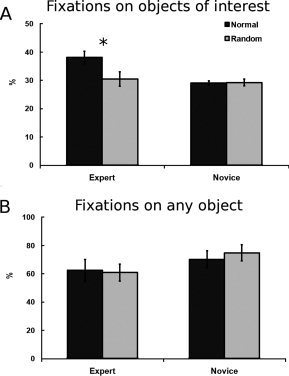
Eye movement data. A: The average percentage of fixations on the objects of interest for experts and novices on normal and random positions across the whole trial. B: The average percentage of fixations on any objects irrespective of interest for experts and novices on normal and random positions across the whole trial. Blue color represents experts; red color novices. Error bars indicate SEM. *P < 0.01 in a t‐test for dependent samples.
MRI Data Analysis
All fMRI data were analyzed using the Statistical Parametric Mapping software package (SPM5; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Data preprocessing involved spatial realignment to the mean image including unwarping, co‐registration of the anatomical image to the mean EPI, and the unified segmentation procedure. The normalization parameters to the MNI‐brain template (Montreal Neurological Institute space; MNI‐template Avg152T1) from segmentation were used for spatial normalization of the functional images at a voxel size of 3 × 3 × 3 mm3 and of the anatomical images with a voxel size of 1 × 1 × 1 mm3. Finally, the data were spatially smoothed, using a Gaussian filter with 8‐mm full‐width‐at‐half maximum.
In order to keep the stimulus time constant across different participants and trials within the task, since reaction times varied, we modeled the first 3 s of each trial separately from the rest of the trial [for a similar approach, see Bilalić et al., 2010; Iacoboni et al., 2004; Koenig et al., 2005]. It is important to note that eye‐movement parameters (number and duration of fixation) were almost identical for experts and novices within the first 3 s of the trial (see Supporting Information). The nominal duration of the trials also did not seem to influence the activation in the regions of interests (see the analysis in Supporting Information). Modeling shorter or longer durations produced similar brain activations. The button press was also explicitly modeled, while the baseline was implicitly modeled in a general linear model (GLM). Modeling of the time series of hemodynamic activation relied upon a canonical response function. Autocorrelation correction was estimated with a AR(1) model and considered by prewhitening the data. A high‐pass filter was applied (DCT with cut‐off of 128 Hz) to eliminate low‐frequency noise components.
We first present the task analysis where both experts and novices and both normal and random positions were contrasted to the baseline (starting position). In the group analysis, we used the parameters (contrast images) of the individual analysis of each participant to perform a 2 × 2 (expertise × type of position) ANOVA. The main effects of expertise and position type, as well as their interaction were based on F‐statistics, corrected for multiple comparisons across the whole brain. We set the significance level at P(FWE) < 0.05 (family‐wise error correction for multiple comparisons, equivalent to an F value of 26.4), and considered clusters with a size of five or more contiguous voxels only. Given the interaction effect is probably more subtle than the main effects and thus difficult to detect in the whole brain analysis with a stringent criterion, we looked for the areas modulated by interaction by (1) lowering threshold to P < 0.0001 uncorrected and (2) by looking for the voxels modulated by interaction in the areas already active in the main effect of expertise using a stringent FWE threshold. The latter approach is particularly relevant because we were interested in possible interaction effects within the areas more active in experts than novices [for a related approach, see Friston et al., 2006].
We used surfrend toolbox in SPM5 to extract statistical maps which were then loaded into FreeSurfer (Athinoula A. Martinos Center for Biomedical Imaging, Harvard, Cambridge, MA; http://surfer.nmr.mgh.harvard.edu/) to graphically present results on an inflated standardized brain. We further treated the peak voxel (highest activated) in each significantly activated area as a region of interest (ROIs). For each participant, we used the MarsBaR SPM Toolbox (Marseille ROI toolbox, Version 041) to extract the Percent Signal Change (PSC) relative to the baseline for the normal and random positions. These results are plotted in Figure 3. This is mainly done for the illustrative and descriptive purposes [see Poldrack and Mumford, 2009]. Extracting the SPC from all the activated voxels in the region instead of a single peak voxel, yield similar results.
Figure 3.
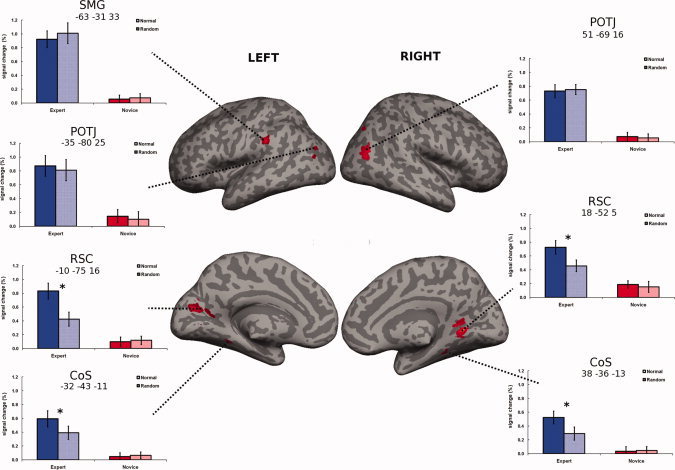
Neuroimaging data (expertise effect). Brain regions more activated in experts than in novices (main effect of expertise; P < 0.05; FWE‐corrected; k = 5), presented on an inflated brain. SMG, supramarginal gyrus; POTJ, parieto‐occipito‐temporal junction; RSC, retrosplenial cortex; CoS, collateral sulcus. The MNI coordinates are presented below the ROI labels. Percent signal change (relative to starting position/baseline) from the most activated voxel in each of the regions was extracted and plotted for descriptive purposes. Blue color represents experts; red color novices. Error bars indicate SEM. *P < 0.01 in a t‐test for dependent samples.
