Abstract
There is consistent evidence that brain volume changes in early and late life. Most longitudinal studies usually only span a few years and include a limited number of participants. In this review, we integrate findings from 56 longitudinal magnetic resonance imaging (MRI) studies on whole brain volume change in healthy individuals. The individual longitudinal MRI studies describe only the development in a limited age range. In total, 2,211 participants were included. Age at first measurement varied between 4 and 88 years of age. The studies included in this review were performed using a large range of methods (e.g., different scanner protocols and different acquisition parameters). We applied a weighted regression analysis to estimate the age dependency of the rate of relative annual brain volume change across studies. The results indicate that whole brain volume changes throughout the life span. A wave of growth occurs during childhood/adolescence, where around 9 years of age a 1% annual brain growth is found which levels off until at age 13 a gradual volume decrease sets in. During young adulthood, between ∼18 and 35 years of age, possibly another wave of growth occurs or at least a period of no brain tissue loss. After age 35 years, a steady volume loss is found of 0.2% per year, which accelerates gradually to an annual brain volume loss of 0.5% at age 60. The brains of people over 60 years of age show a steady volume loss of more than 0.5%. Understanding the mechanisms underlying these plastic brain changes may contribute to distinguishing progressive brain changes in psychiatric and neurological diseases from healthy aging processes. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc
Keywords: MRI, longitudinal, brain volume changes, healthy individuals and plasticity
INTRODUCTION
Comparison of brain weight between people at different ages has suggested that considerable volume change takes place during development in humans. It has been shown in an autopsy study that included more than 4,000 individuals across the full age range that the most pronounced increase in brain weight occurs during the first 3 years of life [Dekaban,1978]. Between age 3 and 18, the brain increases in weight to about 5 times that of a newborn. At ∼45–50 years of age, a progressive decline in brain weight begins and reaches the lowest values after the age of 86. By then, it is about 11% smaller relative to the maximum brain weight attained around 19 years of age. Another postmortem study suggests that it is after the age of 80 that the brain mass is rapidly decreasing [Ho et al.,1980]. Cross‐sectional studies suggest a linear decline in cerebral volume throughout adulthood [Raz et al.,2004] with an estimated volume loss of an average of 14% at age 90 [Jernigan et al.,2001].
Magnetic resonance imaging (MRI) provides us with the opportunity to study brain development within subjects over time. In longitudinal studies, participants can serve as their own control and therefore subtle changes can be identified on an individual level. Longitudinal studies have revealed that brain changes occur in childhood and in adulthood including in old age. However, to date, individual longitudinal MRI studies describe only the development in a limited age range as scanning subjects across their whole life span requires a very long interval. While having shown that brain changes occur during the whole life span, each of these studies provides a keyhole representation of these changes at a limited age span. Specifically, these studies do not allow for a direct comparison between childhood and adult changes and between young adult and old adult changes. It is during these transitions that important changes may take place. Therefore, we reviewed and integrated the findings of longitudinal MRI studies in healthy subjects throughout the life span on whole brain volume change.
METHODS
Data Sources
A systematic search was conducted to identify MRI studies that quantitatively examined longitudinal whole brain volume changes in healthy individuals with at least two MRI scans at different time points. These studies were obtained through the computerized database PUBMED for English‐language articles published until January 1, 2010. The keywords combinations used in the computerized search were “MRI and longitudinal and “whole brain,” “Brain volume change(s) and longitudinal and MRI and healthy subjects.” Articles were examined to investigate whether papers reached the inclusion criteria (see below). Additional studies were obtained by hand search of cross‐references in already identified papers.
From the studies that were found, those that also reported on volume change over time in gray matter (GM) and/or white matter (WM) volume were identified.
Study Selection
Studies were included if they (1) were longitudinal MRI studies with at least two MRI scans, (2) investigated healthy individuals, (3) provided quantitative measures of whole brain volume change, and (4) were published in the English language in scientific peer review journals.
Statistical Analysis and Data Extraction
Whole brain, GM, and WM volume change in percent change per year (Q) was extracted from the studies. The formula for this calculation was
with ΔV = V2−V1 representing the volume change between volume (V1) at baseline (t1) and volume (V2) at follow‐up (t2), and Δt = t2−t1 representing the time between t1 and t2. For each study, mean age of the sample was defined as the mean age halfway the interval. It was measured based on either mean age at t1 or t2 in years (dependent on what was available) and the interval of the longitudinal measurement in years as follows: [t1 + (follow‐up interval)/2] or [t2 − (follow‐up interval)/2].
For whole brain volume change, regression analysis in the form of a locally weighted running‐line smoother [Cleveland and Devlin,1988; Hastie and Tibshirani,1990] was used to obtain the dependence of Q on age. Software for these analyses was developed in house [van Haren et al.,2008]. First, the data were split into two age ranges based on the mean age of the sample, i.e., from 7 to 19 years and from 19 to 84 years. This was done because there are only a limited number of studies available covering the age range between 18 and 21 years, preventing us from making a reliable connection between development during childhood and adolescence and development during the adult age range. The degree of freedom of the fits was set to a conservative value of 3, the lowest number allowing some curvature in the fits, since there is no (statistical) support for higher values.
Smoothed Q was numerically integrated to obtain volume as a function of age: Volume(age) = Volume(age0) * exp(Integrate[Q(age')/100, {age', age0, age}]). The division by 100 is because Q is given in %; the “exp” is because integration of Q leads to the log of Volume, since Q is a relative measure. Age0 and Volume(age0) are taken from the study of 9‐year‐olds by Peper et al. [2009]; the endpoint of the integration of the younger age group was the starting point of the integration of the older age group (19 year).
For GM and WM volume change, percent change per year was calculated according to the same procedure as described previously. For those studies in the adult age range (>19 years), we smoothed the GM and WM data using the locally weighted running‐line smoother (3 dfs). The studies in childhood and adolescence were excluded from the smoothing procedure as there were too few studies that provided data on GM/WM changes.
RESULTS
Fifty‐six studies were identified as suitable for our review. Table I lists the included articles. DeLisi et al. [1992] published the first longitudinal study that included healthy controls. The number of participants per study ranged from 7 [Henley et al.,2006] to 228 [Lenroot et al.,2007]. Minimum interval between the MRI scans was 29 days [Pfefferbaum et al.,1995] while the maximum interval was 10 years [DeLisi et al.,2004]. Out of these 56, nine studies provided data on GM and WM matter volume change over time, and one study provided data on only GM volume change. Table II lists the articles with GM and WM volume change. Whenever it states “no results” in the tables for the column(s) “Relative rate of % brain/GM/WM volume change/year,” it means that there was no information available to extract percentage change per year. These studies were left out from the smoothing analysis.
Table I.
