Abstract
Recent advances in social neuroscience research have unveiled the neurophysiological correlates of race and intergroup processing. However, little is known about the neural mechanisms underlying intergroup empathy. Combining event‐related fMRI with measurements of pupil dilation as an index of autonomic reactivity, we explored how race and group membership affect empathy‐related responses. White and Black subjects were presented with video clips depicting white, black, and unfamiliar violet‐skinned hands being either painfully penetrated by a syringe or being touched by a Q‐tip. Both hemodynamic activity within areas known to be involved in the processing of first and third‐person emotional experiences of pain, i.e., bilateral anterior insula, and autonomic reactivity were greater for the pain experienced by own‐race compared to that of other‐race and violet models. Interestingly, greater implicit racial bias predicted increased activity within the left anterior insula during the observation of own‐race pain relative to other‐race pain. Our findings highlight the close link between group‐based segregation and empathic processing. Moreover, they demonstrate the relative influence of culturally acquired implicit attitudes and perceived similarity/familiarity with the target in shaping emotional responses to others' physical pain. Hum Brain Mapp 34:3168–3181, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: empathy, fMRI, racial bias, insula, autonomic, pupil dilation, categorization
INTRODUCTION
Empathy is the defining feature of social interactions that allow us to share and understand others' feelings and intentions. Recent neuroscientific models posit that empathizing may imply the vicarious mapping of others' experiences onto the neural and physiological circuitries involved in the first‐person experience of the same or similar sensations (Decety, 2011; Decety and Jackson, 2004; Gallese et al., 2004; Keysers and Gazzola, 2009; Preston and de Waal, 2002). For instance, the mere perception and/or imagination of someone in pain induces activity in a neural network, which includes structures involved in processing somatosensory aspects of pain as the primary (SI) and secondary (SII) somatosensory cortices (e.g., Akitsuki, 2009; Aziz‐Zadeh et al., 2012; Betti et al., 2009; Bufalari et al., 2007; Keysers et al., 2010; Valeriani et al., 2008; Voisin et al., 2011) and structures coding the motivational‐affective dimensions of pain, such as the anterior insular (AI) cortex, and anterior and mid‐cingulate cortex (Jackson et al., 2005; Lamm et al., 2011; Saarela et al., 2007; Singer et al., 2007, 2004). This suggests that empathy, or at least some forms of it, may trigger automatic resonance mechanisms that allow the interindividual sharing of sensory and affective states (Avenanti and Aglioti, 2006; Avenanti et al., 2009b; Bastiaansen et al., 2009; Decety, 2011; Fitzgibbon et al., 2010; Gallese, 2006). Embodied empathy is not an all‐or‐none phenomenon but is tuned by a variety of factors that provide the required flexibility to respond to the demanding complexities of human social interactions (de Vignemont et al., 1996). Accordingly, interindividual differences such as the empathizer's personality (Avenanti et al., 2009a; Jabbi et al., 2007; Lawrence et al., 2006; Minio‐Palluelo et al., 2010) and previous experiences with an empathy‐triggering situation (Cheng et al., 2007) may influence empathic reactivity. In addition, empathy‐related neural responses seem sensitive to the social context and the appraisal of it (Akitsuki et al., 2009; Lamm et al., 2007a). Importantly, given its intrinsic interpersonal dimension, the target's characteristics and the relationship with the empathizer are key determinants of the intensity and quality of the empathic responses. The perceived fairness (Singer et al., 2006) and social status (Decety et al., 2010) of the target person, along side with the affective link (Cheng et al., 2010; Singer et al., 2004) and similarity with the empathizer (Lamm et al., 2010) are known to strengthen empathic resonance. Two recent studies demonstrated differential neural responses to others' physical pain (Hein et al., 2010) or success/failure (Cikara et al., 2011a) as a function of group affiliation. Both studies reported stronger activity within empathy‐related brain areas, i.e., AI, in response to negative experiences of individuals belonging to the participants' group relative to those of the rival group.
Indeed, humans are extremely prone to categorize and divide others and the self into in‐groups and out‐groups, in a “Us versus Them” fashion (Amodio, 2008; Tajfel, 1981). People spontaneously classify others according to socially relevant categories, such as race, age, and gender, and based on this classification determine who may be the target of favoritism or derogation. Race represents a powerful salient cue to group membership, especially, in the absence of other affiliation factors. Considerable evidence demonstrates that race affects social categorization and evaluation within milliseconds even when processed subliminally (Amodio, 2008; Ito et al., 2010). Several neuroimaging studies revealed that individual scores in behavioral measures of implicit racial bias predicted increased amygdala reactivity to other‐race faces and increased fusiform activity to own‐race faces (Chiao et al., 2008; Cunningham et al., 2004; Golby et al., 2004; Phelps et al., 2000). These findings are of great importance as they suggest that culturally acquired prejudiced attitudes (Dunham et al., 2008) result in automatic and negative emotional responses and impoverished visual processing of other‐race stimuli. The discovery of such neural and physiological markers encouraged researchers to hypothesize different neural responses in empathy‐eliciting situations according to the target's ethnicity. Indeed, a recent study showed greater neural reactivity within the anterior cingulate cortex in response to painful stimulation applied to same versus other‐race faces (Xu et al., 2009). Additionally, increased activity within the medial prefrontal cortex, likely reflecting self‐evaluative processing, in response to scenes depicting emotional suffering of own‐race relative to other‐race individuals predicted greater empathy and altruistic motivation for in‐group members (Mathur et al., 2010). These studies suggest that different emotional reactions are elicited when perceiving the suffering of own‐race and other‐race individuals. Nevertheless, it is not clear whether reduced affective responses for the pain of other‐race members reflect specific racial attitudes or are a reflection of broader intergroup characterization processes such as reduced familiarity/perceived similarity with the target (Liew et al., 2011; Preston and de Wall, 2000; Valentini et al., 2012).
Here, we sought to investigate how racial group membership and racial attitudes affect neural and autonomic responses associated with empathy for pain. Combining event‐related fMRI with measurements of autonomic reactivity, i.e., pupil dilation, we examined White‐Italian and Black‐African participants' reactivity to the physical pain of white and black models. Crucially, we measured subjects' racial attitudes, using both implicit and explicit methods, as well as judgments of familiarity/similarity with the models to explore the mechanisms underlying possible biased empathy‐related responses to the pain of different‐race individuals. Additionally, we presented to participants the pain of a novel/unknown race, i.e., Violet‐skinned models. In this way, we explored empathy‐related brain responses in conditions of remarkable visual unfamiliarity and perceived dissimilarity with the self, but in the absence of racial cues (Avenanti et al., 2010).
We predicted that the perception of pain, irrespective of the model, would be associated with resonant activation of both the affective and sensory nodes of the pain network, as well as with enhanced pupillary changes. Based on the notion of in‐group bias in empathic reactivity, we expected subjects to empathize preferentially with in‐group members (i.e., own‐race) compared to out‐group (i.e., other‐race and violet) models' pain. Most importantly, we predicted reduced empathic resonance with other‐race compared to own‐race members. We further hypothesized that such impaired reactivity to other‐race models' pain could be predicted by participants' (implicit) racial attitudes. Additionally, the comparison between other‐race and violet conditions also allowed us to assess the relative influence of familiarity/perceived similarity and of other‐race disregard on empathic reactivity with out‐group members. If biased empathic responses are uniquely driven by culturally acquired racial attitudes, greater empathic reactivity should be detected for the culturally unmarked violet models relative to other‐race models. Alternatively, if empathic responses are largely influenced by familiarity/perceived similarity, then greater empathy‐related responses should be found for other‐race relative to the unknown and remarkably unfamiliar and dissimilar violet models. Finally, based on previous imaging research on facial race processing, we expected some brain areas (e.g., fusiform gyrus, amygdala) to respond preferentially to own‐race or out‐group stimuli independently of stimulation type.
