Abstract
The primary aim of this study was to enhance our understanding of the functional architecture of the cortico‐basal ganglia circuitry during motor task execution. Twenty right‐handed female subjects without any history of neuropsychiatric illness underwent fMRI at 3 T. The activation paradigm was a complex motor task completed with the nondominant hand. Analyses of functional connectivity strength were conducted for pairs of structures in input, intrinsic, and output segments of the circuitry. Next, connectivity strengths were correlated with results of neurocognitive testing conducted outside of the scanner, which provided information about both motor and cognitive processes. For input pathways, results indicate that SMA–striatum interactions are particularly relevant for motor behavior and disruptions may impact both motor and cognitive functions. For intrinsic pathways, results indicate that thalamus (VA nucleus) to striatum feedback pathway appears to have an important role during task execution and carries information relevant for motor planning. Together, these findings add to accumulating evidence that the GPe may play a role in higher order basal ganglia processing. A potentially controversial finding was that strong functional connectivity appears to occur across intrinsic inhibitory pathways. Finally, output (thalamus to cortex) feedback was only correlated with motor planning. This result suggests circuit processes may be more relevant for future behaviors than the execution of the current task. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: basal ganglia; motor pathways; caudate nucleus; MRI, functional; thalamus
INTRODUCTION
The basal ganglia, thalamus and cortex (Fig. 1) along with associated anatomical connections form the cortico‐basal ganglia information processing loops [Alexander et al., 1986, 1990]. These circuits are involved in cognitive processing [Casey et al., 2002; Chang et al., 2007; Middleton and Strick, 2000; Ystad et al., 2010, 2011] as well as motor behaviors, including motor learning and adaptation [Doyon and Benali, 2005; Graybiel, 2005; Lehericy et al., 2005; Seidler et al., 2006], action selection [Gurney et al., 2001; Mink, 1996] and motor sequence learning [Lehericy et al., 2005]. Despite extensive research, our understanding of the mechanisms by which these pathways process both cognitive and motor information during motor behaviors remains incomplete. The primary aim of this study was to enhance our understanding of these processes.
Figure 1.
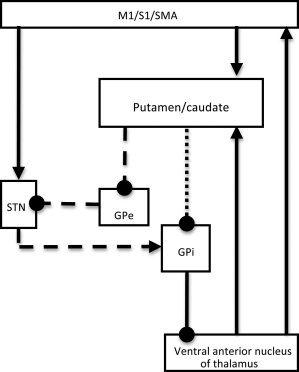
Simplified schematic of cortico‐basal ganglia circuitry based on the original model developed by Alexander et al. (1990, 1986). Arrows = glutamate pathways; circles = GABA pathways; dotted line = direct pathway; dashed lines = indirect pathway.
To that end, we first examined functional connectivity strength between pairs of cortico‐basal ganglia circuit structures. Next we correlated connectivity strengths with performances on neurocognitive tasks performed outside of the scanner. The neurocognitive instruments provided information about both motor and cognitive processing. We have previously shown this approach to provide useful information about cortico‐basal ganglia circuit function [Marchand et al., 2011].
Functional connectivity analyses were focused on three general segments of the circuit; the input, intrinsic, and output pathways. The goal of these analyses was to delineate the relative level of engagement of the various subpathways (e.g., M1 and putamen) during task execution. We reasoned that this information would be relevant for the understanding of both normal function and the neurobiology of basal ganglia disorders. The aim of the correlational analyses was to determine what type of information (cognitive, motor efficiency, or motor planning) was conveyed along subpathways. We anticipated these analyses would also provide information about the relative contribution of each circuit studied to overall task performance.
METHODS
Subjects
Twenty‐five female subjects were originally recruited for the study. Five subjects were removed from the original sample due to extreme values of questionable validity on one or more behavioral variables from neurocognitive testing conducted outside of the scanner (e.g., excessive number of errors suggesting random responding), for the final sample of 20 participants. Potential subjects were excluded if they were either not native English speakers or string or keyboard musicians because of the possible impact on communicating with study staff and motor task execution, respectively. Additional exclusionary criteria were any history of: head injury, neurological disorder or dementia as well as either any medical disorder or current use of medications that could impact the central nervous system. Those with contraindications to fMRI as well as those with any history of psychiatric disorders, substance abuse, treatment with psychiatric medications or any first‐degree relative with any psychiatric disorder were also excluded. All subjects received a study evaluation that included administration of several neuropsychological tasks (described below) as well as the administration of the Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders‐Research Version to rule out psychiatric illness.
Subjects were all female to avoid any possible confound secondary to gender‐specific activation patterns [Bell et al., 2006; Li et al., 2006] and were between the ages of 25 and 33 (mean = 27.70, SD = 2.41) with 12–16 years of formal education (mean = 14.90, SD = 1.33). Estimated IQ of the group ranged from 89 to 125 (mean = 108.05, SD = 11.55). All subjects were strongly right‐handed as evidenced by a score of ≥ 80 (mean = 90.25, SD = 8.19) on the Edinburgh Handedness Inventory [Oldfield, 1971]. After a complete description of the study was given to the subjects, written informed consent was obtained, as approved by both the Institutional Review Board at the University of Utah and the Research Review Committee of the George E. Whalen Veterans Administration Medical Center.
Neurocognitive Testing Instruments and Procedures
All neurocognitive measures were administered on a separate day from and prior to scanning. The tasks used in the present study were administered as part of longer battery used for another study [Marchand et al., 2011]. The entire battery took ∼ 45 min and was conducted in a quite testing room by a trained technician.
Intellectual functioning was estimated using the Wechsler Test of Adult Reading (WTAR), a 3–5 min measure of oral reading consisting of 50 words that have irregular grapheme‐to‐phoneme translation but do not require text comprehension or knowledge of word meaning (The Psychological Corporation 2001). The WTAR was specifically developed as a tool for estimating premorbid intellectual functioning in adults aged 16–89, and was conormed with the Wechsler Adult Intelligence Scale‐III [Wechsler, 1997] to provide direct comparisons between predicted and observed intelligence scores.
Executive and motor functioning was assessed using the Alphanumeric Sequencing and the Push‐Turn‐Taptap subtests from the Behavioral Dyscontrol Scale‐electronic version (BDS‐EV) battery [Suchy et al., 2005]. These are described in turn below.
The Alphanumeric Sequencing (AS) task is an electronic counterpart to the Trail Making Test‐Part B (TMT‐B) [Reitan, 1958], which is a well known and extensively validated measure of executive functioning thought to specifically assess cognitive flexibility [Arbuthnott and Frank, 2000; Moll et al., 2002; Stuss et al., 2001; Zakzanis et al., 2005]. On the AS task, participants are required to push buttons on a specialized response console marked with letters of the alphabet (A through H) and numerals one through nine. The buttons are to be pressed in alphanumeric sequence (i.e., 1A, 2B, 3C, etc.) Speed of performance is measured electronically in ms. The AS task has been found to correlate highly with the original TMT‐B [Suchy et al., 2005], and its reliability has been found to be superior to the original trail making task [Eastvold et al., 2004].
The Push‐Turn‐Taptap (PTT) task is an electronic parallel to the well‐known “Fist‐Edge‐Palm” task developed by Alexander Luria [Luria and Majovski, 1979]. Participants are required to learn four different sequences of increasing length that consist of different permutations of three different hand movements executed on a specialized response consol. The three hand movements are “Push”—pushing the joystick on the response console forward; “Turn” —turning the joystick clockwise; and “Taptap”—double‐tapping on a large dome button on the response console. The task begins with Block 1, in which a two‐movement sequence is presented on the computer screen. The participant performs the indicated task until three correct trials are achieved. Following these three learning trials, participants continue to perform the sequence from memory, until accomplishing five additional correct trials. This completes the Block 1 of the task. After completing Block 1, participants move on to the next block in which they follow the same learning procedures. There is a total of four Blocks, each characterized by different and progressively longer sequences. Mistakes are followed by an audible tone, along with the presentation of the correct sequence on the computer screen and the highlighting of the next movement to be performed. This task was selected for the study because it allows assessment of various discrete components of motor output.
