Abstract
A large body of behavioural research has used the cued task‐switching paradigm to characterize the nature of trial‐by‐trial preparatory adjustments that enable fluent task implementation when demands on cognitive flexibility are high. This work reviews the growing number of fMRI studies on the same topic, mostly focusing on the central hypothesis that preparatory adjustments should be indicated by enhanced prefrontal and parietal BOLD activation in task switch when compared with task repeat trials under conditions that enable advance task preparation. The evaluation of this straight‐forward hypothesis reveals surprisingly heterogeneous results regarding both the precise localization and the very existence of switch‐related preparatory activation. Explanations for these inconsistencies are considered on two levels. First, we discuss methodological issues regarding (i) the possible impact of different fMRI‐specific experimental design modifications and (ii) statistical uncertainty in the context of massively multivariate imaging data. Second, we discuss explanations related to the multidimensional nature of task preparation itself. Specifically, the precise localization and the size of switch‐related preparatory activation might depend on the differential interplay of hierarchical control via abstract task goals and attentional versus action‐directed preparatory processes. We argue that different preparatory modes can be adopted relying either on advance goal activation alone or on the advance resolution of competition within action sets or attentional sets. Importantly, while either mode can result in a reduction of behavioral switch cost, only the latter two are supposed to be associated with enhanced switch versus repeat BOLD activation in prepared trial conditions. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: task preparation, proactive control, cognitive flexibility, reconfiguration, proactive interference, biased competition, task set, action set, attentional set, set shifting, goal representation, task demand
INTRODUCTION
Cognitive control has been broadly defined as the ability to flexibly use and change rules on the basis of advance information or feedback from previous performance [Kok et al., 2006]. Just about 10 years ago, many brain imagers interested in cognitive control processes shared an expectation that the task‐switching paradigm would offer a simple and well‐controlled experimental approach to uncover a distinct set of brain regions that constitute the neuroanatomical basis of some key aspects of cognitive flexibility. Moreover, a special interest emerged into the neural basis of advance task preparation, a process that is often regarded as one of the defining elements of cognitive control [e.g., Meiran, 2010]. The importance of advance preparation was indicated by early performance data, which showed that it can reduce behavioral switch cost (i.e., slower response times and higher error rates when subjects are required to switch to a new task relative to repeating the previously relevant task). This suggests that advance task preparation enables switching to a new task set before the appearance of the imperative target stimulus that requires the concrete implementation of the prepared task rather than other equally possible tasks (for reviews focusing on behavioral data, see Kiesel et al. [ 2010], Meiran [ 2010], Monsell [ 2003b], Vandierendonck et al. [ 2010]). In other words, the human brain seems to be equipped with a control mechanism that operates via proactive adjustment instead of solely relying on reactive adjustment at the time of actual task implementation [cf., Braver et al., 2007; Meiran, 1996; Rogers and Monsell, 1995]. Note that the term “adjustment” is used as a placeholder for processes that enable the fluent implementation of the currently relevant task. Later, in this review, we will elaborate further on the range of processes that constitute switch‐related preparatory adjustment as suggested by the pattern of preparatory BOLD activation under different study conditions.
Principally, an advantage of the use of brain imaging techniques like fMRI is that it can provide measures of proactive control processes itself rather than their impact on subsequent overt behavior as indicated by the reduction of behavioral switch cost. Switch‐related preparatory BOLD activation patterns are characterized by the amplitude of preparation‐related BOLD activation in task switch relative to task repeat trials and by the neuroanatomical localization of such activation. We will discuss the theoretical implications of different types of preparatory switch‐related amplitude patterns (switch only, switch > repeat, switch = repeat) for explaining the reduction of behavioral switch cost in prepared trial conditions. We will also discuss implications of the specific localization of such switch‐related activation patterns. Generally, switch‐related activation patterns that are consistent across different study designs would indicate the operation of core preparatory processes common to different types of task switching, whereas variation in the amplitude of such modulations and/or their localization as a function of particular study design would suggest dissociable subcomponents of preparatory processes.
As this review will show, fMRI activation patterns vary considerably across different task‐switching protocols. Broadly, existing studies show that the precise functional anatomical expression of preparatory control processes in task switching is dependent upon a number of modulatory variables. Some of the variability of BOLD activation patterns between fMRI studies can be attributed to the multidimensional nature of the process itself. However, some of the inconsistency might also be related to fMRI‐specific methodological constraints. Accordingly, one central objective of this work is to critically assess the strength and limitation of inferences that can be derived from different types of fMRI designs and analyses that aim to disentangle BOLD response components associated with cue‐driven proactive control processes when compared with target‐driven reactive control processes (“General fMRI‐methodological issues” section). Keeping in mind these methodological challenges, we attempt to answer three key research questions regarding the signature of preparatory switch‐related BOLD activation in cued task switching and its implications regarding the nature of task preparation.
Key Question 1: Is There Evidence That Some Brain Areas Are Exclusively Activated Proactively on Switch Trials But Not on Repeat Trials?
If yes, this would indicate the operation of an exclusive switch‐only process that is not required when the previous task repeats and that might be, at least partly, responsible for the reduction of behavioral switch cost in prepared trial conditions. Furthermore, it is important to establish what potential modulatory variables (e.g., the degree of task practice or the relative proportion of switch and repeat trials) determine whether a preparatory process engages exclusively in switch trials, or, put differently, disengages in repeat trials. Depending on such modulatory variables, brain regions that would otherwise be associated with switch‐only preparatory processes would instead fall into the scope of the following key question 2.
Key Question 2: Is There Evidence That Some Brain Areas Show Greater Preparatory Activation on Switch Trials Than on Repeat Trials?
Notwithstanding any evidence for switch‐only preparatory processes as addressed by question 1, the identification of brain areas exhibiting relatively stronger activation for switch when compared with repeat trials would be sufficient to infer the operation of proactive adjustment processes, which mediate the reduction of behavioral switch cost in prepared trial conditions. Furthermore, it is important to establish what variables determine the precise localization of switch‐related activation in prepared trial conditions and the relative strength of amplitude enhancements for switch versus repeat trials, or indeed, whether any switch‐related amplitude enhancements can be detected at all.
Key Question 3: Is There Evidence That Distinct Brain Areas Exhibit Switch‐Related Activation in Prepared Versus Unprepared Trial Conditions?
If yes, this would suggest a functional–anatomical segregation of proactive switch‐related adjustment processes (i.e., mostly cue‐driven in prepared trials) and reactive adjustment processes (i.e., mostly target‐driven in unprepared trials). Otherwise, these two control modes might simply reflect temporal differences in the engagement of a common switch‐related adjustment process.
Inclusions Criteria for Task Switching Studies Considered in This Review
Importantly, this work is not a meta‐analysis or even a comprehensive review of all fMRI task‐switching studies. Instead, we attempt to provide an in‐depth selective review of fMRI studies that specifically address the neurocognitive basis of preparatory processes in cued task switching. This present approach complements a previous, highly condensed review, which provided an integrative account of results across different data modalities, including behavioral performance, event‐related brain‐electrical potentials (ERPs), and fMRI [Karayanidis et al., 2010]. The reader is referred to a number of recent behavioral review works that provide detailed analyses of behavioral studies and conceptual issues of task switching [Kiesel et al., 2010; Meiran, 2010; Monsell, 2003b; Vandierendonck et al., 2010].
This review will focus on fMRI studies that are best suited to address the three questions raised earlier. First, because this review deals with preparatory processes, we only included studies that incorporate and separately analyze at least one “prepared trial condition,” that is, studies including a preparation interval >500 ms (roughly 30–50% of all fMRI task‐switching studies). Second, we only included studies that use randomly cued task‐switching procedures—using either “task cues” directly indicating the relevant task or “transition cues” indicating whether to continue with the previous task or to switch to the alternative task. These cued task‐switching studies represent the vast majority of fMRI studies on prepared task switching. Studies using predictable task sequences (i.e., memory‐based instead of randomly cued) [Kimberg et al., 2000; Sohn et al., 2000] and variants of the Wisconsin Card Sorting Test [e.g., Monchi et al., 2001] were excluded due to relatively weak experimental control over the timing of preparatory processes. Third, we restricted our analysis to studies investigating switching between different stimulus–response (S–R) mappings, that is, studies in which participants have to switch between two different S–R rules defined on the same or distinct categories of stimuli (e.g., classifying a number as odd/even vs. </>5; or classifying a number as odd/even and a letter as vowel/consonant). We also included task‐switching studies that require the reversal of S–R mapping (e.g., task A: circle, left hand; triangle, right hand vs. task B: circle, right hand; triangle, left hand). Common to these task‐switching procedures is that a switch trial requires disengagement from the previous S–R mapping and re‐engagement of the alternative S–R mapping. “Attentional set shifting” studies that require switching between stimulus features or dimensions, but involve only a single constant S–R mapping throughout the entire experiment are not included in this review, except where necessary to highlight important similarities or differences with task‐switching studies (for an earlier meta‐analysis including all types paradigms, see Wager et al. [ 2004]).
The section on “General fMRI‐Methodological Issues” will highlight the relevant fMRI‐methodological issues and will be followed by sections addressing each of the three key research questions listed earlier. For each question, we will first provide a broad overview of the main findings and interpretations from relevant studies, largely ignoring study‐specific issues. These more distinct contributions of individual studies, together with a critical assessment of contradictory results, will be addressed subsequently within each section, highlighting promising future research directions. Finally, “General Conclusions” will provide an overall summary of key conclusions and future directions.
GENERAL fMRI‐METHODOLOGICAL ISSUES
In the behavioral research domain, many variants of the task‐switching paradigm have been established to measure indices associated with different underlying cognitive processes [e.g., Meiran et al., 2000]. The comparison of single task blocks with blocks of mixed task trials (mixed task blocks) provides a measure of the global cost (“general switch cost”) of alternating between tasks irrespective of any differential local processes associated with repeating or switching task within the mixed task sequence. This global switch effect can be readily captured by blocked fMRI designs [Dreher et al., 2002]. However, event‐related fMRI designs are necessary to capture the constituent components of this effect. One such component—expressed by the behavioral “switch cost”—provides a more specific measure of the local cost of alternating between tasks by comparing switch trials and repeat trials within the same mixed task block. The other component—expressed in behavioral “mixing cost”—provides a measure of the additional demands of implementing a task in random sequence with a competing task by comparing repeat trials in mixed task blocks with physically identical repeat trials in single task blocks [Rubin and Meiran, 2005]1.
From an fMRI‐methodological perspective, the task‐switching paradigm is a perfect example of the usefulness of event‐related design and analysis. Straightforward rapid event‐related designs can be implemented to determine relative BOLD response differences between switch and repeat trials when the separation of preparatory and target‐related BOLD activation components is not of interest [e.g., Henson and Friston, 2007]. In this case, the original behavioral task‐switching designs can simply be adopted without considerable modifications. Importantly, although, for other more specific research questions, fMRI design issues become more complicated and require considerable modifications of the original task‐switching designs, as discussed below.
Measuring Trial‐Related BOLD Activation Against Baseline
To compare BOLD response estimates of switch and repeat trials against an intertrial interval (ITI) baseline, it is necessary to modify the typical task‐switching procedure by including a variable ITI. This may be required when the aim of the study is to determine whether a brain region is selectively active for switch trials or for both switch and repeat trials. The optimal ITI distribution depends on several, partly antagonistic considerations related to the detectability, and the estimation accuracy of the BOLD response [Birn et al., 2002; Hagberg et al., 2001]. However, irrespective of the specific ITI distribution, the introduction of variable ITIs might vary the level of task set carryover from one trial to the next or the level of task set adaptation as suggested by de Baene et al. [ 2011]. This could in turn impact on the extent to which passively decaying task set activation from the preceding trial would disadvantage a subsequent repeat trial (i.e., less benefit from task set perseveration for longer ITIs) or advantage a subsequent switch trial (i.e., less interference due to task set perseveration for longer ITIs). Indeed, behavioral data suggest that the impact of passive task set decay processes on task‐switching performance depends on the response‐cue interval (RCI), particularly when the RCI is randomly varied [Meiran et al., 2000]. Shifting from a fixed to a variable ITI can also have an effect on expectancy. The ability to accurately predict cue onset will affect the readiness to process the cue and the task‐related information it conveys. In simple and choice reaction time (RT) paradigms, it has been shown that preparatory activity other than anticipatory responses may begin before the onset of the cue, especially in designs with short foreperiods [Niemi and Naatanen, 1981]. Although the effects of ITI/RCI jittering have not been examined systematically in any fMRI task‐switching study, evidence from ERP studies suggests that RCI jittering may change the effectiveness of preparatory control in task‐switching2. So, switch‐related BOLD activation obtained under modified ITI distributions cannot be assumed to be directly comparable to results from studies using short and constant ITIs as is typically the case in behavioral and ERP task‐switching studies.
Segregating Preparation‐Related and Target‐Related BOLD Activation Components
A second considerable modification of the original task‐switching designs is required for the functional imaging of preparatory processes in task switching. The length of the cue‐target‐interval (CTI) is certainly a critically important variable when investigating preparatory processes. Generally, behavioral study results indicate that longer CTIs lead to increasingly prepared states unless intervals become “excessively” long (e.g., >2,000 ms), suggesting that there is an “optimal” long preparation interval, for example, around 600 ms under certain study conditions [Monsell and Mizon, 2006; Nicholson et al., 2005]. Although preparatory state varies continuously as a function of CTI length, for simplicity, preparation intervals are typically categorized according to a “short” versus “long” dichotomy. In this review, “short” preparation intervals are defined as intervals ≤300 ms, resulting in incompletely prepared or fully unprepared states (i.e., when CTI = 0). By contrast, “long” preparation intervals are defined as intervals ≥500 ms. According to this definition, we use the terms “unprepared” trial condition for CTIs ≤ 300 ms and “prepared” trial condition for cases where the CTI is longer than 500 ms.
Clearly, the optimum range of long CTIs for implementing “prepared” task‐switching conditions in behavioral and ERP studies (i.e., 500–2,000 ms) is simply too fast to be directly implemented in the fMRI environment without considerable design modifications. This is especially true when attempting to isolate preparatory BOLD activation from subsequent target‐driven BOLD activation (sections “Constant long CTI design” to “Altered preparatory processes in task switching through design modifications?”). An alternative approach that does not require fMRI‐related design modifications relies on the direct contrast of prepared versus unprepared trial conditions to tap into BOLD correlates of preparatory processes (sections “Examining preparatory processes without explicitly isolating preparatory BOLD activation components” and “Key question 3”). However, this approach comes at the expense of not being able to explicitly disentangle preparation‐related and target‐related BOLD components.