Overview of the longitudinal studies on whole brain volume change in healthy individuals
| Authors, year | Subjects | Age at baseline (yrs: mean(sd)[range]) | Age in between scans (yrs: mean) | Interval (yrs: mean (sd) [range]) | Brain region | Acquisition protocol | Method of analysis | Relative rate of % brain volume change/year (mean (sd) [range]) |
|---|---|---|---|---|---|---|---|---|
| DeLisi et al.,1992 | N = 33 | 28.0 (6.3) | 29.0 | 2 | Cerebrum corrected for baseline brain volume | 1.5T GE, spin echo pulse sequence, 5‐mm slice thickness with 2‐mm gap in coronal and sagittal plane | Manual | No information |
| DeLisi et al.,1995 | N = 5, completely overlapping with DeLisi et al.,1992 | 28.2 (6.0) | 30.2 | 4 | Cerebrum corrected for baseline brain volume | 1.5T GE, spin echo pulse sequence, 5‐mm slice thickness with 2‐mm gap in coronal, axial, and sagittal plane | Manual | No information |
| Pfefferbaum et al.,1995 | N = 58 | 45.3 (14.2) | 45.4 | 0.1 | Cerebrum corrected for baseline brain volume | 1.5T GE, spin echo sequence, 5‐mm slice thickness with 2.5‐mm gap in axial plane | Semiautomated | No information |
| Fox et al., 1996 | N = 11 | 51.3 (5.9) | 51.8 | 1.07 | Whole brain | 1.5T GE, SPGR, 1.5‐mm slice thickness | Semiautomated | Q = −0.05 |
| DeLisi et al.,1997 | N = 20 | 26.5 (5.0) | 28.7 | 4.3 (1.1) | Cerebrum, corrected for sex and baseline hemispheric volume | 1.5T GE, spin echo pulse sequence, 5‐mm slice thickness with 2‐mm gap | Manual | Q = −0.70 |
| Gur et al.,1998 | N = 17 | 31.9 (8.9) | 33.3 | 2.7 | Whole brain corrected for baseline volume | 1.5T GE, multiecho sequence, 5‐mm slice thickness in transaxial plane | Automated | No information |
| Mueller et al.,1998 | N = 46 A: N = 11 B: N = 15 C: N = 20 | A: 87.0(2.2) [85.1–93.1] B: 81.1 (2.8) [75.7–84.8] C: 70.4 (2.4) [66.6–73.7] | A: 72.4 B: 82.8 C: 88.5 | A: 3.10 (1.21) [3–6] B: 3.47 (1.25) [3–6] C: 4.09 (0.83) [3–5] | Cerebrum corrected for IC | 1.5T, multiecho, multiplanar image acquisition, contiguous slices with 4‐mm slice thickness in coronal plane. 14 scans acquired in the first year had a 2.5‐mm gap | Semiautomated | A: Q = −0.44 B: Q = −0.39 C: Q= −0.32 |
| Fox et al.,1999a | N = 15, completely overlapping with Fox et al.,2000 | 55.3 (14.0) | 56.2 | 1.7 (1.2) | Whole brain | 1.5T GE, SPGR, 1.5‐mm slice thickness in coronal plane | Semiautomated (BBSI) | Q = −0.40 (0.70) |
| Fox et al.,1999b | N = 26 | No information | Approx. 1 | Cerebrum | 1.5T, T1‐weighted 1.5‐mm slice thickness | Semiautomated (BBSI) | Median Q = −0.20 | |
| Giedd et al.,1999a | N = 36, complete overlap with Lenroot et al.,2007 | 13.6 (2.6) | 14.6 | Approx. 2 | Cerebrum, corrected for age | 1.5T GE, SPGR, 1.5‐mm slice thickness in axial plane | Semiautomated | Trajectory |
| Giedd et al.,1999b | N = 65, complete overlap with Lenroot et al.,2007 | 4.2–21.6, N = 145 | Approx. 2 | Cerebrum corrected for sex | 1.5T GE, SPGR, 1.5‐mm slice thickness in axial plane | Semiautomated | Trajectory | |
| Fox et al.,2000 | N = 18 | 65.0 (10.5) [52–84] | 65.5 | 0.89 | Cerebrum corrected for baseline volume | 1.5T GE, SPGR, 1.5‐mm slice thickness in coronal plane | Semiautomated (BBSI) | Q = −0.41 (0.47) |
| Chan et al.,2001 | N = 27 | 59.6 (11.7) | 60.2 | 1.06 | Whole brain corrected for sex | 1.5T GE, SPGR, 1.5‐mm slice thickness | Semiautomated (BBSI) | Q = −0.47 (0.40) |
| Cohen et al.,2001, a | N = 25, female only | Weighted mean: 59.8 | 60.8 | 2 | Cerebrum corrected for IC | 1.5T GE, 3D gradient echo sequence with RF spoiling, 1.5‐mm slice thickness | Semiautomated | Weighted mean: Q = −0.19 |
| Hu et al.,2001 | N = 10 | 71.6 (9.5) | 72.4 | 1.59 | Whole brain | 1.0T Picker, 3D, slice thickness 1.6‐mm in sagittal plane | Semiautomated | Q = −0.06 [−0.61–0.34] |
| Liu et al.,2001 | N = 20, complete overlap with Liu et al.,2003 | Median: 35 (18–81) | 36.8 | Median: 3.5 | Whole brain corrected for baseline volume | 1.5T GE, IR‐SPGR, 1.5‐mm slice thickness | Automated | Q = −0.11 |
| Tang et al.,2001 | N = 66 | 78.4 (2.9) [74–87] | 80.6 | 4.4 (3–5) | Cerebrum corrected for baseline volume | 1.5T GE, T2‐weighted images with 5‐mm slice thickness and 1.5‐mm gap in axial plane | Manual with Cavalieri principle | Q = −2.10 (1.6) |
| Wood et al.,2001 | N = 26 | 23.8 (7.9) | 24.9 | 2.2 [0.86–4.18] | Whole brain corrected for IC | 1.5T GE, SPGR, 1.5‐mm slice thickness | Semiautomated | No information |
| Cahn et al.,2002 | N = 36, N = 35 overlap with van Haren et al.,2008 | 24.5 (5.8) [17–40] | 25.0 | 1.04 | Whole brain corrected for IC, age, and sex | 1.5T Philips, FFE, 1.2‐mm slice thickness in coronal plane | Automated | Q = +0.95 |
| James et al.,2002 | N = 16 | 16.0 (2.0) | 16.9 | 1.7 (0.5) | Whole brain | 1.5T GE, spin echo, 5‐mm slice thickness in sagittal plane and volumetric T1‐weighted SPGR with 3‐mm slice thickness in coronal plane | Semiautomated | No information |
| Cardenas et al.,2003 | N = 16 | 76.0 (5.0) | 77.3 | 2.6 (1.0) | Whole brain | 1.5 T Siemens, MP‐RAGE, 3‐mm slice thickness | Semiautomated (BBSI) | Q = −0.20 (0.10) |
| Ho et al.,2003 | N = 23 | 26.9 (5.3) [16–35] | 28.6 | 3.39 (1.60) [0.92–6.67] | Whole brain corrected for sex, height, age, and interval duration | 1.5T GE, spoiled GRASS, 1.5‐mm slice thickness in coronal plane | Manual | Q = +0.12 |
| Liu et al.,2003 | N = 90 A: N(<35y) = 44 B: N(35–54 y) = 37 C: N(>54y) = 9 | A: 24.5 (6.6) [14–34] B: 44.5 (5.7) [35–53] C: 67.9 (6.4) [57–77] | A: 26.3 B: 46.3 C: 69.7 | A: 3.57 B: 3.53 C: 3.52 | Whole brain corrected for baseline MRI‐volume and sex | 1.5T GE, IR‐SPGR, 1.5‐mm slice thickness in coronal plane | Automated | A: Q = −0.06 B: Q = −0.18 C: Q = −0.38 |
| Resnick et al.,2003 | N = 92 | 70.4 (7.0) [59–85] | 72.4 | 2 | Cerebrum corrected for baseline volume, age, and sex | 1.5T GE, SPGR, 1.5‐mm slice thickness | Semiautomated | Q = −0.5 |