First, the PTT task allows assessment of the “motor planning” component of motor output, which has been described as an internal strategy that precedes an intended movement [Banich, 2004]. Specifically, prior to initiating a sequence of coordinated movements, an abstract plan is generated that contains both general information about the intended goal and specific information about the neuromuscular control that will be required [Keele, 1968]. As such, motor planning latencies may provide an indirect indication of motor‐executive integration. Motor planning was assessed by measuring the mean latency before initiation of a correctly executed sequence.
Second, the PTT task allows assessment of performance speed, reflected in the time required to complete a given sequence from start to finish. This “motor speed” variable was measured by computing the mean speed of completing correct sequences across the four blocks. Because motor speed contributes to performance of many tasks purported to measure executive functioning, this variable served not only the purpose of assessing the efficacy of the motor system, but also of controlling for motor speed on the AS task.
Last, the PTT task allows assessment of the “motor learning” aspect of motor output, by computing the total number of errors committed across the four blocks, as participants learn new and progressively more complex sequences. The overall performance on this task has been shown to correlate with measures of executive functioning above and beyond participants' demographic characteristics and simple motor speed, with the motor planning latencies showing the strongest association with executive functions [Kraybill and Suchy, 2008; Suchy et al., 2005; Suchy and Kraybill, 2007; Suchy et al., 2010].
Functional MRI Tasks and Experimental Procedure
A block‐design motor activation paradigm was used to probe cortico‐basal ganglia function. The task, which we have used previously [Lee et al., 2010; Marchand et al., 2011; Marchand et al., 2008], was a self‐paced paradigm performed with the nondominant hand. In this task, subjects alternated pressing buttons with the middle finger alone and then the index and ring finger simultaneously during a four minute run with six blocks of rest and six blocks of activity presented in pseudorandom order (Fig. 2). The task was self‐paced and subjects were instructed to complete repetitions at a consistent but comfortable pace during each run.
Figure 2.
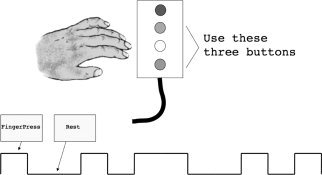
Graphic illustration of the complex motor task.
Visual stimuli for the task were presented on a translucent slide screen at the back of the magnet, which was viewed through a mirror mounted on top of the head coil. Stimulus presentation and response recordings were controlled by E‐prime software (Psychology Software Tools, Pittsburgh, USA; http://www.pstnet.com/eprime).
Subjects were trained on the task immediately prior to scanning utilizing a computer to display the visual stimuli while instructions were given. Subjects practiced the task using the actual button boxes used during the scan. Training and orientation to the scan required approximately 10 min per subject. Task compliance was confirmed during scanning by way of a remote button control box that indicated subject button presses by illuminating a light color coded for each button.
Functional Imaging
Subjects were scanned on a Siemens 3T Trio MR scanner with a 12‐channel head coil. Functional MRI data were acquired with a susceptibility weighted gradient echo EPI sequence (field‐of‐ view 22 cm, matrix 64 x 64, repetition time TR = 2.08 s, echo time TE = 30 ms, slice thickness 3 mm with 10% gap, flip angle 75°). Thirty‐five slices were acquired during each repetition time. The first five image volumes of each task were discarded to ensure signal equilibrium. Distortions caused by variations in magnetic susceptibility were removed during post‐processing using fieldmap data acquired with a separate sequence.
Anatomical
T1‐weighted images were acquired using an MPRAGE sequence (field‐of‐ view 22 cm, matrix 192 x 192, repetition time TR = 1.5 s, inversion time TI = 1.1 s, slice thickness 2 mm, slices = 80, flip angle 8°, signal averages = 2).
Data Processing
fMRI processing
Preprocessing and statistical analyses were carried out with SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Images were realigned to correct for head motion, unwarped to remove susceptibility distortion, and slice‐time corrected. The mean‐realigned EPI image was coregistered with the anatomical image. All images were spatially normalized to the Montreal Neurological Institute (MNI) template, and voxel sizes resampled to 2 × 2 × 2. EPI images were smoothed using isotropic 6 mm Gaussian kernels and statistically analyzed using an epoch design convolved with the hemodynamic response function. Low‐frequency noise was removed with a high‐pass filter with a cutoff period of 128 s and an autoregressive AR (1) model was fit to the residuals to account for temporal autocorrelation.
Regions of interest (ROIs) were selected based upon known components of the cortico‐basal ganglia circuitry involved with motor task execution. In regard to cortical structures, primary motor cortex (M1) and primary somatosensory cortex (S1) were selected because of their well‐known role in motor behavior. The supplementary motor area (SMA) was chosen because of evidence that while this structure is involved with initiating motor responses, there is evidence that contributions continue during motor execution [Brendel et al., 2010] in parallel with M1 [Chen et al., 1991]. The ventral anterior (VA) nucleus of the thalamus was included because of evidence of circuit outflow anatomical connectivity from globus pallidus internal segment (GPi) to SMA through the VA [Sakai et al., 2002; Wiesendanger and Wiesendanger, 1985]. The other regions, subthalamic nucleus (STN), caudate, globus pallidus external segment (GPe) and GPi, are well‐known subcortical components of the circuitry [Albin et al., 1989; Alexander et al., 1986].
ROIs for connectivity analysis were generated by forming the intersection of activation maps from three groups of female subjects (part of a larger study) with anatomical ROIs in the ‘VOI Tool Utility’ available at http://www.ihb.spb.ru/~ pet_lab. Please see Table I for volumes and coordinates of ROIs and Figure 3 for selected ROI images. The activation maps used to generate subcortical ROIs were thresholded at 0.001, uncorrected for multiple comparisons, and restricted to cluster sizes that achieved P < 0.05 according to SPM random field theory [Friston et al., 1993). The activation maps used to generate cortical ROIs were thresholded at a higher P < 0.05 threshold, corrected for multiple comparisons, because of their stronger activation, and a few protruding spikes were eliminated to produce uniform models. In addition, regions of interest for regressors of no interest were obtained by placing 3 mm‐radius seeds in both white matter (MNI coordinates 33, −62, 24) and CSF (MNI coordinates 6, −2, 19), and using the six motion regressors that result from spatial realignment in SPM5. Our method of combining anatomical ROIs with activation maps was chosen to increase specificity. Since we limit attention to those portions of the ROI that are active for the group as a whole, any portions of the ROI that exhibit large variability across the group due to differences in normalization are less likely to produce group‐level activation, and are unlikely to survive the imposed activation threshold. Furthermore, a comparison of hand‐drawn anatomical ROIs to the use of atlases found no substantial difference in the result [Prodoehl et al., 2008] and this approach has been used by others [Boecker et al., 2008].
Table I.