MATERIALS AND METHODS
Participants
The sample comprised 27 subjects: 14 White‐Caucasian (seven males; mean age = 23.57 years, sd = 4.01) and 13 Black‐African (four males, mean age = 24.26 years, sd = 4.35, mean years in Italy = 7.54, sd = 5.08). All White participants were Italian native and all Black subjects were born in African countries—Burundi, Cameroon, Congo, Nigeria, and Tunes—living in Italy for a minimum of 2.5 years, and were fluent in Italian. All subjects were university students with the exception of four preuniversity students (one White male, one White female, and two Black females) and one graduated worker (White male). All had normal or corrected vision, free from any contraindication to fMRI, and with no history of major psychiatric or neurological problems. All subjects gave written informed consent and the study was approved by the independent Ethics Committee of the Santa Lucia Foundation (Scientific Institute for Research Hospitalization and Health Care). Five additional volunteers took part in the experiment but were excluded from the analysis due to excessive head motion during image acquisition, i.e., a displacement within each functional run >2° or 2 mm.
Visual Stimuli
The visual stimuli consisted of video clips showing right male hands being either deeply penetrated by a hypodermic needle (pain condition) or touched by a Q‐tip (touch condition). Stimulation sites were identical in both conditions, namely, in the first dorsal interosseous muscle region, the abductor digiti minimi muscle region, and in the region between the base of the little and ring fingers. Models were either two White Italians or two Black Africans. Additionally, Violet–skinned models were created by digitally coloring the White and Black models' hands. During the experiment, White and Black subjects were presented with the Violet hands stimuli obtained by coloring the other‐race hands (Black and White hand, respectively), thus removing skin color racial cues while maintaining the morphology of the hands. It is worth of noting that the color and morphology of the hands were the only indicators of the models' race. All videos were manipulated frame‐by‐frame in Photoshop 2.0 (Adobe, CA; http://www.adobe.com) and were identical in size, mean luminance, and motion parameters (i.e., speed, trajectory, and angle). However, due to hands' color differences, only stimuli within the same Model type were matched for contrast, luminance distribution, and color. Images were taken from the first‐person perspective so that subjects would not need to perform mental rotation. To minimize habituation effects, needles of three different sizes were filled with different red‐toned liquids, and three different colored Q‐tips were used. Each video had a total duration of 3,133 ms and started showing a static hand and a still needle/Q‐tip positioned slightly above. After 1,000 ms, the needle/Q‐tip moved towards the hand and penetrated or touched it, respectively. The hand remained perfectly still throughout the stimulation and the holder of the syringe/Q‐tip was not visible at anytime.
Procedure
Participants were positioned in the scanner, in a dimly lit environment. The experimental visual stimuli were presented via a mirror mounted on the MRI headcoil (total display size 19.5° × 14.6° of visual angle). The visual stimuli were back‐projected on a screen behind the magnet, from a computer monitor with 1,024 × 768 screen resolution and 60‐Hz refresh rate. Stimulus presentation was controlled with Cogent2000 (http://www.vislab.ucl.ac.uk/Cogent/). Six event types were organized in a 2 × 3 factorial design: Stimulation (pain or touch) × Model (White, Black, or Violet). A fully randomized event‐related design was used. Each subject completed five functional runs, each consisting in the presentation of 72 stimuli (12 per condition) interleaved with a fixation cross (interstimulus interval) of jittered duration (2,500–3,500 ms). Each run lasted ∼8 min for a total experimental duration of about 50 min. Subjects were only instructed to pay maximum attention to the stimuli and were informed that some questions about the stimuli would be asked at the end of the scanning session.
Behavioral Measures
Measures of racial bias
To assess implicit racial bias, subjects performed a computerized version of the racial implicit association test (IAT) (Presentation software; http://www.neurobs.com/). The IAT measures the easiness and strength of automatic associations between pairs of concepts such as social categories (White and Black individuals) and attributes (good or bad) (for further information regarding the IAT refer to Greenwald et al., 2003). Each participant completed two sequences of the IAT with reversed block orders, one before and one after the functional sessions. D scores of each sequence were computed as suggested by Greenwald et al. (2001) and averaged to create a final IAT D score. D values greater than zero reflect implicit preference for own‐race relative to other‐race individuals.
To measure explicit racial bias we used a selection of questions of the Italian version (Arcuri et al., 1996) of the Subtle and Blatant Prejudice Scale by Pettigrew and Meertens (SPML) (Pettigrew et al., 2004). Larger scores reflect greater reports of racial bias (min = 7; max = 35).
Dispositional empathy
To assess empathic dispositional traits, subjects were asked to complete the Italian version (Albiero et al., 2006) of the interpersonal reactivity index (IRI) (Davis, 1996) a 28‐item self‐ report questionnaire comprising four subscales: two emotional, Empathic Concern (EC, which measures the tendency to feel sympathy and concern for others) and Personal Distress (PD, which measures self‐oriented anxiety when experiencing others in distress), and two cognitive, Perspective Taking (PT, which measures the tendency to take the perspective of others) and Fantasy Scale (FS, which measures the tendency to imaginatively transpose oneself into the feelings and actions of fictitious characters and situations).
Subjective ratings
After the fMRI session, subjects reviewed each video clip and were asked to rate the intensity and unpleasantness of the sensation supposedly felt by the model using a 10‐point likert scale, in which 0 indicated no sensation (intensity or unpleasantness) and 10 maximum sensation imaginable. Additionally, subjects were presented with still pictures of the models' hands and were asked to evaluate how familiar to them was the morphology of each hand and how similar was to their own hand. Ratings were made on a 10‐point Likert scale, in which 0 indicated no familiarity/similarity and 10 indicated maximum familiarity/similarity.
Pupil Dilation
Participants' pupil diameter was monitored by means of an ASL eye‐tracking system that was adapted for use in the scanner (Applied Science Laboratories, Bedford, MA; Model 504). Pupil diameter was sampled at 60 Hz from stimuli onset to stimuli offset. For each trial, baseline correction was performed by subtracting the first sample (at trial onset), from each of the following pupil samples. Additionally, an eight‐point moving unweighted average was applied to smooth the data. Although all stimuli had identical mean luminance, because of hands' colors differences, stimuli were only matched in terms of contrast and luminance distribution within model type. Therefore, to account for possible effects due to such differences, pupil changes to touch stimuli were subtracted from the responses to pain stimuli delivered to the same model. To validate this procedure, we performed paired t‐tests at each time point of the average waveforms of responses to pain and touch videos (across Model and Race conditions), and confirmed larger reactivity to pain relative to touch from 1,400 ms after stimuli onset onwards (all ts > 4.2, all ps < 0.05, Bonferroni corrected for the number of time points). Additionally, pupil data was divided into two different time windows, early and late, according to such time point.
Magnetic resonance imaging and data analysis
A Siemens Allegra (Siemens Medical Systems, Erlangen, Germany) operating at 3T and equipped for echo‐planar imaging (EPI) acquired functional magnetic resonance (MR) images. A quadrature volume head coil was used for radio‐frequency transmission and reception. Head movements were minimized by mild restraint and cushioning. Thirty‐two slices of functional MR images were acquired using blood oxygenation level‐dependent imaging (3.0 × 3.0 × 2.5‐mm thick, 50% distance factor, TR = 2.08 s, TE = 30 ms), covering the entire cortex.
