Abstract
We used fMRI to investigate both common and differential neural mechanisms underlying two distinct types of switching requirements, namely switching between stimulus categorizations (color vs. form) and switching between response modalities (hand vs. foot responses). Both types of switching induced similar behavioral shift costs. However, at the neural level, switching between stimulus categorizations led to left‐hemispheric activations including the inferior frontal gyrus as well as the intraparietal sulcus extending to the superior parietal gyrus and the supramarginal gyrus. In contrast, switching between response modalities was associated mainly with left‐hemispheric activation of the intraparietal sulcus and the supramarginal gyrus. A conjunction analysis indicated common activation of the left intraparietal sulcus and the supramarginal gyrus for both types of switching. Together, these results qualify previous claims about a general role of the left prefrontal cortex in task control by suggesting that the left inferior frontal gyrus is specifically involved in switching between stimulus categorizations, whereas parietal cortex is more generally implicated in the selection of action rules. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: action selection, prefrontal cortex, response modalities, functional imaging, cognitive control
INTRODUCTION
One of the most important human skills is the ability to quickly adapt to changing situations, to switch from one task to another, and to correctly select task‐relevant actions ‐ processes usually referred to as “cognitive control.” Task‐switching paradigms allow to examine cognitive control [for reviews see e.g., Kiesel et al., 2010; Koch et al., 2010; Monsell, 2003; Vandierendonck et al., 2010]: converging evidence suggests that performance declines when a task needs to be changed compared to when a task is performed repeatedly [e.g., Allport et al., 1994; Meiran, 1996].
In most studies, task switching has been examined by asking subjects to switch between different stimulus categories (e.g., color vs. form). Yet, a cognitive task representation includes various components ranging from stimulus encoding to response execution [e.g., Rogers and Monsell, 1995; Vandierendonck et al., 2008]. Consequently, switching between motor‐related components, like response modalities, also constitutes a type of task switching. Hence, response‐modality switching constitutes an additional means to investigate task switching [Philipp and Koch, 2005, 2010, 2011] and its underlying neural mechanisms. The present fMRI study was aimed to elucidate stimulus‐categorization switching and response‐modality switching to examine differential neural mechanisms underlying the two types of switching.
Previous fMRI studies suggest that both frontal and parietal regions play a crucial role in task switching [e.g., Braver et al., 2003; Crone et al., 2006; Dove et al., 2000; Liston et al., 2006; Sohn et al., 2000; Sylvester et al., 2003; Yeung et al., 2006]. More specifically, the areas involved consist of prefrontal cortex and inferior parietal cortex – areas known to be involved in cognitive control [see, e.g., Bunge et al., 2002; Collette and Van der Linden, 2002; Stephan et al., 2003; see also Cabeza and Nyberg, 2000, for a review]. While the prefrontal cortex has been suggested to exert top‐down control to maintain or to update task representations [cf. Miller and Cohen, 2001], the inferior parietal cortex is assumed to be relevant for transformations of stimulus representations into associated response codes [cf. Andersen et al., 1997; Culham and Kanwisher, 2001]. This dissociation is supported by fMRI data reported by Bunge et al. [ 2002] who used an Eriksen flanker task. In that study, the (left) parietal cortex activation was associated with stimulus‐based activation of responses, while a bilateral prefrontal activation was attributed to context‐related resolution of conflict.
According to this functional dissociation, the activation pattern observed in task switching is likely to differ depending on the type of switching. Wager et al. [ 2004] addressed this question in a meta‐analysis: Although the typical fronto‐parietal activation pattern was observed in different types of task switching, the location of peak activations differed depending on the type of switching [see also Rushworth et al., 2001b; Rushworth et al., 2002]. Interpretation of these differences is, however, difficult since different types of switching were compared across subjects and/or across studies. Furthermore, the task requirements in the different types of switching were very heterogeneous.
To elucidate the neural mechanisms underlying task switching, we therefore compared different types of switching within an identical setting. In contrast to previous studies, the present study compared two types of task switching within subjects. The experimental design ensured identical numbers of stimulus‐response (SR) or action rules across both types of switching (i.e., between stimulus categorizations and response modalities). Both kinds of switches involved the selection of the correct action rule and of a correct response. The two types of switches differed, however, with regard to whether task‐relevant action rules were either updated in terms of stimulus categorizations or response modalities. Given that updating cognitive components (i.e., stimulus categorization) and motor‐related components (i.e., response modalities) involves different types of cognitive control processes, we hypothesized that the two types of switching would differ with regard to their underlying neural mechanisms (and hence with respect to the brain areas involved). In addition, we also expected processes more generally involved in task‐switching and hence related to both types of task switching. Accordingly, a conjunction analysis was expected to also reveal common activations for the two types of task switching.
METHOD
Subjects
Twenty‐three subjects (15 female, 8 male, mean age = 26 years) were tested and received 30 € remuneration for participation. Written informed consent was obtained from all subjects. The study was approved by the Ethics Committee of the University Hospital of Aachen and conducted in accordance with the Declaration of Helsinki (revised version).
Stimuli, Tasks, Procedures, and Design
Subjects were required to judge the color or the form of a stimulus. The stimulus was either a circle (diameter 1.02° visual angle) or a square (1.02° × 1.02° visual angle), presented in red or blue color. Each stimulus was presented within a rectangular white frame (3.6° × 3.6° visual angle) in the middle of a black screen. Visual stimuli were presented using a Silent Vision fiberoptic system (Avotec, FL). Subjects' responses were given by pressing a response key with the right / left index finger or with the right/left foot via Lumitouch optical keypads (Photon Control, CA).
The experiment included two different types of task switching. In one type (“stimulus categorization”), subjects switched between color and form categorization while the response modality was constant in each block but differed between blocks. In the other type (“response modality”), subjects switched between finger and foot responses while stimulus categorization was constant in each block but differed between blocks. This design resulted in four different block types, which were randomly intermingled: (1) Switching between color and form categorizations, finger responses; (2) Switching between color and form categorizations, foot responses; (3) Color categorization, switching between finger and foot responses; and (4) Form categorization, switching between finger and foot responses. An example trial sequence for block types 1 and 3 is depicted in Figure 1.
