Abstract
Noninvasive brain imaging methods provide useful information on cerebral involution and degenerative processes. Here we assessed cortical degeneration in 20 nondemented patients with Parkinson's disease (PD) and 20 healthy controls using three quantitative neuroanatomical approaches: voxel‐based morphometry (VBM), cortical folding (BrainVisa), and cortical thickness (FreeSurfer). We examined the relationship between global and regional gray matter (GM) volumes, sulcal indices, and thickness measures derived from the previous methods as well as their association with cognitive performance, age, severity of motor symptoms, and disease stage. VBM analyses showed GM volume reductions in the left temporal gyrus in patients compared with controls. Cortical folding measures revealed significant decreases in the left frontal and right collateral sulci in patients. Finally, analysis of cortical thickness showed widespread cortical thinning in right lateral occipital, parietal and left temporal, frontal, and premotor regions. We found that, in patients, all global anatomical measures correlated with age, while GM volume and cortical thickness significantly correlated with disease stage. In controls, a significant association was found between global GM volume and cortical folding with age. Overall these results suggest that the three different methods provide complementary and related information on neurodegenerative changes occurring in PD, however, surface‐based measures of cortical folding and especially cortical thickness seem to be more sensitive than VBM to identify regional GM changes associated to PD. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: Parkinson's disease, voxel‐based morphometry, gray matter volume, cortical folding, brain sulci, cortical thickness, gyral white matter
INTRODUCTION
Magnetic resonance imaging (MRI) has been used in the study in vivo of cerebral degeneration occurring in Parkinson's disease (PD) [Whitwell and Josephs,2007]. There are several methods based on this technique that allow the analysis of global or local volumetric degeneration of the brain. For whole brain volumetric analysis, voxel‐based morphometry (VBM) studies have shown gray matter (GM) reductions in frontal, limbic, and paralimbic areas of nondemented PD patients as well as in temporal associative regions [Beyer et al.,2007; Burton et al.,2004; Nagano‐Saito et al.,2005; Ramirez‐Ruiz et al.,2005; Summerfield et al.,2005]. Moreover, these GM decreases have been related to cognitive impairment in these patients such as visuospatial and visuoperceptual deficits [Pereira et al.,2009].
With the development of powerful three‐dimensional‐based image‐processing techniques, recent studies have proposed sulcal area and cortical folding, a measure that relies on gross anatomical landmarks of the cortical surface describing the burying of the cortex [Cachia et al.,2003,2008], as new indicators of brain degeneration. Previous studies have shown that widening of cortical sulci and decreases of sulcal depth are associated with normal aging [Kochunov et al.,2005,2008], mild cognitive impairment (MCI) [Im et al.,2008a], and Alzheimer's disease (AD) [Bastos‐Leite et al.,2006; Im et al.,2008a]. Specifically, the collateral fissure and parieto‐occipital sulcus suffer the most significant changes during healthy aging [Kochunov et al.,2005], while in MCI patients, these abnormalities particularly affect the frontal, temporal and parietal lobes and in AD spread through the entire cortex [Im et al.,2008a]. Measures of sulcal widening in the anterior cingulate and superior frontal sulci have been related to executive dysfunction in senescing subjects [Kochunov et al.,2009]. Sulcal indices of the cingulate and calcarine sulci have shown to correlate with deficits on verbal fluency and visuospatial tasks respectively in patients with AD [Mega et al.,1998]. In PD, only one study has analyzed sulcal morphological changes using a visually guided method to measure the depth of the olfactory sulcus and its relationship with olfactory deficits [Kim et al.,2007]. In that study no evidences of sulcal alterations were found.
Recently, the width of the cortical GM layer that covers the surface of the brain, referred to as cortical thickness, has been assessed in a variety of disorders. Most consisting findings point to cortical thinning in the lateral and medial temporal lobe of MCI patients [Seo et al.,2007] and to general cortical thinning of the frontal, temporal, parietal and occipital regions in patients with AD [Singh et al.,2006]. Some studies have shown that cortical thickness is able to detect early stages of preclinical AD [Fennema‐Notestine et al.,2009] and is a potential marker to discriminate different clinical diagnosis such as AD and frontotemporal dementia [Du et al.,2007]. Like voxel‐based and sulcal morphological measures, cortical thickness has also been associated with performance in neuropsychological tests. In AD patients, cortical thinning of the perirhinal and parahippocampal cortices was related to episodic memory deficits [Dickerson et al.,2009], while in elderly subjects measures of neuroticism and extraversion correlated with prefrontal thickness [Wright et al.,2007]. To our knowledge, only one study has assessed cortical thickness in a sample of PD patients showing cortical thinning of the inferior parietal, frontal and temporal cortices compared with healthy controls [Lyoo et al.,2010].
All together, these studies indicate that GM volume, cortical folding and thickness are useful measures to assess the neuroanatomical patterns associated with aging, neurodegerative diseases such as AD, and very possibly PD. According to neuropathological studies of PD [Braak and Braak,2000; Braak et al.,2003,2004], in the earliest disease stages neural degeneration occurs in motor nuclei and limbic areas that propagate to the inferior temporal and paralimbic cortex and further reach associative areas like the prefrontal lobes. At this stage, patients become symptomatic, expressing the typical features of PD. Taking into consideration these studies, we hypothesized that nondemented patients with PD would present GM volume decreases in limbic, temporal, paralimbic, and prefrontal areas of the cortex. Similarly, significant reductions of sulcal indices and cortical thickness within the previous areas were expected, showing a regional correspondence of pathological alterations between volume‐based and surface‐based measures.
METHODS
Participants
Twenty patients with PD and 20 normal controls were included. This sample was selected from a pool of subjects recruited in an outpatient Movement Disorders Clinic (Parkinson's Disease and Movement Disorders Unit, Department of Neurology, Hospital Clinic, Barcelona, Spain) during a 6‐month period. Only subjects with MRI scans that could be preprocessed and analyzed using the three methods employed in this study (VBM, cortical folding, and cortical thickness) were included. In line with this, 12 subjects had to be excluded due to masking, segmentation or sulcus labeling errors during the cortical folding image analysis (see Cortical Folding section).
For PD patients the following inclusion criteria were set: diagnosis of idiopathic PD according to the UK Parkinson's disease Society Brain Bank criteria [Daniel and Lees,1993], namely bradykinesia and at least one of the three cardinal signs (resting tremor, muscular rigidity, postural instability); a good initial response to l‐dopa or dopamine agonists; unilateral onset; persistent asymmetry affecting mostly the side of onset; lack of evidence of other medical conditions associated with atypical parkinsonism (i.e. progressive supranuclear palsy or multiple‐system atrophy); lack of diagnostic criteria for dementia associated with Parkinson's disease [Emre et al.,2007]; and absence of clinical depression.
The exclusion criteria for the patient group consisted of: other brain disorders apart from PD; parkinsonism due to antipsychotics or other drugs; suspected dementia with Lewy bodies (signs of cognitive impairment during the first year, transient loss of consciousness, neuroleptic sensitivity); delirium; confusion; amnestic disorder; neuropsychiatric diseases; severe vascular risk factors (heart failure, hypertension, or diabetes); vascular lesions; and past traumatic brain injury on MRI. Written informed consent was obtained from patients and controls after full explanation of the procedures. This investigation was approved by the ethics committee of Hospital Clinic, Barcelona.