The separation of preparation‐related and target‐related BOLD components represents a general fMRI‐analytical problem due to the temporal overlap of successive BOLD responses across at least 20 s. That is, due to the sluggishness of an event‐related BOLD response, the modulation of BOLD activation in a given voxel cannot be easily attributed to a particular time point or an associated distinct event within a cue‐target trial (see Fig. 1). Without taking additional measures, the event‐related analysis of fMRI data yields one single trial‐related BOLD response estimate for a given voxel that integrates all the different potential BOLD subcomponents associated with the successive within‐trial events, including the cue, the delay, delay termination, the target, and the response [cf., Jennings and van der Molen, 2005]. A range of different approaches have been designed to disentangle within‐trial BOLD subcomponents, including the use of long constant CTIs, jittering of CTIs, and partial trials designs.
Figure 1.
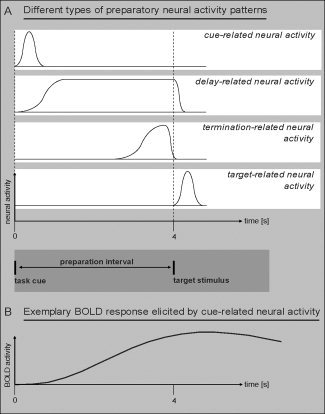
A: Illustration of different types of neural activity that can occur during a cue‐target trial in relation to (B) an exemplary BOLD response elicited by the cue. This schematic drawing highlights the analytical challenges of inferring the precise pattern of preparatory and target‐related neural activity from the associated event‐related BOLD response, which evolves much slower than the underlying neural activity.
Constant long CTI design
A first approach that has been used in cued task switching as well as other paradigms is to use a constant, but “sufficiently long” CTI of, say, five or more seconds (e.g., Barber and Carter [ 2005], who used a CTI of 7.5 s). The trial‐related BOLD activation time course is simply divided into two segments, one ranging from cue onset to target onset, and the other one starting from target onset. Based on this simple analysis, two distinct activation patterns can be interpreted unambiguously. First, BOLD activation measured until target onset can clearly be attributed to preparation. This is possible as with a CTI > 5 s, cue‐related BOLD activation has sufficient time to reach considerable strength before a possible target‐related BOLD component starts contributing to the trial‐related BOLD signal. Second, BOLD activation that starts to rise from a flat baseline after target onset is clearly not related to preparation, but can instead only reflect target‐related processes. Importantly, it is only in this case that target‐related BOLD activation can be determined without contamination from preceding preparation‐related BOLD activation. As soon as there is above‐baseline activation before target onset, target‐related activation cannot be isolated from preceding preparation‐related activation. A second serious problem is that only transient cue‐related activation elicited early within the CTI can be unambiguously interpreted as preparatory activation. Delay‐related activation evolving later during the CTI (i.e., closer to target onset) is largely indistinguishable from target‐related BOLD activation. Finally, as elaborated in “Altered preparatory processes in task switching through design modifications?” section, the use of such atypically long CTIs is likely to have unintended “psychological” side effects in terms of altered preparatory strategy of participants.
Jittered CTI design
A second approach attempts to decorrelate cue‐related, delay‐related, and target‐related BOLD activation by introducing a variable cue‐target interval and including a delay‐related GLM regressor convolved with the length of the delay [e.g., Bunge et al., 2003; Curtis and D'Esposito, 2003; Sakai and Passingham, 2003]. Importantly, the jittered‐CTI approach only works properly when a delay‐related regressor is explicitly included within the model. Otherwise, an undistorted estimation of separate cue‐related and target‐related BOLD activation components rests on the unverifiable assumption that trial‐related activation in a given voxel is independent of the length of the CTI, that is, when no delay‐related activation is present [Serences, 2004]. Furthermore, to be able to adequately capture delay‐related activation, a rather wide range of CTIs might be preferable to increase delay‐related variance (e.g., CTIs ranging between 2 and 12 s). As far as BOLD decomposition is concerned, the jittered CTI design—if properly implemented—is certainly preferable to the constant‐long CTI design. However, as elaborated in “Altered preparatory processes in task switching through design modifications?” section, CTI jittering—like the use of single constant long CTI—might come with its own set of problems with regard to “psychological” side effects in terms of altered preparatory strategy of participants.
The partial‐trial design
A third approach uses the so‐called partial‐trial design that attempts to decompose cue‐related and target‐related BOLD components by including partial “cue‐only” trials intermixed with full “cue plus target” trials [Brass and von Cramon, 2002; Ollinger et al., 2001; Ruge et al., 2009b; Serences, 2004]. More complex versions of this design attempt to additionally determine the contributions of delay‐related and target‐omission‐related BOLD activation [Ruge et al., 2009b]. The main advantage of the basic partial trial design when compared with both constant‐long CTI designs and jittered CTI designs is that a more typical, that is, a relatively short and nonjittered long CTI can be used (e.g., 1,000 ms). Its main drawback is that the omission of the target stimulus in cue‐only trials might itself elicit a BOLD response that cannot easily be distinguished from genuine preparation‐related BOLD activation (for an extensive discussion of this issue, see Ruge et al. [ 2009b]). Also, like CTI jittering, the occasional omission of the target stimulus might affect the participants' preparatory strategy or unintentionally introduce sequence effects in the data (see Jamadar et al. [ 2010c]).
Altered preparatory processes in task switching through design modifications?
Notwithstanding their intended purpose to disentangle preparation‐related and target‐related BOLD components, the above design modifications might inadvertently alter the very preparatory processes they are designed to measure. Thus, preparation‐related BOLD activation obtained in these designs might index partly different preparatory processes than those indexed by the standard cued task‐switching designs typically used in behavioral and ERP studies. Moreover, preparation‐related BOLD activation might indicate partly different preparatory processes across the different fMRI designs [cf., Goghari and MacDonald, 2008; Serences, 2004]. Note that this is not a problem per se. In fact, if a specific fMRI design modification implies certain preparatory processes that are different from more standard designs, this only means that there is more than one general kind of preparatory process involved in task switching. It does not mean that processes involved in different types of designs are more or less “real” or of greater or smaller research interest per se. Nevertheless, it is highly important to explicitly determine the possible process differences implied by different study designs to avoid unnecessary confusion.
Functional MRI design modifications might affect preparatory processes on two levels: general process alterations would affect both switch and repeat trials, whereas switch‐specific process alterations would affect switch and repeat trials differently. From the extensive random foreperiod literature [Niemi and Naatanen, 1981], it can principally be expected that any modification that affects temporal predictability also likely alters the efficiency of preparatory processes. Unfortunately, there has been little systematic work by which to determine the severity of such process alterations specifically in fMRI studies of task switching. Goghari and MacDonald [ 2008] compared a jittered CTI/ITI design to a slow interval design and to a partial trial design using an experimental protocol similar to cued task switching with Stroop‐like stimuli. They analyzed how these different fMRI designs affected performance and BOLD activation both on a general level of preparation (i.e., irrespective of trial type) and depending on cue type (word‐reading vs. color‐naming), but unfortunately not regarding task switching (task repeat vs. task switch). Although the jittered design showed a faster RT relative to the other two designs, this effect did not interact with cue type (word‐reading vs. color‐naming). All three designs activated similar regions during the preparation interval although with slightly different sensitivities for some regions. This suggests that the different designs did not imply completely different preparatory processes.
Goghari and MacDonald [ 2008] did not compare behavioral performance between the modified fMRI designs and a condition with the typical “behavioral” design parameters. Thus, it remains unclear whether any of the three fMRI designs was comparable to the original behavioral design in terms of behavioral performance. Such comparison is sometimes used to validate fMRI design modifications based on the absence of an impact on behavior [e.g., Koch et al., 2003; Ruge et al., 2010]. The rationale behind such a validation is that if performance remained unaffected, one could safely conclude that preparatory processes were also not altered. However, it should be noted that this rationale ignores that different data modalities might tap into different aspects of preparation3. Thus, validating fMRI design modifications by evaluating the impact on behavioral effects might be misleading (see section “Task switching without preceding task implementation,” e.g., for such a cross‐modal dissociation with regard to the impact of target omission on switching‐related processes in the subsequent trial). In conclusion, there is yet no clear‐cut evidence for or against a strong impact of fMRI‐design modifications on the involved preparatory processes, thus highlighting the need for more systematic examination.
Examining Preparatory Processes Without Explicitly Isolating Preparatory BOLD Activation Components
Notably, most cued task‐switching studies that examined prepared trial conditions did not implement one of the above fMRI designs. Instead, many studies implemented prepared trial conditions with a constant long CTI > 0.5 s and ≪5 s. In contrast to the fMRI designs discussed in the preceding section ”Segregating preparation‐related and target‐related BOLD activation components,” these studies cannot explicitly disentangle preparation‐related and target‐related BOLD components within a cue‐target trial. Instead, the compound trial‐related BOLD response that includes both preparation‐related and target‐related BOLD components needs to be related to additional sources of information that may help determine whether this compound activation is more likely to be associated with proactive or reactive processes in task switching. One approach examines cross‐modal relationships between this compound BOLD response and either behavioral or ERP indices of preparatory and target‐related processes (see section “Cross‐modal correlational approaches” below). Another approach directly contrasts the compound BOLD response elicited in prepared trial conditions with the BOLD response elicited in unprepared trial conditions with a CTI ≪ 500 ms (see section “Directly contrasting BOLD responses across prepared and unprepared trial conditions” below). Although both these approaches offer less direct evidence than designs that explicitly disentangle BOLD components associated with proactive and reactive adjustment processes, they have the distinct advantage that they do not require fMRI‐specific design modifications that might unintentionally alter the preparatory processes of interest (see section “Altered preparatory processes in task switching through design modifications?” above).
Cross‐modal correlational approaches
A first cross‐modal correlational approach relates switch‐related compound BOLD activation in prepared trial conditions with the corresponding behavioral residual switch cost [Braver et al., 2003; Jamadar et al., 2010b]. Depending on the direction of this relationship, the compound BOLD effect is either more likely to be associated with proactive adjustment processes or with reactive adjustment processes (for a concrete example, see Key Question 2). Following a similar line of reasoning, by examining whether switch‐related compound BOLD activation in prepared trial conditions is associated with cue‐locked or target‐locked ERP components, it is possible to infer the likely temporal locus of the observed BOLD activation [Jamadar et al., 2010a]. However, it is important to note that the inferences drawn from such cross‐modal correlational approaches about the temporal locus of the observed compound BOLD response are indirect. Rather than indicating a cue‐related process per se, a significant positive relationship between the compound BOLD response and cue‐locked ERP activation might be mediated by a third variable that is only indirectly affected by preparation (e.g., target‐related outcome of preparation). Additionally, the absence of a relationship between these measures must also be interpreted with caution, because ERPs and fMRI BOLD activation capture different types of neuronal activity. It is highly probable that not all ERP activity will be visible in the BOLD signal and, correspondingly, not all BOLD activation will be captured in ERP activity.
Directly contrasting BOLD responses across prepared and unprepared trial conditions
Some studies directly compare the brain activation correlates of proactive versus reactive control adjustment processes by contrasting switch‐related compound BOLD activation for prepared trials and unprepared trials. This contrast yields a number of valuable conclusions, based on two basic assumptions (for a similar rationale in the context of modeling behavioral performance data, see Meiran et al. [ 2008]). Firstly, in prepared trials, switch‐related compound BOLD activation indicates a mixture of potential proactive switch‐related adjustments during the CTI and potential residual reactive adjustments after target presentation [cf., Rogers and Monsell, 1995]. Second, with shorter CTI, there is less time for proactive adjustment, and so the demand for residual reactive adjustment will be larger. Hence, the proportion of proactive and reactive adjustment processes potentially contributing to the compound switch‐related BOLD response is shifted in favor of the reactive component. Based on these assumptions, three relevant activation patterns can be distinguished. First, a brain region that shows larger switch‐related activation for prepared when compared with unprepared or incompletely prepared trial conditions is more likely to be associated with proactive switch‐related adjustment processes (i.e., more time for proactive control). Second, a brain region that shows larger switch‐related activation for unprepared when compared with prepared trial conditions is more likely to be associated with reactive adjustment processes (i.e., less time for proactive control must be compensated by increased reactive control effort). Third, a region that exhibits similar switch‐related activation for both prepared and unprepared trials is likely to be associated with control processes that are common to both proactive and reactive adjustment and are flexibly activated either before or after target onset depending on the time available for preparation. In other words, the longer the CTI, the more proactive adjustment is possible, and, therefore, less reactive adjustment is necessary when the target appears. Conversely, for shorter CTI, less proactive adjustment is possible, and, therefore, more reactive control is necessary when the target appears4. This analytical rationale will be used to evaluate Key Question 3 (see below).
Methodological issues: Concluding remarks
None of the techniques described earlier comes without justified criticism. Thus, as applies more generally in all empirical sciences, design‐specific constraints should be considered when interpreting the results of individual studies. In addition, these differences in design and methodology can provide invaluable information regarding specific effects that remain replicable regardless of paradigm specifications. However, it is also clear that more systematic research is needed to determine the way in which different fMRI design variables alter the nature of preparatory processes involved in task switching
KEY QUESTION 1: IS THERE EVIDENCE THAT SOME BRAIN AREAS ARE EXCLUSIVELY ACTIVATED ON SWITCH TRIALS BUT NOT ON REPEAT TRIALS?