Figure 1.
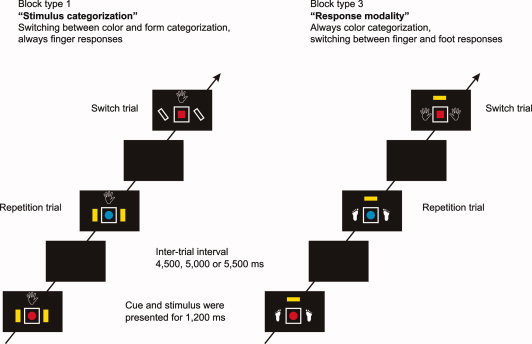
Example trial sequence for block type 1 (switching between color and form, finger responses) and block type 3 (color, switching between finger and foot responses). The stimulus categorization color was indicated by a yellow square, form was indicated by the outline of a parallelogram. Finger responses were indicated by a black and white drawing of a hand, foot responses were indicated by a black and white drawing of a foot. The cues on the right and left side of the frame indicated the variable task component (e.g., the stimulus categorization in stimulus‐categorization switching blocks), the cue above the frame indicated the constant task component (e.g., the response modality in stimulus‐categorization switching blocks). Please note that the cues were always presented 200 ms before the stimulus (i.e., blue or red circle or square). This cue‐stimulus interval is not depicted in the figure.
Before each block, subjects were informed about the constant task component in the next block of trials. In stimulus‐categorization switching blocks, in which the response modality was constant, a written instruction informed subjects to respond with either finger or foot responses in a given block of trials. In response‐modality switching blocks, in which the stimulus categorization was constant, subjects were instructed to attend always to the color or to the form of the stimulus, respectively. Additionally, in each block, the constant task component was indicated by a cue above the stimulus frame. Finger responses were indicated by a black and white drawing of a hand (2.0° × 2.1° visual angle), foot responses were indicated by a black and white drawing of a foot (1.3° × 2.1° visual angle). The color categorization was indicated by a yellow rectangle (0.9° × 1.6° visual angle) and form categorization was indicated by the outline of a parallelogram (1.9° × 1.9° visual angle). Hence, each cue had a perceptual similarity to the task component it indicated. The same cues were used to indicate the variable task component in each trial (i.e., the relevant stimulus categorization in stimulus‐categorization switching blocks and the relevant response modality in response‐modality switching blocks). However, for the variable task component the cues were presented on the right and left side of the stimulus frame (see Fig. 1).
In each trial, the cues and the stimulus were presented with a stimulus onset asynchrony of 200 ms. The stimulus together with the cues remained on the screen for 1,200 ms. The intertrial interval (i.e., a black screen) was either 4,500 ms, 5,000 ms or 5,500 ms. Subjects' responses were registered until 1,000 ms after stimulus offset.
The functional imaging experiment was run in a single session with a duration of ∼ 37 min. In addition, subjects performed eight training blocks outside the MR scanner. This training session was identical to the actual experiment except that subjects were seated in front of a laptop computer and used different response devices. The experiment itself consisted of 16 blocks (i.e., four blocks of each block type) with 24 trials each. The sequence of trials and blocks was pseudo‐random with the restriction that repeat and switch trials in each condition appeared equally often within the experiment. A new sequence was generated for each subject. The SR mapping was counterbalanced between subjects.
For data analyses, we used Transition (switch trial vs. repetition trial) and Type of Switching (stimulus categorization vs. response modality) as within‐subject independent variables. The first two trials of each block were discarded from further data analyses as were trials with an RT below 200 ms. In addition, error trials as well as trials subsequent to error trials were excluded from the analysis.
fMRI Acquisition
Functional images were acquired by means of a 1.5‐Tesla Avanto MRI system (Siemens, Erlangen, Germany), using a T2*‐weighted echo planar (EPI) sequence (TR = 3.0 s, TE=60 ms). Seven hundred forty‐one volumes were obtained each consisting of 30 axial slices, allowing for whole brain coverage. Slice thickness was 4 mm and inter‐slice distance 0.4 mm, with a 20‐cm FOV and a 64 × 64 image matrix, and a voxel size of 3.1 × 3.1 × 4 mm3. Furthermore, for each subject high‐resolution anatomical images (voxel size 1 × 1 × 1 mm3) were acquired using a standard T1‐weighted 3D MP‐RAGE sequence.
Images were spatially realigned to the fifth volume (see below) to correct for inter‐scan head movement and normalized to the Montreal Neurological Institute (MNI) single subject template using the “unified‐segmentation” function in SPM5 (see below). This algorithm is based upon a probabilistic framework that permits image registration, tissue classification, and bias correction to be combined within the same generative model. The resulting parameters of a discrete cosine transform, which define the deformation field necessary to move the subject's data into the space of the MNI tissue probability maps [Evans et al., 1994], were then combined with the deformation field transforming between ‘MNI tissue probability maps’ and the MNI single‐subject template. The resulting deformation was subsequently applied to the individual EPI volumes. The data were then smoothed using a Gaussian kernel of 8‐mm full‐width half‐maximum.
fMRI Data Analysis
fMRI data were analyzed using the Statistical Parametric Mapping software SPM5 (Wellcome Department of Imaging Neuroscience, London; http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). The first four images were excluded from further analyses, as these were acquired within the time period the MR signal needs to reach a steady state. Slow signal drifts across the experiment were removed by applying a high‐pass filter with a cut‐off of 128 s. Onset regressors were separately defined for switch and repetition trials in both stimulus‐categorization and response‐modality switching blocks, indicating the onsets of individual trials. Error trials as well as post‐error trials were modeled in a separate regressor. The hemodynamic response to each type of event was modeled using a canonical synthetic hemodynamic response function (HRF) and its first derivative. The six head movement parameters were included as confounds.
