Abstract
A major challenge in central nervous system (CNS) drug research is to develop a generally applicable methodology for repeated measurements of drug effects on the entire CNS, without task‐related interactions and a priori models. For this reason, data‐driven resting‐state fMRI methods are promising for pharmacological research. This study aimed to investigate whether different psychoactive substances cause drug‐specific effects in functional brain connectivity during resting‐state. In this double blind placebo‐controlled (double dummy) crossover study, seven resting‐state fMRI scans were obtained in 12 healthy young men in three different drug sessions (placebo, morphine and alcohol; randomized). Drugs were administered intravenously based on validated pharmacokinetic protocols to minimize the inter‐ and intra‐subject variance in plasma drug concentrations. Dual‐regression was used to estimate whole‐brain resting‐state connectivity in relation to eight well‐characterized resting‐state networks, for each data set. A mixed effects analysis of drug by time interactions revealed dissociable changes in both pharmacodynamics and functional connectivity resulting from alcohol and morphine. Post hoc analysis of regions of interest revealed adaptive network interactions in relation to pharmacokinetic and pharmacodynamic curves. Our results illustrate the applicability of resting‐state functional brain connectivity in CNS drug research. Hum Brain Mapp, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: morphine, alcohol, resting state fMRI, opioid, functional connectivity, pharmacology, BOLD, dual regression, resting state networks, pharmaMRI
INTRODUCTION
One of the major challenges of central nervous system (CNS) drug discovery is to develop a generally applicable methodology to demonstrate the effects of a compound on the brain across the different phases of drug development. An optimal methodology should be minimally invasive, repeatable, and able to identify overlapping and distinguishable effects of different CNS drugs with little reliance on a priori models for drug effects.
The advances in functional neuroimaging, especially positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), have opened important frontiers for CNS drug research. However, radiation dose restrictions in PET prevent repeated intrasubject measurements within short periods of time. This is a major limitation of PET because repeated measurements allow testing different drug compounds on the same individual and minimize the within‐subject variances. More importantly, repeated neuroimaging acquisitions would allow the assessment of pharmacokinetic/pharmacodynamic (PK/PD) relationships, which form the scientific basis of modern drug development.
Compared with PET, blood‐oxygen‐level‐dependent (BOLD) fMRI is noninvasive, and benefits from high temporal and spatial resolution. Until recently, fMRI was applicable in task‐related designs, where pharmachologically induced changes in regional BOLD signal in response to a hypothesized CNS function (e.g., pain alleviation) were investigated. For examples see [Honey and Bullmore,2004]. One of the major limitations of this approach was the difficulty in controlling for inter‐subject variations in stimulus perception or performance in task‐related experiments. In addition, fMRI tasks had to be chosen to activate regions of interest based on a priori hypotheses about the site of drug effect [Breiter et al.,1997]; or about the PK/PD profile [Stein et al.,1998], which in many cases are unknown.
A recent breakthrough in neuroimaging has resulted from discovering the complex (but consistent and reliable) functional architecture of brain activity from the BOLD fluctuations [Biswal et al.,2010; Bullmore and Sporns,2009; Smith et al.,2009]. Today, a growing body of evidence suggests that the “resting‐state” brain activity (i.e., spontaneous BOLD fluctuations in the absence of any specific stimuli) forms spatially correlated topographies, which represent functional connections that relate to, or even predict, emotional, and cognitive behavioral outcomes [Fox et al.,2007; Seeley et al.,2007] or clinical conditions [Greicius,2008]. These resting‐state networks (RSNs) represent distinct functional systems (e.g., motor, vision, attention, etc.), that are reliable and reproducible [Beckmann et al.,2005; Biswal et al.,2010; Damoiseaux et al.,2006; Zuo et al.,2010]. Hence, if different drugs produce specific and detectable changes in the functional topography of these networks, then RSNs may become an important biomarker for CNS drug research.