Volumes and coordinates of regions of interest
| ROI | Volume (mm3) | Coordinates | |||||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| max | min | max | min | max | min | ||
| SMA | 904 | 2 | 0 | −6 | 4 | 50 | 66 |
| S1 | 5520 | 30 | 58 | −46 | −12 | 30 | 64 |
| M1 | 6136 | 26 | 52 | −32 | −8 | 46 | 70 |
| Caudate | 424 | 6 | 16 | −14 | 22 | −6 | 22 |
| Putamen | 4168 | 14 | 32 | −22 | 18 | −6 | 18 |
| STN | 168 | 8 | 12 | −16 | −10 | −8 | −2 |
| GPe | 752 | 14 | 28 | −20 | 6 | −4 | 10 |
| GPi | 88 | 16 | 20 | −10 | −2 | −2 | 2 |
| VA | 160 | 10 | 14 | −8 | −4 | 6 | 14 |
SMA, supplementary motor area; STN, subthalamic nucleus; GPe, globus pallidus, external segment; GPi, globus pallidus, internal segment, VA, ventral anterior nucleus of the thalamus.
Figure 3.
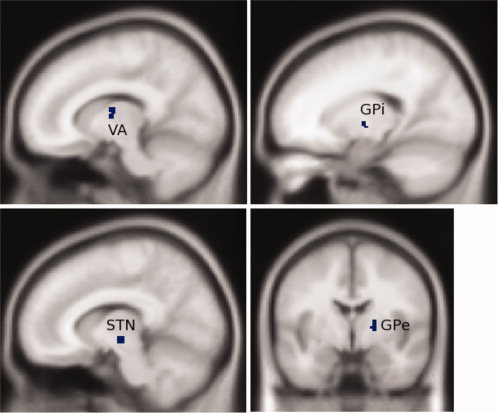
Four subcortical regions of interest. Starting in the lower left corner and proceeding clockwise, regions of interest are subthalamic nucleus, ventral anterior nucleus of the thalamus, globus pallidus internal segment, and globus pallidus external segment. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Waveforms for all ROIs were extracted with Marsbar software (http://marsbar.sourceforge.net), mean corrected, and bandpass‐filtered from 0.008 to 0.1 Hz with a second order butterworth filter. See Figure 4 for graph of ROI timecourses. Partial correlations between pairs of ROIs were carried out using the Matlab Statistics Toolbox (Mathworks, Natick, Massachusetts), partialing out the influence of white matter, CSF and motion. The resulting partial correlations were normalized with Fisher's z transform to produce functional connectivity values. These values representing the strength of connectivity were generated for all possible combinations of cortical and subcortical structures for which direct anatomical connections are known to exist.
Figure 4.
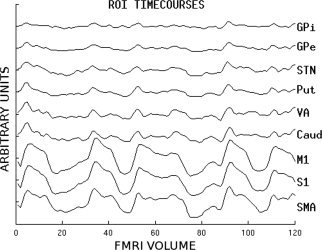
Timecourses of regions of interest.
Signal to noise ratio (SNR) was measured for each ROI (Fig. 5), and correlated with cortical‐subcortical connectivity. The aim of this analysis was to determine whether distinctive SNRs in different ROIs might influence connectivity results. These pathways were chosen because of the high SNR in cortical ROIs. Mean signal was measured in normalized image volumes acquired during rest, so it would not be affected by activation, and noise was calculated as the standard deviation in the outermost edge voxels in acquired images. A scale factor was applied to the acquired image noise to compensate for intensity adjustments applied during the SPM normalization process.
Figure 5.
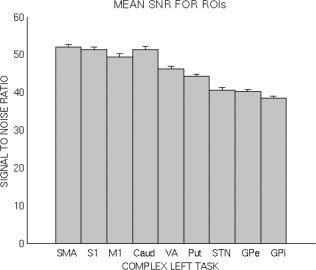
Mean signal to noise ratios for each region of interest.
Functional connectivity comparisons
We first examined the overall pattern of connectivity strengths among the cortical and subcortical regions, using an omnibus repeated measures analysis of variance (ANOVA). This analysis compared connectivity of individual cortical and subcortical regions, using cortical regions (SMA vs. S1 vs. M1) and subcortical regions (STN vs. caudate vs. VA vs. putamen) as two within‐subjects factors. This analysis determined whether (a) specific regions exhibited stronger overall connectivity values than other regions (reflected in statistically significant main effects), and (b) specific pairs exhibited stronger connectivity values than other pairs (reflected in a statistically significant interaction between the two within‐subjects factors). Follow‐up analyses were conducted as needed to further explicate the overall patterns indicated by the omnibus test (see Results). Next, we examined overall pattern of subcortical–subcortical connectivity strengths in an omnibus repeated measures ANOVA. However, because not all subcortical structures connect to all other subcortical structures, a single ANOVA could not accomplish this. To that end, we conducted two omnibus tests. In the first ANOVA, we used connectivity of caudate vs. putamen vs. STN as one within subjects factor, and connectivity of GPe vs. GPi as a second within subjects factor. In the second ANOVA, we used GPe vs. GPi vs. VA as one within‐subjects factor, and caudate vs. putamen as a second within‐subjects factor. Again, follow‐up analyses were conducted as needed (see Results).
Functional connectivity and behavioral data
Finally, analyses were completed to determine if correlations existed between connectivity and either performance on neuropsychological testing completed outside of the scanner. To minimize the number of correlations, we only examined associations of behavioral data with a limited number of connectivity pairs that provided information about all pathway components (input, intrinsic, and output).
BEHAVIORAL RESULTS
Neuropsychological Evaluation and Data Obtained During the Scan
Participants performed all tasks on par with expectations based on our prior research with these tasks [Suchy et al., 2005; Suchy and Kraybill, 2007]. The AS task took on average 28.40 s to complete (SD = 8.53). The mean time to complete a sequence on the PTT task across the four blocks was 2.34 s (SD = 0.47). The mean number of errors across the four PTT blocks was 7.20 (SD = 9.31). The mean motor planning latency across the four PTT blocks was 0.83 s (SD = 0.11). For all variables, higher values reflect poorer (i.e., slower, less accurate) performance. The correlation matrix among the behavioral variables is presented in Table II. As can be seen from the table, motor planning latency correlated with performance on the AS task, consistent with prior research [Kraybill and Suchy, 2008; Suchy et al., 2005; Suchy and Kraybill, 2007; Suchy et al., 2010]. Importantly, although motor speed also correlated with motor planning, the dissociation of the constructs measured by the two variables is evident from the lack of correlation between motor speed and the AS task. This latter finding also suggests that motor speed contributes a negligible amount of variance to the AS task performance. The fact that motor planning was correlated both with motor speed and performance on a measure of executive functioning is consistent with the suggestion that motor planning reflects an integration of motor and cognitive processes.
Table II.
Correlation among behavioral variables
| PTT motor speed | PTT accuracy | PTT motor planning | AS task | IQ estimate | |
|---|---|---|---|---|---|
| Complex left task | −0.017 | −0.414* | −0.550** | −0.438* | 0.038 |
| PTT motor speed | −0.653** | 0.492* | 0.097 | −0.558** | |
| PTT accuracy | 0.173 | 0.267 | 0.153 | ||
| PTT motor planning | 0.549** | −0.343 | |||
| AS task | −0.325 |
PTT, Push‐Turn‐Taptap task; AS, alphanumeric sequencing.
**P= 0.001, *P = < 0.05, one tailed.
For the motor activation paradigm used during scanning, the number of task repetitions per run for each subject was recorded by E‐prime. The mean for the group was 14.06 (SD = 4.54) repetitions (higher values reflecting better performance). As can be seen in Table II, the number of repetitions completed on this task exhibited a trend toward correlation with the AS task and the PTT task accuracy, reflecting that this task requires both motor learning abilities and executive abilities for successful completion.