RESULTS
Behavioral Evidence
Reaction times indicate that the task indeed taps chess skill.
Expert players needed much less time to enumerate the relations between chess objects (i.e., threats) across both types of positions [Fig. 1C, two‐way expertise (experts/novices) × position type (normal/random) ANOVA produced a significant expertise effect—F(1, 21) = 22.8, P < 0.0001]. Normal positions required less time than random positions [position type effect—F(1, 21) = 11, P < 0.003]. Although the interaction between expertise and position type was not significant, experts were faster in enumerating threats in normal positions than in random positions [t‐test for dependent samples for the differences between normal and random positions, t(7) = 7.1, P < 0.0001]. There were no differences among novices.
Experts' shorter RTs were not related to their lesser accuracy. The analysis of errors (Fig. 1D) shows that experts made fewer mistakes than novices [expertise effect—F(1, 21) = 15.1, P = 0.001] and that there were more mistakes on the random than on the normal positions [position type effect—F(1, 21) = 10.9, P = 0.003]. The discrepancy in the experts' performance on normal and random positions was solely responsible for the latter result. Experts made more mistakes on random than on normal positions [t(7) = 3.5, P = 0.01], while there was no difference among novices. This resulted in a significant interaction between expertise and position type [F(1, 21) = 4.9, P < 0.039].
The enumeration of relations between two chess objects is rather easy and even beginners would be able to carry out such a task. However, correctly identifying all inter‐relations is more difficult because the positions contain 15 or more objects. Although the task proved to be rather difficult in the end, the eye‐movement protocols showed that both experts and novices carried out the task with best intentions. This was further corroborated by the fact that there were no significant RT differences between correct and incorrect trials among novices and experts.
Eye‐Movement Evidence
The different pattern of reaction times on normal and random positions among experts and novices indicates that experts use a different strategy to solve the task. In order to shed light on the strategy, we examined the eye movements of experts and novices. Eye movements have proved useful in identifying cognitive mechanisms behind expertise phenomena [Bilalić et al., 2008a, 2010, 2011; Charness et al., 2001; De Groot et al., 1996; Gobet et al., 2004; Reingold and Charness, 2005; Reingold et al., 2001a, b; Reingold and Sheridan, in press].
The average number of fixations needed to complete the task was unsurprisingly in line with the reaction time results (see Supporting Information for this and analysis on fixation duration). We were, however, more interested in the pattern of these fixations. Figure 2A shows that experts fixated more often at the objects of interest than novices [expertise—F(1, 9) = 5.2, P = 0.048]. Similarly, normal positions resulted in more fixation on objects of interest than random ones [position type—F(1, 9) = 13.4, P = 0.005]. This difference was, however, driven solely by experts who fixated more often on the objects of interest on normal than on random positions [normal vs. random positions among experts—t(4) = 4.8, P = 0.008]. This was not the case among novices where there were no differences [which resulted in the expertise × position interaction—F(1, 9) = 15.2, P = 0.004].
These differences were not related to the general pattern of fixations. Figure 2B shows that there were no differences among experts and novices in the amount of fixations falling on objects irrespective of their relevance. Experts nevertheless could focus their fixation more often on the objects of interest. These results indicate that experts' advantage is mainly in focusing their attention to the relevant aspects of the environment and drawing important information from the visual input (see Supporting Information for the analysis of the first 3 s of the trials, which confirms these results).
Neuroimaging Evidence
The eye‐movement analysis showed that experts do not make more fixations than novices, but that their knowledge enables them to fixate more often on the object of interest. The neuroimaging evidence presented here provides the neural basis behind this amazing ability. The main effect of expertise will provide evidence on the brain areas associated with recognition of objects and their functions, while the main effect of position type, and the interaction between expertise and position type, will give insight in the neural basis behind pattern recognition.