| Rusinek et al.,2003 | N = 32 | 68.2 (5.1) | 69.2 | 2.2 | Whole brain corrected for baseline volume, age, sex, and education | 1.5T GE, SPGR,1.3‐mm slice thickness in coronal plane | Automated | Q = −0.58 (0.42) |
| Scahill et al.,2003 | N = 39 A: N = 8 B: N = 10 C: N = 10 D: N = 6 E: N = 5 | Weighted mean: 52.4y [31–84] A: 36.1 (2.5) B: 45.6 (2.9) C: 53.9 (3.5) D: 62.7 (2.3) E: 76.8 (5.5) | 53.3 A: 36.9 B: 46.5 C: 54.9 D: 63.7 E: 77.3 | Weighted mean: 1.72 A: 1.57 B: 1.83 C: 1.91 D: 2.07 E: 0.98 | Whole brain corrected for IC, sex, and age | 1.5T GE, 1.5‐mm slices thickness in coronal plane | Semiautomated (BBSI) | Total: Q = −0.32 [−0.10–−0.54] A: Q = −0.29 B: Q = −0.35 C: Q = −0.26 D: Q = −0.46 E: Q = −0.55 |
| Sporn et al.,2003 | N = 43, complete overlap with Lenroot et al.,2007 | 14.8 (2.2) | 16.6 | 3.6 (1.6) | Cerebrum corrected for baseline brain volume | 1.5T GE, SPGR, 1.5‐mm slice thickness in axial plane | Automated | Q = −0.26 |
| Thompson et al.,2003 | N = 14 | 71.4 (0.9) | 72.7 | 2.6 (0.3) | Cerebrum, corrected for age and sex | 2T Bruker Medspec, 3D MP‐RAGE in oblique plane | Automated | Q = −0.88 (0.15) |
| DeLisi et al.,2004 | N = 10, complete overlap with DeLisi et al.,1997 | At follow‐up: 35.5 (5.38) [24–43] | 30.5 | 10 | Cerebrum, corrected for sex and baseline volume | 1.5T GE, spin echo pulse sequence, 5‐mm slice thickness with 2‐mm gap | Manual | Q = −0.41 |
| Ezekiel et al.,2004 | N = 22 | 76.7 (8.1) | 77.7 | 2.0 (0.7) | Whole brain | 1.5T Siemens, MP‐RAGE, 1.4‐mm slice thickness | Semiautomated (BBSI) | Q = −0.49 (0.39) |
| Gogtay et al.,2004 | N = 38, complete overlap with Lenroot et al.,2007 | 13.3 (3.1) | 14.6 | 2.6 (0.8) | Cerebrum | 1.5T GE, SPGR, 1.5‐mm slice thickness in axial plane | Automated | Q = −0.17 |
| Jack Jr et al.,2004 | N = 40 | 79 (56–93) | 81.2 | 4.3 (2.5–5.2) | Whole brain corrected for baseline MRI‐volume | 1.5T GE, SPGR, 1.6‐mm slice thickness | Automated | Median, Q = −0.4 [−0.5–0.2] |
| Blumberg et al.,2005 | N = 8 | 15.3 (2.8) [11–19] | 16.3 | 2.0 (0.6) | Whole brain | 1.5T GE, SPGR, 1.2‐mm slice thickness in sagittal plane | Automated | No information |
| Fotenos et al.,2005 | N = 38 | At baseline N = 94 78.0 (8) [65–95] | 78.9 | 1.8 (0.5) [1.1–3.9] | Whole brain corrected for IC, age, and sex | 1.5T Siemens, MR‐RAGE, 1.25‐mm slice thickness | Automated | Q = −0.45 (0.53) |
| Goldstein et al.,2005 | N = 121 A: N = 62 B: N = 59 | 66.2 (6.0) A: [55–66] B: [67–79] | 68.7 | 4.9 (0.63) | Whole brain corrected for IC | 1.5T Picker, transverse asymmetrical dual spin‐echo Carr‐Purcell‐Meiboom‐Gill sequence, interleaved 3‐mm slice thickness in axial plane | Manual | Weighted mean: Q = −0.62 A:Q = −0.50 B:Q = −0.73 |
| Lieberman et al.,2005 | N = 44 | 25.5 (4.1) from the baseline sample with 62 participants | 26.0 | 1.00 | Whole brain corrected for IC | 1.5T GE and Philips, IR‐SPGR, 1.5‐mm slice thickness in axial plane | Automated | Q = +0.60 |
| Schott et al.,2005 | N = 19 | 69.3 (7) | 70.3 | 1.00 | Whole brain | 1.5T GE, spoiled fast GRASS, 1.5‐mm slice thickness | Semiautomated (BBSI) and manual | BBSI: Q = −0.72 Manual: Q = −0.97 |
| Whitworth et al.,2005 | N = 20 | At follow‐up: 31.5 (4.9) y | 29.7 | 3.70 (1.63) | Whole brain corrected for age and height | 1.5T Siemens, MP‐RAGE, 0.9–1.4‐mm slice thickness in sagittal plane | Semiautomated | Q = +0.13 |
| Henley et al.,2006 | N = 7 | 40.7 (10.5) | 40.9 | 0.48 | Whole brain corrected for IC, age, and IQ | 1.5T GE, IR prepared fast spoiled GRASS sequence, 1.5‐mm slice thickness in coronal plane | Semiautomated (BBSI) | Q = −0.26 (0.54) |
| Paviour et al.,2006 | N = 18 | 66.8 (5.4) [56–74] | 67.2 | 0.69 | Whole brain corrected for baseline MRI volume | 1.5T GE, SPGR, 1.5‐mm slice thickness | Semiautomated | Q = −0.4 (0.5) |
| Ridha et al.,2006 | N = 25 | 46.5 (10) | 47.3 | 1.5 (0.8) | Whole brain corrected for IC and sex | 1.5 T GE, SPGR, 1.5‐mm slice thickness in coronal plane | Semiautomated (BBSI) | Q = −0.01 (0.57) |
| Chen et al.,2007, b | N = 36 (ApoE e4) | Weighted mean: 56.8 | 57.9 | Weighted mean: 2.17 | Whole brain corrected for IC and sex | 1.5T GE, SPGR, 1.5‐mm slice thickness | Semiautomated (BBSI) | Weighted mean: Q = −0.11 |
| Lenroot et al.,2007 | N = 228 | At follow‐up: 13.0 (3.9) | 12.3 | 1.5 | Cerebrum, corrected for IC and age | 1.5T GE, SPGR, 1.5‐mm slice thickness in axial plane | Automated | Different developmental trajectories for boys and girls |
| Autti et al.,2008 | N = 12 | 12.6 (0.9) | 15.9 | 6.5 (0.5) | Whole brain, corrected for IC and sex | 1.5T Siemens, MP‐RAGE, 1.0‐mm slice thickness | Automated | Q = −0.20 (0.5) |
| Brans et al.,2008a, c | N = 54 twins, N = 29 overlap with van Haren et al.,2008 | Weighted mean: 35.4 | 37.8 | Weighted mean: 4.81 | Whole brain corrected for IC, age, and sex | 1.5T Philips, FFE, 1.2‐mm slice thickness in coronal plane | Automated | Q = −0.07 |
| Brans et al.,2008b | N = 33, N = 32 overlap with van Haren et al.,2008 | 40.2 (8.2) | 42.7 | 5.02 (0.39) | Whole brain corrected for IC, age, and sex | 1.5T Philips, FFE, 1.2‐mm slice thickness in coronal plane | Automated | Q = −0.04 |
| Fisher et al.,2008 | N = 17 | 41.6 (8.1) [32–56] | 43.6 | 4 | Brain parenchymal fraction (BPF) | 1.5T, T1‐weighted spin echo image with and without contrast, 5‐mm slice thickness and T2‐weighted FLAIR | Automated | No information |
| Fotenos et al.,2008 | N = 33, complete overlapping with Fotenos et al.,2005 | Weighted mean: 77.3 | 79.5 | Weighted mean: 4.35 [3.1–6.5] | Whole brain corrected for IC, age, and sex | 1.5T Siemens, MP‐RAGE, 1.25‐mm slice thickness | Automated | Weighted mean: Q = −0.53 |
| Rais et al.,2008 | N = 31, N = 29 overlap with van Haren et al.,2008 | 24.7 (6.7) [16.7–40.2] | 27.3 | 5.21 (0.18) [4.78–5.50] | Whole brain corrected for IC, age, and sex | 1.5T Philips, FFE, 1.2‐mm slice thickness in coronal plane | Semiautomated | Q = −0.07 |
| Silbert et al.,2008 | N = 104 | 85.1 (5.6) [64.6–88.2] | 88.2 | 1.3 (0.7) [0.9–5.5] | Cerebrum | 1.5T, multiecho sequence, 4‐mm slice thickness in sagittal plane | Automated | Q = −0.67 |