We used the statistical parametric mapping package SPM8 (http://www.fil.ion.ucl.ac.uk) implemented in MATLAB (v 7.1, The MathWorks, Natick, MA) for data preprocessing and statistical analyses. For all participants, we acquired 1,090 fMRI volumes, 218 for each of the five functional runs. The first four image volumes of each run were used for stabilizing longitudinal magnetization and were discarded from the analysis. Preprocessing included rigid‐body transformation (realignment) and slice timing to correct for head movement and slice acquisition delay. Residual effects of head motion were corrected for by including the six estimated motion parameters for each subject as regressors of no interest in the statistical multiple regression model. Slice‐acquisition delays were corrected using the middle slice as a reference. All images were normalized to the standard SPM8 EPI template, resampled to 2‐mm isotropic voxel size, and spatially smoothed using an isotropic Gaussian kernel of 8‐mm FWHM. Statistical inference was based on a random effects approach (Penny and Holmes, 2007). The paradigm is based on a 2 × 3 × 2 factorial design: Stimulation (pain/touch) × Model (Own‐race/Other‐race/Violet) × Race (White/Black). For each participant, the data were best‐fitted at every voxel by convolving each of the six conditions (two stimulation × three models) with the SMP8 hemodynamic response function. The hemodynamic function was time‐locked 1,000 ms after stimuli appearance, corresponding to the time point of needle/Q‐tip movement start, until stimuli offset for a total duration of 2,133 ms. For each subject, contrast images were estimated for each of the six individual conditions. For group analysis, the single‐subjects contrast images of parameter estimates were entered into a mixed design ANOVA with stimulation and model as within‐subjects variables and race as between‐subjects variable. Analyses were performed collapsing data from both groups of subjects. The analysis aimed at determining: (1) the brain areas associated with the observation of pain stimuli irrespective of model and group membership, i.e., the main effect of pain (all pain conditions > all touch conditions); (2) the brain areas showing increased activation to the pain of own‐race models compared to that of the remaining models, i.e., in‐group bias [(pain > touch)own‐race] > [(pain > touch)out‐group]; (3) brain activity showing reduced resonance with racially charged members, i.e., [(pain > touch)own‐race] > [(pain > touch)other‐race]; (4) brain activity reflecting the relative influence of culturally driven prejudice and perceptual similarity/familiarity/novelty in out‐group disregard [(pain > touch)other‐race] > [(pain > touch)violet], and [(pain > touch)violet] > [(pain > touch)other‐race]; (5) whether any brain area responds specifically to the models' race independently of stimulation type, i.e., the main effect of in‐group (all own‐race conditions > all other‐models conditions), and the main effect of out‐group (all other‐models conditions > all own‐race conditions). Please note that we use the terms “own‐race” and “in‐group” as synonyms, while we use “out‐group” to refer to “other‐race” and “violet” models averaged together. Except for the main effects of in‐group and out‐group, all analyses are based on the subtraction of touch from pain responses within model type. Touch stimuli provide a baseline for each model type by minimizing responses related to tactile sensation, action, movement, and nonpain responses related to the models (e.g., salience, novelty, aversiveness). Statistical maps were initially thresholded at voxel level at P < 0.001 uncorrected. Results were reported at cluster level at P < 0.05 corrected for multiple comparisons (Family Wise Correction, FWE), except when specified differently.
Region of interest analyses
To further investigate biased empathic resonance with members of different racial groups, we carried out region of interest (ROI) analyses. Three ROIs were identified, one based on our whole‐brain analysis and two on extant literature. First, whole‐brain analysis revealed a cluster within the left anterior insular cortex (lAI) (−30, 20, −4) activated primarily for the observation of pain delivered to in‐group compared to out‐group models, i.e., [(pain > touch)own‐race] > [(pain > touch)out‐group]. Using ROI analyses in this area, we carried out additional tests breaking down the interaction pattern to highlight the effect of pain (vs. touch) separately for each model, and investigate the psychological factors underlying such biased responses. Additionally, a recent meta‐analysis based on 32 empathy for pain fMRI studies revealed two further areas consistently activated when observing others in pain independently of stimuli type: right anterior insular cortex (rAI) (39, 23, −4) and anterior/medial cingulate cortex (aMCC) (−2, 23. 40) (Lamm et al., 2011). Accordingly, ROIs were created with Marsbar 0.4 (MARSeille Boîte À Région d'Intérêt' SPM toolbox) extracting average BOLD signals from voxel activity within a 8 mm of radius sphere (i.e., matching the FWHM of the smooth parameters) centered at the above mentioned coordinates. Within each individual ROI, we explored differential responses according to models' race, i.e., in‐group bias and direct comparisons between the brain responses to the pain (vs. touch) of the different models. Additionally, we proceeded to investigate if any difference can be observed between Black and White subjects in such contrasts. All statistical results presented are Bonferroni corrected for the number of ROIs, and significance threshold set at P < 0.05.
Correlation Analyses
We were interested in understanding if levels of racial bias could explain the differential brain reactivity to the pain of own‐race versus other‐race individuals. Therefore, we carried out correlation analysis between BOLD activity within the ROIs and both implicit and explicit scores of racial bias. As IAT's D score is computed by considering behavioral responses to own‐race and other‐race individuals (not to violet models), correlation analyses were performed using the contrast [(pain > touch)own‐race models] > [(pain > touch)other‐race models]. Additionally, we investigated possible correlations between perceptions of similarity and familiarity with differential activity within the ROIs to the pain of the different models. Finally, we looked for relationships between pupillary and brain responses. To correct for multiple comparisons P values were multiplied by 9 [three ROIs × three independent categories of variables (scales of racial bias; familiarity/similarity ratings; pupil dilation)], and significance threshold set at P < 0.05, except when specified differently.
RESULTS
Behavioral Measures
IAT scores revealed that both groups showed implicit preference toward in‐group members. However, while White subjects revealed a strong bias toward own‐race individuals (t 13= 6.05, P < 0.001), Black subjects showed weaker, and nonsignificant, racial bias (t 12= 1.525, P = 0.15) (Table 1). Consistent with previous literature (Avenanti et al., 2010; Dunham et al., 2008), the socially dominant group (White‐Italians in our study) revealed greater racial bias compared to that of the ethnic minority (i.e., Black‐Africans) (t 25= 2.93, P = 0.007). Conversely, explicit bias scores (SPML) did not differ between groups (t 25= 0.79, P = 0.44) (Table 1). IRI scores revealed similar empathic traits between groups (all ts < 1.46, all ps > 0.16) (Table 1); with the exception of PD scale, for which Black subjects demonstrated greater levels of personal distress to others' suffering (t 25= 2.71, P = 0.012).
Table 1.
Black subjects and White subjects mean (sd) scores on personality trait measures
| IRI | IAT (D score) | SPML | ||||
|---|---|---|---|---|---|---|
| EC | PT | FS | PD | |||
| Black subjects | 20.3 (3.9) | 18.6 (4.7) | 16.7 (2.6) | 16.00 (3.5) | 0.105 (0.25) | 12.7 (3.4) |
| White subjects | 17.9 (2.6) | 18.2 (4.6) | 17.9 (2.6) | 12.00 (4.0) | 0.377 (0.23) | 12.0 (4.3) |
IRI, interpersonal reactivity index; EC, empathic concern subscale; PT, perspective taking subscale; FS, fantasy scale subscale; PD, personal distress subscale; IAT, racial implicit association test; SPML, adapted version of the Subtle and Blatant Prejudice Scale by Pettigrew and Meertens.
Regarding the subjective ratings of the stimuli, both groups of subjects perceived pain videos as more intense and unpleasant than touch videos (all ts > 119.84, all ps < 0.001). No difference between models (all ts < 1.74, all ps > 0.09) or subjects' groups was observed (all ts < 1.59, all ps < 0.14). Own‐race hands were rated as more familiar than other‐race hands (all ts > 5.88, all ps < 0.001), and violet hands as less familiar than both the others (all ts > 4.02, all ps < 0.001). Regarding the similarity judgments, subjects perceived own‐race hands as more similar to their own than the other two groups of models (all ts > 5.32, all ps < 0.001), and judged both other‐race and violet hands as equally dissimilar (t 26= 1.95, P = 0.18). Together these results confirmed our expectations of greater identification with own‐race models in respect to the remaining, and that violet models were perceived as very unfamiliar (Fig. 1).
Figure 1.