FUNCTIONAL IMAGING RESULTS
Functional Connectivity of Cortical‐Subcortical Pathways
Mean connectivity coefficients of the cortical (M1, S1, and SMA) with subcortical regions (caudate, putamen, STN, and VA) are presented in Table III. Omnibus repeated measures analysis of variance was used to first examine the overall pattern of relationships among connectivity pairs, with follow‐up ANOVA conducted to explicate the omnibus results by directly comparing connectivity strengths of individual regions. The initial omnibus analysis (in which connectivity strengths of SMA vs. M1 vs. S1 served as one within‐subjects factor, and connectivity strengths of STN vs. caudate vs. putamen vs. VA served as a second within‐subjects factor) revealed a large main effect of connectivity among the cortical regions [F(2,38) = 13.93, P < 0.001], indicating that the three cortical regions significantly differed from each other with respect to the magnitude of connectivity with subcortical regions. The same analysis also demonstrated a large main effect of connectivity among the subcortical regions [F(3,28) = 12.80, P < 0.001] indicating that the connectivity strengths of the four subcortical regions (with the cortical regions) were significantly different from each other. Follow‐up ANOVAs directly comparing connectivity strengths of the individual cortical regions demonstrated that, overall, SMA showed significantly stronger coactivation with subcortical regions than either M1 [F(1,19) = 20.97, P < 0.001] or S1 [F(1,19) = 7.60, P = 0.013]. Further, S1 showed significantly stronger connectivity than M1 [F(1,19) = 10.94, P = 0.004]. Additional follow‐up ANOVAs directly comparing connectivity strengths of the subcortical regions demonstrated that, overall, putamen showed significantly stronger connectivity (with cortical regions) than any of the three remaining subcortical regions, that is, stronger than STN [F(1,19) = 35.73, P < 0.001], caudate nucleus [F(1,19) = 17.58, P < 0.001], or VA [F(1,19) = 15.61, P = 0.001].
Table III.
Functional connectivity coefficients of cortical with subcortical structures of the cortico‐basal ganglia circuitry
| Caudate | STN | VA | Putamen | |
|---|---|---|---|---|
| SMA | 0.5825 | 0.5231 | 0.5808 | 0.7808 |
| M1 | 0.4703 | 0.4182 | 0.4294 | 0.6130 |
| S1 | 0.5302 | 0.4820 | 0.4824 | 0.6561 |
N = 20.
SMA, supplementary motor area; STN, subthalamic nucleus, VA, ventral anterior nucleus of the thalamus.
However, the initial omnibus ANOVA also revealed an interaction (albeit somewhat weaker than the main effects) between cortical and subcortical connectivity strengths [F(6,14) = 3.47, P = 0.026], suggesting that additional follow‐up analyses (i.e., paired t‐tests) directly comparing individual connectivity pairs were also indicated. These direct comparisons showed that the single strongest connectivity pair was between putamen and SMA, showing a significantly stronger connectivity coefficient than all other examined pairs (t values ranging from 3.91 to 7.46, P values equal or less than 0.001, df = 19). The second strongest connection was that between putamen and S1, yielding significantly stronger connectivity coefficients than all connectivity pairs between S1/M1 and VA/STN/caudate (t values ranging from 3.67 to 5.16, P values equal or less than 0.002, df = 19), as well as significantly stronger connectivity than that between SMA and STN (t = 2.82, df = 19, P = 0.011). Figure 6 shows a summary of all comparisons.
Figure 6.
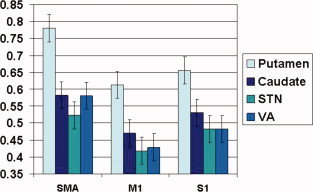
Comparison of Functional connectivity of SMA versus M1 and S1 with subcortical structures of the cortico‐basal ganglia circuitry. Error bars represent +1 standard error of the mean. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Functional Connectivity of Subcortical to Subcortical (Intrinsic) Pathways
To evaluate the coactivation of subcortical structures with other subcortical structures to which they have known anatomical connections, mean connectivity coefficients of the subcortical structures with each other were determined (Table IV). As was done with the cortical–subcortical connectivity, we first conducted an omnibus repeated measures ANOVA to determine the overall pattern of connectivity strengths. However, because not all subcortical structures connect to all other subcortical structures, a single ANOVA could not accomplish this. To that end, we conducted two omnibus tests. In the first ANOVA, we used connectivity of caudate vs. putamen vs. STN as one within subjects factor, and connectivity of GPe vs. GPi as a second within subjects factor (the values included in this analysis are indicated in bold in Table IV). The results yielded a large main effect of external versus internal segment of the pallidum [F(1,19) = 135.48, P < 0.001] demonstrating that GPe connectivity with caudate, putamen and STN was significantly stronger than GPi connectivity with the same structures (Fig. 7). Additionally, the results yielded an interaction between the two factors [F(2,38) = 71.72, P < 0.001]. To explicate this interaction, we conducted follow‐up paired t‐tests, directly comparing connectivity pairs. These analyses demonstrated that putamen—GPe connectivity was significantly stronger than connectivity of either the caudate [t(19) = 6.67, P <.001] or STN [t(19) = 8.28, P <.001] with GPe. In contrast, these results indicated that connectivity strengths of putamen, caudate and STN with GPi were not significantly different from each other (See Fig. 7).
Table IV.
Functional connectivity coefficients of pairs of subcortical structures of the cortico‐basal ganglia circuitry
| GPe | GPi | VA | |
|---|---|---|---|
| Caudate | 0.8568 | 0.4813 | 1.1931 |
| PUT | 1.1326 | 0.4838 | 0.8266 |
| STN | 0.8049 | 0.5033 | — |
| GPi | — | — | 0.5067 |
STN, subthalamic nucleus; GPe, globus pallidus, external segment; GPi, globus pallidus, internal segment, VA, ventral anterior nucleus of the thalamus; PUT, putamen. Values in bold and italic fonts indicate connectivities used in analyses of subcortical to subcortical functional connectivity. Values in bold were included in ANOVA using connectivity of caudate vs. putamen vs. STN as one within subjects factor, and connectivity of GPe vs. GPi as a second within subjects factor. Values in italics were included in ANOVA using GPe vs. GPi vs. VA as one within–subjects factor, and caudate vs. putamen as a second within–subjects factor.
Figure 7.
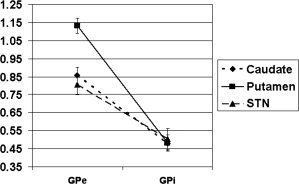
Differential connectivity strength of the putamen with GPe versus GPi, relative to connectivity strengths of other subcortical structures. Error bars represent +1 standard error of the mean.
Next, we conducted a second omnibus test, comparing the strength of the connectivities of the VA with caudate and putamen to the connectivities of the GPe and the GPi with those structures (the values included in this analysis are indicated in italics in Table IV). Specifically, we conducted a repeated measures analysis of variance, using GPe vs. GPi vs. VA as one within‐subjects factor, and caudate vs. putamen as a second within‐subjects factor. The results showed a main effect of the first factor [F(2,18) = 85.76, P <.001], reflecting an overall difference in the connectivity strengths among GPe, GPi, and VA. Follow‐up ANOVAs aimed at directly comparing these three regions' connectivities revealed that both the GPe [F(1,19) = 163.91, P <.001] and the VA [F(1,19) = 125.61, P <.001] have significantly stronger overall connectivities with other subcortical structures than did GPi with other subcortical structures. In contrast, direct comparison of the overall connectivity of the GPe with that of VA to other subcortical regions indicated no significant difference [F(1,19) = 0.156, P = 0.697]. Lastly, because both omnibus tests also showed a significant interaction between their respective within‐subjects factors [F(2,18) = 60.84, P < 0.001 and F(2,18) = 49.08, P < 0.001], we conducted follow‐up paired t‐tests to explicate the results. These direct comparisons of individual connectivity pairs showed that both the caudate‐VA and the putamen‐GPe connectivities were stronger than all the other connectivity pairs (paired t values ranging from 6.03 to 14.15, all P values < 0.001), except than each other (P = 0.213). In contrast, the connectivity strength was comparable across all connectivity pairs between GPi and other subcortical structures. The overall profile of connectivity strengths across the seven regions is shown in Figure 8.