In this study we investigate whether different psychoactive drug compounds (alcohol and morphine) elicit distinguishable changes in the functional topography of the RSNs. The RSN connectivity is determined in terms of similarity of temporal fluctuations in the whole brain in relation to eight networks of interest (NOI). These NOIs represent the most reliably and reproducibly detectable RSNs of functional significance (e.g. visual, somatosensory, motor, attention, working memory) [Beckmann et al.,2005; Damoiseaux et al.,2006]. Drugs are administered under PK‐controlled infusion protocols. Seven resting‐state fMRIs (RS‐fMRI) and several PK and PD assessments are made at controlled intervals (see Fig. 1). We report drug‐specific changes in the profile of RSN connectivity, and show their temporal relation to the profiles of drug concentrations and pharmacodynamic effects in areas where effects of morphine and alcohol are expected.
Figure 1.
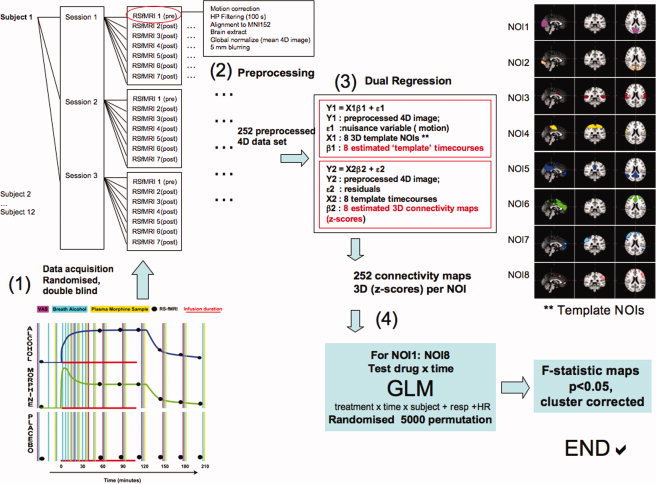
Schematic presentation of study design and data collection. Each subject participated in three randomized sessions. Both examiners and the subject were blind to which drug they received. The expected pharmacokinetic profiles for alcohol and morphibe are plotted (1). The placebo infusion was conducted concurrently with the drug infusion. After preprocessing (2) dual regression on all data sets to estimate whole‐brain connectivity in relation to the eight template RSNs (3), followed by statistical testing (4). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
MATERIALS AND METHODS
Experimental Design
A schematic diagram of the study design and the analyses is provided in Figure 1. This study is randomized, double blind (i.e., both examiner and the participant are unaware of which drugs are given), and double dummy placebo‐controlled (i.e., placebo was used for each treatment, consisting of infusion of the vehicle for treatment A in parallel to the administration of active treatment B or vice versa, and two vehicle infusions in the placebo session). Alcohol and morphine are two of the most commonly used substances in addiction and pain studies with similar analgesic and euphoric effects but they also result in different autonomic responses. In humans, alcohol exerts a wide range of psychoactive effects by interacting with GABAergic, dopaminergic, serotonergic and even opiate neurotransmitter systems [Koob et al.,1998]. In contrast, morphine targets specific receptors (μ‐opioid), which in turn interact with many other neurotransmitter systems [Contet et al.,2004]. This within‐subject design aimed to test whether drug‐specific effects on the RSN connectivity would be detectable and whether their profile corresponded to the PK and PD profiles we measured. However, both alcohol and morphine have dose‐dependent effects on the regional cerebral blood flow [Blaha et al.,2003; Wagner et al.,2001], and pharmacodynamic effects can significantly vary across individuals. Hence, we applied previously validated infusion regimens to achieve approximately steady state serum levels and controlled pharmacodynamic effects. The infusion protocols were based on validated PK models for each of these drugs [Sarton et al.,2000; Zoethout et al.,2008,2009]. This allowed (1) minimizing the between‐ and within subject variations in plasma drug concentration and (2) maintaining each of these drugs' concentrations at a plateau level for 90 min (∼60 min after the onset of experiment).