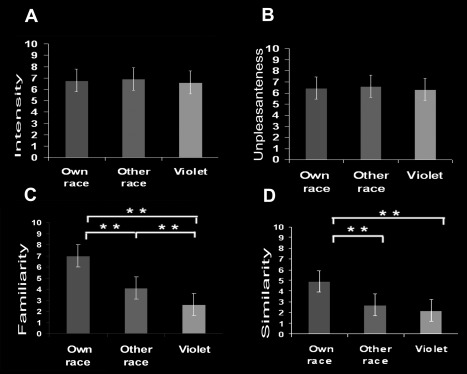
Subjective ratings of familiarity (A) and similarity (B) with the models' hands, and intensity (C) and unpleasantness (D) of the sensation supposedly felt by each group of models. **P < 0.001.
Pupil Dilation
The model × time × race ANOVA on amplitude of pupillary response revealed a main effect of time (F 1,25 = 35.18, P < 0.000003) and of model (F 2,50 = 4.27, P < 0.019) and, importantly, a significant interaction time × model (F 2,50 = 7.62, P < 0.0013). No main effect of race or interaction with this factor was found (Fs < 1.89, all ps < 0.16), suggesting that Black and White individuals reacted in a very similar way to the pain of the three models. In the early time window (when the needle/Q‐tip were approaching the hand and no pain‐related response was detected) response to own‐race models' pain was comparable to other‐race and violet models' pain (P > 0.92); response to other‐race models' pain was slightly but significantly greater than violet models' pain (P = 0.047). A different pattern of results was obtained in the later time window (when the needle was entering the hand and significant pain‐related responses were detected): responses to own‐race were larger than those to other‐race and violet models' pain (all ps < 0.02); moreover, responses to other‐race were larger than responses to violet models' pain (P = 0.013) (Fig. 2).
Figure 2.
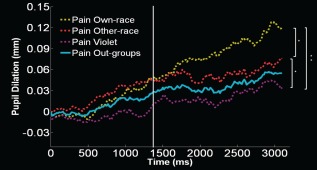
Pupil dilation (mm) over time (ms) in response to the pain (pain > touch) of each model group. The white vertical bar signals the time point defining early and late time windows. Significant statistical differences found only for the late time window. *P < 0.05, **P < 0.001.
fMRI Results
ME pain
We first investigated the hemodynamic responses related to the perception of others in pain, irrespective of model and group membership (i.e., all pain stimuli vs. all touch stimuli). In keeping with previous research, the observation of others in pain resulted in the activation of bilateral clusters in frontoparietal regions (Table 2A; Fig. 3A) known to be involved in action understanding and anticipation, as well as in the evaluation of pain intensity and unpleasantness (Lamm et al., 2007b), including the ventral premotor cortex and the intraparietal sulcus. Notably, parietal lobe activity comprised the postcentral gyri, an area responsible for the sensory representations of pain (i.e., the primary somatosensory cortex, SI). Finally, two bilateral clusters were found in the posterior temporolateral regions encompassing parts of the occipital cortices and cerebellum, likely due to increased visual processing.
Table 2.
Brain areas activated for (A) main effect of pain (i.e., pain stimuli > touch stimuli); (B) main effect of in‐group (i.e., in‐group stimuli > out‐groups stimuli)
| x | y | z | t Value | |
|---|---|---|---|---|
| (A) Main effect of pain | ||||
| Parietal cortex | ||||
| Left postcentral gyrus (BA2)/supramarginal gyrus | −58 | −26 | 38 | 8.54 |
| Left intraparietal sulcus | −38 | −50 | 58 | 4.89 |
| Right postcentral gyrus (BA2) | 62 | −18 | 36 | 7.01 |
| Right intraparietal sulcus | 34 | −58 | 58 | 6.22 |
| Right superior parietal lobe | 28 | −52 | 52 | 5.36 |
| Right inferior parietal cortex | 54 | −32 | 42 | 4.88 |
| Prefrontal cortex | ||||
| Left ventral premotor cortex (BA44) | −60 | 8 | 28 | 4.29 |
| Right ventral premotor cortex (BA44) | 62 | 12 | 16 | 6.48 |
| Temporal cortex, occipital cortex, and cerebellum | ||||
| Left mid‐temporal cortex | −48 | 76 | 4 | 8.6 |
| Left inferior temporal cortex | −44 | −70 | 0 | 8.55 |
| Left cerebellum | −36 | −70 | −20 | 7.11 |
| Right mid‐temporal cortex | 32 | −80 | 26 | 5.08 |
| Right inferior temporal cortex | 48 | −70 | 2 | 12.02 |
| Right cerebellum | 24 | −90 | −14 | 5.3 |
| Right occipital cortex | 32 | −90 | −4 | 5.11 |
| (B) Main effect of in‐group | ||||
| Left occipital cortex | −48 | −76 | −14 | 4.51 |
| Right inferior temporal cortex/inferior occipital gyrus (EBA) | 46 | −70 | −10 | 4.99 |
Initial activation maps were thresholded at voxel level at P < 0.001(uncorrected) and clusters significance set at P < 0.05 (FWE‐corrected). Reported coordinates correspond to local maxima of the respective clusters, and are defined in Montreal Neurologic Institute (MNI) stereotactic space.
Figure 3.
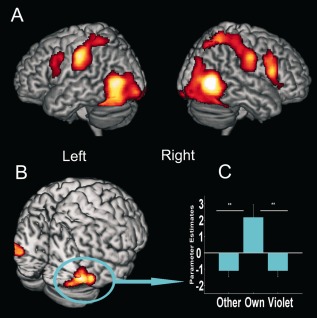
Brain responses associated with (A) main effect of pain (contrast: (all pain stimuli > all touch stimuli), and (B) main effect of group membership (contrast: [(pain + touch) own‐race > (pain + touch) out‐groups]). (C) Parameter estimates extracted from the cluster in the right inferior temporal cortex, including extrastriate body area (EBA), associated with the observation of each model group (other‐race, own‐race, violet) independently of stimulation type (pain + touch). P < 0.05 (FWE) at cluster level.
ME of Group Membership
Two clusters, in the inferior temporal‐occipital region, bilaterally, and including the extrastriate body area (EBA),1 an area known to process body parts, revealed increased activity for own‐race relative to out‐group models irrespective of stimulation type (Table 2B; Fig. 3B,C). No significant activation was found for the inverse comparison, i.e., greater activation for stimuli depicting out‐group models compared to in‐group models.
Own‐race empathy‐related bias
To test our main hypothesis of increased empathic resonance towards own‐race individuals, we tested for areas with greater responses to the pain of own‐race models compared to that of other models [(pain > touch)own‐race > (pain > touch)out‐group)]. Whole brain analysis revealed increased activation in the lAI (t = 4.56, P = 0.030) (Fig. 4A), an area that has been consistently reported as responsible for encoding affective aspects of self and observed pain (Lamm et al., 2011). The inverse contrast [(pain > touch)out‐group) > (pain > touch)own‐race] did not reveal any significant activation.
Figure 4.
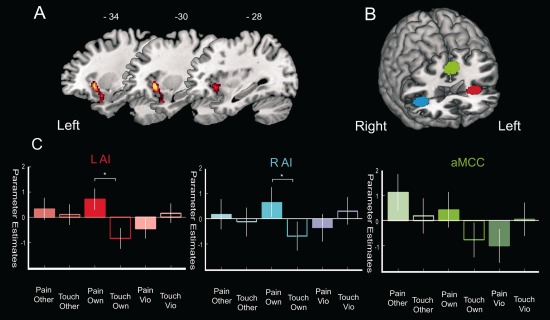
(A) Activation in the left anterior insula reflecting in‐group bias in empathic reactivity (contrast: [(pain > touch) own‐race > (pain > touch) out‐groups)]), P < 0.05 (FEW) at cluster level. (B) 3D rendering of the canonical MNI template showing the location of the three ROIs—left anterior insula (lAI; red), anterior medial cingulate cortex (aMCC; green), and right anterior insula (rAI; blue). (C) Parameter estimates extracted from each region of interest (ROI) when subjects observed pain and touch being delivered to each group of models (other‐race, own‐race, Violet), *P < 0.05.
