Abstract
New episodic memory traces represent a record of the ongoing neocortical processing engaged during memory formation (encoding). Thus, during encoding, deep (semantic) processing typically establishes more distinctive and retrievable memory traces than does shallow (perceptual) processing, as assessed by later episodic memory tests. By contrast, the hippocampus appears to play a processing‐independent role in encoding, because hippocampal lesions impair encoding regardless of level of processing. Here, we clarified the neural relationship between processing and encoding by examining hippocampal–cortical connectivity during deep and shallow encoding. Participants studied words during functional magnetic resonance imaging and freely recalled these words after distraction. Deep study processing led to better recall than shallow study processing. For both levels of processing, successful encoding elicited activations of bilateral hippocampus and left prefrontal cortex, and increased functional connectivity between left hippocampus and bilateral medial prefrontal, cingulate and extrastriate cortices. Successful encoding during deep processing was additionally associated with increased functional connectivity between left hippocampus and bilateral ventrolateral prefrontal cortex and right temporoparietal junction. In the shallow encoding condition, on the other hand, pronounced functional connectivity increases were observed between the right hippocampus and the frontoparietal attention network activated during shallow study processing. Our results further specify how the hippocampus coordinates recording of ongoing neocortical activity into long‐term memory, and begin to provide a neural explanation for the typical advantage of deep over shallow study processing for later episodic memory. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: episodic memory, explicit memory, level of processing, encoding, connectivity, fMRI, psychophysiological interaction
INTRODUCTION
Episodic memory is the ability to remember personally experienced episodes in their spatial and temporal context [Tulving, 2002]. Converging evidence from lesion studies highlights the role of the hippocampus and adjacent medial temporal lobe (MTL) structures in human episodic memory [Scoville and Miler, 1957; Squire et al., 2004; Vargha‐Khadem et al., 1997]. Functional neuroimaging allows one to observe brain activity during encoding and to analyze event‐related brain activity as a function of later remembering versus forgetting, a phenomenon often referred to as the DM effect (difference due to later memory effect; e.g., Paller and Wagner, 2002). Functional magnetic resonance imaging (fMRI) studies of the DM effect have confirmed the role of the MTL in episodic memory and additionally demonstrated that dorsolateral and ventrolateral prefrontal as well as parietal structures participate in successful encoding [e.g., Diana et al., 2007; Kim et al., 2011; Paller and Wagner, 2002; Spaniol et al., 2009; for a review of parietal activations see Uncapher and Wagner, 2009]. Although lesion studies have demonstrated that episodic memory deficits can occur in absence of any other pronounced accompanying cognitive deficits [Scoville and Miler, 1957; Mayes, 2000; Squire et al., 2004; Vargha‐Khadem et al., 1997], studies in healthy participants have demonstrated that ongoing cognitive processes have an important influence on whether an episode will be remembered or forgotten.
Perhaps the best‐replicated behavioral phenomenon in episodic memory research is that inducing participants to attend to the meaning of stimuli presented at encoding (“deep” cognitive processing) typically leads to better retention of the episode than does inducing participants to attend to the perceptual (rather than the semantic) aspects of stimuli presented at encoding (“shallow” cognitive processing). Such level‐of‐processing (LOP) effects can be very large even in the absence of any intention to learn during encoding [e.g., Craik and Lockhart, 1972; Craik and Tulving, 1975].
The most common explanation for LOP effects is that deep study processing leaves behind semantically more elaborate memory traces than shallow processing. Because most episodic recognition and recall tests typically induce participants to rely on semantic, associative, information to retrieve the encoding episode, deep processing therefore leads to better performance than shallow processing over a wide range of episodic memory tests [Craik, 2002]. Furthermore, memory traces established during deep processing are distinctively different from each other and preserve strong connections with a unique encoding context [e.g., Craik and Lockhart, 1972; Fisher and Craik, 1977; Moscovitch and Craik, 1976]. They thus support consciousness at retrieval of specific details of the previous encoding episode, such as what one was thinking at the time a particular item was earlier encountered, which is the hallmark of episodic memory [Gardiner et al., 1996, 1998]. Previous neuroimaging studies have yielded both an overlap between brain regions involved in LOP and those engaged in successful memory formation [Kapur et al., 1994; Wagner et al., 1998], and a largely similar set of areas has been implicated in successful encoding of both deeply and shallowly studied items [Baker et al., 2001; Fletcher et al., 2003; Otten et al., 2001; Schott et al., 2006]. On the other hand, electrophysiological studies have provided evidence of a neural signature of successful encoding that is temporally and topographically separable from LOP or distinctiveness effects [Guderian et al., 2009; Schott et al., 2002]. Most notably, magnetoencephalographic data have identified theta (i.e., 7 Hz) oscillations originating from the MTL that were associated with successful encoding, but did not vary as a function of level of processing [Guderian et al., 2009].
Notably, the typical level‐of‐processing effect can be eliminated or even reversed when the chosen memory test calls on memory for perceptual rather than semantic information from the encoding episode [e.g., Blaxton, 1989; Fisher and Craik, 1977; Moscovitch and Craik, 1976; Morris et al., 1977], making a pure strength interpretation unlikely. Instead, memory traces formed during deep versus shallow processing are now thought to differ qualitatively rather than quantitatively, with their informational record being a by‐product of the kind of cognitive processing engaged during encoding. Thus, level‐of‐processing effects have been integrated within a more general transfer‐appropriate‐processing framework [e.g., Morris et al., 1977; Roediger et al., 2002], in which successful retrieval depends on the degree of overlap between the type of cognitive processing engaged by retrieval cues and the type of cognitive processing engaged earlier during encoding. Consistent with this notion, neuroimaging evidence shows that the same informationally content‐specific brain regions involved in processing information at encoding are reactivated during successful retrieval of that information [e.g., Fenker et al., 2005; Johnson and Rugg, 2007; for reviews see Khader and Rösler, 2009; Nyberg, 2002; Rugg et al., 2008]. In addition, distinct frontoparietal attentional networks have been found to be involved in establishing memory traces during encoding, depending on whether the later memory test calls on semantic or perceptual information [e.g., Wimber et al., 2010].
A theoretical perspective that integrates the concept of an MTL memory system with processing theories is provided by component models like the Component Process Model [Moscovitch, 1992], the related Multiple Trace Theory [Nadel and Moscovitch, 1997], or the concept of complementary learning systems [McClelland et al., 1995]. Although such models differ in some aspects regarding the detailed roles of the participating structures, all suggest that the MTL subserves episodic memory formation by integrating information processed in distributed neocortical regions [e.g., Eichenbaum, 2000; Lavenex and Amaral, 2000; Moscovitch, 2008; Squire et al., 2004; Wang and Morris, 2010]. According to this perspective, MTL areas are responsible for laying down a record of brain‐activity patterns related to ongoing cognitive processing at encoding [e.g., Moscovitch, 2008; Richardson‐Klavehn, 2010a] and for orchestrating the reinstatement of these patterns during successful retrieval [e.g., Guderian and Düzel, 2005; Moscovitch, 2008]. If this integrative perspective is correct, successful episodic encoding should be accompanied by enhanced communication between the MTL and neocortical structures involved in ongoing cognitive processing during encoding.
Such communication between brain structures can be assessed with fMRI measures of functional connectivity [Cordes et al., 2000; Friston, 2002; Rogers et al., 2007]. Some fMRI studies investigating how psychological variables modulate connectivity of distinct brain regions [Das et al., 2005; Egner and Hirsch 2005; Friston et al., 1997] have already demonstrated the hypothesized encoding‐related modulation in functional connectivity of the hippocampus with the neocortex [Ranganath et al., 2005; van Kesteren et al., 2010]. Thus far, however, there have been no fMRI studies that have explicitly examined the relationship between level of processing and hippocampal–cortical functional connectivity during episodic memory formation in humans. Accordingly, in the current experiment, we used fMRI during a verbal memory encoding task with a level‐of‐processing manipulation [Guderian et al., 2009; Schott et al., 2006]. Study phases involving either deep or shallow processing were followed, after distraction, by free recall tests. We examined overall signal changes at encoding related to later remembering and forgetting. We also examined encoding‐related changes in functional connectivity between the hippocampus and neocortical regions. To assess functional connectivity, we used psychophysiological interaction (PPI) analysis, which measures the functional connectivity between distinct brain regions in relation to a psychological variable [Das et al., 2005; Friston et al., 1997; Gitelman et al., 2003]. In the present case, later remembering versus forgetting of study items was the psychological variable, examined separately for deeply and shallowly processed items. Such an approach should lead to a better understanding of the functional neuroanatomy of the relationship between successful encoding and neocortical activity related to ongoing cognitive processing, as engaged here by the deep and shallow study tasks.
Because free recall tests of words require verbal semantic processing of the stimuli, and because successful retrieval depends on a match in cognitive processing between encoding and retrieval, we hypothesized that during successful deep encoding, the left hippocampus might show increased functional connectivity with larger scale semantic networks, which would facilitate later verbal recall. We further hypothesized that successful shallow encoding might reflect, to some extent, incidental semantic processing at encoding, leading to partly similar, albeit weaker, encoding‐related connectivity changes in the shallow study condition. Finally, in part on the basis of electrophysiological studies [e.g., Schott et al., 2002] we tentatively hypothesized that shallow encoding might additionally be associated with qualitatively distinct connectivity changes, reflecting other routes to successful memory like, for example, perceptual distinctiveness.