Subjects
Twelve healthy male subjects (age range, 18–40; BMI 18–26 kg/m2) were selected to participate in the neuroimaging study. (See supplemental material for exclusion criteria.) The study was approved by the Medical Ethics Review Board of Leiden University Medical Centre. Both oral and written informed consents were obtained from all participating subjects. All studies were performed in compliance with the law on clinical trials of the Netherlands (WMO). Each participant was scanned on three separate days, at least seven days apart. Subjects fasted for at least 4 h prior to the start of a study day. After arrival at the hospital a light standardized meal was provided. On each study day, negative scores on alcohol breath tests and a urinary drug screen for amphetamines, cocaine, morphine, and Δ9‐Tetrahydrocannabinol (THC) were required. Three intravenous cannulas (one for morphine or placebo administration, one for alcohol or placebo administration and one for blood sampling) were placed in veins of the arms. Before the start of drug or placebo administration, a baseline resting‐state scan was made and two baseline measurements of each visual analogue scale (VAS) were taken. After the last scan, the intravenous cannulas were removed and a second meal was provided.
Morphine Infusion Protocols
In order to reach stable serum levels of morphine (∼80 nmol/L) an initial bolus of 100 μg/kg/h was infused during 1 min; followed by a continuous infusion of 30 μg/kg/h for 2.5 h. A prior morphine study using an identical infusion paradigm [Sarton et al.,2000] showed that this infusion regimen could be safely applied, without the occurrence of major side effects. This dose was also associated with significant pharmacodynamic CNS effects. The total amount of morphine administered during one occasion using this dosage scheme, was approximately 14.5 mg for an average weighted male subject, infused over a time period of 2.5 h. This is considered to be a safe and rational dose, since it is within the therapeutic range of morphine (i.e., 2.5 mg–15 mg in 4–5 mL in 4–5 min intravenously, for acute pain). To determine the plasma concentration of morphine, venous blood was collected in 5 mL plain tubes (Becton and Dickinson Franklin Lakes, NJ, USA). Blood samples were taken at 0, 15, 30, 50, 60, 90, 120, 150, 180, 210, and 270 min after the start of the placebo or drug administration. All samples were centrifuged for 10 min at 2000g between 30 and 45 min after collection. Plasma samples were stored at −21°C. Plasma concentrations of morphine were determined using liquid chromatography with tandem mass spectrometry [Sarton et al.,2000].
Alcohol Infusion Protocol
Alcohol concentrations were controlled based on an intravenous alcohol clamping paradigm using ethanol 10% in glucose 5% [O'Connor et al.,1998]. We aimed to maintain alcohol levels at 600 mg/L, which barely exceeds the legal limits for driving in the Netherlands (approximately equivalent to two glasses of wine). The alcohol clamp has previously been well‐tolerated at this serum level and produced statistically significant pharmacodynamic CNS effects [Zoethout et al.,2009]. Infusion rates required to maintain stable alcohol levels were computed by a nonblind staff member without any other involvement in the study, based on measurements of breath alcohol (BrAC) at 5‐min intervals between 0 and 30 min, at 10‐min intervals between minutes 30 and 60, and 30‐min intervals between minutes 60 and 300 after the start of the placebo or drug administration. The alcohol placebo condition consisted of a sham‐procedure using a glucose 5% solution, including computer‐driven adaptations of infusion rates and BrAC measurements.
Neuroimaging Acquisition and Processing Protocols
A 3T Achieva scanner (Philips Medical System, Best, The Netherlands) was used for image acquisition. For each subject we obtained 21 resting‐state T2*‐weighted acquisitions (gradient echo EPI with parameters set to a TR = 2180 ms, TE = 30 ms, flip angle = 80; 64 × 64 × 38 isotropic resolution 3,44 mm, 220 frames, 8 min) and a T1‐weighted high resolution scan for anatomical registration. During scanning, a pulse oximeter (INVIVO MRI 4500, Siemens Healthcare, Germany) was used to monitor heart rate and oxygen saturation. The scanner's flexible pressure belt was used to record the respiratory signals. These RS‐fMRI data were preprocessed using the standard procedure including motion correction, brain extraction, Gaussian smoothing with a 5 mm FWHM kernel, mean‐based intensity normalization of all volumes by the same factor (i.e., 4D grand‐mean), and high‐pass temporal filtering (FWHM = 100 s). After preprocessing, the functional scans were affine‐registered to an MNI152 standard space (Montreal Neurological Institute, Montreal, QC, Canada). In all stages Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL 4.0, Oxford, UK; http://www.fmrib.ox.ac.uk/fsl) was used.
