Abstract
Although children with heavy prenatal alcohol exposure may exhibit the distinctive facial dysmorphology seen in full or partial fetal alcohol syndrome (FAS/PFAS), many lack that dysmorphology. This study examined the functional organization of working memory in the brain in three groups of children—those meeting diagnostic criteria for FAS or PFAS, heavily exposed (HE) nonsyndromal children, and healthy controls. A verbal n‐back task (1‐back and 0‐back) was administered to 47 children (17 with FAS/PFAS, 13 HE, and 17 controls) during fMRI. Intra‐group one‐sample t‐tests were used to identify activity regions of interest central to verbal working memory including the dorsal prefrontal cortex (dPFC), inferior frontal gyrus, caudate/putamen, parietal cortex, and cerebellar Crus I/lobule VI and lobule VIIB‐IX. Whereas groups did not differ in task sensitivity, fMRI analyses suggested different patterns of sub‐network recruitment across groups. Controls primarily recruited left inferior frontal gyrus (Broca's area). By contrast, HE primarily recruited an extensive set of fronto‐striatal regions, including left dPFC and left caudate, and the FAS/PFAS group relied primarily on two cerebellar subregions and parietal cortex. This study is, to our knowledge, the first to demonstrate differential recruitment of critical brain regions that subserve basic function in children with different fetal alcohol spectrum disorders compared to controls. The distinct activation patterns seen in the two exposed groups may be related to substantial differences in alcohol dose/occasion to which these groups were exposed in utero. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: working memory, fetal alcohol spectrum disorder, fetal alcohol syndrome, prenatal alcohol exposure, fMRI
INTRODUCTION
Studies spanning four decades have identified a broad range of cognitive and behavioral deficits in children with fetal alcohol spectrum disorders (FASD). Fetal alcohol syndrome (FAS), the most severe form of FASD, is characterized by distinctive craniofacial dysmorphology (short palpebral fissures, thin upper lip, flat, or smooth philtrum), small head circumference, and growth retardation [Stratton et al.,1996]. Partial FAS (PFAS) is diagnosed when there is a history of heavy maternal drinking during pregnancy, the presence of two of the three principal alcohol‐related facial anomalies, and small head circumference, growth retardation, or cognitive and/ or behavioral dysfunction [Hoyme et al.,2005]. Other heavily exposed children lack the distinctive facial anomalies but exhibit cognitive impairment in the same domains as syndromal children that is generally somewhat less severe [Coles et al.,1997; Dodge et al.,2009; Jacobson et al.,2008; Mattson et al.,1998]. In one of the few studies to compare regional brain volumes among syndromal, nonsyndromal HE, and nonexposed children, virtually all the volumetric differences were limited to the children with FAS [Archibald et al.,2001]. Children who meet diagnostic criteria for FAS or PFAS are generally the most heavily exposed [Jacobson et al.,2008].
Although differences among sub‐groups in the FASD spectrum have been examined extensively in the neuropsychological literature, potential differences among these subgroups in the implementation of fundamental processes, such as working memory have not been investigated with fMRI. Most fMRI studies relating to FASD have compared heavily exposed subjects with nonexposed controls [e.g., Fryer et al.,2007; Sowell et al.,2007; Spadoni et al.,2009], presumably because too few children meeting diagnostic criteria for FAS were available to examine separately.
Working memory refers to a cognitive system that provides temporary storage and manipulation of the information required to perform a higher order cognitive task [Baddeley,1992,1996]. Baddeley's model of verbal working memory hypothesizes a phonological loop that permits the short‐term maintenance of verbal information. This loop has two components—a phonological store, which can hold speech‐related items for 1–2 s, and an articulatory control system, which involves subvocal rehearsal to prevent their rapid decay. Working memory is mediated by a well‐characterized cortico‐striatal‐cerebellar network. In both children and adults, the frontal lobe reliably responds when subjects are asked to maintain verbal tokens temporarily, and, in adults, the working memory response in both dorsal prefrontal cortex (dPFC) and Broca's area is load dependent [Cohen et al.,1997]. The working memory network also extends to other cortical and subcortical regions, including the basal ganglia (caudate and putamen), parietal cortex, and cerebellum [Wager and Smith,2003]. Meta‐analyses suggest that the ventral aspects of the prefrontal cortex, particularly Broca's area, are sensitive to demands involving articulatory rehearsal (e.g., verbal n‐back paradigms involving letters), whereas the dorsal aspects are particularly responsive during maintenance and manipulation of information regardless of stimulus type [Chein et al.,2002]. The basal ganglia subserve working memory function by integrating signals with the prefrontal cortex [Chang et al.,2007; Voytek and Knight,2010]. In the cerebellum, several regions have been implicated in working memory‐related processing, including the junction of Crus I/lobule VI, as well as lobule VIIB/VIIIA within the inferior cerebellum, which is believed to be involved in maintenance of information over delays [Marvel and Desmond,2010; Stoodley and Schmahmann,2009].
Working memory has emerged as an important cognitive domain in the study of FASD. Kodituwakku et al. [1995] have suggested that the neuropsychological tasks that best discriminate alcohol‐exposed from nonexposed children are those that depend on the “ability to manage goals in working memory in a flexible manner” (p. 1562). In a factor analytic study, Burden et al. [2005] found that working memory was the component of attention most strongly related to moderate‐to‐heavy prenatal alcohol exposure in our Detroit prospective longitudinal cohort at 7.5 years. Working memory deficits have also been documented in alcohol‐exposed children and adolescents in a prospective longitudinal study conducted at the University of Washington [Olson et al.,1998; Streissguth et al.,1993] and in adults with FASD [Connor et al.,2000; Kerns et al.,1997]. Although working memory is a critical component of the general cognitive competence assessed on IQ tests, the fetal alcohol‐related deficit in working memory is evident even after statistical adjustment for overall IQ [Burden et al.,2005; Connor et al.,2000]. Yet, the neural bases of working memory function in FASD have only been investigated to a limited degree, and differences in neural recruitment between children with FAS or PFAS and other heavily exposed children have never been examined.
Working memory is a potentially informative domain to investigate using fMRI as studies have (a) clarified the cortico‐striatal‐cerebellar bases of its implementation in populations of diverse ages [Wager and Smith,2003], (b) demonstrated its developmental bases [Edin et al.,2007], and (c) provided evidence of alternative functional architectures and compensatory mechanisms associated with alcohol exposure [Pfefferbaum et al.,2001]. In a recent study, O'Hare et al. [2009] used fMRI to study working memory (with a Sternberg task) in 20 children and adolescents with FASD and 20 typically developing controls (mean age = 10.8, range = 7–15 years). Children were instructed to encode an array of letters for 1.5 s and were then shown a series of individual letters and asked to identify whether each was or was not part of the previous array. Because the controls performed more accurately than the exposed children on the trials with one‐letter arrays, only the fMRI data from the more difficult three‐ and six‐letter array trials were examined. Both groups activated essentially the same cortico‐cerebellar networks, including diffuse portions of dorsolateral prefrontal cortex, superior parietal cortex, and cerebellum. However, the exposed children showed greater activation particularly in the dorsal frontal, left inferior parietal, bilateral posterior temporal, and superior cerebellar regions.
