Abstract
Recent studies in cognitive neuroscience have suggested that the integration of information about the internal bodily state and the external environment is crucial for the experience of emotion. Extensive overlap between the neural mechanisms underlying the subjective emotion and those involved in interoception (perception of that which is arising from inside the body) has been identified. However, the mechanisms of interaction between the neural substrates of interoception and emotional experience remain unclear. We examined the common and distinct features of the neural activity underlying evaluation of emotional and bodily state using functional magnetic resonance imaging (fMRI). The right anterior insular cortex and ventromedial prefrontal cortex (VMPFC) were identified as commonly activated areas. As both of these areas are considered critical for interoceptive awareness, these results suggest that attending to the bodily state underlies awareness of one's emotional state. Uniquely activated areas involved in the evaluation of emotional state included the temporal pole, posterior and anterior cingulate cortex, medial frontal gyrus, and inferior frontal gyrus. Also the precuneus was functionally associated with activity of the right anterior insular cortex and VMPFC when evaluating emotional state. Our findings indicate that activation in these areas and the precuneus are functionally associated for accessing interoceptive information and underpinning subjective experience of the emotional state. Thus, awareness of one's own emotional state appears to involve the integration of interoceptive information with an interpretation of the current situation. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: interoception, body, insular cortex, ventromedial prefrontal cortex, precuneus
INTRODUCTION
Understanding the subjective experience of emotion has been a topic of great interest for more than a century. William James [1884] proposed that the experience of emotion results from the perception of specific and unique patterns in the somatovisceral response, a theory that has provoked much discussion [Plutchik and Ax, 1967; Rainville et al., 2006; Schachter and Singer, 1962]. A large number of studies have examined how emotional responses occur, and which parts of the brain regulate these responses. Not only the neural responses but also peripheral autonomic activity specific to particular emotions have been studied using various emotion‐inducing paradigms such as spontaneous recall of emotional episodic memory [Damasio et al., 2000; Rainville et al., 2006], emotional facial expression [Ekman et al., 1983], and emotion‐laden films [Vianna and Tranel, 2006]. A recent study found that emotion‐related neural activity also encodes underlying patterns of peripheral response, and suggested that activity in certain brain areas represents essential relations between subjective emotional feelings and bodily response [Harrison et al., 2010]. Although it remains unresolved which aspects of the neural and peripheral response fundamentally determine our emotional experiences, the results of recent psychological and brain imaging studies indicate that the perception of bodily signals contribute to and at least partially mediates emotional experience [Bechara et al., 1996; Dunn et al., 2010; Pollatos et al., 2005; Werner et al., 2009].
In the current study, we focused on the psychological and neural mechanisms of interoception as key factors for understanding subjective feeling states. Interoception constitutes the perception of afferent information arising from anywhere and everywhere within the body [Cameron, 2001; Sherrington, 1906]. Some studies have suggested that the sensitivity of an individual's interoception represents one's disposition of emotional experience [Critchley et al., 2004; Wiens et al., 2000; Werner et al., 2009]. For instance, Schandry [1981] assessed individual interoceptive sensitivity using a heartbeat detection task, and reported that individuals who could accurately perceive their own heartbeat tended to exhibit higher scores on state of anxiety and emotional lability. Another study reported that individuals with high interoceptive sensitivity exhibited higher arousal in response to emotional visual stimuli [Dunn et al., 2010; Pollatos et al., 2005]. These results suggest that a significant relationship may exist between interoceptive processes and subjective emotional experience. Identifying and examining the shared neural substrates underlying these two types of processing will lead to a greater understanding of the neural system underlying emotional experience.
Elucidating the neural substrates of interoceptive awareness requires an experimental paradigm in which participants are made aware of information from their bodies. Previous studies have used various paradigms, including those involving pain [e.g., Craig et al., 2000; Dunkley et al., 2005; Wiech et al., 2006], distention pressure on the viscera [Hamaguchi et al., 2004], hypoglycemic states [Teves et al., 2004], and air‐hunger states [Evans et al., 2002; Liotti et al., 2001]. These studies typically reported activation in the anterior cingulate cortex (ACC), the insular cortex, the thalamus and the brainstem when bodily states significantly deviated from the steady state. However, several possible interpretations exist regarding the reason for activation in those regions. For example, the detection of deviation of the bodily state from the steady state may result in the activation in these regions. Alternatively, the activation may be related to the subsequent experience of emotional states, such as distress or threat.
Among the regions exhibiting activation related to interoceptive awareness, the insular cortex has been identified by several previous studies as an essential neural substrate for combining information from interoception and subjective emotional experience. For instance, Critchley et al. [2004] used a task in which participants' attention is oriented to their heartbeats and found activations in the ACC and the right anterior insular cortex. In addition, they revealed a statistically significant association between the volume of the right anterior insular cortex and individual anxiety symptoms, and sensitivity to internal bodily states. Their results indicated that the right anterior insular cortex supports awareness of the visceral response, and integrates it with other information to form the basis of the subjective experience of an emotional state. This notion is consistent with the results of a number of other studies [Craig, 2003; Naqvi et al., 2007; Paulus and Stein, 2006]. Studies using electroencephalography (EEG) found the neural index of processing signals from the cardiovascular system known as the heartbeat‐evoked potential, which is observed from 250 to 350 ms after the R‐wave of electrocardiography (ECG) over somatosensory cortex and the frontal/prefrontal cortex [Pollatos and Schandry, 2004]. The insular cortex and somatosensory cortex have been proposed as the possible sources of this neural potential [Aziz et al., 1995; Pollatos and Schandry, 2004]. Another important region for interoceptive awareness is the orbitofrontal (ventromedial) cortex. Lesions in this region can drastically change patients' personality and decision‐making behavior [Bechara et al., 1994]. According to Damasio's somatic marker hypothesis, the ventromedial cortex is crucial for processing changes in internal bodily states according to situation and social context, and supports subjective feeling of emotions and intuitive decision‐making [Bechara, 2004; Northoff et al., 2006]. Taken together, these previous results provide evidence supporting the notion that we have the ability to perceive our internal bodily states, and that this ability enables us to detect changes in the bodily state and to maintain homeostasis.
Many studies have examined the neural correlates of emotional experience in a broad sense. Some studies have asked participants to recall an emotional episodic memory as a way of causing the subjective experience of an emotional state [Damasio et al., 2000; Rudrauf et al., 2009]. One positron emission tomography study reported that activation and deactivation of the cingulate cortex, the somatosensory area and the insular cortex varied qualitatively during the experience of emotions such as fear, anger, and happiness [Damasio et al., 2000]. In addition, a magnetoencephalography study reported that activity in the orbitofrontal cortex, ventromedial frontal cortex, ACC and somatosensory area was specifically related to the subjective experience of emotion [Rudrauf et al., 2009]. Two recent reviews of neuroimaging research focusing on the neural bases of emotional experience drew conclusions about the likely neural locus of the underlying processing involved [Lee and Siegel, 2009; Tsuchiya and Adolphs, 2007]. Both reviews focused on the processing required in the particular experimental task used in each study, and specified certain brain regions as neural correlates of emotional experience, including the anterior and posterior cingulate cortex, the precuneus, the orbitofrontal cortex, and the insular cortex. In particular, the rostral ACC (BA32) and the inferior frontal/insular cortex were specified as neural substrates for evaluating participants' own emotional state. The authors of one review suggested that the results supported the notion that the process of evaluating one's own emotional state includes the evaluation of bodily experience [Lee and Siegel, 2009]. An extensive overlap between the neural mechanisms underlying the experience of emotion and interoception was identified. Those regions are the anterior and posterior cingulate cortex, the somatosensory area, the insular cortex, and the orbitofrontal cortex. This overlapping neural substrate supports the notion that we access to our own bodily state when explicitly evaluating our emotional state. However, direct evidence to support this notion has not yet been reported.