| Sluimer et al.,2008a | N = 23 | 66 (9) | 67.0 | 1.9 (1.0) | Whole brain corrected for age and sex | 1.0T Siemens, MP‐RAGE, 1.5‐mm slice thickness in coronal plane | Automated | Q = −0.6 (0.6) |
| Sluimer et al.,2008b | N = 10 | 69 (7) | 70.2 | 2.3 (0.5) | Whole brain corrected for age and sex | 1.0T Siemens, MP‐RAGE, 1.5‐mm slice thickness in coronal plane | Automated | Q = −0.50 (0.5) |
| van Haren et al.,2008 | N = 113 | 35.3 (12.3) [16.8–56.3] | 37.8 | 4.94 (0.32) | Whole brain corrected for ICV, age, and sex | 1.5T Philips, FFE, 1.2‐mm slice thickness in coronal plane | Automated | Q = −0.15 |
| Driscoll et al.,2009 | N = 120, partly overlapping with Resnick et al.,2003 | 70.6 (6.1) [64–86] | 73.6 | 6.02 (2.91) [1–10] | Cerebrum corrected for ICV, age, and sex | 1.5T GE, SPGR, 1.5‐mm‐slice thickness | Semiautomated | Q = −0.77, MRI baseline volume was read from a graph |
| Ment et al.,2009 | N = 20 | 8.6 (0.7) | 10.4 | 3.5 | Whole brain, corrected for age, sex, and interval | 1.5T GE, SPGR, 1.5‐mm slice thickness in axial plane | Automated | Q = +0.98 |
| Reig et al.,2009 | N = 34 | 15.8 (1.4) [13–18] | 16.8 | 2.02 | Whole brain, corrected for ICV | 1.5T Philips, gradient echo sequence with 1.5‐mm slice thickness | Semiautomated | Weighted mean: Q = −0.17 Boys: Q = −0.29 Girls: Q = +0.01 |
Abbreviations: N: number of subjects; sd: standard deviation; yrs: years; ICV: Intracranial volume; GE: General Electrics; T: tesla; SPGR: spoiled prepared gradient recalled echo sequence; IR: inversion recovery; MP‐RAGE: magnetization prepared rapid gradient echo; GRASS: gradient recalled acquisition in steady state; FFE: fast‐field echo; FLAIR: fluid attenuated inversion recovery.
9 APOE‐e4 allele negative and 16 APOE‐e4 allele positive subjects. Age and relative rate of brain volume change per year are weighted in relation to the population frequency of ApoE‐e4 carriers (Cumming and Robertson,1984).
10 homozygote for ApoE e4, 10 heterozygote for ApoE e4, and 16 noncarriers. Age, follow‐up period, and relative rate of brain volume change per year are weighted in relation to the population frequencies for ApoE‐carriers (Hill et al.,2007).
Age, follow‐up period, and relative rate of brain volume change are weighted for healthy controls in monozygotic and dizygotic group.
“no results” in the column for “Relative rate of % brain volume change/year” indicates that there was no information available to extract percentage brain volume change per year. These studies were excluded from the smoothing analysis.
Table II.
Overview of the longitudinal studies for GM and WM volume changes in healthy individuals
| Authors, year | Subjects | Age at baseline (yrs: mean(sd)[range]) | Age in between scans (yrs: mean) | Interval (yrs: mean (sd) [range]) | Relative rate of % WB volume change/year (mean, sd, range) | Relative rate of % GM volume change/year (mean, sd, range) | Relative rate of % WM volume change/year (mean, sd, [range]) |
|---|---|---|---|---|---|---|---|
| Ment et al.,2009 | N = 20 | 8.6 (0.7) | 10.4 | 3.5 | Q = +0.98 | Q = −2.73 | Q = +7.51 |
| Lenroot et al.,2007 | N = 228 | At follow‐up: 13.0 (3.9) | 12.3 | 1.5 | Different developmental trajectories for boys and girls | Different developmental trajectories for boys and girls | Different developmental trajectories for boys and girls |
| Autti et al.,2008 | N = 12 | 12.6 (0.9) | 15.9 | 6.5 (0.5) | Q = −0.20 (0.5) | Q = −0.30 (0.60) | Q = +0.80 (0.70) |
| Reig et al.,2009 | N = 34 | 15.8 (1.4) [13–18] | 16.8 | 2.0 | Weighted mean: Q = −0.17 Boys: Q = −0.29 Girls: Q = +0.01 | Weighted mean: Q = −0.74 Boys: Q = −1.01 Girls: Q = −0.30 | Weighted mean: Q = +1.03 Boys: Q = +1.26 Girls: Q = +0.65 |
| Lieberman et al.,2005 | N = 44 | 25.5 (4.13) from the baseline sample with 62 participants | 26.0 | 1.0 | Q = +0.60 | Q = +0.59 | Q = +0.62 |
| van Haren et al.,2008 | N = 113 | 35.3 (12.3) [16.8–56.3] | 37.8 | 4.9 (0.3) | Q = −0.15 | Q = −0.15 | Q = +0.30 |
| Liu et al.,2003 | N = 90 A: N(<35y) = 44 B: N(35–54y) = 37 C: N(>54y) = 9 | A: 24.5 (6.6) [14–34] B: 44.5 (5.7) [35–53] C: 67.9 (6.4) [57–77] | 26.3 46.3 69.7 | A: 3.6 B: 3.5 C: 3.5 | A: Q = −0.06 B: Q = −0.18 C: Q = −0.38 | A: Q = −0.11 B: Q = −0.02 C: Q = −0.26 | A: Q = +0.06 B: Q = −0.43 C: Q = −0.57 |
| Driscoll et al.,2009 | N = 120, partly overlapping with Resnick et al.,2003 | 70.6 (6.1) [64–86] | 73.6 | 6.0 (2.9) [1–10] | Q = −0.77, MRI baseline volume was read from a graph | Q = −0.50, MRI baseline volume was read from a graph | Q = −1.07, MRI baseline volume was read from a graph |
| Thompson et al.,2003 | N = 14 | 71.4 (0.9) | 72.7 | 2.6 (0.3) | Q = −0.88 (0.15) | Q = −0.91 (0.92) | Q = −2.72 (1.44) |
| Cardenas et al.,2003 | N = 16 | 76.0 (5.0) | 77.3 | 2.6 (1.0) | Q = −0.20 (0.10) | Q = −0.90 (1.40) | No information |
Abbreviations: N: number of subjects; sd: standard deviation; yrs: years; WB: whole brain; GM: gray matter; WM: white matter.
“no results” in the column for “Relative rate of % brain volume change/year” indicates that there was no information available to extract percentage brain volume change per year. These studies were excluded from the smoothing analysis.
MRI Acquisition and Processing Specifics
The majority of the MRI studies acquired T1‐weighted images (SPGR, FFE, or MPRAGE depending on manufacturer being GE, Philips, or Siemens, respectively) on a 1.5 Tesla scanner. Brain scans were obtained in either axial, sagittal, or coronal plane with slice thickness ranging from 0.9 to 5 mm. Images were segmented using manual, semiautomated, or automated procedures or with a combination of these. For details see Table I.
Study Specific Details
Several studies published data on totally or partly overlapping samples. To prevent bias, these studies were identified, and the study with the highest number of participants (usually the most recent study) was included in the regression plots (see Table I).
