Abstract
Experience‐based neuroplasticity has typically been associated with functional changes, but growing evidence indicates that training can also render dynamic structural alterations in the brain. Although research on training‐induced morphological plasticity has consistently demonstrated rapid increases of gray matter volume in task‐related regions, no studies have examined if local volumetric reductions in gray matter associated with certain psychiatric disorders may be reversible by adequate training. We aimed to assess whether a training program applied to ADHD patients can contravene some of the associated neuroanatomical alterations. High‐resolution anatomical scans were acquired before and after the training period, and a whole‐brain tensor‐based morphometric approach was applied to extract a voxel‐wise estimation of longitudinal changes in regional gray matter volume. Our results show focal volumetric gray matter increases in bilateral middle frontal cortex and right inferior–posterior cerebellum after cognitive training compared with the ADHD control group. The extent of gray matter volume increase in the inferior–posterior cerebellum was associated with attentional performance. These findings illustrate the capacity of the nervous system for rapid morphological adjustments in response to environmental triggers. Moreover, the dorsolateral prefrontal cortex and cerebellum are commonly considered sites of volumetric reduction in ADHD, and the inferior–posterior lobule of the cerebellum is associated with progressive symptom‐related volume loss. Hence, the clusters of volumetric change observed in our study were confined to structures typically characterized by volume reduction in ADHD patients, providing preliminary indications that cognitive training may contravene some of the neuroanatomical deficits associated with the disorder. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: neural plasticity, tensor‐based morphometry, gray matter volume, attention‐deficit/hyperactivity disorder, middle frontal cortex, inferior‐posterior cerebellum, MRI
INTRODUCTION
Plasticity is an intrinsic property of the brain that ensures dynamic modifications at multiple levels of neural organization, allowing the brain to process, encode, and implement new knowledge. Animal studies have demonstrated that sensory experience can render both macro‐ and microscopic changes in the nervous system, including alterations in gene transcription, receptor expression, synaptic density, and cortical map organization [for reviews, see Buonomano and Merzenich,1998]. Concordantly, motor skill training has been associated with modifications in synaptic number, dendritic volume, mitochondrial and vascular density, glial volume and larger‐scale morphological features such as gyral size [Poldrack,2000]. Neurogenesis has also been related to training‐induced plasticity, and research suggests that training on associative learning tasks in adult rats enhances the survival rates of newly generated neurons in the dentate gyrus [Gould et al.,1999; Shors et al.,2001].
More recently, studies have commenced to investigate learning‐induced plasticity in the human brain. Techniques of functional neuroimaging applied to human samples have revealed signal changes after training, thought to reflect enhanced neuronal responsiveness or cortical map expansion [Callan et al.,2003; Poldrack and Gabrieli,2001]. In addition, a few studies have assessed structural plasticity in the human brain, establishing alterations in brain morphology after training. Draganski et al. [2004] demonstrated transient and selective volumetric expansions of gray matter in visuomotor regions following 3‐month of juggling practice in the adult human brain [Draganski et al.,2004], which were already manifested after 7 days of practice [Driemeyer et al., 2008]. Focal morphological gray matter increases were also observed after a 2‐week training of mirror reading [Ilg et al.,2008]. Besides explicit training programs, studying for an exam can affect the morphological characteristics of the nervous system, as demonstrated by Draganski et al. [2006], who observed learning‐induced parietal gray matter expansions and a continuous volumetric increase in the hippocampal formation. Two weeks of self‐study is sufficient for gray matter adjustments to surface [Ceccarelli et al.,2009]. Interestingly, a 5‐day application of repetitive transcranial magnetic stimulation can mimic training‐induced morphological changes, yielding local alterations in gray matter volume and cortical evoked potentials [May et al.,2007].
These findings consistently demonstrate rapid increases of gray matter volume after training in task‐related regions, postulating the possibility that local gray matter deficits associated with certain disorders may be reversible by adequate training programs. We aimed to test this hypothesis, using a program of cognitive training applied to ADHD patients. We have previously assessed the effects of cognitive training on neural activity and observed focal increases in BOLD signal in frontal, temporal and cerebellar regions [Hoekzema et al.,2009]. However, it remains to be determined whether cognitive training can alter brain morphology and contravene some of the neuroanatomical deficits associated with the disorder. To our knowledge, this is the first study to assess if training of cognitive functions applied to psychiatric patients can render structural alterations in the brain.