Figure 8.
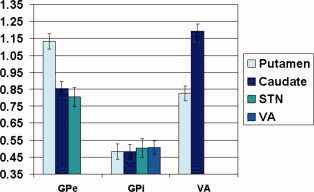
Comparison of functional connectivity of subcortical structures of the cortico‐basal ganglia circuitry. Error bars represent +1 standard error of the mean. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Analyses of Signal‐to‐Noise Ratios
As described above, it is possible that dissimilarities of SNRs among the various ROIs might confound the connectivity results. Thus, the connectivity of all 12 cortical–subcortical pathways were correlated with the square root of the sum of the squares of the SNR for the two ROIs involved, to ascertain the influence of SNR on connectivity strength. The resulting significance values were submitted to a False Discovery Rate multiple comparisons procedure with a threshold of 0.05. None of the correlations were significant. Thus, we found no evidence that region‐specific differences in SNR impacted our results.
Correlations of Strength of Functional Connectivity With Behavioral Data
Significant negative correlations were found between performance on the neurocognitive testing completed outside the magnet and strength of functional connectivity (Table V). Higher values on the PTT and AS task reflect slower and less accurate performance, thus the negative correlations mean that better performances were associated with stronger connectivities. These analyses demonstrated that most cortical inputs to the circuitry (SMA connectivity with caudate, putamen, and STN) were correlated with both better performance on PTT motor speed and planning as well as the AS task. In regard to intrinsic connections, GPe connectivity with all input nuclei was correlated with better performance on PTT motor planning, but motor speed and the AS task only correlated with the STN‐GPe pair. The caudate‐VA intrinsic connection was correlated with better performance on the motor planning only. Finally, circuit output to the cortex (VA to SMA pair) was also correlated with motor planning.
Table V.
Correlations of strength of functional connectivity with performance on neurocognitive testing
| Pathways | Specific connectivity pairs | Performance on neurocognitive testing | ||
|---|---|---|---|---|
| PTT motor speed | PTT motor planning | AS task | ||
| Input | SMA‐caudate | −0.440* | −0.657** | −0.592** |
| (cortex and input nuclei) | SMA‐STN | −0.391* | −0.437* | −0.616** |
| SMA‐putamen | −0.067 | −0.447* | −0.683** | |
| Intrinsic | Putamen‐GPe | −0.270 | −0.641** | −0.250 |
| (GPe and input nuclei) | Caudate‐GPe | −0.141 | −0.495* | −0.285 |
| STN‐GPe | −0.519** | −0.603** | −0.615** | |
| Intrinsic | ||||
| (caudate and VA) | ||||
| Caudate‐VA | −0.001 | −0.666** | −0.330 | |
| Output | ||||
| (thalamus and cortex) | VA‐SMA | −0.308 | −0.533** | −0.372 |
N = 21 *p<0.05, **p<0.01, one tailed.
AS, alphanumeric sequencing task; PTT, push‐turn‐taptap task; SMA, supplementary motor area; STN, subthalamic nucleus; GPe, globus pallidus, external segment; GPe, globus pallidus, internal segment, VA, ventral anterior nucleus of the thalamus.
Higher values on the PTT and AS task reflect poorer (i.e., slower, less accurate) performance.
To determine whether the four observed significant correlations between specific connectivity pairs and executive functioning (as assessed by the AS task) could be explained by the necessary motor output associated with this task, we re‐examined these correlations after controlling for three aspects of motor performance assessed by the PTT task. The results showed the following partial correlation values between the AS and the connectivity pairs: −0.454 for SMA‐caudate, −0.592 for SMA‐STN, −0.751 for SMA‐putamen, and −0.563 for STN‐GPe. These findings demonstrate that motor output alone cannot explain the relationship between AS task performance and components of both circuit input (SMA—caudate and STN pairs) and intrinsic (STN‐GPe) connectivity.
DISCUSSION
The primary aim of this study was to enhance our understanding of the functional architecture of the cortico‐basal ganglia circuitry during motor behavior. Key findings are discussed in the following subsections.
Input Pathways
Our results provide the first evidence of the relative engagement of circuit input pathways during motor task execution. It was previously unknown whether subpathways between cortical regions (M1, S1, and SMA) and the striatum (caudate and putamen) were equally engaged during motor behaviors. Direct comparisons revealed significant differences in connectivity strength (Fig. 6) with the strongest connectivity pair being SMA and putamen. Furthermore, SMA connectivity with caudate and STN was stronger than that of M1 and S1 (with the exception of putamen connectivities). These results suggests that SMA—striatum interactions, especially SMA—putamen, are particularly relevant for motor behavior.
Correlations between connectivity pairs and behavioral data (Table V) provide further support for the critical role of SMA—input nuclei interactions in motor output, and indicate these subpathways contribute to both motor and cognitive domains of motor execution. All SMA subpathways were correlated with better performance on the PTT (motor efficacy and planning) and AS (cognition) tasks, with the exception of the SMA‐putamen pair, which was not correlated with motor speed.
These findings are consistent with evidence of SMA—putamen anatomical [Lehericy et al., 2004] and functional [Postuma and Dagher 2006] connectivity in humans. Further, SMA—basal ganglia interactions have been shown to play a role in motor learning [Ma et al., 2010]. The SMA is also known to be involved with initiating and preparing motor responses; however, its contributions continue during motor execution [Brendel et al., 2010] and are thought to occur in parallel with M1 [Chen et al., 1991]. Our results suggest that at least some of these parallel operations involve providing input to the cortico‐basal ganglia circuitry.
Enhancing our understanding of SMA—input nuclei interactions has clinical relevance. For example, abnormalities of SMA—putamen connectivity have been found during motor behavior in Parkinson's disease [Wu et al., 2010a, b] and these likely contribute to impaired task performance. Our findings in healthy subjects provide additional support for the critical role of SMA—striatal interactions during task execution. Further, these results indicate that disruptions of SMA—caudate/STN communication may specifically impact both motor planning and motor speed as well as cognitive functions, while aberrant SMA—putamen connectivity may impact cognitive and motor planning domains only.
Intrinsic Pathways
Direct comparisons revealed that both the caudate‐VA and the putamen‐GPe connectivities were significantly stronger than all the other subcortical connectivity pairs (Fig. 8). With respect to the caudate—VA feedback pathway, VA to caudate projections have been demonstrated in animal studies [Tanaka et al., 1986], and are known to provide a major source of excitatory input to the striatum, inducing burst firing in striatal output neurons [McFarland and Haber, 2000]. However, the function of this pathway in motor execution has not been well characterized. The strong connectivity of the caudate—VA pathway evidenced during motor task execution supports the notion that thalamic feedback to the striatum may play an important role in motor behavior. Importantly, correlational analysis (Table V) confirmed this role and suggested a specific motor planning function.