One study included only female subjects [Cohen et al.,2001], and two studies included only male subjects [Pfefferbaum et al.,1995; Withworth et al.,2005]. Eighteen studies measured cerebrum volume, and the remainder of the studies (N = 38) measured whole brain volume. As we were interested in relative brain volume change, this was not a problem in this study. Findings from 20 studies were corrected for intracranial volume (ICV) and 17 studies for baseline brain volume. Seventeen studies reported brain volume change after correcting for age and sex. An additional four studies only corrected for age, while another six studies corrected only for sex. Here, the rate of change for whole brain, GM, and WM volumes is based on the uncorrected data (if present).
In the study by Lieberman et al. [2005], the follow‐up interval with 52 weeks with N = 44 was used in the review, but baseline volumes of N = 52 were used to calculate the rate of change per year.
Two studies were designed to investigate the effect of Apolipoprotein Epsilon e4+ (ApoE e4+) on whole brain volume change [Chen et al.,2007; Cohen et al.,2001]. Groups were either defined as ApoE e4+ carrier or noncarrier [Cohen et al.,2001], or subjects were divided in three groups being either homozygous (noncarriers or carriers) or heterozygous for ApoE e4+ [Chen et al.,2007]. The frequency of ApoE e4+ carriers in the total population of white Caucasians is 15% [Cumming and Robertson,1984]. In addition, the frequency of being homozygous or heterozygous for ApoE e4+ is ∼2% and 26.5%, respectively [Hill et al.,2007]. Weighted means of rate of whole brain volume change per year were calculated using these known frequency distributions. For ApoE e4+ carriers [Cohen et al.,2001], the formula is as follows: (0.15 * Q in ApoE 4+ carriers) + (0.85 * Q in ApoE e4+ noncarriers). The formula for the study by Chen et al. [2007] is as follows: (homozygous: 0.02 * Q) + (heterozygous: 0.27 * Q) + (noncarriers: 0.71 * Q).
Schott et al. [2005] compared two segmentation methods, i.e., brain boundary shift integrals (BBSIs) and manual segmentation. Here, the findings from the manual segmentation were chosen as this is still considered to be the golden standard.
One study included monozygotic (MZ) and dizygotic (DZ) twin pairs and reported whole brain volume change for each group separately [Brans et al.,2008a]. For our purpose, relative rate of volume change per year was weighted according to the number of DZ and MZ twins.
In Figure 1, all studies for which relative rate of brain volume change per year was calculated are shown (N = 33). Eight studies did not present sufficient information to extract relative rate of whole brain volume change per year. Fifteen studies were excluded as they reported on overlapping samples.
Figure 1.
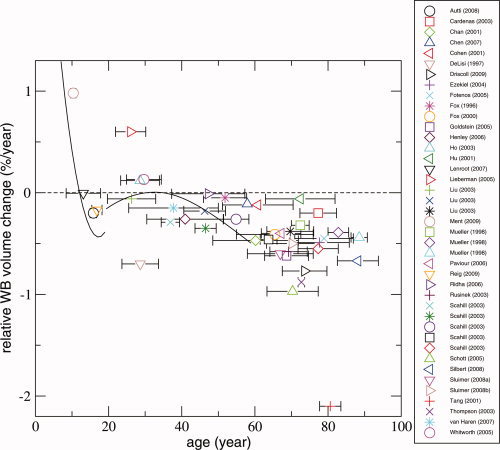
Longitudinal magnetic resonance brain imaging studies measuring whole brain volume change with age in humans. The total number of studies, after excluding overlapping samples, was 33. Each data point represents a study or a particular age group from an individual study. The relative whole brain volume change in %/year (Q) was set out against the mean age in between the two time points. The horizontal bars represent the standard deviation for age at baseline for all subjects included in the study. The zero‐line indicates no whole brain volume change. Above zero indicates an increase in the whole brain volume while below zero represents a decrease in whole brain volume. Several studies reported results for different age groups [Liu et al.,2003; Mueller et al.,1998; Scahill et al.,2003]. These individual age groups are depicted separately in Figure 1; therefore, 41 data points are shown. Two studies reported their data as nonlinear trajectories [Lenroot et al.,2007; van Haren et al.,2008], and their respective trajectories are also shown here.
Several studies reported results for different age groups [Liu et al.,2003; Mueller et al.,1998; Scahill et al.,2003], and these individual age groups are depicted separately. As a result 41 data points are shown. Two studies reported their data as nonlinear trajectories [Lenroot et al.,2007; van Haren et al.,2008], and their respective trajectories are also shown here.
Figure 2 shows the relative rate of brain volume change per year for each individual study as circles. The area of the circle scales with the number of included subjects. One study is excluded from this analysis since it deviates from all the other studies [Tang et al.,2001]. Therefore, fits were based on 32 studies, including 1,393 participants.
Figure 2.
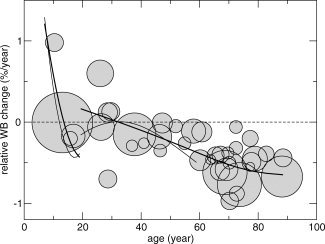
Fits that show the association between relative rate of whole brain volume change and age. Whole brain volume change data from the individual studies are shown as circles. The area of the circles scales with the number of subjects in the study (a larger area of the circle corresponds to more participants). Fits (with 3 degrees of freedom) were calculated to the data below and above age 19 separately (thick lines). Two studies reported their data as trajectories [Lenroot et al.,2007; van Haren et al.,2008], and these are made visible (thin lines).
In Figure 3, whole brain volume is presented as a function of age, obtained by integration of the fits from Figure 2 with respect to age. As starting volume mean, whole brain volume was used from a study of N = 210 nine‐year‐old twins [Peper et al.,2009].
Figure 3.
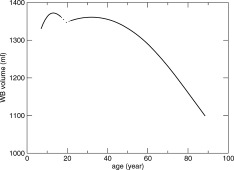
Whole brain volume across the life span between 4 and 88 years of age. Whole brain volume as a function of age is obtained by numerical integration of the whole brain volume change fits with respect to age from Figure 2. As starting volume, the mean whole brain volume from a study of N = 210 nine‐year‐old twins was used [Peper et al.,2009]. The curves are dashed around age 18–21, indicating the uncertainty in this area, since only few data were available for fitting this age range. Two separate fits were calculated for the younger (<19 years) and older (>19 years) group.
The results indicate that a wave of growth in whole brain volume occurs during childhood and adolescence, i.e., around 9 years of age, a 1% annual brain growth is found which levels off until at age 13 a gradual volume decrease sets in. During young adulthood, between ∼18 and 35 years of age, possibly another wave of growth occurs or at least a period of no brain tissue loss. After age 35 years, a steady volume loss is found of 0.2% per year, which accelerates gradually to an annual brain volume loss of 0.5% at age 60.
Figure 4a,b shows the relative rate of GM and WM volume change per year for each individual study as circles. The area of the circle scales as the number of included subjects. A decrease is found in percent GM volume per year in all studies except for one [Lieberman et al.,2005]. Those studies that included individuals below the age of ∼45 years show an increase in percent WM volume per year while studies with subjects older than 45 years of age show decreases. Two studies provided age‐related trajectories of GM and WM changes with age [Lenroot et al.,2007; van Haren et al.,2008]. These are added to Figure 4a,b.
Figure 4.