First‐level linear baseline contrasts were calculated comparing each onset regressor with the implicit baseline (i.e., those time periods that were not explicitly modeled and where no event occurred). These contrasts were then taken to the second level, where they were subjected to a within‐subject repeated‐measures analyses of variance (ANOVA) with the variable Transition (switch trials vs. repetition trials) and the variable Type of Switching (stimulus categorization vs. response modality), using a corrected threshold of P < 0.05 at the cluster level (P < 0.001 cut‐off at the voxel level). In addition, the contrasts testing for an interaction are reported with a lowered threshold of P < 0.001 uncorrected (see below). Unequal variances between subjects and conditions were compensated for by non‐sphericity correction. Differential contrasts were calculated comparing switch trials with repetition trials separately for the stimulus‐categorization switching blocks and the response‐modality switching blocks. To test for common neural activations, the resulting (thresholded) T‐maps were subjected to a minimal statistics analysis, testing for the conjunction null hypothesis [Nichols et al., 2005; Friston et al., 2005].
To test whether switching between stimulus categorizations was different from switching response modalities, the interaction contrasts were calculated at a statistical threshold of P < 0.001 uncorrected (i.e. [Stimulus‐categorization switches minus Stimulus‐categorization repetitions] compared to [Response‐modality switches minus Response‐modality repetitions] and vice versa).
To test for a differential involvement of frontal and parietal brain areas in switching between stimulus categorizations and response modalities, BOLD‐amplitudes changes (as estimated by beta‐parameters) at different locations were further examined. Therefore, beta‐parameters elicited by the four different conditions at two locations in frontal and parietal cortex were subjected to a three‐way repeated measures ANOVA with the variables Brain regions (frontal vs. parietal), Transition (switch trial vs. repetition trial) and Type of Switching (stimulus categorization vs. response modality).
RESULTS
Behavioral Data
The RT and error rate data were examined by ANOVAs, with the variables Transition (switch trial vs. repetition trial) and Type of switching (stimulus categorization vs. response modality). For the results of the RT and error analyses see Table I.
Table I.
RT (in ms) and error percentage as a function of type of switching (stimulus categorization vs. response modality) and transition (switch vs. repetition)
| Transition | ||||
|---|---|---|---|---|
| Switch | Repetition | |||
| Mean | SD | Mean | SD | |
| RT (in ms) | ||||
| Stimulus‐categorization switching | 1183 | 326 | 1069 | 266 |
| Response‐modality switching | 1037 | 222 | 949 | 231 |
| Error percentage | ||||
| Stimulus‐categorization switching | 4.6% | 3.9% | 3.8% | 4.3% |
| Response‐modality switching | 6.3% | 6.7% | 5.0% | 6.0% |
The RT analysis revealed a main effect of Transition [F(1, 22) = 42.87; MSe = 5417; P < 0.001], indicating shift costs, and a main effect of Type of Switching [F(1, 22) = 23.891; MSe = 16993; P < 0.001], demonstrating higher RTs in the stimulus‐categorization switching blocks (1,126 ms) than in the response‐modality switching blocks (993 ms). The interaction of Transition and Type of Switching was not significant [F(1, 22) = 0.65; MSe = 6032; n.s]. That is, significant shift costs were observed in both types of switching. This result was confirmed in post hoc two‐tailed t‐tests comparing switch and repeat trials in stimulus‐categorization switching blocks [shift costs of 114 ms, t(22) = 4.4; P < 0.001] and in response‐modality switching blocks [shift costs of 88 ms, t(22) = 4.9; P < 0.001].
An analogous ANOVA of the error rates failed to reveal any significant effects: Transition: F(1, 22) = 3.75; MSe = 0.06307; n.s. (P = 0.065); Type of Switching: F(1, 22) = 3.15; MSe = 0.1489; n.s. (P = 0.089); Transition × Type of Switching: F(1, 22) = 0.16; MSe = 0.06845; n.s.. The trend for an effect of Transition supported the RT data, but the trend for an effect of Type of Switching opposed the RT data, suggesting a speed‐accuracy trade‐off (i.e., faster but less accurate responses in response‐modality switching than in stimulus‐categorization switching).
Taken together, the behavioral results showed comparable shift costs in both types of task switching. That is, a switch from one stimulus categorization to another resulted in higher RTs and error rates than the repetition of the relevant stimulus categorization [cf. Meiran, 1996; Rogers and Monsell, 1995]. Please note that with respect to the stimulus‐categorization switching blocks the stimulus always contains information for both categorizations so that two different responses may be activated by the stimulus. Consequently, “congruent” trials, in which the stimulus requires the same response in both categorizations, can be differentiated from “incongruent” trials, in which the stimulus requires different responses in both categorizations. However, congruency had no significant influence on switch costs in stimulus‐categorization switching blocks.1
Like the shift costs observed in stimulus‐categorization switching blocks, responding in a different response modality than in the previous trial resulted in higher RTs and more errors than a repetition of the response modality. The data thus support our assumption that switching between response modalities is a type of task switching – comparable to more established types of task switching like switching between stimulus categorizations [see also Philipp and Koch, 2005, 2010, 2011].
Furthermore, it is interesting to note that shift costs were not influenced by either the stimulus categorization (color vs. form in stimulus‐categorization blocks) or the response modality (hand vs. foot in response‐modality switching blocks).2 That is, both types of switching did not reveal shift‐cost asymmetries [cf. Allport et al., 1994], suggesting that we can cautiously conclude that also cognitive control demands in stimulus‐categorization switching and response‐modality switching are comparable.
Functional Imaging Data
We conducted whole brain analyses for the two types of task switching (stimulus categorization vs. response modality) individually as well as a conjunction analysis. The locations of significant activation clusters are shown in Table II.
Table II.