In this work, we report findings from an fMRI study of working memory in a more heavily exposed cohort of children than that examined by O'Hare et al. [2009], using a simpler working memory task. This is the first fMRI study to use an n‐back task to examine working memory effects in FASD. This is also, to our knowledge, the first study to compare regional brain activations in three groups of children—a group diagnosed with FAS or PFAS, a nonsyndromal heavily exposed (HE) group, and a healthy control group whose mothers abstained or drank no more than minimally during pregnancy. This study was conducted in Cape Town, South Africa, where there is a very high prevalence of heavy alcohol use during pregnancy in the Cape Coloured (mixed ancestry) community [Croxford and Viljoen,1999; Jacobson et al.,2008] and the incidence of FAS is 18–141 times greater than in the United States and among the highest in the world [May et al.,2000,2007]. On the basis of the previous neuropsychological studies, we predicted that the alcohol‐exposed children would perform adequately on a simple 1‐back task but would probably perform more poorly than controls on a more challenging 2‐back paradigm. Given the heavier exposure and unique dysmorphology in the children with FAS or PFAS, we examined whether, in an fMRI study, the FAS/PFAS group might exhibit different patterns of neural activation than those seen in the nonsyndromal HE group.
METHODS
Participants
Participants were 47 children (27 males and 20 females) from a prospective longitudinal cohort in Cape Town, South Africa, who ranged in age from 8.9 to 10.6 years [Jacobson et al.,2008]. Their mothers were recruited between July 1999 and January 2002 at the antenatal clinic of a midwife obstetric unit that serves an economically disadvantaged, predominantly Cape Coloured population. This population, composed mainly of descendants of white European settlers, Malaysian slaves, Khoi‐San aboriginals, and black African ancestors, historically comprised the large majority of workers in the wine‐producing and fruit‐growing region of the Western Cape Province of South Africa. The high prevalence of FAS is a consequence of the very heavy maternal drinking during pregnancy commonly found in this community, due to poor psychosocial circumstances and the traditional dop system, in which farm laborers were paid, in part, with wine. Although the dop system has been outlawed, heavy alcohol consumption persists in certain sectors in urban and rural Cape Coloured communities [Carter et al.,2005; Jacobson et al.,2006], and weekend binge drinking is a major source of recreation for many in the community.
Each gravida was interviewed at her first antenatal visit regarding her alcohol consumption during pregnancy, using a timeline follow‐back interview [Jacobson et al.,2002,2008]. Any woman averaging at least 1.0 oz absolute alcohol (AA) per day, the equivalent of two standard drinks, or reporting at least two incidents of binge drinking (five standard drinks/occasion) during the first trimester of pregnancy was considered a heavy drinker and invited to participate. Women initiating antenatal care who drank < 0.5 oz AA/day and did not binge drink during the first trimester were invited to participate as controls. All women who reported drinking during pregnancy were advised to stop or reduce their intake and offered referral for treatment. Women <18 years of age and those with diabetes, epilepsy, or cardiac problems requiring treatment were not included. Religiously observant Muslim women were also excluded, because their religious practices prohibit alcohol consumption, and they would, therefore, have been disproportionately represented among the controls. Infant exclusionary criteria were major chromosomal anomalies, neural tube defects, multiple births, and seizures.
Each mother was transported to our UCT laboratory by a staff driver and research nurse once during mid‐pregnancy and again at 1‐month postpartum and interviewed using the timeline follow‐back approach regarding her alcohol, smoking, and drug use during the middle and later parts of pregnancy, respectively. Volume was recorded for each type of beverage consumed and converted to oz of AA. The data from the antenatal clinic, antenatal laboratory, and postpartum visits were averaged to provide three summary measures of alcohol use during pregnancy: average oz AA/day and AA/drinking day (dose/occasion) and frequency (days/week).
Each child was examined for growth and FAS dysmorphology at 5 years of age by two expert US‐based dysmorphologists using a standard protocol [Hoyme et al.,2005] during a 6‐day clinic held in September 2005 at a neighborhood church [Jacobson et al.,2008]. There was substantial agreement between the two dysmorphologists regarding assessment of all the dysmorphic features, including the three principal facial anomalies—philtrum and vermilion measured on the Astley and Clarren [Astley and Clarren,2001] rating scales and palpebral fissure length (r's = 0.80, 0.84, and 0.77, respectively)—and between them and a Cape Town‐based dysmorphologist, who examined a small subset of children who could not attend the clinic (median r = 0.78). The dysmorphologists, SJ, JJ, and CM, subsequently conducted a case conference to reach consensus regarding which children met criteria for the FAS and PFAS diagnoses.
PROCEDURE
Working memory was assessed at 9 years on an n‐back task administered as part of a neuropsychological test battery at our UCT Child Development Research Laboratory and subsequently using fMRI at the Cape Universities Brain Imaging Centre. The examiners who conducted the assessments were blind with regard to maternal alcohol history and the child's diagnostic status, except in the most severe cases where FAS status was obvious. Written informed consent was obtained from the mothers and oral assent from the children. Approval for human research was obtained from the Human Investigation Committee at Wayne State University and the Faculty of Health Sciences Research Ethics Committee at the University of Cape Town. Mothers and children were given breakfast, lunch, and a snack during the morning at each visit. At the end of each visit, the mother received a small monetary compensation and photograph of her child, and the child was given a small gift.
Neuropsychological Assessment
n‐Back
The n‐back task was adapted from Casey et al. [1995]. The child sat in front of a computer, on which a series of letters was displayed. The “F” and “J” keys on the computer were each covered with a white sticker. In the 1‐back condition, which was administered first, the child was instructed to press the left key if the letter that appeared on the screen was the same as the previous letter; the right key if it was not. Four consecutive 12‐letter blocks were administered. Each letter appeared on the screen for 500 ms, followed by a 2500‐ms interstimulus interval (ISI). A fixation cross was presented for 15 s between each block. In the 2‐back condition, the child was instructed to press the left button if the letter on the screen was the same as the one that appeared two letters before; the right key, if it was not. Four consecutive 2‐back blocks were administered using the same timing as in the 1‐back condition. Behavioral data are missing for the 1‐back task from six children due to a computer malfunction and from one who did not complete the task. The principal behavioral outcome was d‐prime, which measures number of correct button presses, adjusted for false alarms, to correct for any tendency to press the same button regardless of whether the stimulus was seen previously.
IQ assessment and handedness
All the children were administered the Wechsler Intelligence Scale for Children, fourth edition (WISC‐IV) at the 9‐year visit in English or Afrikaans, depending on the language used in the child's elementary school classroom. The WISC‐IV was translated into Afrikaans by a clinical psychologist whose first language is Afrikaans. At the 5‐year follow‐up of this cohort, we had administered the Junior South African Intelligence Scale [JSAIS; Madge et al.,1981], which is available in Afrikaans and English and has been normed for South African children. Seventy‐one of the children from that follow‐up were administered the WISC‐IV at 9 years. IQ scores obtained using the JSAIS at 5 years were strongly correlated with the 9‐year WISC scores, r = 0.79, P < 0.001. Handedness was assessed on the Annett (1970) Behavioral Handedness Inventory.
Neuroimaging Assessment
Each child was scanned on a 3 T Allegra MR scanner (Siemens, Erlangen Germany). A magnetization‐prepared rapid gradient echo structural image was acquired in a sagittal orientation with the following parameters: TR = 2,300 ms, TE = 3.93 ms, TI = 1,100 ms, 160 slices, flip angle 12°, voxel size = 1.3 × 1.0 × 1.0 mm3, and scan time = 6:03 min. During the fMRI protocol, 180 functional volumes sensitive to blood oxygen level dependent contrast were acquired with a T2*‐weighted gradient echo, echo planar imaging sequence [TR = 2,000 ms, TE = 30 ms, 34 interleaved slices, 3 mm thick, gap 0.9 mm, 200 × 200 mm field of view (in‐plane resolution 3.125 × 3.125 mm2)]. The first four volumes were discarded from all analyses in order to allow the signal to reach steady state.