Whole‐brain analysis exploring empathic bias towards culturally charged racial members, i.e., [(pain > touch)own‐race > (pain > touch)other‐race)], and those exploring the importance of racial attitudes vs similarity and familiarity/novelty in out‐group disregard, i.e., [(pain > touch)other‐race models] > [(pain > touch)violet models] and [(pain > touch)violet models] > [(pain > touch)other‐race models], did not show any significant activation at the selected threshold. Data inspection at uncorrected level revealed activity in main areas related to empathic responding to pain stimuli (Lamm et al., 2011; see Methods section). Specifically, in the lAI (30; 20; −2) (t = 3.36) for the contrast own‐race vs. other‐race and in the aMCC (0; 34; 28) (t = 3.78) for the contrast other‐race vs. violet models. Additionally, we restricted the search volume (using small volume correction SPM function) to the brain areas responding to pain stimuli (i.e., main effect of pain) but found no further significant activity for the contrasts of interest.
To further investigate differential empathy‐related responses to the pain of the different models, we carried out ROI analyses on the lAI, rAI, and aMCC (Fig. 4B,C; Table 3). As expected from whole‐brain analysis, in‐group bias was observed in the lAI, with the dissection of activity pattern in the lAI revealing decreased responses to pain delivered to both other‐race and violet models. Additionally, in‐group bias was also found in the rAI, but not in the aMCC, being this difference mostly explained by decreased response to violet models pain compared to that of own‐race. On the other hand, the aMCC mirrored whole‐brain (uncorrected) activity levels showing enhanced reactivity to own‐race and other‐race pain relative to violet models. Finally, analysis on both groups separately revealed in‐group bias in the lAI (White: t = 3.64, P < 0.001; Black: t = 2.77, P = 0.011). Only in the white group however this difference reached significance in the rAI (t = 2.74, P = 0.012). No significant activations emerged from the between group analyses.
Table 3.
Biased empathy‐related brain responses within each region of interest (ROI)
| Coordinates | own > (other and violet) | own > other | own > violet | other > violet | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | Z | t‐score | P‐corr | t‐score | P‐corr | t‐score | P‐corr | t‐score | P corr | |
| lAI | −30 | 20 | −4 | 4.54 | <0.001 | 2.96 | 0.006 | 4.28 | <0.001 | – | ns |
| rAI | 39 | 23 | −4 | 2.43 | 0.025 | – | ns | 2.71 | 0.012 | – | ns |
| MCC | −2 | 23 | 40 | – | Ns | – | ns | 2.36 | 0.029 | 2.39 | 0.027 |
Anatomical coordinates of ROIs, t‐scores, and P values (Bonferroni corrected) for each contrast of interest reflecting biased hemodynamic responses to the pain (vs. touch) of the different models.
Correlation Analyses
We found a linear relationship between IAT scores and increased activity in the lAI for own‐race pain compared to other‐race pain (r = 0.577, P = 0.006) (Fig. 5A). These finding support the notion that implicit racial bias inhibits empathic‐related brain responses towards other‐race individuals. No correlations were found with explicit measures of bias (all ps > 0.74).2
Figure 5.
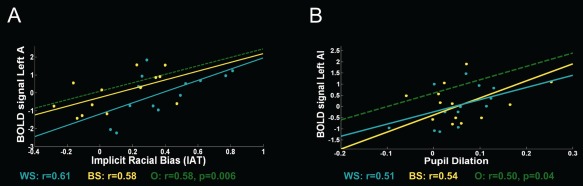
(A) Correlation between the mean activity within the lAI ROI for the contrast [(pain > touch) own‐race > (pain > touch) other‐race)] and individual scores in the racial implicit association test (IAT). Greater activity for own‐race models was associated with higher racial bias. (B) Correlation between the mean activity within the lAI ROI and pupil dilation (mean averaged values) for the contrast [(pain > touch) own‐race > (pain > touch) out‐groups)]. WS, white subjects; BS, Black subjects; O, overall correlation.
No correlation related to subjective ratings remained significant after the corrections adopted for multiple comparisons. Importantly, we observed a positive relation between activity in the lAI [contrast: (pain > touch)own‐race) > (pain > touch)other‐race] and familiarity ratings (r = 0.465, P = 0.011, uncorrected; P = 0.096, corrected); and a negative correlation between activity in the aMCC (same contrast as for lAI) and differences in perceived similarity (r = −0.428, P = 0.013, uncorrected; P = 0.12, corrected). We then carried out a subject‐based parametric regression with the IAT and both similarity and familiarity ratings to explore the relative importance of each variable predicting biased empathic reactivity with other‐race members. When accounting for all the variables, IAT still significantly predicted increased resonance with own‐race members (vs. other‐race) in the lAI (P = 0.018, corrected). On the other hand, the correlations with similarity and familiarity ratings were no longer present, P = 0.17 (uncorrected) and P = 0.095 (uncorrected), respectively.
Finally, a positive correlation was found between enhanced activity within the lAI and pupil responses to the pain of own‐race models compared to that of out‐group models (r = 0.501, P = 0.036) (Fig. 5B). Such relationship supports both the finding of empathic resonance modulation by group membership and the use of pupil dilation as a measure of autonomic reactivity in empathy eliciting situations.
DISCUSSION
In the present study, we compared the BOLD and autonomic responses associated with the observation of pain stimuli delivered own‐race, other‐race, or violet‐skinned hands. We observed increased autonomic activity and brain activations in the anterior insula, which is an important empathy‐related area, in response to the pain of own‐race members compared to that of the remaining models. This result expands the notion of in‐group bias in empathic reactivity. Most importantly, we provide evidence that impaired resonance with culturally marked racial groups in the lAI can be predicted by the levels of negative implicit attitudes towards that specific race. Additionally, the pain of models of an unknown race, with no social connotations, elicited less activity in the bilateral insula and aMCC, suggesting less motivation to resonate with these remarkably unfamiliar and dissimilar models. Together, we demonstrate the relative influence of different intergroup segregation features, when resonating with the pain of individuals of distinct racial groups.
Consistent with previous research, the perception of pain in others brought about activation of sensorimotor and affective areas involved in first‐person experience of pain (Bastiaansen et al., 2009; Lamm et al., 2011). In particular, watching a needle entering the hand of strangers models activated a frontoparietal network that is known to be involved in action anticipation and understanding (Aglioti and Pazzaglia, 2011; Avenanti and Urgesi, 2011; Cattaneo and Rizzolatti, 2009; Gallese, 2006), as well as in the evaluation of pain stimuli intensity and unpleasantness (Avenanti et al., 2007; Lamm et al., 2007b). Additionally, increased SI activation in pain compared to touch conditions, confirms the vicarious mapping of the sensory qualities of the stimulation (e.g., Avenanti et al., 2007; Bufalari et al., 2007; Keysers et al., 2010; Lamm et al., 2007b; Valeriani et al., 2008; Voisin et al., 2011). While motor and somatic regions were similarly activated by seeing the pain of in‐group or out‐group members, the structures encoding the motivational‐affective aspects of pain, i.e., bilateral AI and aMCC showed model‐related selectivity. Confirming the hypothesis of in‐group bias, the bilateral anterior insula was found more active in response to the pain of own‐race members than to that of out‐group individuals. Consistently, the pattern of concurrent autonomic activity also revealed larger pupillary responses to the pain of in‐group vs out‐group individuals. Such in‐group bias paralleled activity in the lAI, confirming greater emotional engagement with the pain of same‐race members.