Patients were clinically assessed by the motor subsection of the Unified Parkinson's Disease Rating Scale (UPDRS) [Fahn and Elton,1987] and the Hoehn and Yahr scale [1967]. They were further screened for dementia and depression using the mini‐mental state examination (MMSE) [Folstein et al.,1975] and the Beck's Depression Inventory (BDI) [Beck et al.,1996], respectively. Five patients from our sample had visual hallucinations.
Demographic information including age and gender was collected from the entire sample. Furthermore, all participants underwent a neuropsychological test battery that comprised: Semantic and Phonemic Fluency tests, the Rey's Auditory Verbal Learning Test (RAVLT), the Stroop test and the forward and backward Digits subtests from WAIS‐III. The procedures for neuropsychological assessment are described in Lezak et al. [2004]. PD patients were evaluated on the clinical and neuropsychological tests while on medication. Antiparkinsonian treatments were recorded and the total daily equivalent dose of levodopa was calculated for each patient. All subjects underwent neuropsychological testing in first place and MRI scanning in the same week.
Image Acquisition
Anatomical MRI scans were obtained on a 3.0T Magnetom Trio Tim Siemens (Erlangen, Germany) at the Center of Imaging Diagnosis Clinic (CDIC) of Hospital Clinic, Barcelona. MRI parameters of the three‐dimensional MPRAGE Saggital ISO sequence were as follows: repetition time (TR) = 2300 ms; echo time (TE) = 2.98/3.01 ms; inversion time (TI) = 900 ms; 1 mm thickness; field of view (FOV) = 24 × 24 mm; 256 × 256 matrix. Inspection of anatomical abnormalities on MRI scans was carried out by an expert neuroradiologist (N.B.).
Brain Structural Analyses
Voxel‐based morphometry
Image preprocessing was conducted using the VBM8 toolbox (available at: http://dbm.neuro.uni-jena.de/vbm) implemented in SPM8 (available at: http://www.fil.ion.ucl.ac.uk/spm/). In brief, images were classified into GM, white matter (WM) and cerebrospinal fluid (CSF) [Ashburner and Friston,2005; Cuadra et al.,2005; Rajapaske et al.,1997; Tohka et al.,2004], the tissue classified GM maps were applied a high‐dimensional DARTEL normalization [Ashburner et al.,2007] modulating for nonlinear effects only and finally smoothed using a Gaussian smoothing kernel of 12‐mm full width at half‐maximum. The resulting final voxel size was 1.5 mm3.
At the second statistical level, smoothed GM images were compared between PD patients and controls using a two‐sample t test, in which age and gender were included as nuisance variables. Because normalization of nonlinear effects only had been applied during VBM8 preprocessing, which corrects for differences in GM, WM, and intracranial volumes (ITV) [Buckner et al.,2004], there was no further need to control for these variables in the statistical model. Additionally, an absolute threshold mask of 0.1 was used. The final results were corrected for multiple comparisons using a family wise error (FWE) rate correction set at P < 0.05. The MNI coordinates of significant clusters were converted into Talairach coordinates using an automated nonlinear match method that adjusts for differences between the two atlases (available at: http://imaging.mrc-cbu.cam.ac.uk/). The Marsbar toolbox [Brett et al.,2002] was used to extract the mean GM values from significant clusters. Differences between groups in local volume reductions identified in patients compared with controls were further assessed using analyses of variance (ANOVA), with age, gender and ITV as covariates.
Description of VBM methods, statistical analyses and results were performed following the rules for reporting VBM studies by Ridgway et al. [2008].
Cortical folding
We removed 12 MRI scans (23%) from this study, seven of which belonged to patients with PD and five to healthy controls due to failure in computing a brain mask for each hemisphere (three), segmentation problems (two), errors in computation of cortical fold graphs (three), and sulcal labeling errors (four). The final MRI images from 20 patients and 20 controls were imported into the BrainVisa database (available at: http://brainvisa.info/) [Mangin et al.,2004]. Briefly, image preprocessing included: registration to the Talairach frame; inhomogeneity bias correction [Mangin,2000]; segmentation into GM, WM, and CSF [Mangin et al.,2004]; extraction and labeling of brain sulci [Mangin et al.,1995; Riviere et al.,2002]; and calculating global and local sulcal indices [Cachia et al.,2008; Penttila et al.,2009]. Global sulcal index was estimated for each hemisphere as the percentage ratio between total sulcal area and the outer cortex area, while local sulcal indices were calculated as the percentage ratio between the area of a labeled sulcus and the outer cortical area. For local sulcal measurements, we selected six sulci based on our study's hypothesis that PD patients would present GM reductions in temporal, limbic, paralimbic, and prefrontal areas: the inferior temporal sulcus, the collateral fissure, the occipitotemporal sulcus, the anterior cingulate sulcus, the anterior sylvian fissure, and the superior frontal sulcus (see Fig. 1). Differences in global and local sulcal indices from the selected sulci were assessed separately using multivariate ANOVA with sulcal measures as intrasubjects factors, group (PD patients, controls) as an intersubject factor and age, gender, and brain volume as covariates. The choice of including brain volume as a covariate was based on a previous report by Toro et al. [2008], in which a strong relationship was found between cortical surface area and brain volume and so we wanted to control for the confounding effects of this variable. In order to correct for multiple comparisons, a significance level of P < 0.004 (0.05/12 sulci) was applied to the previous analyses.
Figure 1.
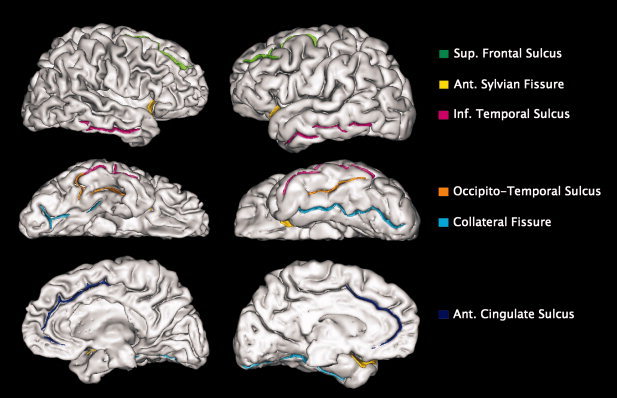
Sulcal structures selected for measuring local cortical folding: the superior frontal sulcus, anterior sylvian fissure, inferior temporal sulcus, occipitotemporal sulcus, collateral fissure, and anterior cingulate sulcus. On the left: outer cortical surface of a PD patient. On the right: outer cortical surface of a control subject.