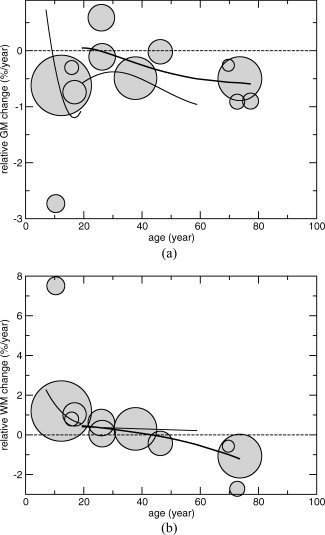
a and b. GM and WM volume change with age. Total number of studies that presented data on GM (after excluding overlapping studies) was 10, while 9 studies presented data on WM. The relative GM and WM volume change in %/year (Q) was set out against the mean age in between the two time points. The zero‐line indicates no volume change. Above zero indicates an increase in the volume, whereas below zero represents a decrease in volume. The area of the circles scales as the number of subjects in the study. Fits for both GM and WM with age (with 3 degrees of freedom) were calculated to the data above age 19 (thick line). Two studies reported their GM and WM data as trajectories [Lenroot et al.,2007; van Haren et al.,2008], and these are made visible (thin lines). Liu et al. [2003] reported results for different age groups. These individual age groups are depicted separately; therefore, 12 data circles are shown for GM, while 11 data circles are shown for WM.
DISCUSSION
In this review, findings from longitudinal MRI studies on whole brain volume change in healthy individuals over the full life span are integrated. Fifty‐six studies were selected, including a total of 2,211 healthy individuals. We find that brain volume changes throughout life, not only in childhood and adolescence but also in adulthood. More specifically, the results indicate that brain volume increases during childhood and young adolescence until the age of ∼13 years. After age 13 years, a decrease in whole brain volume sets in. The main finding from the current study is that we provide evidence for a possible second wave of brain volume growth or at least a stable period in early adulthood preceding a brain volume decrease from the age of 35 years with accelerating tissue loss occurring with increasing age.
As is shown in Figure 1, there are three studies that show brain growth in young adulthood [age 20–30 years; Ho et al.,2003; Lieberman et al.,2005; Whitworth et al.,2005; symbols above zero], and two studies showing a decrease [DeLisi et al.,1997; Liu et al.,2003; symbols below zero]. Fitting the data from these studies suggests that brain volume slightly increases over time in this age range, but given the fact that there is no agreement between the different studies, we interpret this result with caution and suggest a possible growth or a plateau period where no change in volume takes place.
Most likely these whole brain volumes changes are a net consequence of many different factors such as focal growth and shrinkage of GM and WM. Until now, only a limited number of studies have investigated GM and WM volume change over time in longitudinal studies. Our findings suggest an increase in GM volume in childhood after which a decrease sets in, while WM increases till the age of ∼45 years and thereafter starts to decrease.
Indeed, the NIMH group showed that cerebral GM volume increases in preadolescence [Giedd et al.,1999a,b; Gogtay et al.,2004; Lenroot et al.,2007; Sporn et al.,2003], after which it starts to decrease in postadolescence, while cerebral WM continues to increase in volume [Giedd et al.,1999b]. It has been suggested that GM is replaced by WM in childhood and adolescence [Giedd et al.,1999b; Jernigan et al.,1991]. In line with our findings, others have suggested that GM shows a linear age‐related decrease in adulthood but an increase in WM until midlife which started to decrease after that in an inverted U‐shaped curve [Taki et al.,2009; van Haren et al.,2008]. If indeed there is subtle brain growth in young adulthood as is suggested by the plots in the current review, this might then be explained by WM increasing more than GM is decreasing.
Most studies did not provide sufficient data to investigate differential effects between males and females while those that did report inconsistent findings. One study showed a greater decline in cerebral volume in girls as compared with boys in cerebral volume during the 2nd decade of life [Lenroot et al.,2007]. The developmental trajectories suggest that brain volume in girls peaked at 10.5 years while that in boys peaked at 14.5 years. One study, however, was not able to replicate this in children between the ages of 8 and 12 years [Ment et al.,2009]. In older age, a significant sex difference with more pronounced volume loss in males relative to females has been reported [Driscoll et al.,2009]. However, most studies suggested that in adulthood and old age rate of change in whole brain volume is similar in males and females [Autti et al.,2008; Chan et al.,2001; DeLisi et al.,2004; Fotenos et al., 2005; Fotenos et al.,2008; Liu et al.,2003; Resnick et al.,2003; Ridha et al.,2006; Scahill et al.,2003; Tang et al.,2001]. Here, we focus on volume change in global measures such as whole brain or GM and WM. Males do indeed have larger brains, but the available literature in adults on gender differences suggests that males and females have similar rates of change in global brain volume measures.
As to the mechanisms underlying the brain changes throughout life, we can only speculate at this point. Our finding of brain changes over the entire life span suggests continuous brain plasticity throughout life. Brain plasticity can be referred to as the changing of neurons, the reorganization of their networks, and their change in function as a consequence of new experiences. Obviously and fortunately, experiencing new events does not stop at reaching adulthood. In the aging brain, it has been proposed that cell shrinkage, degeneration of key neurons, and circuits could explain the age‐related decrease in brain volume [Morrison and Hof,1997]. We recently found that genes are implicated in brain structure changes in adulthood [Brans et al.,2010]. In contrast, there is no evidence that it is synaptic density that explains the adult volume change since a postmortem study showed that the synaptic density is constant throughout adult life (ages 16–72 years) [Huttenlocher,1979]. Disentangling the processes underlying brain plasticity is important for healthy development and also provides insight into what may be arrested in brain disorders that have their first symptoms at a particular age.
There are several limitations to the study that have to be considered when interpreting its findings. A limited number of studies were available covering the age range between 18 and 21 years, preventing us from making a reliable connection between development during childhood and adolescence and development during the adult age range. For this reason, the data were split into two age ranges. However, since the (mathematical) integration was determined over the full age range, and only the integrand (i.e., the rate of change) was derived from two separate regressions, we can conclude that after the growth wave during childhood and adolescence, a second wave of growth or at least a plateau period occurs in young adulthood. Since there remains some uncertainty as to what exactly happens between 18 and 21 years, this finding requires confirmation from longitudinal study in subjects between 16 and 25 years.
A few studies did not fit the regression line well and can be considered as outliers. One study included subjects over the age of 80 years and found remarkable losses in brain volume of 2.1% per year [Tang et al.,2001]. An explanation for this finding may be that it is the only study that used the Cavalieri method to estimate the whole brain volume. One study included subjects with a mean age of ∼25 years and reported a relatively large decrease of −0.70% per year [DeLisi et al.,1997]. Consideration mentioned by the authors was possible artifacts in the scanner over time [see DeLisi et al.,1997].
The differences in acquisition methods and study design may have affected the results, specifically the regression lines. Studies with high‐resolution scans are expected to be more sensitive to brain volume changes than studies using thicker MRI slices. The high/low‐resolution studies were more or less evenly distributed over the age range. Studies with large age ranges have to be dealt with carefully, since they tend to average out the change‐rate‐per‐age values. In our analysis, we assume that the scan intervals are short enough (≤ 6.5 year) so that between the first and the last scan, the rate of change will not differ much. For rapidly changing rates and for large age ranges, this assumption is violated. In two studies, this assumption could be violated [Lenroot et al.,2007; van Haren et al.,2008]. However, these two studies applied an age‐dependent analysis, which we incorporated in full in our analysis. Another six studies that had an age range >20 years: [Driscoll et al.,2009; Fotenos et al.2005; Fox et al.,2000; Goldstein et al.,2005; Liu et al.,2003; Silbert et al.,2008]. After inspection, these studies do not seem to violate the assumption of piecewise constant change rate.