Intergroup categorization is an automatic feature of human behavior and a powerful source of self‐identification (Amodio, 2008; Tajfel, 1981). In the present experiment, group categorization cues were only based on the physical features of the models' hands, i.e., skin color, and previous associations with racial groups. Although we cannot determine whether judgments of similarity with the models are the cause or the consequence of group categorization, similarity ratings and hemodynamic responses to models independently of simulation type clearly suggest that subjects identified themselves with the models of their own‐race. The pattern of activation in the inferior temporal cortex (Fig. 3B,C), most importantly within the EBA, an area know to process body parts with preference to the self body and/or emotional body expressions (Downing et al., 2001; Peelen et al., 2007; Urgesi et al., 1981, 2004; Vocks et al., 2010), indicates advantaged processing of same‐race stimuli. Activity within EBA may reflect top‐down modulation due to increased attention toward same‐race bodies no matter whether innocuously or painfully stimulated (Downing and Peelen, 2011; Urgesi and Avenanti, 2011). In keeping with this notion, greater orienting response and faster autonomic reactivity (as indexed by heart beat and skin conductance response) was previously found when seeing stimuli delivered to same‐race body (Avenanti et al., 2010). Notably, previous imaging research has demonstrated increased activation in the fusiform face area in response to in‐group faces compared to out‐group faces, likely reflecting increased perceptual expertise or enhanced motivation (Golby et al., 2001; Van Bavel et al., 2008, 2008). We extended these findings by showing a similar processing bias for in‐group body parts, and provide further support to the notion that self‐identification and intergroup distinction occur at an early processing stage.
Studies on empathy for pain showed biased neural empathic responses according to racial group membership (Mathur et al., 2010; Xu et al., 2009). However, previous imaging research did not establish whether such biased empathic brain responses are linked to specific racial attitudes or to broader intergroup categorization processes.
In the present study, we observed diminished autonomic responses and decreased activity in the lAI to the pain of other‐race race members compared to that of own‐race. Most importantly, we observed that individual IAT scores could predict the left insular cortex BOLD responses evoked by the observation of pain stimuli delivered to own‐race relative to other‐race members, even when accounting for perceived similarity/familiarity ratings. The IAT scores reflect implicit preferences, i.e., often without awareness, about social groups that are believed to be mainly a product of cultural influences and personal experiences.3 Previous research has shown that such racial evaluations could predict amygdala activity to other‐race faces (Cunningham et al., 2004; Phelps et al., 2000) and reduced sensorimotor empathic resonance with other‐race pain (Avenanti et al., 2010). We extend these findings by demonstrating that IAT scores can predict affective‐motivational brain responses to the pain of different race individuals. Interestingly, no relationship was found with the levels of explicit bias, in keeping with the notion that racial prejudice influences interpersonal reactivity at an unconscious level (Amodio, 2008; Avenanti et al., 2010; Dunham et al., 2008; Ito et al., 2010).
The comparison of the hemodynamic responses to the pain experienced by other‐race and violet models, allowed us to highlight the relative effect of perceived familiarity/similarity in shaping empathy‐related responses to the pain of out‐group individuals in the absence of previous racial associations. We observed greater aMCC activity and autonomic responses to the pain of other‐race models relative to violet models, supporting the key role of familiarity/similarity in empathic resonance with members of an unknown race. Such pattern, however, was not found in the AI that, together with the aMCC, is involved in processing the motivational‐affective aspects of self and others' pain (Lamm et al., 2011). A possible explanation for these differential responses may be related to the different roles of the insular and cingulate cortices in emotional processing and in the experience of pain. The insular cortex, is an interoceptive cortex, involved in mapping internal bodily states and in representing emotional arousal and feelings (Critchley, 2005). In particular, activity in the AI is believed to be the final product of the integration of physiological signals with motivational and social conditions represented in other parts of the brain, providing a meta‐representation of the “global emotional moment” (Craig, 2009). In other words, the AI is most likely the brain region that better reflects the subjective feeling state associated with the vicarious experience of pain. In the present experiment, the subjective experience of resonating with the models' pain is likely to be the result of the integration of the vicarious autonomic responses with the social and perceptual features associated with each model. We found no difference in activity in the AI for the pain of other‐race and violet models, suggesting similarly reduced empathy‐related feelings for both out‐groups. In keeping with the notion of in‐group bias (Avenanti et al., 2010; Hein et al., 2010; Liew et al., 2011; Mathur et al., 2011, 2010; Van Bavel et al., 2011; Xu et al., 2009) subjects resonated preferentially with models more similar to themselves and tended to resonate less with the models associated to negative social connotation or unfamiliar features. It is worth noting that, the cingulate cortex is believed to be mostly related with the motivational and volitional aspects of pain processing, such as preparation and regulation of associated motor and autonomic responses (Craig, 2002; Critchley, 2005; Fan et al., 2011; Medford et al., 2011; Vogt et al., 2003). Thus, decreased aMCC and autonomic activity to violet models' pain suggest less motivation to respond to the pain of models with particularly dissimilar and unfamiliar/implausible features. Another non‐exclusive possibility is that increased activity in the aMCC for other‐race relative to violet pain might reflect an effort to inhibit unwanted race‐biased responses (Bartholow et al., 2010; Ito et al., 2010). Indeed, we found a seemingly counterintuitive relationship between reported judgements of similarity and activity in the aMCC for the contrast pain of own versus other‐race members. Although not significant at a corrected threshold, this relationship seems to suggest the influence of a top‐down mechanism where the larger the perceived dissimilarity between these two groups of models the less bias could be observed in the aMCC. In sum, that differences between other‐race and violet conditions were particularly visible in the aMCC prompt us to suggest that by coloring other‐race hands in violet, we decreased the motivation to resonate with this novel/strange race and/or decreased the motivation to inhibit unwanted biased responses toward these unknown models. Nevertheless, future studies designed to disentangle the functional roles of the insular and cingulate cortices in empathy‐eliciting situations (Gu et al., 2010; Valentini, 2010), or using more sensitive measures of the motivation to inhibit bias (e.g., Plant et al., 1998), are needed to elucidate this further.
Increased resonance with own‐race members' pain seems to arise from the interaction between the responses to pain and touch stimuli and not only from increased response to pain per se. This pattern was previously reported (e.g., Gou et al., 20011; Perry et al., 2010) and suggests that vicarious experiences are differently mapped according to perceived valence (Bufalari et al., 2007; Jabbi et al., 2007; Morisson et al., 2011) and as a function of the target (Cikara et al., 2011a). Interestingly, Cikara et al. (2011a) recently found opposite patterns of AI responses to positive, neutral, and negative events occurring to differently socially charged targets. Future studies with extra control conditions and with designs allowing a better estimation of baseline BOLD activity might help to reach a better understanding of the meaning of touch or neutral conditions in empathy‐related studies.
By showing greater emotional responding to the pain of in‐group members, the present fMRI study extents the results of our previous TMS study where greater sensorimotor resonance to others' pain was found in‐group relative to out‐group individuals (Avenanti et al., 2010). Thus, when facing the physical pain of others, both emotional and sensorimotor brain regions may show a bias in empathic responding as a function of racial membership and implicit racial bias. However, in contrast to our previous study, no race‐related modulation of sensorimotor activity was found in the present experiment. The lack of such modulation may be due to difference in the experimental design (event‐related vs. block design) and/or to the different techniques (fMRI vs. TMS) used in the two studies. Studies suggest that sensorimotor regions are less consistently modulated than emotional brain regions in empathy for pain (Lamm et al., 2011). Moreover, TMS may be particularly adept in detecting weak effects in sensorimotor cortices (Avenanti et al., 2009b; Fourkas et al., 2008; Singer and Frith, 2005).
It is also worth noting that the pattern of autonomic activity paralleled brain responses of in‐group biased reactions to others' pain. These findings complement and extend previous studies showing that during social perception facial mimicry and autonomic measures, such as heart beat and skin conductance response (Avenanti et al., 2010; Brown et al., 2006; Yabar et al., 2006), may be modulated by group membership, and reveal pupil dilation as an important physiological marker of intergroup empathic processing.