In the current study, we, thus, examined the neural mechanisms underlying the evaluation of emotional and bodily state, and identified common and distinct areas of activation during the different types of processing. Previous studies have examined the neural substrates of these processes separately, but to our knowledge, no studies have addressed the common and distinct areas of activation together. This study could thus provide an important insight into the relationship between interoception and the subjective experience of emotion. As we aimed to identify the neural substrate of experience of the emotional state itself, we did not administer any emotion‐eliciting procedures, unlike previous studies. In most cases, prominent changes in emotional state are accompanied by changes in bodily states such as cardiac or respiratory activity. Clear segregation of the neural substrates of evaluating emotional state is difficult when using emotion‐eliciting procedures in neuroimaging study, because neural substrates that represent accompanying bodily responses are activated simultaneously. Therefore, our methods enabled us to focus exclusively on the neural substrates for evaluating emotional state. If accessing to the internal bodily state is an intrinsic property of emotional experience, the accessing process would be expected to occur whenever we are aware of our emotional state, even if a salient bodily state change is absent. In addition, for the purposes of identifying the neural substrates representing the subjective experience of bodily and emotional states in the immediate present, we used two temporal constraint conditions: “now” and “usual”. This manipulation was designed to provide data bearing on the relation between experience of emotion and consciousness [Craig, 2009; Damasio, 1999; Tsuchiya and Adolphs, 2007].
Evidence from recent cognitive neuroscience studies on emotion suggest the constructive view which assumes that emotional experience is determined by integration of interoception of bodily state and exteroception and evaluation of environmental information [Barrett and Bliss‐Moreau, 2009]. Viewing from the constructive hypothesis, we could identify the essential regions for interoception as commonly activated areas for evaluating emotional and bodily state. As subjective emotion is an integrated entity of interoception and environmental information, some other regions are expected to activate selectively when participants evaluate their own emotional state in addition to the interoceptive regions. Specifically related regions for evaluating emotional state in this study would support to experience interoception as subjective emotion by comparing and integrating present situations and context of past analogous experience. Thus, some regions are expected to activate prominently for emotion‐now condition, such as medial prefrontal cortex, ventromedial frontal cortex, temporal pole, ACC, and superior temporal sulcus. The activity of these regions essentially relates to evaluation of self and other's mental and emotional state [Amodio and Frith, 2006; Calder et al., 2002; Frith and Frith, 2003; Mobbs et al., 2006; Olson et al., 2007].
METHOD
Participants
Eighteen undergraduate and graduate students (five males and 13 females) participated in our study (mean 22.9 years ± 2.11 SD). All participants were right handed and reported no history of neurological or psychiatric diseases. The experiment was performed with the approval of the Keio University Research Ethics Committee (No. 09006). Before participation, all participants read and signed a written informed consent form explaining (i) the purpose and procedure of the study and (ii) that they were able to cease their participation in the study at any time. All participants completed the experiment.
Procedure and Materials
In the MRI scanner, participants were required to answer questions on three topics: emotional awareness, bodily awareness, and personal possessions. The emotional awareness and bodily awareness conditions were designed to identify the neural regions related to the evaluation of emotion or the body, respectively. On the other hand, the possession condition was designed as a control condition to identify the regions of activation associated with general task components related to responding to the questions about the self. Besides the sentence‐type conditions, we prepared temporal constraint conditions: now and usual. The now condition required participants to perform online monitoring, whereas the usual condition required them to perform offline monitoring.
After presenting a fixation point for 4–6 s on a monitor placed in the MRI scanner, we presented a cue (now or usual), followed by a statement as “I'm happy (for emotional awareness),” “I have a fast pulse (for bodily awareness),” and “I have money (for possessions).” The cue was presented for 3 s, and the sentence was presented for 10 s. In the now condition, participants evaluated the appropriateness of the statement as a description of their current state. In the usual condition, they evaluated the appropriateness of the statement as a description for their usual disposition. Participants' evaluation regarding the appropriateness of the statement was chosen from four options, such as “not at all, somewhat, very and definitely.” Stimuli were generated by a control computer located outside the MRI room using Cogent 2000 (http://www.vislab.ucl.ac.uk/cogent_2000.php) implemented in MATLAB (MathWorks). Participants responded using a four‐button MRI compatible keypad connected to the control computer, which recorded the responses and reaction times (RTs).
Sentences for the emotional awareness conditions were selected from the Positive and Negative Affect Scale [Watson et al., 1988] and translated into Japanese based on Sato and Yasuda [2001]. For the bodily awareness condition, sentences were selected from the Body Perception Questionnaire [Porges, 1993] and Modified Somatic Perceptions Questionnaire [Main et al., 1992]. Items used in possession condition were selected by interviewing undergraduate students about what they were carrying. We prepared 16 sentences for each condition. Thus, there were 96 trials in total (two cues, three conditions, and 16 sentences). The full list of stimuli used in each condition is shown in Appendix Table AI. The 96 trials were divided into three blocks; therefore, a block comprised 32 trials. The order of the blocks and trials was randomized and counterbalanced across participants.
Functional Magnetic Resonance Imaging Data Acquisition and Analysis
fMRI scanning used a 3T Siemens Tim Trio scanner with an 8‐channel headcoil for data acquisition. Scanning consisted of three experimental functional runs and a high‐resolution T1‐weighted structural scan (1‐mm isotropic resolution 3D MPRAGE). Each functional run consisted of 274 whole‐brain T2* weighted single‐shot gradient‐echo planar imaging (EPI) images, collected in an oblique axial orientation (TR 2.35 s, TE 30 ms, FA 90 degrees, voxel size 3.5 × 3.5 × 2 mm, 44 slices (descending), 1 mm slice gap). The first six functional volumes were discarded to allow for equilibration of net magnetization. The structural scan was coregistered to the subject's mean EPI image.
The data were preprocessed and analyzed using statistical parametric mapping (SPM5) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). Functional images from each participant were spatially corrected for head movement, and temporally corrected for slice timing (using the middle slice acquired in time as a reference), spatially normalized to the Montreal Neurological Institute (MNI) template with a resample voxel size of 1 × 1 × 1 mm and spatially smoothed with a three‐dimensional Gaussian filter (8‐mm full width half maximum). In addition, high‐pass temporal filtering with a cut‐off of 128 s was applied to remove low‐frequency drifts in signal, and global changes were removed by proportional scaling.
First‐level analyses were performed to determine each subject's voxel‐wise activation while participants were choosing the appropriate options. The analyses were performed using an event‐related model with six trial types: “body‐now,” “body‐usual,” “emotion‐now,” “emotion‐usual,” “possession‐now,” and “possession‐usual.” A canonical set of three functions, the hemodynamic response function (HRF), its temporal derivatives, and its dispersion derivatives [Friston et al., 1998], characterized the neural response. Effects of questionnaire type and the now/usual condition on neural activity were analyzed on a voxel‐wise basis for each participant, in the form of statistical parametric maps of discrete contrasts. The general linear model was used to create statistical parametric maps. Subsequent second‐level group random effects analyses were performed on the SPM contrast images of the first‐level canonical HRF responses. In accordance with previous fMRI studies, statistical threshold of P < 0.001, uncorrected, with an extent threshold of 10 voxel for multiple spatial comparisons across the whole brain was used, except for conjunction analysis in which threshold was set at P < 0.00001 uncorrected.
RESULTS
Behavioral Results
RTs were recorded from the time of presentation of the sentences until the time the appropriate response button was pressed and statistically analyzed. The mean RT and standard error of the mean (SEM) for each condition were as follows; emotion‐now: 2,762.5 (±275.81) ms, emotion‐usual: 2,933.3 (±280.9) ms, body‐now: 2,613.7 (±244.1) ms, body‐usual: 2,720.3 (±275.2) ms, possession‐now: 2,558.3 (±240.6) ms, possession‐usual: 2,826.0 (±244.9) ms. We performed a two‐way analysis of variance (ANOVA) to investigate differences in RTs among the three question types (emotional awareness, bodily awareness, and possession) and two cue conditions (now/usual). The ANOVA revealed a statistically significant main effect of cue condition [F (1, 17) = 11.28, P < 0.01]. Participants required more time to answer questions in the usual condition. There was no significant main effect of question type [F (2, 34) = 1.39, ns].
