Abstract
Behavioural and neurophysiological studies of task‐switching have tended to employ ‘bivalent’ stimuli (which afford responses in two tasks). Using brain potential recordings, we investigated task‐switching with ‘univalent’ stimuli affording responses in only one of the tasks, and compared the outcomes to those recently obtained with bivalent stimuli (Lavric et al. [2008]: Eur J Neurosci 1‐14), in order to examine two phenomena. First, when only univalent stimuli are presented, the processing of task cues becomes optional. Our results showed that in these circumstances linguistic (but not pictorial) cues were still effective in eliciting at least some degree of preparation for a task‐switch, as evidenced by the reduction in the error cost of switching at the longer preparation interval and by a posterior switch‐induced ERP positivity at about 450–800 ms in the cue‐stimulus interval. Second, single affordance stimuli not only reduced behavioural switch costs relative to bivalent stimuli; they also produced a smaller post‐stimulus switch‐induced negativity, consistent with the latter being a marker of conflict between task‐sets. However, using stimuli not associated with responses in the alternative task did not completely eliminate the negativity. We speculate that the residue reflects other sources of conflict: attention to the irrelevant perceptual dimension and/or persistence of task goals. Hum Brain Mapp, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: cognitive control, task‐switching, ERPs, reconfiguration, task‐set conflict
INTRODUCTION
Performing a cognitive task requires an appropriate configuration of the cognitive system. Adopting such a configuration (‘task set’) is under voluntary control and can be started in expectation of an upcoming stimulus. But task‐sets can also be triggered by external stimuli, as the Stroop effect demonstrates. [Text evokes unwanted reading in a colour naming task, Stroop,1935]. Changing task typically prolongs the response times (RTs) and results in more errors than repeating it, a ‘switch cost’ that can be reduced if sufficient time is given prior to stimulus onset [Rogers and Monsell,1995]. Task‐cueing paradigms investigate these phenomena by presenting before each stimulus a cue specifying the upcoming task [Meiran,1996]. The reduction in switch cost (RISC) associated with increasing the cue‐stimulus interval (CSI) up to about one second suggests that the additional time allows the task‐set to be at least partially reconfigured before stimulus onset [Meiran,1996; Monsell and Mizon,2006]. However, even ample time to prepare does not usually eliminate the switch cost1, with the “residual” switch cost suggesting that there is a component of the switch cost which preparation cannot overcome. One likely source of the residual switch cost is that the persistence of the previous task‐set ‐ “task‐set inertia” ‐ results in competition at several levels of processing, including response conflict [Allport et al.,1994; Yeung et al.,2006; Yeung and Monsell,2003]. Recent fMRI work by Yeung et al. [2006] showed that increased activation in brain areas associated with the irrelevant task on switch relative to repeat trials was associated with increased behavioural (RT) switch costs. Another possibility is the competing task set does not merely persist from the previous trial, but is re‐activated associatively by the stimulus due to earlier learning of stimulus‐task associations [Waszak et al.,2003].
Electrophysiological Correlates of Preparation for a Task Switch
The excellent temporal resolution of brain event‐related potentials (ERPs) makes them a technique of choice for distinguishing effects of a task switch on (post‐stimulus) processes associated with task execution from effects on (pre‐stimulus) processes of task‐set preparation. An increasing body of studies has examined ERP differences between otherwise identical task‐switch and task‐repeat trials [e.g. Astle et al.,2006,2008a,b; Karayanidis et al.,2003; Kieffaber and Hetrick,2005; Lavric et al.,2008; Nicholson et al.,2005; Rushworth et al.,2002; Swainson et al.,2006; Tieges et al.,2006]. These and other studies have documented several differences between ERP waveforms on switch and repeat trials during the pre‐stimulus interval. Relative to repeat trials, switch trials show: (1) a posterior positive deflection ∼400 ms or more into the preparation interval, [e.g. Astle et al.,2006; Karayanidis et al.,2003; Lavric et al.,2008; Nicholson et al.,2005; Rushworth et al.,2002]; (2) an anterior negativity in the same latency range [e.g. Astle et al.,2006; Lavric et al.,2008; Mueller et al.,2007]; (3) a longer‐latency anterior‐central negativity [Tieges et al.,2006]; and (4) a brief early (∼150‐350 ms) anterior positivity [Lavric et al.,2008; Rushworth et al.,2002,2005]. Our recent work has shown that the first two of the above effects were the likely electrophysiological substrate of advance task‐set reconfiguration: their amplitude co‐varied with the effectiveness of preparation over trials (within subjects), as well as over subjects [Lavric et al.,2008]. Decomposition of ERPs by means of temporal Principal Components Analysis indicated that the protracted anterior negativity (2 above) was not independent of the posterior positivity (1 above), suggesting that the two may reflect the two poles of the underlying dipolar generators (though see Astle et al.,2008a, and Mueller et al.,2007, for an alternative view).
Contrasting Linguistic and Nonlinguistic Cues
ERP researchers have increasingly relied on the task‐cueing paradigm [e.g. Nicholson et al.,2005; Lavric et al.,2008; Swainson et al.,2006], as it allows one to unconfound effects of endogenous preparation (triggered by the cue) from any passive dissipation of task‐set inertia from the previous trial by varying the cue‐stimulus interval independently of the interval since the previous response [Meiran,1996]. It also allows one to address the effectiveness of linguistic vs. non‐linguistic cues in eliciting task‐set preparation.
There is evidence that linguistic self‐instruction can play a critical role in task‐set control: a verbal secondary task [Goschke,2000], or concurrent articulation [Miyake et al.,2004] during the preparation interval has been found to eliminate or attenuate the RISC effect. If verbalizing the task goal is important, an appropriate verbal cue should be particularly effective, obviating the need for the subject to generate their own covert verbal self‐instruction. Recent evidence is consistent with this expectation [Lavric et al.,2008]. When subjects were cued to identify the shape or colour of a stimulus (as one of four), word cues (“shape”, “colour”) were associated with a smaller switch cost than pictorial cues (composites of the possible shapes or colours ‐ Fig. 1A) and the cortical source estimation of the posterior positivity pointed to substantial activity in the left temporal cortex on picture‐cued trials, consistent with self‐generation of a verbal task label. In the present investigation, the superior efficacy of the verbal cues (relative to picture cues) is further tested in a design in which the processing of the cues was not obligatory.
Figure 1.
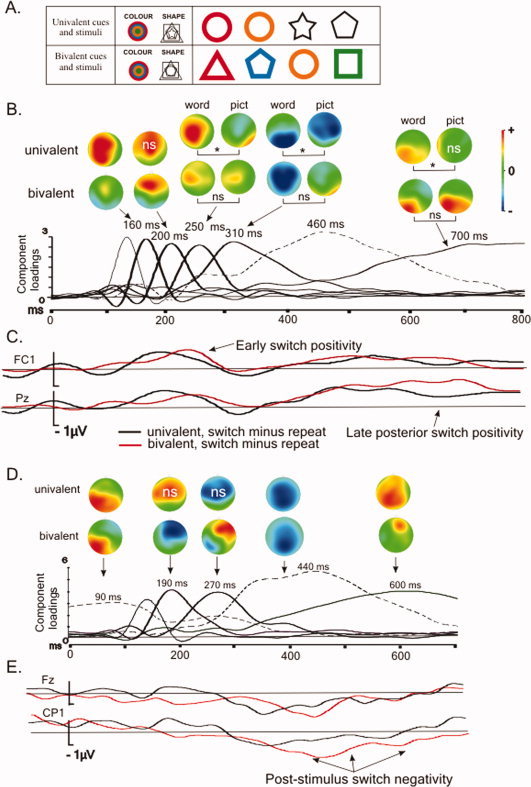
A: The course of a trial (top) and illustrations of the cues and stimuli used in the current (univalent) study and in the bivalent study by Lavric et al. [2008]. In the univalent experiment, only the circle was used in the colour task (it did not appear in the shape task) and only the black colour was used in the shape task (black did not appear in the colour task). In the bivalent experiment, each of four shapes was presented in each of four colours, with each stimulus used equally in both tasks. B: Results from the PCA‐based analysis of the long CSI. To facilitate description, the latency at which temporal PCA components reached maximal amplitude (largest component loading), is given for switch‐sensitive components rounded to the nearest tens of ms. For components revealing significant interactions between switch and valence (plotted using thicker lines), the spline‐interpolated scalp distribution of the switch‐repeat difference in component scores is shown above (‘ns’ on the maps refers to the absence of significant switching effects in the ANOVA). The scales for all maps were symmetric. The scales were different across components but equivalent for all maps within components (there are two exceptions to the latter: the scales for the bivalent picture map in the component peaking at 310 ms and for the univalent picture map in the last component had to be ‘blown up’ by a factor of 2 in order to make smaller switch‐repeat differences visible). Where switch and valence interacted with cue type, scalp distributions are given by cue (‘ns’ and ‘*’ refer to non‐/significant switch by cue interactions in follow‐up ANOVAs). The component sensitive to switching but not valence is plotted in a dashed line. C: ERP difference waves of the long CSI in two representative electrodes. D: Results from the PCA‐based analysis of the stimulus interval, long CSI condition and E: corresponding ERP difference waves. The graphic conventions are the same as in (B) and (C).