Finally, we advocate some caution in the interpretation of some of the results of this study. First, empathy is a complex phenomenon that cannot be fully grasped by individual studies alone. While we provide evidence for brain and autonomic reactivity to others' pain, our study does not deal with all the possible aspects of empathy. Second, it is worth noting that by coloring other‐race hands in violet we decreased the perception of familiarity/similarity with the models. However, we cannot exclude the possibility that the bias found for violet hand models is partially due to the novelty/implausibility of this stimulus. Given their intrinsic relation, the race effect cannot be differentiated from the effects of perceived familiarity/similarity. Therefore, we may only conclude that biased responses to these models' pain are due to remarkable dissimilarity and unfamiliarity/novelty with this “new” race. In any case, the role of perceived familiarity/similarity in shaping empathy‐related brain responses finds support in the correlation analyses with other‐race member's pain.
In sum, we provide neural and autonomic evidence of in‐group bias in empathic reactivity and demonstrate that both perceived familiarity/similarity and racial attitudes modulate motivational and affective responses to out‐group members' pain. Although humans may be hard‐wired to empathize with everyone, they seem to preferentially resonate with the pain of individuals belonging to the same social group. Advantaged resonance with relevant others may be crucial to maintain and strengthen the bonds that unite people particularly in situations of potential threat, like pain, or competition over resources, when favoring close others may be of great value. In conclusion, our findings suggest that in‐group and out‐group segregation may be at the core of intergroup empathic processing. Moreover, automatic and unconscious attitudes, such as implicit racial bias, play a key role modulating the neural correlates of interpersonal reactivity.
Footnotes
Confirmed by masking whole brain activation with a sphere with a 8 mm of radius (i.e., matching the FWHM of the smooth parameters) centered on mean coordinates of peak activity in independent studies (right: 47.6, −69.3, 0.8; left: −48.5, −72.7, 4; Moro et al., 2008) (all ts > 4.24, all ps < 0.001, FWE voxel level).
To explore the possibility of conflict between implicit and explicit measures of bias, i.e., dissonance between automatic and controlled attitudes, we created an index of conflict consisting in the product of the IAT and SPML scores (see Cunningham et al., 2004). However, we found no relation between this measure and brain activity.
We adopted the IAT's standard approach (Greenwald et al., 2003) and assume that D scores simply reflect automatic preferences toward Black or White individuals. However, there are other emerging views on the IAT's interpretation. For instance, some authors argue for multiple processes underlying performance on the IAT and social attitudes in general (Conrey et al., 2005). Also, there is now some conflicting evidence suggesting that the IAT either reflects specific bias towards the targeted groups (e.g., Dasgupta et al., 2009) or instead a general preference for in‐group/out‐group members (van Ravenzwaaij et al., 2011). For further discussion on the interpretation, limitations and future directions of implicit measurements see: Nosek and colleagues (2011); De Houwer and colleagues (2009).
REFERENCES
- Akitsuki Y, Decety J (2009): Social context and perceived agency modulate brain activity in the neural circuits underpinning empathy for pain: An event‐related fMRI study. NeuroImage 47:722–734. [DOI] [PubMed] [Google Scholar]
- Albiero P, Ingoglia S, Lo Coco A (2006): Contributo all “adattamento italiano dell” interpersonal reactivity index. Testing Psychometr Methodol Appl Psychol 13:107–125. [Google Scholar]
- Amodio DM (2008): The social neuroscience of intergroup relations. Eur Rev Soc Psychol 19:1–54. [Google Scholar]
- Arcuri L, Boca S (1996): Pregiudizio e affiliazione politica: Destra e sinistra di fronte all'immigrazione dal terzo mondo In: Legrenzi P, Girotto V. editors.Psicologia e Politica.Milan:Raffaello Cortina; pp.241–274. [Google Scholar]
- Aglioti SM, Pazzaglia M (2011): Sounds and scents in (social) action. Trends Cogn Sci 15:47–55. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Aglioti SM (2006): The sensorimotor side of empathy In: Mancia M, editor.Psychoanalysis and Neuroscience.Milan:Springer‐Verlag Italia; pp235–256. [Google Scholar]
- Avenanti A, Bolognini N, Maravita A, Aglioti SM (2007): Somatic and motor components of action simulation. Curr Biol 17:2129–2135. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Minio‐Paluello I, Bufalari I, Aglioti SM (2009a): The pain of a model in the personality of an onlooker: Influence of state‐reactivity and personality traits on embodied empathy for pain. Neuroimage 44:275–283. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Minio‐Paluello I, Sforza A, Aglioti SM (2009b): Freezing or escaping? Opposite modulations of empathic reactivity to the pain of others. Cortex 45:1072–1077. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Sirigu A, Aglioti SM (2010): Racial bias reduces empathic sensorimotor resonance with other‐race pain. Curr Biol 20:1018–1022. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Urgesi C (2011): Understanding “what” others do: Mirror mechanisms play a crucial role in action perception. Soc Cogn Affect Neurosci 6:257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz‐Zadeh L, Sheng T, Liew SL, Damasio H (2012): Understanding otherness: The neural bases of action comprehension and pain empathy in a congenital amputee. Cereb Cortex 22:811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Henry EA (2010): Response conflict and affective responses in the control and expression of race bias. Soc Pers Psychol Compass 4:871–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Keysers C (2009): Evidence for mirror systems in emotions. Philos Trans R Soc Lond B Biol Sci 364:2391–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti V, Zappasodi F, Rossini PM, Aglioti SM, Tecchio F (2009): Synchronous with your feelings: Sensorimotor {gamma} band and empathy for pain. J Neurosci 29:12384–12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Bradley MM, Lang PJ (2006): Affective reactions to pictures of ingroup and outgroup members. Biol Psychol 71:303–311. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM (2007): Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex 17:2553–2561. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Rizzolatti G. (2009): The mirror neuron system. Arch Neurol 66:557–560. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, Hung D, Decety J (2007): Expertise modulates the perception of pain in others. Curr Biol 17:1708–1713. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chen C, Lin CP, Chou KH, Decety J (2010): Love hurts: An fMRI study. Neuroimage 51:923–929. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Iidaka T, Gordon HL, Nogawa J, Bar M, Aminoff E, Sadato N, Ambady N (2008): Cultural specificity in amygdala response to fear faces. J Cogn Neurosci 20:2167–2174. [DOI] [PubMed] [Google Scholar]
- Cikara M, Botvinick MM, Fiske ST (2011a): Us versus them: Social identity shapes neural responses to intergroup competition and harm. Psychol Sci 1: 22:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikara M, Fiske ST (2011b): Bounded empathy: neural responses to outgroup targets' (mis)fortunes. J Cogn Neurosci 23:3791–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD (2005): Forebrain emotional asymmetry: A neuroanatomical basis? Trends Cogn Sci 9:566–571. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel‐now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD (2005): Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493:154–166. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR (2004): Separable neural components in the processing of black and white faces. Psychol Sci 15:806–813. [DOI] [PubMed] [Google Scholar]
- Davis MH (1996):Empathy: A Social Psychological Approach.Madion:Westview Press. [Google Scholar]
- de Vignemont F, Singer T (2006): The empathic brain: How, when and why? Trends Cogn Sci 10:435–441. [DOI] [PubMed] [Google Scholar]
- Decety J (2011): Dissecting the neural mechanisms mediating empathy. Emotion Rev 3:92–108. [Google Scholar]
- Decety J, Jackson PL (2004): The functional architecture of human empathy. Behav Cogn Neurosci Rev 3:71–100. [DOI] [PubMed] [Google Scholar]
- Decety J, Echols S, Correll J (2010): The blame game: The effect of responsibility and social stigma on empathy for pain. J Cogn Neurosci 22:985–997. [DOI] [PubMed] [Google Scholar]
- Downing PE, Peelen MV (2011): The role of occipitotemporal body‐selective regions in person perception. Cogn Neurosci 2:3–4, 186–203. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N (2001): A cortical area selective for visual processing of the human body. Science 293:2470–2473. [DOI] [PubMed] [Google Scholar]