Another issue concerns the definition of “healthy individual,” which differed between studies. Some studies excluded participants with a psychiatric history or hypertension, while others did not obtain this information or performed population‐based studies. In addition, even when using the same scale (such as the MMSE) as a screening, the cutoff for inclusion differed between studies (e.g., 24 for the MMSE in two studies [Mueller et al.,1998; Silbert et al.,2008] and 28 for one other study [Rusinek et al.,2003]. Finally, with increasing age, the incidence of hypertension, diabetes, WM hyperintensities (WMH), and cognitive decline most likely increases, so that the inclusion of healthy participants at an older age may be limited to a shrinking population of people that remain (extremely) healthy throughout life. As can be seen from Figure 2, there appears to be a flattering of the decrease in brain volume after the age of 75 years. This might be explained by the inclusion bias mentioned. It is likely that extremely healthy participants are selected in the age range over 75 years of age. Only those who are physically healthy (can) participate while functionally impaired subjects cannot.
When interpreting longitudinal follow‐up studies using complex techniques such as MRI, one always has to take into consideration that noise is introduced by changes in the measurement over time. However, it is unlikely that such limitations of the study significantly influenced the results of our analysis. Because of the large number of studies that were included, one would expect noise to be largely leveled out. We were able to plot the trajectories of whole brain volume change with age that were present in two studies onto our plot based on all studies. Comparison of these fits with the overall fit shows that both trajectories, i.e., one during childhood and adolescence [Lenroot et al.,2007] as well as one in adulthood [van Haren et al.,2008], agree nicely with the brain volume change based on all the included longitudinal MRI studies.
In conclusion, we reviewed and integrated the findings of 56 studies investigating longitudinal whole brain volume change. The results indicate that whole brain volume change is an ongoing process throughout the full life span with increasing brain volume in childhood and adolescence in the age of ∼13 years after which a rapid volume decrease sets in. We found suggestive evidence of a second period of growth or at least stability in brain volume. It is only after the age of 35 years, the brain starts to decrease in adulthood. Longitudinal studies that include subjects between 15 and 25 years of age are needed to confirm our finding of a stable or growing whole brain volume during this possibly critical stage of brain development. The results may help in understanding the mechanisms of normal brain changes and may contribute to distinguishing psychiatric and neurodegenerative diseases from healthy aging processes.
REFERENCES
- Autti TH, Hämäläinen J, Mannerkoski M, Van Leemput KV, Aberg LE ( 2008): JNCL patients show marked brain volume alterations on longitudinal MRI in adolescence. J Neurol 255: 1226–1230. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, Pittman B, Martin A, Peterson BS, Fulbright RK, Krystal JH ( 2005): Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord 7: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brans RG, van Haren NE, van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE ( 2008a): Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry 65: 1259–1268. [DOI] [PubMed] [Google Scholar]
- Brans RG, van Haren NE, van Baal GC, Staal WG, Schnack HG, Kahn RS, Hulshoff Pol HE ( 2008b): Longitudinal MRI study in schizophrenia patients and their healthy siblings. Br J Psychiatry 193: 422–423. [DOI] [PubMed] [Google Scholar]
- Brans RG, Kahn RS, Schnack HG, van Baal GC, Posthuma D, van Haren NE, Lepage C, Lerch JP, Collins DL, Evans AC, Boomsma DI, Hulshoff Pol HE ( 2010): Brain plasticity and intellectual ability are influenced by shared genes. J Neurosci 30: 5519–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn W, Hulshoff Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, Schothorst PF, van Engeland H, Kahn RS ( 2002): Brain volume changes in first‐episode schizophrenia: A 1‐year follow‐up study. Arch Gen Psychiatry 59: 1002–1010. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Du AT, Hardin D, Ezekiel F, Weber P, Jagust WJ, Chui HC, Schuff N, Weiner MW ( 2003): Comparison of methods for measuring longitudinal brain change in cognitive impairment and dementia. Neurobiol Aging 24: 537–544. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Jenkins R, Scahill RI, Crum WR, Rossor MN ( 2001): Rates of global and regional cerebral atrophy in AD and frontotemporal dementia. Neurology 57: 1756–1763. [DOI] [PubMed] [Google Scholar]
- Chen K, Reiman EM, Alexander GE, Caselli RJ, Gerkin R, Bandy D, Domb A, Osborne D, Fox N, Crum WR, Saunders AM, Hardy J ( 2007): Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. Am J Psychiatry 164: 916–921. [DOI] [PubMed] [Google Scholar]
- Cleveland WS, Devlin SJ ( 1988): Locally weighted regression: An approach to regression analysis by local fitting. J Am Statistical Assoc 83: 596–610. [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T ( 2001): Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology 57: 2223–2228. [DOI] [PubMed] [Google Scholar]
- Cumming AM, Robertson FW ( 1984): Polymorphism at the apoprotein‐E locus in relation to risk of coronary disease. Clin Genet 25: 310–313. [DOI] [PubMed] [Google Scholar]
- Dekaban AS ( 1978): Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Ann Neurol 4: 345–356. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Stritzke P, Riordan H, Holan V, Boccio A, Kushner M, McClelland J, Van Eyl O, Anand A ( 1992): The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol Psychiatry 31: 241–254. Erratum in: Biol Psychiatry 31: 1172. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Tew W, Xie S, Hoff AL, Sakuma M, Kushner M, Lee G, Shedlack K, Smith AM, Grimson R ( 1995): A prospective follow‐up study of brain morphology and cognition in first‐episode schizophrenic patients: Preliminary findings. Biol Psychiatry 38: 349–360. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R ( 1997): Schizophrenia as a chronic active brain process: A study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res 74: 129–140. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Maurizio AM, Relja M, Hoff AL ( 2004): Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Res 130: 57–70. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM ( 2009): Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 72: 1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel F, Chao L, Kornak J, Du AT, Cardenas V, Truran D, Jagust W, Chui H, Miller B, Yaffe K, Schuff N, Weiner M ( 2004): Comparisons between global and focal brain atrophy rates in normal aging and Alzheimer disease: Boundary Shift Integral versus tracing of the entorhinal cortex and hippocampus. Alzheimer Dis Assoc Disord 18: 196–201. [PMC free article] [PubMed] [Google Scholar]
- Fisher E, Lee JC, Nakamura K, Rudick RA ( 2008): Gray matter atrophy in multiple sclerosis: A longitudinal study. Ann Neurol 64: 255–265. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL ( 2005): Normative estimates of cross‐sectional and longitudinal brain volume decline in aging and AD. Neurology 64, 1032–1039. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL ( 2008): Brain volume decline in aging: Evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol 65: 113–120. [DOI] [PubMed] [Google Scholar]
- Fox NC, Freeborough PA, Rossor MN. Visualisation and quantification of rates of atrophy in Alzheimer's disease. Lancet. 1996 Jul 13;348(9020):94–97. [DOI] [PubMed] [Google Scholar]
- Fox NC, Scahill RI, Crum WR, Rossor MN ( 1999a): Correlation between rates of brain atrophy and cognitive decline in AD. Neurology 52: 1687–1689. [DOI] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Rossor MN ( 1999b): Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer's disease. Lancet 353: 2125. [DOI] [PubMed] [Google Scholar]
- Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN ( 2000): Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: Power calculations and estimates of sample size to detect treatment effects. Arch Neurol 57: 339–344. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Jeffries NO, Blumenthal J, Castellanos FX, Vaituzis AC, Fernandez T, Hamburger SD, Liu H, Nelson J, Bedwell J, Tran L, Lenane M, Nicolson R, Rapoport JL ( 1999a): Childhood‐onset schizophrenia: Progressive brain changes during adolescence. Biol Psychiatry 46: 892–898. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL ( 1999b): Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2: 861–863. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Sporn A, Clasen LS, Nugent TF III, Greenstein D, Nicolson R, Giedd JN, Lenane M, Gochman P, Evans A, Rapoport JL ( 2004): Comparison of progressive cortical gray matter loss in childhood‐onset schizophrenia with that in childhood‐onset atypical psychoses. Arch Gen Psychiatry 61: 17–22. [DOI] [PubMed] [Google Scholar]