Letters were projected in sequence (presentation time: 500 ms; ISI: 2,500 ms). In the zero‐back (control) condition, subjects pressed the left button with their right index finger every time they saw a letter other than the letter “D” and their right middle finger for the letter “D.” In the 1‐back condition, they pressed the left button with their right index finger for every letter they saw except if the letter was the same as the one shown previously in the sequence, in which case they pressed the button under their right middle finger. Conditions were blocked (12 letters per block and 36 s duration). Each active block was preceded by an instruction slide displayed for 5 s indicating either a 0‐back (“Target = D') or 1‐back (“1‐BACK”) condition. Subjects were presented with six active blocks (three of each condition) in randomized order. A fixation cross was presented for 15 s preceding each active block and following the final active block. Total task duration was 5 min and 51 s and was triggered by the scanner to start automatically after the acquisition of the first four dummy scans. Each child practiced the task initially in a mock scanner built for this study, which was important in reducing anxiety and facilitating completion of the MRI scans. The experimental task was programmed using E‐Prime software (Psychology Software Tools, Pittsburgh, PA) and presented using a data projector positioned in the control room behind the scanner in line with the bore of the magnet. Images were projected through a waveguide onto a rear projection screen mounted behind the scanner, which subjects viewed using the standard mirror system that mounts to the single channel head coil. Responses were recorded using a Lumitouch response system (Photon Control, Burnaby, Canada). The child was able to talk to the examiner using an intercom that is built into the scanner and could stop the scan at any time by squeezing a ball held in his/her left hand.
fMRI processing
MR images were preprocessed and analyzed using SPM8 (Statistical Parametric Mapping, Wellcome Department of Imaging and Neuroscience, London, UK). For fMRI, all images were manually oriented to the AC‐PC line, realigned to correct for head movement, spatially normalized to the Montreal Neurological Institute template brain, resliced (2 mm3), and smoothed spatially by a Gaussian filter of 8‐mm full‐width half maximum. An autoregressive AR(1) model was used to account for serial correlation, and regressors modeled as a 36‐s box‐car vectors (for each of the task‐related conditions) were convolved with a canonical hemodynamic reference wave form, with the six motion parameters included as covariates of no interest. To isolate memory‐related processing, first level contrasts were based on 1‐back > 0‐back contrasts for each subject.
The a priori working memory network, including Broca's Area (inferior frontal gyrus), dPFC, basal ganglia (caudate + putamen), parietal cortex, and Crus I/lobule VI and lobules VII‐IX of the cerebellum, was defined based on previously published methods [Maldjian et al.,2003; Tzourio‐Mazoyer et al.,2002] (see Supporting Information Fig. 1). Cluster level significance to detect minimum extent thresholds of contiguous voxels (P < 0.01) was set at P FWE < 0.05 [Ward, 2000]. Intragroup one‐sample t tests were used to identify clusters of significant activation in three groups of children: children who met diagnostic criteria for FAS or PFAS, nonsyndromal HE children, and controls. The first level contrasts (1‐back > 0‐back) for each of the clusters were then subjected to second‐level random effects analyses of variance with group as the single factor, and the eigenvalues (betas) for each subject were extracted for use in subsequent analyses.
Figure 1.
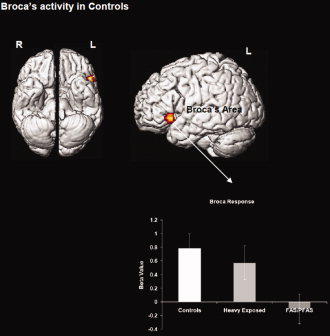
Regional activations (P < 0.05, cluster level) from the intra‐group analysis for the control group are projected to ventral and lateral cortical surfaces. Control subjects increased activity in Broca's area more in the 1‐back relative to the 0‐back condition. The radiating graph depicts the between‐group comparison of beta values drawn from the main effect of group in Broca's area indicating the preferential activation of this region in controls, with markedly reduced activation in FAS/PFAS. Error bars are ± SD. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Statistical Analysis
Eleven control variables were assessed for consideration as potential confounders of the effect of alcohol exposure group on n‐back performance: maternal socioeconomic status, years of education, marital status, age at delivery, parity, and smoking during pregnancy, and child gender, total intracranial volume, age at assessment, IQ, and postnatal lead exposure based on a venous blood lead sample obtained from the child at 5 years of age. Each control variable was examined in relation to each behavioral outcome and cluster of interest. Any control variable that related at P < 0.10 to a behavioral or neuroimaging outcome was considered a potential confounder of the effect of exposure group on that endpoint. Analysis of covariance (ANCOVA) was used to determine whether the effect of exposure on each outcome remained significant after adjustment for potential confounders.
We first report the results of ANCOVAs comparing the performance of the three groups (FAS/PFAS, HE, and controls) on the two behavioral tasks. Next, we present contrast maps indicating the regions recruited under working memory conditions (1‐back > 0‐back) for each group separately. We then report the results of ANCOVAs comparing the beta values (derived as noted above) for the three groups on the clusters of interest that emerged in the intragroup analyses, adjusted, where necessary, for potential confounders.
RESULTS
Sample Characteristics
Demographic and background characteristics are summarized in Table I. Six (20.0%) children born to the 30 heavy drinking mothers met the revised Institute of Medicine criteria [Hoyme et al.,2005] for full FAS: at least two of the principal dysmorphic features (short palpebral fissures, thin upper lip, flat, or smooth philtrum), small head circumference (bottom 10th percentile), and low weight or short stature (bottom 10th percentile); 11 met criteria for PFAS. Thirteen children, the nondysmorphic HE group, were born to heavy drinking mothers but did not meet criteria for FAS or PFAS, and 17 children, the healthy control group, were born to women who abstained or drank at very low levels during pregnancy.
Table 1.
Sample characteristics
| FAS/PFAS (N = 17) | HE (N = 13) | Controls (N = 17) | F or χ2 | ||||
|---|---|---|---|---|---|---|---|
| M or % | SD | M or % | SD | M or % | SD | ||
| Maternal characteristics | |||||||
| Education (years) | 7.4 | 1.9 | 8.5 | 3.2 | 10.5 | 1.6 | 8.20* |
| Marital status (% married) | 23.5 | 23.1 | 52.9 | 4.24 | |||
| Age at delivery | 27.1 | 7.2 | 25.2 | 5.4 | 27.8 | 4.7 | 0.68 |
| Parity | 2.8 | 1.6 | 1.9 | 0.8 | 1.7 | 0.9 | 4.16** |
| Socioeconomic status | 35.1 | 31.1 | 26.9 | 20.9 | 30.7 | 16.5 | 0.44 |
| Child characteristics | |||||||
| Blood lead concentration (μg/dL)a | 10.8 | 5.1 | 9.9 | 4.1 | 7.9 | 2.9 | 2.04 |
| Age at scan | 9.3 | 0.2 | 9.7 | 0.6 | 9.3 | 0.4 | 3.92** |
| Gender (% male) | 52.9 | 53.8 | 41.2 | 0.64 | |||
| Handedness (% right‐handed) | 94.1 | 100.0 | 100.0 | 1.80 | |||
| IQ | 66.8 | 6.3 | 72.2 | 8.4 | 75.8 | 8.6 | 5.64*** |
| Total intracranial volume (×106 mm3)b | 1.3 | 0.2 | 1.4 | 0.1 | 1.4 | 0.1 | 5.25*** |
| Prenatal alcohol exposure | |||||||
| oz AA/day | 0.9 | 0.8 | 0.5 | 0.5 | 0.008 | 0.03 | 11.72* |
| oz AA/drinking day | 4.0 | 2.1 | 2.7 | 1.7 | 0.2 | 0.4 | 25.82* |
| Drinking days/week | 1.4 | 0.9 | 1.2 | 0.9 | 0.03 | 0.1 | 19.00* |
| Prenatal cigarette exposure | 8.0 | 5.3 | 8.7 | 7.5 | 3.5 | 9.6 | 2.17 |
Missing for 1 FAS/PFAS, and 1 HE.