To assess participants' rating response, we calculated mean rating value by the participants' average response by replacing the rating options with numerical quanta. That is, “not at all” was replaced by 0, “somewhat” by 1, “very” by 2, and “definitely” by 3. The mean rating value and SEM for each condition were as follows: emotion‐now, 1.51 (±0.15); emotion‐usual, 1.68 (±0.17); body‐now, 1.58 (±0.18); body‐usual, 1.70 (±0.17); possession‐now, 1.97 (±0.19); and possession‐usual, 2.10 (±0.19). We performed a two‐way ANOVA to investigate differences in rating value among the three question types (emotional awareness, bodily awareness, and possession) and two cue conditions (now/usual), and observed a statistically significant main effect of question types [F (2, 34) = 28.40; P < 0.01]. Subsequent analysis revealed that participants gave higher rating in possession, rather than emotional and bodily awareness trials. There was no significant main effect of cue condition [F (1, 17) = 3.815; ns] and interaction [F (2, 34) = 0.165; ns]. In this study, we intentionally eliminated factors that evoke prominent emotional or bodily responses in all conditions. The participants' responses show that they did not feel prominent changes in emotional or bodily state.
fMRI Data
In the first step, we analyzed the fMRI data in a mixed‐effects group analysis (n = 18). To identify the regions underlying participants' evaluations of their own emotions or bodies, we compared the activated areas for the emotion and the body condition, versus the possession condition, respectively (see Table I). The body‐now contrast versus possession‐now contrast revealed greater blood‐oxygen level‐dependent (BOLD) responses in the lingual gyrus, the inferior/middle frontal gyrus [ventromedial prefrontal cortex (VMPFC)], the insular cortex, the middle cingulate cortex, and the cerebellum. The emotion‐now contrast versus possession‐now contrast revealed greater BOLD responses in the lingual gyrus, the temporal pole, the inferior frontal cortex, and the superior temporal cortex. We also analyzed the fMRI data for the usual conditions. The body‐usual contrast versus possession‐usual contrast revealed greater BOLD responses in the cuneus, the middle occipital gyrus, the cerebellum, and the lingual cortex. The emotion‐usual versus possession‐usual contrast revealed greater BOLD responses in the precentral gyrus, the precuneus, the lingual gyrus, the supramarginal gyrus, the fusiform gyrus, the cuneus, the postcentral gyrus, the superior temporal cortex, and the inferior parietal cortex. Activations in some regions were not reported in Table I because of insufficient number of activated voxels. Bilateral anterior insular cortex, the region of greatest interest in this study, was found to be activated with <10 voxels in all four contrasts. Also, higher left ventromedial cortex activation was observed in emotion‐now and body‐now compared with possession‐now condition, and right inferior frontal cortex was more activated in emotion‐usual and body‐usual condition compared with possession‐usual condition.
Table I.
Anatomical locations and coordinates of activations
| Region of activation | L/R | BA | Number of voxels in cluster | t Value | MNI | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Body now > possession now | |||||||
| Lingual gyrus | L | 17/18 | 148 | 5.06 | −12 | −72 | −12 |
| Insula/inferrior frontal gyrus | R | 13 | 24 | 4.87 | 39 | 21 | 6 |
| Cerebellum | R | — | 18 | 4.43 | 3 | −48 | −9 |
| R | 14 | 4.32 | 15 | −72 | −39 | ||
| Insula/claustrum | L | 13 | 18 | 4.41 | −24 | 12 | 24 |
| Middle cingulate gyrus | L | 23/24 | 12 | 4.22 | −3 | −18 | 33 |
| Inferior/middle frontal gyrus | R | 10/46/47 | 15 | 3.81 | 51 | 45 | −3 |
| Emotion now > possession now | |||||||
| Cerebellum (Crus1) | L | — | 41 | 4.76 | −9 | −84 | −18 |
| Temporal pole/inferior frontal gyrus | L | 38 | 78 | 4.37 | −57 | 12 | −12 |
| L | 47 | 4.36 | −51 | 33 | −3 | ||
| Temporal pole | R | 38 | 14 | 4.41 | 51 | 18 | −15 |
| Lingual gyrus | R | 18 | 119 | 4.35 | 21 | −84 | −15 |
| Superior temporal gyrus | L | 22 | 10 | 3.81 | −60 | −48 | 9 |
| Body usual > possession usual | |||||||
| Cerebellum | R | — | 47 | 5.07 | 2 | −60 | −18 |
| Lingual gyrus | L | 18 | 261 | 4.93 | −9 | 75 | −9 |
| Cuneus | L | 18 | 30 | 4.35 | −18 | −84 | 15 |
| Middle occipital gyrus | R | 19/39 | 17 | 4.04 | 42 | −75 | 12 |
| Emotion usual > possession usual | |||||||
| Precentral gyrus | R | 4/6 | 74 | 5.05 | 39 | −18 | 54 |
| L | 4 | 16 | 4.25 | −36 | −15 | 51 | |
| Precuneus | L | 7 | 49 | 4.89 | −18 | −54 | 60 |
| R | 7 | 14 | 3.78 | 15 | −51 | 54 | |
| Lingual gyrus | L | 18 | 268 | 4.79 | −9 | −72 | −6 |
| L | 9 | −84 | −6 | ||||
| Supra marginal gyrus | R | 2 | 22 | 4.57 | 60 | −27 | 36 |
| Precentral gyrus | L | 4 | 60 | 4.36 | −63 | −9 | 27 |
| Fusiform gyrus | R | 19 | 21 | 4.46 | 27 | −60 | −15 |
| 37 | 10 | 3.62 | −42 | −63 | −15 | ||
| Cuneus | L | 18 | 37 | 4.44 | −18 | −84 | 12 |
| Insula | L | 13 | 73 | 4.43 | −39 | −3 | 21 |
| Post central gyrus | R | 2 | 65 | 4.36 | 36 | −39 | 69 |
| Superior temporal gyrus | R | 38 | 14 | 4.08 | 54 | 15 | −9 |
| Postcentral gyrus | L | 3 | 29 | 4.08 | −51 | −15 | 45 |
| Inferior parietal gyrus | L | 2/40 | 19 | 4.04 | −54 | −27 | 45 |
| L | 40 | 16 | 3.94 | −60 | −39 | 24 | |
BA, Brodmann area; MNI, coordinates referring to the standard brain of the Montreal Neurological Institute; L, left hemisphere; R, right hemisphere.
Clusters of maximally activated voxels that survived statistical thresholding at t = 3.35 (P < 0.001, uncorrected, extent threshold of 10 voxel).
To identify the commonly activated regions, we examined the overlap between the images for the body‐now (body‐now vs. possession‐now) and emotion‐now (emotion‐now vs. possession‐now) contrasts. The MatLab toolbox xjView 8 was used for this analysis (http://www.alivelearn.net/xjview8/index2.html). The right anterior insular cortex (BA 13), the left VMPFC (BA11) and the bilateral lingual gyrus (BA17) were identified as commonly activated regions (Fig. 1a). To examine the precise activation pattern of common activated areas, we calculated parameter estimates of right anterior insula and VMPFC for emotion‐now, body‐now, and possession‐now condition (Fig. 1b,c). The right anterior insula was selectively activated for emotion‐now and body‐now condition, and similar trend was observed at VMPFC. We also conducted a conjunction analysis for the emotion‐now and body‐now conditions, and identified the same areas as commonly activated regions in the emotion‐now and body‐now conditions (see Table II). These regions are critically involved in the online monitoring of emotional and bodily states.
Figure 1.
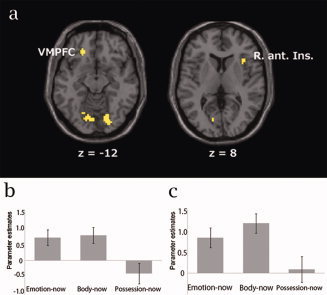
(a) Commonly activated areas for the emotion‐now and body‐now conditions (P < 0.001, uncorrected). VMPFC, ventromedial prefrontal cortex (x = −20, y = 36, z = −12); R. ant. Ins, right anterior insular cortex (x = 36, y = 18, z = 6). (b) Parameter estimates in VMPFC. (c) Parameter estimates in right anterior insular cortex.
Table II.