MATERIALS AND METHODS
Participants
The participants were 64 young (age range 18 ‐ 38; 41 female), right‐handed native speakers of German, who were paid for participation in the study. All participants underwent routine clinical interview to exclude neurological or psychiatric illness and had normal T1‐weighted MR images. Participants were checked for MRI contraindications and gave written informed consent to participate, in accordance with the Declaration of Helsinki. The study was approved by the local ethics committee. The cohort participating here overlapped in part with the cohort of a previously reported imaging genetics study [Schott et al., 2006].
Paradigm
Brain‐activity patterns during study of visually presented words (equal proportions of nouns, verbs, and adjectives) were compared as a function of remembering and forgetting in a later oral free recall task, together with a manipulation of deep versus shallow study processing during study‐list presentation. This paradigm elicits robust hippocampal activation during study of later recalled when compared with later forgotten items [Schott et al., 2006], and a highly reliable behavioral LOP effect [Guderian et al., 2009; Schott et al., 2006].
The experiment consisted of three fMRI runs, each comprising three study‐lists with a deep study task and three study‐lists with a shallow study task, giving nine lists per study task in total. The two study tasks alternated within a given participant, with the order of study tasks being counterbalanced across participants (i.e., deep‐shallow‐deep, etc., vs. shallow‐deep‐shallow, etc.). In the deep study task, participants were instructed to judge whether the meaning of a word was pleasant or unpleasant and to respond with the index finger of one hand for pleasant words and with the index finger of the other hand for unpleasant words. This task has previously been used in numerous LOP studies [Guderian et al., 2009; Richardson‐Klavehn and Gardiner, 1996, 1998; Schott et al., 2002]. Participants were encouraged to think of personal associations with the meaning of the word if it initially seemed neither pleasant nor unpleasant. In the shallow study task, participants were instructed to judge whether or not a word had exactly two syllables and to respond with one index finger for two syllables and with the other index finger for any other number of syllables. The syllable range of the words was 1–5, with approximately half having two syllables. Participants were asked to rely on the sound of the word rather than its orthography, because such phonemic processing elicits similar later perceptual priming, but poorer later episodic memory, in comparison with semantic processing as engaged by the pleasantness rating task [e.g., Richardson‐Klavehn and Gardiner, 1996, 1998; Schott et al., 2002; for review, see Richardson‐Klavehn, 2010b]. We could thus be confident that encoding‐related differences in brain activity between the deep and shallow study conditions should relate to episodic memory and not to perceptual priming. Response hand was counterbalanced across participants.
Twenty German words were presented per study list (giving 180 words per study task across the entire experiment). Trials consisted of a central fixation cross (+) for 500 ms, a word presented for 1,000 ms, and a fixation asterisk (*) for 1,250 ms. Study lists were followed by a 20 s distractor task consisting of four moderately difficult arithmetic problems, together with correct or incorrect solutions. Participants judged whether the solution was correct or not and responded via button press. The distractor task precluded recall from working memory, thus removing the recall advantage for later list items (i.e., recency effect) that would occur with immediate free recall. After the distractor task, a cue (Please speak) prompted participants for a 90 s free‐recall phase, in which they orally recalled, in any order, as many studied words as possible from the immediately preceding study list. Oral responses were recorded with a microphone placed at the bottom of the head coil. Overt responses were scored offline, both for accuracy and for time of oral response onset. The session structure and trial timings are summarized in Fig. 1.
Figure 1.
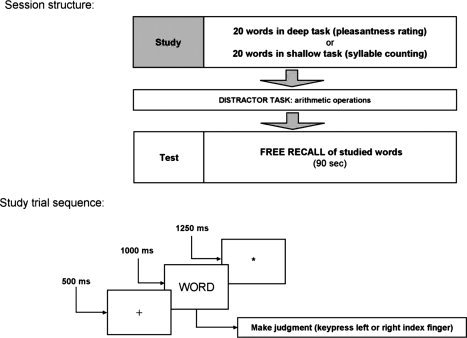
Experimental session and trial structure. Top: Session structure. A total of three fMRI sessions with the structure displayed were performed by each participant. For each participant, each session consisted of three study lists with deep study processing and three study lists with shallow study processing (making a total of six lists per fMRI session, and a total of 18 lists over the three sessions). Order of study condition was counterbalanced across participants (i.e., deep‐shallow‐deep vs. shallow‐deep‐shallow, etc.). Bottom: Structure of a single study trial. Each study list comprised 20 such study trials, followed by a 20 s distractor task and a 90 s oral recall period.
Because participants knew of the upcoming recall tests, they were explicitly instructed not to intentionally memorize the words during the study phases, but simply to focus on the judgment task at hand. Moreover, the study tasks were highly attention‐demanding within the time allowed (2,750 ms per word in total). Although we cannot rule out some participants having tried to memorize the words during study, the highly significant LOP effect on later recall (see Results) strongly suggests that the LOP manipulation was effective in directing cognitive processing resources to different aspects of the study words.
Functional MRI Scanning
The fMRI experiment was conducted on a GE 1.5 T Signa MRI system (General Electric Medical Systems) using a standard quadrature head coil. Before the fMRI experiment, a sagittal T1‐weighted 3D anatomical MR image [fast spoiled gradient echo pulse sequence, FSPGR; time to repetition (TR) = 24 ms; time to echo (TE) = 8 ms; flip angle = 30°; 60 slices, in‐plane resolution = 256 × 256; voxel size = 2.8 × 0.9 × 0.9 mm3] was acquired and used for slice positioning and later normalization (see below). Three sessions of 544 T2*‐weighted echo planar images [EPIs; TR = 2.0 s; TE = 35 ms; 23 axial slices, parallel to the AC‐PC line; image matrix = 64 × 64; voxel size = 3.13 × 3.13 × 6 mm3 (slice thickness = 5 mm; interslice gap = 1 mm)] were then acquired in an interleaved odd–even slice order with ascending acquisition direction during the study tasks, distractor tasks, and recall tests. The first four volumes of each session were discarded to allow for steady‐state magnetization.
Data Processing and Analysis
Data analysis employed Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, London, UK). EPIs were corrected for acquisition delay and motion artifacts. The individual FSPGR images of each participant were coregistered to the individual mean EPI, segmented using the automated segmentation algorithm provided by SPM8, and EPIs were normalized into a common stereotactic reference space (International Consortium for Brain Mapping; http://www.loni.ucla.edu/ICBM/) using the normalization parameters determined from segmentation of the FSPGR image (isotropic voxel = 3 × 3 × 3 mm3), and spatially smoothed using an isotropic Gaussian kernel (full width half maximum = 8 mm; see Mikl et al., 2008). A high pass filter with a cut off frequency of 1/128 Hz was applied to the data. Statistical analysis was performed in a two‐stage mixed effects model. In the first stage, neural activity was modeled by a delta function at stimulus onset. The blood‐oxygen‐level‐dependent (BOLD) response was modeled by convolving these delta functions with the canonical hemodynamic response function (HRF) as implemented in SPM. The resulting time courses were downsampled for each scan to form the explanatory variables of a general linear model (GLM). The resulting GLM comprised four regressors for the four event‐types of interest (later recalled deep, later forgotten deep, later recalled shallow, later forgotten shallow), plus one for the distractor task (20 s epoch), one for the speech events in the 90 s free recall task (consisting of delta functions at the onset of each speech event, convolved with the canonical HRF), one for each of the six rigid‐body movement parameters determined from realignment, and a single constant representing the mean over scans. Weighting parameters for the GLM were estimated by restricted maximum likelihood fit. Linear contrast images were computed for the conditions of interest for each participant. For these contrast images second level random effects analyses were conducted. To assess the correlates of LOP and later memory performance, we computed a two‐way analysis of variance (ANOVA) for repeated measures with LOP (deep, shallow) and later memory (recalled, forgotten) as within‐participant factors. Data were scaled to the grand mean to control for unspecific global effects. F and T contrasts were computed on the resulting parameter estimates to assess main effects of LOP and LOP‐specific correlates of successful memory formation. The significance level for all voxelwise comparisons was set to P < 0.05 (whole‐brain‐corrected for familywise error, FWE), with minimal cluster size of 20 adjacent voxels (540 mm3). The cluster extent threshold had been used to counteract the risk for false positives resulting from the increased number of voxels after normalization. For anatomical localization and report, all coordinates were transformed into Talairach space [Talairach and Tournoux, 1988].
Functional Connectivity Analysis
To investigate how LOP modulates functional connectivity between the hippocampus and distant brain regions, we used the PPI approach [Das et al., 2005; Friston et al., 1997; Gitelman et al., 2003]. Because of our a priori knowledge of the predominant role of the left hemisphere in processing verbal material in right‐handed humans [e.g., Kelley et al., 1998], we focused our PPI analysis on the left hippocampus as seed region [see also Schott et al., 2006], but exploratory analyses were also performed for the right hippocampus. For each participant, a sphere with a radius of 6 mm was centered on the individual local maximum of the effects of interest contrast [unthresholded; Ranganath et al., 2005] within the anterior portion of a hippocampus region of interest (ROI) obtained from the SPM Anatomy Toolbox [Eickhoff et al., 2005] [−30 < y < −7]. First eigenvariate time series from the resulting spheres were extracted, adjusted for the effects of interest (corrected for variance explained by distractor and overt recall events as well as head movements) and deconvolved with the canonical HRF. The resulting time courses were convolved with a psychological function P of the time t, which was set to +1 if t was the onset of a later recalled word, −1 if t was the onset of a later forgotten word, and 0 in all other cases. Separate P functions were computed for the deep and shallow study conditions. Reconvolution of the resulting function with the HRF yielded the vectors X deep and X shallow, which formed the primary regressors of interest in the design matrix of a new GLM. P deep and P shallow were also convolved with the HRF to form additional regressors. The fifth explanatory variable was the original BOLD eigenvariate. The distractor‐task and recall‐task regressors, the six rigid‐body movement parameters determined from realignment, and a constant representing the mean over scans were included in the design matrix as covariates of no interest (see above). Model estimation was performed as described above, separately for the left and right hippocampus. Voxelwise linear contrast images for X deep and X shallow were computed for all participants, and submitted to second‐level random effects analyses, using grand mean scaling and the same a priori statistical thresholds as described above. To assess which connectivity increases were modulated by LOP, T‐contrasts of X deep were masked exclusively with the X shallow T‐contrast (statistically thresholded at P < .01, uncorrected), and vice versa. To validate the results obtained by this masking procedure, an exploratory direct t‐test‐based statistical comparison of the PPIdeep and PPIshallow contrasts was performed (P < 0.005, uncorrected, minimum cluster size = 10 voxels).