Acquired with an magnetization‐prepared rapid gradient echo sequence using a real‐time motion correction procedure developed by van der Kouwe and colleagues [Tisdall et al., 2009] and measured with FreeSurfer [Fischl et al., 2002].
P < 0.001.
P < 0.05.
P < 0.01.
Although the women in the two alcohol consuming groups concentrated their drinking on 1.2–1.4 days/week on average and the women in both groups met criteria for the revised NIAAA criterion for binge drinking by women (four or more drinks/occasion), the mothers in the FAS/PFAS group averaged substantially more alcohol intake per occasion—8.0 drinks/occasion, compared to 5.4 for the HE group, P = 0.036. By contrast, all but two women in the control group abstained; one woman drank only three times during pregnancy (two drinks/occasion); the other drank three drinks/occasion twice/month. None of the women reported using cocaine or methaqualone (“mandrax”), and two mothers of children with PFAS reported light marijuana use (1–3 days/month) during pregnancy. There were no between‐group differences for social class, marital status, maternal age at delivery, smoking, child gender, or blood lead levels, all P's > 0.10 (Table I). Mothers of children in the FAS/PFAS and HE groups completed fewer years of school than control mothers and had more children than those in the other two groups. The children in the HE group were slightly older than those in the two other groups at scan. As expected, the IQ scores of the children in the FAS/PFAS group were lower than the controls, P = 0.002, and tended to be lower than those in the HE group, P = 0.073; IQ scores of the HE and control groups were not significantly different, P > 0.20.
Behavioral Findings
The three groups did not differ on any of the behavioral measures for the 1‐back task (Table II). The 2‐back task was much more difficult for all the children; nine (22.5%) of the 40 who completed the 1‐back task were not able to perform the 2‐back. The d‐prime scores for those who completed both tasks were markedly lower for the 2‐back task, t(30) = 9.60, and P < 0.001. The d‐prime scores for the 2‐back did not differ significantly among the exposure groups, primarily because four of the controls also performed poorly on that task. In a secondary analysis, we examined how many of the exposed children performed as well as the better performing children in the control group, defined as a d‐prime score in the top tercile for that group. Based on this criterion, only one (4.8%) of the alcohol‐exposed children performed well on the 2‐back task, compared to 36.4% of the children in the control group, χ2 = 5.58, P < 0.025. All three groups performed well on the 1‐back task in the scanner.
Table II.
n‐Back performance inside and outside of scanner
| FAS/PFAS | HE | Controls | F | ||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Outside scanner | |||||||
| 1‐Back | N = 14 | N = 11 | N = 15 | ||||
| Hit rate | 0.71 | 0.20 | 0.70 | 0.15 | 0.70 | 0.20 | 0.02 |
| False alarm rate | 0.05 | 0.04 | 0.08 | 0.06 | 0.07 | 0.06 | 0.74 |
| d′ | 2.41 | 0.78 | 2.12 | 0.75 | 2.25 | 1.01 | 0.35 |
| 2‐Back | N = 12 | N = 9 | N = 11 | ||||
| Hit rate | 0.53 | 0.19 | 0.52 | 0.21 | 0.56 | 0.14 | 0.13 |
| False alarm rate | 0.17 | 0.11 | 0.23 | 0.21 | 0.14 | 0.12 | 0.43 |
| d′ | 1.13 | 0.67 | 0.99 | 0.47 | 1.39 | 0.72 | 1.05 |
| Inside scanner 1‐Back | N = 17 | N = 13 | N = 17 | ||||
| Hit rate | 0.73 | 0.19 | 0.71 | 0.18 | 0.76 | 0.17 | 0.22 |
| False alarm rate | 0.07 | 0.05 | 0.06 | 0.06 | 0.07 | 0.06 | 0.02 |
| d′ | 2.14 | 0.81 | 2.12 | 0.80 | 2.26 | 0.81 | 0.13 |
Neuroimaging Findings
Intra‐group one‐sample t‐tests revealed highly distinct patterns of network recruitment across groups during the 1‐back relative to the 0‐back. The control group increased activity in Broca's area (with no other regions reaching significance). The HE group increased activity in a fronto‐striatal network that included bilateral dPFC, caudate, and putamen. Finally, the FAS/PFAS group increased activity in Crus I/lobule VI and lobule VIIB/VIIIA of the cerebellum and the inferior parietal cortex. Individual peaks and cluster extents for each of the analyses are depicted in Table III. Surface projections of thresholded clusters for each of these analyses are depicted in Figures 1–3.
Table III.
Regional activations from the intragroup analyses (1‐back vs. 0‐back)
| Mean x | Mean y | Mean Z | Number of voxelsa | Max t | |
|---|---|---|---|---|---|
| Control group | |||||
| L inferior frontal gyrus/Broca's area | −50 | 16 | −2 | 161 | 6.4 |
| HE group | |||||
| R dorsolateral prefrontal cortex | 50 | 6 | 40 | 169 | 5.4 |
| L caudate | −12 | −10 | 20 | 78 | 5.0 |
| R putamen | 26 | −22 | 4 | 96 | 4.8 |
| L dorsolateral prefrontal cortex | −34 | 30 | 40 | 145 | 4.1 |
| L putamen | −40 | 18 | 2 | 82 | 4.0 |
| FAS/PFAS group | |||||
| R parietal | 40 | −36 | 34 | 723 | 5.3 |
| L cerebellum Crus I/lobule VI | −30 | −36 | −36 | 212 | 4.0 |
| L inferior cerebellum (lobule VIIB–VIIIA) | −24 | −40 | −44 | 200 | 4.1 |
P (uncorrected) < 0.01.
To compare potential differences in the fMRI response across groups, we modeled group differences in second‐level random effects analyses of variance. Analyses of the overall main effect of group examined functionally defined region of interest masks created based on the intragroup analyses (Figs. 1, 2, 3; Table III). This hierarchical approach allowed us to identify preferred working memory networks within each of the groups (using identical statistical thresholds) and then to explore group differences in activation in those networks.
Figure 2.