In contrast to the highly heterogeneous pattern of results concerning the other two key questions, the quest for brain regions exhibiting preparatory BOLD activation exclusively in switch trials yields a rather consistent picture. We are not aware of studies that have unambiguously confirmed the existence of switch‐only preparatory BOLD activation, that is, brain areas that are significantly activated in switch trials but are not reliably activated in repeat trials when contrasting each condition against the intertrial interval baseline (for an exception, see below). The studies that report enhanced BOLD activation for switch relative to repeat trials in prepared trial conditions and also separately analyzed switch and repeat trials against baseline also typically report above‐baseline activation for repeat trials [Barber and Carter, 2005; Braver et al., 2003; Crone et al., 2006; Shi et al., 2010]. Additionally, as discussed below (Key Questions 2 and 3), many studies report above‐baseline activation in prepared repeat trials in the absence of significantly enhanced activation in switch trials [Brass and von Cramon, 2002, 2004; Bunge et al., 2003; Cavina‐Pratesi et al., 2006; Luks et al., 2002; Ruge et al., 2005]. To our knowledge, the only exception to this pattern is a recent study by Chiu and Yantis [ 2009], which does report switch‐only preparatory BOLD activation in the medial superior parietal lobule (SPL) in a conjunction analysis of spatial versus categorization switching. Importantly, though, this study used a special protocol in which the intertrial‐interval baseline was made up of a series of rapidly presented distracter stimuli. This is not comparable to the typical “empty” intertrial‐interval baseline used by the other event‐related task‐switching studies. Thus, the hemodynamic response during baseline in the relevant brain areas might have been higher than usual, obscuring a relatively weak repeat‐related activation5.
Together, the consistent absence of switch‐only BOLD activation in cued task‐switching suggests that switch trials rely on the recruitment of the same basic neural regions and thus likely the same preparatory processes that are also involved in repeat trials. This conclusion is consistent with models derived from behavioral studies [e.g., Gilbert and Shallice, 2002; Koch and Allport, 2006; see review by Kiesel et al. [ 2010]) and is partly supported by ERP studies ([Wylie et al., 2009; but see Karayanidis et al. [ 2011]). On a theoretical level, these converging results are not consistent with the notion of a stage‐like process specifically inserted for implementing a task switch. Such a switch‐only process has often been associated with the so‐called task set reconfiguration (TSR) metaphor (not necessarily by the authors who popularized the term) and has as such most clearly been conceptualized in terms of a goal shifting process by Rubinstein et al. [ 2001].
Open Questions and Promising Directions
Relative proportion of intermixed switch and repeat trials and its impact for task automatization
A possible caveat to the conclusion that there is no strong evidence for switch‐only BOLD activation is that many of the above studies used relatively high proportions of switch trials of at least 50% [e.g., Braver et al., 2003; Crone et al., 2006; Ruge et al., 2005]. Thus, one might argue that these studies lack switch‐only activation, because repeat trials are treated similarly to switch trials under such circumstances [cf., Monsell and Mizon, 2006; Monsell et al., 2003]. For instance, when the relative proportion of switch and repeat trials is 1:1, a task repetition relative to trial N‐1 will have been a task switch relative to trial N‐2 on roughly half the trials. Thus, memory traces of the N‐2 task might still be sufficiently strong to necessitate proactive control processes in repeat trials that are supported by the same brain areas that are also engaged proactively in switch trials. An interesting follow‐up question is whether such brain regions might become increasingly disengaged in task repeat trials when switch and repeat trials are made more dissimilar by increasing the relative proportion of repeat trials (at the extreme end this would be repeat‐only, that is, single task blocks). A possible hypothesis is that a higher proportion of repeat trials would increase, on average, the extent of short‐term task automatization across longer sequences of repeat trials. Another approach to examine the impact of short‐term task automatization would be to compare different lengths of repeat and switch sequences within blocks of intermixed repeat and switch trials (e.g., RS vs. RRRS or RR vs. RRRR; see Wilkinson et al. [ 2001]). A very recent task‐switching study indeed showed that repeat trial BOLD activation progressively decreased with increasing repetition run length, suggesting that the size of activation differences between switch and repeat trials is strongly affected by short‐term adaptation of task sets [De Baene et al., in press]. Consistent with these results, Slagter et al. [ 2006] found that increasing the relative proportion of repetitions (25, 50, and 100%) resulted in a decrease in preparatory activation for repeat trials in all areas that also showed switch‐related activation, that is, presupplementary motor area (Pre‐SMA), premotor cortex (PMC), and posterior SPL (pSPL). Yet, even in the 100% repetition condition, these areas still showed significant preparatory activation. Note that this activation pattern was restricted to areas outside the prefrontal cortex (PFC) and that there was little evidence for any PFC involvement in this study, possibly due to the use of low‐level attentional attribute switching. Using a paradigm that did involve higher‐level task set switching, De Baene et al. [ 2011] showed that the engagement of prefrontal “cognitive control” areas decreased with increasing repetition run length. Future research needs to examine how such patterns of short‐term task‐automatization compare to other types of paradigms investigating task automatization on different time scales [Cole et al., 2010; Hartstra et al., in press; Ruge and Wolfensteller, 2010; Schneider and Chein, 2003]. For instance, Ruge and Wolfensteller [ 2010] found that large parts of prefrontal cortex disengaged after only four repetitions of the same arbitrary S–R link, suggesting that the recruitment of the lateral PFC is restricted to the initial practice of nonroutine behavior. Importantly though, stimuli in the Ruge and Wolfensteller study were not associated with multiple competing tasks as is the case in most task‐switching studies. Thus, it remains to be tested whether the observed rapid decline of PFC recruitment also holds for longer sequences of task repetitions when stimuli are ambiguously linked to two competing tasks (e.g., the same digit stimulus is mapped onto a response in the magnitude task as well as in the parity task).
KEY QUESTION 2: IS THERE EVIDENCE THAT SOME BRAIN AREAS ARE RELATIVELY MORE ACTIVATED PROACTIVELY ON SWITCH TRIALS THAN ON REPEAT TRIALS?
The absence of strong evidence for a switch‐only preparatory BOLD activation speaks against an all (switch) or none (repeat) implementation of proactive control processes. Rather, it supports a less radical view that proactive adjustments in switch trials may instead rely on the relatively greater recruitment of the same basic control processes that are also required for successful performance in repeat trials. Accordingly, the operation of proactive adjustment processes would be evidenced by relatively stronger preparation‐related activation in the same brain areas for switch when compared with repeat trials without the need to assume the absence of any activation in repeat trials. The identification of such brain regions would explain the reduction of behavioral switch cost in prepared trials vs. unprepared trials in terms of increased opportunity to use proactive control in switch relative to repeat trials. In fact, this view is common to many prominent theoretical accounts in the task‐switching literature. Specifically, accounts linked to the TSR metaphor postulate a process of preparatory adjustment/reconfiguration of task representations [Meiran, 1996; Rogers and Monsell, 1995], whereas most accounts linked to the notion of “proactive task set interference” (PI) postulate the preparatory resolution of between‐task competition [Badre and Wagner, 2006; Gilbert and Shallice, 2002; Koch and Allport, 2006; Wylie et al., 2009; Yeung and Monsell, 2003]. It is difficult to pinpoint precisely the differences between “re‐configuring task set” and “resolving proactive task interference.” One distinction that has often been made is that TSR‐like processes are engaged in switch trials only [e.g., Kiesel et al., 2010]. As there is little evidence for such switch‐only preparatory processes (see key question 1 in this review), the two accounts become conceptually hard to distinguish (see also the General Conclusions). Thus, it seems fair to state that both views would commonly imply the need for increased preparatory control effort in switch trials when compared with repeat trials to establish the currently relevant task set, conceptualized as either reconfiguring versus configuring task sets (TSR) or as resolving increased competition from the currently irrelevant task when it was the more recently performed one (PI). Accordingly, the greater recruitment of cognitive control processes in anticipation of a switch compared to a repeat should be indicated by greater activation for prepared switch than prepared repeat trials within a generic “cognitive control brain network” that would include prefrontal and parietal cortical regions [e.g., Cole and Schneider, 2007; Dosenbach et al., 2008]. Moreover, the identification of switch‐related activation in lower‐level task‐specific brain regions would reflect the outcome of these adjustment processes within the putative target areas of cognitive control.
There remains considerable discrepancy between fMRI studies as to whether there is any evidence in support of such proactive switch‐related adjustment processes and, if so, which precise brain regions might be involved. In support of proactive switch‐related adjustment processes, many studies have found evidence for switch‐related activation in prefrontal and parietal brain regions for prepared trials with CTIs >500 ms [Badre and Wagner, 2006; Barber and Carter, 2005; Braver et al., 2003; Chiu and Yantis, 2009; Crone et al., 2006; Forstmann et al., 2005; Jamadar et al., 2010a; Ruge et al., 2010; Rushworth et al., 2001, 2002; Wylie et al., 2006]. The most consistently reported brain region exhibiting switch‐related activation in these studies is the posterior parietal cortex (PPC), but most of the above studies also report switch‐related activation in more anteriorly located parietal, premotor, and lateral and medial prefrontal regions. As discussed below (section “Does switch‐related preparatory activation reflect stimulus‐directed or response‐directed adjustment processes?”), the precise localization and strength of switch‐related activation especially within the frontal cortex seems to depend strongly on the specific type of task switching required, whereas switch‐related activation in the PPC occurs more broadly.
Importantly, though, many other studies have reported general trial‐related activation for both switch and repeat trials in frontal and parietal areas, but no reliable activation differences between switch and repeat trials in any of these areas [Brass and von Cramon, 2002, 2004; Bunge et al., 2003; Cavina‐Pratesi et al., 2006; Gruber et al., 2006; Luks et al., 2002; Ruge et al., 2005, 2009a]. These latter “null‐results” are rather irritating from the perspective of both TSR and PI accounts of preparation‐related reduction of behavioral switch cost. Thus, one might be inclined to simply explain away these null‐findings in terms of “misses” due to insufficient statistical power (see sections “Statistical uncertainty” and “Discrepancies between fMRI and ERP findings” below). However, as elaborated on in “Key Question 3”, it is theoretically possible to explain the reduction of proactive task interference during subsequent target processing (as indexed by reduced RT switch cost) without necessarily assuming competition resolution during the CTI (as indexed by common preparation‐related activation for switch and repeat trials).
Does Switch‐Related Preparatory Activation Reflect Stimulus‐Directed or Response‐Directed Adjustment Processes?
Studies that have found significant switch‐related preparatory BOLD activation typically report activation in “some” lateral and medial frontal regions and “some” parietal regions within a generic “fronto‐parietal cognitive control network.” Yet, a closer look reveals a surprising heterogeneity concerning the precise localization of switch‐related activation especially within the frontal cortex. To reconcile the discrepancies between the reported studies, it is essential to consider additional task parameters that may determine the nature of advance preparatory processes recruited in task switching.
One key task parameter that has been manipulated extensively in behavioral and ERP studies relates to whether the task requires switching between stimulus‐directed (attentional) or response‐directed (intentional) processes. Classical task‐switching paradigms involve switching between two different, but consistent, S–R mappings defined either on different stimulus dimensions of a single item (e.g., magnitude vs. parity of a single digit; Allport et al. [ 1994]) or on each dimension of a compound stimulus (e.g., vowel/consonant or odd/even for a letter/number combination; Rogers and Monsell [ 1995]). Thus, as suggested by Meiran [ 2000], preparation likely involves shifting attentional focus from one stimulus dimension to another (e.g., focus on magnitude but not parity or focus on letter not number). By contrast, other studies have used “rule switching,” defined in terms of S–R reversal (circle = left, square = right vs. circle = right, square = left) or switching between abstract categorization rules (e.g., match vs. nonmatch) that cannot rely on attentional (i.e., stimulus‐directed) preparatory mechanisms. Rather, these tasks rely on relinking the same set of stimuli to different responses—processes that exclusively involve a change of “action set” or “response meaning” (i.e., R1 is used to indicate presence of S1 or S2) rather than “stimulus set” [Brass et al., 2003; Meiran, 2000]. Thus, one might expect partially different brain regions for switching paradigms that rely on attentional control (i.e., adjustment of stimulus set) versus intentional control (i.e., adjustment of action set). Indeed, using an S–R reversal task with a constant CTI of 1.5 s, Crone et al. [ 2006] found differential switch‐related activation in mid‐dorsolateral PFC (mid‐DLPFC)/mid‐ventrolateral PFC (mid‐VLPFC), amongst other areas, when switching between bivalent targets (i.e., targets that include features of both S–R rules and are thus likely to involve intentional preparation) when compared with switching between univalent targets (i.e., targets that are linked to only one S–R mapping and are thus likely to involve attentional preparation only). Similarly, using a partial trial design, Ruge et al. [ 2010] also found that the mid‐DLPFC was associated with intentional switch‐related preparatory control. Specifically, there was additional switch‐related preparatory activation in mid‐DLPFC (amongst others) when compared with Pre‐SMA only (amongst others) for an “intention‐based” preparatory condition, which differed from a purely “attention‐based” preparatory condition with respect to the explicitness of task‐specific response meanings. Rushworth et al. [ 2001, 2002] compared an S–R reversal condition with an attentional switching condition using a constant CTI of 1 s. These two types of switching were associated with activation in different areas of the medial PFC and the PPC, but, unfortunately, the authors did not examine whether there was differential involvement of mid‐DLPFC in intentional versus attentional task control.
The involvement of mid‐DLPFC in response‐related adjustment processes in task switching is consistent with results from other research paradigms, which suggest a general role of this region for action‐directed control processes [e.g., Lau et al., 2004; Pochon et al., 2001; Rowe et al., 2000]. However, despite this intriguing overlap of mid‐DLPFC involvement across paradigms, the above task‐switching studies are not as consistent with regard to switch‐related activations in other brain regions. This may be partly due to the fact that these studies use very different experimental manipulations to tease apart attentional and intentional processes and implemented different fMRI designs (constant CTI vs. partial trial). Alternatively, different activation patterns across task‐switching procedures may suggest the existence of multiple action‐directed control processes. This is particularly likely with regard to fronto‐medial regions that are implicated in models that postulate parallel and inter‐related hierarchical organization of lateral PFC regions and medial PFC regions in cognitive control [Kouneiher et al., 2009]. Specifically, the rostral cingulate zone (RCZ) is implicated in intentional switch‐related adjustment processes in both Rushworth et al. [ 2002] and Ruge et al. [ 2010]. Furthermore, these same studies implicated the Pre‐SMA/SMA region in switch‐related adjustment processes irrespective of specific intentional or attentional requirements. More importantly, though, transcranial magnetic stimulation application over that region disrupted only intention‐related, but not attention‐related adjustment processes [Rushworth et al., 2002], a finding that is consistent with Crone et al. [ 2006] who report an involvement of Pre‐SMA/SMA in intention‐related adjustment process. To further clarify this complex pattern of results, future research needs to tease apart different aspects of action‐directed preparatory adjustment processes, an endeavor that will certainly benefit from more precisely conceptualizing the notion of “response intention.”