Generation of Probabilistic Temporoparietal Regions of Interest
We created MRI‐literature‐based probabilistic ROIs for the temporoparietal junction (DLPFC, VLPFC) bilaterally using a previously described algorithm [Schubert et al., 2008]. Thus, the right parietal activations were selected [Uncapher and Wagner, 2009]. The coordinates of all memory‐related activations in these areas reported by the authors were pooled and—if necessary—transformed to the montreal neurological institute (MNI) space, using the affine algorithm proposed by Brett et al. (2001). On the basis of this data set, we create the ROIs in a three‐step process.
- The probability that a voxel at a given position lay within the area of interest was estimated by calculating a 3D normal (Gaussian) distribution G(x, y, z) as follows [Turkeltaub et al., 2002]:
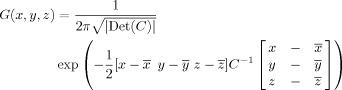
where C is the covariance matrix for all coordinate triples x, y, and z from the underlying literature and
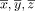 are the mean values of the x, y, and z coordinates, respectively [Nielsen and Hansen, 2002].
are the mean values of the x, y, and z coordinates, respectively [Nielsen and Hansen, 2002].Because the resulting distribution also contains voxels located in white matter and extracerebral space, we restrict the 3D distribution only to those voxels, that belong to gray matter with a probability of at least 50%. To this end we used the gray matter probability map as provided by SPM8.
The outer limits of the finally used ROI were defined by a threshold of n standard deviations of the resulting 3D distribution. Finally a binary mask including all surviving voxels was formed.
RESULTS
Behavioral Results
The mean proportions of items in the deep and shallow study conditions correctly recalled at test are displayed in Table I. There was a strong, significant effect of LOP at study on the proportion of correctly recalled items at test (F 1,63 = 134.15, P < 0.0001; one‐way ANOVA for repeated measures). At study, reaction times (RTs) were significantly shorter for shallowly studied items than for deeply studied items (main effect of LOP: F 1,63 = 16.82, P < 0.001; two‐way ANOVA for repeated measures), as is typically found [e.g., Craik and Tulving, 1975; Schott et al., 2002]. However, there was no significant RT difference as a function of later recalled versus forgotten status, and no significant interaction of later recalled versus forgotten status with LOP (both P > 0.186).
Table I.
Behavioral results
| Measure | LOP | |
|---|---|---|
| Deep | Shallow | |
| Proportion recalled | 0.37 (±0.098) | 0.28 (±0.102) |
| RT later recalled | 1424 (±254) | 1344 (±245) |
| RT later forgotten | 1433 (±242) | 1336 (±238) |
Mean proportions of correctly recalled words during free recall, and mean reaction times (RT) in milliseconds during study for later recalled and later forgotten words, as a function of level of processing (LOP) at study. All data are means ± standard deviations.
fMRI Results
Brain activity differences related to level of processing (LOP)
Irrespective of later memory, deep and shallow study processing engaged extensive, largely nonoverlapping cortical networks. Deep processing, when compared with shallow study processing, was associated with activations in the bilateral medial frontal cortex [Brodmann Area (BA) 6], the left inferior frontal gyrus (IFG; BA 45, 47) and left DLPFC, as well as portions of the left parietal and temporal cortex (Fig. 2, orange). Notably, the activations showed overlap with the default mode network (DMN) (Supporting Information Table SI). The results thus replicate the effects of LOP with the same paradigm [Schott et al., 2006] and with different study tasks [Kapur et al., 1994; Otten et al., 2001]. Conversely, shallow when compared with deep processing was associated with bilateral but predominantly right‐sided activation of a frontoparietal network including right DLPFC and bilateral superior and inferior parietal lobules (Fig. 2, blue; Supporting Information Table SII).
Figure 2.
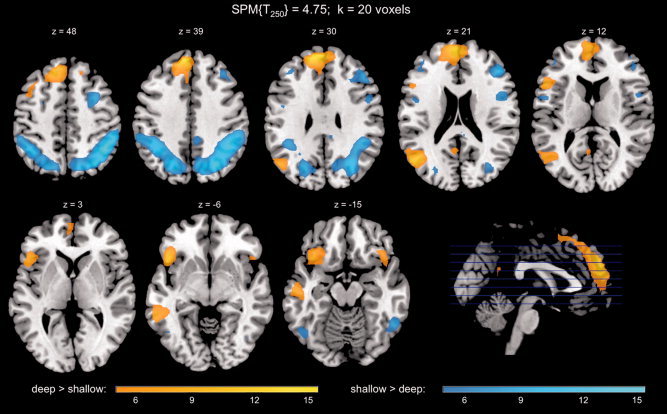
Level‐of‐processing effect. Regions with increased activity during deep versus shallow study included medial and left inferior prefrontal cortex and the left temporoparietal junction (orange‐yellow), whereas shallow study processing was associated with activation of dorsolateral prefrontal and posterior lateral brain structures (blue‐cyan). Activations above a threshold of T250 = 4.75 (P < 0.05, FWE‐corrected; minimal cluster size k = 20 voxels) are displayed. The scale bars display the T value.
LOP effects on brain activity related to successful encoding during deep and shallow study processing
Similar to previous observations [Otten et al., 2001; Schott et al., 2006; Wagner et al., 1998], successful encoding of words (i.e., later remembered vs. later forgotten) was associated with activations of the MTL. When this later memory contrast (DM effect) was tested separately for the deep and shallow study conditions, successful encoding was associated with significant activations in the bilateral hippocampus in both study conditions (Fig. 3A).
Figure 3.
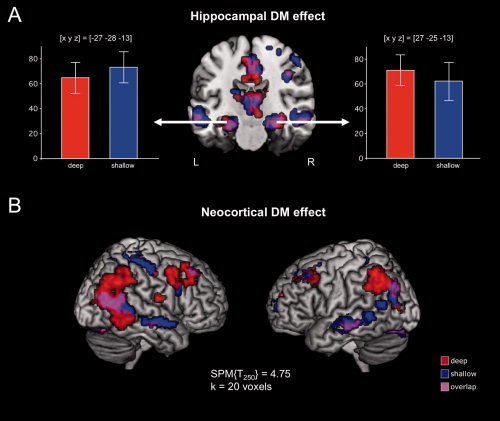
Activations related to successful episodic memory encoding (DM effects: later recalled > later forgotten) in the deep and shallow study conditions.
A: The bilateral hippocampus (arrows) showed robust activations for later recalled relative to later forgotten words during both deep and shallow study processing. Bar plots depict contrasts of parameter estimates ± standard errors in the left (L) and right (R) hippocampus (x, y, z = peak voxel coordinates in Talairach reference space). B: Bilateral prefrontal and parietal cortices showed extensive activations for later recalled relative to later forgotten words during both deep and shallow study processing. Activations above a threshold of T250 = 4.75 (P < 0.05, whole‐brain FWE‐corrected; minimal cluster size k = 20 voxels) are displayed.
In addition to the hippocampal DM effects, successful encoding during both deep and shallow study processing also elicited pronounced activations of the dorsolateral and ventrolateral PFC (DLPFC, VLPFC), particularly the middle frontal gyrus, bilaterally (Supporting Information Table SIII), replicating previous results that suggested a task‐independent role of the PFC in episodic encoding [Baker et al., 2001]. These activations were more pronounced during deep when compared with shallow study processing. Furthermore, activations of bilateral superior and inferior parietal cortices were more extensive during deep than shallow study processing, although they were also observed during shallow study processing. On the other hand, lateral temporal activations were more extensive during shallow relative to deep study processing (Fig. 3B; Supporting Information Tables SIII, IV, and V).
Functional connectivity related to successful encoding during deep and shallow study processing
PPI analyses were conducted in order to investigate encoding‐related changes in functional connectivity between the hippocampus, which was robustly activated bilaterally in relation to successful memory encoding during both deep and shallow study processing, and neocortical brain structures. As with the overall activation patterns during successful encoding, which were essentially similar in the deep and shallow study conditions (see Fig. 2), changes in functional connectivity between the hippocampus and distant brain regions during successful encoding showed some degree of overlap between the two study conditions, but also several differences as a function of study condition (Fig. 4; Tables IV and V).IV, V
Figure 4.