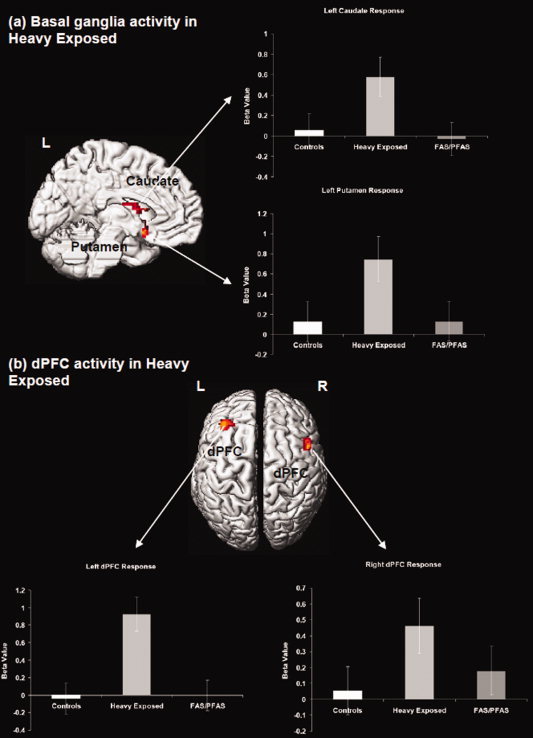
Regional activations (P < 0.05, cluster level) from the intra‐group analysis for the heavily exposed nonsyndromal group (HE) are projected to the left medial and bilateral axial surfaces of the brain. (a) The figure depicts recruitment of the basal ganglia (including the caudate and the putamen). (b) Bilateral recruitment of the dorsal prefrontal cortex is shown. HE subjects increased activity in these areas more in the 1‐back relative to the 0‐back condition. The radiating graphs depict between‐group comparison of beta values in the depicted clusters. Error bars are ± SD. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
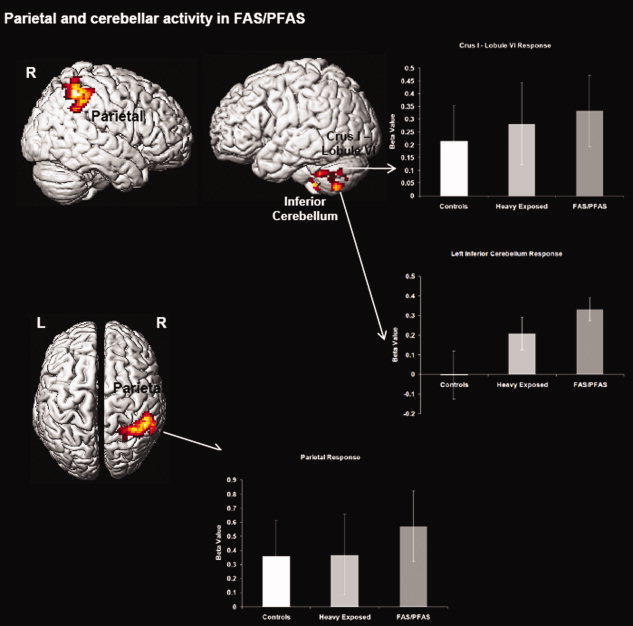
Regional activations from the intra‐group analysis for the FAS/PFAS group are projected to the bilateral sagittal and axial surfaces. As seen, FAS/PFAS subjects increased activity in parietal and cerebellar areas more in the 1‐back relative to the 0‐back condition. Radiating graphs depict between‐group comparisons of beta values for those regions (see also Supporting Information Fig. 2). Error bars are ± SD. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table IV presents the results of the ANCOVAs examining the fMRI responses for each of the clusters of interest generated in the intra‐group analyses across the three subgroups adjusted, where necessary, for potential confounders. Post hoc comparisons show that activations in the left dPFC, caudate, and putamen were significantly greater in the HE group than in either the FAS/PFAS or control groups. The children in the FAS/PFAS group exhibited significantly less activation in the region of Broca's area that is known to mediate subvocal rehearsal than either of the other groups and significantly more activation than the controls in lobule VIIB/VIIIB, which has been implicated in maintenance of information over delays.
Table IV.
Between‐group comparisons of the regions of interest from the intragroup analyses (1‐back vs. 0‐back)
| Group means | F | Post hoc comparisons (P values) | |||||
|---|---|---|---|---|---|---|---|
| Controls | HE | FAS/PFAS | HE versus controls | HE versus FAS/PFAS | Controls versus FAS/PFAS | ||
| L inferior frontal gyrus/ Broca's area | 0.780 (0.586) | 0.570 (1.303) | −0.106 (0.792) | 4.40* | n.s. | 0.048 | 0.006 |
| R dorsolateral prefrontal cortex | 0.054 (0.733) | 0.463 (0.340) | 0.181 (0.672) | 1.61 | 0.083 | n.s. | n.s. |
| L caudate | 0.057 (0.628) | 0.578 (0.583) | −0.031 (0.723) | 3.17* | 0.048 | 0.024 | n.s. |
| R putamen | 0.154 (0.621) | 0.323 (0.423) | 0.179 (0.619) | 1.14 | n.s. | n.s. | n.s. |
| L dorsolateral prefrontal cortex | −0.038 (0.511) | 0.927 (0.984) | −0.004 (0.639) | 8.33** | 0.001 | 0.001 | n.s. |
| L putamen | 0.123 (0.692) | 0.746 (0.791) | 0.130 (0.936) | 2.72† | 0.044 | 0.046 | n.s. |
| R parietal | 0.356 (1.192) | 0.370 (1.307) | 0.572 (0.532) | 0.22 | n.s. | n.s. | n.s. |
| L cerebellum Crus I/lobule VI | 0.248 (0.748) | 0.264 (0.495) | 0.325 (0.389) | 0.09 | n.s. | n.s. | n.s. |
| L inferior cerebellum (lobule VIIB/VIIIA) | −0.004 (0.508) | 0.207 (0.301) | 0.253 (0.241) | 2.24 | n.s. | n.s. | 0.051 |
Values are mean (standard deviation) beta values. None of the control variables met the P < 0.10 criterion for inclusion as covariates in any of these analyses, except that child's age at assessment was related to the activation of L caudate and R putamen. The values and post hoc comparisons shown for those ROIs, therefore, adjust for child's age at assessment in this table.
n.s., not significant.
P < 0.05.
P < 0.001.
P < 0.10.
DISCUSSION
This is the first study to examine working memory in FASD using an n‐back task and the first to explore differences between subgroups in the FASD spectrum in cortico‐striatal‐cerebellar recruitment during basic working memory processing. Behaviorally, both groups of exposed children performed more poorly than controls on a challenging 2‐back task, but all three groups performed well on a simple 1‐back paradigm both in and outside the scanner. Intra‐group analyses of the fMRI data indicated that the three groups recruited distinct elements of the cortico‐striatal‐cerebellar network known to subserve working memory function. Whereas controls increased activity in Broca's area more during the simple 1‐back (relative to the 0‐back) task, the HE group showed this pattern of activity increase in an extensive fronto‐striatal network that is usually activated for performing more challenging 2‐back paradigms. Finally, the most heavily exposed children—those with FAS or PFAS—increased activity in the parietal and cerebellar regions. These differential patterns of intragroup functional recruitment were partially corroborated by between‐group differences in activation; significant intergroup activation differences in 1‐back relative to 0‐back were observed in Broca's area, the basal ganglia, and the dPFC. Notably, none of the FASD subgroups were behaviorally impaired on the 1‐back task, indicating that the observed differences in network recruitment were not confounded by performance deficits in the exposed groups. These findings confirm the utility of using a simplified task that all three groups can perform well in the scanner. Differences in brain regional activation are thus not confounded with differences in performance, making it possible to study a cognitive domain in which group differences are clearly evident outside the scanner.
The recruitment of working memory networks in the brain is highly adaptive, reflecting multiple constraints including memory load, sensory modality of processing, attentional demands, and neurodevelopment [Bledowski et al.,2010; Edin et al.,2007]. In general, the meta‐network is thought to include frontal cortex, basal ganglia, parietal lobe, and cerebellum, all of which have been consistently implicated in meta‐analyses of working memory tasks, such as the n‐back.