ADHD bears a considerable impact on brain structure and function, and converging research findings appoint the frontal lobes, striatum, and cerebellum as the primary sites of impairment in the ADHD brain, harboring a predominant share of the established syndrome‐associated deficits [for reviews, see Biederman and Faraone,2005; Giedd et al.,2001; Krain and Castellanos,2006; Nigg and Casey,2005]. Accordingly, volumetric studies assessing brain anatomy in ADHD patients have detected gray matter volume reductions in these regions [Biederman and Faraone,2005; Carmona et al.,2009; Giedd et al.,2001; Krain and Castellanos,2006; Nigg and Casey,2005; Tremols et al.,2008]. While ADHD is commonly treated with psychostimulant medication, training programs are often provided to complement pharmacological treatment. One of the standard training programs applied to ADHD patients in the clinical practice is cognitive training, a program directly targeting cognitive skills, which was originally developed to enhance rehabilitation after brain damage [Cicerone et al.,2000].
We used high‐resolution T1‐weighted MRI scans of the brain obtained from 18 unmedicated ADHD children of the combined subtype before and after a 2‐week period of training. To assess regional alterations in brain structure, we applied tensor‐based morphometry, a computational technique that renders a voxel‐wise map of longitudinal volumetric tissue changes. We hypothesized that cognitive training would contravene some of the local reductions in gray matter volume associated with ADHD.
METHODS
Subjects and Treatment Protocol
The subjects were referred from outpatient clinics at Vall d'Hebron hospital. All subjects met the DSM‐IV diagnostic criteria for ADHD combined subtype, assessed by means of semi‐structured diagnostic interviews. Our exclusion criteria comprised comorbidity with neurological disorders, cerebral damage, extreme prematurity, other psychiatric disorders, and low IQ's (<80, WISC‐R). The subjects had never been exposed to cognitive training, and they were either medication‐naive or medication‐free for at least 15 days prior to their participation. For this study, 27 children were recruited. However, five subjects had to be excluded from the sample due to inability to attend all training and MRI sessions. In addition, a radiologist performed a visual inspection of the obtained T1‐weighted MRI scans to check for sufficient quality in both the pre and post‐training scans for inclusion in a longitudinal anatomical analysis. Thereupon, five subjects were removed from the data, reducing our final subject group to 18 ADHD children for the tensor‐based morphometric analysis. The sample consisted of two ADHD groups, with matching demographic characteristics for age, IQ, Conner's scores, and prior exposure to medication (Independent Samples T‐test. M ± SD. Contr: age: 11.22 ± 3.11; IQ: 110.67 ± 16.4; 8 M/1 F; Conners: 87.46 ± 12.13; 3 medication‐naive patients. Exp: age: 11.33 ± 2.60; IQ: 109.11 ± 19.70; 7 M/2 F; Conners: 90.00 ± 3.71; 3 medication‐naive patients. All P‐values are >0.6).
A standard Stroop color and word test was administered before and after the training period, to obtain a pre and post‐training measure of cognitive performance on a task that was not included in the training program and has been associated with altered performance in ADHD children [for review, see Lansbergen et al.,2007], for implementation in behavioral and regression analyses. The Stroop test comprised a condition of color words printed in black, a condition of “XXXX” printed in color, and a condition of color words printed in incongruent colors [Golden,2001; Stroop,1935]. The pre‐training Stroop interference values were subtracted from the post‐training values to obtain a difference variable for the regression analyses.
Our subjects participated in a 2‐week training program, provided in an ambulatory setting. The experimental group was subjected to cognitive training, a training method directly targeting cognitive skills, which was originally developed to enhance cognitive rehabilitation after cerebral damage [Cicerone et al.,2000]. This method comprises one of the standard training programs provided to ADHD patients, and behavioral studies have observed improvement in symptoms and cognitive performance in ADHD patients after cognitive training [for review, see Toplak et al.,2008]. Treatment was conducted by 1 of 2 trained therapists, and the program comprised daily 45‐min training sessions. We chose a program of cognitive training that has been extensively implemented in the collaborating clinics to treat children with ADHD [García‐Sánchez,2001; García‐Sánchez and Estévez‐González,2002]. The cognitive training program comprised the implementation of paper and pencil exercises designed to stimulate several areas of higher‐order cognitive functioning. Before each exercise, the child was asked to read the accompanying task instructions and explain the task to the therapist, receiving further directions from the therapist if needed. The processes targeted by the cognitive training program are working memory, cognitive flexibility, attention, planning, and problem solving [García‐Sánchez,2001; García‐Sánchez and Estévez‐González,2002]. Some examples of tasks used during these sessions are labyrinths (planning), word list recall (memory), detecting the missing numbers from numerical lists (attention), creating lists of objects sharing certain characteristics (cognitive flexibility), and code deciphering (problem solving). The treatment manuals incorporated tasks designed for different age groups, and the tasks were selected accordingly [García‐Sánchez,2001; García‐Sánchez and Estévez‐González,2002]. We implemented the tasks in the order as proposed in the treatment manuals. The tasks were not arranged according to the targeted cognitive process, but were presented intermixedly to enhance the diversity of the session and maintain the interest of the subjects. Most of the tasks relied on multiple cognitive functions, and the implementation of the tasks was balanced to stimulate each of the indicated cognitive processes rather than unevenly bearing on one of the target functions. The duration of the individual tasks was variable and depended on the extent of the specific task and the performance of the subject.