Assessment of Drug Effects on RSN Connectivity
We defined RSN connectivity in terms of the similarity of the BOLD fluctuations in each brain voxel in relation to characteristic fluctuation in eight predefined networks of interest (NOI). These networks are obtained from a weighted mask of networks that are most reliably (and reproducibly) identified from a model‐free analysis of the spatio‐temporal structure of the resting‐state BOLD fluctuations [Beckmann et al.,2005]. These template NOIs include over 80% of the total brain volume and comprise: medial and lateral visual systems (NOIs 1 and 2, respectively), auditory and somatosensory system (NOI3), sensorimotor system (NOI4), the default mode network (NOI5), executive salience network (NOI6), and visual‐spatial and working memory networks (NOIs 7 and 8, which represent almost mirrored networks). Each NOI represents shared neurophysiological fluctuations of the anatomical locations it includes.
To measure connectivity, we used the dual‐regression method [Beckmann et al.,2009]. Briefly, dual‐regression is based on first extracting the temporal pattern of resting‐state signal fluctuations within an RSN‐for each resting fMRI dataset; and next regressing these “fitted time courses” against fluctuations in the entire brain. Dual‐ regression analysis generates statistical maps of z‐scores that represent connectivity to the given NOI. In other words, the higher the absolute value of the z‐score, the stronger the connectivity to an NOI. These statistical maps can then be used in voxel‐wise mixed model analyses of complex experimental designs to obtain a statistical representation of where the drug interactions with any particular network connectivity are significant. The statistical sensitivity of the dual‐regression method has been successfully demonstrated in a study that reported distinct differences in brain connectivity in young carriers of the APOE4 gene [Filippini et al.,2009].
Here, dual‐regression resulted in 252 statistical parametric maps of whole brain connectivity to each of NOIs (12 subjects × 7 RSfMRIs × 3 treatments × 8NOIs) (See Figure 1). For each NOI, the respective RSN connectivity maps were entered in a mixed‐effect generalized linear model (GLM) to identify which brain regions and which networks were most significantly affected by the drug × time interactions. We used fixed factors treatment and time, and random factor subject. Particularly, we tested the difference between morphine‐placebo and alcohol‐placebo, while accounting for the variance across time (six post‐injection time points vs. the first preinfusion). The effects of morphine versus placebo; alcohol versus placebo, each of the time points 2 to 7 versus the pre‐drug time point, and an intercept for each subject were modeled as covariates.
Pharmacologically induced variations in heart rate and respiration rate were expected. Previous studies have shown that both heart rate [Chang et al.,2009; Shmueli et al.,2007] and respiration [Birn et al.,2008; Chang and Glover,2009b; Wise et al.,2004] fluctuations introduce variance in the resting‐state BOLD signal. We controlled for global effects of respiration and heart rate by examining the linear association of average heart rate and average respiration rate with resting‐state connectivity of each network. We also tested separate models, with and without respiration and heart rates as covariates to examine the robustness of detected effects to nuisance variables.
Permutation‐based statistical inference [Nichols and Holmes,2002] (5,000 permutation tests) was used. Statistical significance was set at P < 0.05, after cluster‐based correction for family wise errors (based on the null distribution of the max cluster size across the image). In all stages FSL 4.0 was used.
Pharmacodynamic Assessment
Computerised Visual Analogue Scales (VAS) were used to measure subjective CNS effects of drugs at baseline and repeated at 30, 60, 90, 120, 150, 180, and 210 min after infusion started. The assessments were performed outside the scanner.
The VAS Bond and Lader [Bond and Lader,1974] was used for the subjective assessment of the state of mind at that moment. Three factors corresponding to “alertness,” “mood,” and “calmness” can be derived from the VAS Bond and Lader. The VAS Bond and Lader scores are expressed in millimeter (mm), in which 50 mm indicates a normal feeling.