Significant neural activations in the whole brain analyses
| Structure | Cluster size (voxel) | T‐score | MNI |
|---|---|---|---|
| Cluster level 0.05 (P < 0.001 cutoff at the voxel level) | |||
| (a) Stimulus‐categorization switch > stimulus‐categorization repetition | |||
| Inferior frontal sulcus | 355 | 4.56 | Left (−44, 4, 30) |
| Superior parietal cortex | 905 | 4.85 | Left (−14, −66, 46) |
| (b) Response‐modality switch > response‐modality repetition | |||
| Intraparietal sulcus/Supramarginal gyrus | 152 | 4.17 | Left (−30, −46, 44) |
| (c) Conjunction: a ∩ b | |||
| Intraparietal sulcus/Supramarginal gyrus | 16 18 | 3.6 3.55 | Left (−44, −38, 46) Left (−40, −50, 46) |
| Interaction contrasts p < 0.001 uncorrected (voxel>10) | |||
| (d) Stimulus‐categorization (switch minus repetition) vs. Response‐modality (switch minus repetition) | |||
| Lateral orbital gyrus | 13 | 3.61 | Right (30, −56, 52) |
| Inferior frontal sulcus | 17 | 3.66 | Left (−46, 24, 34) |
| Temporoparietal junction | 56 | 4.57 | Left (−58, −50, 26) |
| 59 | 4.25 | Right (56, −52, 30) | |
| (e) Response‐modality (switch minus repetition) vs. Stimulus‐categorization (switch minus repetition) | |||
| Cingulate gyrus | 61 | 5.07 | Left (−12, −8, 32) |
| Long gyrus of the insula | 25 | 3.89 | Right (26, −16, 18) |
| Medial superior frontal gyrus | 13 | 3.8 | Left (−14, −12, 48) |
With respect to stimulus categorizations, the effect of switching was assessed by comparing functional imaging data related to stimulus‐categorization switch trials with stimulus‐categorization repetition trials (within stimulus‐categorization switching blocks, averaged across response modalities). This revealed a left lateralized pattern of activations involving two distinct clusters. One cluster was located in the left inferior frontal sulcus extending (in a posterior direction) to the junction with the precentral gyrus with its peak maximum located at the inferior frontal junction (−44, 4, 30). A large cluster of activation was observed in the left superior parietal lobe extending to the intraparietal sulcus and the supramarginal gyrus (Fig. 2).
Figure 2.
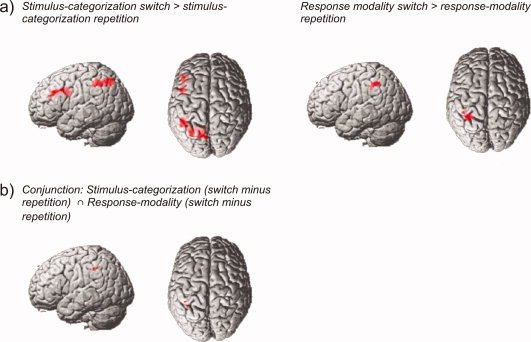
(a) Surface renderings of functional contrasts for brain areas showing increased activity along with switch compared to repetition trials, separately shown for stimulus‐categorization (left) and response‐modality blocks (right). (b) Surface renderings showing those brain areas that were significantly activated by switches in both the stimulus‐categorization as well as in the response‐modality blocks.
Switching between different response modalities was analysed by comparing trials where subjects had to switch their response modality (relative to a previous trial) with trials where subjects responded within the same modality (within response‐modality switching blocks, averaged across stimulus categorizations). This contrast revealed one large cluster of activation in left parietal cortex. This activation was centred upon the left intraparietal sulcus, extending to inferior parietal cortex (Fig. 2).
A minimum statistics conjunction analysis [Nichols et al., 2005] of the activations reported above revealed significant activation in the left intraparietal sulcus extending in an inferior direction to the supramarginal gyrus associated with switching both between different response modalities and between stimulus categorizations (Fig. 2).
In addition, the interaction contrasts [Stimulus‐categorization (switch minus repetition) vs. Response‐modality (switch minus repetition)] and [Response‐modality (switch minus repetition) vs. Stimulus‐categorization (switch minus repetition)] were calculated to test whether switch‐related activation was different for both types of task switching (locations of significant activation clusters are also shown in Table II). Applying a corrected threshold (P < 0.05 corrected at the cluster‐level), no significant activation was observed for any of the interaction contrasts.
Lowering the threshold to an uncorrected level (P < 0.001 with voxel threshold of 10 consecutive voxels) revealed several clusters of activation. Switches between stimulus categories (relative to repetitions) elicited higher signal changes than switches of the response modality (relative to repetitions) at the temporoparietal junction (TPJ) bilaterally. In addition stronger signal changes were observed in the right lateral orbital gyrus and in the left inferior frontal sulcus. The latter overlapped with the left inferior frontal sulcus activation observed in switches of stimulus categories (−46, 24, 34).
The reverse contrast indicating stronger signal changes related to switches of the response modality (relative to repetitions) than to switches of the stimulus category (relative to repetitions) activated medial brain regions involving the middle cingulate gyrus as well as left medial superior frontal gyrus and insular cortex at the right long insular gyrus (Fig. 3).
Figure 3.
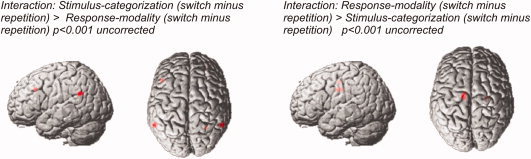
Surface renderings of functional contrasts reflecting the interaction between the variables transition (switch vs. repetition) and type of switching (stimulus categorization vs. response modality). Brain areas showing a stronger change related difference for Stimulus categorization compared to Response‐modality (i.e. [Stimulus‐categorization switches minus Stimulus‐categorization repetitions] compared to [Response‐modality switches minus Response‐modality repetitions]) are depicted on the left. The reverse contrast (i.e. [Response‐modality switches minus Response‐modality repetitions] compared to [Stimulus‐categorization switches minus Stimulus‐categorization repetitions]) is shown on the right. The interaction contrasts were calculated at a statistical threshold of P < 0.001, uncorrected.