In addition, we implemented a control training program to account for the effects of participation in a training program per se. The control training was provided with identical frequency and duration as the experimental training sessions. This allows us to control for attendance to a 2‐week training program, although we cannot exclude an effect of some remaining variability between the groups in the implementation method, like a more interactive approach and less task‐oriented methodology in the control training as compared with the cognitive program. During the control training sessions, the children were provided with strategies for social problems. By implementing this program, we aimed to include a potential benefit for the ADHD children assigned to the control group without directly stimulating cognitive functions via the performance of cognitively challenging tasks. The main goal of this program was to aid in the differentiation between aggressive, passive, and assertive behavior, to explain the meaning and value of interpersonal skills, and to address the expression of negative emotions such as aggravation or uneasiness. The sessions included the presentation of information on social rules and standards, for instance by watching an informative video or reading a situation sketch, and examples were implemented to illustrate these concepts, e.g., via discussion or role‐playing. The examples focussed on situations that may provide problems in everyday life for ADHD children.
The study was approved by the Hospital Universitari Vall d'Hebron Ethics Committee, and informed consent was obtained from the children as well as the parents or legal guardians.
Acquisitions and Analyses
MRI acquisitions were performed on a Philips head‐only 1.5T scanner, equipped with a standard quadrature radiofrequency coil. A high‐resolution T1‐weighted image was acquired using a fast spoiled gradient recalled pulse sequence (TR = 20 ms, TE = 4.6 ms, FA = 30°, matrix size 256 × 256, 100 slices, voxel size 0.86 × 0.86 × 1.4 mm3).
Behavioral analyses were performed in SPSS, and MRI image processing and statistical analyses were conducted with Statistical Parametric Mapping software (SPM2; http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab (version 7.0; http://www.mathworks.com). Changes in regional gray matter volume were analyzed using a voxel‐based morphometric approach optimized for longitudinal analyses. Voxel‐based morphometry is a fully automated technique designed to evaluate differences in local brain anatomy [Ashburner and Friston,2000]. We applied a voxel‐based morphometric procedure incorporating tensor‐based morphometric elements, which is optimized for the analysis of longitudinal MRI studies, and has proved a sensitive technique for capturing within‐subject changes in brain structure [e.g., Agosta et al.,2009; Brambati et al.,2007; Moorhead et al.,2009]. This procedure involves deriving a voxel‐wise estimation of regional tissue volume change from the deformation field of the warp between the pre and post‐training images, allowing the evaluation of longitudinal volumetric differences. A schematic illustration of the processing steps applied to the data is provided in Supporting Information Figure 1.
First, the T1‐weighted postimage was registered to the preimage, using a rigid body registration. Subsequently, high‐dimensional warping was performed to model the deformations between the images per subject. This step rendered a deformation field that reflects the contractions and expansions required to provide a voxel‐by‐voxel mapping between the two images. The high‐dimensional warping involved a regularization parameter of four, and eight iterations of the algorithm were performed. To quantify volumetric differences between these images based on the high dimensional warp, we extracted the map of jacobian determinants, which provides the deformation gradient at each voxel. The pre‐image was then segmented, rendering gray matter, white matter, and cerebrospinal fluid partitions. During the segmentation step, sample‐specific tissue prior probability maps were applied to replace the default adult‐based tissue priors, which are less suitable for pediatric samples. Next, we used a 12‐parameter affine transformation algorithm followed by nonlinear warping to register the gray matter segment to a gray matter ADHD children template created for this study [based on the subject sample described in Carmona et al.,2009], aiming to optimize the normalization for the tissue of interest and ensure high‐resolution transformation. The resulting transformation matrix was then applied to the preimage and to the jacobian determinants obtained from the high‐dimensional warp. Subsequently, a product image was calculated by multiplying the gray matter tissue segments (i1) voxel by voxel by the high‐dimensional warp jacobian determinant (i2) according to the formula i1*(i2‐1), producing images that represent a measure of the tissue‐specific volume changes between the two longitudinal scans. We applied a correction to the product images to preserve the original tissue volume, by multiplying the intensity value of each gray matter voxel by the jacobian determinants obtained in the normalization step, allowing us to make comparisons of gray matter volume rather than concentration. Finally, the modulated product images were smoothed by imposing a 12‐mm full‐width half maximum isotropic Gaussian kernel and a tissue‐specific binary mask on the space domain [Brambati et al.,2007].