Our analyses also provided information about the intrinsic pathways between the GPe and input nuclei. As described above, putamen‐GPe and caudate‐VA connectivities were both significantly stronger than those for all the other subcortical pairs (Fig. 8). Further, GPe connectivity with caudate, putamen, and STN was significantly stronger than GPi connectivity with the same structures (Fig. 7). The GPe is an intrinsic nucleus of the cortico‐basal ganglia circuitry. However, it is unclear whether it serves primarily as a relay or if it may also have other functions. Since it is reciprocally connected to input nuclei (striatum and STN) and projects to the GPi/SNr, it has been suggested that the GPe could control some activity of other basal ganglia structures [Bolam et al., 2000]. A recent study found that deep brain stimulation of the GPe in Huntington's disease impacted activity and connectivity within the cortico‐basal ganglia circuitry as well as cortical networks [Ligot et al., 2011], which suggests the possibility of fairly wide ranging control functions. However, it is conceivable those results were related to aberrant connectivity secondary to disease. Our finding of strong connectivity with all other subcortical structures in healthy subjects indicates that the GPe plays an important role in at least motor task execution. However, we also found that GPe connectivity was significantly stronger than GPi and comparable to VA connectivity with other subcortical structures (Fig. 8). GPe and GPi have been thought to have relay functions; therefore one interpretation of these findings is that GPe may have a fundamentally different role than GPi and might be involved in more complex processing.
Correlations with neurocognitive testing performance (Table V) also support the possibility of higher order processing by the GPe. Connectivity of this structure with all input nuclei (caudate, putamen and STN) was correlated with motor planning. Further, STN‐GPe connectivity was also correlated with motor efficiency (PTT motor speed) and cognition (AS task). These findings indicate that further research into possible higher order processing by the GPe is warranted.
Connectivity between the GPe and STN reported herein warrants comment. The STN receives short‐latency input from SMA and other cortical regions [Fujimoto and Kita, 1993; Monakow et al., 1978] and sends excitatory output to both the GPi/SNr and GPe [Smith et al., 1990]. This is the so‐called cortico‐subthalamo‐pallidal “hyperdirect” pathway that is proposed to have an inhibitory function [Aron et al., 2007; Aron and Poldrack, 2006; Ballanger et al., 2009; Nambu et al., 2002]. However, there is evidence of more complex processing by the STN. For example, there is evidence that the human STN is involved in feedback‐based learning [Brown et al., 2006] and when a new motor program is solicited independently of the choice of strategy [Monchi et al., 2006]. Further, a study of response inhibition found greater STN activation during stop trials as compared with go trials but also found greater activity during stop errors, compared to stop successes [Li et al., 2008]. The authors of that study reviewed a number of studies in addition to their own and concluded that STN activity might be more related to attentional or visuomotor processing of the stop signal than to the processes that determine the outcome of a stop trial.
Our finding that greater speed of task completion (PTT motor speed) is associated with the strength of connectivities within intrinsic (GPe‐STN) and input (SMA‐STN) pathways supports the argument that the STN hyperdirect pathway is not directly inhibitory and that other regions may be more relevant for the outcome of the stop signal [Duann et al., 2009; Li et al., 2008]. Additionally, the correlation we found between STN‐GPe connectivity strength and both motor planning (PTT) and cognition (AS) further supports the hypothesis of higher order processing along the STN hyperdirect pathway.
In addition to the GPe‐STN pathway discussed above, projections from the striatum (caudate and putamen) to the GPe and from GPe back to striatum are also inhibitory [Bolam et al., 2000] and would be expected to result in negative connectivity. In contrast, we found that putamen‐GPe connectivity was among the strongest and caudate—GPe connectivity was relatively strong (Fig. 8). Similarly, inhibitory striatum to GPi fibers would predict negative connectivity while excitatory STN to GPi inputs would predict strong connectivity. Instead, we found no significant differences in the strength of connectivity of these regions with GPi (Fig. 8). These results raise the intriguing question of why would structures that are connected by inhibitory neurons seem to exhibit strong coactivation.
Connectivity results presented herein provide a “big picture” view of cortico‐basal ganglia circuit function across the duration of task execution. Although it could be argued that these results are an artifact of the low temporal resolution of fMRI, the results on the whole suggest otherwise. In particular, growing evidence suggests that nuclei connected by inhibitory fibers do in fact exhibit strong functional connectivity. For example, we have previously found that basal ganglia intrinsic structures receiving primarily inhibitory input activate during motor task execution [Marchand et al., 2007b]. Further, we have reported [Marchand et al., 2007d] failure to find evidence of deactivation among basal ganglia intrinsic structures that receive inhibitory input during motor behavior. Finally, we have provided evidence of strong functional connectivity across the striatum—GPe inhibitory pathway in response to a motor activation paradigm in a separate study [Marchand et al., 2008]. The results reported herein both replicate and extend our previous findings.
In this study, we provide the first evidence that functional connectivity across intrinsic inhibitory pathways is not an artifact of fMRI by demonstrating that connectivity is positively correlated with motor task performance (Table V). Negative correlations would be expected if the interactions were predominately of an inhibitory nature. That said, there is no doubt that the fibers connecting these structures are inhibitory. Thus, we must consider whether it is possible to explain this unexpected result.
One possibility is that positive information transfer, rather than inhibition, occurs by way of cortico‐basal ganglia inhibitory pathways, at least in some instances. There is evidence for this sort of information transfer in an avian model of basal ganglia function [Leblois et al., 2009] where fast signaling through disinhibition, rather than excitatory drive, may result in an increased firing probability in postsynaptic neurons. In other words, firing of inhibitory presynaptic neurons results in firing of postsynaptic neurons through the mechanism of postinhibitory rebound [Leblois et al., 2009]. Postinhibitory rebound is not directly testable using fMRI and it is unknown if this mechanism exits in the human basal ganglia. Nonetheless, our findings could be consistent with this type of information transfer. Certainly, other currently unknown mechanisms might be involved as well.
Output Pathways
Strength of functional connectivity along the VA‐SMA output pathway was correlated with our measure of motor planning, but not with either motor efficiency or cognition (Table V). One interpretation of this finding is that during motor behavior, circuit feedback to the cortex is more relevant for planning subsequent behaviors than execution of the current movement. Also, the PTT motor planning task may serve as an indirect measure of cognitive/motor integration. Thus, our finding may add some support to the argument that one function of these circuits is to integrate motor and cognitive information, for review see [Marchand, 2010]. Further our results are in agreement with the general concept of motor and cognitive integration [Koechlin et al., 2003; Suchy and Kraybill, 2007]. There is increasing evidence that motor networks in general may directly support executive processing [Hikosaka and Isoda, 2010; Kim et al.; Koechlin et al., 2003]. Further, given that motor and executive performances often correlate [Kraybill and Suchy, 2008; Suchy et al., 1997, 2010], it has been suggested that executive functions may represent an evolutionary extension of the motor system [Suchy and Kraybill, 2007]. Further studies should examine motor and cognitive processing within the cortico‐basal ganglia circuitry with a focus on the interface between the two domains.
Implications for Future Research
We have previously found motor paradigms to serve as effective probes of cortico‐basal ganglia circuitry in both bipolar [Marchand et al., 2007a, c] and panic [Marchand et al., 2009] disorder. We hope to continue to use motor tasks in fMRI studies of psychiatric disorders because of robust activation [Marchand et al., 2008], excellent group reliability [Lee et al., 2010] and sensitivity to age‐related changes in the functional architecture of the cortico‐basal ganglia circuitry [Marchand et al., 2011]. However, it was previously unclear whether activation and connectivity associated with this task reflected only motor function. This study indicates that the complex motor task demonstrates connectivities associated with both motor and cognitive domains and possibly the integration of these at the level of the cortico‐basal ganglia circuitry.