ROI Analysis
Contrasting switch and repetition trials separately within stimulus‐categorization blocks and response‐modality blocks suggests that left inferior frontal and left superior parietal regions are differently involved in task switching. While left intraparietal sulcus was activated by both types of task switching, as indicated by the conjunction analysis, the left inferior frontal junction showed switching related effects only in stimulus categorization blocks. Additionally, the interaction contrasts (in which the threshold was lowered to an uncorrected level) indicated stronger signal changes in the left inferior frontal sulcus for stimulus‐categorization switching than for response modality switching, whereas no difference was found with respect to the left intraparietal sulcus.
Thus, to test whether these two regions indeed respond differently to different types of task switching, a three‐way ANOVA was conducted on the beta‐parameters of all four conditions, extracted at two different locations. Beta parameters in left intraparietal cortex were extracted at the maximum peak location of the activation revealed by the conjunction analysis (−44, −38, 46). Significant activations in left inferior frontal cortex were present in the switch vs. repetition contrast of the stimulus categorization blocks only. Accordingly, the coordinates for the inferior frontal location were chosen on the basis of this contrast (−44, 4, 30).
The three‐way ANOVA conducted in order to test whether the Type of Switching (stimulus categorization vs. response modality) affects Transition (switch trials vs. repetition trials)‐related activations specifically in different Brain regions (frontal vs. parietal) revealed a significant main effect of Brain regions [F(1, 22) = 22.492; MSe = 78.427; P < 0.001] and a significant main effect of Transition [F(1, 22) = 29.743; MSe = 4.1038; P < 0.001] confirming the involvement of these regions in switch‐related processing.
Furthermore a significant three‐way interaction of Type of Switching × Transition × Brain regions [F(1, 22) = 7.326; MSe = 0.97008; P < 0.05] was observed, indicating differential switch‐related processing within these brain areas (Fig. 4). To qualify this interaction, separate two‐way ANOVAs were calculated for the two brain regions with the variables Transition and Type of Switching. These analyses revealed significant main effects for the variable Transition in both brain regions [parietal: F(1, 22) = 37.563; MSe = 2.892; P < 0.001; frontal: F(1, 22) = 12.459; MSe = 1.356; P < 0.01]. However, while no evidence was found for a Transition × Type of Switching two‐way interaction in the intraparietal sulcus [F(1, 22) = 0.0331; MSe = 0.004; n.s.], a significant two‐way interaction was observed in inferior frontal sulcus [F(1, 22) = 5.3207; MSe = 1.759; P < 0.05]. To further specify the Transition × Type of switching interaction, post hoc t‐tests were performed, comparing switch and repetition trials separately for stimulus‐categorization blocks and response‐modality blocks. This was done for both the inferior frontal sulcus as well as the intraparietal sulcus. In the inferior frontal sulcus, switches of a stimulus category elicited significantly stronger signal changes than repetitions [t(22) = 3.54; P < 0.001]. In contrast, signal changes related to switches of a response modality were not observed as different from those elicited by repetitions [t(22) = −0.2606; P = 0.80, n.s.]. Furthermore, switch‐related changes of beta‐values (Switch‐Repetition) were significantly higher for stimulus categories relative to response modalities [t(22) = 2.3067; P < 0.05]. In the intraparietal sulcus, switch trials generated significantly larger signal changes than the respective repetition trials for both the stimulus‐category blocks and the response‐modality blocks [stimulus categorization switch vs. repetitions: t(22) = 3.61; P < 0.01; response modality switch vs. repetition: t(22) = 3.79; P < 0.01]. Consequently, switch‐related changes of beta‐values (Switch‐Repetition) were not different for stimulus categorizations compared to response modalities [t(22) = −0.1818; P = 0.85, n.s.]. These results suggest that the intraparietal cortex is generally involved in task switching, whereas the switch‐related activation difference in the inferior frontal cortex was restricted to switching between stimulus categorizations.
Figure 4.
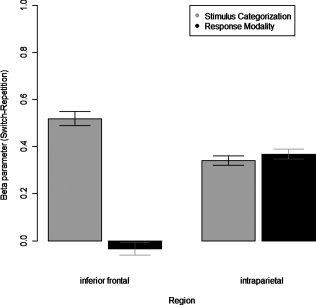
Switch‐related increases of beta parameters (switch–repetition) shown separately for stimulus‐categorization and response‐modality blocks at two different locations: left inferior frontal cortex (−44, 4, 30) and left intraparietal cortex (−44, −38, 46).
DISCUSSION
In the present study, we aimed at elucidating the neural mechanisms underlying switching between stimulus categorizations and switching between response modalities. Therefore, the most important finding is that we observed a dissociation between both types of task switching at the neural level. Switching between stimulus categorizations activated the left inferior frontal sulcus and the left superior parietal cortex. In contrast to switching between stimulus categorizations, switching between response modalities activated a single cluster in the left intraparietal sulcus/supramarginal gyrus only. Consequently, the conjunction analysis revealed activation common to both types of task switching in the left intraparietal sulcus/supramarginal gyrus.
At first sight, not observing a prefrontal activation in the conjunction analysis appears to be at odds with a number of studies in which the relevant stimulus categorization remained the same for all trials and in which an activation of the prefrontal cortex was observed [see, e.g., Dove et al., 2000; Rushworth et al., 2002]. It is important to note, however, that the data pattern observed in the present study does not support the notion that prefrontal cortex is relevant only for switches between different stimulus categorizations. As we compared only two types of task switching, the present results cannot be generalized to the involvement of the prefrontal cortex in other types of task switching. Yet, the current study provides evidence that left prefrontal cortex is involved in switches between stimulus categories, whereas we did not observe prefrontal involvement in switches of response modalities. This notion is supported by an interaction in left inferior frontal junction (whole brain: P < 0.001 uncorrected) and by the three‐way interaction observed in the ROI analysis. Consequently, these results indicate that the magnitude of the prefrontal activation depends on the task requirements. The data thus suggest that a substantial part of the prefrontal activation observed in previous task‐switching experiments depended on switching between stimulus categorizations.