Ascribing precise roles to each of the regions involved in working memory has proved challenging. In normally developing children and adults, working memory tasks with verbal or phonological demands result in robust recruitment of Broca's area [Casey et al.,1995; Smith and Jonides,1998; Strand et al.,2008]. Chein et al. [2002] identified two distinct subregions of the left inferior frontal gyrus that are activated—a dorsal region (pars opercularis, BA 45), related to response organization and automation, and a ventral region (pars triangularis, BA 44), sensitive to sublexical phonological processing, which is the site where we observed activation in controls. In our control subjects, this activation appears to be sufficient to sustain working memory at the relatively low level of load in the 1‐back task.
As seen in Figure 2, the HE group activated a fronto‐striatal subnetwork that includes the dPFC, caudate, and putamen. On more complex verbal working memory tasks, such as the Sternberg task, responses in the dPFC, and basal ganglia are highly sensitive to increases in load, particularly during the encoding, maintenance, and manipulation of memoranda [Chang et al.,2007; Lewis et al.,2004], and fronto‐striatal synchrony appears essential in subserving more complex working memory processing. Activation of the basal ganglia also appears to facilitate attentional control of the extended working memory network by filtering of task irrelevant information [McNab and Klingberg,2008] and through explicit synchronization of activity [Chang et al.,2007]. Therefore, fronto‐striatal activation in the HE group suggests a neural response of increased complexity to this simple task, leading to the recruitment of a compensatory pathway that is typically activated only under complex memory conditions in controls.
The FAS/PFAS group activated cerebellar and parietal regions and within the former recruited the two principal regions known to be involved in verbal working memory. One of these regions, which is located in the superior cerebellum at the junction of Crus I and lobule VI, is notable for its anatomical connectivity with the inferior frontal gyrus and premotor cortex [Chen and Desmond,2005]. The superior cerebellum has been proposed as a supplementary region for phonological rehearsal [Desmond et al.,1997; Durisko and Fiez,2010] and is known to subserve covert speech [Marvel and Desmond,2010] and verbal and nonverbal auditory working memory [Salmi et al.,2009; Stoodley et al.,2010]. The connectivity of this region to Broca's area is believed to facilitate the rapid translation of letters into an articulatory trajectory required to initiate the phonological loop [Chen and Desmond,2005; Marvel and Desmond,2010]. Crus I has also been implicated in rapid naming difficulties in children with dyslexia [Eckert et al.,2003] and is activated more in high‐load working memory tasks by alcoholics, suggesting a compensatory increase in a cerebro‐cerebellar circuit that may be compromised by chronic alcohol consumption [Desmond et al.,2003]. The second cerebellar region activated by the FAS/PFAS group is located in lobule VIIB/VIIIA within the inferior cerebellum, a region that receives inputs originating in parietal and temporal regions and is believed to facilitate short‐term phonological storage [Desmond et al.,1997; Kirschen et al.,2005]. The area of parietal activation seen in the FAS/PFAS group corresponds to Ravizza et al.'s [2004] dorsal inferior parietal cortex, which is highly sensitive to memory load in both verbal and nonverbal working memory tasks. This region has been characterized as part of a “frontal‐executive system” that is believed to be important in focusing attention on items in working memory, retaining temporal order, and rapidly switching attention.
These differences in the activation of regions across groups were largely corroborated by differences in BOLD signal change between groups. Signal change in FAS/PFAS subjects was significantly reduced in the basal ganglia and the dPFC compared to HE and in Broca's area compared to controls. However, with the exception of lobule VIIB/VIIIA, significant intergroup differences were not observed in the parieto‐cerebellar regions preferentially recruited in the FAS/PFAS group. To explore possible bases for the relative lack of significant group differences in these regions, we conducted a conjunction analysis using the minimum inference statistic [Nichols et al.,2005] to investigate potential overlap in working memory‐related activation across groups. The results of this analysis (see Supporting Information Fig. 2) suggest significant overlap in activation principally in the parieto‐cerebellar subnetwork. This overlap indicates significant common bases of activation in the parietal cortex and Crus I/lobule VI within the cerebellum, though the preferential recruitment in the FAS/PFAS group at robustly employed statistical thresholds confirms a cerebellar compensatory mechanism that has been hypothesized to characterize working memory implementation in alcoholic adults [Chen and Desmond,2005].
Pathology in childhood and adulthood affects working memory network function in highly variable ways. For example, in both children and adults with ADHD, hypo‐recruitment of frontal, striatal, and cerebellar structures during working memory has been reported [Dickstein et al.,2006; Wolf et al.,2009]. Similar patterns have been reported in adolescents with dyslexia [Vasic et al.,2008]. In schizophrenia, working memory appears to result in inefficient cortical recruitment, particularly of prefrontal regions [Callicott et al.,2003; Jansma et al.,2004], and these patterns of cortico‐cortical inefficiency have been observed in adolescent at‐risk offspring of patients as well [Bakshi et al.,2011; Diwadkar et al., 2012].
Several of the brain regions known to be involved in working memory appear to be particularly vulnerable to fetal alcohol exposure. Volumetric MRI studies have reported disproportionate size reductions in caudate nucleus [Archibald et al.,2001; Astley et al.,2009b; Nardelli et al.,2011], putamen [Astley et al.,2009a,b; Nardelli et al.,2011], and in the parietal lobe and cerebellum [Archibald et al.,2001]. Differences in cortical thickness and brain surface area have been reported in frontal regions for children with FASD [Sowell et al.,2002,2008].
Our data suggest that the brain in FASD is differentially sensitive to comparable levels of memory load, with similar levels of demand resulting in differential patterns of recruitment across the memory network depending on the severity of the disorder. The distinctly different activation patterns seen in the FAS/PFAS and HE groups are likely related to the substantial differences in average alcohol dose/occasion to which these two groups were exposed during gestation. The differential recruitment of structures in these two subgroups is suggestive of alternative developmental trajectories that may characterize these different forms of fetal alcohol disorder. Thus, if very high levels of alcohol exposure during pregnancy negatively impact striatal dopamine [Schneider et al.,2005], which is strongly associated with working memory [Landau et al.,2009], the children in the FAS/PFAS group, who are the most heavily exposed, may rely on working memory subcircuits that exclude the basal ganglia. In groups that are less exposed, such as HE, the striatal deficit may not reach the threshold of a functional impairment. In this group, the increased activation of the fronto‐striatal network compared to controls likely reflects an increased sensitivity to task difficulty. Thus, simple tasks, such as those used in this study, allow us to probe the basic integrity of functional networks in this clinical phenotype. Patterns of regional recruitment in these subgroups may reveal latent functional deficits in the brain, the full impact of which may not be fully expressed until later in life, when marked increases in cognitive competence are demanded [Luna,2009]. Recently, important demonstrations that network engagement in children exposed to methamphetamine is partially distinct from that seen in children exposed to alcohol [Roussotte et al.,2011] further highlight the complex and distal effects of fetal alcohol exposure on this fundamental cognitive domain.
The exposure group differences in the response for the nine ROIs that emerged from the intragroup analyses were not attributable to group differences in maternal age at delivery, parity, smoking during pregnancy, or years of education; family socioeconomic status; or child gender, age at assessment, or postnatal lead exposure. Given that the responses were adjusted for IQ, the group differences in brain activation were also not attributable to the poorer overall intellectual function of the FAS/PFAS group. Thus, these findings are consistent with the hypothesis that fetal alcohol exposure may specifically target the neural regions and pathways mediating verbal working memory.