Post‐Stimulus Switch‐Induced ERP Effects
Following the stimulus, a more negative ERP deflection on task‐switch than ‐repeat trials, with the difference showing a broad central‐parietal scalp distribution, has been consistently reported [e.g. Karayanidis et al.,2003; Nicholson et al.,2005; Lavric et al.,2008; Swainson et al.,2006]. This ‘switch‐related post‐stimulus negativity’ has been interpreted as a manifestation of active task‐set consolidation on repeat trials [Swainson et al.,2006]. We [Lavric et al.,2008] proposed that it may instead reflect enhanced conflict on switch trials due to task‐set inertia from the previous trial, also manifest in the behavioural residual switch cost. This account was in part motivated by the observation of a conflict‐related negativity, with a similar latency and distribution, in ERP studies of Stroop interference (N450; West,2003]. A related interpretation of the post‐stimulus switch negativity in terms of greater response conflict on switch trials was proposed by Nicholson et al. [2005].
A Task‐Cueing Study With ‘Univalent’ Stimuli
Electrophysiological studies to date have tended to use stimuli that afford responses in all the tasks ‐ ‘bivalent stimuli’ in a two‐task experiment. The present study aimed to examine ERP correlates of task‐cueing with ‘univalent’ stimuli (which afford responses only in one task) and compare them directly with the ERP effects previously documented with bivalent stimuli by Lavric et al. [2008]. To enable a quantitative (statistical) comparison, the current study was closely modelled on Lavric et al.'s [2008] experiment, which used cueing of colour or shape identification, with four values of each attribute mapped to a common set of four responses. The same tasks, responses, timing parameters and EEG set‐up were used, but the stimuli and task cues were modified to yield, instead of 16 bivalent stimuli (4 colours × 4 shapes) 8 univalent stimuli: a single shape (circle) combined with four colours for the colour task, and a different single colour (black) combined with four different shapes for the shape task (see Fig. 1A for examples). Thus the shape seen on the colour task trials was associated with no response in the shape task, and the colour seen on the shape task trials with no response in the colour task.
What insights might univalent stimuli offer concerning preparatory processes? In the task‐cueing paradigm, one must attend to the task cue to know how to respond to bivalent stimuli. But when all stimuli are univalent, cue processing becomes optional: the stimulus alone unambiguously specifies the task and response. To the extent that cue processing and task‐preparation are deliberate and effortful, using only univalent stimuli should reduce the behavioural and ERP indices of preparation. But if the processing of transparent verbal cues is relatively automatic, we might see clearer differences between the impact of linguistic and nonlinguistic cues: trials with word cues should show more evidence of task set preparation, both in behaviour (the RISC effect) and in the posterior switch‐induced ERP positivity. We might also see a differentiation between word and picture cue trials in the earliest switch‐sensitive ERP component seen in the CSI ‐ the positivity over the central‐anterior scalp at 150−350 ms following the cue (see above), which, given its latency and apparent lack of relationship to behavioural performance, was suggested by Lavric et al. [2008] to reflect detection of the need to reconfigure task‐set, rather than the process itself.
Turning to the post‐stimulus effects of a task switch, bivalent (but not univalent) stimuli contain perceptual attributes associated with responses in the competing task‐set, so one would expect any switch‐related increase in task‐set conflict to be stronger following bivalent stimuli. A greater behavioural switch cost in the bivalent condition was indeed found by Rogers and Monsell [1995]. They examined switching between classifying the letter and classifying the digit contained in bivalent (e.g., G4) and univalent (e.g., G% or #4) stimuli using alternation between predictable runs of two trials in each task. (They examined the effect of valence both across experimental blocks, as in the present study, and within blocks). Karayanidis et al. [2003] employed the same tasks as Rogers and Monsell [1995] and acquired ERP data during univalent and bivalent blocks. They confirmed the smaller RT switch cost in the univalent condition, and found that the post‐stimulus switch‐induced ERP negativity at electrode Pz was reduced in amplitude in the univalent relative to the bivalent condition. An ERP study by Poulsen et al. [2005] used the same tasks in a task‐cueing design. Their within‐block comparison between univalent and bivalent stimuli revealed greater frontopolar and centroparietal negativity at around 200−600 ms following bivalent stimuli. Although the switch by foil type [valence] interaction approached significance (P < 0.07) at 200−300 ms following stimulus onset, other analyses (time‐window and peak amplitude analyses in the P3 range) found no reliable modulation of the effects of switching by valence.
One feature of Poulsen et al.'s [2005] design that may have led to an underestimation of the switch by valence interaction was the CSI of 450 ms. A protracted cue‐locked switch‐related positivity widely reported in the literature would have likely extended into the stimulus interval (as reported in short CSI analyses by Lavric et al. [2008] and Nicholson et al., [2005], thus potentially masking the post‐stimulus switch related negativity and its modulation by valence. One aim of this study was to clarify the influence of stimulus valence on the switch‐induced post‐stimulus negativity in the task‐cueing paradigm. If the post‐stimulus switch‐related negativity obtained with bivalent stimuli is driven (at least in part) by conflict between competing task‐sets, this negativity should be reduced by using univalent stimuli. We expected the longer of the two CSIs we used [200 and 800 ms, as in Lavric et al.,2008] to allow the assessment of the post‐stimulus switch‐related negativity in conditions of maximal preparation, whose effectiveness could be assessed by the behavioural contrast with the shorter CSI.
METHOD
Participants
Sixteen right‐handed students from the University of Exeter (12 female, 4 male; aged between 18 and 34, mean = 22) were paid £5 per hour for participation, supplemented by a bonus payment (max £2) calculated on the basis of their performance (see Procedure). Participants gave informed consent following the guidelines set by the University of Exeter School of Psychology ethics committee.
Stimuli and Procedure
On each trial the participant was required to identify the colour or shape of an outline coloured shape, subtending about 4.5° in the centre of the screen, by pressing one of four keys in a row. For the shape task four shapes were mapped to the keys, and for the colour task four colours were mapped to the same keys. Participants were trained on these mappings in single‐task blocks at the beginning of the experiment.
In the Lavric et al. [2008] experiment, the four shapes assigned to the keys (left to right) were circle, triangle, square, and pentagon; the four colours were red, orange, green and blue, and all 16 combinations were presented so that each stimulus afforded a response in both tasks (Fig. 1A); one quarter of the stimuli were mapped to the same response for both tasks (congruent stimuli) and three quarters to a different response (incongruent stimuli). For the shape task in the present experiment the four shapes (star, triangle, square and pentagon) were presented in a colour (black) not assigned to a response in the colour task. Similarly, for the colour task the four colours (red, orange, green, blue) filled the outline (0.4° thick) of a circle, a shape not assigned to a response in the shape task. Hence these stimuli were neither congruent nor incongruent; they had a value on the irrelevant dimension, but it was not associated with a key response.
Apart from these changes in stimuli and their mapping to responses, the parameters of the new experiment were as in Lavric et al. [2008]. Each stimulus was preceded by a cue presented 200 or 800 ms before the stimulus (Fig. 1A); with bivalent stimuli, Lavric et al. [2008] found a substantial reduction in switch cost at the longer interval. Each task could be cued by either of two cues: the shape task either by the word “SHAPE” or by a collage of the four relevant shapes; the colour task by the word “COLOUR” or by a collage of the four relevant colours (Fig. 1A). The cue type changed on every trial from collage to word or vice versa, to avoid confounding cue change with task change [cf. Monsell and Mizon,2006]. The task changed randomly on only one third of the trials [to discourage preparation for a task change before the cue‐ cf. Monsell and Mizon2006]. The stimulus was presented surrounding the cue, and both remained on the screen until a response was made. For the four responses, four adjacent keys on the bottom letter row of a standard computer keyboard (v to m) were pressed by index or middle fingers of the two hands. If the wrong key was pressed, the word “ERROR” was displayed for an extra 2000 ms. Otherwise, the interval between each response and the next stimulus was a constant 1650 ms, to avoid confounding CSI with the opportunity for passive dissipation of task‐set from the previous trial [Meiran et al.,2000].
Participants were trained in a session without EEG recording; it comprised 4 single‐task blocks of 32 trials (two blocks for each task), followed by 4 blocks of 97 trials of task‐switching. On the subsequent day, the EEG was acquired while participants performed 12 blocks of 97 trials. Ignoring an initial lead‐in trial in each block, there were equal numbers of trials for each combination of task, stimulus, cue, and each combination had a 1:2 ratio of task‐switch to task‐repeat trials. Subject to these constraints, the order of trials was randomized anew for each block and participant.
Participants were instructed to use the cue to prepare for the following task. To encourage effective preparation, an incentive scheme was employed: a score (mean RT/10 + errors x 5) was computed for each block and a bonus payment was made for blocks on which the score was lower than a running average of previous blocks with the same CSI, adjusted for the marked improvement in performance from Day 1 to Day 2.