Coactivated areas for body and emotion conditions
| Region of activation | L/R | BA | Number of voxels in cluster | t Value | MNI | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Coactivated areas for body now and emotion now | |||||||
| Lingual gyrus | L | 18 | 2,350 | 10.19 | −3 | −75 | −3 |
| Middle frontal gyrus | L | 9 | 203 | 7.57 | −48 | 6 | 36 |
| L | 6 | 173 | 7.41 | −33 | −3 | 63 | |
| Superior frontal gyrus | L | 6 | 109 | 7.45 | −6 | 9 | 57 |
| Superior temporal gyrus | L | 38 | 18 | 6.25 | −51 | 18 | −12 |
| L | 22 | 12 | 5.90 | −57 | −42 | 6 | |
| Superior parietal lobule | R | 7 | 18 | 6.24 | 54 | 18 | −6 |
| Postcentral gyrus | L | 2 | 20 | 6.16 | −51 | −21 | 30 |
| Inferior frontal gyrus | R | 47 | 9 | 6.09 | 54 | 18 | −6 |
| Middle temporal gyrus | L | 21 | 32 | 6.08 | −51 | −27 | 3 |
| Insula | R | 13 | 10 | 5.88 | 36 | 18 | 6 |
| L | 13 | 8 | 5.56 | −33 | 18 | 6 | |
| Coactivated areas for body usual and emotion usual | |||||||
| Lingual gyrus | L | 18 | 2,296 | 9.68 | −9 | −81 | 0 |
| R | 17 | 14 | 6.15 | 9 | −87 | 3 | |
| Postcentral gyrus | L | 1 | 446 | 8.29 | −42 | −27 | 57 |
| Medial frontal gyrus | L | 6 | 84 | 7.38 | −3 | −3 | 57 |
| Middle frontal gyrus | L | 6 | 30 | 6.39 | −48 | 6 | 42 |
| Superior parietal lobule | L | 7 | 63 | 6.00 | −24 | −66 | 51 |
| Middle occipital gyrus | R | 18 | 11 | 5.70 | 42 | −87 | 3 |
| Inferior frontal gyrus | L | 47 | 12 | 5.59 | −48 | 21 | −9 |
Note. BA = Brodmann area, MNI = coordinates referring to the standard brain of the Montreal Neurological Institute, L = left hemisphere, R = right hemisphere. Clusters of maximally activated voxels that survived statistical thresholding at t = 4.95 (P < 0.00001, uncorrected).
Psychophysiological interaction (PPI) analyses were performed to examine the change in effective connectivity between common activated regions (right anterior insula [6 mm sphere centered x = 36, y = 18, z = 6] and VMPFC [6 mm sphere centered x = –20, y = 36, z = –12]) and other brain regions while participants evaluated their own immediate emotional state versus bodily state. The analyses revealed that the activity of precuneus (MNI coordinates = 12, –54, 45) and left anterior insula (–33, 27, 9) functionally interacted with those of right anterior insula and VMPFC in emotion‐now condition (P < 0.001, uncorrected, extent threshold value = 10). On the other hand, in the body‐now condition, only the left anterior and posterior insular cortex (anterior, MNI coordinates = –36, 6, –9: posterior, MNI coordinates = –36, –6, 3) activity was associated with the activity of the right anterior insula and VMPFC (P < 0.001, uncorrected, extent threshold value = 10).
The intersection of contrast images for body‐usual (body‐usual vs. possession‐usual) and emotion‐usual (emotion‐usual vs. possession‐usual) conditions also revealed some commonly activated regions. We used xjView and conjunction analysis to identify commonly activated regions, which included the medial frontal gyrus, the middle and inferior frontal gyrus, the bilateral temporal pole, the left superior temporal gyrus, postcentral gyrus, the left posterior insular cortex, and the bilateral lingual gyrus (see Table II). These regions, thus, appear to be critically involved in knowledge‐based self‐related judgments about participants' own emotional and bodily states.
To disentangle the relationship between emotional and interoceptive processing, we focused on the neural substrates uniquely activated during the two types of processing of interest. Uniquely activated regions in the emotion‐now and body‐now conditions were identified by examining the contrast images with the emotion‐now and body‐now conditions. Uniquely activated regions for emotion‐now condition included the left superior temporal gyrus (BA22/38), the bilateral posterior cingulate cortex (BA31), the bilateral ACC (BA24/32), the right superior medial frontal gyrus (BA9/10), the bilateral inferior frontal gyrus (BA45, 47), the left supramarginal gyrus, and the superior frontal gyrus (BA8) (Fig. 2).
Figure 2.
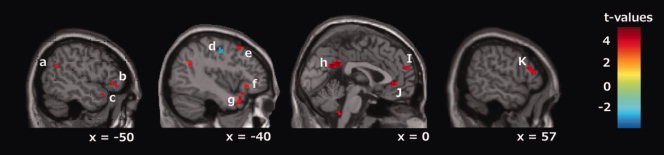
Emotion‐ versus body‐now condition. Areas showing stronger activation in the emotion‐now condition (emotion‐now vs. body‐now) are depicted in red, and areas showing stronger activation in the body‐now condition are depicted in blue (P < 0.001, uncorrected). The areas of activation are as follows; (a) supramarginal gyrus (x = −48, y = −57, z = 26), (b and f) left inferior frontal gyrus (x = −51, y = 33, z = −3), (c and g) temporal pole (x = −54, y = 18, z = −14), d) supplementary motor area (x = −35, y = −14, z = 52), (e) superior frontal gyrus (x = −42, y = 18, z = 54), (h) posterior cingulate cortex (PCC) (x = −1, y = −49, z = 28), (i) superior medial frontal gyrus (x = −1, y = 58, z = 21), (j) anterior cingulate cortex (ACC) (x = −1, y = 39, z = −1), (k) right inferior frontal gyrus (x = 57, y = 30, z = 10).
In contrast, the left supplementary motor area (BA6) and the inferior parietal gyrus were the only regions that were more strongly activated in the body compared to the emotion‐now trials (Fig. 2).
DISCUSSION
This study sought to disentangle the relationship between interoception and the subjective experience of emotion by examining the neural mechanisms underlying the evaluation of emotional and bodily states. To address this question, we tested which neural areas were commonly activated between both processes, and which areas were activated uniquely for only one of the two processes. We identified several commonly activated regions related to both emotional and bodily awareness, and some of these were strongly related to on‐going monitoring processes (now condition). We also found several regions that were uniquely activated for only emotional awareness or bodily awareness. The findings support the hypothesis which posits the integration of interoceptive awareness and an interpretation of the current situation underpins subjective experience of emotion.
Commonly Activated Regions for Emotion‐Now and Body‐Now Conditions
The present results revealed that the right anterior insular cortex (BA13), the VMPFC (BA11) and the bilateral lingual gyrus (BA17) were strongly activated in both the emotion‐now and body‐now trials. The insular cortex is traditionally considered a viscerosensory region, and neuropsychological studies have provided evidence that the insula is a crucial region for recognizing visceral sensations, such as pain [Greenspan et al., 1999] and nausea [Penfield and Faulk, 1955]. In addition, the insular cortex is considered an essential area for regulating autonomic responses and maintaining physiological homeostasis [Abboud et al., 2006; Laowattana et al., 2006]. On the other hand, recent neuroimaging studies have revealed that the insula, especially the anterior portion, plays an important role in emotional processing and social interaction [Damasio et al., 2000; Iaria et al., 2008; Winston et al., 2002]. A number of studies have identified activation in this region when participants experience emotion while recalling emotional episodic memories [Damasio et al., 2000], or while they undertake decision‐making that is highly uncertain or risk‐related [Feinstein et al., 2006; Paulus et al., 2003]. These studies have typically reported that anterior insular cortex activity reflects the subjective intensity of emotional experience. Neuropsychological studies have revealed that damage to this brain region leads to impairments in the experience of emotion [Adolphs et al., 2003; Calder et al., 2000]. In addition, the right anterior portion of the insula is related not only to subjective emotion but also to interoceptive awareness [Critchley et al., 2004]. Activation in this region has been observed when participants made an effort to attend to their internal bodily states. These studies have accumulated evidence indicating that the anterior insular cortex supports awareness of visceral responses and provides important information for the subjective feeling state [Critchley et al., 2004].