Although many areas were related to the task performance (see Supporting Information), only a few of them were related to expertise. Figure 3 shows the areas that were significantly more activated in expert than in novices (main effect of expertise). The network included lateral brain areas: bilateral area slightly posterior of pMTG, which we will call the parieto‐occipito‐temporal junction (POTJ) as we did in our previous work [Bilalic et al., 2010, 2011], and left SMG (see upper part of Fig. 3). The MNI coordinates of the areas are presented in the figures. The other areas related to the expertise effect were the area where the calcarine fissure meets the parieto‐occipital sulcus at the medial left side and the slightly anterior point at the right hemisphere (see middle part of Fig. 3). We will call these areas retrosplenial cortex (RSC) following the terminology used by others [Epstein, 2008]. Finally, a part of parahipocampal cortex in the middle part of the CoS was more active in experts than in novices in both hemispheres (see lower part of Fig. 3). No areas showed significantly more activation in novices than in experts. No other areas survived the stringent threshold in the main effect of position type. The same was the case for the expertise and position type interaction. We then analyzed the areas sensitive to main effect of expertise for possible interaction effects and found the bilateral RSC and bilateral CoS sensitive to interaction. The same areas were revealed when we used a less stringent threshold specified in the method section.
In order to illustrate this and other effects in brain areas, we extracted the PSC relative to baseline (starting position) in these areas and plotted the averages for both groups on both types of positions in Figure 3 [for this approach, see Poldrack and Mumford, 2009]. This provides an overview of the activation levels in the significant regions, but also gives additional cues about potential interaction between the factors of expertise and position type. As we already mentioned, only two regions showed such tendencies—bilateral RSC and bilateral CoS.
Interplay Between Different Levels of Evidence
Our study investigated object and pattern recognition using the expertise approach of comparing experts with novices. We were interested in the differences between experts and novices in those cognitive processes and we devised a task where the differences were evident. We do not hold behavioral performance constant as we believe that it would not enable us to effectively investigate the expertise effects. Rather, we treat all three dependent variables (RT, eye movements, and neuroimaging) as different consequences of the same underlying expertise processes [for a discussion and justification of this view, see Henson, 2005: pp. 212–213, 2006: p. 68]. This approach has a long tradition in cognitive psychology and neuropsychology [De Groot, 1978; Shallice, 1988] and offers the possibility to directly falsify the conclusion obtained from experts' findings by comparing them with those of novices [Bilalic et al., 2010]. The question about interaction between different levels of evidence, nevertheless, remains. It is reasonable to assume that the fMRI measurement is influenced by, say, visual input, which is seemingly different among experts and novices. The fMRI differences in experts and novices could be a consequence of different viewing patterns of experts and novices. We want to stress here that both experts and novices watched the same visual stimuli and that their eye‐movement patterns were largely similar when it comes to fixations and their durations (see Supporting Information). Both groups fixated at objects (i.e., chess pieces) with the difference that the objects fixated by experts carried more task‐relevant information. Hence, the fMRI differences have more likely the same cause as the behavioral and eye‐movement differences than to be directly influenced by them. We further minimized the fMRI differences by evaluating only the first 3 s of each trial where the differences were smaller than during the whole trial (see eye movement analysis in Supporting Information).
A related danger in comparing different groups is possible different motivational and attentional influence on results. Unlike in some other studies, participants in our study did not passively observe stimuli or execute a simple sequential matching of stimuli. Participants were engaged in clearly specified tasks which they obviously executed given the behavioral and eye‐movement evidence. We have no reason to believe that the differences in the interest and attention paid to the chess task could possibly explain the results. If anything, this task was more difficult for novices and required more concentration. And yet, it was the experts who displayed higher activation levels in the areas sensitive to expertise. The different positions were also presented randomly and not in a block, which also reduce the expectation and attentional effects. The areas associated with attentional processes such as superior parietal lobe (SPL) and intraparietal sulcus [IPS; Corbetta and Shulman, 2002], were required during the execution of the task but were not modulated by expertise or position type (see Supporting Information). Even if there were such attentional effects, it would be difficult to explain different patterns of activation on normal and random positions among experts and novices. Finally, the same chess stimuli produced different activation patterns in the same areas in a control task not related to chess processes in our previous study [Bilalić et al., 2010]. If attention were automatically drawn to particular type of stimuli, that is position type, we would have expected similar patterns with activation on the same stimuli without regard on the task at hand.
DISCUSSION
Expert chess players were faster than novices in enumerating threats in chess positions. This performance was a consequence of their highly specialized chess knowledge as shown by the difference between normal and random positions. Expert players were particularly fast on normal positions where the common relations between chess objects were intact, while their performance suffered when the relations were disturbed on random positions. In contrast, novice players, who do not possess sophisticated chess knowledge, did not display much difference between normal and random positions. Eye movements showed that experts' advantage lies in the ability to immediately focus on relevant objects and relations between them in the environment and ignore the irrelevant ones. Novices, on the other hand, inspected irrelevant objects and their relations too. Neuroimaging data demonstrated involvement of numerous areas in the task execution reflecting its complexity (see Supporting Information). Only a few areas, however, were related to expertise—left SMG and bilateral POTJ laterally, bilateral CoS ventrally, and bilateral RSC medially (see Fig. 3). These areas reflect the processes necessary for skilled performance and in the following sections we will relate the processes with particular areas.