EEG/ERPs
As in Lavric et al. [2008], the EEG was sampled continuously at 500 Hz with a bandpass of 0.016‐100 Hz, the reference at Cz and the ground at AFz using a 64−Ag/AgCl‐electrode cap (ElectroCap International Inc., Eaton, Ohio) connected to BrainAmp amplifiers (Brain Products, Munich, Germany). There were 58 electrodes on the scalp in a 10−10 configuration, two on the outer canthi of the eyes, one above and one below the orbit of the right eye and one on each earlobe. Electrode locations were adjusted using a CMS ultrasound digitizer (Zebris Medical, Isny, Germany) and impedances were kept below 10kΩ.
EEG data were filtered offline with a 20 Hz low‐pass filter (24 dB/oct) and re‐referenced to the linked ears. Ocular artefacts were corrected using Independent Component Analysis (Infomax ICA, Bell and Sejnovski,1995]. Following the ICA‐based correction, the EEG was segmented into a 1500 ms epoch for the long CSI and a 900 ms epoch for the short CSI, time‐locked to the cue and baseline‐corrected relative to the average of the 100 ms preceding the cue. Segments associated with incorrect responses were discarded, as were segments following errors. As exact repeats of stimuli from the previous trial may lead participants to shortcut processing on task‐repeat trials, trials containing immediate stimulus repetitions were also discarded. The resulting segments were visually inspected for muscle, drift and other non‐ocular artifact, as well as residual ocular artifact, and segments containing such artifacts removed. The remaining EEG segments were averaged for every participant and experimental condition.
Temporal Principal Components Analysis
To identify temporal components underlying the evolution of the ERP, we employed temporal principal components analysis (PCA) [Donchin and Heffley,1978]. PCA was run on the covariance matrix with time points as variables and subjects/electrodes/conditions as observations, eigenvalue ≥1 as component extraction criterion, and Varimax rotation of the solution to yield uncorrelated components. The extraction of uncorrelated components is especially useful in the current paradigm for separating stimulus‐related activity from pre‐stimulus preparatory activity that carries over into the post‐stimulus interval [e.g. Karayanidis et al.,2003]. Another virtue of the PCA is that it serves as a robust data‐driven procedure for determining the temporal ranges (‘windows’) to be subjected to statistical tests. Each PCA component is effectively a ‘virtual time‐window’, whose component scores—which represent the amplitude of the component in each subject/electrode/condition—can be submitted to statistical analysis, e.g. by means of ANOVA [for a similar PCA‐based approach to ERP analysis, see Weber and Lavric,2008, Wills et al.,2007, and in the task‐switching domain, Kieffaber and Hetrick,2005, Lavric et al.,2008]. Separate PCAs were run for the long and short CSI ERPs. Furthermore, in the long CSI separate PCAs were run for the pre‐ and post‐stimulus intervals (0–800 ms and 800–1500 ms), which allowed the separation of pre‐stimulus preparation‐related activity and its post‐stimulus carry‐over. This resulted in three PCA analyses—one for the short CSI and two for the long CSI.
To explore the interaction between task‐switching and valence in the long CSI, the two PCAs for this condition included data from subjects of the current (univalent) study and the Lavric et al. [2008] (bivalent) study. To enable direct statistical comparisons between the two studies, the data from the Lavric et al. study were re‐processed in exactly the same way as the present (univalent) data2. Prior to the statistical analysis by means of ANOVA, PCA component scores3 were averaged for 5 groups of electrodes in each hemisphere, ignoring the midline electrodes, to yield average scores for 5 regions on the left: anterior frontal (FP1, AF3, F1, F3, F5, F7), posterior frontal (FC1, FC3, FC5, C1, C3, C5), temporal (T7, TP7, CP5, P7), parietal (CP1, CP3, P1, P3, P5), and parieto‐occipital (PO1, PO3, PO7, O1), and the corresponding regions on the right. This grouping of electrodes has several useful features: it enables simultaneous and straightforward tests of anterior vs. posterior and left vs. right effects, while using over 86% of scalp electrodes, has good correspondence to gross brain anatomy, and increases the signal‐to‐noise ratio by spatial smoothing (the smoothing is relatively uniform due to similar group sizes). Region and hemisphere were factors in the ANOVA along with switch/repeat, cue type (word/picture), and (for the long CSI condition) stimulus valence (bivalent, univalent). Reliable interactions involving factors switch/repeat and valence were further investigated by separate ANOVAs for the univalent and bivalent data‐sets. Significance levels were adjusted using the Huynh‐Feldt correction for violations of sphericity (but unadjusted degrees of freedom are reported).
RESULTS
Behavioural Results
Mean correct RTs and error rates for each combination of switch/repeat, CSI and cue type are given in Table I. (As in the ERP analyses, trials preceded by errors and those associated with stimulus repetitions were discarded).
Table I.
Mean response times (ms) and error rates (%), with standard deviations in parentheses
| CSI and cue type | Trial type | Univalent | Bivalent | ||
|---|---|---|---|---|---|
| RT | Errors | RT | Errors | ||
| Short CSI Word | Switch | 646 (90) | 5.9 (7) | 914 (244) | 6.3 (4) |
| Repeat | 618 (76) | 3.4 (4) | 826 (208) | 3.3 (3) | |
| Switch cost | 28 (27) | 2.5 (5) | 88 (66) | 3.0 (4) | |
| Short CSI Picture | Switch | 632 (83) | 3.8 (4) | 1,056 (264) | 9.0 (5) |
| Repeat | 612 (72) | 4.0 (3) | 939 (241) | 4.2 (2) | |
| Switch cost | 20 (26) | −0.3 (2) | 117 (57) | 4.9 (4) | |
| Long CSI Word | Switch | 631 (84) | 2.9 (3) | 831 (223) | 5.8 (3) |
| Repeat | 602 (75) | 2.7 (3) | 780 (235) | 3.4 (2) | |
| Switch cost | 29 (16) | 0.3 (2) | 34 (49) | 2.4 (3) | |
| Long CSI Picture | Switch | 629 (96) | 3.8 (5) | 863 (254) | 8.8 (4) |
| Repeat | 602 (70) | 3.2 (2) | 793 (214) | 3.8 (2) | |
| Switch cost | 27 (33) | 0.7 (4) | 70 (81) | 5.0 (4) | |
For the present study with univalent stimuli, a switch (2) by cue (2) by CSI (2) by task (2) ANOVA on the RTs found a significant main effect of switch [F(1,15) = 45.59; P < 0.001], but there was no reliable reduction in RT switch cost between the short CSI (switch cost 24 ms) and the long CSI (28 ms)—i.e., no switch by CSI interaction [F(1,15) = 0.6; P = 0.45]. For error rates, the main effect of switch failed to reach significance over all trials [F = 3.13; P = 0.1], but was reliable for trials with word cues [F(1,15) = 4.8; P < 0.05]. There was a significant switch by task interaction [F(1,15) = 7.6; P < 0.05], reflecting greater switch cost in the colour task (colour 2.3%, shape 0.6%). There was no overall reliable reduction in the error switch cost at the longer CSI [switch × CSI, F(1,15) = 0.99; P = 0.33], however with word cues this reduction was marginally greater than with picture cues [switch × CSI × cue, F(1,15) = 3.29; P = 0.09]. Follow‐up pairwise tests found a reliable reduction in the error switch cost for word cue trials [t(15) = −1.89; P <0.05, one‐tailed], but not for picture cue trials [t(15) = 0.78; P = 0.23].
To compare the above data for univalent stimuli to the data for bivalent stimuli from Lavric et al. [2008], ANOVAs were run on RT and errors with an additional between‐experiment factor. The RT switch cost was reliably larger for bivalent stimuli (77.2 ms) than for univalent stimuli (25.5 ms) [F(1,30) = 18.03, P < 0.001; Fig. 2]. The reduction in switch cost from short to long CSI was reliably larger in the experiment with bivalent stimuli compared to the one with univalent stimuli, as revealed by the reliable interaction between CSI, switch and stimulus valence, [F(1,30) = 14.25, P < 0.01]. For bivalent stimuli, RT switch cost reduced reliably from 103 ms in the short to 52 ms in the long CSI [F(1,15) = 17.07, P < 0.01], while for univalent stimuli there was no such reduction, as indicated above.
Figure 2.
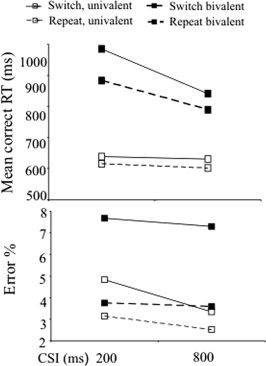
Response times and error rates in the two experiments.
The error switch cost was reliably larger for bivalent stimuli (3.8%) than for univalent stimuli (0.8%) [F(1,30) = 12.2, P < 0.01]. For errors there was no reliable three way interaction between CSI, switch and stimulus valence [F(1,30) = 0.41, P = 0.5].