Also, the VMPFC (BA11) has been found to exhibit activation while participants attend to themselves, such as during the monitoring of moment‐to‐moment experience in response to presented adjectives [Farb et al., 2007]. During self‐focusing trials, strong functional connectivity between this area and the right insular cortex was observed, suggesting that viscerosomatic signals are associated with activation in the VMPFC. While some studies emphasis its function in representing reward value and reward‐related learning, it has been noted that activation of VMPFC represents subjective but not monetary or objective value of the stimuli. Wright et al., [2007] obtained VMPFC activity associated with participants' attitudes to evaluate the stimuli value. In addition, self‐relatedness of stimuli induces neural activity in VMPFC and other reward systems [de Greck et al., 2008; Phan et al., 2004]. In addition, similar to the insular cortex, this area is significantly associated with self‐related emotional memory [Hennig‐Fast et al., 2008]. These reports and our findings imply that VMPFC underpins the evaluating stimuli value by integrated with person's past and possible future, and this evaluating system includes emotional processing based on accessing bodily states.
Interestingly, activation in the insular cortex and VMPFC were found in this study, even though no intentional emotion‐eliciting procedures were used. The activation we found in these brain areas suggests that interoceptive processes are essential for monitoring and evaluating subjective emotional states. Some review articles regarding the neural substrates of emotion have identified these brain regions as critical for the regulation of internally oriented attention [Menon and Uddin, 2010], suggesting that the evaluation of one's own emotional state may involve the evaluation of bodily experience, which is executed by internally oriented attention [Lee and Siegel, 2009]. Our results provide direct evidence to support the notion that interoceptive processes play an important role in emotional evaluation. Craig [2009] suggested that anterior insular cortex provides a unique neural substrate that integrates all subjective feelings from body and feelings of emotion in the immediate present. Although our findings partially support this claim, we also identified uniquely activated regions for emotion‐now condition and depicted the distinct aspect of neural substrates for interoception and subjective emotion. Our findings indicate that emotional experience comprises implicit accessed bodily state, and appraises the state by comparing and integrating current situation and context of past analogous experience. The results are consistent with the core affect theory that suggests core affect is realized by integrating incoming sensory information from the external world with homeostatic and interoceptive information from the body [Barrett and Bliss‐Moreau, 2009].
The bilateral lingual gyrus was identified as another commonly activated area. This area is known to be involved in visual word recognition [Fu et al., 2002; Petersen et al., 1998]. The Japanese sentences presented in the emotion and body conditions were longer (i.e., a greater number of graphemes) than those in the possession condition. Five to ten graphemes were presented in the emotion and body conditions, but three to seven graphemes were presented in the possession condition. Although the RTs in the three conditions were equivalent, the difference in number of graphemes may have led to differences in activation in the lingual gyrus between conditions. Also, the frequency of use of presented words was equivalent across three conditions [Ikehara et al., 1999]. The homogeneity of frequency of words and RTs indicate equivalent level of difficulty to understand statements. On the other hand, imageability of the words used in possession condition was higher than those in emotion and body conditions. All words used in possession trials were nouns, whereas adjectives were included in emotion and body trial. Possession condition required participants to imagine particular objects, but emotion and body condition required them to imagine particular states. To imagine certain states would require more effort than to imagine certain objects; thus strong activation in the lingual cortex may represent this difference.
Uniquely Activated Areas for Emotion‐Now Condition
Several regions were identified as uniquely activated areas for the emotion‐now condition. These regions included the left temporal pole (BA22/38), the bilateral posterior cingulate cortex (BA31), the bilateral ACC (BA24/32), the right superior medial frontal gyrus (BA9/10), the bilateral inferior frontal gyrus (BA45, 47), the left supramarginal gyrus (BA40), and the superior frontal gyrus (BA8). Almost all of these areas have been previously reported to be related to feeling and understanding one's own or others' mental states.
The importance of the bilateral ACC (BA24/32) in emotion and cognitive control is broadly accepted in cognitive neuroscience [Botvinick et al., 2001; Etkin et al., 2011]. The rostral ventral portion of ACC was strongly activated in the emotion‐now condition; this area is known as affective/rostral division [Vogt, 2005] and this and the adjacent prefrontal area are primarily related to the evaluation of emotional and reward‐related information as well as monitoring of somatic states [Bechara et al., 2000; Devinsky et al., 1995; di Pellegrino et al., 2007; Phillips et al., 2003; Whalen et al., 1998]. Simmons et al. [2006] reported strong activation in this area when processing ambiguous affective stimuli and suggested that demanding cognitive and emotional processing recruits this area. Also, this area effects attention shift from external to internal environment [Wager et al., 2005]. As our experimental design did not involve the emotion‐eliciting procedure, emotion‐now trial was a type of demanding emotional processing and required participants to orient their attention internally to responses in each trial. One would expect activity in this area to be altered if an emotion‐eliciting procedure is used.
The bilateral posterior cingulate cortices (PCC) are consistently activated by emotionally salient stimuli, in the context of word comprehension, and also episodic memory retrieval [Maddock, 1999]. In addition, previous studies have reported that activation in the PCC is closely related to decision‐making based on participants' subjective evaluations, monetary reward [Kable and Glimcher, 2007], or the impression of others [Schiller et al., 2009]. The present finding of strong activation in this area in the emotion‐now condition is consistent with these previous reports. Moreover, this area is an essential part of the default mode network [Gusnard and Raichle, 2001], and is thought to be a part of the neural substrate of consciousness [Laureys et al., 2004; Laureys et al., 2006]. In accord with this, many thalamic neurons project to the PCC to subserve cognitive function [Parvizi et al., 2006]. Our results, thus, suggest that explicitly attending to one's own emotional state involves one of the highest states of consciousness.
As mentioned above, the subjective emotional value of objects and activity in the rostral ACC, PCC, and inferior frontal gyrus (BA45, 47) are closely related [Kable and Glimcher, 2007]. The orbitofrontal cortex, including BA47, projects information from areas related to multisensory processing, and is known as an area related to monitoring one's own state [Damasio, 1999]. The current results are consistent with this hypothesis, and suggest that the orbitofrontal cortex (OFC) plays an important role when people are attending to their emotional state using monitored sensory information.
Converging evidence from neuroimaging and neuropsychological studies suggests that the medial frontal gyrus (BA9/10) is related to the processing of knowledge about self and others, as well as mentalizing [Amodio and Frith, 2006; Umeda et al., 2010]. Some studies have reported that activation in this area is associated with self‐relevant evaluations, such as the evaluation of personal traits [Johnson et al., 2002], or one's own emotional state [Lane et al., 1997; Ochsner et al., 2004]. Activation in the supramarginal gyrus is also thought to be related to mentalizing [Grosbras and Paus, 2006; Malhi et al., 2008]. Our results are consistent with previous studies indicating that the medial frontal gyrus, supramarginal gyrus, and PCC are the key regions for representing and evaluating the mental states of the self and others [Moran et al., 2009]. The current results indicate that these areas support the evaluation of emotion or the mental states of the self and others in reference to contextual, situational information. In this study, these areas and the PCC and its adjacent area precuneus appeared to integrate interoceptive and situational information, as participants became aware of their current mental state.
Neuroimaging studies concerned with socioemotional processing show activation in temporal pole. Activation in this region was observed during autobiographical memory retrieval [Fink et al., 1996; Piefke et al., 2005] and inferring other minds [Brunet et al., 2000; Calarge et al., 2003]. Anatomically, primate temporal pole receives and projects to the three sensory systems represented in the temporal lobe [Kondo et al., 2003]. Olson et al. [2007] reviewed these studies and defined this area as a region of integration between perceptual inputs and previously experienced social knowledge and emotion. In the present study, both the emotion‐usual and body‐usual conditions were found to recruit the activation of the bilateral temporal poles and the left superior temporal gyrus. These conditions, as well as the emotion‐now condition, commonly required participants to recall episodic or semantic memory related to each question. As such, the activation in the temporal pole and superior temporal gyrus may correspond to this recall process. These findings indicate that this area support the evaluation of emotional state, by combining interoceptive information with information about the environmental situation in which the participants were involved.