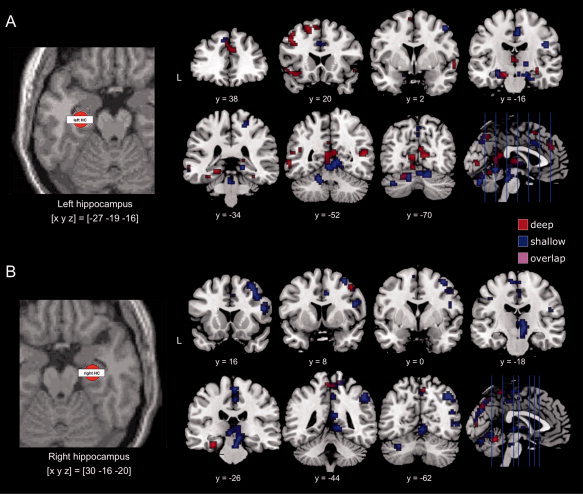
Functional connectivity of the left and right hippocampus related to successful episodic memory encoding in the deep and shallow study conditions.
A: Left panel: Representative volume of interest (VOI) from the left hippocampus of a single participant. VOIs were spheres with a radius of 6 mm and contained between 20 and 33 voxels. Right panel: In the deep study condition, the left hippocampus showed increased functional connectivity with the dorsolateral and ventrolateral prefrontal cortex as well with an extensive midline and parietal network, largely overlapping with the default mode network. These connectivity changes were less pronounced in the shallow condition.
B: Left panel: Representative volume of interest (VOI) from the right hippocampus of a single participant. Right panel: The right hippocampus showed increased functional connectivity with the dorsal and ventral attention networks of the right hemisphere the shallow study condition, whereas connectivity increases of the right hippocampus during deep study were sparse. All displayed activations are thresholded at P < 0.05, whole‐brain FWE‐corrected.
Table IV.
Encoding‐related connectivity changes with the right hippocampus during deep study processing
| Brodmann area (BA) | x y z | SPM{T 125} | |
|---|---|---|---|
| Left parahippocampal gyrus | 36 | −30 −28 −15 | 6.68 |
| Right paracentral lobule | 4, 5 | 6 −45 58 | 6.35 |
| 12 −41 64 | 5.51 | ||
| Right precuneus | 7 | 6 −65 51 | 5.98 |
| Right cerebellum, anterior lobe | 3 −51 −9 | 5.93 | |
| Left cerebellum, anterior lobe | −6 −51 −12 | 5.47 | |
| Left cerebellum, posterior lobe | −33 −72 −23 | 5.88 | |
| −12 −78 −18 | 5.7 |
Local maxima of the PPI contrast in the deep study condition. All coordinates are given in Talairach space (Talairach and Tournoux, 1988).
Table V.
Encoding‐related connectivity changes with the right hippocampus during shallow study processing
| Brodmann area (BA) | x y z | SPM{T 125} | |
|---|---|---|---|
| Right medial frontal gyrus | 6 | 3 −21 60 | 7.45 |
| Left medial frontal gyrus | 6 | −9 −10 62 | 6.8 |
| Right middle frontal gyrus | 6 | 33 13 53 | 7.16 |
| 39 5 53 | 6.17 | ||
| Right inferior frontal gyrus | 9 | 50 15 20 | 6.26 |
| Right precentral gyrus | 6, 8 | 48 −6 23 | 6.85 |
| 36 19 41 | 5.79 | ||
| Right paracentral lobule | 5 | 6 −93 61 | 6.98 |
| Right inferior parietal lobule | 7, 40 | 39 −60 43 | 6.54 |
| 48 −43 39 | 6.13 | ||
| 48 −54 40 | 5.6 | ||
| Right amygdala | 30 −5 −22 | 5.66 | |
| Right posterior cingulate | 29 | 6 −44 17 | 5.7 |
| Right superior tempral gyrus | 39 | 48 −58 20 | 5.52 |
| Right supramarginal gyrus | 40 | 53 −50 20 | 6.34 |
| Left precuneus | 7 | 0 −72 35 | 5.75 |
| Right precuneus | 7 | 6 −65 51 | 6.51 |
| Left cuneus | 19 | 0 −78 30 | 5.6 |
| Right thalamus | 6 −21 −1 | 6.63 | |
| Left thalamus | −12 −30 −3 | 6.38 | |
| Right cerebellum, anterior lobe | 6 −28 −10 | 6.37 | |
| Right cerebellum, posterior lobe | 15 −77 −15 | 6.98 | |
| Left cerebellum, posterior lobe | −6 −77 −13 | 6.62 | |
| −27 −75 −28 | 6.41 |
Local maxima of the PPI contrast in the shallow study condition. All coordinates are given in Talairach space (Talairach and Tournoux, 1988).
PPI of the left hippocampus
In both study conditions, the PPI capturing encoding‐related activations of the left anterior hippocampus revealed significant encoding‐related functional connectivity increases between the hippocampus and bilateral anterior MTL structures (amygdala, parahippocampal cortex), extrastriate visual cortex, the right DLPFC, the medial PFC (extending into the anterior cingulate) and subcortical structures, including the thalamus and the cerebellum (Fig. 4A Tables II and III). In the deep study condition, activity‐dependent left hippocampal–cortical connectivity was generally more pronounced. The left hippocampus showed extensive increases in functional connectivity with a large medial frontoparietal network, including bilateral ventrolateral and dorsolateral PFC (superior frontal gyrus, middle frontal gyrus, and IFG; BA 6, 9, 45, 47) as well as inferior parietal cortex regions and the posterior cingulate (Fig. 4A, top panel; Table II). The observed pattern showed considerable overlap with the DMN [Raichle and Snyder, 2007]. To test for specificity of this increased functional connectivity of the left hippocampus and frontoparietal networks during successful memory formation in the deep study condition, the PPI contrast of the deep condition (thresholded at P < 0.05, whole‐brain FWE‐corrected) was exclusively masked with the PPI contrast of the shallow study condition (thresholded at P < 0.01, uncorrected). Exclusive masking revealed three clusters with preferentially increased hippocampal–cortical connectivity during deep study processing: the left and right VLPFC (Fig. 5A), and the right temporoparietal junction (TPJ; Fig. 5B, left panel). No preferential increases of left hippocampal–cortical connectivity were observed during shallow study processing when the PPIshallow contrast (P < 0.05, whole‐brain FWE‐corrected) was exclusively masked with the PPIdeep contrast (P < 0.01, uncorrected). The exploratory direct t‐test‐based statistical comparison of the PPIdeep and PPIshallow contrasts (P < 0.005, uncorrected, minimum cluster size = 10 voxels) revealed activation clusters that essentially overlapped with those observed in the masking‐based analysis.
Table II.
Encoding‐related connectivity changes with the left hippocampus during deep study processing
| Brodmann area (BA) | x y z | SPM{T 125} | |
|---|---|---|---|
| Left inferior frontal gyrus | 47 | −33 16 −10 | 6.00 |
| −48 19 −8 | 5.91 | ||
| Left medial frontal gyrus | 6, 9 | −6 5 59 | 5.72 |
| −3 39 32 | 5.78 | ||
| Left middle frontal gyrus | 9 | −42 24 33 | 6.07 |
| Left superior frontal gyrus | 6, 9 | −9 16 53 | 7.67 |
| −18 22 50 | 6.46 | ||
| −15 50 29 | 6.03 | ||
| Left precentral gyrus | 6, 9 | −50 −5 29 | 6.14 |
| −42 18 39 | 6.05 | ||
| Right inferior frontal gyrus | 45 | 56 26 8 | 5.97 |
| Right medial frontal gyrus | 9 | 3 41 24 | 6.14 |
| Right middle frontal gyrus | 6, 8 | 45 10 42 | 6.59 |
| 39 2 51 | 5.29 | ||
| Left parahippocampal gyrus/amygdala | 28, 36 | −15 −13 −14 | 8.13 |
| −21 −30 −8 | 5.87 | ||
| −33 −34 −13 | 5.71 | ||
| Right parahippocampal gyrus/amygdala | 35 | 27 −25 −13 | 7.59 |
| 33 −13 −6 | 5.60 | ||
| Left middle temporal gyrus | 21, 37 | −59 −45 −7 | 6.23 |
| −62 −27 −5 | 6.16 | ||
| −50 −42 −5 | 6.62 | ||
| Right superior temporal gyrus | 22, 39 | 56 11 −2 | 5.84 |
| 50 −50 14 | 6.55 | ||
| −53 −55 18 | 6.41 | ||
| Right middle temporal gyrus | 21 | 62 −7 −4 | 6.77 |
| Left posterior cingulate | 29 | −6 −50 12 | 6.60 |
| Right posterior cingulate | 30 | 12 −62 10 | 6.52 |
| Right precuneus | 7 | 3 −57 45 | 5.78 |
| 6 −45 50 | 5.64 | ||
| Left putamen | −24 11 −2 | 5.19 | |
| Right putamen | 30 −1 −7 | 5.45 | |
| Left thalamus | −15 −27 8 | 6.15 | |
| −18 −18 13 | 5.25 | ||
| −3 −18 7 | 6.58 | ||
| Right thalamus | 18 −30 8 | 6.11 | |
| 6 −24 10 | 5.59 | ||
| Left cerebellum, posterior lobe | −21 −83 −18 | 7.15 | |
| Right cerebellum, anterior lobe | 27 −57 −22 | 5.63 | |
| Right cerebellum, posterior lobe | 24 −78 −18 | 6.68 | |
| 6 −80 −13 | 5.61 |
Local maxima of the PPI contrast in the deep study condition. All coordinates are given in Talairach space (Talairach and Tournoux, 1988).