Object Recognition
The essential component of the task was recognition of objects. A good part of the ventral stream associated with recognition of shapes and objects in general [Grill‐Spector and Malach, 2004; Peissig and Tarr, 2007] was relevant for the task performance (see Supporting Information). Similarly, the involvement of the dorsal stream related to functional properties of objects [Noppeney, 2008] was also evident in the task performance. Only a part of the dorsal stream (OPTJ), however, distinguished between skilled performance of experts and nonskilled of novices (see Fig. 3). The involvement of the dorsal stream brain regions makes sense because chess pieces are manmade objects. Manmade objects have a specific function, which is inextricably related to movement. Seeing a manmade object, or even hearing or reading its name, activates the left area around the occipito‐parieto‐temporal junction, in particular pMTG [Brambati et al., 2006; Damasio et al., 1996; Noppeney, 2008].
The absence of the differences on normal and random position in the POTJ indicates the involvement in object recognition—object recognition is necessary in both kinds of positions. Further evidence about POTJ involvement in skilled object recognition is provided in similar studies. Our previous study [Bilalić et al., 2010], which involved a similar visual search task, connected these areas to the identification of chess pieces. Similarly, the study by Campitelli et al. [2007] found these areas to be active when players were recalling a briefly seen chess position. Both studies, however, used positions with numerous objects where it is difficult to pinpoint these activations to recognition processes of isolated objects. In a more recent study, we [Bilalić et al., 2011] used recognition of isolated chess objects and showed that the same lateral areas bilaterally around POTJ were more activated in experts than novices.
Pattern Recognition
The medial and ventral areas more activated in experts (bilateral RSC and bilateral CoS) also showed different activation on normal and random positions among experts (see Fig. 3). Given that the difference between these two kinds of positions was a mere presence or absence of common relations, we can conclude that these areas are related to experts' advantage in orientation in the environment based on common placements of objects and their common relations. In other words, the CoS and RSC are associated with pattern recognition processes. These processes, however, also include not only knowledge about common placement of objects in environment, but also about common relations that these objects form [Gobet et al., 2001; Gobet and Simon, 1998; McGregor and Howes, 2002]. The threats task required both aspects and it may not be easy to exactly relate brain activations to a particular aspect of the performance.
Other studies using similar material and tasks [Bilalić et al., 2010, 2011; Campitelli et al., 2007; Righi et al., in press] also found similar areas. In the study by Bilalić et al. [2010], a similar visual search task was used but it did not require explicit processing of relations between objects. Campitelli et al. study [2007] used a recall task that benefits from explicit extraction of relations between objects. In contrast, Righi et al. [in press] required players to judge normal and random positions without explicit involvement of relations. All three studies identified CoS to be related to experts' advantage on normal positions, but only the study by Campitelli et al. reveals more activation on normal positions among experts in the RSC. We thus can suppose that the CoS is mainly related to fast orientation processes while the RSC to explicit parsing relations in the environment. The other study that used isolated chess objects [Bilalić et al., 2011] did not reveal expertise‐modulated activations in RSC or CoS, indirectly supporting the involvement in pattern recognition of these areas.
These results have implication for the functional properties of these brain areas. The CoS is a part of the medial temporal lobe, which is associated with episodic memory [Diana et al., 2007]. The part of the CoS activated in our study also belongs to the parahippocampal place area [PPA; Epstein and Kanwisher, 1998], which is supposed to be involved in the perception of complex scenes made of several elements and in particular of their spatial layout [Epstein, 2008]. Our results, however, suggest that this part of the PPA may be related to processing of associations between objects and thus responsible for fast orientation in the environment [see also Bar, 2004, 2009; Bilalić et al., 2010].
The RSC is activated during scene viewing, imagining scenes, and navigations through them, in particular if they are familiar [Ino et al., 2002; O'Craven and Kanwisher, 2000]. The same area, however, is engaged during autobiographic and episodic memory [Hassabis et al., 2007; Svoboda et al., 2006] as well as in supporting associations related to scene context [Bar, 2009]. Our study shows that the bilateral RSC are also related to explicitly parsing relations between objects.
Recognition of Relations
Our study explicitly investigated an important aspect of expertise—recognition of relations between chess objects (threats). We already saw that RSC is an important area for (spatial) parsing relations between objects in a complex environment. RSC is, however, also inextricably connected to pattern recognition (i.e., orientation among numerous objects and relations that those objects make). The remaining lateral area, left SMG, is more likely related to the cognitive processes responsible for explicit identification of relations between two objects. The SMG is generally thought to support the retrieval of actions associated with objects [Noppeney, 2008]. It is activated when people are explicitly instructed to retrieve a function‐related action with an object [Kellenbach et al., 2003; Martin et al., 1996] and its activation is modulated through actual execution of an action [Boronat et al., 2005; Canessa et al., 2008].