Almost all of the uniquely activated areas in the emotion‐now condition were regions that have been previously related to feeling and understanding one's own or others mental state. For evaluating emotional state, the emotion condition required participants to imagine the emotional situations described by the presented sentences, and to compare the situation to their own ongoing state. The activation we observed in emotion‐related areas may have been related to the recruitment of similar neural substrates during the imagination of emotional situations, and the experience of emotion while empathizing with others' emotional states [Carr et al., 2003; Singer et al., 2004].
Uniquely Activated Areas in the Body‐Now Condition
In contrast to the emotion‐now condition, only the supplementary motor area (BA6) and the inferior parietal gyrus were identified as uniquely activated areas in the body‐now condition. The supplementary motor area is traditionally classified as a part of motor system, and is considered as an important area for executing sequential complex movements [Hikosaka et al., 1996]. On the other hand, activation in this region has been found to induce the phantom limb illusion, suggesting that this area may influence the conscious perception of the body [McGonigle et al., 2002]. Sensory integration, body image, the concept of self and executive function are considered the major functions of the inferior parietal gyrus, and these functions are impaired in individuals with schizophrenia [Torrey, 2007]. To respond to each trial in the body‐now condition, it was necessary for participants to imagine certain bodily sensations corresponding to the presented sentences. The activation we found in the supplementary motor area and inferior parietal gyrus indicates that these areas may underlie the comparison between imagined and actually felt sensations in certain parts of the body.
We were unable to specify any other regions as uniquely activated areas in the body‐now condition. This result indicates that the neural substrates involved in evaluating bodily states are mostly included in the regions concerning evaluating emotional states, and that attending bodily states is a fundamental process for awareness of one's own emotional state.
How Does Interoception Relate to the Subjective Experience of Emotion?
Recent studies in cognitive neuroscience have suggested that the integration of information about bodily states, and the current situation is an essential factor in experiencing emotion. These studies provide support for the classical peripheral theory of emotion, which argues that perception of bodily responses is essential for experiencing emotions [Critchley et al., 2004; Naqvi et al., 2007]. Although classical theories are insufficient for explaining the mechanisms underlying the subjective experience of emotions, the main claims of these theories are still accepted in current research [Craig, 2008; Gray and Critchley, 2007; Rudrauf et al., 2009]. For example, the claim that integrative processing of visceral or sensory information and environmental information such as social context leads to subjective feelings remains commonly accepted [Lane, 2008]. Our findings are consistent with this constructive claim, suggesting that core affect is realized by integrating incoming sensory information from the external world with homeostatic and interoceptive information from the body [Barrett and Bliss‐Moreau, 2009].
Previous studies examining the neural mechanisms underlying emotional processing have focused on the function of the insular cortex and VMPFC, those were identified as shared neural substrates for the awareness of bodily and emotional states in current research. Our findings indicate that activation in these areas was related to accessing the bodily state, underlying awareness of the emotional state of the self. These findings imply that we access to bodily states implicitly when we evaluate our own emotional state, even if dynamic emotional bodily responses are absent. In the body condition, the evaluations were determined by the coactivation of interoception‐related areas and sensory integration‐related areas. On the other hand, in the emotion condition, the evaluations were determined by the integrated inference of information from interoception and information related to the understanding of the ongoing situation based on experience.
Though the some areas found to be commonly activated in the body‐now and emotion‐now conditions were also activated during the body‐usual and emotion‐usual conditions, activation in the anterior insular cortex and VMPFC was observed only in the now condition. Both in the now and usual conditions, participants were required to respond to questions about their own emotion or body. Thus, self‐referential thought would be activated during trials in both conditions. Therefore, the areas found to be commonly activated in both the now and usual conditions are likely to have been related to self‐referential thought or more general task components. However, there was a clear difference between the now and usual conditions in the associated object of self‐referential thought. In the usual conditions, the participants made evaluations based on knowledge about themselves and were thus required to attend to self‐related semantic or episodic memories. In the now condition, on the other hand, participants were required to focus on the immediate moment.
Consequently, the anterior insula and VMPFC, the areas significantly activated in the now condition, were not recruited for self‐referential thought generally but instantiating subjective feelings from body and feeling emotion in the immediate present. Neuroimaging studies have reported increased activity in anterior insular cortex associated with increasing task instability, complexity, and ambiguity with decision‐making context, and propose that this area integrates exteroceptive and interoceptive signals concerning uncertainty and improves learning and guiding our choice of behavior [Feinstein et al., 2006; Huettel et al., 2005; Singer et al., 2009]. Our findings imply that activity in anterior insular cortex increases when we access bodily internal states; thus, we strongly seek interoceptive signals to choose the options preceding desirable outcomes when we face highly uncertain situations, rather than certain ones. The VMPFC is another area thought to be critically involved in ongoing processing [Farb et al., 2007]. This area has been proposed to provide a mechanism for accessing thinking to on‐going reality by suppressing currently irrelevant memories [Schnider and Ptak, 1999; Treyer et al., 2003]. As mentioned above, this area underpins the evaluation of a stimulus for oneself by accessing one's own bodily state. The results may help to disentangle the question of why activation in insula cortex has been reported in association with disparate cognitive, affective, and regulatory functions [Menon and Uddin, 2010].
PPI analyses revealed the activity of right anterior insular cortex and VMPFC were functionally interacted with precuneus and left anterior insular cortex in emotion‐now condition, but only left anterior and posterior insular cortex was identified as functionally interacted with these two brain areas in body‐now condition. The results indicate that precuneus exclusively interacted with neural substrates of accessing bodily state in emotion‐now condition. Critchley et al. [2001] reported decreased activity in this area in patients with pure autonomic failure (PAF). PAF patients show impairment in peripheral autonomic regulation and also report attenuation of subjective emotional experience. These findings suggest that the autonomic response and subjective emotion are closely related and precuneus is an essential area for linkage between these two processes. Data from Critchley et al., [2001] and our findings suggest that precuneus takes a crucial role to transform interoceptive information into subjective emotion. This area was selectively activated when participants tried to evaluate their emotional state; therefore, participants' subjective awareness impacted the activity in this area. The precuneus may be seen as an integrating area of interoceptive information represented in insula and VMPFC and contextual information in uniquely activated areas for emotion‐now condition, and modulates our subjective experience.
Recent studies reported high‐glucose metabolism in precuneus and consider precuneus as essential neural substrate of consciousness [Gusnard and Raichle, 2001; Laureys et al., 2004]. Functionally, activity of precuneus is broadly related to human cognitive and emotional functions such as visuospatial imagery, episodic memory retrieval, and self‐consciousness [Cavanna and Trimble, 2006]. Distinct parts in this area have reciprocal projections connected with various cortical and thalamic areas, and the divergence of neural connectivity in this area would underpin the various mental processing. Moreover, precuneus is consistently reported part of the neural substrates of inferring others' emotions [Atique et al., 2011; Van Overwalle, 2009]. These evidences support our hypothesis that precuneus is an integrating area of interoceptive information and contextual information. To test our hypothesis, it might be insightful to conduct more detailed studies using functional and effective connectivity analyses among right anterior insula, VMPFC, precuneus, and emotion specifically activated areas during participants experiencing subjective emotion.
There are some limitations with our findings. First, the main methodological limitation of this study was not eliciting emotion or bodily sensation. This limitation stems from our decision to focus on evaluating process of emotional and bodily state in this study. As participants rating responses indicate, they were not aware of prominent emotion or bodily sensation during the task. Although our findings show neural substrates underpinning evaluating emotion or bodily sensation, future studies are necessary to examine the validity of our findings in emotion‐eliciting context. Second, this study was conducted in Japanese native speakers. Although the semantic structure of emotion terms in English and Japanese has been discussed [Uchida and Kitayama, 2009], how the differences in semantic structure affect evaluation of self‐emotional state is unresolved. To generalize our findings into English speakers, for example, verifying the findings using identical method in English speakers is required. Another limitation is the statistical threshold we used: P < 0.001 uncorrected. This is a less strict threshold level of fMRI study and we obtained a few activated regions when used correction for multiple comparisons. However, we performed another fMRI study by analogous procedure, and obtained replicated results of this study with more strict statistical criteria. Further replicated results in another study would support the validity of our present findings.