Table III.
Encoding‐related connectivity changes with the left hippocampus during shallow study processing
| Brodmann area (BA) | x y z | SPM{T 125} | |
|---|---|---|---|
| Right middle frontal gyrus | 6, 8 | 42 16 42 | 6.16 |
| 48 4 45 | 5.84 | ||
| Left parahippocampal gyrus/amygdala | 28 | −24 −7 −14 | 7.15 |
| −21 −16 −13 | 6.09 | ||
| −30 −27 −3 | 5.34 | ||
| Right cingulate gyrus | 24 | 3 12 33 | 5.71 |
| Midbrain | 0 −31 −18 | 5.83 | |
| 3 −22 −13 | 5.98 | ||
| 6 −25 −5 | 5.60 | ||
| Left cerebellum, anterior lobe | −30 −57 −19 | 6.22 | |
| −3 −51 −2 | 6.00 | ||
| Left cerebellum, posterior lobe | −18 −66 −16 | 6.34 | |
| −3 −75 −16 | 6.27 | ||
| Right cerebellum, anterior lobe | 6 −45 −2 | 6.14 | |
| 9 −53 4 | 5.81 | ||
| Right cerebellum, posterior lobe | 6 −74 −13 | 6.95 |
Local maxima of the PPI contrast in the shallow study condition. All coordinates are given in Talairach space (Talairach and Tournoux, 1988).
Figure 5.
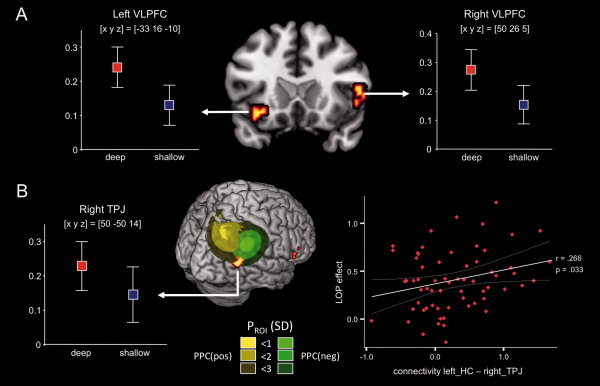
Preferential increases in functional connectivity with the left hippocampus related to successful episodic memory encoding in the deep study condition. Brain regions that showed increased connectivity with the hippocampus in the deep study condition, exclusively masked with the PPI contrast from the shallow study condition (P < 0.01, uncorrected), are displayed.
A: Preferential functional connectivity of the bilateral ventrolateral prefrontal cortex (VLPFC; arrows) with the left hippocampus during deep study processing.
B: Left: Preferential functional connectivity of the right temporoparietal junction (TPJ; arrow) with the left hippocampus during deep study processing. The local maximum was at the intersection of previously reported positive (yellow) and negative (green) DM effects (Uncapher and Wagner, 2009). PPC: posterior parietal cortex DM effect. Right: Increased functional connectivity of these brain regions during deep study processing was positively correlated with the behavioral LOP effect. X‐axis: contrasts of parameter estimates in the TPJ for individual participants. Y‐axis: behavioral LOP effect on later recall for individual participants [(later remembereddeep ‐ later rememberedshallow)/later rememberedshallow]. plots display contrasts of parameter estimates ± standard error (x, y, z = peak voxel coordinates in Talairach reference space).
To identify to which extent connectivity increases in the right parietal cortex overlapped with previously reported DM effects in this region, we generated probabilistic ROIs based on coordinates of previously reported positive and negative subsequent memory effects, which were published in a recent metaanalysis [Uncapher and Wagner, 2009]. The preferential connectivity increase in the right TPJ during deep study processing was located at the inferior intersection of the ROIs generated from previously reported positive and negative DM effects (Fig. 4B, left panel). Using the activation map of the masked PPI contrasts as a mask for the original DM contrast revealed that a positive DM effect was observed in the right TPJ during both deep and shallow study processing (deep: T 250 = 7.77, P < 0.001, whole‐brain FWE‐corrected; shallow: T 250 = 6.81, P < 0.001, whole‐brain FWE‐corrected).
Notably, the preferential functional connectivity of the left hippocampus and the right TPJ during deep study processing was positively correlated, across participants, with the behavioral LOP effect [(later remembered deep − later remembered shallow)/later remembered shallow], as indexed by a relatively higher later recall rate for deep when compared with shallow study processing (r = 0.266; P = 0.033, two‐tailed; Fig. 5B, right panel). A positive across‐participants correlation with the behavioral LOP effect was also observed for the unnormalized difference of the parameter estimates (PPIdeep − PPIshallow: r = 0.259; P = 0.047, two‐tailed). These correlations were specific to connectivity increases during deep study processing, because connectivity increases during shallow study processing did not significantly correlate with the behavioral LOP effect.
PPI of the right hippocampus
Functional connectivity increases of the right hippocampus during successful encoding showed little overlap with those of the left hippocampus (Fig. 4B). Notably, right hippocampal functional connectivity was more pronounced and more widespread during shallow when compared with deep study processing. As in our analysis of left hippocampal functional connectivity, we conducted exclusive masking analyses of the PPIshallow and PPIdeep contrasts. Regions that showed preferential functional connectivity increases with the right hippocampus during shallow study processing included bilateral, but predominantly right, dorsolateral prefrontal and parietal structures, whereas no brain structures exhibited stronger connectivity with the right hippocampus during deep study processing. This network included a portion of the TPJ that was, however, located superiorly to the one that showed increased functional connectivity with the left hippocampus during deep study processing (see Fig. 6). When conducting the exploratory t‐test comparison of the PPIshallow and PPIdeep contrasts (P < 0.005, uncorrected, extent threshold = 10 voxels), a pattern largely overlapping with the masking results was also observed.
Figure 6.
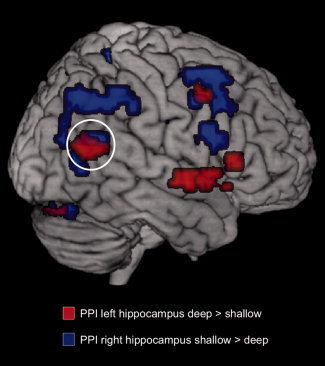
Right temporoparietal connectivity changes during deep and shallow encoding. The right hippocampus showed increased functional connectivity with a region in the right TPJ that was more superiorly located relative to the region that showed increased functional connectivity with the left hippocampus during deep encoding. All displayed activations are thresholded at P < 0.05, whole‐brain FWE‐corrected.
To test how left versus right hippocampal connectivity changes related to main effects of LOP, we calculated the overlap of the LOP contrasts (deep vs. shallow, shallow vs. deep, P < 0.05, FWE‐corrected) and the PPI contrasts (left vs. right hippocampus × deep vs. shallow, P < 0.05, FWE‐corrected). There was considerable overlap between the deep versus shallow contrast and the PPI of the left hippocampus during deep encoding (N = 238 voxels; V = 6.43 cm3) and between the shallow versus deep contrast and the PPI of the right hippocampus during shallow encoding (N = 265 voxels/V = 7.16 cm3), whereas all other overlaps were considerably smaller (all N < 31; all V < 0.84 cm3). This inhomogeneity in the distribution of overlaps was statistically significant (χ2(3) = 478.28; P < 0.0001).
DISCUSSION
Our results complement recent research by showing that successful encoding for later episodic memory is associated with increased functional connectivity between the hippocampus and neocortical brain regions. They also extend previous research by showing that successful encoding during deep study processing, compared with successful encoding during shallow study processing, is accompanied by greater connectivity increases between the left hippocampus and ventral prefrontal regions involved in semantic processing, and midline and inferior parietal brain structures related to social and self‐referential processing and possibly distinctiveness. The results thus begin to provide an idea of the neural basis for the usual superiority of deep over shallow study processing for later episodic memory tests that rely upon semantic‐associative processing, suggesting that this increased connectivity may represent the physiological reason for the more distinctive and semantically elaborate memory traces formed during deep when compared with shallow study processing.
More generally, the results are consistent with an integrative theoretical perspective suggesting that the hippocampus is involved, during encoding, in establishing a record of the ongoing cognitive processing subserved by neocortical regions, which is the precondition for the later reinstatement of that neocortical processing during successful retrieval. The results thus help to reconcile systems perspectives, which view a medial–temporal‐lobe memory system as subserving episodic memory, with processing perspectives, which view memory traces as residing in neocortical areas responsible for ongoing cognitive processing, with memory traces being left behind in these areas as a by‐product of that processing. According to the integrative theoretical perspective, patients with hippocampal damage may have normal ongoing neocortical information processing, but are unable to leave behind a viable record of that processing, owing to the critical coordinatory role of the hippocampus, as revealed here by hippocampal–neocortical connectivity increases during successful encoding.
Functional Connectivity Between the Prefrontal Cortex and Hippocampus During Successful Episodic Encoding
In agreement with the well‐established role of both the PFC and the MTL in episodic memory formation [Buckner et al., 2000; Rugg et al., 2002; Wagner et al., 1998], successful encoding of word stimuli during both deep and shallow study processing was associated with activation of the PFC and large portions of the MTL bilaterally, particularly the anterior hippocampus. Considerable overlap of activations during deep and shallow study processing was observed in the left DLPFC, whereas deep study processing elicited more widespread activations of the right DLPFC. Note that some of the prefrontal areas showing DM effects irrespective of LOP were located more dorsally than the prefrontal areas that typically show DM effects as assessed by later recognition memory tests [Otten et al., 2001; Wagner et al., 1998], although other more ventral areas did also show a DM effect. This pattern accords with the role that the DLPFC plays for memory encoding when the later test benefits from organization (or “chunking”) of the study material [Blumenfeld and Ranganath, 2007], as is the case with the free recall test that we used. Participants may have noticed semantic relationships between studied words despite the study tasks, which emphasized processing of the words as independent units.