The general linear model was used for statistical inference, and the output images for each group were entered into the design matrix, representing the regressors of interest. Optimal parameter estimates were computed using a least squares function. The linear contrasts “experimental group > control group” and “control group > experimental group” were applied to estimate effect sizes and generate statistical parametric maps, incorporating an extent threshold of 10 contiguous voxels. All whole‐brain statistical maps were constructed by applying a per‐voxel probability below 0.001 (uncorrected). Randomization experiments have supported the validity of uncorrected statistical tests with 12 mm smoothed data in whole‐brain voxel‐based morphometric analyses [Ashburner and Friston,2000], and this threshold is frequently applied in anatomical MRI studies [e.g., Cheng et al.,2009; Harvey et al.,2007; Newman et al.,2007].
To assess if the changes in gray matter volume observed after cognitive training were associated with demographic and behavioral variables (age, IQ, post‐pre Stroop performance), we performed regression analyses on the data and applied regions of interest of the clusters significantly affected by cognitive training in the whole‐brain analyses (indicated in Table I). These regression analyses incorporated the output images of the experimental group, reflecting the individual longitudinal volume changes in the brain between the pre and post‐training scans. To correct for multiple comparisons, we applied FWE‐correction to the regression analyses.
Table I.
Results of whole‐brain tensor‐based morphometric analysis
| Brain region | Talairach | Cluster size (mm3) | t | P | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Experimental > control | R middle frontal cortex | 28 | 6 | 52 | 114 | 4.47 | <0.001 |
| L middle frontal cortex | −26 | 3 | 60 | 61 | 4.14 | <0.001 | |
| R inferior–posterior cerebellum | 14 | −54 | −48 | 21 | 3.75 | <0.001 | |
| Control > experimental | – | ||||||
The general linear model incorporated the individual output images, representing a measure of regional longitudinal volume change in brain morphology between the pre‐ and post‐training scans. The table indicates regions of increased gray matter volume after the cognitive training period compared with the ADHD control group, obtained by applying the contrast “experimental group > control group”. The contrast “control group > experimental group”, assessing if there are voxels that show an increase of gray matter volume after the training period in the group receiving control training compared with the group receiving cognitive training, did not render significant results.
RESULTS
To chart local changes in gray matter volume, we applied a tensor‐based morphometric approach to the high‐resolution anatomical MRI images. The resulting output images, reflecting subject‐specific longitudinal volumetric changes in brain structure, were entered into a general linear model, and whole‐brain analyses were performed to map the differences between the groups. For the contrast “experimental > control”, comparing the volumetric changes after the training period between the groups, we observed selective focal clusters in bilateral middle frontal cortex and right inferior‐posterior cerebellum (Table I, Fig. I). These results indicate a volumetric increase in frontal and cerebellar gray matter in the group subjected to cognitive training compared with the control group. The contrast “control > experimental” did not yield significant results.
Figure 1.
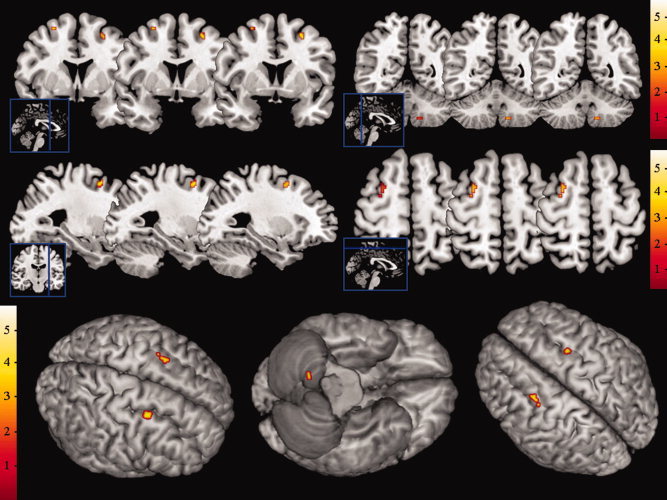
Statistical parametric maps depicting focal increases in gray matter volume after cognitive training compared with the ADHD control group, super‐imposed on anatomical slices and brain surface maps. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To assess if the changes in gray matter volume after the cognitive training period were associated with cognitive or demographic variables, we performed regression analyses on the data obtained from the experimental group. These regression analyses incorporated the tensor‐based morphometric output images, reflecting the subject‐specific morphological alterations after the cognitive training period. Regions of interest representing the suprathreshold clusters of the whole‐brain tensor‐based morphometric analyses were applied to restrict the results to the regions that were significantly affected by the training.