In our study with isolated chess objects [Bilalić et al., 2010], SMG was modulated by the task demands. If players were explicitly asked to identify whether two objects form a relation, SMG was more active in experts than novices. If the task was to solely identify the chess object without explicit instruction to relate the two objects, SMG activity did not differentiate between experts and novices. Similarly, the other studies that did not feature explicit identification of relations [Bilalić et al., 2010; Campitelli et al., 2007; Righi et al., in press] did not find the SMG modulated by expertise.
Development of Expertise
Our results provide insight into the nature of the expertise development. Many areas were related to the task performance but only the areas connected to domain‐specific knowledge distinguished between experts and novices. It is a well‐known finding in expertise research that acquired knowledge, and not general abilities, enable expert performance [for a review, see Ericsson and Lehmann, 1996]. Our results indicate that this is might also be the case in the brain implementation of expertise.
Our study also gives clues about the relative importance of different cognitive processes in expertise. The activation in the areas related to pattern and relation recognition (SMG, RSC, and CoS) was not different from the baseline (i.e., the chess board with pieces in the starting positions; see Fig. 3). This means that the processes of chess pattern and relation recognition do not seem to be developed among novices. In contrast, the object recognition areas, at least the left POTJ, were activated in novices. The implication is that the processes of pattern and relation recognition, which are more complex than processes of object recognition, are the cornerstone of expertise [Chase and Simon, 1973; De Groot, 1978; Gobet and Simon, 1996a; Saariluoma, 1995]. This is in accordance with theoretical perspectives and a large body of behavioral evidence [for reviews, see Ericsson and Lehmann, 1996; Gobet et al., 2004].
The other important aspect is the bilateral activation among experts in the areas related to object and pattern recognition (POTJ, RSC, and CoS). In contrast, novices either do not show any activation in the pattern and relation recognition areas (RSC, CoS, and SMG) or show it only in one hemisphere in the object recognition related areas (POTJ). On the one hand, this pattern of results confirms the common finding of left lateralization in object recognition [Lewis, 2006], as both experts and novices engaged the left temporo‐lateral areas. However, it is also points out that for highly skilled object recognition found among experts, not only higher efficiency of the same left temporo‐lateral areas is necessary, but also additional homologous brain areas on the right side. This indicates that the nature of cognitive processes among experts, not only in pattern and relation recognition, but also in object recognition, is qualitatively different from those found in novices.
Behavioral and, in particular, eye movement studies of expertise point out to a similar conclusion [for a review, see Ericsson and Lehmann, 1996]. Experts are faster and more efficient in behavioral tasks because they can automatically and in parallel retrieve the knowledge necessary for the execution of the task [Reingold and Charness, 2005; Reingold et al., 2001a; Reingold and Sheridan, in press]. This parallel and automatic processing may be too demanding to accommodate within a single hemisphere and the additional analogous areas are necessary to meet the demands of experts' cognitive processing [for a review on interhemispheric communication, see Banich, 1998].
Conclusions
We showed that experts' superior performance is powered by their extensive knowledge that enabled them to focus immediately on the important aspects of the environment. The neural basis of this superior skill is related to both ventral and dorsal visual streams. The bilateral temporo‐lateral areas of the dorsal streams were associated with recognition of objects, while the left SMG was particularly involved in the retrieval of object functions and their relations to other objects. The areas in the middle part of the CoS together with RSC were associated with pattern recognition processes. The results show that the developmental changes in specific object and pattern processing are also reflected in the way they are accommodated in the brain. Experts' superior processing is accommodated in additional brain areas which do not seem to be relevant for the functioning among novices. Skilled object and pattern recognition thus require qualitatively different cognitive processes that are differently accommodated in the brain. This finding underlines the importance of experience and expertise on recognition. Our research, thus, suggests that, since in real life we are all experts and deal with meaningful stimuli in a typical context, studying expert‐like cognitive processing adds value to laboratory studies that aim to generalize to everyday‐life cognition.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
Authors thank Mathias Röger for the help with participants and measurement, as well as Jakob Erb for the help with the eye movement analysis. The cooperation of chess players is greatly appreciated.
REFERENCES
- Amidzic O, Riehle HJ, Fehr T, Wienbruch C, Elbert T ( 2001): Pattern of focal gamma‐bursts in chess players. Nature 412: 603. [DOI] [PubMed] [Google Scholar]
- Atherton M, Zhuang J, Bart WM, Hu X, He S ( 2003): A functional MRI study of high‐level cognition. I. The game of chess. Cogn Brain Res 16: 26–31. [DOI] [PubMed] [Google Scholar]
- Banich MT ( 1998): The missing link: The role of interhemispheric interaction in attentional processing. Brain Cogn 36: 128–157. [DOI] [PubMed] [Google Scholar]
- Bar M ( 2004): Visual objects in context. Nat Rev Neurosci 5: 617–629. [DOI] [PubMed] [Google Scholar]
- Bar M ( 2009): The proactive brain: Memory for predictions. Phil Trans R Soc B 364: 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman I, Mezzanotte RJ, Rabinowitz JC ( 1982): Scene perception: Detecting and judging objects undergoing relational violations. Cogn Psychol 14: 143–177. [DOI] [PubMed] [Google Scholar]
- Bilalić M, McLeod P, Gobet F ( 2008a): Why good thoughts block better ones: The mechanism of the pernicious Einstellung (set) effect. Cognition 108: 652–661. [DOI] [PubMed] [Google Scholar]
- Bilalić M, McLeod P, Gobet F ( 2008b): Inflexibility of experts—Reality or myth? Quantifying the Einstellung effect in chess masters. Cogn Psychol 56: 73–102. [DOI] [PubMed] [Google Scholar]
- Bilalić M, Turella L, Erb M, Grodd W ( 2008c): Neural correlates of perception in chess. Poster presented at the 2008 HBM Annual Meeting in Melbourne, Australia.