A related question is whether the same proactive adjustment processes are involved for task switching that involves a change in stimulus set (e.g., switching between letter and digit tasks without S–R reversals) and “lower‐level” attribute switching. Direct evidence in support of such a notion comes from Chiu and Yantis [ 2009], who found activation in pSPL for both location‐based attribute switching and classical task switching (digit categorization). This is consistent with pSPL activation in lower‐level attentional shifting situations irrespective of target modality [Behrmann et al., 2004]. Interestingly, there is also evidence that attentional switch‐related preparatory processes interact with content‐specific sensory areas for color versus motion processing ([Wylie et al., 2006; see also Yeung et al. [ 2006] for similar material‐specific effects in a blocked fMRI design).
Task Switching Without Preceding Task Implementation
A distinctly different approach to identify switch‐related preparatory activation was pursued by Brass and von Cramon [ 2004], who developed the “double‐cue” paradigm. In this paradigm, two different task cues are presented consecutively during the preparation period and signal either the same task (cue meaning repeat) or different tasks (cue meaning switch). Contrasting these two cue conditions yielded significant switch‐related activation in the posterior LPFC within the so‐called inferior frontal junction (IFJ) area (IFJ, Derrfuss et al. [ 2005, 2009]) as well as in the parietal cortex6. This result may seem surprising in the light of behavioral findings [Schuch and Koch, 2003], which suggest that switch‐related task adjustment processes depend on the actual implementation of the previously cued task and therefore would not be expected to have been completed to the first cue in the Brass and von Cramon paradigm (for further elaboration on the distinction between task instruction and task implementation, see Brass et al. [ 2009]). These contrasting outcomes suggest that switch‐related adjustment processes as indicated by brain activation measures may not always be reflected in behavioral performance. If this conclusion is valid, an important caveat of the partial trial design appears less problematic. Specifically, the fact that trials after partial cue‐only trials require a task switch without previous task implementation might not critically reduce the chance to detect switch‐related preparatory BOLD activation when analyzed together with trials following full cue‐target trials. Indeed, the size of switch‐related preparatory activation reported in Ruge et al. [ 2010] was not influenced by the presence or absence of previous task implementation as suggested by a separate analysis of switch‐related preparatory activation following partial trials versus full trials [Ruge and Braver, 2008]. Similar results were obtained in a recent ERP study that implemented the Schuch and Koch [ 2003] paradigm and showed that switch‐related preparatory brain activation was not influenced by completeness of task implementation in the preceding trial [Jamadar et al., 2010c].
Preparatory Activation Irrespective of Switching or Repeating
Many fMRI studies have focused on preparatory BOLD activation in anticipation of the upcoming target irrespective of whether switching or repeating tasks [Brass and von Cramon, 2002; MacDonald et al., 2000; Ruge et al., 2009a; Sakai and Passingham, 2003; Shi et al., 2010]. Other studies have included only switch trials making a comparison with repeat trials inherently impossible [Sakai and Passingham, 2006]. Instead of examining possible differences in preparatory BOLD activation between switch and repeat trials, these studies examine differences in preparatory brain activation depending on the type of advance information that is provided (e.g., the specific task that is cued).
Generally, these studies can be grouped according to the specific type of preparatory activation they examine (see Fig. 1 for different possible types of preparatory neural activation). Some studies focus on delay‐related (i.e., working‐memory‐related) preparatory activation and, thus, mostly use jittered CTI designs for explicitly extracting delay‐related BOLD components. Other studies are interested in preparatory processes under minimized working memory (WM) load and thus use partial trial designs with comparably short CTIs. Interestingly, preparatory activation within prefrontal cortex upon presentation of a typical task cue (e.g., a more or less abstract symbol denoting the currently relevant task) appears to systematically vary for partial trial designs and jittered CTI designs. In partial‐trial studies, there is a consistent overlap of preparatory activation related to task cue presentation in more posterior regions of PFC in the vicinity of the IFJ and the Pre‐SMA [Brass and von Cramon, 2002; Ruge et al., 2009a; Shi et al., 2010]. By contrast, studies based on wide CTI jittering report additional delay‐related preparatory BOLD activation within more anterior VLPFC regions extending into frontopolar cortex [Bengtsson et al., 2009; Bunge et al., 2003; Chiu and Yantis, 2009; Sakai and Passingham, 2003, 2006]. A plausible explanation for this striking difference is related to the fact that CTI jittering involves much longer CTIs (e.g., 4–12 s) than partial trial designs (e.g., 2 s), which have, in principle, no lower CTI limit. Additionally, these paradigms differ in that the cue remains on screen during the CTI in the partial trial studies but not in the jittered CTI studies, thus reducing the requirement for the active maintenance of cue identity. Thus, delay‐related preparatory activation extending into more anteriorly located VLPFC regions likely reflects the need for active maintenance of task‐related representations.
In addition to this general regional dissociation regarding the type of preparatory activation (i.e., cue‐related vs. delay‐related), the above studies yield a number of interesting findings that may further constrain the role of prefrontal subregions for different aspects of task preparation. One such prefrontal subdivision in the context of high WM demands was described by Sakai and Passingham [ 2003, 2006]. Specifically, they showed that anterior LPFC activation (including BA10) was maintained during the delay irrespective of the currently cued task. However, this activation correlated in a task‐specific manner with BOLD activation within more posteriorly located LPFC areas—both, during the delay and during subsequent task implementation (i.e., at the time of target presentation) as well as with behavioral task performance (for an extensive review of related research, see Sakai [ 2008]). Studies investigating task preparation under minimal WM demands and focusing on cue‐related BOLD activation (instead of delay‐related activation) do not typically examine the possible task‐specificity of preparation‐related activation. In fact, these studies often seem to maximize task similarity (e.g., letter vs. digit categorization or horizontal vs. vertical placement) instead of using tasks that are maximally distinct in terms of processing modality (e.g., verbal vs. spatial tasks or color vs. motion tasks; but see previous section for Wylie et al. [ 2006], who compared color and motion tasks within a non‐WM design). Thus, in these non‐WM task preparation studies, the detection of task‐specific preparatory activation might have been difficult in any case.
Another prefrontal subdivision was described in a partial‐trials study by Ruge et al. [ 2009a], which contrasted preparatory activation elicited by standard advance task cues (followed by target stimuli) with preparatory activation elicited by advance target stimuli (followed by task cues). On one hand, the results suggested a strong functional–anatomical overlap between cue‐related and target‐related processes within posterior prefrontal (IFJ, Pre‐SMA, and dorsal PMC) and posterior parietal areas (posterior intraparietal sulcus (IPS) and pSPL). Specifically, these areas were engaged with advance task cues, re‐engaged with the subsequent target, and also showed preparatory activation when the target stimulus appeared in advance of the cue. Moreover, there was no other brain region that was engaged exclusively for advance task cues. On the other hand, a number of more anterior brain regions within the PFC (DLPFC and RCZ) as well as regions in the parietal cortex (anterior IPS) were exclusively engaged with advance target stimuli. This pattern of results is consistent with a conceptual distinction between two “task set” components, that is, abstract task goals that specify “what to do next” and concrete task implementation rules that specify “how to do it” [cf., Rubinstein et al., 2001]. Accordingly, the advance activation of abstract task goals might have occurred for both conditions in the Ruge et al. [ 2009a] study, that is, cue first (single goal) and target first (two potential goals). By contrast, advance activation of implementation rules might have occurred exclusively for the target first condition and is supposed to indicate that a concrete target stimulus directly activates the respective task‐specific implementation rules. The involvement of mid‐DLPFC in preparatory activation of implementation rules is also directly supported by a recent study, which found preparatory mid‐DLPFC activation specifically for advance “rule cues” that explicitly denoted the currently relevant S–R rule when compared with standard advance task cues [Shi et al., 2010]. Notably, this involvement of mid‐DLPFC in the preparation of “implementation rules” is consistent with the findings of preparatory switch‐related activation in very similar brain regions reported in studies that examined the preparatory adjustment of “action sets” (see section “Does switch‐related preparatory activation reflect stimulus‐directed or response‐directed adjustment processes?” above). Finally, as elaborated further below (“Multiple preparatory modes?” section), the conceptual distinction between abstract task goals and implementation rules (i.e., action sets) might ultimately serve to explain why some studies did and some other studies did not observe switch‐related preparatory activation indicative of proactive task adjustment processes.
Open Questions and Promising Directions
In sections “Does switch‐related preparatory activation reflect stimulus‐directed or response‐directed adjustment processes?” through “Preparatory activation irrespective of switching or repeating,” we identified a number of variables that might explain some of the heterogeneity of fMRI results regarding the precise localization of preparation‐related BOLD activation in task switching. As most of these variables are identified by integrating results across different studies, these conclusions need to be interpreted with caution, and future research needs to systematically examine the influence of each of these variables within studies while controlling for confounding variables.
One fundamental question that remains largely unresolved is why some studies do and others do not find reliably enhanced activation for switch versus repeat trials in prepared trial conditions. In part, this discrepancy might simply be due to low statistical power in those studies that fail to detect significant switch‐related BOLD activation (for further elaboration, see sections “Statistical uncertainty” and “Discrepancies between fMRI and ERP findings” below). Alternatively, it may be partly due to the fact that a large proportion of studies that do report significant switch‐related activation in prepared trial conditions used designs and analysis procedures that do not allow for disentangling cue‐related and target‐related BOLD components [Badre and Wagner, 2006; Braver et al., 2003; Crone et al., 2006; Jamadar et al., 2010a; Rushworth et al., 2001, 2002; Wylie et al., 2006]. Consequently, as discussed in section “Examining preparatory processes without explicitly isolating preparatory BOLD activation components” earlier, switch‐related activation in these studies may be attributable to reactive (i.e., post‐target) adjustment processes rather than proactive adjustments completed during the CTI. However, this explanation is unlikely to fully account for the variability of switch‐related activation across studies for two reasons. First, a subset of studies that have attempted to explicitly disentangle cue‐related and target‐related BOLD response components (by use of partial trial designs, jittered CTI designs, or constant‐long CTI designs; see section “Segregating preparation‐related and target‐related BOLD activation components”) still show substantial inconsistencies; some studies report reliable switch‐related activation clearly linked to the preparation interval [Barber and Carter, 2005; Chiu and Yantis, 2009; Ruge et al., 2010] and others do not [Bunge et al., 2003; Cavina‐Pratesi et al., 2006; Ruge et al., 2009a]. Second, some of the studies that could not explicitly disentangle the compound cue‐target BOLD response have examined the relationship between the overall BOLD response and behavioral/ERP measures to try and constrain the timing of the BOLD effect. In these studies, RT‐fMRI and ERP‐fMRI relationships (see section “Cross‐modal correlational approaches”) suggest that the compound switch‐related BOLD activation they observed may be associated with preparatory switch‐related processes. Specifically, Braver et al. [ 2003] found that smaller residual behavioral switch cost was associated with greater switch‐related activation in pSPL (for a similar finding, see Chiu and Yantis [ 2009]). This suggests that smaller residual switch cost in performance was caused by stronger proactive adjustment processes as indicated by greater switch‐related pSPL activation during preparation. Alternatively, if pSPL activation reflected reactive adjustments, it should have shown greater switch‐related activation for larger residual switch cost, that is, when proactive adjustment processes had been relatively ineffective. Similarly, Jamadar et al. [ 2010a] reported that switch‐related BOLD activation in a pSPL subregion correlated positively with switch‐repeat differences in a cue‐locked but not target‐locked ERP component, a finding that is consistent with the interpretation that pSPL is involved in switch‐related proactive preparatory processes.
Statistical uncertainty
Some of the inconsistencies concerning switch‐related preparatory BOLD activation could simply be related to statistical uncertainty. That is, some studies may report significant effects that have emerged by pure chance, whereas other studies may have failed to find significant effects because of insufficient statistical power, a problem prevalent in many domains of imaging research [Yarkoni et al., 2010]. This problem is often further complicated by the fact that studies apply different significance thresholds for dealing with multiple comparisons on the whole‐brain level, thus differentially biasing the rejection of the null hypothesis7. Because the absence of a significant effect never justifies the acceptance of the null‐hypothesis, studies that failed to find significant switch‐related activation might be underrepresented in published reports as such negative results can easily be refuted by postulating insufficient statistical power. Furthermore, null results with respect to switch‐related preparatory activation in fMRI studies are challenged by the highly consistent finding of significant switch‐related preparatory activation in ERP studies. The validity of this latter argument will be critically assessed in a separate subsection “Discrepancies between fMRI and ERP findings” below.
The size of switch‐related preparatory activation in previous studies, and hence its statistical detectability, is likely to be affected by a host of study‐specific procedural parameters. However, as mentioned earlier, the existing fMRI studies differ in too many features at once to be able to pin down, which distinct procedural parameters might determine the presence or absence of significant switch‐related preparatory activation. This current state of affairs dramatically highlights the interpretative caveats of assessing main effects of trial type (i.e., switch > repeat). Assessing interaction effects between trial type and conditions designed to differentially modulate the size of any basic trial type effect (i.e., differential size of switch > repeat effect in condition A vs. condition B) is likely to lead to stronger conclusions. Importantly, rather than focusing on the absolute size of switch‐related activation per se, future studies should assess the relative size of switch‐related preparatory activation under experimentally varied conditions.
The relative size of switch‐related preparatory activation certainly depends on parameters that generally affect the efficiency of proactive adjustment processes. As discussed in sections “Measuring trial‐related BOLD activation against baseline” and “Altered preparatory processes in task switching through design modifications?,” preparatory efficiency might be reduced by modifications to the task‐switching paradigm that are designed to make it more amenable to certain types of fMRI data analysis. Besides fMRI design considerations, there are certainly other parameters that might potentially affect preparatory efficiency, or even the type of preparatory processes engaged. The most direct way to assess the relative size of switch‐related preparatory activation is to directly compare prepared and unprepared conditions without necessarily being interested in the significance of switch‐related activation in the prepared trial condition per se. Indeed, a fair number of studies have followed this approach as addressed extensively in Key Question 3 below. On the basis of these studies, we will propose a theoretical framework of multiple preparatory modes that can help to generate working hypotheses regarding the parameters that determine the type of preparatory mode adopted and the degree to which (and whether) increased preparatory control is likely to be required for switch relative to repeat trials (“Multiple preparatory modes?” section).