Although the two subject groups were carefully matched for age and IQ (M ± SD. Independent Samples T‐test. Age: exp: 11.33 ± 2.60, contr: 11.22 ± 3.11; T = 0.08, P = 0.94. IQ: exp: 109.11 ± 19.70, contr: 110.67 ± 16.42; T = 0.18, P = 0.86), we aimed to assess if within‐group differences in age and IQ were associated with the extent of the training‐induced effects on brain morphology.
A regression analysis incorporating the age of the subjects revealed a positive correlation with the gray matter volume changes in the left and right middle frontal cortex (Left frontal middle: −25x3y59z, T = 2.78, 28 mm3, P < 0.05, FWE‐corrected. Right frontal middle: 28x8y53z, T = 3.67, 28 mm3, P < 0.05, FWE‐corrected, Fig. 2), indicating that the changes in bilateral frontal gray matter after cognitive training within the experimental group were most pronounced in the older children.
Figure 2.
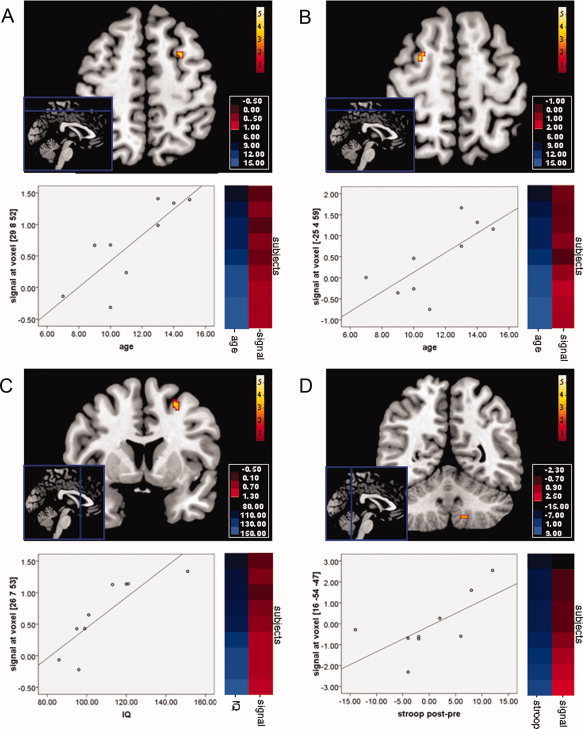
Results of the regression analyses between the individual output images, representing subject‐specific longitudinal volume changes in brain morphology after cognitive training, and age (panel A and B), IQ (panel C), and Stroop performance (panel D). Each panel depicts a statistical parametric map, a scatterplot, and an intensity color plot that illustrates the distribution of the variables across subjects (Cluster 3.0, Java Treeview). Shades of blue indicate the subjects' age, IQ, or change in Stroop performance after the training period, while red colors reflect the individual MRI signal change after cognitive training in this region. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In addition, we found a positive correlation between IQ and right middle frontal gray matter volume change (25x7y53z, T = 5.24, 87 mm3, P < 0.05, FWE‐corrected, Fig 2), which suggests that cognitive training manifested stronger morphological alterations in the right frontal cortex in children with higher IQ's.
We also performed a regression analysis involving a measure of cognitive performance, namely the interference rates of the Stroop task that was performed before and after the training period. We entered the post‐pre difference variable of Stroop performance obtained from the children in the experimental group into the regression model to assess if, although we did not observe significant differences in Stroop performance (M ± SD. Paired Samples T‐test. Pre: 49.56 ± 8.41, Post: 49.78 ± 12.10. t = −0.09., P > 0.05), we could identify an association between within‐group differences in cognitive performance and regional volumetric increase. We found a positive correlation between Stroop performance and gray matter volume change in the inferior–posterior cerebellum (16x‐54y‐47z, T = 2.37, 22 mm3, P < 0.05, FWE‐corrected, Fig. 2), suggesting that children with relative improvement in Stroop performance were characterized by more pronounced morphological increases in the inferior–posterior lobule of the cerebellum after cognitive training.
DISCUSSION
Our whole‐brain analyses indicate focal increases in gray matter volume after cognitive training in the right posterior–inferior cerebellar lobule and bilateral dorsolateral prefrontal cortex, more specifically left and right middle frontal cortex, in comparison with the control group. Frontal lobe changes after training were more pronounced in the children in the experimental group of higher IQ and age, and the extent of gray matter expansion in the inferior‐posterior cerebellum was associated with Stroop performance.