The present results, on the one hand, qualify previous claims about the general role of the left prefrontal cortex in task control and, on the other hand, suggest a general role of parietal cortex in the selection of action rules. This suggestion is supported by the fact that parietal cortex was consistently activated in previous task‐switching studies [Braver et al., 2003; Crone et al., 2006; Dove et al., 2000; Le et al., 1998; Liston et al., 2006; Rushworth et al., 2001b; Sohn et al., 2000; Yeung et al., 2006] even if no prefrontal activation occurred [see, e.g., Kimberg et al., 2000; Gurd et al., 2002]. Furthermore, a recent study by Chiu and Yantis [ 2009] identified the medial superior parietal cortex as the only brain region which was activated both in shifting attention between different locations and stimulus‐categorization switching [see also Esterman et al., 2009]. This finding strongly supports the proposed general role of the parietal cortex in cognitive control.
We assume that the general role of the parietal cortex in task switching is related to the selection of SR or action rules. This selection of actions rules is relevant for all different types of task switching as a specific stimulus attribute is always mapped to a specific response. Yet, action rules certainly differ with respect to the type of task switching. Thus, one might also speculate that different peak activations within the parietal cortex can be observed for different types of task switching [see, e.g., Rushworth et al., 2001b]. However, it is important to note that we neither expected nor observed effector‐specific activations in parietal (or premotor) cortex. This is because we always addressed switch‐specific activations, while keeping primary effector‐specific attributes comparable (i.e. switch and repetition trials both involved hand as well as foot responses).
While parietal areas show a more general involvement in the selection of SR or action rules, we also observed in the (uncorrected) interaction contrasts that medial frontal areas seem to be more specifically involved in switching between different response modalities. This finding is consistent with the notion that the medial superior frontal gyrus plays a crucial role in selecting superordinate sets of action‐selection rules or selection sets [Rushworth et al., 2004]. These data indicate that medial frontal areas are more involved in selecting responses than in selecting SR associations.
With regard to the differential role of prefrontal and parietal cortex in task switching, our findings are consistent with the idea that the prefrontal activation is mainly associated with a stimulus‐related switching component, whereas the parietal activation mainly corresponds to a response‐related switching component. Empirical evidence for the prefrontal activation as a stimulus‐driven component can be found, for example, in studies using the Wisconsin Card Sorting Test (WCST). Shifting attention between different stimulus categorizations in the WCST is typically associated with activation of the prefrontal cortex [see, e.g., Konishi et al., 1998; Lie et al., 2006; Rogers et al., 2000]. Further support for a role of the prefrontal cortex in stimulus categorization stems from studies on monkeys [Freedman et al., 2001]. Finally, research on hierarchical representations in the prefrontal cortex [for reviews see Badre, 2008; Botvinick, 2008] demonstrates a role of the prefrontal cortex in switching between categorization rules of varying complexity [Yoshida et al., 2010].
Empirical evidence for the parietal activation reflecting response‐related task components is derived from studies on apraxia [e.g., De Renzi et al., 1968]. That is, patients with a lesion of the left parietal cortex often suffer from the difficulty or failure to perform an action in response to a visual stimulus. Further, the parietal cortex also plays a crucial role for the intention to move [Desmurget et al., 2009]. These observations are in good accordance with the notion that one prominent aspect of parietal cortex is its function as an association area that integrates visual, cognitive, and motor information [Bremmer et al., 2001; Grefkes et al., 2002; for a review see Gottlieb, 2007]. This suggests that the parietal cortex is generally relevant for the visuo‐motor transformation of stimuli into corresponding responses [cf. Andersen et al., 1997; Culham and Kanwisher, 2001; Rumiati et al., 2004]. This association of the parietal cortex to a response‐related component in task switching is further supported by lesion work and TMS studies indicating that the parietal cortex is critical in switching from one movement to another [Rushworth et al., 1997; Rushworth et al., 2001a].
A differentiation between stimulus‐related and response‐related components is also consistent with a variety of models in task switching [see, e.g., Kiesel et al., 2010, for a review]. For example, Rubinstein and colleagues [ 2001] proposed a two‐stage model of task switching. In a first stage, the relevant task goal or task representation is updated. In a second stage, the activation and application of specific SR rules takes place. In accordance with this idea, activation of the prefrontal cortex (associated with the updating of an abstract task representation in terms of the stimulus categorization) should occur before activation of the inferior parietal cortex (associated with rule activation). This sequence of events was indeed observed in an event‐related potential (ERP) study of Brass et al. [ 2005]. Consequently, these authors suggested that activation of the prefrontal cortex is related to the updating of an abstract task representation (for which stimulus categorizations play a role) and influences the activation of specific response‐related action rules stored in parietal cortex. Yet, in contrast to the observation of Brass and colleagues, Bode and Haynes [ 2009] reported an earlier peak in the left parietal cortex than in the left frontal cortex with respect to the representation of task rules. It is important to note, however, that this early peak in the left parietal cortex was even present before the onset of the imperative stimulus – thus further indicating a more abstract and global role of parietal cortex in task switching.
Although we did not manipulate the cue‐stimulus interval, it is interesting to relate the present results to task preparation. This is because models on task switching distinguish between the preparation of stimulus‐related and of response‐related components [Meiran, 2000; see also Meiran et al., 2008]. Furthermore, previous fMRI studies demonstrated that task preparation resulted in an activation of both the prefrontal cortex and the parietal cortex [Brass and von Cramon, 2002; Gruber et al., 2006; Ruge et al., 2005; Sohn et al., 2000; for a review see Ruge et al., 2011]. Brass and von Cramon [ 2004] suggested that frontal cortex supports more abstract aspects of task preparation, including preparation for specific stimulus categorizations, while parietal cortex is related in preparing mainly response‐related action rules [see also Ruge et al., 2011]. Thus, the distinction between the prefrontal cortex and stimulus‐related components of task switching on the one hand and parietal activation and response‐related components on the other hand also appears to emerge with respect to task preparation. In line with the data from our experiment, these observations again strongly suggest a relatively global and response‐related role of parietal cortex in task switching.