ERP Results
The inspection of the ERP waveforms for the long CSI condition presented in Figures 3 (univalent stimuli) and 4 (bivalent stimuli) reveals considerable similarity between the switch‐repeat differences in the ERPs for the two data sets (henceforth for brevity referred to as ‘univalent ERPs’ and ‘bivalent ERPs’). In both sets of ERPs, one can see previously documented effects of task‐switching: the early anterior and late posterior positivities in the CSI and the protracted post‐stimulus negativity with a posterior maximum. However, some non‐trivial differences between the switch‐repeat contrasts in the two valences are also apparent (see also Figs. 1C and 1E for examples of switch‐repeat difference waves for the CSI and the stimulus interval, respectively). The switch‐induced posterior positivity late in the CSI and the post‐stimulus switch negativity seem both reduced in amplitude in the univalent ERPs; furthermore this reduction appears to be a function of cue type (e.g., the posterior positivity seems to be particularly strongly attenuated for the picture cue trials).
Figure 3.
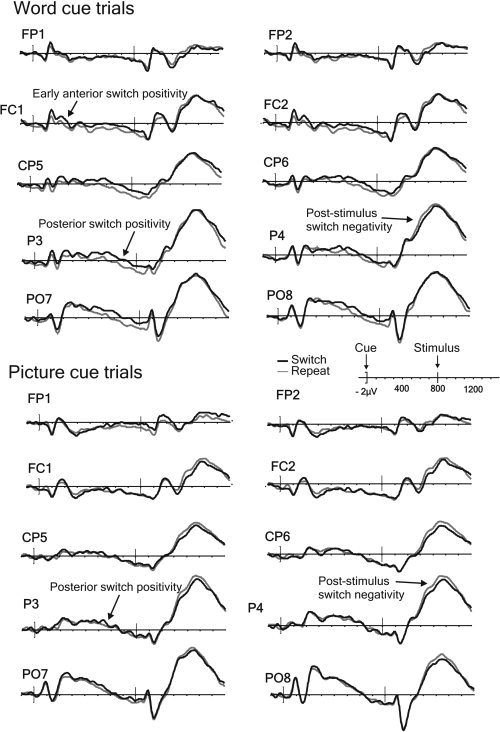
Long CSI ERPs from the univalent data‐set time‐locked to the word or the picture cue onset in a representative sample of electrodes. Arrows point to reliable switch vs. repeat differences. (See Fig. 4 for comparison with the bivalent ERPs.)
Figure 4.
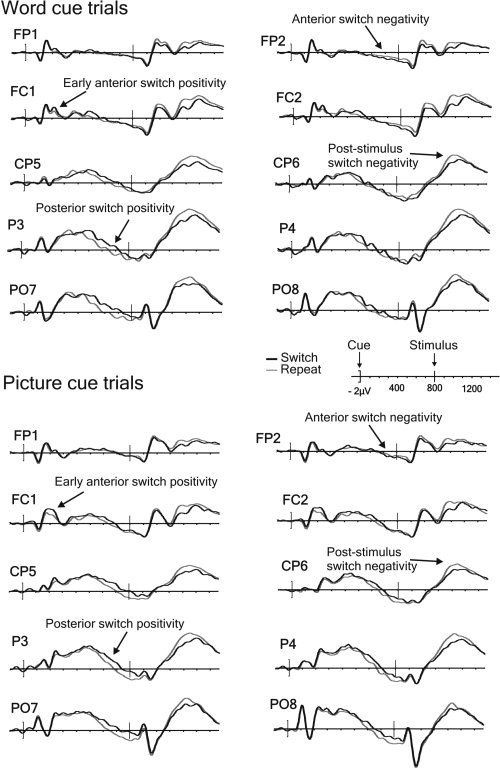
Long CSI ERPs from the bivalent data‐set [Lavric et al.,2008] time‐locked to the word or the picture cue onset in a representative sample of electrodes.
The inspection of the short CSI ERP waves for the univalent condition (see Fig. 7) also reveals familiar switch‐repeat differences. Between 450 ms and 600 ms following the cue onset, there is switch‐induced positivity in posterior electrodes, reminiscent of the positivity seen in the long CSI [cf. Nicholson et al.,2005; Lavric et al.,2008], followed by more negative voltages over the anterior‐central scalp for switches relative to repeats.
Figure 7.
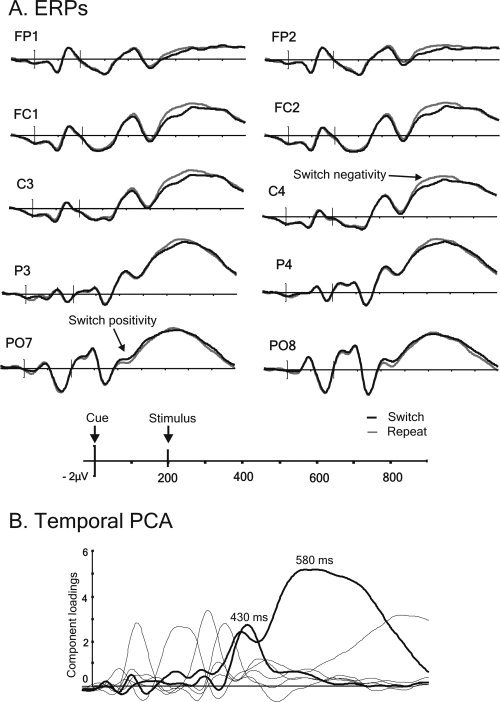
Short CSI ERPs from the univalent data‐set time‐locked to the cue (A) along with the PCA components (B); switch‐sensitive components are plotted using thicker lines.
We relate (but do not limit) our exposition of the PCA‐based analysis to the above switching effects.
Long CSI trials: pre‐stimulus ERPs
Early switch‐related positivity
Of the seven PCA components that explained over 3% variance (each), three were in the range of the early anterior switch‐related positivity (∼150–300 ms) (see Fig. 1B). The first, which peaked at ∼160 ms and explained 4.6% of the total variance of the ERP, showed a reliable main effect of switch [F(1,30) = 4.91, P < 0.05] and a reliable switch by region interaction [F(4,120) = 12.2, P < 0.01]. An inspection of the topography of the switch‐repeat difference in this component shows a broad positivity with a left anterior‐central maximum, which has greater amplitude in the univalent condition [switch by valence interaction, F(1,30) = 10.5, P < 0.01], though follow‐up ANOVAs found reliable effects of switching for both valences [univalent: main effect of switch, F(1,15) = 15.51, P < 0.01; switch by hemisphere interaction, F(1,15) = 8.21, P < 0.05; bivalent: switch by region interaction F(1,15) = 3.68, P < 0.05].
The second switch‐sensitive component (peak at ∼200 ms; 4.9% variance explained) showed a significant switch by region interaction [F(4,120) = 7.16, P < 0.05]. There were also two reliable interactions involving switch and valence (one marginal): switch by valence by region by hemisphere [F(4,120) = 2.75, P = 0.054], and switch by valence by cue type by region by hemisphere [F(4,120) = 3.0, P < 0.05]. Follow‐up ANOVAs found a significant switch by region interaction in the bivalent condition [F(4,60) = 10.98, P = 0.001], reflecting switch‐induced positivity over the anterior scalp, but no significant effects of switch in the univalent condition.
The third switch‐sensitive [main effect of switch, F(1,15) = 6.18, P < 0.05] PCA component in the ∼150–300 ms range, which peaked at 250 ms and explained 6.1% variance, showed two reliable interactions involving switch, valence and cue: switch by valence by cue type by hemisphere [F(1,15) = 4.21, P < 0.05]; and switch by valence by cue type by region by hemisphere [F(4,120) = 4.73, P < 0.01]. ANOVAs by valence revealed a main effect of switch in the bivalent ERPs [F(1,15) = 5.64, P < 0.05], reflecting switch‐related positivity with a left central maximum. In the univalent ERPs, a switch positivity with a similar topography was present for the word cue trials, but not for the picture cue trials—these showed a more posterior switch positivity [switch by cue type by region interaction, F(4,60) = 4.51, P < 0.05].
Late switch‐related positivity
In the late portion of the long CSI, PCA found a component which rose slowly from ∼450 ms until the end of the CSI (peaking at ∼700 ms), explained 23% of variance, and was amplified on switch trials [main effect of switch, F(1,30) = 6.51, P < 0.05; switch by region by hemisphere interaction, F(4,120) = 3.93, P < 0.01]. It seemed to capture the extensively reported switch‐induced posterior positivity accompanied (in the bivalent ERPs) by a switch‐related anterior negativity. To examine the relationship between the switch‐repeat difference in this PCA component and the behavioural index of anticipatory task‐set reconfiguration (the RISC effect), the two measures were correlated over participants. Specifically, we correlated the switch‐repeat difference in three scalp regions (two containing the maximum of the positivity: left parietal and left parieto‐occipital, and one containing the maximum of the anterior negativity: right frontal anterior) with the RISC effect in RT and errors (separately). To disentangle the effects of individual differences and those of the (between‐subjects) valence manipulation on the correlations, the latter were run separately for the uni‐ and bivalent data‐sets. There were statistically significant correlations between the RT RISC effect and the switch‐induced positivity in the bivalent data in both posterior regions under scrutiny [left parietal, r(14) = 0.77; P = 0.001; left parieto‐occipital, r(30) = 0.73; P = 0.001]4; no correlation approached significance in the univalent data (all Ps > 0.5) (see Fig. 5).