We have previously demonstrated [Marchand et al., 2011] that the correlation of functional connectivity strength with neurocognitive task performance is useful in neuroimaging studies of the basal ganglia. Herein, we provide replication of the method using the same instruments and activation paradigm. This method has the potential to greatly enhance our understanding of cortico‐basal ganglia circuit function by providing evidence of how specific circuit components contribute to behavioral output in basal ganglia disorders. Perhaps more importantly, the finding that different behavioral variables correlated with different connectivity pairs raises the possibility that performance profile on the PTT and AS may serve as measures of circuit integrity that could be developed to the point of being clinically useful in detecting subtle clues to the onset of basal ganglia pathology, before overt neurological or psychiatric symptoms are present. Further, this performance profile used in conjunction with functional connectivity might provide a more robust measure of integrity than either method alone. Other methods, such as diffusion tensor imaging [Ystad et al., 2011] and independent component analysis combined with resting state fMRI [Ystad et al., 2010] have been shown to provide important information about basal ganglia function. Adding these approaches along with voxel based morphometry and the evaluation of cortico‐cerebellar connectivity may benefit future studies of PTT and AS performance profile.
Limitations
There are several limitations of this work that must be acknowledged. The first is that we only studied female subjects who were strongly right handed. It is unknown whether our results will generalize to males, since to our knowledge, comparisons of male and female responses have not been published for either the PTT and AS or the activation paradigm we used. However, gender‐specific activation patterns have been reported in response to fMRI using a cognitive paradigm [Bell et al., 2006]. Also, activation associated with stop signal inhibition has been shown to have gender‐specific activation in the basal ganglia [Li et al., 2006]. Thus, further studies comparing genders are warranted. Also, our activation paradigm was only completed with the nondominant hand and we did not analyze connectivity data from the opposite hemisphere. Because of length considerations, we felt analyses of the opposite hemisphere data were most appropriate for a follow‐up paper. Additional studies will need to assess results using the dominant hand. Another limitation is that our sample size was relatively small given the number of statistical comparisons made. However, examination of p values reveals that the majority of reported results were highly significant (P < 0.001), suggesting that the probability of spurious findings is quite low. Finally, anatomical precision is always a concern in studies of the basal ganglia. As previously mentioned, a comparison of hand‐drawn anatomical ROIs to the use of atlases found no substantial difference in the result [Prodoehl et al., 2008] and this approach has been used by others [Boecker et al., 2008]. Nonetheless, technical limitations inherent in studies such as this must be considered when interpreting results.
CONCLUSIONS
Main findings of this study include evidence of the relative importance of interactions between the SMA and striatum as well as between the VA nucleus of the thalamus and the striatum during motor task execution. Further, we demonstrate that both the GPe and the STN hyperdirect pathway may play a role in higher order processing. Potentially controversial findings indicate that functional connectivity may exist across inhibitory pathways and that this coactivation may be important for motor behavior. Finally, our results support the use of functional connectivity and neurocognitive testing methods as probes of these circuits in both healthy subjects and those with neuropsychiatric disorders.
REFERENCES
- Albin RL, Young AB, Penney JB ( 1989): The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR ( 1990): Basal ganglia‐thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85: 119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL ( 1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381. [DOI] [PubMed] [Google Scholar]
- Arbuthnott K, Frank J ( 2000): Trail making test, part B as a measure of executive control: Validation using a set‐switching paradigm. J Clin Exp Neuropsychol 22: 518–528. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA ( 2007): Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27: 3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA ( 2006): Cortical and subcortical contributions to Stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci 26: 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanger B, van Eimeren T, Moro E, Lozano AM, Hamani C, Boulinguez P, Pellecchia G, Houle S, Poon YY, Lang AE, Strafella AP ( 2009): Stimulation of the subthalamic nucleus and impulsivity: Release your horses. Ann Neurol 66: 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich M ( 2004): Cognitive Neuroscience and Neuropsychology. Boston, MA: Houghton Mifflin; 636 p. [Google Scholar]
- Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH ( 2006): Males and females differ in brain activation during cognitive tasks. Neuroimage 30: 529–538. [DOI] [PubMed] [Google Scholar]
- Boecker H, Jankowski J, Ditter P, Scheef L ( 2008): A role of the basal ganglia and midbrain nuclei for initiation of motor sequences. Neuroimage 39: 1356–1369. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD ( 2000): Synaptic organisation of the basal ganglia. J Anat 196( Part 4): 527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel B, Hertrich I, Erb M, Lindner A, Riecker A, Grodd W, Ackermann H ( 2010): The contribution of mesiofrontal cortex to the preparation and execution of repetitive syllable productions: An fMRI study. Neuroimage 50: 1219–1230. [DOI] [PubMed] [Google Scholar]
- Brown P, Chen CC, Wang S, Kuhn AA, Doyle L, Yarrow K, Nuttin B, Stein J, Aziz T ( 2006): Involvement of human basal ganglia in offline feedback control of voluntary movement. Curr Biol 16: 2129–2134. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Fossella J ( 2002): Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Dev Psychobiol 40: 237–254. [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz‐Herbette S, Menon V ( 2007): Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage 34: 1253–1269. [DOI] [PubMed] [Google Scholar]
- Chen DF, Hyland B, Maier V, Palmeri A, Wiesendanger M ( 1991): Comparison of neural activity in the supplementary motor area and in the primary motor cortex in monkeys. Somatosens Mot Res 8: 27–44. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H ( 2005): Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15: 161–167. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS ( 2008): Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci 28: 7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS ( 2009): Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci 29: 10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastvold A, Derbidge C, Cope C, Suchy Y ( 2004): Test‐retest reliability of an electronic alternative to the Trail Making Test. Arch Clin Neuropsychol 19: 901. [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak R, Mazziotta JC, Evans AC ( 1993): Assessing the significance of focal activation using their spatial extent. Hum Brain Mapp 1: 210–220. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kita H ( 1993): Response characteristics of subthalamic neurons to the stimulation of the sensorimotor cortex in the rat. Brain Res 609( 1‐2): 185–192. [DOI] [PubMed] [Google Scholar]
- Graybiel AM ( 2005): The basal ganglia: Learning new tricks and loving it. Curr Opin Neurobiol 15: 638–644. [DOI] [PubMed] [Google Scholar]
- Gurney K, Prescott TJ, Redgrave P ( 2001): A computational model of action selection in the basal ganglia. I. A new functional anatomy. Biol Cybern 84: 401–410. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Isoda M ( 2010): Switching from automatic to controlled behavior: Cortico‐basal ganglia mechanisms. Trends Cogn Sci 14: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele S ( 1968): Movement control in skilled motor performance. Psychol Bull 70: 387–403. [Google Scholar]
- Kim J‐H, Lee J‐M, Jo HJ, Kim SH, Lee JH, Kim ST, Seo SW, Cox RW, Na DL, Kim SI, Saad ZS ( 2010): Defining functional SMA and pre‐SMA subregions in human MFC using resting state fMRI: Functional connectivity‐based parcellation method. Neuroimage 49: 2375–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody Cl, Kounelher FDR ( 2003): The architecture of cognitive control in the human prefrontal cortex. Science 302: 1181–1185. [DOI] [PubMed] [Google Scholar]