Discrepancies between fMRI and ERP findings
In contrast to the rather heterogeneous results across different fMRI studies, virtually, every ERP study reports switch‐related modulation of cue‐locked ERP components [Karayanidis et al., 2010]. The highly consistent switch‐related preparatory activation in ERP studies might simply be due to the fact that ERP studies do not have to rely on design modifications (CTI and ITI lengths; target omission) to extract cue‐related and target‐related ERP components (see sections “Measuring trial‐related BOLD activation against baseline” and “Altered preparatory processes in task switching through design modifications?” above). Yet, this is unlikely to fully account for the discrepancy as those fMRI studies that have relied most heavily on fMRI‐specific design modifications have found switch‐related preparatory BOLD activation in prefrontal and parietal brain regions [Barber and Carter, 2005; Chiu and Yantis, 2009; Ruge et al., 2010]. Conversely, despite relatively modest modifications of the original behavioral task‐switching design, Ruge et al. [ 2005] did not find switch‐related activation for prepared trials when compared with significant switch‐related activation in unprepared trials.
Alternatively, differences in statistical power between fMRI and ERP methodologies may also contribute to the discrepancy regarding switch‐related preparatory activation. This is particularly likely as standard fMRI and ERP statistical analysis procedures apply different statistical thresholding and correction procedures. Typically, ERP studies apply local thresholds of P < 0.05 and either do not correct for multiple comparisons across electrodes and/or timepoints or apply simple family‐wise error rate correction. Therefore, ERP studies are more likely to detect small effect sizes, yet at the cost of increased risk of false positives. Thus, traditional ERP research relies highly on replication to validate such small effects (for a promising alternative ERP analysis approach, see Murray et al. [ 2008] and Wylie et al. [ 2009]). Functional MRI studies involve an exponentially larger data set and typically control for the massive spatial multiple comparisons problem in imaging data sets, thus leading to higher local thresholds and increasing the likelihood of missing relatively weak true effects. Moreover, switch‐related activation in circumscribed brain regions examined in fMRI studies could be expected to be weaker than suggested by switch‐related activation in certain ERP components. The reason is that an ERP component is likely to results from the summation of switch‐related signals elicited in spatially segregated, but functionally associated brain regions, each of which exhibits only weak activation on its own. These relatively weak activation effects within each constituent brain region might be missed by the localized measure of brain activation obtained in fMRI. One way to reduce the difference in statistical power between ERP and fMRI studies would be to rely more heavily on fMRI region of interest (ROI) analyses, which may detect smaller activation effects that would not survive more conservative whole‐brain‐corrected thresholds.
KEY QUESTION 3: IS THERE EVIDENCE THAT DISTINCT BRAIN AREAS EXHIBIT SWITCH‐RELATED ACTIVATION IN PREPARED VERSUS UNPREPARED TRIAL CONDITIONS?
Some studies directly compare proactive versus reactive control adjustments by contrasting switch‐related BOLD activation for long CTI (i.e., “prepared” trials) versus short CTI (“unprepared” or incompletely prepared trials). As discussed earlier (section “Directly contrasting BOLD responses across prepared and unprepared trial conditions” above), a brain region primarily engaged in proactive control adjustments should exhibit stronger switch‐related activation in prepared than in unprepared trials, whereas a brain region primarily engaged in reactive control adjustments should exhibit stronger switch‐related activation in unprepared trials than in prepared trials. By extension, a region that exhibits similar switch‐related activation for both prepared and unprepared trials is likely to be associated with control processes that are common to both proactive and reactive adjustment and are activated either before or after target onset depending on the time available for preparation. Interestingly, studies that have directly compared prepared and unprepared trial conditions have found evidence for all three activation patterns partly even within the same brain regions.
In support of reactive switch‐related control adjustments, Ruge et al. [ 2005] and Brass and von Cramon [ 2004] found widespread switch‐related activation for unprepared trials (CTI = 100 ms) when compared with prepared trials (CTIs > 800 ms) in multiple brain regions including parietal cortex (aIPS and pIPS/pSPL) and frontal cortex (mid‐DLPFC, IFJ, Pre‐SMA, and dPMC). A similar, but regionally more confined reactive control pattern was found by Badre and Wagner [ 2006] specifically for the mid‐VLPFC—a region not reported in the above two studies. The involvement of these brain regions in reactive adjustment processes is further supported by studies that included only unprepared trial conditions (i.e., no direct comparison with prepared trial conditions), most of which report switch‐related activation in similar parietal and frontal regions [e.g., Brass et al., 2003; Dove et al., 2000; Hyafil et al., 2009; Liston et al., 2006; Smith et al., 2004]. Importantly, a subset of these regions (IFJ, Pre‐SMA, PMC, and pIPS/pSPL) also showed general preparation‐related BOLD activation, that is, similar levels of activation for switch and repeat trials in long CTI conditions [e.g., Brass and von Cramon, 2002; Ruge et al., 2005, 2009a]. Thus, these areas do seem to play a role in proactive control—just not in terms of a substantially stronger recruitment in switch than repeat trials. However, to further complicate matters some of the same regions (IFJ, Pre‐SMA, mid‐DLPFC, and pIPS/pSPL) that showed stronger switch‐related activation for unprepared trials than for prepared trials in Ruge et al. [ 2005] and Brass and von Cramon [ 2004] exhibited similar switch‐related activation levels in both prepared and unprepared trials in Badre and Wagner [ 2006]. This latter result is consistent with a flexible engagement of these regions in both proactive and reactive switch‐related adjustment processes depending on the time for advance preparation. As discussed in “Key Question 2” above, the involvement in proactive switch‐related adjustment processes is further supported by some studies that implemented prepared trial conditions only [e.g., Crone et al., 2006; Ruge et al., 2010; Rushworth et al., 2002]. Finally, a recent study by Jamadar et al. [ 2010a] found a third pattern of switch‐related activation when comparing prepared and unprepared conditions. Specifically, there was switch‐related activation in the pIPS/pSPL for prepared trials but not for unprepared trials (and no other areas showed switch‐related activation for unprepared trials), suggesting an exclusive role in proactive switch‐related adjustments. This conclusion was also supported by analyses of fMRI‐ERP correlations discussed in “Key Question 2” earlier.
Open Questions and Promising Directions
The complex pattern of the above results raises one particularly puzzling question: how can the same areas (IFJ, Pre‐SMA, mid‐DLPFC, and pIPS/pSPL) appear to be involved in switch‐related adjustment processes either only proactively ([Jamadar et al., 2010a, specifically the pSPL), only reactively [Ruge et al., 2005], or both proactively and reactively [Badre and Wagner, 2006]. One immediately obvious implication is that none of these areas is likely to be specialized in either proactive or reactive switch‐related adjustments. Instead, they seem to be able to play either role depending on the current experimental context.
An intriguingly simple explanation for the flexible recruitment of these brain areas in either proactive or reactive adjustment processes is that long CTI trials are not really “prepared trials” under all particular study conditions. Just as occasional failures to engage proactive adjustment processes on a few long CTI trials might explain residual behavioral switch cost despite sufficient preparation time [DeJong, 2000], a relatively high proportion of unprepared long CTI trials might explain the absence of significant switch‐related preparatory BOLD activation in long CTI trials as observed in Ruge et al. [ 2005] or Brass and von Cramon [ 2004]. In other words, switch‐related preparatory neural activation associated with relatively few fully prepared long CTI trials might be too small to be detected when analyzed together with the many unprepared long CTI trials. However, this explanation seems implausible for two reasons. First, studies that fail to identify any brain area associated with proactive adjustment processes [Brass and von Cramon, 2004; Ruge et al., 2005] still show a reduction in behavioral switch cost in long CTI trials when compared with short CTI trials, suggesting that proactive adjustment is likely to have been undertaken on a considerable proportion of trials. Second, if subjects did not substantially adjust proactively during the long CTI in those studies, they must have adjusted reactively after the target arrived. This compensatory target‐driven reactive adjustment in effectively unprepared long CTI trials should have been reflected by switch‐related activation more or less equivalent to that observed for similarly unprepared short CTI trials. Yet, this was not the case in either Ruge et al. [ 2005] or Brass and von Cramon [ 2004]. Thus, paradoxically, although reduced behavioral switch cost and reduced switch‐related activation in long when compared with short CTI trials suggests that subjects are prepared for the upcoming switch trial, long CTI trials show no sign of substantial switch‐related enhancement of preparation‐related BOLD activation. This suggests that under certain study conditions, the need for switch‐related reactive adjustments at the time of target presentation can be reduced via preparatory processes that are similarly engaged for both switch and repeat trials. Under other study conditions, however, the reduction of reactive adjustment demands in long CTI trials seems to rely on a type of preparation that does involve an increased recruitment of neural resources in switch relative to repeat trials as indicated by sizable switch‐related BOLD activation in long CTI trials [Badre and Wagner, 2006; Jamadar et al., 2010a]. Below, we attempt to specify more clearly these different types of preparatory processes.
Multiple preparatory modes?
Many models of task‐switching assume that preparation to repeat or switch tasks involves a number of different processes operating on different task set components. Rubinstein et al. [ 2001] distinguished between abstract task goals (i.e., a representation of “what to do next”) and concrete task implementation rules (i.e., task‐related action sets that determine “how to reach the activated task goal”; see also Mayr and Bryck [ 2005]. Arrington et al. [ 2007] proposed that cue encoding leads to an abstract task goal (cue “mediator”) representation that is subsequently integrated with target information to complete a compound cue‐target representation that determines the correct response. Meiran et al. [ 2000, 2008] distinguished between abstract task goals, action sets and, as an additional third component, attentional sets that determine which stimulus dimension should be selectively processed. Rubinstein et al. [ 2001] and Meiran et al. [ 2000] agree that abstract goal representations can be activated and switched in advance, but that action sets are not typically adjusted according to the active task goal in advance of target presentation. Additionally, Meiran et al. [ 2000] suggested that attentional sets are typically adjusted proactively in accordance with changed task goals. Notably, there are reasons to believe that the timing and sequence of these processes can be flexibly aligned depending on the particular study conditions. Specifically, in section “Does switch‐related preparatory activation reflect stimulus‐directed or response‐directed adjustment processes?,” we reviewed evidence from fMRI studies, suggesting that action sets can in fact be adjusted proactively under certain conditions—as evidenced by switch‐related BOLD activation in long CTI conditions within a distinct set of brain regions (most reliably including mid‐DLPFC and RCZ). By contrast, under other conditions, task preparation seems to rely primarily on adjustments of attentional sets as reflected by switch‐related BOLD activation in long CTI conditions most reliably within pIPS/pSPL. From the perspective of previous PI theory, preparation of attentional set and/or action set according to the currently activated task goal implies more effortful resolution of increased competition in switch relative to repeat trials and is thus consistent with the observed increase of switch‐related BOLD activation in the aforementioned brain areas.
In addition to these two preparatory modes (i.e., proactive adjustment of attentional sets and proactive adjustment of action sets), we propose a third preparatory mode that relies on goal activation alone (for conceptual considerations that might imply similar conclusions, see Arrington et al. [ 2007], Badre and Wagner [ 2006], Gilbert and Shallice [ 2002], and Goschke [ 2000]). This goal‐activation‐only mode explains the preparation‐related reduction of residual reactive adjustment demands (as indicated by reduced switch cost in prepared vs. unprepared trials) without assuming substantial preparatory competition resolution within attentional set and/or action set. This mode is hence compatible with the absence of reliable preparation‐related enhancement of BOLD activation in switch relative to repeat trials. Importantly, this account rests on the central assumption that the perseverative tendency to repeat the previous task is not related to lingering goal activation and a resulting difficulty in establishing the new task goal in switch trials (otherwise, we would expect goal interference and an associated increase in switch‐related activation). Note that the neutralization of goal activation between trials is common to most computational models of task switching [Badre and Wagner, 2006; Brown et al., 2007; Gilbert and Shallice, 2002]. Because perseveration is not mediated by lingering goal activation, it is instead assumed to be linked to lingering activation within “action set” and/or “attentional set”8. On unprepared switch trials, this results in greater interference as reflected by strong switch cost and strongly increased BOLD activation relative to repeat trials. On prepared trials, reduced interference during task implementation is explained by the interaction of different biases at the time of target presentation: top‐down bias from the proactively activated task goal and “bottom‐up” (here: not proactively resolved) biases resulting from lingering activation of the previously active action set and attentional set. Importantly, due to the temporal priority of advance task goal activation in prepared trials and due to the putative hierarchical dominance of this top‐down bias, the target‐evoked bottom‐up biases cannot develop a considerable impact. In other words, when the correct goal representation has sufficient time to settle into a stable state during the CTI, bottom‐up biases in favor of the currently irrelevant task are dampened right from the start of target processing. Envisioning an implementation via neuronal network models of task switching (see Badre and Wagner [ 2006], Brown et al. [ 2007], and Gilbert and Shallice [ 2002]), the impact of bottom‐up biases during target processing would depend on the strength of lateral inhibition between competing attentional sets and/or action sets and on the strength of top‐down afferents from the goal layer. On unprepared trials, the correct goal representation has not yet settled, and hence initial target processing would be much more vulnerable to the impact of lingering bottom‐up biases. In turn, this would lead to more effortful competition resolution in favor of the current task goal as indicated by a greater increase in switch‐related BOLD activation. Note that according to this goal‐activation‐only preparatory mode, prolonged preparation time does not reduce task interference simply because the previous goal state has more time to decay passively. Instead, we assume that previous goal activation decays rapidly during the ITI before the next task cue is presented (see Badre and Wagner [ 2006] and Gilbert and Shallice [ 2002]). Therefore, activating the currently relevant goal representation during a long CTI is expected to be the same for repeat and switch trials.
The above considerations are not meant to imply mutually exclusive preparation modes. Rather, the relative preparatory involvement of abstract goal representations, action sets, and attentional sets is envisioned as depending on task conditions. Thus, even if the absence of significant switch‐related BOLD activation in long CTI conditions implies a predominant engagement of the goal‐activation‐only mode, this does not categorically exclude the additional, though comparably weak, involvement of proactive switch‐related adjustment processes operating on attentional or action sets. These adjustment processes might, however, be too weak to be detected via measures of BOLD activation, whereas more sensitive ERP measures may reveal even this comparably small switch‐related enhancement of neural activity (see Discrepancies between fMRI and ERP findings).