Figure 5.
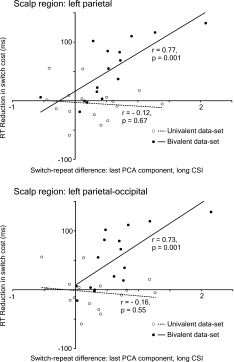
The correlation between the switch‐repeat difference in the scores of the PCA component that comprised the late posterior switch‐induced positivity in the CSI (peaking at ∼700 ms in Fig. 1B) and the reduction in the RT switch cost in the long CSI relative to the short CSI. The correlation (run separately for the bivalent and univalent data‐sets) is shown for the two posterior scalp regions where it was statistically significant in the bivalent data‐set.
This PCA component also showed reliable interactions: switch by valence by region [F(4,120) = 5.25, P < 0.01], reflecting differences in switch‐related effects between uni‐ and bivalent stimuli, and switch by valence by cue type [F(1,30) = 6.31, P < 0.05]. Follow‐up ANOVAs by valence found a significant switch by region interaction for the bivalent ERPs [F(4,60) = 17.0, P < 0.001]. In the univalent ERPs switch interacted reliably with hemisphere [F(1,30) = 5.31, P < 0.05], cue type [F(1,15) = 4.7, P < 0.05] and cue type and region [F(1,15) = 3.8, P < 0.05], the last two interactions reflecting greater positivity on switch trials following a word cue [main effect of switch, F(1,15) = 12.1, P < 0.01], but not following a picture cue (no effects of switch approached significance).
Analyses of RT distributions have previously reported smaller switch costs for trials with fast responses than for trials with slow responses [De Jong,2000; Nieuwenhuis and Monsell,2002; Lavric et al.,2008], suggesting more effective preparation on the ‘fast’ trials. In Lavric et al. [2008]; the bivalent data‐set) the late switch‐related positivity in the CSI followed this pattern: it was substantial for the fastest third of trials and statistically undetectable for the slowest third. We performed a similar analysis based on RT distributions for the univalent ERPs to determine whether the cue type associated with greater switch‐related posterior positivity and greater error RISC (word cue) would also show greater fast‐slow differentiation. An ANOVA with factors switch, cue type, fast‐slow (based on a 50% split‐ smaller quantiles could not be used due to low trial numbers in ERP averages) was run on the ERPs averaged within the 400–700 ms time‐window following cue onset5. Despite the relatively low contrast between fast and slow responses achievable with a 50% split, the analysis revealed robust fast vs. slow differentiation in the switch‐induced positivity only for word cues (see Fig. 6): the ANOVA interaction between switch, cue type and fast‐slow was marginally reliable [F(1,15) = 3.71, P = 0.07]; switch interacted reliably with fast‐slow for word cues [F(1,15) = 8.18, P < 0.05], but not for picture cues [F(1,15) = 0.3, ns].
Figure 6.
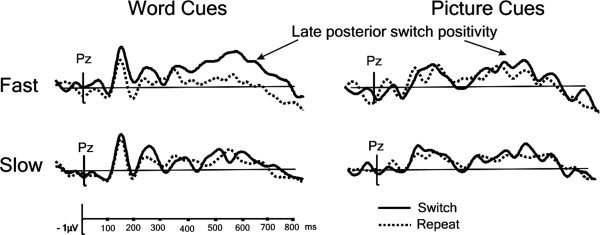
The grand‐average univalent ERPs corresponding to the trials with the fastest and slowest responses (50% split) presented by switch/repeat transition and word/picture cue.
Other switch‐sensitive components.
The PCA found two other switch‐sensitive components during the long CSI. One peaked at ∼310 ms, explained 12% of the variance and showed robust effects of switching [main effect of switch, F(1,30) = 5.48, P < 0.05; switch by cue by region, F(4,120) = 4.74, P < 0.01]. It also showed a reliable switch by valence by cue by region interaction [F(4,120) = 3.20, P < 0.05], reflecting a switch‐induced negativity, which was broadly distributed in the bivalent ERPs [main effect of switch, F(1,15) = 6.18, P < 0.05], whilst in the univalent ERPs the distribution reliably varied as a function of cue type [switch by cue by region, F(4,60) = 6.94, P < 0.01], with a posterior topography for the word cue and a broad central‐anterior distribution for the picture cue. A further switch‐sensitive (but not valence‐sensitive) PCA component seemed to capture the P3b wave in the ERP. Given its broad time‐course with a maximum at ∼460 ms, its topography (pooled over switch and repeat) showing the characteristic P3b parietal maximum and the fact that it explained a very large proportion of the overall ERP variance (31%). This component showed reliably more positive amplitudes for switch than repeat [F(1,30) = 4.86, P < 0.05], mainly due to word cue trials [switch by cue, F(1,30) = 5.25, P < 0.05; main effect of switch for the word cue [F(1,15) = 7.87, P < 0.01], whilst no effect of switch was found for the picture cue.
To summarise the effects of a task switch and their interaction with stimulus valence during the long CSI
a. Three components seemed to capture the early anterior switch‐related positivity observable in the ERP waveforms. At its onset (the first of these PCA components, peaking at 160 ms post cue onset) this positivity seemed to be greater in the univalent ERPs, whereas in the component peaking at ∼200 ms it was only reliable in the bivalent ERPs, suggesting an earlier onset in the univalent condition. In the component peaking at ∼250 ms, a switch‐related positivity (with a more central distribution) was present in the bivalent ERPs, whereas in the univalent ERPs it was present only following word cues; picture cues were associated with an entirely different topography.
b. A marked differentiation between switch‐related activity following word and picture cues in the univalent condition persisted for the rest of the CSI and was particularly evident in the PCA component that captured the late posterior switch‐related positivity. The extra positivity on switch trials reaching a maximum shortly before stimulus onset (∼700 ms into the CSI), correlating over subjects with the RT RISC effect for the bivalent experiment, was not reliably detected in the univalent ERPs for the picture cue, and was overall more robust in the bivalent ERPs than in the univalent ERPs. The late positivity on a switch trial was, in the present univalent experiment, clearly detectable only for the trials with the fastest responses containing a word cue.
Long CSI trials: post‐stimulus ERPs
Overspill of the preparation‐related switch positivity
The first of five PCA components that explained over 3% of the variance in the stimulus interval had high loadings immediately after stimulus onset and peaked at 90 ms (Fig. 1D). It explained 11% of variance, was greater on switch trials [main effect of switch, F(1,30) = 7.83, P < 0.05; switch by hemisphere, F(1,30) = 18.35, P < 0.01; switch by region, F(4,120) = 12.75, P < 0.01; and switch by hemisphere by region, F(4,120) = 4.41, P < 0.05], and showed a switch‐repeat difference that was topographically very similar to the switch‐induced positivity observed late in the CSI (Fig. 1B). We assume it reflects extra activity associated with preparation on switch trials continuing through early stages of stimulus processing. A switch by cue type by valence interaction [F(1,30) = 6.13, P < 0.05] in this component suggested divergent patterns for the cues in the two valence conditions, but separate ANOVAs for the uni‐ and bivalent data sets found no effects of cue type [bivalent: switch by region, F(4,60) = 7.3, P = 0.003; switch by hemisphere, F(1,15) = 11.61, P = 0.004; univalent: main effect of switch, F(1,15) = 7.96, P = 0.013; switch by region, F(4,60) = 5.87, P = 0.01; switch by hemisphere, F(1,15) = 7.75, P = 0.014; switch by region by hemisphere, F(4,60) = 2.89, P = 0.049].
The post‐stimulus switch‐induced negativity
There were four switch‐sensitive PCA components (Fig. 1D) in the range of the switch‐repeat differential negativity that can be seen in the ERP at ∼200–600 ms (Fig. 1E). The first peaked at ∼190 ms, explained 7% of the variance and was sensitive to interactions involving switch and valence [switch by valence, F(1,30) = 8.58, P < 0.01; switch by region by valence, F(4,60) = 5.83, P < 0.01]. Separate ANOVAs by valence revealed a reliable switch‐related right central‐anterior negativity in the bivalent ERPs [main effect of switch, F(1,15) = 5.34, P < 0.05], whilst in the univalent condition the switch‐induced positivity was not confirmed by any reliable effect involving switch.
The second switch‐sensitive component, which peaked at 270 ms and explained 9% of the variance, showed a reliable interaction between switch, region and valence [F(4,120) = 3.52, P < 0.05]. Follow‐up ANOVAs by valence found a reliable interaction between switch and hemisphere in the bivalent condition [F(1,30) = 4.45, P = 0.05] indicative of a switch‐induced left‐posterior negativity accompanied by a switch‐related right anterior positivity, and no reliable effects of switching in the univalent condition.