Cortical thickness
Measurements of cortical thickness were performed using the FreeSurfer software package (version 4.3.1, available at: http://surfer.nmr.harvard.edu), [Fischl and Dale,2000; Fischl et al.,1999b]. The implemented processing stream involved: removal of nonbrain tissue; transformation to the Talairach reference space; segmentation into GM and WM [Dale et al.,1999]; correction of topological defects [Fischl et al.,2001]; intensity normalization [Dale et al.,1999]; and subvoxel representation of the GM/WM boundary and pial surfaces [Dale et al.,1999; Fischl et al.,1999a]. Cortical thickness was calculated as the shortest distance between the previous surfaces at each vertex across the cortical mantle. Maps were smoothed using a circularly symmetric Gaussian kernel across the surface with a FWHM of 15 mm and averaged across subjects. Moreover, estimated total intracranial volume (eTIV) was calculated from the Freesurfer processing stream. To assess cortical thickness differences between the two groups at each vertex of the surface we performed a general linear model controlling for the effects of age, gender, and intracranial volume. In order to correct for multiple comparisons, Monte Carlo simulations were performed to identify significant vertex‐wise group cluster differences. Furthermore, in order to quantify brain alterations, regional cortical thickness values were extracted from significant clusters of the previous analysis for each subject and between group differences were assessed by means of ANOVA with age, gender and ITV as covariates.
Regional WM volumes
Using FreeSurfer (available at: http://surfer.nmr.harvard.edu), additional measurements were performed to assess the potential role of regional WM volumes underlying GM volumes, brain sulci and cortical thickness changes occurring in PD. Following the software's processing stream, after topological corrections, the cortical surface was parcellated according to procedures described by Fischl et al. [2004]. Each vertex was assigned a neuroanatomical label based on: (a) the probability of each label at each location in a surface‐based atlas, based on a manually parcellated training set; (b) local curvature information; and (c) contextual information, encoding spatial neighborhood relationships between labels. Using this automated labeling system [Desikan et al.,2006; Fischl et al.,2004] the cortical surface was divided in 33 gyral‐based areas in each hemisphere, which were manually inspected for accuracy. By means of a recently developed algorithm [Fjell et al.,2008; Salat et al.,2009] the WM volume in the gyri underneath each cortical label was calculated. Based on our study's hypotheses, the following WM labels were included for analysis: medial frontal, superior frontal, superior temporal, inferior temporal and insula. Differences in WM volume between patients and controls were assessed using ANOVA with volumes as intrasubjects factors, group (PD patients, controls) as an intersubject factor and age, gender, and ITV as covariates.
Statistical Analyses
To assess the relationship between whole brain indices derived from each method we performed correlation analyses using whole‐brain GM volumes (VBM), global sulcal index and average cortical thickness in the following groups separately: whole sample, controls, and PD patients.
Correlation analyses were also carried out to examine the association between the previous global indices and stage of the disease, severity of motor symptoms, global cognitive performance, and age in the three groups, when appropriate. Severity of motor symptoms was indexed by the motor section (part III) of the UPDRS and the patient's disease stage was assessed by the HY rating scale. According to these scales, higher scores represent greater motor deficits and more advanced disease stage, respectively. Regarding cognitive performance, scores from all neuropsychological tests were subjected to a factor analysis to produce noncollinear estimates of global cognitive performance.
Additional analyses were performed to assess the correlation between regional changes detected by the three methods in each brain hemisphere, their relationship with demographic and clinical variables and the role of WM reductions in local GM, sulcal, and thickness changes in controls and PD patients. Correlations in the whole sample were also carried out and have been included as Supporting Information.
For correlation analyses associated with disease stage as indexed by the HY rating scale, which is an ordinal variable, Spearman correlations were used while the rest of correlation analyses were based on Pearson coefficients. Moreover, in order to compare correlation coefficients from the previous analyses between controls and PD patients we applied Fisher's Z transformation and the difference between coefficients was computed following: z = (Z r 1 − Z r2)/√(1/(20 − 3) + 1(20 − 3)).
Importantly, all the analyses performed in the PD patient's group included age and gender as nuisance variables or covariates in order to exclude their potential confounding effects from disease‐related processes.
A Bonferroni‐adjusted significance level of P < 0.001 was applied in order to correct for multiple comparisons. Statistical analyses were carried out using SPSS 16.0 (SPSS for Windows, Version 16.0.1, 2008, SPSS Inc., 1989–2007).
RESULTS
Clinical and neuropsychological data of the whole sample are presented and were analyzed as raw scores (Table I). There were no significant differences in age or gender between groups. PD patients had a lower MMSE score than controls, although none scored less than 26. Furthermore, they performed significantly worse than controls in all neuropsychological tests.
Table I.
Clinical and neuropsychological data of the sample
| PD patients | Controls | x 2/t | P | |
|---|---|---|---|---|
| Subjects | 20 | 20 | — | — |
| Gender (male/female) | 13/7 | 14/6 | 0.11 | 0.739 |
| Mean age (yr) | 64 (9.53) | 59.1 (10.9) | −1.52 | 0.136 |
| MMSE score | 28.5 (1.9) | 29.8 (0.7) | 2.90 | 0.008 |
| HY stage | 2.4 (0.9) | — | — | — |
| UPDRS III | 24.9 (15.5) | — | — | — |
| Duration of disease (yr) | 6.8 (5.8) | — | — | — |
| Dopaminergic dosis (mg) | 627 (487.7) | — | — | — |
| Semantic fluency | 15 (6.7) | 20.4 (5.3) | 2.80 | 0.008 |
| Phonemic fluency | 11.9 (6.1) | 18.1 (5.8) | 3.30 | 0.002 |
| Rey's auditory verbal learning test | ||||
| Learning score | 34.3 (11.7) | 48.4 (10) | 4.10 | 0.0002 |
| Delayed recall score | 6.7 (3.8) | 10.3 (1.9) | 3.73 | 0.001 |
| Recognition score | 17 (10.6) | 25.8 (6.3) | 3.18 | 0.003 |
| STROOP | ||||
| Word reading | 79.9 (31.7) | 107.6 (17) | 3.36 | 0.002 |
| Colour naming | 48.7 (18.1) | 69 (12.4) | 4.03 | 0.0003 |
| Words + colours | 24.8 (10.5) | 41.3 (9.8) | 5.04 | 0.00001 |
| Interference score | −5.5 (8.5) | 0.4 (7.8) | 2.24 | 0.032 |
| Digits | ||||
| Forward | 7.5 (1.5) | 8.7 (1.6) | 2.40 | 0.022 |
| Backward | 4.2 (1.9) | 6.9 (1.8) | 4.53 | 0.00007 |
Values are given in means followed by (standard deviations).
MMSE score, mini‐mental state examination score; HY stage, Hoehn and Yahr stage; UPDRS III, unified Parkinson's disease rating scale III (motor subscale).
Regional VBM Differences
For the VBM analyses, PD patients showed significant GM volume reductions in the left superior temporal gyrus (BA 41) while controlling for age and gender effects (Fig. 2, Table II). No GM decreases were observed in controls compared with PD patients.
Figure 2.
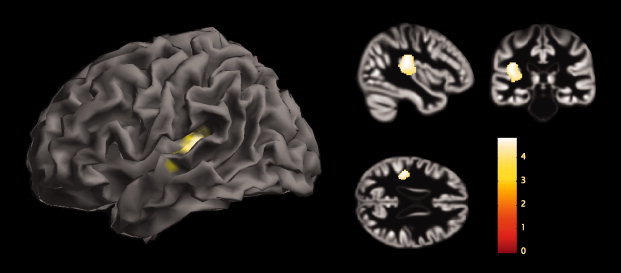
Group VBM differences. Results from the analysis of VBM8 showing gray matter volume reductions in PD patients compared with healthy elderly controls are displayed in the form of statistical maps superimposed on the surface of a standardized brain and on the sample's template created during Dartel normalization with peak intensity located at the global maxima.