These data are consistent with the findings from more complex working memory tasks in the literature, providing further support for the speculation that the exposed sub‐groups found the simplest level of the n‐back to be more difficult than the control task. Often when task difficulty increases, regional recruitment extends bi‐laterally, reflecting dynamic cortical responses to demand‐sensitive variations. Our intragroup differences in the right parietal, right prefrontal, and left superior cerebellum may, in part, reflect contra‐lateral activations that are reflective of the increased demand in the exposed samples.
Our findings of preferential activation of different components of the working memory network in children with different FASD phenotypes differ from those reported by O'Hare et al. [2009]. One important difference between these studies is that the 1‐back paradigm we used is a much simpler task, which our controls could perform by relying primarily on Broca's area. By contrast, the more challenging Sternberg task in the O'Hare et al. [2009] study required much more extensive fronto‐parieto‐temporal activations even by the control children. Because of the relatively small number of syndromal children in their cohort, they were not able to examine differences in patterns of activation between FAS/PFAS and heavily exposed nonsyndromal children. A strength of our approach is that it allowed us to compare correlates of working memory recruitment in different alcohol‐related disorders in the context of the simplest and most basic level of working memory processing.
To our knowledge, only one prior FASD study has compared BOLD responses among dysmorphic, nondysmorphic exposed, and control subjects, but the results of that study are difficult to interpret, because behavioral performance also differed among the groups [Santhanam et al.,2009]. Previous fMRI studies of alcohol‐exposed groups comprised both syndromal and nonsyndromal individuals have reported increased frontal cortical activation across a broad range of cognitive domains, including learning and memory [Sowell et al.,2007], response inhibition [Fryer et al.,2007], and spatial working memory [Spadoni et al.,2009]. In these studies, the exposed children appeared to rely on prefrontal cortical activations to compensate for less efficient processing in neural networks specialized for the tasks in question, a pattern that Halperin and Schulz [2006] have also described in ADHD. By contrast, in a study of arithmetic comparing a group of children with FAS or PFAS with nonexposed controls, in which the control children activated the fronto‐parietal network specialized for number processing, the syndromal children did not show an increased frontal BOLD response but instead showed extensive parietal activations (angular gyrus, posterior cingulate, and precuneus) to perform a simple magnitude comparison task [Meintjes et al.,2010]. For simple addition problems, these very heavily exposed children exhibited cerebellar activations that have been reported in adults only when more complex and rapid arithmetic processing is required [Menon et al.,2000]. Thus, the data in the present study provide additional evidence for the recruitment of more extensive neural networks, which are normally engaged in more challenging problems, when there is impairment in the neural circuitry that is most efficient for performance of a relatively simple cognitive task.
CONCLUSIONS
This is the first study to examine working memory in FASD using an n‐back task and the first to explore differences between subgroups in the FASD spectrum in cortico‐striatal‐cerebellar recruitment during basic working memory processing. These data suggest that the effects of exposure across subgroups may differentially impair critical brain regions that subserve basic function. Notably, neither FASD subgroup was behaviorally impaired on the 1‐back task, indicating that the observed differences in network recruitment were not confounded by performance deficits in the exposed groups. The distinctly different activation patterns seen in the FAS/PFAS and HE groups are likely related to the substantial differences in average alcohol dose/occasion to which these two groups were exposed during gestation. This study is, to our knowledge, the first to demonstrate that children who meet diagnostic criteria for FAS and PFAS may use qualitatively different compensatory strategies than heavily exposed nonsyndromal children to perform a simple cognitive task. Future studies in FASD may need to account for phenotypic variations in the spectrum that may have differential effects on behavior and functional brain organization.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figures
Acknowledgements
We thank two anonymous reviewers for their helpful suggestions for improving the manuscript. We thank the three dysmorphologists H.E. Hoyme, L.K. Robinson, and N. Khaole, who performed the dysmorphology examinations of the children, M. September for her major contribution in cohort maintenance, M. Pienaar for her work on data collection, and Renee Sun on data scoring. We also express our gratitude to the staff at the Cape Universities Brain Imaging Centre (CUBIC), and the mothers and children who have participated in the Cape Town Longitudinal Study.
Contributor Information
Vaibhav A. Diwadkar, Email: vdiwadka@med.wayne.edu.
Joseph L. Jacobson, Email: joseph.jacobson@wayne.edu.
REFERENCES
- Annett M ( 1970): A classification of hand preference by association analysis. Br J Psychology 61: 303–321. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema‐Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL ( 2001): Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol 43: 148–154. [PubMed] [Google Scholar]
- Astley SJ, Clarren SK ( 2001): Measuring the facial phenotype of individuals with prenatal alcohol exposure: Correlations with brain dysfunction. Alcohol Alcohol 36: 147–159. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T ( 2009a): Functional magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. J Neurodev Disord 1: 61–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T ( 2009b): Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 33: 1671–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A ( 1992): Working memory. Science 255: 556–559. [DOI] [PubMed] [Google Scholar]
- Baddeley A ( 1996): The fractionation of working memory. Proc Natl Acad Sci USA 93: 13468–13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi N, Pruitt P, Radwan J, Keshavan MS, Rajan U, Zajac‐Benitez C, Diwadkar VA ( 2011): Inefficiently increased anterior cingulate modulation of cortical systems during working memory in young offspring of schizophrenia patients. J Psychiatr Res 45: 1067–1076. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Kaiser J, Rahm B ( 2010): Basic operations in working memory: Contributions from functional imaging studies. Behav Brain Res 214: 172–179. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL ( 2005): Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res 29: 443–452. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR ( 2003): Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. Am J Psychiatry 160: 2209–2215. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson SW, Molteno CD, Chiodo LM, Viljoen D, Jacobson JL ( 2005): Effects of prenatal alcohol exposure on infant visual acuity. J Pediatr 147: 473–479. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, Kaysen D, Hertz‐Pannier L, Rapoport JL ( 1995): Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage 2: 221–229. [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz‐Herbette S, Menon V ( 2007): Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage 34: 1253–1269. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fissell K, Jacobs S, Fiez JA ( 2002): Functional heterogeneity within Broca's area during verbal working memory. Physiol Behav 77: 635–639. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE ( 2005): Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage 24: 332–338. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE ( 1997): Temporal dynamics of brain activation during a working memory task. Nature 386: 604–608. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind‐Hood CL, Brown RT, Falek A, Smith IE ( 1997): A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res 21: 150–161. [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP ( 2000): Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol 18: 331–354. [DOI] [PubMed] [Google Scholar]