- Goldstein IB, Bartzokis G, Guthrie D, Shapiro D ( 2005): Ambulatory blood pressure and the brain: A 5‐year follow‐up. Neurology 64: 1846–1852. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC ( 1998): A follow‐up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry 55: 145–152. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R ( 1990): Exploring the nature of covariate effects in the proportional hazards model. Biometrics 46: 1005–1016. [PubMed] [Google Scholar]
- Henley SM, Frost C, MacManus DG, Warner TT, Fox NC, Tabrizi SJ ( 2006): Increased rate of whole‐brain atrophy over 6 months in early Huntington disease. Neurology 67: 694–696. [DOI] [PubMed] [Google Scholar]
- Hill JM, Bhattacharjee PS, Neumann DM ( 2007): Apolipoprotein E alleles can contribute to the pathogenesis of numerous clinical conditions including HSV‐1 Corneal disease. Exp Eye Res 84: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KC, Roessmann U, Straumfjord JV, Monroe G ( 1980): Analysis of brain weight. I. Adult brain weight in relation to sex, race, and age. Arch Pathol Lab Med 104: 635–639. [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M ( 2003): Progressive structural brain abnormalities and their relationship to clinical outcome: A longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 60: 585–594. [DOI] [PubMed] [Google Scholar]
- Hu MT, White SJ, Chaudhuri KR, Morris RG, Bydder GM, Brooks DJ ( 2001): Correlating rates of cerebral atrophy in Parkinson's disease with measures of cognitive decline. J Neural Transm 108: 571–580. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR ( 1979): Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Res 163: 195–205. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr, Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC ( 2004): Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AC, Javaloyes A, James S, Smith DM ( 2002): Evidence for non‐progressive changes in adolescent‐onset schizophrenia: Follow‐up magnetic resonance imaging study. Br J Psychiatry 180: 339–344. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA ( 1991): Maturation of human cerebrum observed in vivo during adolescence. Brain 114: 2037–2049. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema‐Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR ( 2001): Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22: 581–594. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN ( 2007): Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M, HGDH Study Group ( 2005): Antipsychotic drug effects on brain morphology in first‐episode psychosis. Arch Gen Psychiatry 62: 361–370. [DOI] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Bartlett PA, Sander JW, Sisodiya SM, Shorvon SD, Duncan JS ( 2001): A longitudinal quantitative MRI study of community‐based patients with chronic epilepsy and newly diagnosed seizures: Methodology and preliminary findings. Neuroimage 14: 231–243. [DOI] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JW, Duncan JS ( 2003): A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage 20: 22–33. [DOI] [PubMed] [Google Scholar]
- Ment LR, Kesler S, Vohr B, Katz KH, Baumgartner H, Schneider KC, Delancy S, Silbereis J, Duncan CC, Constable RT, Makuch RW, Reiss AL ( 2009): Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics 123: 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR ( 1997): Life and death of neurons in the aging brain. Science 278: 412–419. [DOI] [PubMed] [Google Scholar]
- Mueller EA, Moore MM, Kerr DC, Sexton G, Camicioli RM, Howieson DB, Quinn JF, Kaye JA ( 1998): Brain volume preserved in healthy elderly through the eleventh decade. Neurology 51: 1555–1562. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Price SL, Jahanshahi M, Lees AJ, Fox NC ( 2006): Longitudinal MRI in progressive supranuclear palsy and multiple system atrophy: Rates and regions of atrophy. Brain 129: 1040–1049. [DOI] [PubMed] [Google Scholar]
- Peper JS, Schnack HG, Brouwer RM, van Baal GC, Pjetri E, Székely E, van Leeuwen M, van den Berg SM, Collins DL, Evans AC, Boomsma DI, Kahn RS, Hulshoff Pol HE ( 2009): Heritability of regional and global brain structure at the onset of puberty: A magnetic resonance imaging study in 9‐year‐old twin pairs. Hum Brain Mapp 30: 2184–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO ( 1995): Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res 19: 1177–1191. [DOI] [PubMed] [Google Scholar]
- Rais M, Cahn W, Van Haren N, Schnack H, Caspers E, Hulshoff Pol H, Kahn R ( 2008): Excessive brain volume loss over time in cannabis‐using first‐episode schizophrenia patients. Am J Psychiatry 165: 490–496. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning‐Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD ( 2004): Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol Aging 25: 377–396. [DOI] [PubMed] [Google Scholar]
- Reig S, Moreno C, Moreno D, Burdalo M, Janssen J, Parellada M, Zabala A, Desco M, Arango C ( 2009): Progression of brain volume changes in adolescent‐onset psychosis. Schizophr Bull 35: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C ( 2003): Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci 23: 3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridha BH, Barnes J, Bartlett JW, Godbolt A, Pepple T, Rossor MN, Fox NC ( 2006): Tracking atrophy progression in familial Alzheimer's disease: A serial MRI study. Lancet Neurol 5: 828–834. [DOI] [PubMed] [Google Scholar]
- Rusinek H, De Santi S, Frid D, Tsui WH, Tarshish CY, Convit A, de Leon MJ ( 2003): Regional brain atrophy rate predicts future cognitive decline: 6‐year longitudinal MR imaging study of normal aging. Radiology 229: 691–696. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC ( 2003): A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 60: 989–994. [DOI] [PubMed] [Google Scholar]
- Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC ( 2005): Measuring atrophy in Alzheimer disease: A serial MRI study over 6 and 12 months. Neurology 65: 119–124. [DOI] [PubMed] [Google Scholar]
- Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA ( 2008): Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 71: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluimer JD, Bouwman FH, Vrenken H, Blankenstein MA, Barkhof F, van der Flier WM, Scheltens P ( 2008a): Whole‐brain atrophy rate and CSF biomarker levels in MCI and AD: A longitudinal study. Neurobiol Aging 31: 758–764. [DOI] [PubMed] [Google Scholar]
- Sluimer JD, van der Flier WM, Karas GB, Fox NC, Scheltens P, Barkhof F, Vrenken H ( 2008b): Whole‐brain atrophy rate and cognitive decline: Longitudinal MR study of memory clinic patients. Radiology 248: 590–598. [DOI] [PubMed] [Google Scholar]
- Sporn AL, Greenstein DK, Gogtay N, Jeffries NO, Lenane M, Gochman P, Clasen LS, Blumenthal J, Giedd JN, Rapoport JL ( 2003): Progressive brain volume loss during adolescence in childhood‐onset schizophrenia. Am J Psychiatry 160: 2181–2189. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H ( 2009): A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiol Aging 32: 907–915. [DOI] [PubMed] [Google Scholar]
- Tang Y, Whitman GT, Lopez I, Baloh RW ( 2001): Brain volume changes on longitudinal magnetic resonance imaging in normal older people. J Neuroimaging 11: 393–400. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW ( 2003): Dynamics of gray matter loss in Alzheimer's disease. J Neurosci 23: 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Brans R, Carati I, Rais M, Kahn RS ( 2008): Progressive brain volume loss in schizophrenia over the course of the illness: Evidence of maturational abnormalities in early adulthood. Biol Psychiatry 63: 106–113. [DOI] [PubMed] [Google Scholar]
- Whitworth AB, Kemmler G, Honeder M, Kremser C, Felber S, Hausmann A, Walch T, Wanko C, Weiss EM, Stuppaeck CH, Fleischhacker WW ( 2005): Longitudinal volumetric MRI study in first‐ and multiple‐episode male schizophrenia patients. Psychiatry Res 140: 225–237. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Velakoulis D, Smith DJ, Bond D, Stuart GW, McGorry PD, Brewer WJ, Bridle N, Eritaia J, Desmond P, Singh B, Copolov D, Pantelis C ( 2001): A longitudinal study of hippocampal volume in first episode psychosis and chronic schizophrenia. Schizophr Res 52: 37–46. [DOI] [PubMed] [Google Scholar]