Table II.
Significant gray matter volume reductions in PD patients compared with controls
| Brain region (Brodmann area) | Cluster size (mm3) | Coordinates | PD patients, mean (SD) | Controls, mean (SD) | Main effect, F (P value) | t | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| L superior temporal gyrus (BA 41) | 1,752 | −34.7 | −34.5 | 10 | 0.36 (0.04) | 0.44 (0.04) | 27.397 (<0.001) | 4.28 |
| −50.5 | −17.6 | −1.6 | 4.27 | |||||
The reported cluster is corrected at P < 0.05 FWE. The coordinates x, y, and z refer to the anatomical location, indicating standard stereotactic space as defined by Talairach and Tournoux [1988].
L, left.
Regional Cortical Folding Differences
Concerning sulcal indices, ANOVA analyses showed significant local reductions in the left superior frontal sulcus (left: F (1,39) = 9.493, P < 0.004) and the right collateral fissure (F (1,39) = 10.399, P < 0.003) in patients compared with controls, controlling for multiple comparisons (Fig. 3, Table III). No significant differences were found for the other brain sulci or global sulcal indices.
Figure 3.
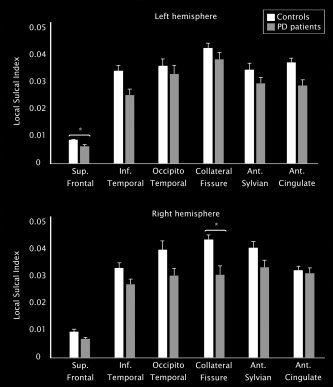
Graphic presenting data distribution with average means and standard errors of local sulcal indices of PD patients and controls. (*) Indicates significant results after correction for multiple comparisons.
Table III.
Results from the ANOVA analysis of global and local sulcal indices differences between PD patients and controls
| PD patients, mean (SD) | Controls, mean (SD) | Main effect, F (P value) | |
|---|---|---|---|
| Left hemisphere | |||
| Global sulcal index | 1.29 (0.2) | 1.39 (0.1) | 1.571 (0.219) |
| Local sulcal indices | |||
| Sup. frontal sulcus | 0.006 (0.003) | 0.009 (0.001) | 9.493 (0.004)a |
| Inf. ant. temporal sulcus | 0.025 (0.01) | 0.034 (0.01) | 6.763 (0.013) |
| Collateral fissure | 0.033 (0.01) | 0.043 (0.01) | 2.187 (0.148) |
| Ant. cingulate sulcus | 0.029 (0.01) | 0.038 (0.01) | 7.756 (0.008) |
| Ant. sylvian fissure | 0.029 (0.008) | 0.035 (0.008) | 2.557 (0.119) |
| Occipitotemporal sulcus | 0.033 (0.01) | 0.035 (0.01) | 0.116 (0.735) |
| Right hemisphere | |||
| Global sulcal index | 1.25 (0.2) | 1.36 (0.1) | 0.763 (0.389) |
| Local sulcal indices | |||
| Sup. frontal sulcus | 0.006 (0.002) | 0.009 (0.004) | 4.910 (0.033) |
| Inf. ant. temporal sulcus | 0.027 (0.01) | 0.033 (0.01) | 5.147 (0.029) |
| Collateral fissure | 0.031 (0.01) | 0.044 (0.01) | 10.399 (0.003)a |
| Ant. cingulate sulcus | 0.031 (0.01) | 0.032 (0.01) | 0.021 (0.885) |
| Ant. sylvian fissure | 0.032 (0.01) | 0.04 (0.01) | 3.272 (0.079) |
| Occipitotemporal sulcus | 0.029 (0.01) | 0.037 (0.01) | 3.667 (0.063) |
Ant., anterior; Sup., superior; Inf., inferior.
Corrected for multiple comparisons.
Regional Cortical Thickness Differences
PD patients showed significant cortical thinning in the left lateral occipital cortex (maxima) that extended to inferior and superior parietal areas, with respect to controls. In addition, significant cortical thinning was also found in the right hemisphere, concretely in the inferior parietal cortex, extending into various regions of the lateral occipital, supramarginal, inferior, middle, and superior temporal cortex. Finally, cortical thinning was also observed in the right frontal cortex, comprising the pars opercularis, triangularis, precentral, and postcentral areas in PD patients (Fig. 4, Table IV). No cortical thickness reductions were found in controls compared with patients.
Figure 4.
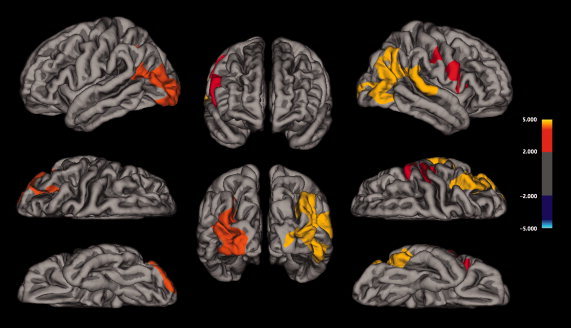
Group cortical thickness differences. Results from the analysis of cortical thickness showing cortical thinning in PD patients compared with healthy elderly controls are displayed at each vertex of the surface of a standardized brain (averaged over all subjects) in terms of t statistical maps.
Table IV.
Significant clusters for the left and right hemispheres, mean cortical thickness, and P values from the Monte Carlo simulation resulting from the vertex‐wise comparison of cortical thickness between PD patients and controls
| Cortical area | Cluster size (mm2) | Coordinates | PD patients, mean (SD) | Controls, mean (SD) | Main effect, F (P value) | CWP | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Left hemisphere | ||||||||
| Lateral occipital | 4,136.89 | −24.7 | −92.9 | 4.5 | 2.23 (0.13) | 2.42 (0.08) | 28.892 (<0.001) | 0.0027 |
| Right hemisphere | ||||||||
| Inferior parietal | 6,513.06 | 45.8 | −53.2 | 21.5 | 2.42 (0.14) | 2.61 (0.11) | 18.880 (<0.001) | 0.0001 |
| Frontal pars opercularis | 2,306.74 | 40 | 17.7 | 7.9 | 2.14 (0.15) | 2.29 (0.1) | 9.298 (0.004) | 0.0153 |
The reported clusters have been corrected for multiple comparisons. The coordinates x, y, and z refer to the anatomical location, indicating standard stereotactic space as defined by Talairach and Tournoux [1988].
CWP: Cluster‐wise P value. P value: significant between group differences.
Regional WM Volume Differences
No statistically significant differences were found between PD patients and controls in regional or global WM volumes (Supporting Information, Table I).