Activity‐dependent increases in functional connectivity between the left hippocampus and the PFC accompanying successful encoding during deep study processing were extensive and included bilateral DLPFC and VLPFC as well as medial prefrontal regions. Notably, both the VLPFC and the medial PFC also exhibited a pronounced LOP effect regardless of later memory (see Fig. 1). When the connectivity contrast for successful encoding in the deep study condition was exclusively masked with the corresponding contrast for the shallow study condition, preferential connectivity increases of the hippocampus during deep study processing were observed in the bilateral VLPFC (see Fig. 5). Activations of VLPFC structures, particularly the left IFG, have been commonly observed during successful encoding of verbal stimuli [Buckner et al., 2000; Otten et al., 2001; Rugg et al., 2002; Wagner et al., 1998]. The left IFG is closely linked to and partly overlaps with Broca's language area, and is involved in higher level processing of verbal material [Hagoort, 2005]. Its consistent activation in verbal memory encoding tasks might be related to increased semantic or contextual processing, and enhanced encoding‐related functional connectivity of the left IFG with MTL structures, as revealed here, might reflect integration of the information processed by the left IFG into complex memory episodes [Badre and Wagner, 2007].
Consistent with our results, van Kesteren et al. (2010) found increased functional connectivity of the hippocampus with the VLPFC during during an encoding task in which extensive cognitive processing was required to render new information consistent with a nonobvious knowledge schema. The role of the VLPFC in successful memory encoding has recently also been highlighted by a combined fMRI/diffusion tensor imaging (DTI) study [Schott et al., 2011]. By using DTI‐based fiber tracking between the VLPFC regions activated in the DM paradigm used here and the rhinal cortex, a key input structure of the MTL, it could be demonstrated that the anatomical strength of left VLPFC‐rhinal connections was strongly associated with successful recall performance. Notably, in that study, the relationship between VLPFC‐rhinal fiber tract strength and memory performance was more pronounced in the deep when compared with the shallow study condition. It should be noted, though, that the VLPFC region showing the strongest LOP effect and most pronounced functional connectivity increase during deep study largely consisted of BA 47 and was located inferior to the region identified in the fiber tracking study (which consisted primarily of BA 45). We therefore suggest that semantic processing enhanced semantic processing in BA 47 might facilitate further processes more specifically related to successful encoding in more dorsally located structures of the VLPFC.
A very large behavioral literature in humans [for review, see Richardson‐Klavehn and Bjork, 2002], together with recent results from animal research [Squire, 2007; Tse et al., 2007], show that the integration of novel events into pre‐existing organized knowledge schemas promotes the rapid and effective encoding of new information. Consistent with these findings, LOP effects reflect increased retrieval of organized pre‐existing semantic information relevant to studied items during deep when compared with shallow study processing [e.g., Craik and Tulving, 1975; Richardson‐Klavehn and Bjork, 2002], thus forming more elaborate, distinctive, and retrievable new memory traces as assessed by the majority of episodic memory tests [e.g., Craik, 2002; Fisher and Craik, 1977; Lockhart, 2002; Moscovitch and Craik, 1976]. Our connectivity results with respect to LOP thus complement and extend knowledge regarding the hypothesized role of the VLPFC, and its interaction with the hippocampus, in schematic integration and successful encoding.
Temporoparietal Cortex Involvement in Successful Episodic Encoding
Whereas the increased functional connectivity of the bilateral PFC with the left hippocampus during deep study processing might reflect the hippocampus‐dependent integration of semantically processed information, the significance of the increased connectivity of the left hippocampus and the right TPJ in the deep study condition, and its correlation with the behavioral LOP effect on later recall (see Fig. 5), seem less straightforward. The right TPJ as part of the ventral attention network plays a central role in attentional orienting [for reviews, see Corbetta and Shulman, 2002; Decety and Lamm, 2007]. It activates when attention is captured by behaviorally relevant stimuli [Serences et al., 2005], and tends to deactivate during cognitively demanding tasks like visual search [Shulman et al., 2007] or working memory [Anticevic et al., 2010; Todd and Marois, 2005]. This deactivation goes along with activation in the dorsal attentional control system [Anticevic et al., 2010], and appears to predict successful task performance [Shulman et al., 2007; Todd and Marois, 2005]. The exact role of the TPJ for long‐term memory is still unclear. A recent meta‐analysis [Uncapher and Wagner, 2009] revealed that positive DM effects in this area were only found when the interval between encoding and test was short (as was indeed the case in the present study, with recall after only 20 s of distraction). This pattern led the authors to suggest that the TPJ might be involved in processing superficial (e.g. perceptual) stimulus attributes that are only available for conscious episodic recollection for a restricted time interval [Uncapher and Wagner, 2009]. In line with this notion, right TPJ activation at encoding positively predicts later perceptual priming [Richardson‐Klavehn, 2010b; Wimber et al., 2010]. Such an explanation is well in line with the observation that a TPJ region adjacent to the one that exhibited preferential functional connectivity with the left hippocampus during deep study procesing, showed increased functional connectivity with the right hippocampus during shallow study processing, in which stimulus features such as perceptual distinctiveness are indeed likely to capture attention and thereby promote encoding (see, e.g., Sommer et al., 2006, for distinctiveness effects).
An perhaps more likely, but not necessarily conflicting, explanation of the preferential connectivity increase of the right TPJ and the left hippocampus during deep study processing, and its correlation with the behavioral LOP effect, is that it reflects the role of the right TPJ in mentalizing and social cognition [Decety and Lamm, 2007]. The deep study task was to rate the pleasantness of a word's meaning, which involves reference to internal semantic and emotional representations, and participants were also instructed to perform the task, if necessary, using self‐reference (see Methods). Such self‐referential processing typically recruits medial PFC and posterior cingulate structures [Kelley et al., 2002] that were also active during the deep study task (see Fig. 2). In fact, the network activated by the deep study condition showed considerable overlap with the so‐called DMN, a frontoparietal network that includes midline and bilateral temporoparietal brain structures and is often observed during the “rest” conditions of attention or working memory tasks [Raichle and Snyder, 2007]. Activations of the DMN have been related to self‐reference [Kelley et al., 2002], social cognition [Schilbach et al., 2008] and episodic retrieval [Cansino et al., 2002; Kim and Cabeza, 2007; Schott et al., 2005; Yonelinas et al., 2005]. It must be noted that we observed considerable overlap between the main effect of deep versus shallow study and the connectivity increases of the left hippocampus during successful deep encoding. Enhanced communication of the hippocampus and the right TPJ during deep study processing might therefore reflect hippocampus‐dependent encoding of novel episodes into a larger scale self‐referential and social metacognitive network, namely the DMN. Consistent with this hypothesis, episodic memory retrieval often involves consciousness that the retrieved items reminded one of personally significant events at the time they were earlier encoded [Gardiner et al., 1998].
Recent studies suggest that different subregions within and around the TPJ are involved in attentional and mentalizing functions [Scholz et al., 2009; Young et al., 2010], with the peak activation locations for these different functions differing only within the range of 8–10 mm. TPJ activations related to attention, however, appear to have, on average, higher z coordinates than those related to social processing or mentalizing [Scholz et al., 2009]. Compatible with that observation, the TPJ region that showed increased functional connectivity with the right hippocampus during shallow study processing was located more superiorly than the region that showed increased connectivity with the left hippocampus during deep study processing (Fig. 6). For now, we tentatively suggest that hippocampal–TPJ functional connectivity during successful episodic memory encoding might differ qualitatively for deep and shallow processing by representing social/DMN activity in the former and attentional capture in the latter case.
Laterality and Network Effects During Successful Deep Versus Shallow Encoding
Although functional connectivity of the left hippocampus with ventrolateral prefrontal and temporoparietal brain structures was more pronounced in the deep study condition, a different pattern emerged for the right hippocampus, which showed stronger connectivity with right DLPFC and bilateral (right > left) posterior parietal cortices during shallow when compared with deep encoding. Notably, these brain regions showed considerable overlap with those activated during shallow study processing irrespective of later memory, whereas the main overlap with brain activity related to deep processing was found for the PPI of the left hippocampus during deep encoding (see Fig. 2 and Fig. 4). The brain regions showing increased functional connectivity overlapped largely with the (right‐lateralized) ventral attention network and with the right portion of the dorsal attention network [Corbetta and Shulman, 2002; Fox et al., 2006].