- Bilalić M, McLeod P, Gobet F ( 2009): Specialization effect and its influence on memory and problem solving in expert chess players. Cogn Sci 33: 1117–1114. [DOI] [PubMed] [Google Scholar]
- Bilalić M, Langner R, Erb M, Grodd W ( 2010): Mechanisms and neural basis of object and pattern recognition: A study with chess experts. J Exp Psychol G 139: 728–742. [DOI] [PubMed] [Google Scholar]
- Bilalić M, Kiesel A, Pohl C, Erb M, Grodd W ( 2011): It takes two‐skilled recognition of objects engages lateral areas in both hemispheres. PLoS One 6: e16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Myers D, Wilson A, Rankin KP, Allison SC, Rosen HJ, Miller BL, Gorno‐Tempini ML ( 2006). The anatomy of category‐specific object naming in neurodegenerative diseases. J Cogn Neurosci 18: 1644–1653. [DOI] [PubMed] [Google Scholar]
- Brockmole JR, Hambrick DZ, Windisch DJ, Henderson JM ( 2008): The role of meaning in contextual cueing: Evidence from chess expertise. Q J Exp Psychol 61: 1886–1896. [DOI] [PubMed] [Google Scholar]
- Bukach CM, Gauthier I, Tarr MJ ( 2006): Beyond faces and modularity: The power of an expertise framework. Trends Cogn Sci 10: 159–166. [DOI] [PubMed] [Google Scholar]
- Boronat CB, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, Kimberg DY, Detre JA ( 2005): Distinctions between manipulation and function knowledge of objects: Evidence from functional magnetic resonance imaging. Cogn Brain Res 23: 361–373. [DOI] [PubMed] [Google Scholar]
- Campitelli G, Gobet F, Parker A ( 2005): Structure and stimulus familiarity: A study of memory in chess‐players with functional magnetic resonance imaging. Span J Psychol 8: 238–245. [DOI] [PubMed] [Google Scholar]
- Campitelli G, Gobet F, Head K, Buckley M, Parker A ( 2007): Brain localization of memory chunks in chessplayers. Int J Neurosci 117: 1641–1659. [DOI] [PubMed] [Google Scholar]
- Campitelli G, Parker A, Head K, Gobet F ( 2008): Left lateralization in autobiographical memory: An fMRI study using the expert archival paradigm. Int J Neurosci 118: 191–209. [DOI] [PubMed] [Google Scholar]
- Canessa N, Borgo F, Cappa SF, Perani D, Falini A, Buccino G, Tettamanti M, Shallice T ( 2008): The different neural correlates of action and functional knowledge in semantic memory: An FMRI study. Cereb Cortex 18: 740–751. [DOI] [PubMed] [Google Scholar]
- Charness N ( 1992): The impact of chess research on Cogn science. Psychol Res 54: 4–9. [Google Scholar]
- Charness N, Reingold EM, Pomplun M, Stampe DM ( 2001): The perceptual aspect of skilled performance in chess: Evidence from eye movements. Mem Cogn 29: 1146–1152. [DOI] [PubMed] [Google Scholar]
- Chase WG, Simon HA ( 1973): Perception in chess. Cogn Psychol 4: 55–81. [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR ( 1996): A neural basis for lexical retrieval. Nature 380: 499–505. [DOI] [PubMed] [Google Scholar]
- De Groot AD ( 1978/1946 (originally published 1946)): Thought and Choice in Chess, 2nd ed. New York: Mouton De Gruyter. [Google Scholar]
- De Groot AD, Gobet F, Jongman RW ( 1996): Perception and Memory in Chess: Studies in the Heuristics of the Professional Eye. Assen: Van Gorcum. [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C ( 2007): Imaging recollection and familiarity in the medial temporal lobe: A three‐component model. Trends Cogn Sci 11: 379–386. [DOI] [PubMed] [Google Scholar]
- Downing P, Jiang Y, Shuman M, Kanwisher N ( 2001): A cortical area selective for visual processing of the human body. Science 293: 2470–2473. [DOI] [PubMed] [Google Scholar]
- Elo AE ( 1978): The Rating of Chess Players, Past and Present. New York: Arco. [Google Scholar]
- Epstein RA ( 2008): Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci 12: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N ( 1998): A cortical representation of the local visual environment. Nature 392: 598–601. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Kintsch W ( 1995): “Long‐term working memory”. Psychological Review 102: 211–245. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Lehmann AC ( 1996): Expert and exceptional performance: Evidence of maximal adaptation to task constraints. Ann Rev Psychol 47: 273–305. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Rotshtein P, Geng JJ, Sterzer P, Henson RN ( 2006): A critique of functional localisers. NeuroImage 30: 1077–1087. [DOI] [PubMed] [Google Scholar]