Correlations Between Global Cerebral Indices
Analyses performed separately in controls and PD patients did not show any significant correlations between global anatomical indices in these groups. In healthy controls, GM volumes and global sulcal index significantly correlated with age (r = −0.714, P < 0.001; r = −0.708, P < 0.001, respectively). On the other hand, in PD patients, GM volumes and cortical thickness correlated with disease stage (r = −0.684, P < 0.001; r = −0.627, P < 0.001) (Fig. 5), while controlling for age and gender effects. Moreover, correlations with age were also assessed in patients and, as opposed to controls, were found to be significant for all global indices in the following order: brain sulci (r = −0.752, P < 0.001), GM volumes (r = −0.692, P < 0.001) and cortical thickness (r = −0.680, P < 0.001) (Fig. 5). Differences in correlation coefficients between controls and PD patients were not significant for the correlation between age and GM volume (P < 0.899) or between age and brain sulci (P < 0.783). No significant correlations were found between global indices and cognitive performance or severity of motor symptoms. Correlations in the whole sample have been included as Supporting Information.
Figure 5.
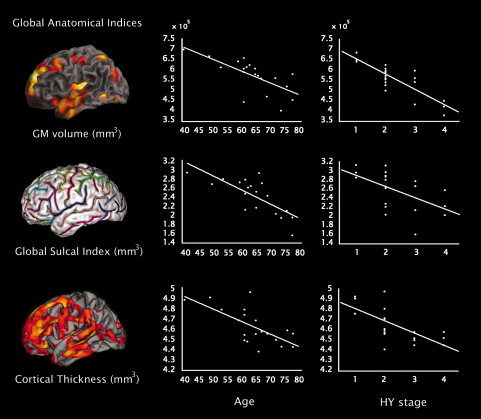
Correlations between whole‐brain GM volume, global sulcal index and average cortical thickness plotted versus patient's age and disease stage as indexed by the Hoehn & Yahr scale. Correlations with Hoehn & Yahr stage have not been corrected for age and gender effects only for picture purposes; the corrected values can be found in the text. All correlations were statistically significant except the one between cortical folding and Hoehn & Yahr stage.
Correlations Between Regional Cerebral Indices
No significant correlations were found between local changes in GM volume, brain sulci, and thickness in PD patients or controls. Concerning the relationship between local changes and demographic/clinical variables, only the patient's age was found to correlate with reductions of the left collateral fissure (r = −0.741, P < 0.001).
We also performed additional analyses to assess the potential influence of regional WM reductions in local changes of GM volume, sulci, and mean thickness. We observed significant correlations in PD patients between the left anterior cingulate sulcus and right medial frontal (r = 0.754, P < 0.001), as well as right superior frontal WM regions (r = 0.719, P < 0.001) while controlling for age and gender effects.
No significant correlations were found between any local measures in the control's group. Correlations between regional anatomical measures in the whole sample are provided as Supporting Information.
DISCUSSION
In this study we compared voxel‐based morphometry, cortical folding and cortical thickness in order to determine specific measures contributing to brain degeneration in patients with PD. Our findings suggest that these three different methods detect alterations of the GM layer in PD patients compared with healthy controls and furthermore correlate with disease stage and age, providing both complementary and related information.
To our knowledge, this is the first study to analyze patterns of regional neuroanatomical changes in PD using three widely used technical approaches. Studies using VBM have identified GM reductions in the superior temporal gyrus in nondemented PD patients [Beyer et al., 2006; Ramirez‐Ruiz et al., 2007; Summerfield et al.,2005]. Our results are in accordance with these studies in that GM decreases of superior temporal areas were found as well in our sample. Moreover, compared with previous VBM studies, which have mainly used uncorrected levels of statistical significance, our study has the advantage of including a Family Wise Error (FWE) rate correction to control for multiple comparisons, reducing the rate of false positives and thus providing true information on the comparisons that were tested.
Analyses of cortical folding showed that, in this study, PD patients presented lower sulcal indices in the left superior frontal and right collateral fissure compared with controls. To date, the only study assessing sulcal morphology in PD used a visually guided method to measure the depth of the olfactory sulcus, failing to find significant differences between patients and controls or a correlation between olfactory deficits and sulcal depth [Kim et al.,2007]. Our study has the advantage of using a method with robust computer‐based identification algorithms that allows for a precise automatic labeling of brain sulci and avoiding biases introduced by visual identification methods. Moreover, it has been previously suggested that cortical complexity is more related to cortical folding and convolutions of sulcal shape than sulcal depth [Im et al.,2006,2008b].
Concerning cortical thickness, our study showed that PD patients presented significant cortical thinning in lateral occipital and inferior parietal regions of the right hemisphere as well as left parietal, occipital, frontal, temporal and premotor areas. To our knowledge, only one study has assessed cortical thickness in PD [Lyoo et al.,2010] showing thinning of inferior parietal, latero‐occipital, middle temporal, supramarginal, and frontal regions like we found in our study, but also in fusiform and lingual areas. However, the authors from the previous study considered cortical areas with an uncorrected significance level, while in our study Monte Carlo simulations were performed to control for multiple comparisons, which are far more restrictive. Hence, the differences in the results from both studies are most likely related to the applied statistical corrections.
When comparing regional cortical changes identified by the different methods, analyses of VBM seemed to be the most conservative, followed by cortical folding and cortical thickness. These differences between methods could be related to several issues. For instance, cortical folding and thickness are fundamentally surface‐based methods measuring differences in the GM layer based on cortical surface geometry. On the contrary, VBM performs whole‐brain voxel‐based comparisons of the local GM between groups of subjects [Ashburner and Friston,2000,2001]. Previous studies combining VBM with cortical thickness show that these techniques tend to lead to different findings. For instance, in a study performed in adults with autism, Hyde et al. [2010] found cortical thickness increases in several bilateral areas of frontal, temporal, parietal and occipital lobes compared with controls. However, when VBM analyses were performed in the same data set, GM increases were only identified in the midbrain and frontal gyrus. In another study with autistic subjects, Jiao et al. [2010] constructed diagnostic models based on cortical thickness and compared them with diagnostic models relying on volumetric neurodegeneration. The authors from this study found that, similarly to our study, thickness‐based diagnostic models were superior to those based on VBM, achieving the best classification performance between autistic subjects and controls. Finally, it has been reported that cortical thickness methods detect more changes in the GM layer in normal aging compared with VBM [Hutton et al.,2009]. This difference in sensitivity between these two techniques has been related to limitations of cortical GM assessment by VBM in that it merges information about morphology, size, and position [Ashburner and Friston,2001] and the final measures include a mixture of thickness and cortical folding [Park et al.,2009; Voets et al.,2008], being therefore less specific. Moreover, VBM analyses are particularly sensitive to misregistration errors across different brains and incorrect classification of tissue classes during the segmentation process, which can be misinterpreted as cortical folding or thickness reductions, ultimately providing essentially false results [Ashburner,2009]. By contrast, cortical thickness provides a more direct index of cortical morphology that is less susceptible to positional variance given that the extraction of the cortex follows the GM surface despite local variations in its position [Kim et al.,2005; MacDonald et al., 2001]. This method differentiates between cortices of opposing sulcal walls within the same sulcal bed, enabling more precise measurement in deep sulci [Lerch and Evans,2005]. Moreover, one of the main advantages of cortical thickness measures is that they allow for subvoxel precision because thickness values are assigned to individual vertices instead of voxels [Fischl and Dale,2000]. Finally, an additional issue that could be related to the differences in our results is that these different techniques require different levels of smoothing to increase the validity of the final statistical results. For instance, in VBM analyses a 12‐mm smoothing kernel was selected as it has been described as the ideal compromise to improve the validity of statistical inferences, reduce interindividual variation and to obtain good spatial resolution in VBM [Salmond et al.,2002]. On the contrary, cortical folding analyses with BrainVisa do not apply smoothing during image preprocessing [Cachia et al.,2008; Kochunov et al.,2005,2008,2009; Liu et al.,2010; Pentilla et al.,2009]. Finally, in cortical thickness analysis, FreeSurfer calculates the level of smoothing that must be applied during the Monte Carlo simulation, which is necessary in order to correct for multiple comparisons and in the case of this study was 15 mm.