An adapted version of the VAS Bowdle [Bowdle et al.,1998] was used for subjective assessment of psychedelic effects. From the VAS Bowdle, three factors corresponding to “internal perception” and “external perception” (two modalities of psychedelic effects) and “feeling high” can be derived. “External perception” consist of the VAS scores changing of body parts, changes of surrounding, altered passing of time, difficulty controlling thought, changes in color intensity and changes in sound intensity. It indicates a misperception of an external stimulus or a change in the awareness of the subject's surroundings. “Internal perception” reflects inner feelings that do not correspond with the reality, e.g. hearing of unrealistic voices or sounds, unrealistic thoughts, paranoid feelings and anxious feelings. “Feeling high” is a separate item of the VAS Bowdle. The minimum score for the VAS Bowdle (absence of psychedelic effects) is 0 mm. We also used VAS nausea [Mearadji et al.,1998] and VAS alcohol effect (intoxication), each consisting of a single scale in which the extreme left side (0 mm) corresponds to “not nauseous/drunk at all” and the extreme right side (100 mm) to “maximum nauseous/drunk”.
Repeatedly measured pharmacodynamic and physiological data were compared with a mixed model analysis of variance with fixed factors treatment, period, time, and treatment by time and random factor subject, subject by treatment and subject by time and the average prevalue (average over all measurements at or before time = 0) as covariate (SAS for windows V9.1.2 ; SAS Institute, Inc., Cary, NC). Images were generated using Prism 5, GraphPad Software Inc., La Jolla, CA).
RESULTS
Controlled Pharmacokinetic Profiles
To reduce inter‐ and intrasubject variability, we examined variations in resting‐state connectivity under controlled pseudo‐steady state plasma drug concentrations, using a target‐controlled infusion regimen for morphine and an intravenous clamp for alcohol. Figure 2 illustrates the concentrations over time for each subject. Sixty minutes after the start of the infusion at the onset of RS‐fMRI 2, the between‐subject averaged (±SD) morphine level was 67.17 ± 10.22 (nmol/L), which remained in the same range in the following RS‐fMRIs 3, 4, and 5 (68.63 ± 8.0; 66.88 ± 5.8; 68.04 ± 8.8, respectively). Average alcohol concentrations were also stable, with low between‐subject variability at RS‐fMRI 2, 3, 4, and 5 (0.60 ± 0.05, 0.60 ± 0.056; 0.58 ± 0.069, and 0.63 ± 0.038 (g/L), respectively.)
Figure 2.
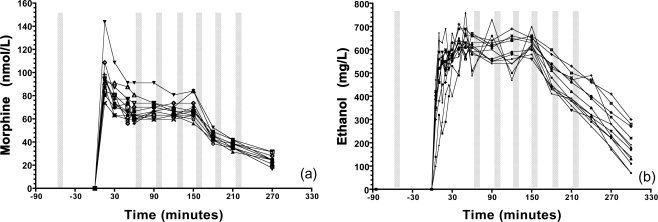
Pharmacokinetic effects: (a) Plasma morphine concentrations for each subject; (b) Breath alcohol readouts. The bars illustrate when the RS‐fMRI acquisition took place.
Pharmacodynamic Effects of Morphine and Alcohol
Figure 3 and Table I summarize the pharmacodynamic effects.
Figure 3.
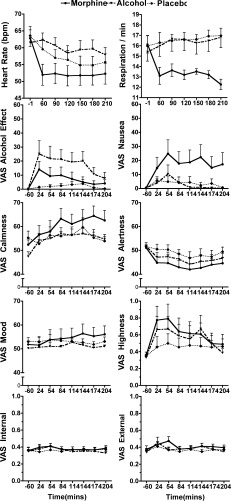
Pharmacodynamic effects over time (mean ± SEM). See Table I for details of main effects.
Table I.