Converging evidence on ADHD has detected a constellation of focal biochemical, morphological and functional aberrations in the ADHD brain, predominantly localized to the frontal lobes, striatum and cerebellum [for reviews, see Biederman and Faraone,2005; Giedd et al.,2001; Krain and Castellanos,2006; Nigg and Casey,2005]. The frontal lobes play a central role in the disease, and anatomical studies measuring frontal lobe volume have observed volumetric reductions in ADHD children, predominantly pinpointed to dorsolateral prefrontal and orbitofrontal regions [Nigg and Casey,2005; Sowell et al.,2003; Van't Ent et al.,2007]. The cerebellum is also characterized by robust volumetric gray matter reductions, thought to be directly related to ADHD pathophysiology [Castellanos et al.,2002; Giedd et al.,2001; Krain and Castellanos,2006; Mackie et al.,2007; Nigg and Casey,2005; Valera et al.2007]. Moreover, the inferior–posterior lobule of the cerebellum is a site of progressive volume loss, and its volumetric properties are associated with the clinical outcome of the patients [Mackie et al.,2007].
Along with a constellation of other brain regions, the frontal lobes and cerebellum have been associated with the functions targeted by our cognitive training program. The frontal lobes support a multitude of higher‐order cognitive processes, and play a central role in executive functions like attention, working memory, and planning. With respect to the cerebellum, although this structure has traditionally been regarded as a neural device dedicated to motor control, research findings indicate a role for the cerebellum in the acquisition and modulation of cognitive processes by remodulating cortical connections and adjusting the responsiveness in other brain systems via cerebello‐thalamico and cortico‐pontine‐cerebellar feedback and feed‐forward loops [Akshoomoff et al.,1997; Steinlin,2007].
Although learning‐based plasticity has typically been associated with changes in synaptic strength, increasing evidence shows that training can also render dynamic structural alterations in the nervous system [for review, see Holtmaat and Svoboda,2009]. Although few studies have assessed training‐induced structural plasticity in the human brain, results have consistently demonstrated local increases of gray matter volume in task‐related regions, which already surface after very short training periods [Ceccarelli et al.,2009; Draganski et al.,2004,2006; Driemeyer et al., 2008; Ilg et al.,2008]. This capacity for training‐induced plastic changes in gray matter volume suggests that local gray matter deficits associated with certain disorders may be partially contravened by training, and our study assessed if a training program applied to a psychiatric patient group can target structures of gray matter volume reduction. The results are in line with previous studies of morphological plasticity, observing localized clusters of relative gray matter volume expansion after training. These findings demonstrate the proneness of the brain to undergo rapid changes in morphology when triggered by cognitive learning.
Moreover, the clusters of volumetric change in our study were confined to brain regions typically characterized by volume reduction in ADHD patients, including a cerebellar substructure associated with symptom‐related progressive volume loss. These results suggest that cognitive training may contravene some of the structural brain deficits associated with the disorder. However, an important limitation of our study resides in the relatively modest sample size, and future research should replicate and further elucidate the current findings, potentially also including follow‐up sessions to assess whether the detected morphological alterations are of a transient, stable or continuous nature.
A previous functional MRI study from our group assessing the effects of cognitive training on neural activity in ADHD children also detected signal increases in frontal and cerebellar regions [Hoekzema et al.,2010]. However, it should be noted that, although adjacent, the clusters of gray matter volumetric increase observed in the current study did not overlap with the fronto‐cerebellar regions of enhanced neural activity, which were confined to the right inferior frontal cortex, left superior frontal cortex, orbitofrontal cortex and right superior posterior cerebellum. We can conjecture that the clusters of volumetric increase reflect the regions principally targeted by the cognitive training program, while the fMRI study detected changes in the regions most strongly stimulated by the fMRI tasks, which were slightly different from the cognitive training tasks to restrict the repetition bias in the fMRI results. However, we can only speculate on the source of the observed discrepancy. Perhaps future studies that further define the cognitive contributions of distinct frontal and cerebellar substructures may help explain this lack of overlap. Moreover, further research is needed to assess whether training‐induced volumetric changes underpin symptom relief or cognitive improvement, an important issue that was not addressed in the current study.
The changes in gray matter volume detected by anatomical MRI studies reflect modifications at the cellular level, but the histological processes underlying these macroscopic findings remain to be elucidated. A cellular process that can be manifested over very short periods of time and has been associated with learning‐based plasticity is spino and synaptogenesis [Basarsky et al.,1994; Holtmaat and Svoboda,2009]. Increasing evidence indicates that, in addition to adjustments in synaptic strength, experience‐based plasticity involves rapid turnover of dendritic spines and axonal boutons, accompanied by the formation of new synapses [Holtmaat and Svoboda,2009]. In line with previous anatomical MRI studies on structural plasticity [Draganski et al.,2006; Ilg et al.,2008; May et al.,2007], we hypothesize that the changes in gray matter volume observed in our study reflect changes in neuronal morphology, such as sprouting of dendritic and axonal arbors, accompanied by synaptic remodeling within the task‐related regions. However, these speculations remain to be confirmed, and animal research combining histological measures with in‐vivo imaging results should further define the microscopic processes underlying the observed changes in gray matter volume.