Although the dorsal attention network can typically be observed bilaterally [Fox et al., 2006], little connectivity increase was found between the right hippocampus and left portions of this network. Converging evidence from fMRI, Wada testing and callosotomy patients points to a stimulus‐specific lateralization of episodic memory, with verbal memory episodes being preferentially encoded via the left hemisphere [Kelley et al., 1998, 2002; Miller et al., 2002]. Another laterality phenomenon in episodic memory has been observed in patients with temporal lobe epilepsy (TLE). Although patients with left TLE show primarily familiarity‐based recognition, but deficits in recollection, patients with right TLE exhibit the opposite pattern, suggesting a preferential role for the left hippocampus in recollection‐based memory, even for nonverbal stimuli [Blaxton and Theodore, 1997]. Because the free recall task used in the present study requires recollection‐based retrieval of verbal material, it is conceivable that the stored information might be relatively easily retrieved when the DMN, which is active during recollection [Cansino et al., 2002; Kim and Cabeza, 2007; Schott et al., 2005; Yonelinas et al., 2005], shows increased functional coupling with the left hippocampus during encoding. Along the same line, information might be less readily accessible if shallow encoding engages primarily connectivity changes between the right hippocampus and a right frontoparietal attention network, which is, if at all, rather involved in familiarity‐based recognition memory [Kim and Cabeza, 2007; Yonelinas et al., 2005]. This notion is well compatible with the transfer‐appropriate‐processing model according to which episodes are more likely to be retrieved when the same, or at least related, stimulus properties are processed during encoding and retrieval [Morris et al., 1997; Roediger et al., 2002].
The notion that two distinct patterns of connectivity were observed during shallow study processing—a weak, but present connectivity increase between the left hippocampus and DMN structures and a strong, specific connectivity increase between the right hippocampus and a right frontoparietal attention network—is in good agreement with findings from human electrophysiology. Event‐related potential (ERP) studies of episodic encoding have shown distinct DM effects during deep as opposed to shallow encoding of word stimuli. Specifically, deep encoding has been associated with a late frontal DM effect only, whereas shallow encoding showed an earlier parietal ERP deflection as a function of later remembering that was sensitive to perceptual distinctiveness [Fabiani et al., 1990; Fabiani and Donchin, 1995; Schott et al., 2002]. Notably in one of the studies, these two DM effects were negatively correlated for shallowly studied items [Schott et al., 2002]. Together with our present connectivity findings, these results suggest that successful shallow encoding can occur via two alternative routes, one primarily via incidental deep processing and one via enhanced stimulus processing due to, for example, distinctiveness.
CONCLUSIONS
The present results show that brain activity associated with later free recall engages similar brain regions during deep and shallow study processing, and engages similar connectivity patterns with the hippocampus, consistent with a processing‐independent role for the hippocampus in coordinating and recording neocortical activity patterns. On the other hand, functional coupling of the left hippocampal formation with VLPFC and temporoparietal cortex was relatively enhanced during deep when compared with shallow study processing, whereas the right hippocampus shows enhanced functional connectivity with a right‐lateralized frontoparietal network during shallow study processing. Our findings confirm that connectivity measures can provide important additional information to standard BOLD contrasts. Our PPI approach was directed at activity‐dependent measures of connectivity, but future studies might help to elucidate the influence of level of study processing on more sustained patterns of functional connectivity, possibly by using resting‐state fMRI approaches [Rogers et al., 2007].
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supplementary Tables.
Acknowledgements
We thank Corinna Lauer, Ulrike Malecki, Adrienn Gasde, Kerstin Möhring, Ilona Wiedenhöft, and Claus Tempelmann for assistance with MRI scanning and analysis, and Christine Esslinger for her helpful comments on the manuscript.
Contributor Information
Björn H. Schott, Email: bjoern.schott@med.ovgu.de.
Alan Richardson‐Klavehn, Email: alan.richardson-klavehn@med.ovgu.de.
REFERENCES
- Anticevic A, Repovs G, Shulman GL, Barch DM ( 2010): When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage 49: 2638–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD ( 2007): Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45: 2883–2901. [DOI] [PubMed] [Google Scholar]
- Baker JT, Sanders AL, Maccotta L, Buckner RL ( 2001): Neural correlates of verbal memory encoding during semantic and structural processing tasks. Neuroreport 12: 1251–1256. [DOI] [PubMed] [Google Scholar]
- Blaxton TA ( 1989): Investigating dissociations among memory measures: Support for a transfer‐appropriate processing framework. J Exp Psychol Learn Mem Cognit 15: 657–668. [Google Scholar]
- Blaxton TA, Theodore WH ( 1997): The role of the temporal lobes in recognizing visuospatial materials: Remembering versus knowing. Brain Cogn 35: 5–25. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C ( 2007): Prefrontal cortex and long‐term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 13: 280–291. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J ( 2001): Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage 14: 486–500. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Logan J, Donaldson DI, Wheeler ME ( 2000): Cognitive neuroscience of episodic memory encoding. Acta Psychologica 105: 127–139. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD ( 2002): Brain activity underlying encoding and retrieval of source memory. Cereb Cortex 12, 1048–1056. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME ( 2000): Mapping functionally related regions of brain with functional connectivity MR imaging. Am J Neuroradiol 21: 1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Lockhart R ( 1972): Levels of processing: A framework for memory research. J Verb Learn Verb Behav 11: 671–684. [Google Scholar]
- Craik FIM ( 2002): Levels of processing: Past, present. and future? Memory 10: 305–318. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Tulving E ( 1975): Depth of processing and the retention of words in episodic memory. J Exp Psychol 104: 268–294. [Google Scholar]
- Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, Gordon E, Williams LM ( 2005): Pathways for fear perception: Modulation of amygdala activity by thalamo‐cortical systems. Neuroimage 26: 141–148. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C ( 2007): The role of the right temporoparietal junction in social interaction: How low‐level computational processes contribute to meta‐cognition. Neuroscientist 13: 580–593. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Need AC, LaBar KS, Waters‐Metenier S, Cirulli ET, Kragel J, Goldstein DB, Cabeza R ( 2010): COMT val108/158 met genotype affects neural but not cognitive processing in healthy individuals. Cerebral Cortex 20: 672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C ( 2007): Imaging recollection and familiarity in the medial temporal lobe: A three‐component model. Trends Cogn Sci 11: 379–386. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J ( 2005): The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage 24: 539–547. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H ( 2000): A cortical‐hippocampal system for declarative memory. Nat Rev Neurosci 1: 41–50. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Donchin E ( 1995): Encoding processes and memory organization: A model of the von Restorff effect. J Exp Psychol Learn Mem Cogn 21: 224–240. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Karis D, Donchin E ( 1990): Effects of mnemonic strategy manipulation in a Von Restorff paradigm. Electroencephalogr Clin Neurophysiol 75: 22–35. [DOI] [PubMed] [Google Scholar]
- Fenker DB, Schott BH, Richardson‐Klavehn A, Heinze HJ, Düzel E ( 2005): Recapitulating emotional context: Activity of amygdala, hippocampus and fusiform cortex during recollection and familiarity. Eur J Neurosci, 21: 1993–1999. [DOI] [PubMed] [Google Scholar]
- Fisher R, Craik FIM ( 1977): Interaction between encoding and retrieval operations in cued recall. J Exp Psychol Hum Learn Mem 3: 701–711. [Google Scholar]
- Fletcher PC, Stephenson CME, Carpenter TA, Donovan T, Bullmore ET ( 2003): Regional brain activations predicting subsequent memory success: An event‐related fMRI study of the influence of encoding tasks. Cortex 39: 1009–1026. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME ( 2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A 103: 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Friston K ( 2002): Beyond phrenology: What can neuroimaging tell us about distributed circuitry? Annu Rev Neurosci 25: 221–250. [DOI] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM ( 2004): Brain areas underlying visual mental imagery and visual perception: An fMRI study. Brain Res Cogn Brain Res 20: 226–241. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Java R, Richardson‐Klavehn A ( 1996): How level of processing really influences awareness in recognition memory. Can J Exp Psychol 50: 114–122. [Google Scholar]
- Gardiner JM, Ramponi C, Richardson‐Klavehn A ( 1998): Experiences of remembering, knowing, and guessing. Conscious Cogn 7: 1–26. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ ( 2003): Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. Neuroimage 19: 200–207. [DOI] [PubMed] [Google Scholar]
- Greicius M ( 2008): Resting‐state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 21: 424–430. [DOI] [PubMed] [Google Scholar]
- Guderian S, Düzel E ( 2005): Induced theta oscillations mediate large‐scale synchrony with mediotemporal areas during recollection in humans. Hippocampus 15: 901–912. [DOI] [PubMed] [Google Scholar]
- Guderian S, Schott BH, Richardson‐Klavehn A, Düzel E ( 2009): Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci U S A 106: 5365–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P ( 2005): On Broca, brain, and binding: A new framework. Trends Cogn Sci 9: 416–423. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD ( 2007): Recollection and the reinstatement of encoding‐related cortical activity. Cerebral Cortex 17: 2507–2515. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM ( 1994): Neuroanatomical correlates of encoding in episodic memory: Levels of processing effect. Proc Natl Acad Sci U S A 91: 2008–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE ( 1998): Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron 20: 927–936. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Ojemann JG, Wetzel RD, Derdeyn CP, Moran CJ, Cross DT, Dowling JL, Miller JW, Petersen SE ( 2002): Wada testing reveals frontal lateralization for the memorization of words and faces. J Cogn Neurosci 14: 116–125. [DOI] [PubMed] [Google Scholar]