- Kraybill ML, Suchy Y ( 2008): Evaluating the role of motor regulation in figural fluency: Partialing variance in the Ruff Figural Fluency Test. J Clin Exp Neuropsychol 1–10. [DOI] [PubMed] [Google Scholar]
- Leblois A, Bodor AL, Person AL, Perkel DJ ( 2009): Millisecond timescale disinhibition mediates fast information transmission through an avian basal ganglia loop. J Neurosci 29: 15420–15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JN, Hsu EW, Rashkin E, Thatcher JW, Kreitschitz S, Gale P, Healy L, Marchand WR ( 2010): Reliability of fMRI motor tasks in structures of the corticostriatal circuitry: Implications for future studies and circuit function. Neuroimage 49: 1282–1288. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini‐Issac M, Waechter T, Ugurbil K, Doyon J ( 2005): Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA 102: 12566–12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Krainik A, Francois C, Van de Moortele PF, Ugurbil K, Kim DS ( 2004): 3‐D diffusion tensor axonal tracking shows distinct SMA and pre‐SMA projections to the human striatum. Cereb Cortex 14: 1302–1309. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R ( 2006): Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage 32: 1918–1929. [DOI] [PubMed] [Google Scholar]
- Li CS, Yan P, Sinha R, Lee TW ( 2008): Subcortical processes of motor response inhibition during a stop signal task. Neuroimage 41: 1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligot N, Krystkowiak P, Simonin C, Goldman S, Peigneux P, Van Naemen J, Monclus M, Lacroix SF, Devos D, Dujardin K, Delmaire C, Bardinet E, Delval A, Delliaux M, Defebvre L, Yelnik J, Blond S, Destee A, De Tiege X ( 2011): External globus pallidus stimulation modulates brain connectivity in Huntington's disease. J Cereb Blood Flow Metab 31: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria AR, Majovski LV ( 1979): A frontal lobe syndrome due to a cerebrovascular accident affecting the corpus callosum. Clinical Neuropsychol 1: 17–19. [Google Scholar]
- Ma L, Wang B, Narayana S, Hazeltine E, Chen X, Robin DA, Fox PT, Xiong J ( 2010): Changes in regional activity are accompanied with changes in inter‐regional connectivity during 4 weeks motor learning. Brain Res 1318: 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand WR ( 2010): Cortico‐basal ganglia circuitry: A review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct Funct 215: 73–96. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Healy L, Thatcher JW, Rashkin E, Starr J, Hsu E ( 2009): An fMRI motor activation paradigm demonstrates abnormalities of putamen activation in females with panic disorder. J Affect Disord 116( 1‐2): 121–125. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Suchy Y, Garn C, Johnson S, Wood N, Chelune G ( 2011): Age‐related changes of the functional architecture of the cortico‐basal ganglia circuitry during motor task execution. Neuroimage 55: 194–203. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Thatcher GW, Jensen C, Stewart D, Dilda V, Thatcher J, Creem‐Regehr SH ( 2007a): A functional MRI study of a paced motor activation task to evaluate frontal‐subcortical circuit function in bipolar depression. Psychiatry Res 155: 221–230. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Thatcher J, Thatcher GW, Jensen C, Starr J ( 2007b): An fMRI study of frontal–subcortical skeletomotor circuit and dorsolateral prefrontal cortex function using a paced motor activation paradigm. Brain Imaging Behav 1: 58–67. [Google Scholar]
- Marchand WR, Lee JN, Thatcher J, Thatcher GW, Jensen C, Starr J ( 2007c): A preliminary longitudinal fMRI study of frontal‐subcortical circuits in bipolar disorder using a paced motor activation paradigm. J Affect Disord 103( 1‐3): 237–241. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Thatcher JW, Hsu EW, Rashkin E, Suchy Y, Chelune G, Starr J, Barbera SS ( 2008): Putamen coactivation during motor task execution. Neuroreport 19: 957–960. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Thatcher JW, Thatcher GW, Jensen C, Starr J ( 2007d): Motor deactivation in the human cortex and basal ganglia. Neuroimage 38: 538–548. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN ( 2000): Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci 20: 3798–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL ( 2000): Basal ganglia output and cognition: Evidence from anatomical, behavioral, and clinical studies. Brain Cogn 42: 183–200. [DOI] [PubMed] [Google Scholar]
- Mink JW ( 1996): The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira‐Souza R, Moll FT, Bramati IE, Andreiuolo PA ( 2002): The cerebral correlates of set‐shifting: An fMRI study of the trail making test. Arquivos de Neuro‐Psiquiatria. Brazil: Arquivos de Neuro‐Psiquiatria, Brazil; p 900–905. [DOI] [PubMed] [Google Scholar]
- Monakow KH, Akert K, Kunzle H ( 1978): Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Experimental brain research. Experimentelle Hirnforschung 33( 3‐4): 395–403. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J ( 2006): Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol 59: 257–264. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M ( 2002): Functional significance of the cortico‐subthalamo‐pallidal ‘hyperdirect’ pathway. Neurosci Res 43: 111–117. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A ( 2006): Basal ganglia functional connectivity based on a meta‐analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 16: 1508–1521. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE ( 2008): Region of interest template for the human basal ganglia: Comparing EPI and standardized space approaches. Neuroimage 39: 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM ( 1958): Trail Making Test: Manual for Administration, Scoring, and Interpretation. Indianapolis, IN: Indiana University Medical Center. [Google Scholar]
- Sakai ST, Inase M, Tanji J ( 2002): The relationship between MI and SMA afferents and cerebellar and pallidal efferents in the macaque monkey. Somatosens Mot Res 19: 139–148. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Chintalapati P ( 2006): Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res 175: 544–555. [DOI] [PubMed] [Google Scholar]
- Smith Y, Hazrati LN, Parent A ( 1990): Efferent projections of the subthalamic nucleus in the squirrel monkey as studied by the PHA‐L anterograde tracing method. J Compar Neurol 294: 306–323. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D ( 2001): The trail making test: A study in focal lesion patients. Psychol Assess 13: 230–239. [PubMed] [Google Scholar]
- Suchy Y, Blint A, Osmon DS ( 1997): Behavioral dyscontrol scale: Criterion and predictive validity in an inpatient rehabilitation unit population. Clin Neuropsychol 11: 258–265. [Google Scholar]
- Suchy Y, Derbidge C, Cope C ( 2005): Behavioral dyscontrol scale‐electronic version: First examination of reliability, validity, and incremental utility. Clin Neuropsychol 19: 4–26. [DOI] [PubMed] [Google Scholar]
- Suchy Y, Kraybill M ( 2007): The relationship between motor programming and executive abilities: Constructs measured by the Push‐Turn‐Taptap task from the Behavioral Dyscontrol Scale‐Electronic Version. J Clin Exp Neuropsychol 29: 648–659. [DOI] [PubMed] [Google Scholar]
- Suchy Y, Kraybill M, Larson JGL ( 2010): Understanding design fluency: Motor and executive contributions. J Int Neuropsychol Soc 16: 26–37. [DOI] [PubMed] [Google Scholar]
- Tanaka D,Jr. , Isaacson LG, Trosko BK ( 1986): Thalamostriatal projections from the ventral anterior nucleus in the dog. J Comp Neurol 247: 56–68. [DOI] [PubMed] [Google Scholar]
- The Psychological Corporation ( 2001): Wechsler Test of Adult Reading: Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D ( 1997): WAIS‐III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wiesendanger R, Wiesendanger M ( 1985): The thalamic connections with medial area 6 (supplementary motor cortex) in the monkey (macaca fascicularis). Exp Brain Res 59: 91–104. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P ( 2010a): Effective connectivity of brain networks during self‐initiated movement in Parkinson's disease. Neuroimage 55:204–215. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Li K, Chan P ( 2010b): Neural correlates of bimanual anti‐phase and in‐phase movements in Parkinson's disease. Brain 133( Part 8): 2394–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M, Eichele T, Lundervold AJ, Lundervold A ( 2010): Subcortical functional connectivity and verbal episodic memory in healthy elderly—A resting state fMRI study. Neuroimage 52: 379–388. [DOI] [PubMed] [Google Scholar]
- Ystad M, Hodneland E, Adolfsdottir S, Haasz J, Lundervold AJ, Eichele T, Lundervold A ( 2011): Cortico‐striatal connectivity and cognition in normal aging: A combined DTI and resting state fMRI study. Neuroimage 55: 24–31. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ ( 2005): An fMRI study of the trail making test. Neuropsychologia 43: 1878–1886. [DOI] [PubMed] [Google Scholar]