The next component, which explained 42.5% of the variance and was maximal at ∼440 ms, seemed to capture the P3b peak in the ERP waveform. No interactions involving switch and valence were significant for this component, which showed a robust switch‐repeat difference with a central posterior negative topography [main effect of switch, F(1,30) = 42.55, P < 0.001; switch by region interaction, F(4,120) = 7.91, P < 0.01].
The last PCA component in the stimulus interval rose from ∼400 ms (peaking at ∼600 ms) and explained 23% of the variance. It was sensitive to switching [main effect of switch, F(1,30) = 5.37, P < 0.05; switch by region by hemisphere interaction, F(1,30) = 2.85, P < 0.05] and its interaction with valence [switch by valence by hemisphere, F(1,30) = 5.65, P < 0.05], reflecting a topographic difference between the switching effects in the two valences: in the univalent ERPs there was a broad positivity for switch with a posterior maximum [F(1,15) = 6.34, P < 0.05], whereas in the bivalent ERPs, there was a very circumscribed right frontal positivity [switch by region by hemisphere, F(4,60) = 3.37, P < 0.05].
To summarise the post‐stimulus analyses, the PCA separated the spill‐over of the preparation‐related switch‐repeat differences originating in the CSI (the posterior positivity) from other effects in the stimulus interval. The modulation of the magnitude of the switch‐repeat negativity by stimulus valence (greater for bivalent stimuli, Fig. 1E) arose from the summation of at least three temporally overlapping but topographically distinct effects: greater anterior‐central (at ∼190 ms) and left posterior (at ∼270 ms) negativities in the bivalent condition, followed (at ∼400–700 ms) by greater posterior positivity for the univalent condition.
Short CSI trials: post‐stimulus ERPs
Two of the seven components extracted in the short CSI were sensitive to switching (Fig. 7). The first peaked at ∼430 ms after cue onset, explained 4% of the variance, and comprised a switch‐related posterior positivity [switch by region, F(1,30) = 11.22, P < 0.01; switch by hemisphere, F(1,30) = 10.65, P < 0.01]. The second reached maximum at 500–750 ms following cue onset (300–500 ms following stimulus onset), explained 51% of the variance, and comprised a broad switch‐induced negativity with a central maximum [main effect of switch, F(1,30) = 23.22, P < 0.001].
DISCUSSION
The exclusive use of univalent stimuli in the present experiment enabled us to examine preparation for a switch in circumstances when the stimulus itself cues the task and uniquely specifies the response and the processing of the task cue is optional, to compare it to preparation for bivalent stimuli for which processing of the cue is essential, and to compare the impact of verbal and pictorial task cues in these two conditions.
With bivalent stimuli, Lavric et al. [2008] reported an early switch‐induced positivity around 150–300 ms following the cue (see Fig. 4). Rushworth et al. [2002] observed a similar component following a cue indicating reversal of a simple binary response mapping (red/green to left/right), with onset at about 250–300 ms and dipole sources in medial frontal cortex [see also Rushworth et al.,2005]. They suggested that it could reflect the initiation of response re‐mapping by the prefrontal cortex. Two features of the switch‐repeat difference in our bivalent experiment (Lavric et al.,2008) suggested that it may reflect an early registration of the need to reconfigure task‐set rather than the process itself. First, its latency was short. Second, its magnitude, unlike that of the later posterior positivity, did not appear to be related to the efficacy of preparation, being present at equal magnitude in trials with good preparation (fast responses and small switch costs) and in less well‐prepared trials (slow responses and large switch costs).
The early switch‐related positivity observed in the present experiment (see Fig. 3) with essentially identical cues, but univalent stimuli, differed in two respects from that found by Lavric et al. with bivalent stimuli. First, it seemed to emerge earlier (Fig. 1C): the earliest switch‐sensitive PCA component (maximal at ∼160 ms) showed greater effects of switching in the univalent condition than in the bivalent condition, and this changed in the following PCA component, peaking at ∼200 ms, which showed reliable switch effects only in the bivalent condition. Second, at ∼250 ms into the CSI univalent word cue ERPs contained a switch‐induced positivity topographically similar to that in the bivalent ERPs, while picture cue trials showed a topographically distinct effect of switch (Fig. 1B). If one views the early positivity with an anterior‐central scalp distribution as representing an early ‘need to switch’ signal, the present data suggest that when there is less incentive to process the cue, word cues elicit a more robust and/or automatic ‘need to switch’ signal than picture cues.
With univalent stimuli, the cue was redundant. This reduced (see Fig. 3) the late switch‐induced posterior positivity, seen 500–800 ms after the cue on trials with bivalent stimuli (see Fig. 4), which we [Lavric et al.,2008] and others [e.g. Karayanidis et al.,2003; Nicholson et al.,2005] have interpreted as a correlate of advance task‐set reconfiguration. The difference was especially marked with picture cues. With univalent stimuli, the ERPs following word cues contained a reliable switch‐induced positivity at about 450‐800 ms resembling the effect with bivalent stimuli, both in scalp distribution and in having a substantial amplitude only for the ‐ presumably better‐prepared ‐ trials with fast responses (see Fig. 6). However, for picture cues the late positivity was barely visible, nor was it statistically reliable in the univalent ERPs (Figs. 1B, 1C and 3), nor did it show any evidence of differentiation between fast‐ and slow‐response trials (see Fig. 6). The behavioural data from the present study also suggested relatively ineffective preparation for a change of task, with no reduction in RT switch cost at the longer CSI, and only a modest reduction in the error cost (Fig. 2) limited to word cues. The evidence of a correlation between the behavioural (RT RISC) and neurophysiological (switch‐repeat difference in the PCA component that comprised the posterior switch positivity) indices of preparation over individuals was also confined to the bivalent data (see Fig. 5).
The above effects of cue type are consistent with the claim that a verbal cue naming the dimension to be processed is more effective in eliciting task‐set reconfiguration than a cue that explicitly illustrates the stimulus values to be discriminated, perhaps because the former maps directly on to a linguistic self‐instruction, which in turn facilitates task‐set preparation [Goschke,2000; Miyake et al.,2004]. Both early and late ERP positivities, and the error rate data, indicate that word cues are more effective in triggering preparation for a switch than picture cues even when the cue is redundant, suggesting that processing of these verbal cues is relatively automatic, perhaps triggering a shift of attention to the named perceptual dimension regardless of intention. This automatic processing of a verbal cue may lead to extra demands when little time is given before the stimulus (short CSI), but results in benefits when the time provided for preparation is adequate (long CSI): the error switch cost was larger on word than on picture‐cued trials at the short CSI but smaller at the long CSI (Table I).
Astle et al. [2008b] also examined the effect of different kinds of task cues, contrasting spatial and non‐spatial cues. Using identical stimuli (letters presented in one of two locations and one of two colours) in two groups of participants, task was consistently associated either with a stimulus location (e.g. classify a letter on the left as vowel/consonant, on the right as upper‐/lowercase) or with a colour of the letter. In addition the symbols ‘ = ’ and ‘< >’ were used as advance cues to repeat and switch tasks, respectively. Because they found a posterior switch positivity and an anterior switch negativity in the CSI only when the tasks were distinguished by colour, but not by location, Astle et al. argued that the posterior positivity and anterior negativity may not be markers of an obligatory preparatory process. However, Nicholson et al. [2005], who also cued their tasks by location (left‐right or up‐down), found a robust posterior switch positivity in their long CSI conditions, despite using explicit spatial cues (though note that each task was associated with two locations in Nicholson et al.'s paradigm and only one location in Astle et al.'s design, and that locations were much closer in the former than the latter).
Two other aspects of the pre‐stimulus ERPs merit comment. First, Lavric et al. [2008] found the late positivity to be accompanied by an anterior negativity (Fig. 4) and their PCA analysis found one component that explained the variance in both the posterior positivity and the anterior negativity [Lavric et al.,2008]. In the present study we observed no switch‐related anterior negativity (Figs. 1B and 3), possibly because the intra‐cerebral generators of the switch‐repeat difference in this component were somewhat more anterior in the univalent data‐set. However, we do not suggest that the absence of an anterior negativity in the univalent data is an index of reduced task‐set competition [cf. Mueller et al.,2007] or reduced preparation, because we have recently observed preparation‐related posterior positivities not accompanied by anterior negativities in ERP data from bivalent stimuli and accompanied by robust behavioural indices of preparation [Elchlepp et al.,2009].
Second, previous temporal PCA analyses of ERPs acquired in long CSIs reached divergent conclusions concerning the sensitivity of the P3b peak to switching. Kieffaber and Hetrick [2005] reported effects of switching in the PCA component that corresponded to P3b, but we found no such effects in our previous PCA analysis of the bivalent ERPs [Lavric et al.,2008]. In the present analysis, using data from both bivalent and univalent studies (and more EEG segments/trials from the former, due to a different artifact correction procedure), we did find that switching modestly but reliably amplified the PCA component likely to correspond to P3b, albeit only for word cues; this effect did not interact reliably with valence. P3b is known to be sensitive to a variety of factors/manipulations, including probability, subjective salience, task difficulty, etc.)‐ all or any of these could be responsible for the modulation of P3b here. Neverthetheless, we can reiterate our previous conclusion that the substantial index of differential task‐set preparation on task‐switch and ‐repeat trials is not P3b but the later, more protracted component, which is probably time‐locked to the expected stimulus onset rather than cue onset.