What are our results reflecting with respect to PD? Our study's hypotheses were based on the well‐established staging of brain pathology by Braak et al. [2000,2003,2004], according to which PD patients become symptomatic in the last disease stages 4, 5, and 6. Briefly, in stage 4, neural damage occurs in temporal and paralimbic cortices while in stage 5 the lesions extend to the superior temporal gyrus, parietal cortex, and prefrontal areas. Finally, in stage 6, cortical pathology spreads into first order association areas, premotor fields and even, occasionally, to primary sensory and motor regions. Patients that participated in this study were all in the symptomatic stages of PD, however, our main question regarded in which of the previously described stages they could be placed. On one hand, VBM results showed GM reductions in superior temporal areas, which is fairly consistent with pathological changes occurring in stage 5. Analyses of cortical folding revealed sulcal decreases in medial inferior temporal and frontal regions, which are usually affected during stages 4 and 5. Finally, cortical thickness analyses showed thinning of regions consistent with stages 4 and 5 and furthermore, first order areas and premotor fields, which are only compromised in the last stage of brain pathology of the disease. Overall, these findings seem to suggest that cortical thickness might be more sensitive to pathological damage occurring in PD compared with cortical folding and finally VBM. This result is interesting and shows agreement with previous reports of cortical thickness in AD in that cortical thinning mirrors pathological changes identified with histology [Lerch et al.,2005; Singh et al.,2006; Seo et al.,2007]. For instance, in a study by Lerch et al. [2005], AD patients presented greater thinning in the medial temporal lobes as well as the posterior cingulate region, parietal and orbitofrontal cortex, showing agreement with progression of senile plaques and neurofibrillary tangles according to Braak's staging for AD [Braak et al.,1991,1996]. Cortical thickness is related to neuronal structural complexity features such as neuronal size, presynaptic terminals, and complexity of dendritic arborizations. In postmortem studies, frontal and temporal neocortical regions show evidence of cortical thinning with increasing age in the absence of neuronal number or density loss [Freeman et al.,2008]. Hence, changes in cortical thickness could be occurring much before than neuronal death takes place, indicating preclinical stages of a disease as suggested by Fennema‐Notestine et al. [2009]. In line with this, and similarly to our study, the only previous study assessing cortical thickness in PD [Lyoo et al.,2010] also showed cortical thinning consistent with later stages of brain pathology in these patients, suggesting that cortical thickness changes could occur early in the clinical course of PD. However, future longitudinal studies with wider sample sizes of patients as well as pathological postmortem confirmation would be required to assess the relationship between cortical thickness and Braak's staging.
Although no significant relationships were found between global cerebral indices, both GM volumes and cortical thickness correlated with disease stage in PD patients, indicating they are sensitive to disease progression. Moreover, all global anatomical measures were significantly related to age and specifically local reductions of the left collateral fissure correlated with age in the patient's group. It is well known that age is a strong predictive factor for development of dementia [Hobson and Meara,2004] and is associated with greater neurodegenerative and pathological changes in PD [Bouchard et al., 2007; Emre et al.,2003]. Thus, our findings seem to confirm the importance of this variable in PD‐related progression.
Interestingly, even though no significant WM decreases were found in PD patients with respect to controls, WM volume was generally reduced in most regions in patients and in particular, medial and superior frontal WM showed to be significantly related to one particular brain sulcus, the left anterior cingulate. Recent studies have shown that widening of cortical sulci has been primarily associated with decreased gyral WM volume in MCI, AD [Im et al.,2008a] and normal aging [Kochunov et al.,2009]. According to the well known tension‐based theory of morphogenesis [Hilgetag and Barbas,2006; Van Essen,1997], cortical folding processes and development of brain sulci are the result of mechanical tension produced by WM fibers that pull anatomical regions that are strongly connected towards one another. In our study, we did not find a significant relationship between WM volumes with any other anatomical measures but brain sulci, suggesting that, in agreement with the tension‐based theory, regional WM volume reductions could be playing a role in the genesis of cortical folding alterations in PD, similarly to previous reports in other diseases [Im et al.,2008a]. However, future studies including more sensitive measures of WM such as WM connectivity with FA would be required to understand better the relationship between folding indices and gyral WM volumes in PD.
Overall, our results suggest that surface‐based methods of cortical folding and especially of cortical thickness are sensitive to PD‐related neural degeneration. According to Panizzon et al. [2009], in genetic studies, using GM volume as an endophenotype for a disorder may actually confound the underlying architecture of brain structure given that it conflates the contributions of thickness and surface area and therefore may not capture the basic structural elements of the cortex. Our results seem to confirm these findings and strongly encourage the use of folding and thickness analyses in future studies assessing cortical atrophy in PD.
One of the limitations of this study was the small sample size, which reduced the statistical power of our findings. This factor also limits the interpretation of our results in the wider context of the neuropathology of Parkinson's disease, since we did not include newly diagnosed patients or advanced patients with Parkinson's disease and dementia (PDD). Moreover, although cortical thickness, folding and VBM detected changes in the cortical GM layer of PD patients with respect to controls, one would need pathological postmortem confirmation of our findings to know the true GM changes occurring in PD. Therefore, future studies assessing wider samples of PD patients in the different stages of the disease and involving postmortem pathological confirmation, would be essential to further assess the progression of the anatomical reductions and relationship between the methods used in this study with pathological changes.
CONCLUSIONS
We found that, compared with healthy controls, PD patients presented significant reductions of GM volume, sulcal indices and cortical thickness that correlated with the disease stage and age. However, surface‐based methods of cortical folding and especially cortical thickness seemed to be more sensitive to GM changes occurring in PD compared with VBM, indicating that these methods provide relevant information on cortical degeneration in PD.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors thank the Professors Arnaud Cachia, Peter Kochunov, and Domique Gefroy for their help in issues related to BrainVisa cortical analyses. The authors also thank Roser Sala‐Llonch and Silvia Juanes for their help in cortical thickness and statistical analyses.