- Croxford J, Viljoen D ( 1999): Alcohol consumption by pregnant women in the Western Cape. S Afr Med J 89: 962–965. [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV ( 2003): Increased frontocerebellar activation in alcoholics during verbal working memory: An fMRI study. Neuroimage 19: 1510–1520. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH ( 1997): Lobular patterns of cerebellar activation in verbal working‐memory and finger‐tapping tasks as revealed by functional MRI. J Neurosci 17: 9675–9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP ( 2006): The neural correlates of attention deficit hyperactivity disorder: An ALE meta‐analysis. J Child Psychol Psychiatry 47: 1051–1062. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Pruitt P, Zhang A, Radwan J, Keshavan MS, Murphy E, Rajan U, Zajac‐Benitez C ( 2012): The neural correlates of performance in adolescents at risk for schizophrenia: Inefficiently increased cortico‐striatal responses measured with fMRI. J Psychiatr Res 46: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge NC, Jacobson JL, Molteno CD, Meintjes EM, Bangalore S, Diwadkar V, Hoyme EH, Robinson LK, Khaole N, Avison MJ, Jacobson SW ( 2009): Prenatal alcohol exposure and interhemispheric transfer of tactile information: Detroit and Cape Town findings. Alcohol Clin Exp Res 33: 1628–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durisko C, Fiez JA ( 2010): Functional activation in the cerebellum during working memory and simple speech tasks. Cortex 46: 896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW ( 2003): Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain 126: 482–494. [DOI] [PubMed] [Google Scholar]
- Edin F, Macoveanu J, Olesen P, Tegner J, Klingberg T ( 2007): Stronger synaptic connectivity as a mechanism behind development of working memory‐related brain activity during childhood. J Cogn Neurosci 19: 750–760. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP ( 2007): Prenatal alcohol exposure affects frontal‐striatal BOLD response during inhibitory control. Alcohol Clin Exp Res 31: 1415–1424. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP ( 2006): Revisiting the role of the prefrontal cortex in the pathophysiology of attention‐deficit/hyperactivity disorder. Psychol Bull 132: 560–581. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK ( 2005): A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 institute of medicine criteria. Pediatrics 115: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Molteno CD, Odendaal H ( 2006): A prospective examination of the incidence of heavy drinking during pregnancy among Cape Coloured South African women. Alcohol Clin Exp Res 30: 233A.16441272 [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL ( 2002): Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics 109: 815–825. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL ( 2008): Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res 32: 365–372. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS ( 2004): Working memory capacity in schizophrenia: A parametric fMRI study. Schizophr Res 68: 159–171. [DOI] [PubMed] [Google Scholar]
- Kerns KA, Don A, Mateer CA, Streissguth AP ( 1997): Cognitive deficits in nonretarded adults with fetal alcohol syndrome. J Learn Disabil 30: 685–693. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Schraedley‐Desmond P, Desmond JE ( 2005): Load‐ and practice‐dependent increases in cerebro‐cerebellar activation in verbal working memory: An fMRI study. NeuroImage 24: 462–472. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD ( 1995): Specific impairments in self‐regulation in children exposed to alcohol prenatally. Alcohol Clin Exp Res 19: 1558–1564. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ ( 2009): Striatal dopamine and working memory. Cereb Cortex 19: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM ( 2004): Striatal contributions to working memory: A functional magnetic resonance imaging study in humans. Eur J Neurosci 19: 755–760. [DOI] [PubMed] [Google Scholar]
- Luna B ( 2009): Developmental changes in cognitive control through adolescence. Adv Child Dev Behav 37: 233–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madge EM, vandenberg AR, Robinson M, Landman J ( 1981): Junior South African Individual Scales. Pretoria: Human Sciences Research Council. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE ( 2010): Functional topography of the cerebellum in verbal working memory. Neuropsychol Rev 20: 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL ( 1998): Neuropsychological comparison of alcohol‐exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology 12: 146–153. [DOI] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D ( 2000): Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Public Health 90: 1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL ( 2007): The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend 88: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Klingberg T ( 2008): Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci 11: 103–107. [DOI] [PubMed] [Google Scholar]
- Meintjes EM, Jacobson JL, Molteno CD, Gatenby JC, Warton C, Cannistraci CJ, Hoyme HE, Robinson LK, Khaole N, Gore JC, Jacobson SW ( 2010): An FMRI study of number processing in children with fetal alcohol syndrome. Alcohol Clin Exp Res 34: 1450–1464. [DOI] [PubMed] [Google Scholar]
- Menon V, Rivera SM, White CD, Glover GH, Reiss AL ( 2000): Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage 12: 357–365. [DOI] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C ( 2011): Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 35: 1404–1417. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB ( 2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Mattson SN, O'Connor MJ, Sowell ER ( 2009): Altered frontal‐parietal functioning during verbal working memory in children and adolescents with heavy prenatal alcohol exposure. Hum Brain Mapp 30: 3200–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL ( 1998): Neuropsychological deficits in adolescents with fetal alcohol syndrome: Clinical findings. Alcohol Clin Exp Res 22: 1998–2012. [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV ( 2001): Reorganization of frontal systems used by alcoholics for spatial working memory: An fMRI study. NeuroImage 14: 7–20. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA ( 2004): Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage 22: 562–573. [DOI] [PubMed] [Google Scholar]
- Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, O'Connor MJ, Bookheimer SY, Sowell ER ( 2011): Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: The effects of methamphetamine, alcohol, and polydrug exposure. NeuroImage 54: 3067–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, Carlson S ( 2009): Cognitive and motor loops of the human cerebro‐cerebellar system. J Cogn Neurosci 22: 2663–2676. [DOI] [PubMed] [Google Scholar]
- Santhanam P, Li Z, Hu X, Lynch ME, Coles CD ( 2009): Effects of prenatal alcohol exposure on brain activation during an arithmetic task: An fMRI study. Alcohol Clin Exp Res 33: 1901–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Barnhart TE, Larson JA, DeJesus OT, Mukherjee J, Nickles RJ, Converse AK, Roberts AD, Kraemer GW ( 2005): Moderate‐level prenatal alcohol exposure alters striatal dopamine system function in rhesus monkeys. Alcohol Clin Exp Res 29: 1685–1697. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J ( 1998): Neuroimaging analyses of human working memory. Proc Natl Acad Sci USA 95: 12061–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW ( 2002): Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex 12: 856–865. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O'Hare ED, McCourt ST, Mattson SN, O'Connor MJ, Bookheimer SY ( 2007): Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. Neuroreport 18: 635–639. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW ( 2008): Abnormal cortical thickness and brain‐behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex 18: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, Bazinet AD, Fryer SL, Tapert SF, Mattson SN, Riley EP ( 2009): BOLD response during spatial working memory in youth with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 33: 2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD ( 2009): Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. Neuroimage 44: 489–501. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD ( 2010): An fMRI study of intra‐individual functional topography in the human cerebellum. Behav Neurol 23: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand F, Forssberg H, Klingberg T, Norrelgen F ( 2008): Phonological working memory with auditory presentation of pseudo‐words—An event related fMRI Study. Brain Res 1212: 48–54. [DOI] [PubMed] [Google Scholar]
- Stratton KM, Howe C, Battaglia F ( 1996): Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, DC: National Academy Press. [Google Scholar]
- Streissguth AP, Bookstein FL, Sampson PD, Barr HM ( 1993): The Enduring Effects of Prenatal Alcohol Exposure on Child Development, Birth Through 7 Years: A Partial Least Squares Solution. Ann Arbor: University of Michigan Press. [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Vasic N, Lohr C, Steinbrink C, Martin C, Wolf RC ( 2008): Neural correlates of working memory performance in adolescents and young adults with dyslexia. Neuropsychologia 46: 640–648. [DOI] [PubMed] [Google Scholar]
- Voytek B, Knight RT ( 2010): Prefrontal cortex and basal ganglia contributions to visual working memory. Proc Natl Acad Sci USA 107: 18167–18172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE ( 2003): Neuroimaging studies of working memory: A meta‐analysis. Cogn Affect Behav Neurosci 3: 255–274. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Plichta MM, Sambataro F, Fallgatter AJ, Jacob C, Lesch KP, Herrmann MJ, Schonfeldt‐Lecuona C, Connemann BJ, Gron G, Vasic N ( 2009): Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention‐deficit/hyperactivity disorder. Hum Brain Mapp 30: 2252–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figures