- Khader P, Rösler F ( 2009): Content specificity of long‐term memory representations In: Rösler F, Ranganath C, Röder B, Kluwe RH, editors. Neuroimaging of Human Memory: Linking Cognitive Processes to Neural Systems. New York: Oxford University Press; pp. 283–298. [Google Scholar]
- Kim H ( 2010): Neural activity that predicts subsequent memory and forgetting: A meta‐analysis of 74 fMRI studies. Neuroimage 54: 2446–2461. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R ( 2007): Trusting our memories: Dissociating the neural correlates of confidence in veridical versus illusory memories. J Neurosci 27: 12190–12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H (2011): Neural activity that predicts subsequent memory and forgetting: a meta‐analysis of 74 fMRI studies. Neuroimage 54: 2446–2461. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG ( 2000): Hippocampal–neocortical interaction: A hierarchy of associativity. Hippocampus 10: 420–430. [DOI] [PubMed] [Google Scholar]
- Mayes A ( 2000): Selective memory disorders In: Tulving E, Craik FIM, editors. Oxford Handbook of Memory. New York: Oxford University Press; pp. 427–440. [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC ( 1995): Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102: 419–457. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ ( 2005): The rewards of music listening: Response and physiological connectivity of the mesolimbic system. Neuroimage 28: 175–184. [DOI] [PubMed] [Google Scholar]
- Mikl M, Marecek R, Hlustík P, Pavlicová M, Drastich A, Chlebus P, Brázdil M, Krupa P ( 2008): Effects of spatial smoothing on fMRI group inferences. Magn Reson Imaging 26: 490–503. [DOI] [PubMed] [Google Scholar]
- Miller MB, Kingstone A, Gazzaniga MS ( 2002): Hemispheric encoding asymmetry is more apparent than real. J Cogn Neurosci 14: 702–708. [DOI] [PubMed] [Google Scholar]
- Morris C, Bransford J, Franks J ( 1977): Levels of processing versus transfer appropriate processing. J Verb Learn Verb Behav 16: 519–533. [Google Scholar]
- Moscovitch M ( 1992): Memory and working‐with‐memory: A component process model based on modules and central systems. J Cogn Neurosci 4: 257–267. [DOI] [PubMed] [Google Scholar]
- Moscovitch M ( 2008): The hippocampus as a “stupid,” domain‐specific module: Implications for theories of recent and remote memory, and of imagination. Can J Exp Psychol 62: 62–79. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Craik FIM ( 1976): Depth of processing, retrieval cues and uniqueness of encoding as factors in recall. J Verb Learn Verb Behav 15: 447–458. [Google Scholar]
- Nadel L, Moscovitch M ( 1997): Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7: 217–227. [DOI] [PubMed] [Google Scholar]
- Nielsen FA, Hansen LK ( 2002): Modeling of activation data in the BrainMap database: Detection of outliers. Hum Brain Mapp 15: 146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L ( 2002): Levels of processing: A view from functional brain imaging. Memory 10: 345–348. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD ( 2001): Depth of processing effects on neural correlates of memory encoding: Relationship between findings from across‐ and within‐task comparisons. Brain 124: 399–412. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD ( 2002): Observing the transformation of experience into memory. Trends Cogn Sci 6: 93–102. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. ( 2007) A default mode of brain function: A brief history of an evolving idea. Neuroimage 37: 1083–1090 (discussion 1097–1099). [DOI] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J ( 2005): Functional connectivity with the hippocampus during successful memory formation. Hippocampus 15: 997–1005. [DOI] [PubMed] [Google Scholar]
- Richardson‐Klavehn A ( 2010a): Prestimulus neural oscillations and their haemodynamic correlates elucidate the cognitive and neural processes of memory formation. Clin Neurophysiol 41: 137–146. [Google Scholar]
- Richardson‐Klavehn A ( 2010b): Priming, automatic recollection, and control of retrieval: Toward an integrative retrieval architecture In: Mace JH, editor. The Act of Remembering: Toward an Understanding of How We Recall the Past. Oxford, UK: Wiley‐Blackwell; pp. 111–179. [Google Scholar]
- Richardson‐Klavehn A, Bjork RA ( 2002). Memory, long‐term In: Nadel L, editor. Encyclopedia of Cognitive Science, Vol. 2 London, UK: Nature Publishing Group; pp. 1096–1105. [Google Scholar]
- Richardson‐Klavehn A, Gardiner J ( 1996): Cross‐modality priming in stem completion reflects conscious memory, but not voluntary memory. Psychon Bull Rev 3: 238–244. [DOI] [PubMed] [Google Scholar]
- Richardson‐Klavehn A, Gardiner JM ( 1998): Depth‐of‐processing effects on priming in stem completion: Tests of the voluntary‐contamination, conceptual‐processing, and lexical‐processing hypotheses. J Exp Psychol Learn Mem Cogn 24: 593–609. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Gallo DA, Geraci L ( 2002): Processing approaches to cognition: The impetus from the levels‐of‐processing framework. Memory 10: 319–332. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, Gore JC ( 2007): Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging 25: 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Johnson JD, Park H, Uncapher MR ( 2008): Encoding‐ retrieval overlap in human episodic memory: A functional neuroimaging perspective In: Sossin WS, Lacaille JC, Castellucci VF, Belleville S, editors. Progress in Brain Research, Vol. 169 Amsterdam, Netherlands: Elsevier; pp. 339–352. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RNA ( 2002): The neural basis of episodic memory: Evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci 357: 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska‐Jagiela A, Fink GR, Vogeley K ( 2008): Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn 17: 457–467. [DOI] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield‐Gabrieli S, Brown EN, Saxe R ( 2008): Distinct regions of right temporo‐parietal junction are selective for theory of mind and exogenous attention. PLoS One 4: e4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Niklas C, Kaufmann J, Bodammer NC, Machts J, Schutze H, Düzel E ( 2011): Fiber density between rhinal cortex and activated ventrolateral prefrontal regions predicts episodic memory performance in humans. Proc Natl Acad Sci U S A 108: 5408–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Henson RN, Richardson‐Klavehn A, Becker C, Thoma V, Heinze HJ, Düzel E ( 2005): Redefining implicit and explicit memory: The functional neuroanatomy of priming, remembering, and control of retrieval. Proc Natl Acad Sci U S A 102: 1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Niehaus L, Wittmann BC, Schütze H, Seidenbecher CI, Heinze HJ, Düzel E ( 2007): Ageing and early‐stage Parkinson's disease affect separable neural mechanisms of mesolimbic reward processing. Brain 130: 2412–2424. [DOI] [PubMed] [Google Scholar]
- Schott B, Richardson‐Klavehn A, Heinze HJ, Düzel E ( 2002): Perceptual priming versus explicit memory: Dissociable neural correlates at encoding. J Cogn Neurosci 14: 578–592. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein H‐G, Tischmeyer W, Gundelfinger ED, Heinze H‐J, Düzel E ( 2006): The dopaminergic midbrain participates in human episodic memory formation: Evidence from genetic imaging. J Neurosci 26: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert R, Ritter P, Wüstenberg T, Preuschhof C, Curio G, Sommer W, Villringer A ( 2008): Spatial attention related SEP amplitude modulations covary with BOLD signal in S1—A simultaneous EEG–fMRI study. Cerebral Cortex 18: 2686–700. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B (1957): Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S ( 2005): Coordination of voluntary and stimulus‐driven attentional control in human cortex. Psychol Sci 16: 114–122. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, McAvoy MP, D'Avossa G, Corbetta M ( 2007): Right TPJ deactivation during visual search: Functional significance and support for a filter hypothesis. Cerebral Cortex 17: 2625–2633. [DOI] [PubMed] [Google Scholar]
- Sommer T, Rose M, Büchel C ( 2006): Dissociable parietal systems for primacy and subsequent memory effects. Neurobiol Learn Mem 85: 243–251. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL ( 2009): Event‐related fMRI studies of episodic encoding and retrieval: Meta‐analyses using activation likelihood estimation. Neuropsychologia 47: 1765–1779. [DOI] [PubMed] [Google Scholar]
- Squire LR ( 2007): Rapid consolidation. Science 316: 57–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE ( 2004): The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL ( 2009): Correlated low‐frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category‐preferential visual regions. Cerebral Cortex 20: 1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain. Thieme. [Google Scholar]
- Todd JJ, Marois R ( 2005): Posterior parietal cortex activity predicts individual differences in visual short‐term memory capacity. Cogn Affect Behav Neurosci 5: 144–155. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM ( 2007): Schemas and memory consolidation. Science 316: 76–82. [DOI] [PubMed] [Google Scholar]
- Tulving E ( 2001). Does memory encoding exist? In: Naveh‐Benjamin M, Moscovitch M, Roediger HL, III, editors. Perspectives on Human Memory and Cognitive Aging: Essays in Honor of Fergus Craik. New York: Psychology Press; pp. 6–27. [Google Scholar]
- Tulving E ( 2002): Episodic memory: From mind to brain. Annu Rev Psychol 53: 1–25. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA ( 2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. NeuroImage 16: 765–80. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD ( 2009): Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual‐attention theory. Neurobiol Learn Mem 91: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren MTR, Fernández G, Norris DG, Hermans EJ ( 2010): Persistent schema‐dependent hippocampal–neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci U S A 107: 7550–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha‐Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M ( 1997): Differential effects of early hippocampal pathology on episodic and semantic memory. Science 277: 376–380. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL ( 1998): Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science 281: 1188–1191. [DOI] [PubMed] [Google Scholar]
- Wang S‐H, Morris RGM ( 2010): Hippocampal–neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol 61: 49–79. [DOI] [PubMed] [Google Scholar]
- Wimber M, Heinze HJ, Richardson‐Klavehn A ( 2010): Distinct fronto‐parietal networks set the stage for later perceptual identification priming and episodic recognition memory. J Neurosci 30: 13272–13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD ( 2005): Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci 25: 3002–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Dodell‐Feder D, Saxe R ( 2010): What gets the attention of the temporoparietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia 48: 2658–2664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supplementary Tables.