CONCLUSION
The results of the present study contribute to the functional differentiation between the prefrontal and inferior parietal cortex in task switching by comparing two different types of task switching (i.e., stimulus‐categorization switching and response‐modality switching). Our findings strongly suggest that the prefrontal activation is largely related to switching the relevant stimulus categorization, whereas the activation of the inferior parietal cortex reflects a more general selection of the relevant action rule.
Footnotes
A 2 × 2‐factorial repeated measures ANOVA of RTs with the variables Transition (switch trial vs. repetition trial) and Congruency (incongruent trial vs. congruent trial), revealed both main effects but not the interaction as significant [Transition: F(1, 22) = 18.84; MSe = 14885; P < 0.01; Congruency: F(1, 22) = 11.34; MSe = 21147; P < 0.01; Transition × Congruency: F(1, 22) = 0.0017; MSe = 9262.6; n.s. P = 0.97]. The results of an analogous ANOVA for error rates revealed only the main effect for Congruency as significant [Congruency: F(1, 22) = 24.15; MSe = 0.11; P < 0.01; Transition: F(1, 22) = 1.38; MSe = 0.13; P = 0.25; Transition × Congruency: F(1, 22) = 0.08; MSe = 0.16; n.s. P = 0.77]. Thus, for behavioral data, congruency provided an additive effect, as participants responses were slower and more erroneous to incongruent (1171 ms, 5.9%) as compared to congruent trials (1069 ms, 2.5%), but it did not interact with stimulus‐categorization switching.
For stimulus‐categorization switching blocks, two way ANOVAs were calculated with the variables Stimulus categorization (color vs. form) and Transition (switch trial vs. repetition trial) for RTs and Errors. In the RT analysis both main effects were significant [Stimulus categorization: F(1, 22) = 16.345; P < 0.001; Transition: F(1, 22) = 21.257; P < 0.001]. The interaction between both variables was not significant [F(1, 22) = 0.4448; n.s.; P = 0.52]. An analogous ANOVA of the error rates failed to reveal any significant effects [Stimulus categorization: F(1, 22) = 1.3184; n.s.; P = 0.26; Transition: F(1, 22) = 1.1374; n.s.; P = 0.30; Stimulus categorization × Transition: F(1, 22) = 1.9025; n.s.; P = 0.18]. In response‐modality switching blocks, 2 × 2‐factorial repeated measures ANOVAs on Transition (switch trial vs. repetition trial) and Response modality (hand vs. foot) were performed separately for RTs and Errors. For the RT analysis, the main effects of Transition [F(1, 22) = 33.44; P < 0.001] and of Response modality [F(1, 22)= 19.332; P < 0.001] were significant. The interaction between both variables was not significant [F(1, 22) = 2.1973; n.s.; P = 0.15]. An analogous ANOVA of the error rates failed to reveal any significant effects [Transition: F(1, 22) = 2.692; n.s.; P = 0.11; Response modality: F(1, 22) = 0.1031; n.s.; P = 0.75; Transition × Response modality: F(1, 22) = 0.985; n.s.; P = 0.33].
REFERENCES
- Allport DA, Styles EA, Hsieh S ( 1994): Shifting intentional set: Exploring the dynamic control of tasks In: Umiltà C, Moscovitch M, editors. Attention and Performance XV: Conscious and Nonconscious Information Processing. Cambridge, MA: MIT Press; pp 421–452. [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J ( 1997): Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 303–330. [DOI] [PubMed] [Google Scholar]
- Badre D ( 2008): Cognitive control, hierarchy, and the rostro‐caudal organization of the frontal lobes. Trends Cogn Sci 12: 193–199. [DOI] [PubMed] [Google Scholar]
- Bode S, Haynes DJ ( 2009): Decoding sequential stages of task preparation in the human brain. NeuroImage 45: 606–613. [DOI] [PubMed] [Google Scholar]
- Botvinick MM ( 2008): Hierarchical models of behavior and prefrontal function. Trends Cogn Sci 12: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann KP, Zilles K, Fink GR ( 2001): Polymodal motion processing in posterior parietal and premotor cortex. Neuron 29: 287–296. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI ( 2003): Neural mechanisms of transient and sustained cognitive control during task switching. Neuron 39: 713–726. [DOI] [PubMed] [Google Scholar]
- Brass M, Ullsperger M, Knoesche TR, von Cramon DY, Phillips NA ( 2005): Who comes first? The role of the prefrontal and parietal cortex in cognitive control. J Cogn Neurosci 17: 1367–1375. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY ( 2002): The role of the frontal cortex in task preparation. Cereb Cortex 12: 908–914. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY ( 2004): Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci 16: 609–620. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD ( 2002): Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage 17: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Chiu Y‐C, Yantis S ( 2009): A domain‐independent source of cognitive control for task sets: Shifting spatial attention and switching categorization rules. J Neurosci 29: 3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van der Linden M ( 2002): Brain imaging of the central executive component of working memory. Neurosci Biobehav Rev 26: 105–125. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA ( 2006): Neural evidence for dissociable components of task‐switching. Cereb Cortex 16: 475–486. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG ( 2001): Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol 11: 157–163. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Pieczuro A, Vignolo LA ( 1968): Ideational apraxia: A quantitative study. Neuropsychologia 6: 41–52. [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A ( 2009): Movement intention after parietal cortex stimulation in humans. Science 324: 811–813. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY ( 2000): Prefrontal cortex activation in task switching: An event‐related fMRI study. Cogn Brain Res 9: 103–109. [DOI] [PubMed] [Google Scholar]
- Esterman M, Chiu Y‐C, Tamber‐Rosenau BJ, Yantis S ( 2009): Decoding cognitive control in human parietal cortex. Proc Natl Acad Sci USA 106: 17974–17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, Kamber M, Collins DL, MacDonald D ( 1994): An MRI‐based probabilistic atlas of neuroanatomy. Magn Reson Scanning Epilepsy 264: 263–274. [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK ( 2001): Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291: 312–316. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE ( 2005): Conjunction revisited. NeuroImage 25: 661–667. [DOI] [PubMed] [Google Scholar]