To resume our findings, we report focal gray matter volume increases after cognitive training in bilateral middle frontal cortex and right inferior–posterior lobule of the cerebellum compared to the control group. These findings are in line with previous studies on morphological brain plasticity, and illustrate the capacity of the nervous system for rapid morphological adjustments in response to changes in cognitive demands. Moreover, the clusters of volumetric increase observed in our study were confined to brain structures commonly associated with volumetric reduction in ADHD children. These findings provide preliminary indications that training of cognitive functions may contravene some of the neuroanatomical deficits associated with the disorder. Our results stress the need for further research on the potency of training programs to target local reductions in gray matter volume.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1: Schematic representation of tensor‐based morphometric approach. This figure illustrates the preprocessing steps (explained in detail in the main manuscript) that were applied to our data. Orange arrows represent computational processing steps, while the blue arrows reflect output images of a previous processing step.
Acknowledgements
The authors thank Joanna Kyra for providing training sessions to the participants.
REFERENCES
- Agosta F, Gorno‐Tempini ML, Pagani E, Sala S, Caputo D, Perini M, Bartolomei I, Fruguglietti ME, Filippi M ( 2009): Longitudinal assessment of grey matter contraction in amyotrophic lateral sclerosis: A tensor based morphometry study. Amyotroph Lateral Scler 10: 168–174. [DOI] [PubMed] [Google Scholar]
- Akshoomoff NA, Courchesne E, Townsend J ( 1997): Attention coordination and anticipatory control. Int Rev Neurobiol 41: 575–598. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2000): Voxel‐based morphometry—The methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Basarsky TA, Parpura V, Haydon PG ( 1994): Hippocampal synaptogenesis in cell culture: Developmental time course of synapse formation, calcium influx, and synaptic protein distribution. J Neurosci 14: 6402–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV ( 2005): Attention‐deficit hyperactivity disorder. Lancet 366: 237–248. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Renda NC, Rankin KP, Rosen HJ, Seeley WW, Ashburner J, Weiner MW, Miller BL, Gorno‐Tempini ML ( 2007): A tensor based morphometry study of longitudinal gray matter contraction in FTD. Neuroimage 35: 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM ( 1998): Cortical plasticity: From synapses to maps. Annu Rev Neurosci 21: 149–186. [DOI] [PubMed] [Google Scholar]
- Callan DE, Tajima K, Callan AM, Kubo R, Masaki S, Kahane‐Yamada R ( 2003): Learning‐induced neural plasticity associated with improved identification performance after training of a difficult second‐language phonetic contrast. Neuroimage 19: 113–124. [DOI] [PubMed] [Google Scholar]
- Carmona S, Proal E, Hoekzema EA, Gispert JD, Picado M, Moreno I, Soliva JC, Bielsa A, Rovira M, Hilferty J, Bulbena A, Casas M, Tobeña M, Vilarroya O ( 2009): Ventro‐striatal reductions underpin symptoms of hyperactivity and impulsivity in attention‐deficit/hyperactivity disorder. Biol Psychiatry 66: 972–977. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL ( 2002): Developmental trajectories of brain volume abnormalities in children and adolescents with attention‐deficit/hyperactivity disorder. JAMA 288: 1740–1748. [DOI] [PubMed] [Google Scholar]
- Ceccarelli A, Rocca MA, Pagani E, Falini A, Comi G, Filippi M ( 2009): Cognitive learning is associated with gray matter changes in healthy human individuals: A tensor‐based morphometry study. Neuroimage 48: 585–589. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chou KH, Decety J, Chen IY, Hung D, Tzeng OJ, Lin CP ( 2009): Sex differences in the neuroanatomy of human mirror‐neuron system: A voxel‐based morphometric investigation. Neuroscience 158: 713–720. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, Felicetti T, Giacino JT, Harley JP, Harrington DE, Herzog J, Kneipp S, Laatsch L, Morse PA ( 2000): Evidence‐based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil 81: 1596–1615. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A ( 2004): Neuroplasticity: Changes in grey matter induced by training. Nature 427: 311–312. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A ( 2006): Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 26: 6314–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A ( 2008): Changes in gray matter induced by learning–revisited. PLoS One 3: e2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Sánchez C ( 2001): El Juego de la Atención. Barcelona: Lebon. [Google Scholar]
- García‐Sánchez C, Estévez‐González A ( 2002): Estimulación Cognitiva‐II. Barcelona: Lebon. [Google Scholar]
- Giedd JN, Blumenthal J, Molloy E, Castellanos FX ( 2001): Brain imaging of attention deficit/hyperactivity disorder. Ann N Y Acad Sci 931: 33–49. [DOI] [PubMed] [Google Scholar]