- Gobet F, Simon HA ( 1996a): Templates in chess memory: A mechanism for recalling several boards. Cogn Psychol 31: 1–40. [DOI] [PubMed] [Google Scholar]
- Gobet F, Simon HA ( 1996b): Recall of random and distorted positions: Implications for the theory of expertise. Mem Cogn 24: 493–503. [DOI] [PubMed] [Google Scholar]
- Gobet F, Simon HA ( 1998): Expert chess memory: Revisiting the chunking hypothesis. Memory 6: 225–255 [DOI] [PubMed] [Google Scholar]
- Gobet F, Lane PCR, Croker S, Cheng PC‐H, Jones G, Oliver I, Pine JM ( 2001): Chunking mechanisms in human learning. TRENDS in Cognitive Sciences 5: 236–243. [DOI] [PubMed] [Google Scholar]
- Gobet F, Waters AJ ( 2003): The role of constraints in expert memory. J Exp Psychol Learn Mem Cogn 29: 1082–1094. [DOI] [PubMed] [Google Scholar]
- Gobet F, Lane PCR, Croker S, Cheng PCH, Jones G, Oliver I, Pine JM ( 2001): Mechanisms in human learning. Trends Cogn Sci 5: 236–243. [DOI] [PubMed] [Google Scholar]
- Gobet F, de Voogt A, Retschitzki J ( 2004): Moves in Mind: The Psychology of Board Games. Hove: Psychology Press. [Google Scholar]
- Goodale MA, Milner D ( 1992): Separate visual pathways for perception and action. Trends Neurosci 15: 20–25. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Malach R ( 2004): The human visual cortex. Ann Rev Neurosci 27: 649–677. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA ( 2007): Using imagination to understand the neural basis of episodic memory. J Neurosci 27: 14365–14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R ( 2005): What can functional neuroimaging tell the experimental psychologist? Q J Exp Psychol A 58: 193–233. [DOI] [PubMed] [Google Scholar]
- Henson R ( 2006): What has (neuro)psychology told us about the mind (so far)? A reply to Coltheart (2006). Cortex 42: 387–392. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar‐Szakacs I, Moritz M, Throop CJ, Fiske AP ( 2004): Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. NeuroImage 21: 1167–1173. [DOI] [PubMed] [Google Scholar]
- Ino T, Inoue Y, Kage M, Hirose S, Kimura T, Fukuyama H ( 2002): Mental navigation in humans is processed in the anterior bank of the parieto‐occipital sulcus. Neurosci Lett 322: 182–186. [DOI] [PubMed] [Google Scholar]
- Johnson‐Frey SH ( 2004): The neural bases of complex tool use in humans. Trends Cogn Sci 8: 71–78. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K ( 2003): Actions speak louder than functions: The importance of manipulability and action in tool representation. J Cogn Neurosci 15: 30–46. [DOI] [PubMed] [Google Scholar]
- Kiesel A, Kunde W, Pohl C, Berner MP, Hoffmann J ( 2009): Playing chess unconsciously. J Exp Psychol Learn Mem Cogn 35: 292–298. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Glosser G, DeVita C, Moore P, McMillan C, Gee J, Grossman M ( 2005): The neural basis for novel semantic categorization. NeuroImage 24: 369–383. [DOI] [PubMed] [Google Scholar]
- Lewis JW ( 2006): Cortical networks related to human use of tools. Neuroscientist 12: 211–231. [DOI] [PubMed] [Google Scholar]
- Mahon B Z, Caramazza A ( 2009): Concepts and categories: A cognitive neuropsychological perspective. Ann Rev Psychol 60: 27–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Milleville SC, Negri GAL, Rumiati RI, Caramazza A, Martin A ( 2007): Action‐related properties shape object representations in the ventral stream. Neuron 55: 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG ( 1995): Discrete cortical regions associated with knowledge of color and knowledge of action. Sci 270: 102–105. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV ( 1996): Neural correlates of category‐specific knowledge. Nature 379: 649–652. [DOI] [PubMed] [Google Scholar]
- McGregor SJ, Howes A ( 2002): The role of attack and defense semantics in skilled players' memory for chess positions. Mem Cogn 30: 707–717. [DOI] [PubMed] [Google Scholar]
- Miceli G, Fouch E, Capasso R, Shelton JR, Tomaiuolo F, Caramazza A ( 2001): The dissociation of color from form and function knowledge. Nat Neurosci 4: 662–667. [DOI] [PubMed] [Google Scholar]
- Newell A, Shaw JC, Simon H ( 1963): Chess‐playing programs and the problem of complexity In Feigenbaum E, Feldman J, editors, Computers and Thought, pages 109–133. McGraw Hill. [Google Scholar]