For the notion of multiple preparatory modes to have strong explanatory power, two critical questions need to be addressed by future research. First, what are the critical variables that determine the relative preparatory involvement of each mode? Second, are these context variables indeed associated with different strengths of switch‐related preparatory activation? Because no fMRI studies have directly examined these two inter‐related questions, we can only speculate on possibly relevant variables.
The explicitness with which the task cue indicates the current S–R rule may be one important variable [cf., Meiran et al., 2008]. A cue that is explicitly mapped to the concrete S–R rule might encourage preparation in anticipation of the upcoming stimulus category (i.e., attentional set) and the associated responses (i.e., action set), whereas an abstract cue that is not explicitly mapped to the S–R rule would not. Thus, explicit rule cues might provide a strong trigger for preparatory resolution of proactive interference (PI) between different attentional and/or different action sets. In fact, a recent partial‐trial fMRI study contrasted preparatory and target‐related activation for standard advance task cues (here: “gender” vs. “color”) versus rule cues that explicitly denoted the currently relevant implementation rule, that is, for instance, male: left; female: right [Shi et al., 2010]. Mid‐DLPFC regions were sensitive to cue type in a way that suggested an involvement in preparatory action set activation rather than in preparatory task goal activation. However, switch‐related preparatory activation was not systematically analyzed for each cue type (except for a predefined Pre‐SMA ROI that yielded no significant differences in switch‐related activation). Although this study represents an important first step in examining this issue, further work is required to specifically address whether advance rule cues might entail stronger switch‐related proactive adjustment processes than more standard advance task cues.
Similar to the notion that explicit advance task rule information might stimulate preparatory adjustments of action sets, there is evidence that S–R reversal might encourage this preparatory mode as well. As outlined in section “Does switch‐related preparatory activation reflect stimulus‐directed or response‐directed adjustment processes?”, virtually, all existing S–R reversal studies do report significant switch‐related activation in prepared trial conditions (specifically within mid‐DLPFC and RCZ); inconsistent results are only found in task‐switching paradigms that require switching between constant S–R mappings. In these latter paradigms, additional variables might determine whether action set will be activated and adjusted during the CTI. For instance, overpracticed or intrinsically more intuitive S–R mappings might make cue‐triggered retrieval of the relevant task rule easier and thus automatically trigger preparatory activity and the adjustment of action sets if required. Also, more difficult rule retrieval (i.e., less practiced and less intuitive S–R mappings) may lead to strategic (here: voluntary, effortful) adaptations that entail the preparatory activation and adjustment of action sets. Similar preparatory strategies might be elicited by other factors that increase the difficulty of rule implementation (e.g., rule complexity, but see Rubinstein et al. [ 2001]). Furthermore, variables that increase proactive task interference may encourage similar strategic adjustments. For instance, repetition run length (i.e., micropractice; Rogers and Monsell [ 1995]) should increase rule perseveration and might stimulate compensatory competition resolution during the CTI particularly in switch trials. It may even be the case that, under certain conditions, such a strategy would be engaged exclusively in switch trials (see also “Key question 1”). Note that the strategic preparatory adjustment of action sets would be expected to be, where possible, accompanied by preparatory adjustments of attentional set. There are additional parameters that might specifically affect preparatory adjustments of attentional set: like rule complexity might induce the strategic preparatory adjustment of actions set, factors affecting perceptual discriminability or the familiarity of the presented target stimuli might influence preparatory strategy with regard to attentional set.
Finally, preparatory adjustment of attentional and/or action sets always depends on establishing a representation of the currently relevant task goal in the first place. So, it should be expected that variables that modulate the efficiency of preparatory task goal activation should also indirectly impact switch‐related preparatory BOLD activation. Potentially relevant are variables that affect random foreperiod effects associated with CTI/ITI length and distribution (see sections “Measuring trial‐related BOLD activation against baseline” and “Altered preparatory processes in task switching through design modifications?”). Other variables include the duration of cue presentation [Verbruggen et al., 2007], the transparency of cue‐task associations (i.e., arbitrary vs. meaningful cues; Logan and Bundesen [ 2004] and Monsell and Mizon [ 2006]), types of cues (e.g., transition vs. task cues, Forstmann et al. [ 2005], the ratio of cue‐task mapping [Logan and Bundesen, 2004; Mayr and Kliegl, 2003; Nicholson et al., 2005], or the general motivational state [Savine and Braver, 2010].
GENERAL CONCLUSIONS
What Kinds of Processes Are Reflected by Preparatory BOLD Activation in Task Switching?
Much of the task‐switching literature for the last 15 years has been revolving around the notion of TSR—a concept encompassing executive control processes that serve to establish the currently relevant task set under task‐switching conditions. The reduction of behavioral switch cost with increasing preparation time is sometimes interpreted as straight‐forward evidence for the operation of such executive control processes. In functional‐anatomical terms, executive control function in general is often ascribed to the PFC in concert with the PPC [e.g., Cole and Schneider, 2007; Dosenbach et al., 2008]9. However, the precise brain areas within this large generic control network that specifically support preparatory control in task switching have not been clearly delineated. Moreover, it has been difficult to determine the degree of PFC involvement in preparing to switch tasks and possible subspecializations within this or other brain structures. Beyond providing a comprehensive overview of the functional anatomical expressions of preparatory task control, the present review also aimed at evaluating how fMRI results might contribute to the clarification of conceptual issues concerning the specific properties of preparatory processes in task switching and whether the TSR metaphor might adequately capture the nature of these processes.
One conceptual issue concerns whether preparatory control (see Key Question 1) is exclusively engaged in switch trials to establish the currently relevant task set—an implication that has often been derived from the “re‐”configuration metaphor [cf., Kiesel et al., 2010]. Accordingly, if a brain area existed (within PFC) for implementing such a switch‐only preparatory mechanism, we should expect significant preparation‐related BOLD activation in switch but not in repeat trials. The available fMRI data are rather clear in refuting this strong prediction (at least with regard to preparation‐related activation), both for PFC regions as well as other regions.
Alternatively, one might argue that a process that is defined as switch‐only in procedural terms (indeed, nothing has to be re‐configured in repeat trials) does not logically also imply the exclusive engagement of a particular (set of) brain region(s). In this sense, it is easily conceivable that an advance task cue triggers the engagement of certain higher‐level brain regions that “guide the configuration” (i.e., “bias”) processing pathways in accordance with the currently relevant task demands. There is no reason to assume that such a general “task set configuration” process is exclusive to switch trials and cannot be activated on repeat trials as well. The only difference is that in switch trials, it effectively results in a re‐configured state of the brain and that changing the brain state is likely to be associated with relatively stronger preparatory neural activity when compared with just “refreshing” the brain state as required in repeat trials. According to this more moderate view of the TSR metaphor, task preparation should be reflected by switch‐related (i.e., relatively stronger for switch vs. repeat) preparatory BOLD activation within the fronto‐parietal control network. Notably, as mention earlier, this interpretation of the TSR metaphor seems conceptually indistinguishable from PI accounts of reduced behavioral switch cost and thus makes the same prediction of switch‐related preparatory brain activation [Gilbert and Shallice, 2002; Koch and Allport, 2006; Wylie et al., 2009; Yeung and Monsell, 2003]. This prediction, however, has not received ubiquitous support from the fMRI studies reviewed here, as only about half of the studies have reported significant switch‐related BOLD activation in prepared trial conditions (see Key Question 2). A similarly mixed (and even more complex) picture of results emerges when comparing the size of switch‐related BOLD activation in prepared versus unprepared trial conditions (see Key Question 3). Specifically, the same brain regions (i.e., pIFS/IFJ, Pre‐SMA, mid‐DLPFC/mid‐VLPFC, and pIPS/pSPL) have been found to be activated under conditions that promote proactive switch‐related adjustment, reactive adjustment, or a mixture of both. We argue that these apparently inconsistent results can be reconciled by invoking multiple preparatory modes that may all lead to a preparation‐related reduction of behavioral switch cost. So, under some task conditions, behavioral switch cost may be reduced via a proactive goal activation process common to both switch and repeat that serves as an “advance bias in anticipation of subsequent competition.” Such a mechanism may counteract or prevent the built‐up of target‐driven interference originating from lingering activation of previously active task set components (including action set and/or attentional set). By contrast, under other task conditions, the reduction of behavioral switch cost may be (additionally) mediated by advance adjustment of action sets and/or attentional sets according to the currently active task goal. These proactive adjustments would be associated with enhanced preparation‐related BOLD activation in switch trials relative to repeat trials.
At present, it is unclear which particular task parameters might be responsible for a preference of one over the other preparation mode. In “Multiple preparatory modes?” section, we speculate on the possible role of a number of variables. For instance, this might include (i) the explicitness of implementation rules cues, (ii) the amount of prescanning task practice, (iii) general pre‐experimental instructions (e.g., concerning speed/accuracy), or (iv) overall biases towards more or less controlled information processing similar to that assumed in the conflict adaptation literature [Botvinick et al., 2004; Brown et al., 2007, Gratton et al., 1992]. Research tools that are especially suited to tap possible preparatory strategy differences are discussed extensively in a previous work focusing on multimodal analysis approaches to examining preparatory processes in task switching [Karayanidis et al., 2010].
Which Brain Regions Are Involved in Which Preparatory Subprocesses in Task Switching?
Despite a decade of research, it remains premature to make strong claims about one‐to‐one mapping between specific BOLD activation signatures and distinct components of preparatory task control. Nevertheless, we present a tentative summary of region‐to‐function assignments (from anterior to posterior) that might serve as working hypotheses for future studies:
Anterior LPFC (BA10): Domain‐independent maintenance of task goal information, especially when WM demands are high (as in widely jittered CTI designs); see especially “Preparatory Activation Irrespective of Switching or Repeating”.
Mid‐DLPFC: Preparatory activation of task‐specific response intentions (i.e., action sets); implementation‐directed preparation; an often observed parallel activation of the RCZ may reflect a mobilization of the motivational/energizing force that drives mid‐DLPFC engagement; see especially “Does Switch‐Related Preparatory Activation Reflect Stimulus‐Directed or Response‐Directed Adjustment Processes?”.
Posterior LPFC/IFJ: Activation of abstract task goals; the often observed parallel activation of the Pre‐SMA might reflect a mobilization of the motivational/energizing force that drives IFJ engagement; see especially “Key question 3”.
Posterior IPS/pSPL: Preparatory activation of attentional set; see especially “Does Switch‐Related Preparatory Activation Reflect Stimulus‐Directed or Response‐Directed Adjustment Processes?”.
Importantly, and consistent with the notion of multiple preparatory modes, the engagement of most of these regions in preparatory task switching is clearly not restricted to one distinct type of process. Instead, different regions might better be characterized in terms of the type of information or the type of task set component they are supposed to handle (except maybe anterior LPFC). Thus, the specific experimental context seems to determine (i) whether a specific brain region may be involved differentially with regard to switching/repeating (i.e., exhibit enhanced switch‐related activation vs. similar activation levels for switch and repeat), (ii) the temporal locus of this engagement (i.e., exhibit preparatory and/or target‐related activation), and (iii) its involvement in prepared and/or unprepared trial conditions. This diversity of processes within a certain region is illustrated here using the IFJ region as an example.
General preparatory processes irrespective of switching or repeating; IFJ preparatory activation for advance task cues may reflect advance activation of abstract task goals in the context of a goal‐activation‐only preparatory mode that does not involve the proactive adjustment of action sets or attentional sets.
Supporting proactive adjustment of task‐related representations; switch‐related preparatory activation in IFJ may reflect the imposition of goal‐driven bias under increased control demands in switch relative to repeat trials during the proactive adjustment of action sets or attentional sets, which, in turn, is assumed to be associated with concurrent switch‐related preparatory activation in mid‐DLPFC and pSPL, respectively.
Nonpreparatory processes during task implementation; target‐related IFJ activation that may reflect reactivation of previously encoded goal information to ensure correct task implementation.
Supporting reactive adjustment of implementation‐related representations in unprepared trial conditions; switch‐related activation in IFJ may reflect the imposition of goal‐driven bias under increased control demands in switch relative to repeat trials during the reactive (here: unprepared) adjustment of action sets or attentional sets.
This functional characterization of the IFJ region may appear like an arbitrary collection of different processes, hence bearing little explanatory power. On the contrary, however, we believe that such a characterization appeals through its parsimony and integrative power by postulating a single representational basis of abstract task goals. Depending on the current functional context, this representational account can explain the involvement of the IFJ area in a diversity of different processes.
Issues That Need to be Especially Addressed by Future Research
Functional MRI design has come a long way since the first studies using the task‐switching paradigm. Many clever design manipulations have been developed to overcome the tyranny of different timescales between fast, strategic, and therefore variable cognitive processes and the relatively slow BOLD activation signal. However, a number of stubborn issues have remained resistant to these manipulations, and future work is needed to systematically study the relationship between behavioral and BOLD activation phenomena.
A first issue is related to the fact that the consequences of certain fMRI design modifications necessary to isolate preparatory BOLD activation are not yet sufficiently understood (see Section “General fMRI‐Methodological Issues”). This issue requires further systematic examination [e.g., Goghari and MacDonald, 2008; Koch et al., 2003]—not least to better understand the apparent discrepancies between fMRI and EEG results. Currently, emerging innovative fMRI analysis techniques might alleviate some of the limitations faced by more classical analyses. One such technique relies on multivariate pattern classification procedures [Hanke et al., 2010] that have already yielded preliminary results in task‐switching studies [Bode and Haynes, 2009; Esterman et al., 2009]. Another technique relies on independent component analysis (ICA), a multivariate approach that assumes that the fMRI data are a linear mixture of independent sources. ICA algorithms estimate an unmixing matrix to identify statistically independent components of the source signal. The strength of the approach lies in its potential to identify spatially or temporally coherent networks across the entire brain (“functional connectivity”). ICA may therefore be useful in the task‐switching paradigm to extract networks related to temporally overlapping but apparently independent cognitive processes such as proactive and reactive control. This approach has been useful in extracting independent networks of activity in other cognitive paradigms, including WM [Meda et al., 2009], auditory oddball [Meda et al., 2010], and semantic priming [Assaf et al., 2009]. Recent advances in the development of algorithms for data fusion (e.g., joint ICA, Moosmann et al. [ 2008]; parallel ICA, Liu et al. [ 2009]) are also promising future directions that may build upon earlier attempts to multimodal imaging with this paradigm [Jamadar et al., 2010a; Karayanidis et al., 2010].