Stimulus Valence and the Post‐Stimulus Switch‐Repeat Negativity
The other aim of the present investigation was to clarify the functional significance of the switch‐induced ERP negativity seen after the stimulus in task‐switching paradigms. We [Lavric et al.,2008] have suggested that this negativity may reflect greater conflict on switch trials due to task‐set ‘inertia’ from the previous trial [Allport et al.,1994; Yeung et al.,2006; Yeung and Monsell,2003]. With bivalent stimuli, extra conflict on switch trials, due to persistence/re‐activation of the competing task‐set, especially on task‐switch trials, can happen both at task level and response level. (The presence of task‐level conflict explains the longer RT even for congruent bivalent than for univalent stimuli when bivalent and univalent stimuli are mixed; see Aron et al.,2004; Rogers and Monsell,1995; Steinhauser and Hübner,2009]. For the univalent stimuli used in the present study, both colour and form were present in all stimuli and relevant on half the trials, affording the possibility of attending to the wrong dimension (or other forms of task‐level conflict), but there should be no conflict at the response level, as each colour or form was associated with only one response. We therefore expected a reduction in both the switch cost and the post‐stimulus switch‐induced negativity.
As in previous studies [Rogers and Monsell,1995; Karayanidis et al.,2003], both RT and error switch cost were greater for bivalent than for univalent stimuli (Fig. 1B and Table I). Inspection of the ERP waveforms shows that, although the use of univalent stimuli did indeed reduce the post‐stimulus switch‐related negativity (Fig. 1E), the negativity was not eliminated (Figs. 1D, 1E and 2). Indeed, the largest (in terms of variance explained) post‐stimulus PCA component (peaking at ∼440 ms, Fig. 1D) showed robust switch‐related negativity in both bivalent and univalent ERPs and no statistically detectable difference between the two valence conditions (though the negativity was numerically larger in the bivalent ERPs). As we have already noted, because our univalent stimuli had values on both dimensions, they afforded task‐set (but not response) conflict: i.e. the irrelevant dimension attracting attention especially when it was relevant on the previous trial. In addition (or instead of) conflict at the level of attentional selection, task‐set conflict may have arisen on univalent trials from the activation of the conflicting ‘task‐goal’ representation [typically implemented in the computational models of task‐switching as ‘task units’, e.g., Brown et al.,2007]. The presence of the competing stimulus dimension may lead to greater activation of the competing ‘task units’ on switch trials than on repeat trials.
However, other post‐stimulus PCA components did show reliable switch by valence interactions. It is important that the PCA effectively isolated the protrusion of the preparation‐related switch positivity into the stimulus interval (which occurred for both valence conditions). Hence the post‐200 ms univalent vs. bivalent differences did not result from this overspill, but from (weighted) summation of the switch effects in three subsequent PCA components (Fig. 1D). Two of them contained switch‐related effects in the early part of the stimulus interval, significant only for bivalent stimuli: broad anterior‐central negativity maximal at ∼190 ms and left posterior negativity coupled with right anterior positivity peaking at ∼270 ms. The third was a longer‐latency, more protracted (∼400–700 ms) effect that represented a switch‐related, spatially widespread positivity with a posterior maximum for the univalent trials and a confined frontal positivity for the bivalent trials.
That the earlier effects were reliable only following bivalent stimuli might reflect the presence of response conflict for these but not the univalent stimuli. However, given their short latency, it seems more likely that the greater magnitude of these negativities in the bivalent ERPs reflects greater attentional conflict in the bivalent condition. Both bivalent and univalent stimuli had a value on the irrelevant dimension. For example on shape task trials, the univalent stimuli were black, and the bivalent stimuli were red, blue, orange, or green (the four values requiring discrimination for, and associated with responses in, the colour task). As well as not being associated with a response, the colour black was also not associated with (the goal of) attending to and discriminating colour. A consequent weaker attentional “pull” of black versus the other colours may be what is reflected in the weaker early ERP switch‐related negativities for univalent stimuli (particularly the posterior negativity at ∼270 ms post‐stimulus onset), rather than the lack of response conflict. (The same argument applies, mutatis mutandis, to the shape values). Turning to the late post‐stimulus effect (switch‐related posterior positivity at ∼600 ms, Fig. 1D), this could be interpreted as a more‐lasting persistence of switch related negativity with bivalent stimuli, consistent with a contribution of response conflict to the negativity. But effects this late are hard to interpret because of the large difference in mean RT between univalent (∼620 ms) and bivalent (∼820 ms) conditions in the CSI condition under scrutiny (long CSI).
Further Considerations and Conclusions
The contrast between bivalent and univalent stimulus conditions achieved here has its limitations. The cues and stimuli employed in the univalent and bivalent conditions were not exactly equivalent, and the RTs were very different. This was hardly a concern for the CSI (pre‐stimulus) analysis: three of the four cues were identical in the two experiments and one (the picture cue for the shape task) was different only in one of four shapes it contained in the collage (Fig. 1A). The stimuli in the two experiments also differed somewhat, due to the need to add an extra colour and shape, and to reduce the total number of stimuli, to achieve univalent stimuli while keeping the S‐R rules identical. However, it is unlikely that physical differences affected the switch vs. repeat contrast and its interaction with valence, because the same physical differences were present in both switch and repeat conditions. Furthermore, one would expect differences in perceptual processing or discriminability to manifest themselves in early ‘exogenous’ ERP components, such as P1 and N1.
A potentially more serious issue is that the differences found in preparation for a task switch before the stimulus in the two experiments complicates the interpretation of the ERP effects following the stimulus (the switch‐related negativity). There would seem to be two possibilities. First, one may argue that the reduced post‐stimulus negativity in the univalent ERPs relative to the bivalent ERPs might have been a direct marker of sub‐optimal/delayed task‐set preparation in the former. If this were the case, one would have expected greater reduction in the negativity on the trials for which there is little/no evidence of preparation (the picture cue trials), relative to those for which there is some evidence of preparation (the word cue trials). However, the comparison of the word and picture trials is not consistent with this interpretation: if anything the post‐stimulus negativity was larger for picture cue trials, though not reliably so (see Fig. 3). Importantly, the temporal PCA effectively disentangled the components of the ERP signal containing the overspill of the pre‐stimulus switch positivity from subsequent components containing effects of switching. That the overspill could not cause (or inflate) the subsequent switch by valence interactions is indicated by the fact that the effects of switching did not differentiate between the two valence conditions in the component capturing the overspill (Fig. 1D).
Second, it can plausibly be argued that sub‐optimal task‐set preparation in the univalent condition may have led to greater susceptibility to task‐conflict after the stimulus. This interpretation, moreover, is entirely consistent with the claim that the attenuation in the ERP negativity in the present experiment is a manifestation of reduced conflict. It merely raises the possibility that we may have underestimated the reduction in conflict achievable by contrasting univalent to bivalent stimuli.
In conclusion, the univalent vs. bivalent contrast provides new insights into the electrophysiological correlates and mechanisms of task‐set control. Both early and late switch‐related ERP positivities during the preparation interval were modulated in a way consistent with their association with detection of, and preparation for, a task switch. The evidence for differential processing of word and picture cues supports the claim that a verbal cue naming the relevant dimension task has a special efficacy in eliciting advance task‐set reconfiguration, and adds evidence that such a cue triggers attention to the relevant dimension relatively automatically. The modulation of early (∼200–300 ms) post‐stimulus switching negativities by valence suggests an attentional locus of the valence effects, possibly due to attention being attracted by attribute values associated with the need to discriminate in the bivalent condition. The later residual switch‐related negativity following univalent stimuli (which afford no responses in the competing task) points to additional task‐level conflict in the univalent condition.
Footnotes
The exceptions are rare and, so far, problematic. Verbruggen et al. [2007] claimed that displaying the cue only briefly forced people to use it early and hence largely overcome the switch cost. However, they used only one cue per task. The resulting confound between cue and task change means that we cannot be sure there was a robust task‐switch cost to eliminate [see Monsell and Mizon, 2006]. In our laboratory, using brief cues (two per task) with other tasks and stimuli has not eliminated the switch cost. Lien et al. [2005] found no residual cost for the first of 3 S‐R pairs in a left‐right sequence, but Monsell and Mizon [2005, Exps 4 and 5) did for all 4 S‐R pairs in a left‐right sequence. Meanwhile, efforts at eliminating the residual cost with strong incentives to prepare have failed [Nieuwenhuis and Monsell, 2002].
This included off‐line filtering, re‐referencing, artifact correction and rejection, and ERP segmentation. Immediate stimulus repetitions were excluded as it was done in the univalent data‐set. The EEG set‐up and acquisition parameters (EEG hardware, electrode number and positioning, on‐line filtering, sampling rate and A/D conversion) were identical to those used by Lavric et al. [2008].
Only components explaining at least 3% of the overall ERP variance were subjected to statistical analysis. This cut‐off was chosen on the basis of prior experience with PCA‐based analyses of ERP data in our laboratory.