- Gottlieb J ( 2007): From thought to action: The parietal cortex as a bridge between perception, action, and cognition. Neuron 53: 9–16. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Weiss PH, Zilles K, Fink GR ( 2002): Crossmodal processing of object features in human anterior intraparietal cortex: An fMRI study implies equivalencies between humans and monkeys. Neuron 35: 173–184. [DOI] [PubMed] [Google Scholar]
- Gruber O, Karch S, Schlueter EK, Falkai P, Goschke T ( 2006): Neural mechanisms of advance preparation in task switching. NeuroImage 31: 887–895. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR ( 2002): Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: An fMRI study with clinical implications. Brain 125: 1024–1038. [DOI] [PubMed] [Google Scholar]
- Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I ( 2010): Control and interference in task switching—A review. Psychol Bull 136: 849–874. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D'Esposito M ( 2000): Modulation of task‐related neural activity in task‐switching: An fMRI study. Cogn Brain Res 10: 189–196. [DOI] [PubMed] [Google Scholar]
- Koch I, Gade M, Schuch S, Philipp AM ( 2010): The role of inhibition in task switching—A review. Psychon Bull Rev 17: 1–14. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y ( 1998): Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci 1: 80–84. [DOI] [PubMed] [Google Scholar]
- Le TH, Pardo JV, Hu X ( 1998): 4 T‐fMRI study of nonspatial shifting of selective attention: Cerebellar and parietal contributions. J Neurophysiology 79: 1535–1548. [DOI] [PubMed] [Google Scholar]
- Lie C‐H, Specht K, Marshall JC, Fink GR ( 2006): Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. NeuroImage 30: 1038–1049. [DOI] [PubMed] [Google Scholar]
- Liston C, Malaton S, Hare TA, Davidson MC, Casey BJ ( 2006): Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task‐switching paradigm. Neuron 50: 643–653. [DOI] [PubMed] [Google Scholar]
- Meiran N ( 1996): Reconfiguration of processing mode prior to task performance. J Exp Psychology: Learn Mem Cogn 22: 1423–1442. [Google Scholar]
- Meiran N ( 2000): Modeling cognitive control in task‐switching. Psychol Res 63: 234–249. [DOI] [PubMed] [Google Scholar]
- Meiran N, Kessler Y, Adi‐Japha E ( 2008): Control by action representation and input selection (CARIS): A theoretical framework for task switching. Psychol Res 72: 473–500. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Monsell S ( 2003): Task switching. Trends Cogn Sci 7: 134–140. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J ( 2005): Valid conjunction inference with the minimum statistic. NeuroImage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Philipp AM, Koch I ( 2005): Switching of response modalities. Quart J Exp Psychol 58A: 1325–1338. [DOI] [PubMed] [Google Scholar]
- Philipp AM, Koch I ( 2010): The integration of task‐set components into cognitive task representations. Psychol Belgica 50: 383–411. [Google Scholar]
- Philipp AM, Koch I ( 2011): The role of response modalities in cognitive task representations. Adv Cogn Psychology 7: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW ( 2000): Contrasting cortical and subcortical activations produced by attentional‐set shifting and reversal learning in humans. J Cogn Neurosci 12: 142–162. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S ( 1995): Costs of a predictable switch between simple cognitive tasks. J Exp Psychology: General 124: 207–231. [Google Scholar]
- Rubinstein JS, Meyer DE, Evans JE ( 2001): Executive control of cognitive processes in task switching. J Exp Psychology: Hum Percept Perform 27: 763–797. [DOI] [PubMed] [Google Scholar]
- Ruge H, Brass M, Koch I, Rubin O, Meiran N, Cramon DY ( 2005): Advance preparation and stimulus‐induced interference in cued task switching: Further insights from BOLD fMRI. Neuropsychologia 43: 340–355. [DOI] [PubMed] [Google Scholar]
- Ruge H, Jamadar S, Zimmermann U, Karayanidis F ( 2011): The many faces of preparatory control in task switching: Reviewing a decade of fMRI research. Hum Brain Mapp. DOI: 10.1002/hbm.21420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Nixon PD, Passingham E ( 1997): Parietal cortex and movement: I. Movement selection and reaching. Exp Brain Res 117: 292–310. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Ellison A, Walsh V ( 2001a): Complementary localization and lateralization of orienting and motor attention. Nat Neurosci 4: 656–661. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Paus T, Sipila PK ( 2001b): Attention systems and the organization of the human parietal cortex. J Neurosci 21: 5262–5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Hadland KA, Paus T, Sipila PK ( 2002): Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. J Neurophysiology 87: 2577–2592. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM ( 2004): Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 9: 410–417. [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, Fink GR ( 2004): Neural basis of pantomiming the use of visually presented objects. NeuroImage 21: 1224–1231. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS ( 2000): The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97: 13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, Fink GR ( 2003): Lateralized cognitive processes and lateralized task control in the human brain. Science 301: 384–386. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J ( 2003): Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia 41: 357–370. [DOI] [PubMed] [Google Scholar]
- Vandierendonck A, Christiaens E, Liefooghe B ( 2008): On the representation of task information in task switching: Evidence from task and dimension switching. Mem Cogn 36: 1248–1261. [DOI] [PubMed] [Google Scholar]
- Vandierendonck A, Liefooghe B, Verbruggen F ( 2010): Task switching: Interplay of reconfiguration and interference control. Psychol Bull 136: 601–626. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S ( 2004): Neuroimaging studies of shifting attention: a meta‐analysis. NeuroImage 22: 1679–1693. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD ( 2006): Between‐task competition and cognitive control in task switching. J Neurosci 26: 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W, Funakoshi H, Ishii S ( 2010): Hierarchical rule switching in prefrontal cortex. NeuroImage 50: 314–322. [DOI] [PubMed] [Google Scholar]