- Nichelli P, Grafman J, Pietrini P, Alway D, Carton JC, Miletich R ( 1994): Brain activity in chess playing. Nature 369: 191. [DOI] [PubMed] [Google Scholar]
- Noppeney U ( 2008): The neural systems of tool and action semantics: A perspective from functional imaging. J Physiol 102: 40–49. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Kanwisher N ( 2000): Mental imagery of faces and places activates corresponding stimulus‐specific brain regions. J Cogn Neurosci 12: 1013–1023. [DOI] [PubMed] [Google Scholar]
- Onofrj M, Curatola L, Valentini G, Antonelli M, Thomas A, Fulgente T ( 1995): Non‐dominant dorsal‐prefrontal activation during chess problem solution evidenced by single photon emission computerized tomography (SPECT). Neurosci Lett 198: 169–172. [DOI] [PubMed] [Google Scholar]
- Palmeri TJ, Gauthier I ( 2004): Visual object understanding. Nat Rev Neurosci 5: 291–303. [DOI] [PubMed] [Google Scholar]
- Peissig JJ, Tarr MJ ( 2007): Visual object recognition: Do we know more now than we did 20 years ago? Ann Rev Psychol 58: 75–96. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA ( 2009): Independence in ROI analysis: Where is the voodoo? Soc Cogn Affect Neurosci 4: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K ( 1998): Eye movements in reading and information processing: 20 years of research. Psychol Bull 124: 372–422. [DOI] [PubMed] [Google Scholar]
- Reingold EM, Charness N ( 2005): Perception in chess: Evidence from eye movements In: Underwood G, editor. Cognitive Processes in Eye Guidance. Oxford, England: Oxford University Press; pp 325–354. [Google Scholar]
- Reingold EM, Sheridan H: Eye movements and visual expertise in chess and medicine 2001. In: Liversedge SP, Gilchrist ID, Everling S, editors. Oxford Handbook on Eye Movements. Oxford, UK: Oxford University Press (in press). [Google Scholar]
- Reingold EM, Charness N, Pomplun M, Stampe DM ( 2001a): Visual span in expert chess players: Evidence from eye movements. Psychol Sci 12: 48–55. [DOI] [PubMed] [Google Scholar]
- Reingold EM, Charness N, Schultetus RS, Stampe DM ( 2001b): Perceptual automaticity in expert chess players: Parallel encoding of chess relations. Psychon Bull Rev 8: 504–510. [DOI] [PubMed] [Google Scholar]
- Righi G, Tarr MJ, Kingon A (in press): Category‐Selective recruitment of the fusiform gyrus with chess expertise 2001. In Staszewski JJ, editor. Expertise and Skill Acquisition: The Impact of William G. Chase. New York, NY: Psychology Press. [Google Scholar]
- Saariluoma P ( 1990): Chess players' search for task‐relevant cues: Are chunks relevant? In: Brogan D, editor. Visual Search. London: Taylor and Francis; pp. 115–121. [Google Scholar]
- Saariluoma P ( 1995): Chess Players' Thinking: A Cognitive Psychological Approach. London: Routledge. [Google Scholar]
- Saariluoma P, Karlsson H, Lyytinen H, Teras M, Geisler F ( 2004): Visuospatial representations used by chess experts: A preliminary study. Eur J Cogn Psychol 16: 753–766. [Google Scholar]
- Shallice T ( 1988): From Neuropsychology to Mental Structure. Cambridge: Cambridge University Press. [Google Scholar]
- Shallice T ( 2003): Functional imaging and neuropsychology findings: How can they be linked? NeuroImage 20: S146–154. [DOI] [PubMed] [Google Scholar]
- Shank RC, Abelson RP ( 1977): Scripts. Plans, Goals, and Understanding. Hillsdale: Erlbaum. [Google Scholar]
- Simon HA, Chase WG ( 1973): Skill in chess. American Scientist 61: 394–403. [Google Scholar]
- Svoboda E, McKinnon MC, Levine B ( 2006): The functional neuroanatomy of autobiographical memory: a meta‐analysis. Neuropsychologia 44: 2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood GD ( 2005): Cognitive Processes in Eye Guidance. USA: Oxford University Press. [Google Scholar]
- Ungerleider LG, Mishkin M ( 1982): Two cortical visual systems In: Ingle J, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: The MIT Press; pp 549–586. [Google Scholar]
- Vicente KJ, Wang JH ( 1998): An ecological theory of expertise effects in memory recall. Psychol Rev 105: 33–57. [DOI] [PubMed] [Google Scholar]
- Weisberg J, van Turennout M, Martin A ( 2007): A neural system for learning about object function. Cereb Cortex 17: 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D, Halligan P ( 2004): The relevance of behavioural measures for functional‐imaging studies of cognition. Nat Rev Neurosci 5: 67–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