A second issue involves the fact that due to the inherently multivariate nature of BOLD activation data, whole‐brain analyses that use a massively univariate approach such as the general linear model [Friston, 2004] need to rely on statistical tools for handling the immense multiple‐comparison problem. As a consequence, statistical power is limited for detecting small activation effects in circumscribed brain regions. This might explain some inconsistencies across fMRI studies and across data modalities (especially when comparing fMRI and EEG). Thus, future studies should rely more on ROI‐based approaches to increase statistical power. Ideally, these ROIs should be informed by previous findings, which in turn highlights the need for better data‐sharing solutions [e.g., Derrfuss and Mar, 2009; Yarkoni et al., 2010] and the use of advanced meta‐analysis solutions that focus on the replicability of results [e.g., Wager et al., 2009].
A third issue is related to the lack of comparability across fMRI task‐switching studies. Although some inconsistencies across fMRI studies might be due to statistical uncertainty or fMRI‐methodological limitations, many inconsistencies are likely due to differences in procedural parameters that affect the efficiency of preparation or that might even determine the type of preparatory processes used. However, it is difficult to pinpoint the specific factors that might drive the observed differences by integrating existing data across different studies as any two studies differ in multiple procedural features. Thus, future studies need to conduct systematic and tightly controlled within‐study manipulation of parameters that might impact the general effectiveness of preparatory processes as well as determine which preparatory mode will be adopted. Taking into account the theoretical perspective of the proposed multiple‐preparatory modes account, “Multiple preparatory modes?” section concludes with listing a few relevant variables.
Final Conclusions
Ten years of fMRI research have made it clear that the functional neuroanatomy of preparatory control in task switching is multifaceted. The available imaging data suggest that different aspects of task preparation are mediated through distinct nodes of a generic fronto‐parietal control network. This involves different “task set” components, including “goal state” (posterior LPFC), “attentional set” (pSPL), “action set” (mid‐DLPFC), and “set maintenance” across longer time intervals (anterior LPFC). Moreover, the pattern of BOLD activation as a function of task transition (switch vs. repeat) and preparation time is generally consistent with a biased competition account of cognitive control in task switching and refutes the notion of “switch‐only” control processes. Perseverative tendencies and hence switch cost can be explained by bottom‐up biases within attentional set and action set that are carried over from preceding trials. In line with many computational models, imaging data suggest that the current goal state itself is not carried over to the next trial and is hence not a source of proactive task interference. Within a trial, however, the currently active goal state exerts a top‐down bias that serves to resolve task competition in favor of the relevant task. A current working hypothesis is that there are different ways in which advance task preparation can result in a reduction of increased proactive task interference in switch trials and hence reduced switch cost. One way is based on preparatory goal activation alone without directly interacting with attentional set or action set during the preparation interval, yet indirectly dampening the influence of bottom‐up biases after target presentation. Alternatively, the current goal state can also directly interact with attentional set and/or action set during the preparation interval, hence “actively” eliminating bottom‐up biases in the course of preparatory competition resolution. These different preparatory modes can potentially account for the strikingly different sizes of BOLD activation increase for switch versus repeat trials in long preparation intervals that have been found across fMRI studies. Future research needs to systematically examine the variables that might determine the way task preparation is implemented.
Footnotes
So‐called mixed block/event‐related designs can be used to distinguish sustained BOLD activation maintained across a mixed task block from transient event‐related BOLD activation for repeat and switch trials occurring within this block of mixed trials [Braver et al., 2003].
Indirect evidence that jittered RCI may affect switch‐related preparatory processing can be obtained by comparing results across different task‐switching studies using ERPs. In cued paradigms with RCI jittering, the amplitude of the switch‐positivity is much reduced, ranging between 0.3 and 1.5 μV (Jamadar et al. [ 2010c] [0.3 μV); Jamadar et al. [ 2010a] (∼ 1.5 μV); Jamadar et al. [ 2010b] (∼ 0.8 μV), when compared with nonjittered paradigms where the amplitude generally ranges from 2 to 5 μV {Nicholson et al. [ 2005] (2.5–5 μV); Kieffaber and Hetrick [ 2005] (∼ 4 μV); Karayanidis et al. [ 2009] (∼ 3 μV); Astle et al. [ 2008] (∼ 3 μV); Goffaux et al. [ 2006] (∼ 2 μV)}.
In fact, preliminary results from an ERP task‐switching study comparing jittered versus blocked CTI manipulations show that CTI jittering had dissociable effects on ERP components and behavior [Karayanidis et al., 2008]. Although CTI jittering did not affect preparatory switch‐positivity in the cue‐locked ERP, it eliminated the preparation‐related reduction in behavioral switch cost (but see Monsell and Mizon [ 2006]).
Such an activation pattern might alternatively indicate the operation of a task adjustment process that is inserted strictly serially between cue and target processing (i.e., even with CTI = 0 the adjustment process needs to be completed before target processing can be initiated). Such a process is therefore assumed to be exactly the same in prepared and unprepared trials. Yet, such a strictly serial process model that has often been associated with the TSR metaphor is rather unrealistic [Kiesel et al., 2010].
There are two studies that report switch‐specific BOLD activation elicited by the target stimulus (i.e., not during the preparation interval). Barber and Carter [ 2005] found switch‐only target‐related activation in an inferior parietal cortex region. Another study using a predictable (i.e., not randomly cued) task‐switching procedure reports switch‐only target‐related BOLD activation in the posterior SPL [Kimberg et al., 2000].
Interestingly, these activations were specific for switching the task indicated by the cues, as mere cue identity switches (i.e., the second cue was a different symbol as the first cue, but denoting the same task) did not activate these regions. Together with similar findings from other studies that disentangle cue switching and task switching [Jamadar et al., 2010a; Wylie et al., 2006], this suggests that switch‐related activations reported in fMRI studies are rather not reflecting mere cue switching effects (for a debate of this issue in the behavioral literature, see Logan and Bundesen [ 2003], Mayr and Kliegl [ 2003], and Monsell and Mizon [ 2006]).
The study by Bunge et al. [ 2003] is a good example for how the type of threshold can decisively influence the detection of preparatory switch‐related activation. No significant results were obtained at a whole‐brain threshold of P < 0.001 (uncorrected) and at an ROI‐based threshold of P < 0.05 (uncorrected) restricted to regions exhibiting preparatory activation for an independently defined contrast within the same study. When lowering the whole‐brain threshold (uncorrected) down to P < 0.005 or even down to P < 0.05, a number of significant voxels were found in various brain regions. Unfortunately, only few studies provide such transparent reports of stepwise thresholding procedures, which would enable the reader to pick the results that appear most appropriate for a particular purpose[0].
Such a conceptualization is quite plausible in the light of Luria's classical characterization of prefrontal dysexecutive patients who often exhibit perseverative behavior despite their preserved ability to verbally report the theoretically correct task goal [Luria, 1973]. Accordingly, cognitive control is not so much required for establishing a new goal representation (patients' preserved ability to represent the task goal), but to effectively use an active goal representation for task implementation in the face of interference from the competing task in cases where the target stimulus is bivalent (problems in applying the respective task implementation rules in dysexecutive patients).
In the behavioral task‐switching literature such a characterization of prepared task switching has not remained undisputed. There has been a longstanding dispute about whether executive control processes need to be invoked at all to explain performance on the task‐switching paradigm [Altmann, 2003; Logan and Bundesen, 2003; Monsell, 2003a]. This issue seems to be mostly settled in the sense that it is commonly agreed that the reduction of behavioral switch cost likely reflects both automatic cue encoding processes and executive control processes [Kiesel et al., 2010].
REFERENCES
- Allport DA, Styles EA, Hsieh S ( 1994): Shifting intentional set: Exploring the dynamic control of tasks In: Umilta C, Moscovitch M, editors. Conscious and Nonconscious Information Processing: Attention and Performance. Cambridge, MA: Oxford University Press; pp 421–452. [Google Scholar]
- Altmann EM ( 2003): Task switching and the pied homunculus: Where are we being led? Trends Cogn Sci 7: 340–341. [DOI] [PubMed] [Google Scholar]
- Arrington CM, Logan GD, Schneider DW ( 2007): Separating cue encoding from target processing in the explicit task‐cuing procedure: Are there “true” task switch effects? J J Exp Psychol Learn Mem Cogn 33: 484–502. [DOI] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun V, Kraut M, Hart J Jr, Pearlson G ( 2009): Temporal sequence of hemispheric network activation during semantic processing: A functional network connectivity analysis. Brain Cogn 70: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle DE, Jackson GM, Swainson R ( 2008): Fractionating the cognitive control required to bring about a change in task: A dense‐sensor event‐related potential study. J Cogn Neurosci 20: 255–267. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD ( 2006): Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci USA 103: 7186–7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Carter CS ( 2005): Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cereb Cortex 15: 899–912. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S ( 2004): Parietal cortex and attention. Curr Opin Neurobiol 14: 212–217. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Haynes JD, Sakai K, Buckley MJ, Passingham RE ( 2009): The representation of abstract task rules in the human prefrontal cortex. Cereb Cortex 19: 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA ( 2002): Detection versus estimation in event‐related fMRI: Choosing the optimal stimulus timing. Neuroimage 15: 252–264. [DOI] [PubMed] [Google Scholar]
- Bode S, Haynes JD ( 2009): Decoding sequential stages of task preparation in the human brain. Neuroimage 45: 606–613. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS ( 2004): Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci 8: 539–546. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY ( 2002): The role of the frontal cortex in task preparation. Cereb Cortex 12: 908–914. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY ( 2004): Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci 16: 609–620. [DOI] [PubMed] [Google Scholar]
- Brass M, Ruge H, Meiran N, Rubin O, Koch I, Zysset S, Prinz W, von Cramon DY ( 2003): When the same response has different meanings: Recoding the response meaning in the lateral prefrontal cortex. Neuroimage 20: 1026–1031. [DOI] [PubMed] [Google Scholar]
- Brass M, Wenke D, Spengler S, Waszak F ( 2009): Neural correlates of overcoming interference from instructed and implemented stimulus‐response associations. J Neurosci 29: 1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI ( 2003): Neural mechanisms of transient and sustained cognitive control during task switching. Neuron 39: 713–726. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC ( 2007): Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation of Working Memory. Oxford University Press; 76–106. [Google Scholar]
- Brown JW, Reynolds JR, Braver TS ( 2007): A computational model of fractionated conflict‐control mechanisms in task‐switching. Cogn Psychol 55: 37–85. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD ( 2003): Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol 90: 3419–3428. [DOI] [PubMed] [Google Scholar]
- Cavina‐Pratesi C, Valyear KF, Culham JC, Kohler S, Obhi SS, Marzi CA, Goodale MA ( 2006): Dissociating arbitrary stimulus‐response mapping from movement planning during preparatory period: Evidence from event‐related functional magnetic resonance imaging. J Neurosci 26: 2704–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YC, Yantis S ( 2009): A domain‐independent source of cognitive control for task sets: Shifting spatial attention and switching categorization rules. J Neurosci 29: 3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W ( 2007): The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage 37: 343–360. [DOI] [PubMed] [Google Scholar]
- Cole MW, Bagic A, Kass R, Schneider W ( 2010): Prefrontal dynamics underlying rapid instructed task learning reverse with practice. J Neurosci 30: 14245–14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA ( 2006): Neural evidence for dissociable components of task‐switching. Cereb Cortex 16: 475–486. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M ( 2003): Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7: 415–423. [DOI] [PubMed] [Google Scholar]
- De Baene W, Kuhn S, Brass M: Challenging a decade of brain research on task switching: Brain activation in the task‐switching paradigm reflects adaptation rather than reconfiguration of task sets. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong R ( 2000): An intention‐activation account of residual switch costs In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press; pp 357–376. [Google Scholar]
- Derrfuss J, Mar RA ( 2009): Lost in localization: The need for a universal coordinate database. Neuroimage 48: 1–7. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY ( 2005): Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Hum Brain Mapp 25: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY, Lohmann G, Amunts K ( 2009): Neural activations at the junction of the inferior frontal sulcus and the inferior precentral sulcus: Interindividual variability, reliability, and association with sulcal morphology. Hum Brain Mapp 30: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE ( 2008): A dual‐networks architecture of top‐down control. Trends Cogn Sci 12: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY ( 2000): Prefrontal cortex activation in task switching: An event‐related fMRI study. Brain Res Cogn Brain Res 9: 103–109. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Koechlin E, Ali SO, Grafman J ( 2002): The roles of timing and task‐order during task‐switching. Neuroimage 17: 195–109. [DOI] [PubMed] [Google Scholar]
- Esterman M, Chiu YC, Tamber‐Rosenau BJ, Yantis S ( 2009): Decoding cognitive control in human parietal cortex. Proc Natl Acad Sci USA 106: 17974–17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Brass M, Koch I, von Cramon DY ( 2005): Internally generated and directly cued task sets: An investigation with fMRI. Neuropsychologia 43: 943–952. [DOI] [PubMed] [Google Scholar]
- Friston KJ ( 2004): Introduction: Experimental design and statistical parametric mapping In: Friston KJ, Frith CD, Dolan RJ, Price CJ, editors. Human Brain Function: Academic Press; 193–210. [Google Scholar]
- Gilbert SJ, Shallice T ( 2002): Task switching: A PDP model. Cogn Psychol 44: 297–337. [DOI] [PubMed] [Google Scholar]
- Goffaux P, Phillips NA, Sinai M, Pushkar D ( 2006): Behavioural and electrophysiological measures of task switching during single and mixed‐task conditions. Biol Psychol 72: 278–290. [DOI] [PubMed] [Google Scholar]
- Goghari VM, MacDonald AW III ( 2008): Effects of varying the experimental design of a cognitive control paradigm on behavioral and functional imaging outcome measures. J Cogn Neurosci 20: 20–35. [DOI] [PubMed] [Google Scholar]
- Goschke T ( 2000): Involuntary persistence and intentional reconfiguration in task‐set switching In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press; pp 331–355. [Google Scholar]
- Gratton G, Coles MG, Donchin E ( 1992): Optimizing the use of information: Strategic control of activation of responses. J Exp Psychol Gen 121: 480–506. [DOI] [PubMed] [Google Scholar]
- Gruber O, Karch S, Schlueter EK, Falkai P, Goschke T ( 2006): Neural mechanisms of advance preparation in task switching. Neuroimage 31: 887–895. [DOI] [PubMed] [Google Scholar]
- Hagberg GE, Zito G, Patria F, Sanes JN ( 2001): Improved detection of event‐related functional MRI signals using probability functions. Neuroimage 14: 1193–1205. [DOI] [PubMed] [Google Scholar]
- Hanke M, Halchenko YO, Haxby JV, Pollmann S ( 2010): Statistical learning analysis in neuroscience: Aiming for transparency. Front Neurosci 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartstra E, Kühn S, Verguts T, Brass M: The implementation of verbal instructions: An fMRI study. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Friston KJ ( 2007): Efficient experimental design for fMRI In: Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD, editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London, UK: Academic Press; 193–210. [Google Scholar]
- Hyafil A, Summerfield C, Koechlin E ( 2009): Two mechanisms for task switching in the prefrontal cortex. J Neurosci 29: 5135–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar S, Hughes M, Fulham WR, Michie PT, Karayanidis F ( 2010a): The spatial and temporal dynamics of anticipatory preparation and response inhibition in task‐switching. Neuroimage 51: 432–449. [DOI] [PubMed] [Google Scholar]
- Jamadar S, Michie P, Karayanidis F ( 2010b): Compensatory mechanisms underlie intact task‐switching performance in schizophrenia. Neuropsychologia 48: 1305–1323. [DOI] [PubMed] [Google Scholar]
- Jamadar S, Michie PT, Karayanidis F ( 2010c): Sequence effects in cued task switching modulate response preparedness and repetition priming processes. Psychophysiology 47: 365–386. [DOI] [PubMed] [Google Scholar]
- Jennings JR, van der Molen MW ( 2005): Preparation for speeded action as a psychophysiological concept. Psychol Bull 131: 434–459. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Heathcote A, Provost A, Sanday D, Jamadar S ( 2008): Strategic and decision processes in task‐switching: Integrating behavioral and ERP measures. Proceedings of the 10th International Conference on Cognitive Neuroscience. Bodrum, Turkey: Frontiers in Neuroscience.