- Dunham Y, Baron AS, Banaji MR (2008): The development of implicit intergroup cognition. Trends Cogn Sci 12:248–253. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G (2011): Is there a core neural network in empathy? An fMRI based quantitative meta‐analysis. Neurosci Biobehav Rev 35:903–911. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon BM, Giummarra MJ, Georgiou‐Karistianis N, Enticott PG, Bradshaw JL (2010): Shared pain: From empathy to synaesthesia. Neurosci Biobehav Rev 34:500–512. [DOI] [PubMed] [Google Scholar]
- Fourkas AD, Bonavolontà V, Avenanti A, Aglioti SM (2008): Kinesthetic imagery and tool‐specific modulation of corticospinal representations in expert tennis players. Cereb Cortex 18:2382–2390. [DOI] [PubMed] [Google Scholar]
- Gallese V (2006): Intentional attunement: A neurophysiological perspective on social cognition and its disruption in autism. Brain Res 1079:15–24. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G (2004): A unifying view of the basis of social cognition. Trends Cogn Sci 8:396–403. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Gabrieli JD, Chiao JY, Eberhardt JL (2001): Differential fusiform responses to same‐ and other‐race faces. Nat Neurosci 4:845–850. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR (2003): Understanding and using the implicit association test: I. An improved scoring algorithm. J Pers Soc Psychol 85:197–216. [DOI] [PubMed] [Google Scholar]
- Gu X, Liu X, Guise KG, Naidich TP, Hof PR, Fan J (2010): Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. J Neurosci 30:3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G., Silani G., Preuschoff K, Batson CD, Singer T (2010): Neural responses to ingroup and outgroup members' suffering predict individual differences in costly helping. Neuron 68:149–160. [DOI] [PubMed] [Google Scholar]
- Ito TA, Bartholow BD (2009): The neural correlates of race. Trends Cogn Sci 13:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C (2007): Empathy for positive and negative emotions in the gustatory cortex. Neuroimage 34:1744–1753. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J (2005): How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage 24:771–779. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V (2009): Expanding the mirror: Vicarious activity for actions, emotions, and sensations. Curr Opin Neurobiol 19:666–671. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V (2010): Somatosensation in social perception. Nat Rev Neurosci 11:417–428. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J (2007a): The neural substrate of human empathy: Effects of perspective‐taking and cognitive appraisal. J Cogn Neurosci 19:42–58. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J (2007b): What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE 12:e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Meltzoff AN, Decety J (2010): How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. J Cogn Neurosci 22:362–376. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T (2011): Meta‐analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54:2492–2502. [DOI] [PubMed] [Google Scholar]
- Lawrence EJ, Shaw P, Giampietro VP, Surguladze S, Brammer MJ, David AS (2006): The role of ‘shared representations’ in social perception and empathy: An fMRI study. Neuroimage 29:1173–1184. [DOI] [PubMed] [Google Scholar]
- Liew SL, Han S, Aziz‐Zadeh L (2011): Familiarity modulates mirror neuron and mentalizing regions during intention understanding. Hum Brain Mapp 32:1986–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur VA, Harada T, Lipke T, Chiao JY (2010): Neural basis of extraordinary empathy and altruistic motivation. Neuroimage 51:1468–1475. [DOI] [PubMed] [Google Scholar]
- Mathur VA, Harada T, Chiao JY (2011): Racial identification modulates default network activity for same and other races. Hum Brain Mapp. doi: 10.1002/hbm.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD (2010): Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Struct Funct 214:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minio‐Paluello I, Baron‐Cohen S, Avenanti A, Walsh V, Aglioti SM (2009): Absence of embodied empathy during pain observation in Asperger syndrome. Biol Psychiatry 65:55–62. [DOI] [PubMed] [Google Scholar]
- Moro V, Urgesi C, Pernigo S, Lanteri P, Pazzaglia M, Aglioti SM (2008): The neural basis of body form and body action agnosia. Neuron 60:235–246. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Atkinson AP, Andersson F, Vuilleumier P (2007): Emotional modulation of body‐selective visual areas. Soc Cogn Affect Neurosci 2:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny W, Holmes AP (2004): Random‐effects analysis In: Frackowiak RSJ, Ashburner JT, Penny WD, Zeki S, Friston KJ, Frith CD, Dolan RJ, Price CJ, editors.Human Brain Function.San Diego:Elsevier; pp843–850. [Google Scholar]
- Pettigrew TF, Meertens RW (1995): Subtle and blatant prejudice in Western Europe. Eur J Soc Psychol 25:57–75. [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, Banaji M R (2000): Performance on indirect measures of race evaluative predicts amygdala activation. J Cogn Neurosci 12:729–738. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB (2002): Empathy: Its ultimate and proximate bases. Behav Brain Sci 25:1–20. [DOI] [PubMed] [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R (2007): The compassionate brain: Humans detect intensity of pain from another's face. Cereb Cortex 17:230–237. [DOI] [PubMed] [Google Scholar]
- Singer T, Frith C (2005): The painful side of empathy. Nat Neurosci 8:845–846. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD (2004): Empathy for pain involves the affective but not sensory components of pain. Science 303:1157–1162. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD (2006): Empathic neural responses are modulated by the perceived fairness of others. Nature 439:466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Miltner WH (2011): Attention to aversive emotion and specific activation of the right insula and right somatosensory cortex. Neuroimage 54:2534–2538. [DOI] [PubMed] [Google Scholar]
- Tajfel H (1981):Human Groups and Social Categories.Cambridge [Cambridgeshire]:Cambridge University Press; p132–134. [Google Scholar]
- Urgesi C, Berlucchi G, Aglioti SM (2004): Magnetic stimulation of extrastriate body area impairs visual processing of nonfacial body parts. Curr Biol 14:2130–2134. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Avenanti A (2011): Functional and epiphenomenal modulation of neural activity in body selective visual areas. Cogn Neurosci 2:3–4, 212–214. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Calvo‐Merino B, Haggard P, Aglioti SM (2007): Transcranial magnetic stimulation reveals two cortical pathways for visual body processing. J Neurosci 27:8023–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini E (2010): The role of anterior insula and anterior cingulate in empathy for pain. J Neurophysiol 104:584–586. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Betti V, Le Pera D, De Armas L, Miliucci R, Restuccia D, Avenanti A, Aglioti SM (2008): Seeing the pain of others while being in pain: A laser evoked potentials study. Neuroimage 40:1419–1428. [DOI] [PubMed] [Google Scholar]
- Valentini E, Liang M, Aglioti SM, Iannetti GD (2012): Seeing touch and pain in a stranger modulates the cortical responses elicited by somatosensory but not auditory stimulation. Hum Brain Mapping. doi: 10.1002/hbm.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bavel JJ, Packer DJ, Cunningham WA (2008): The neural substrates of in‐group bias: A functional magnetic resonance imaging investigation. Psychol Sci 19:1131–1139. [DOI] [PubMed] [Google Scholar]
- Van Bavel JJ, Packer DJ, Cunningham WA (2011): Modulation of the fusiform face area following minimal exposure to motivationally relevant faces: Evidence of in‐group enhancement (not out‐group disregard). J Cogn Neurosci 23:3343–3354. [DOI] [PubMed] [Google Scholar]
- Vocks S, Busch M, Grönemeyer D, Schulte D, Herpertz S, Suchan B (2010): Differential neuronal responses to the self and others in the extrastriate body area and the fusiform body area. Cogn Affect Behav Neurosci 10:422–429. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW (2003): Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci 18:3134–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin JI, Marcoux LA, Canizales DL, Mercier C, Jackson PL (2011): I am touched by your pain: Limb‐specific modulation of the cortical response to a tactile stimulation during pain observation. J Pain 12:1182–1189. [DOI] [PubMed] [Google Scholar]
- Yabar Y, Johnston L, Miles L, Peace V (2006): Implicit behavioral mimicry: Investigating the impact of group membership. J Nonverbal Behav 30:97–113. [Google Scholar]
- Xu X, Zuo X, Wang X, Han S (2009): Do you feel my pain? Racial group membership modulates empathic neural responses. J Neurosci 29:8525–8529. [DOI] [PMC free article] [PubMed] [Google Scholar]