CONCLUSIONS
We examined the common and distinct features of the neural activity underlying interoception and subjective experience of emotion, using fMRI. We identified some commonly and distinctively activated regions for evaluating emotional and bodily state, and the findings support the constructive theory of emotion. The right anterior insular cortex and VMPFC were identified as shared neural substrates for the awareness of bodily and emotional states. Our findings indicate that activation in these areas and precuneus are functionally associated for accessing interoceptive information and underpinning subjective awareness of the emotional state. These findings provide direct evidence that we access to bodily states implicitly when we evaluate our own emotional state, even if dynamic emotional bodily responses are absent. Several regions were identified as uniquely activated areas for evaluating emotional state. Almost all of these areas have been previously reported to be related to feeling and understanding one's own or others' mental states. Thus, awareness of one's own emotional state appears to involve the integration of interoceptive information with an interpretation of the current situation.
Table AI.
Stimuli list used in each condition
| Emotion | Body | Possession | |
|---|---|---|---|
| 1 | Interested | Heart rate increase | Money |
| 2 | Distressed | Feeling hot all over | Keys |
| 3 | Excited | Sweating all over | Watches |
| 4 | Upset | Pounding in head | Reward cards |
| 5 | Strong | Dizziness | Foods |
| 6 | Guilty | Blurring of vision | Cosmetics |
| 7 | Hostile | Nausea | Books |
| 8 | Enthusiastic | Stomach and gut pains | Pens |
| 9 | Proud | Mouth becoming dry | Tissues |
| 10 | Irritable | Back pain | Electronic device |
| 11 | Alert | Muscle tension in my arms and legs | Tobacco |
| 12 | Ashamed | Rapid breathing | Medicines |
| 13 | Nervous | Feeling hungry | Credit cards |
| 14 | Dtermined | Swelling of my body | Signets |
| 15 | Attentive | Goose bumps | Accessories |
| 16 | Afraid | Being exhausted | Documents |
REFERENCES
- Abboud H, Berroir S, Labreuche J, Orjuela K, Amarenco P ( 2006): Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol 59: 691–699. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR ( 2003): Dissociable systems for recognizing emotions. Brain Cogn 52: 61–69. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Atique B, Erb M, Gharabaghi A, Grodd W, Anders S ( 2011): Task‐specific activity and connectivity within the mentalizing network during emotion and intention mentalizing. Neuroimage 55: 1899–1911. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Furlong PL, Barlow J, Hobson A, Alani S, Bancewicz J, Ribbands M, Harding GF, Thompson DG ( 1995): Topographic mapping of cortical potentials evoked by distension of the human proximal and distal oesophagus. Electroencephalogr Clin Neurophysiol 96: 219–228. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bliss‐Moreau E ( 2009): Affect as a Psychological Primitive. Adv Exp Soc Psychol 41: 167–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A ( 2004): The role of emotion in decision‐making: Evidence from neurological patients with orbitofrontal damage. Brain Cogn 55: 30–40. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW ( 1994): Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50–: 7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR ( 1996): Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex 6: 215–225. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR ( 2000): Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10: 295–307. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD ( 2001): Conflict monitoring and cognitive control. Psychol Rev 108: 624–652. [DOI] [PubMed] [Google Scholar]
- Brunet E, Sarfati Y, Hardy‐Bayle MC, Decety J ( 2000): A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage 11: 157–166. [DOI] [PubMed] [Google Scholar]
- Calarge C, Andreasen NC, O'Leary DS ( 2003): Visualizing how one brain understands another: A PET study of theory of mind. Am J Psychiatry 160: 1954–1964. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW ( 2000): Impaired recognition and experience of disgust following brain injury. Nat Neurosci 3: 1077–1078. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Keane J, Scott SK, Owen AM, Christoffels I, Young AW ( 2002): Reading the mind from eye gaze. Neuropsychologia 40: 1129–1138. [DOI] [PubMed] [Google Scholar]
- Cameron OG ( 2001): Interoception: The inside story–a model for psychosomatic processes. Psychosom Med 63: 697–710. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL ( 2003): Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA 100: 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129( Pt 3): 564–583. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2003): Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol 13: 500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD. 2008. Interoception and Emotion In: Lewis M, Haviland‐Jones J, Barrestt LM, editors. Handbook of Emotions, 3rd ed New York: The Guilford Press; p 272–288. [Google Scholar]
- Craig AD ( 2009): How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM ( 2000): Thermosensory activation of insular cortex. Nat Neurosci 3: 184–190. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ ( 2001): Neuroanatomical basis for first‐ and second‐order representations of bodily states. Nat Neurosci 4: 207–212. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ ( 2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- Damasio AR. 1999. The Feeling of What Happens. Orlando, Florida, USA: Harcourt. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD ( 2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- de Greck M, Rotte M, Paus R, Moritz D, Thiemann R, Proesch U, Bruer U, Moerth S, Tempelmann C, Bogerts B and others ( 2008): Is our self based on reward? Self‐relatedness recruits neural activity in the reward system. Neuroimage 39: 2066–2075. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA ( 1995): Contributions of anterior cingulate cortex to behaviour. Brain 118 ( Pt 1): 279–306. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Ciaramelli E, Ladavas E ( 2007): The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J Cogn Neurosci 19: 275–286. [DOI] [PubMed] [Google Scholar]
- Dunkley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I ( 2005): A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci 25: 7333–7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, Cusack R, Lawrence AD, Dalgleish T ( 2010): Listening to your heart: How interoception shapes emotion experience and intuitive decision making. Psychol Sci 21: 1835–1844. [DOI] [PubMed] [Google Scholar]
- Ekman P, Levenson RW, Friesen WV ( 1983): Autonomic nervous system activity distinguishes among emotions. Science 221: 1208–1210. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R ( 2011): Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RSJ, Corfield DR ( 2002): BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol 88: 1500–1511. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK ( 2007): Attending to the present: Mindfulness meditation reveals distinct neural modes of self‐reference. Soc Cogn Affect Neurosci 2: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Stein MB, Paulus MP ( 2006): Anterior insula reactivity during certain decisions is associated with neuroticism. Soc Cogn Affect Neurosci 1: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD ( 1996): Cerebral representation of one's own past: Neural networks involved in autobiographical memory. J Neurosci 16: 4275–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD ( 2003): Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci 358: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Chen Y, Smith S, Iversen S, Matthews PM ( 2002): Effects of word form on brain processing of written Chinese. Neuroimage 17: 1538–1548. [DOI] [PubMed] [Google Scholar]
- Gray MA, Critchley HD ( 2007): Interoceptive basis to craving. Neuron 54: 183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Lee RR, Lenz FA ( 1999): Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain 81: 273–282. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T ( 2006): Brain networks involved in viewing angry hands or faces. Cereb Cortex 16: 1087–1096. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME ( 2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Hamaguchi T, Kano M, Rikimaru H, Kanazawa M, Itoh M, Yanai K, Fukudo S ( 2004): Brain activity during distention of the descending colon in humans. Neurogastroenterol Motil 16: 299–309. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Gianaros PJ, Critchley HD ( 2010): The embodiment of emotional feelings in the brain. J Neurosci 30: 12878–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig‐Fast K, Meister F, Frodl T, Beraldi A, Padberg F, Engel RR, Reiser M, Moller HJ, Meindl T ( 2008): A case of persistent retrograde amnesia following a dissociative fugue: Neuropsychological and neurofunctional underpinnings of loss of autobiographical memory and self‐awareness. Neuropsychologia 46: 2993–3005. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Putz B ( 1996): Activation of human presupplementary motor area in learning of sequential procedures: A functional MRI study. J Neurophysiol 76: 617–621. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G ( 2005): Decisions under uncertainty: Probabilistic context influences activation of prefrontal and parietal cortices. J Neurosci 25: 3304–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Committeri G, Pastorelli C, Pizzamiglio L, Watkins KE, Carota A ( 2008): Neural activity of the anterior insula in emotional processing depends on the individuals' emotional susceptibility. Hum Brain Mapp 29: 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S, Miyazaki M, Shirai S, Yokoo A, Nakaiwa H, Ogura K, Ooyama Y, Hayashi Y. 1999. Goi‐Taikei—A Japanese Lexicon CDROM. Tokyo: Iwanami Shoten. [Google Scholar]