- Karayanidis F, Mansfield EL, Galloway KL, Smith JL, Provost A, Heathcote A ( 2009): Anticipatory reconfiguration elicited by fully and partially informative cues that validly predict a switch in task. Cogn Affect Behav Neurosci 9: 202–215. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Jamadar S, Ruge H, Phillips NA, Heathcote A, Forstmann BU ( 2010): Advance preparation in task‐switching: Converging evidence from behavioral, brain activation and model‐based approaches. Front Psychol 1: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayanidis F, Provost A, Brown S, Paton B, Heathcote A ( 2011): Switch‐specific and general preparation map onto different ERP components in a task‐switching paradigm. Psychophysiology 48: 559–568. [DOI] [PubMed] [Google Scholar]
- Kieffaber PD, Hetrick WP ( 2005): Event‐related potential correlates of task switching and switch costs. Psychophysiology 42: 56–71. [DOI] [PubMed] [Google Scholar]
- Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I ( 2010): Control and interference in task switching—A review. Psychol Bull 136: 849–874. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D'Esposito M ( 2000): Modulation of task‐related neural activity in task‐switching: An fMRI study. Brain Res Cogn Brain Res 10: 189–196. [DOI] [PubMed] [Google Scholar]
- Koch I, Allport A ( 2006): Cue‐based preparation and stimulus‐based priming of tasks in task switching. Mem Cogn 34: 433–444. [DOI] [PubMed] [Google Scholar]
- Koch I, Ruge H, Brass M, Rubin O, Meiran N, Prinz W ( 2003): Equivalence of cognitive processes in brain imaging and behavioral studies: Evidence from task switching. Neuroimage 20: 572–577. [DOI] [PubMed] [Google Scholar]
- Kok A, Ridderinkhof KR, Ullsperger M ( 2006): The control of attention and actions: Current research and future developments. Brain Res 1105: 1–6. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E ( 2009): Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 12: 939–945. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE ( 2004): Willed action and attention to the selection of action. Neuroimage 21: 1407–1415. [DOI] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ ( 2006): Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task‐switching paradigm. Neuron 50: 643–653. [DOI] [PubMed] [Google Scholar]
- Liu JY, Pearlson G, Windemuth A, Ruano G, Perrone‐Bizzozero NI, Calhoun V ( 2009): Combining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Hum Brain Mapp 30: 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Bundesen C ( 2003): Clever homunculus: Is there an endogenous act of control in the explicit task cuing procedure. J Exp Psychol Hum Percept Perform 29: 575–599. [DOI] [PubMed] [Google Scholar]
- Logan GD, Bundesen C ( 2004): Very clever homunculus: Compound stimulus strategies for the explicit task‐cuing procedure. Psychon Bull Rev 11: 832–840. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WL ( 2002): Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. Neuroimage 17: 792–802. [PubMed] [Google Scholar]
- Luria AR ( 1973): The Working Brain: An Introduction to Neuropsychology. New York: Basic Books. [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, Carter CS ( 2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Mayr U, Bryck RL ( 2005): Sticky rules: Integration between abstract rules and specific actions. J Exp Psychol Learn Mem Cogn 31: 337–350. [DOI] [PubMed] [Google Scholar]
- Mayr U, Kliegl R ( 2003): Differential effects of cue changes and task changes on task‐set selection costs. J Exp Psychol Learn Mem Cogn 29: 362–372. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Folley BS, Calhoun VD, Pearlson GD ( 2009): Evidence for anomalous network connectivity during working memory encoding in schizophrenia: An ICA based analysis. PLoS One 4: e7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Jagannathan K, Gelernter J, Calhoun VD, Liu J, Stevens MC, Pearlson GD ( 2010): A pilot multivariate parallel ICA study to investigate differential linkage between neural networks and genetic profiles in schizophrenia. Neuroimage 53: 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiran N ( 1996): Reconfiguration of processing mode prior to task performance. J Exp Psychol Learn Mem Cogn 22: 1423–1442. [Google Scholar]
- Meiran N ( 2000): Reconfiguration of stimulus task‐sets and response task‐sets during task‐switching In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press; pp 377–400. [Google Scholar]
- Meiran N ( 2010): Task switching: Mechanisms underlying rigid vs. flexible self control In: Hassin R, Ochsner K, Trope Y, editors. Self Control in Society, Mind, and Brain. New York: Oxford University Press. [Google Scholar]
- Meiran N, Chorev Z, Sapir A ( 2000): Component processes in task switching. Cogn Psychol 41: 211–253. [DOI] [PubMed] [Google Scholar]
- Meiran N, Kessler Y, Adi‐Japha E ( 2008): Control by action representation and input selection (CARIS): A theoretical framework for task switching. Psychol Res 72: 473–500. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A ( 2001): Wisconsin Card Sorting revisited: Distinct neural circuits participating in different stages of the task identified by event‐related functional magnetic resonance imaging. J Neurosci 21: 7733–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S ( 2003a): Task‐set reconfiguration processes do not imply a control homunuculus: Reply to Altmann. Trends Cogn Sci 7: 341–342. [DOI] [PubMed] [Google Scholar]
- Monsell S ( 2003b): Task switching. Trends Cogn Sci 7: 134–140. [DOI] [PubMed] [Google Scholar]
- Monsell S, Mizon GA ( 2006): Can the task‐cuing paradigm measure an endogenous task‐set reconfiguration process? J Exp Psychol Hum Percept Perform 32: 493–516. [DOI] [PubMed] [Google Scholar]
- Monsell S, Sumner P, Waters H ( 2003): Task‐set reconfiguration with predictable and unpredictable task switches. Mem Cogn 31: 327–342. [DOI] [PubMed] [Google Scholar]
- Moosmann M, Eichele T, Nordby H, Hugdahl K, Calhoun VD ( 2008): Joint independent component analysis for simultaneous EEG‐fMRI: Principle and simulation. Int J Psychophysiol 67: 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M, Brunet D, Michel C ( 2008): Topographic ERP Analyses: A step‐by‐step tutorial review. Brain Topogr 20: 249–264. [DOI] [PubMed] [Google Scholar]
- Nicholson R, Karayanidis F, Poboka D, Heathcote A, Michie PT ( 2005): Electrophysiological correlates of anticipatory task‐switching processes. Psychophysiology 42: 540–554. [DOI] [PubMed] [Google Scholar]
- Niemi P, Naatanen R ( 1981): Foreperiod and simple reaction‐time. Psychol Bull 89: 133–162. [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M ( 2001): Separating processes within a trial in event‐related functional MRI I. Neuroimage 13: 210–217. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Poline JB, Crozier S, Lehericy S, Pillon B, Deweer B, Le Bihan D, Dubois B ( 2001): The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: An fMRI study. Cereb Cortex 11: 260–266. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S ( 1995): Costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen 124: 207–231. [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE ( 2000): The prefrontal cortex: Response selection or maintenance within working memory? Science 288: 1656–1660. [DOI] [PubMed] [Google Scholar]
- Rubin O, Meiran N ( 2005): On the origins of the task mixing cost in the cuing task‐switching paradigm. J Exp Psychol Learn Mem Cogn 31: 1477–1491. [DOI] [PubMed] [Google Scholar]
- Rubinstein JS, Meyer DE, Evans JE ( 2001): Executive control of cognitive processes in task switching. J Exp Psychol Hum Percept Perform 27: 763–797. [DOI] [PubMed] [Google Scholar]
- Ruge H, Braver TS ( 2008): Dissociable neural networks for attention and intention during preparation in task switching. Cognitive Neuroscience Annual Meeting, San Fransisco, USA.
- Ruge H, Wolfensteller U ( 2010): Rapid formation of pragmatic rule representations in the human brain during instruction‐based learning. Cereb Cortex 20: 1656–1667. [DOI] [PubMed] [Google Scholar]
- Ruge H, Brass M, Koch I, Rubin O, Meiran N, von Cramon DY ( 2005): Advance preparation and stimulus‐induced interference in cued task switching: Further insights from BOLD fMRI. Neuropsychologia 43: 340–355. [DOI] [PubMed] [Google Scholar]
- Ruge H, Braver T, Meiran N ( 2009a): Attention, intention, and strategy in preparatory control. Neuropsychologia 47: 1670–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge H, Goschke T, Braver TS ( 2009b): Separating event‐related BOLD components within trials: The partial‐trial design revisited. Neuroimage 47: 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge H, Muller SC, Braver TS ( 2010): Anticipating the consequences of action: An fMRI study of intention‐based task preparation. Psychophysiology 47: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, Sipila PK ( 2001): Attention systems and the organization of the human parietal cortex. J Neurosci 21: 5262–5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK ( 2002): Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. J Neurophysiol 87: 2577–2592. [DOI] [PubMed] [Google Scholar]
- Sakai K ( 2008): Task set and prefrontal cortex. Annu Rev Neurosci 31: 219–245. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE ( 2003): Prefrontal interactions reflect future task operations. Nat Neurosci 6: 75–81. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE ( 2006): Prefrontal set activity predicts rule‐specific neural processing during subsequent cognitive performance. J Neurosci 26: 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, Braver TS ( 2010): Motivated cognitive control: Reward incentives modulate preparatory neural activity during task‐switching. J Neurosci 30: 10294–10305. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schneider W, Chein JM ( 2003): Controlled and automatic processing: Behavior, theory, and biological mechanisms. Cogn Sci 27: 525–559. [Google Scholar]
- Schuch S, Koch I ( 2003): The role of response selection for inhibition of task sets in task shifting. J Exp Psychol Hum Percept Perform 29: 92–105. [DOI] [PubMed] [Google Scholar]
- Serences JT ( 2004): A comparison of methods for characterizing the event‐related BOLD timeseries in rapid fMRI. Neuroimage 21: 1690–1700. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhou X, Muller HJ, Schubert T ( 2010): The neural implementation of task rule activation in the task‐cuing paradigm: An event‐related fMRI study. Neuroimage 51: 1253–1264. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Weissman DH, Giesbrecht B, Kenemans JL, Mangun GR, Kok A, Woldorff MG ( 2006): Brain regions activated by endogenous preparatory set shifting as revealed by fMRI. Cogn Affect Behav Neurosci 6: 175–189. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K ( 2004): Neural correlates of switching set as measured in fast, event‐related functional magnetic resonance imaging. Hum Brain Mapp 21: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS ( 2000): Inaugural article: The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97: 13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandierendonck A, Liefooghe B, Verbruggen F ( 2010): Task switching: Interplay of reconfiguration and interference control. Psychol Bull 136: 601–626. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Liefooghe B, Vandierendonck A, Demanet J ( 2007): Short cue presentations encourage advance task preparation: A recipe to diminish the residual switch cost. J Exp Psychol Learn Mem Cogn 33: 342–356. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S ( 2004): Neuroimaging studies of shifting attention: A meta‐analysis. Neuroimage 22: 1679–1693. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX ( 2009): Evaluating the consistency and specificity of neuroimaging data using meta‐analysis. Neuroimage 45: S210–S221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DT, Halligan PW, Marshall JC, Buchel C, Dolan RJ ( 2001): Switching between the forest and the trees: Brain systems involved in local/global changed‐level judgments. Neuroimage 13: 56–67. [DOI] [PubMed] [Google Scholar]
- Wylie G, Javitt DC, Foxe JJ ( 2006): Jumping the gun: Is effective preparation contingent upon anticipatory activation in task‐relevant neural circuitry? Cereb Cortex 16: 394–404. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Murray MM, Javitt DC, Foxe JJ ( 2009): Distinct neurophysiological mechanisms mediate mixing costs and switch costs. J Cogn Neurosci 21: 105–118. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Van Essen DC, Wager TD ( 2010): Cognitive neuroscience 2.0: Building a cumulative science of human brain function. Trends Cogn Sci 14: 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Monsell S ( 2003): The effects of recent practice on task switching. J Exp Psychol Hum Percept Perform 29: 919–936. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD ( 2006): Between‐task competition and cognitive control in task switching. J Neurosci 26: 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]