- Golden CJ ( 2001): Stroop, Test de Colores y Palabras. Madrid: TEA Ediciones. [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ ( 1999a): Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2: 260–265. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, Lepage M ( 2007). Individual differences in trait anhedonia: A structural and functional magnetic resonance imaging study in non‐clinical subjects. Mol Psychiatry 12: 767–775. [DOI] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Tremols V, Gispert JD, Guitart M, Fauquet J, Rovira M, Bielsa A, Soliva JC, Tomas X, Bulbena A, Ramos A, Casas M, Tobeña A, Vilarroya O ( 2010): Enhancement of frontal and cerebellar circuits after cognitive training in children with attention‐deficit/hyperactivity disorder. Hum Brain Mapp. DOI: 10.1002/hbm.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K ( 2009): Experience‐dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10: 647–658. [DOI] [PubMed] [Google Scholar]
- Ilg R, Wohlschlager AM, Gaser C, Liebau Y, Dauner R, Woller A, Zimmer C, Zihl J, Muhlau M ( 2008): Gray matter increase induced by practice correlates with task‐specific activation: A combined functional and morphometric magnetic resonance imaging study. J Neurosci 28: 4210–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX ( 2006): Brain development and ADHD. Clin Psychol Rev 26: 433–444. [DOI] [PubMed] [Google Scholar]
- Lansbergen MM, Kenemans JL, van Engeland H ( 2007): Stroop interference and attention‐deficit/hyperactivity disorder: A review and meta‐analysis. Neuropsychology 21: 251–262. [DOI] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF III, Sharp WS, Giedd JN, Rapoport JL ( 2007): Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry 164: 647–655. [DOI] [PubMed] [Google Scholar]
- May A, Hajak G, Ganssbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P ( 2007): Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cereb Cortex 17: 205–210. [DOI] [PubMed] [Google Scholar]
- Moorhead TW, Stanfield A, Spencer M, Hall J, McIntosh A ( 2009): Progressive temporal lobe grey matter loss in adolescents with schizotypical traits and mild intellectual impairment. Psychiatry Res 174: 105–109. [DOI] [PubMed] [Google Scholar]
- Newman LM, Trivedi MA, Bendlin BB, Ries ML, Johnson SC ( 2007): The relationship between gray matter morphometry and neuropsychological performance in a large sample of cognitively healthy adults. Brain Imaging Behav 1: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ ( 2005): An integrative theory of attention‐deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol 17: 785–806. [DOI] [PubMed] [Google Scholar]
- Poldrack RA ( 2000): Imaging brain plasticity: Conceptual and methodological issues—A theoretical review. Neuroimage 12: 1–13. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JD ( 2001): Characterizing the neural mechanisms of skill learning and repetition priming: Evidence from mirror reading. Brain 124: 67–82. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E ( 2001): Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372–376. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS ( 2003): Cortical abnormalities in children and adolescents with attention‐deficit hyperactivity disorder. Lancet 362: 1699–1707. [DOI] [PubMed] [Google Scholar]
- Steinlin M ( 2007): The cerebellum in cognitive processes: Supporting studies in children. Cerebellum 6: 237–241. [DOI] [PubMed] [Google Scholar]
- Stroop JR ( 1935): Studies of interference in serial verbal reactions. J Exp Psychol 18: 643–662. [Google Scholar]
- Toplak ME, Connors L, Shuster J, Knezevic B, Parks S ( 2008): Review of cognitive, cognitive‐behavioral, and neural‐based interventions for Attention‐Deficit/Hyperactivity Disorder (ADHD). Clin Psychol Rev 28: 801–823. [DOI] [PubMed] [Google Scholar]
- Tremols V, Bielsa A, Soliva JC, Raheb C, Carmona S, Tomas X, Gispert JD, Rovira M, Fauquet J, Tobeña A, Bulbena A, Vilarroya O ( 2008): Head and body caudate differential abnormalities in ADHD: A MRI manual ROI‐based study. Psychiatr Res Neuroimaging. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ ( 2007): Meta‐analysis of structural imaging findings in attention‐deficit/hyperactivity disorder. Biol Psychiatry 61: 1361–1369. [DOI] [PubMed] [Google Scholar]
- Van't Ent D, Lehn H, Derks EM, Hudziak JJ, Van Strien NM, Veltman DJ, De Geus EJ, Todd RD, Boomsma DI ( 2007): A structural MRI study in monozygotic twins concordant or discordant for attention/hyperactivity problems: Evidence for genetic and environmental heterogeneity in the developing brain. Neuroimage 35: 1004–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1: Schematic representation of tensor‐based morphometric approach. This figure illustrates the preprocessing steps (explained in detail in the main manuscript) that were applied to our data. Orange arrows represent computational processing steps, while the blue arrows reflect output images of a previous processing step.