In our previous analysis of the bivalent experiment [Lavric et al., 2008], the correlation between RISC and the switch‐repeat difference in the ERP was reliable for the right anterior scalp region and the correlations in the left posterior regions, though substantial, did not reach significance. However, there are important differences between the previous and present correlational analyses: Lavric et al. [2008] correlated the ERP data with RISC computed on the composite measure including RT and errors, whereas here separate correlations were run for RT and error RISC; in the present analysis the ERPs were locally referenced, whereas Lavric et al. correlated RISC with effects in average‐referenced ERPs, which may affect the scalp region where the correlations are manifested.
The fast‐slow analysis was not based on PCA component scores, because the original PCA was run on ERP averages (meaning that PCA component scores were not available for each ERP trial) and it did not include ‘fast vs. slow’ as a condition in the PCA matrix. To avoid duplication we decided not to run another PCA only to include ‘fast vs. slow’ as a condition.
REFERENCES
- Allport DA, Styles EA, Hsieh S ( 1994): Shifting intentional set: Exploring the dynamic control of tasks In: Umiltà C, Moscovitch M, editors. Attention and Performance XV: Conscious and Nonconscious Information Processing. Cambridge, MA: MIT Press; pp 421–452. [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW ( 2004): A componential analysis of task‐switching deficits associated with lesions of left and right frontal cortex. Brain 127: 1561–1573. [DOI] [PubMed] [Google Scholar]
- Astle DE, Jackson GM, Swainson R ( 2006): Dissociating neural indices of dynamic cognitive control in advance task‐set preparation: An ERP study of task switching. Brain Res 1125: 94–103. [DOI] [PubMed] [Google Scholar]
- Astle DE, Jackson GM, Swainson R ( 2008a): Fractionating the cognitive control required to bring about a change in task: A dense‐sensor event‐related potential study. J Cogn Neurosci 20: 255–267. [DOI] [PubMed] [Google Scholar]
- Astle DE, Jackson GM, Swainson R ( 2008b): The role of spatial information in advance task‐set control: An event‐related potential study. Eur J Neurosci 28: 1404–1418. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ ( 1995): An information‐maximation approach to blind separation and blind deconvolution. Neural Comput 7: 1129–1159. [DOI] [PubMed] [Google Scholar]
- Bregadze N, Lavric A ( 2006): ERP differences with vs. without concurrent fMRI. Int J Psychophysiol 62: 54–59. [DOI] [PubMed] [Google Scholar]
- Brown, JW , Reynolds, JR , Braver, TS ( 2007): A computational model of fractionated conflict‐control mechanisms in task‐switching. Cognit Psychol 55: 37–85. [DOI] [PubMed] [Google Scholar]
- De Jong R ( 2000): An intention‐activation account of residual switch costs In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press; pp 357–376. [Google Scholar]
- Donchin E, Heffley EF ( 1978): Multivariate analysis of event‐related potential data: a tutorial review. In Otto D, editors. Multidisciplinary Perspectives in Event‐Related Brain Potential Research. Washington, DC: Government Printing Offic; pp 555–572. [Google Scholar]
- Elchlepp H, Monsell S, Lavric A ( 2009): Partitioning switch cost with ERP. Meeting of the European Society of Cognitive Psychology (ESCOP), Krakow. [Google Scholar]
- Goschke T ( 2000): Intentional reconfiguration and involuntary persistence in task set switching In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press; pp 331–355. [Google Scholar]
- Karayanidis F, Coltheart M, Michie PT, Murphy K ( 2003): Electrophysiological correlates of anticipatory and poststimulus components of task switching. Psychophysiol 40: 329–348. [DOI] [PubMed] [Google Scholar]
- Kieffaber PD, Hetrick WP ( 2005): Event‐related potential correlates of task switching and switch costs. Psychophysiol 42: 56–71. [DOI] [PubMed] [Google Scholar]
- Lavric A, Pizzigalli D, Forstmeier S ( 2004): When ‘go’ and ‘nogo’ are equally frequent: ERP components and cortical tomography. Eur J Neurosci 20: 2483–2488. [DOI] [PubMed] [Google Scholar]
- Lavric A, Mizon GA, Monsell S ( 2008): Neurophysiological signature of effective anticipatory task‐set control: A task‐switching investigation. Eur J Neurosci 28: 1016–1029. [DOI] [PubMed] [Google Scholar]
- Lien M‐C, Ruthruff E, Remington RW, Johnston JC ( 2005): On the limits of advance preparation for a task switch: Do people prepare all the task some of the time or some of the task all the time? J Exp Psychol Hum Percept Perform 31: 299–315. [DOI] [PubMed] [Google Scholar]
- Logan GD, Bundesen C ( 2003): Clever homunculus: Is there an endogenous act of control in the explicit task‐cuing procedure? J Exp Psychol Hum Percept Perform 29: 575–599. [DOI] [PubMed] [Google Scholar]
- Meiran N ( 1996): Reconfiguration of processing mode prior to task performance. J Exp Psychol Learn Mem Cogn 22: 1423–1442. [Google Scholar]
- Meiran N, Chorev Z, Sapir A ( 2000): Component processes in task switching. Cognit Psychol 41: 211–253. [DOI] [PubMed] [Google Scholar]
- Miyake A, Emerson MJ, Padilla F, Ahn JC ( 2004): Inner speech as a retrieval aid for task goals: The effects of cue type and articulatory suppression in the random task cuing paradigm. Acta Psychol 115: 123–142. [DOI] [PubMed] [Google Scholar]
- Monsell S, Mizon GA ( 2006): Can the task‐cueing paradigm measure “endogenous” task‐set reconfiguration? J Exp Psychol Hum Percept Perform 32: 493–516. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Swainson S, Jackson GM ( 2007): Neurophysiological correlates of switch‐related activity in bivalent but not univalent response conditions in task switching. Brain Res 1157: 56–65. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes G ( 2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R, Karayanidis F, Poboka D, Heathcote A, Michie PT ( 2005): Electrophysiological correlates of anticipatory task‐switching processes. Psychophysiol 42: 540–554. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Monsell S ( 2002): Residual costs in task switching: Testing the failure‐to‐engage hypothesis. Psychon Bull Rev 9: 86–92. [DOI] [PubMed] [Google Scholar]
- Poulson C, Luu P, Davey C, Tucker DM ( 2005): Dynamics of task‐sets: Evidence from dense‐array event‐related potentials. Cogn Brain Res 24: 133–154. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S ( 1995): The costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen 124: 207–231. [Google Scholar]
- Rushworth MFS, Passingham RE, Nobre AC ( 2002): Components of switching intentional set. J Cogn Neurosci 14: 1139–1150. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Passingham RE, Nobre AC ( 2005): Components of attentional set‐switching. Exper Psychol 52: 83–98. [DOI] [PubMed] [Google Scholar]
- Steinhauser, M , Hübner R ( 2009): Distinguishing response conflict and task conflict in the Stroop task. J Exp Psychol Hum Percept Perform 35: 1398–1412. [DOI] [PubMed] [Google Scholar]
- Stroop JR ( 1935): Studies of interference in serial verbal reactions. J Exp Psychol 12: 643–662. [Google Scholar]
- Swainson R, Jackson SR, Jackson GM ( 2006): Using advance information in dynamic cognitive control: An ERP study of task‐switching. Brain Res 1105: 61–72. [DOI] [PubMed] [Google Scholar]
- Tieges Z, Snel J, Kok A, Wijnen JG, Lorist MM, Ridderinkhof KR ( 2006): Caffeine improves anticipatory processes in task switching. Biol Psychol 73: 101–113. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Liefooghe B, Vandierendonck A, Demanet J ( 2007): Short cue presentations encourage advance task preparation: A recipe to diminish the residual switch cost. J Exp Psychol Learn Mem Cogn 33: 342–356. [DOI] [PubMed] [Google Scholar]
- Weber K, Lavric A ( 2008): Syntactic anomaly elicits a lexico‐semantic (N400) ERP effect in the second but not in the first language. Psychophysiol 45: 920–925. [DOI] [PubMed] [Google Scholar]
- West R ( 2003): Neural correlates of cognitive control and conflict detection in the Stroop and digit‐location tasks. Neuropsychologia 41: 1122–1135. [DOI] [PubMed] [Google Scholar]
- Wills AJ, Lavric A, Croft GS, Hodgson TL ( 2007): Predictive learning, prediction errors, and attention: Evidence from event‐related potentials and eye tracking. J Cogn Neurosci 19: 843–854. [DOI] [PubMed] [Google Scholar]
- Yeung N, Monsell S ( 2003): Switching between tasks of unequal familiarity: The role of stimulus‐attribute and response‐set selection. J Exp Psychol Hum Percept Perform 29: 455–469. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD ( 2006): Between‐task competition and cognitive control in task switching. J Neurosci 26: 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszak F, Hommel B, Allport A ( 2003): Task‐switching and long term priming: Role of episodic S‐R bindings in task‐shift costs. Cognit Psychol 46: 361–413. [DOI] [PubMed] [Google Scholar]