- James W ( 1884): What is an emotion? Mind 19: 188–205. [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP ( 2002): Neural correlates of self‐reflection. Brain 125( Pt 8): 1808–1814. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW ( 2007): The neural correlates of subjective value during intertemporal choice. Nat Neurosci 10: 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL ( 2003): Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 465: 499–523. [DOI] [PubMed] [Google Scholar]
- Lane RD ( 2008): Neural substrates of implicit and explicit emotional processes: A unifying framework for psychosomatic medicine. Psychosom Med 70: 214–231. [DOI] [PubMed] [Google Scholar]
- Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM ( 2006): Left insular stroke is associated with adverse cardiac outcome. Neurology 66: 477–483; discussion 463. [DOI] [PubMed] [Google Scholar]
- Laureys S, Owen AM, Schiff ND ( 2004): Brain function in coma, vegetative state, and related disorders. Lancet Neurol 3: 537–546. [DOI] [PubMed] [Google Scholar]
- Laureys S, Boly M, Maquet P ( 2006): Tracking the recovery of consciousness from coma. J Clin Invest 116: 1823–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Siegel GJ ( 2009): Common and distinct brain networks underlying explicit emotional evaluation: A meta‐analytic study. Soc Cogn Affect Neurosci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa FE, Fox PT, Denton D ( 2001): Brain responses associated with consciousness of breathlessness (air hunger). Proc Natl Acad Sci USA 98: 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ ( 1999): The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends Neurosci 22: 310–316. [DOI] [PubMed] [Google Scholar]
- Main CJ, Wood PL, Hollis S, Spanswick CC, Waddell G ( 1992): The distress and risk assessment method. A simple patient classification to identify distress and evaluate the risk of poor outcome. Spine (Phila Pa 1976) 17: 42–52. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Das P, Moss K, Berk M, Coulston CM ( 2008): A functional MRI study of Theory of Mind in euthymic bipolar disorder patients. Bipolar Disord 10: 943–956. [DOI] [PubMed] [Google Scholar]
- McGonigle DJ, Hanninen R, Salenius S, Hari R, Frackowiak RS, Frith CD ( 2002): Whose arm is it anyway? An fMRI case study of supernumerary phantom limb. Brain 125( Pt 6): 1265–1274. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ ( 2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214–: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Weiskopf N, Lau HC, Featherstone E, Dolan RJ, Frith CD ( 2006): The Kuleshov effect: The influence of contextual framing on emotional attributions. Soc Cogn Affect Neurosci 1: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM ( 2009): Modulation of cortical midline structures by implicit and explicit self‐relevance evaluation. Soc Neurosci 4: 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A ( 2007): Damage to the insula disrupts addiction to cigarette smoking. Science 315: 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Grimm S, Boeker H, Schmidt C, Bermpohl F, Heinzel A, Hell D, Boesiger P ( 2006): Affective judgment and beneficial decision making: Ventromedial prefrontal activity correlates with performance in the Iowa Gambling Task. Hum Brain Mapp 27: 572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC ( 2004): Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 16: 1746–1772. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y ( 2007): The enigmatic temporal pole: A review of findings on social and emotional processing. Brain 130( Pt 7): 1718–1731. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL ( 2000): The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10: 206–219. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A ( 2006): Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA 103: 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB ( 2003): Increased activation in the right insula during risk‐taking decision making is related to harm avoidance and neuroticism. Neuroimage 19: 1439–1448. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB ( 2006): An insular view of anxiety. Biol Psychiatry 60: 383–387. [DOI] [PubMed] [Google Scholar]
- Penfield W, Faulk ME Jr, ( 1955): The insula; further observations on its function. Brain 78: 445–470. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME ( 1998): The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA 95: 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I ( 2004): Neural correlates of individual ratings of emotional salience: A trial‐related fMRI study. Neuroimage 21: 768–780. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R ( 2003): Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54: 504–514. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Markowitsch HJ, Fink GR ( 2005): Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Hum Brain Mapp 24: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutchik R, Ax AF ( 1967): A critique of “Determinants of emotional state” (by Schachter and Singer, 1962). Psychophysiology 4: 79–82. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Kirsch W, Schandry R ( 2005): On the relationship between interoceptive awareness, emotional experience, and brain processes. Brain Res Cogn Brain Res 25: 948–962. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R ( 2004): Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat‐evoked brain potential. Psychophysiology 41: 476–482. [DOI] [PubMed] [Google Scholar]
- Porges S. 1993. Body Perception Questionnaire: Laboratory of Developmental Assessment, University of Maryland.
- Rainville P, Bechara A, Naqvi N, Damasio AR ( 2006): Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int J Psychophysiol 61: 5–18. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, Lachaux JP, Damasio A, Baillet S, Hugueville L, Martinerie J, Damasio H, Renault B ( 2009): Enter feelings: Somatosensory responses following early stages of visual induction of emotion. Int J Psychophysiol 72: 13–23. [DOI] [PubMed] [Google Scholar]
- Sato A, Yasuda A ( 2001): Development of the Japanese version of Positive and Negative Affect Schedule (PANAS) scales. Jpn J Pers 9: 138–139. [Google Scholar]
- Schachter S, Singer JE ( 1962): Cognitive, social, and physiological determinants of emotional state. Psychol Rev 69: 379–399. [DOI] [PubMed] [Google Scholar]
- Schandry R ( 1981): Heart beat perception and emotional experience. Psychophysiology 18: 483–488. [DOI] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA ( 2009): A neural mechanism of first impressions. Nat Neurosci 12: 508–514. [DOI] [PubMed] [Google Scholar]
- Schnider A, Ptak R ( 1999): Spontaneous confabulators fail to suppress currently irrelevant memory traces. Nat Neurosci 2: 677–681. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. 1906. The integrative action of the nervous system. New Haven: Yale University Press. [Google Scholar]
- Simmons A, Stein MB, Matthews SC, Feinstein JS, Paulus MP ( 2006): Affective ambiguity for a group recruits ventromedial prefrontal cortex. Neuroimage 29: 655–61. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K ( 2009): A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13: 334–340. [DOI] [PubMed] [Google Scholar]
- Teves D, Videen TO, Cryer PE, Powers WJ ( 2004): Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci USA 101: 6217–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treyer V, Buck A, Schnider A ( 2003): Subcortical loop activation during selection of currently relevant memories. J Cogn Neurosci 15: 610–618. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Adolphs R ( 2007): Emotion and consciousness. Trends Cogn Sci 11: 158–167. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Kitayama S ( 2009): Happiness and unhappiness in east and west: Themes and variations. Emotion 9: 441–456. [DOI] [PubMed] [Google Scholar]
- Umeda S, Mimura M, Kato M ( 2010): Acquired personality traits of autism following damage to the medial prefrontal cortex. Soc Neurosci 5: 19–29. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F ( 2009): Social cognition and the brain: A meta‐analysis. Hum Brain Mapp 30: 829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna EP, Tranel D ( 2006): Gastric myoelectrical activity as an index of emotional arousal. Int J Psychophysiol 61: 70–76. [DOI] [PubMed] [Google Scholar]
- Vogt BA ( 2005): Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Smith EE, Nichols TE ( 2005): Toward a taxonomy of attention shifting: Individual differences in fMRI during multiple shift types. Cogn Affect Behav Neurosci 5: 127–143. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A ( 1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Werner NS, Duschek S, Marttern M, Schandry R ( 2009): Interoceptive sensitivity modulates anxiety during public speaking. J Psychophysiol 23: 85–94. [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL ( 1998): The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44: 1219–1228. [DOI] [PubMed] [Google Scholar]
- Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ ( 2006): Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci 26: 11501–11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens S, Mezzacappa ES, Katkin ES ( 2000): Heartbeat detection and the experience of emotions. Cognit Emotion 14: 417–427. [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ ( 2002): Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci 5: 277–283. [DOI] [PubMed] [Google Scholar]
- Wright P, Albarracin D, Brown RD, Li H, He G, Liu Y ( 2008): Dissociated responses in the amygdala and orbitofrontal cortex to bottom‐up and top‐down components of emotional evaluation. Neuroimage 39: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]