REFERENCES
- Ashburner J, Friston KJ ( 2000): Voxel‐based morphometry—The methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2001): Why voxel‐based morphometry should be used. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2005): Unified segmentation. Neuroimage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- Ashburner J ( 2007): A fast diffeomorphic image registration algorithm. Neuroimage 38: 95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J ( 2009): Computational anatomy with the SPM software. Magn Res Imaging 27: 1163–1174. [DOI] [PubMed] [Google Scholar]
- Bastos‐Leite AJ, van Waesberghe JH, Oen AL, va der Flier WM, Scheltens P, Barkhof F ( 2006): Hippocampal sulcus width and cavities: Comparison between patients with Alzheimer disease and nondemented elderly subjects. Am J Neuroradiol 27: 2141–2145. [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK ( 1996): Beck Depression Inventory‐II. Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beyer M, Janvin CC, Larsen JP, Aarsland D ( 2007): A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel‐based morphometry. J Neurol Neurosurg Psychiatry 78: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TP, Malykhin N, Martin WRW, Hanstock CC, Emery DJ, Fisher NJ, Camicioli RM ( 2008): Age and dementia‐associated atrophy predominates in the hippocampal head and amygdale in Parkinson's disease. Neurobiol Aging 29: 1027–1039. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1991): Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol (Berl) 82: 239–259. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1996): Evolution of the neuropathology of Alzheimer's disease. Acta Neuropathol Scand Suppl 165: 3–12. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 2000): Pathoanatomy of Parkinson's disease. J Neurol 247: II/3–II/10. [DOI] [PubMed] [Google Scholar]
- Braak H, Tredici KD, Rub U, Vos RAI, Steur ENHJ, Braak E ( 2003): Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197–211. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Tredici KD ( 2004): Stages in the development of Parkinson's disease‐related pathology. Cell Tissue Res 318: 121–134. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J‐L, Valabregue R, Poline J‐B ( 2002): Region of interest analysis using an SPM toolbox. In: Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, Sendai, Japan.
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ ( 2004): A unified approach for morphometric and functional data analysis in young, old and demented adults using automated atlas‐based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage 23: 724–738. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT ( 2004): Cerebral atrophy in Parkinson's disease with and without dementia: A comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain 127: 791–800. [DOI] [PubMed] [Google Scholar]
- Cachia A, Mangin J‐F, Riviere D, Kherif F, Boddaert N, Andrade A, Papadopoulos‐Orfanos D, Poline J‐B, Bloch I, Zilbovicius M, Sonigo P, Brunelle F, Regis J ( 2003): A primal sketch of the cortex mean curvature: A morphogenesis based approach to study the variability of the folding patterns. Trans Med Imaging 22: 754–765. [DOI] [PubMed] [Google Scholar]
- Cachia A, Paillere‐Martinot M‐L, Galiniwski A, Januel D, de Beaurepaire R, Bellivier F, Artiges E, Andoh J, Bartres‐Faz D, Duchesnay E, Riviere D, Plaze M, Mangin J‐F, Martinot J‐L ( 2008): Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage 39: 927– 935. [DOI] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP ( 2005): Comparison and validation of tissue modelization and statistical classification methods in T1‐weighted MR brain images. IEEE Trans Med Imaging 24: 1548–1565. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI ( 1999): Cortical surface‐based analysis. I: Segmentation and surface reconstruction. Neuroimage 9: 7709–7717. [DOI] [PubMed] [Google Scholar]
- Daniel SE, Lees AJ ( 1993): Parkinson's Disease Society Brain Bank, London, overview and research. J Neural Transm Suppl 39: 165–172. [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ ( 2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: 968–980. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischle B, Buckner RL ( 2009): Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging 30: 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno‐Tempini ML, Rankin K, Miller BL, Weiner MW ( 2007): Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain 130: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M ( 2003): Dementia associated with Parkinson's disease. Lancet Neurol 2: 229–237. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B ( 2007): Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 22: 1689– 1707. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL; Members of the UPDRS Development Committee ( 1987): Unified Parkinson's disease rating scale In: Fanh S, Marsden CD, Calne D, Goldstein M, editors: Recent Development's in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information; pp 153–164. [Google Scholar]
- Fennema‐Notestine C, Hagler DJ Jr, McEvoy LK, Fleisher AS, Wu EH, Karow DS, Dale AM; Alzheimer's Disease Neuroimaging Initiative ( 2009): Structural MRI biomarkers for preclinical and mild Alzheimer's disease. Hum Brain Mapp 30: 3238–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM ( 1999a): Cortical surface‐based analysis. II: Inflation, flattening and a surface‐based coordinate system. Neuroimage 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM ( 1999b): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM ( 2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu AK, Dale AM ( 2001): Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20: 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Golstein J, Kennedy D ( 2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14: 11–22. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Greve DN, Fischl B, Benner T, van der Kouwe AJW, Salat D, Bjornerud A, Due‐Tonessen P, Walhovd KB ( 2008): The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuroimage 42: 1654–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, NcHugh PR ( 1975): “Mini‐mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Freeman SH, Kandel R, Cruz L, Rozkalne A, Newell K, Frosch MP, Hedley‐Whyte ET, Locascio JJ, Lipsitz L, Hyman BT ( 2008): Preservation of neuronal number despite age‐related cortical brain atrophy in elderly subjects without Alzheimer's disease. J Neuropathol Exp Neurol 67: 1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H ( 2006): Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comp Biol 2: 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD ( 1967): Parkinsonism: Onset, progression and mortality. Neurology 17: 427–442. [DOI] [PubMed] [Google Scholar]
- Hobson P, Meara J ( 2004): Risk and incidence of dementia in a cohort of older subjects with Parkinson's disease in the United Kingdom. Mov Disord 19: 1043–1049. [DOI] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N ( 2009): A comparison between voxel‐based cortical thickness and voxel‐based morphometry in normal aging. Neuroimage 48: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Samson F, Evans AC, Mottron L ( 2010): Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel‐based morphometry. Hum Brain Mapp 31: 556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee J‐M, Yoon U, Shin Y‐W, Hong SB, Kim IY, Kwon JS, Kim SI ( 2006): Fractal dimension in human cortical surface: Multiple regression analysis with cortical thickness, sulcal depth and folding area. Hum Brain Mapp 27: 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Seo SW, Kim SH, Kim SI, Na DL ( 2008a): Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer's disease. Neuroimage 43: 103–113. [DOI] [PubMed] [Google Scholar]
- Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI ( 2008b): Brain size and cortical structure in the adult human brain. Cereb Cortex 18: 2181–2191. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Chen R, Ke X, Chu K, Lu Z, Herskovits EH ( 2010): Predictive models of autism spectrum disorder based on brain regional cortical thickness. Neuroimage 50: 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad'Dabbagh Y, MacDonald D, Lee JM, Kim SI, Evans AC ( 2005): Automated 3‐D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage 27: 210–211. [DOI] [PubMed] [Google Scholar]
- Kim JY, Lee WY, Chung EJ, Dhong HJ ( 2007): Analysis of olfactory function and the depth of olfactory sulcus in patients with Parkinson's disease. Mov Disord 22: 1563–1566. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Mangin J‐F, Coyle T, Lancaster J, Thompson P, Riviere D, Cointepas Y, Regis J, Schlosser A, Royall DR, Zilles K, Mazziotta J, Toga A, Fox PT ( 2005): Age‐related morphology trends of cortical sulci. Hum Brain Mapp 26: 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Coyle TR, Lancaster JL, Kochunov V, Royall D, Mangin JF, Riviere D, Fox PT ( 2008): Relationship among neuroimaging indices of cerebral health during normal aging. Hum Brain Mapp 29: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Robin DA, Royall DR, Coyle T, Lancaster J, Kochunov V, Schlosser AE, Fox PT ( 2009): Can structural MRI indices of cerebral integrity track cognitive trends in executive control function during normal maturation and adulthood? Hum Brain Mapp 30: 2581–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Evans AC ( 2005): Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 24: 163–173. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC ( 2005): Focal decline of cortical thickness in Alzheimer's disease identified by computational neuroanatomy. Cereb Cortex 15: 995–1001. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW ( 2004): Neuropsychological Assessment, 4th Edition New York: Oxford University Press. [Google Scholar]
- Liu T, Wen W, Zhu W, Trollor J, Reppermund S, Crawford J, Lin JS, Luo S, Bordaty H, Sachdev P ( 2010): The effects of age and sex on cortical sulci in the elderly. Neuroimage 51: 19–27. [DOI] [PubMed] [Google Scholar]
- Lyoo CH, Ryu YH, Lee MS ( 2010): Topographical distribution of cerebral cortical thinning in patients with mild Parkinson's disease without dementia. Mov Disord 25: 496–499. [DOI] [PubMed] [Google Scholar]
- Mangin JF, Frouin V, Bloch I, Regis J, Lopez‐Krahe J ( 1995): From 3D magnetic resonance images to structural representations of the cortex topography using topology preserving deformations. J Math Imaging Vis 5: 21. [Google Scholar]
- Mangin JF ( 2000): Entropy Minimization for Automatic Correction of Intensity Non Uniformity. Hilton Head Island, SC: IEEE Press; p 8. [Google Scholar]
- Mangin JF, Rivière D, Cachia A, Duchesnay E, Cointepas Y, Papadopoulos‐Orfanos D, Scifo P, Ochiai T, Brunelle F, Regis J ( 2004): A framework to study the cortical folding patterns. Neuroimage 23: S129–S138. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC ( 2000): Automated 3‐D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage 12: 340–356. [DOI] [PubMed] [Google Scholar]
- Mega MS, Thompson PM, Cummings JL, Back CL, Xu ML, Zohoori S, Goldkorn A, Moussai J, Fairbanks L, Small GW, Toga AW ( 1999): Sulcal variability in the Alzheimer's brain: Correlations with cognition. Neurology 50: 145–151. [DOI] [PubMed] [Google Scholar]
- Nagano‐Saito A, Washimi Y, Arahata Y, Kachi T, Lerch, JP , Evans AC, Dagher A, Ito K ( 2005): Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 64: 224–229. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema‐Notestine C, Eyler LT, Jernigan TL, Prom‐Wormley E, Neale M, Jacobson K, Lyons MJ, Garnt MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS ( 2009): Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 19: 2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H‐J, Lee JD, Kim EY, Park B, Oh M‐K, Lee S, Kim J‐J ( 2009): Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. Neuroimage 47: 98–106. [DOI] [PubMed] [Google Scholar]
- Penttila J, Martinot MLP, Martinot JL, Ringuenet D, Wessa M, Houenou J, Gallarda T, Bellivier F, Galinowski A, Bruguiere P, Pinabel F, Leboyer M, Olie JP, Duchesnay E, Artiges E, Mangin JF, Cachia A ( 2009): Cortical folding in patients with bipolar disorder or unipolar depression. J Psychiatry Neurosci 34: 127–135. [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Junque C, Marti MJ, Ramirez‐Ruiz B, Bargallo N, Tolosa E ( 2009): Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson's disease. Mov Disord 24: 1193–1199. [DOI] [PubMed] [Google Scholar]
- Ramirez‐Ruiz B, Marti MJ, Tolosa E, Bartres‐Faz D, Summerfield C., Salgado‐Pineda P, Gomez‐Anson B, Junque C ( 2005): Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol 252: 1345–1352. [DOI] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL ( 1997): Statistical approach to segmentation of single‐channel cerebral MR images. IEEE Trans Med Imaging 16: 176–186. [DOI] [PubMed] [Google Scholar]
- Ridgway GR, Henley SMD, Rohrer JD, Scahill RI, Warren JD, Fox NC ( 2008): Ten simple rules for reporting voxel‐based morphometry studies. Neuroimage 20: 1429–1435. [DOI] [PubMed] [Google Scholar]
- Riviere D, Mangin JF, Papadopoulos‐Orfanos D, Martinez JM, Frouin V, Regis J ( 2002): Automatic recognition of cortical sulci of the human brain using a congregation of neural networks. Med Image Anal 6: 77–92. [DOI] [PubMed] [Google Scholar]
- Salat DH, Greve DN Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B ( 2009): Regional white matter volume differences in non‐demented aging and Alzheimer's disease. Neuroimage 44: 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha‐Khadem F, Conelly A, Gadian DG, Friston KJ ( 2002): Distributional assumptions in voxel‐based morphometry. Neuroimage 17: 1027–1030. [PubMed] [Google Scholar]
- Seo SW, Im K, Lee J‐M, Kim Y‐H, Kim ST, Kim SY, Yang DW, Kim SI, Cho YS, Na DL ( 2007): Cortical thickness in single‐ versus multiple‐domain amnestic mild cognitive impairment. Neuroimage 36: 289–297. [DOI] [PubMed] [Google Scholar]
- Singh V, Chertkow H, Lerch JP, Evans AC, Dorr AE, Kabani NJ ( 2006): Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer's disease. Brain 129: 2885–2893. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Junqué C, Tolosa E, Salgado‐Pineda P, Gómez‐Ansón B, Marti MJ, Pastor P, Ramirez‐Ruiz B, Mercader J ( 2005): Structural brain changes in Parkinson disease with dementia. Arch Neurol 62: 281–285. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain. Thieme, NY. [Google Scholar]
- Tohka J, Zijdenbos A, Evans A ( 2004): Fast and robust parameter estimation for statistical partial volumes models in brain MRI. Neuroimage 23: 84–97. [DOI] [PubMed] [Google Scholar]
- Toro R, Perron M, Pike B, Richer L, Veillette S, Pausova Z, Paus T ( 2008): Brain size and folding of the human cerebral cortex. Cereb Cortex 18: 2352–2357. [DOI] [PubMed] [Google Scholar]
- Van Essen D ( 1997): A tension‐based theory of morphogenesis and compact wiring in the central nervous system. Nature 385: 313–318. [DOI] [PubMed] [Google Scholar]
- Voets NL, Hough MG, Douaud G, Mattthews PM, James A, Winmill L, Webster P, Smith S ( 2008): Evidence for abnormalities of cortical development in adolescent‐onset schizophrenia. Neuroimage 43: 665–675. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA ( 2007): Voxel‐based morphometry and its application to movement disorders. Parkinsonism Relat Disord 13: S406–S416. [DOI] [PubMed] [Google Scholar]
- Wright CI, Feczko E, Dickerson B, Williams D ( 2007): Neuroanatomical correlates of personality in the elderly. Neuroimage 35: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