Pharmacodynamic effects
| Parameter | Least square means | Treatment P‐value | (mean, 95% CI, P) Contrasts | ||||
|---|---|---|---|---|---|---|---|
| Placebo | Alcohol | Morphine | Alcohol vs. Placebo | Morphine vs. Placebo | Morphine vs. Alcohol | ||
| VAS Alcohol effects (mm) | 1.7 | 19.1 | 7.2 | 0.0096 | 17.4 (6.7, 28.2) P = 0.003 | 5.5 (−5.1, 16.2) P = 0.291 | −11.9 (−22.7, −1.1) P = 0.032 |
| VAS Alertness (mm) | 48.6 | 46.0 | 43.7 | 0.1843 | −2.6 (−8.0, 2.8) P = 0.3236 | −5.0 (−10.3, 0.4) P = 0.0696 | −2.3 (−7.7, 3.0) P = 0.374 |
| VAS Calmness (mm) | 53.4 | 58.2 | 60.6 | 0.0428 | 4.7 (−1.5, 11.0) P = 0.132 | 7.2 (1.6, 12.7) P = 0.014 | 2.5 (−3.3, 8.2) P = 0.386 |
| VAS Mood (mm) | 51.7 | 52.8 | 54.4 | 0.5171 | 1.1 (−4.1, 6.2) P = 0.662 | 2.8 (−2.2, 7.8) P = 0.2618 | 1.7 (−3.4, 6.7) P = 0.4982 |
| VAS Nausea (mm) | 3.7 | 3.0 | 18.6 | 0.0320 | −0.6 (−13.5, 12.2) P = 0.918 | 14.9 (2.1, 27.8) P = 0.025 | 15.6 (2.8, 28.4) P = 0.020 |
| VAS External log (mm) | 0.371 | 0.398 | 0.406 | 0.4601 | 0.027 (−0.035, 0.089) P = 0.365 | 0.035 (−0.026, 0.097) P = 0.241 | 0.008 (−0.054, 0.071) P = 0.783 |
| VAS Internal log (mm) | 0.359 | 0.366 | 0.382 | 0.4592 | 0.007 (−0.033, 0.046) P = 0.725 | 0.023 (−0.017, 0.062) P = 0.236 | 0.016 (−0.024, 0.056) P = 0.393 |
| VAS feeling high log (mm) | 0.467 | 0.575 | 0.635 | 0.5275 | 0.107 (−0.202, 0.417) P = 0.477 | 0.168 (−0.141, 0.476) P = 0.271 | 0.060 (−0.251, 0.371) P = 0.691 |
| Heart beats (per minute) | 55 | 61 | 52 | 0.0004 | 5.2 (1.4, 9.0) P = 0.009 | −3.8 (−7.5, 0) P = 0.049 | −9.0 (−12.7, −5.2) P < 0.0001 |
| Respiration (per minute) | 16.6 | 16.6 | 13 | <0.0001 | 0.0 (−1.4 1.4) P = 0.966 | −3.6 (−5, −2.2) P < 0.0001 | −3.6 (−5, −2.2) P < 0.0001 |
Morphine significantly reduced the respiration and heart rates and increased calmness and sensation of nausea compared with placebo. Alcohol significantly increased the heart rate and feelings of intoxication. Other effects did not reach statistical significance.
Effects of Physiological Factors on RSN connectivity
Figure 4 shows overlaid F‐stat maps for each network, at P < 0.05 (cluster corrected). Simple regression shows significant correlation between respiration rate and connectivity patterns of NOI4, NOI5, NOI6, and NOI7. In contrast, heart rate correlated with connectivity of several areas including the cerebellum, the brainstem, and the amygdala in relation to NOI3. Overlapping heart rate modulation of connectivity of a region close to major arteries (and extending to the amygdala) in relation to NOI 4, NOI5, NOI6, and NOI8 was also present. However, when effects of treatment by time were modeled in the GLM, the patterns of correlation between physiological factors and connectivity were diminished. Including respiration and heart rate, the topography of morphine effects were changed (Supporting Information Fig.‐a), but alcohol effects were unchanged (Supporting Information Fig.‐b). With the exception of the default mode network, it seems drug effects on resting‐state connectivity were independent of global physiological variations of heart rate and respiratory depression (due to morphine).
Figure 4.
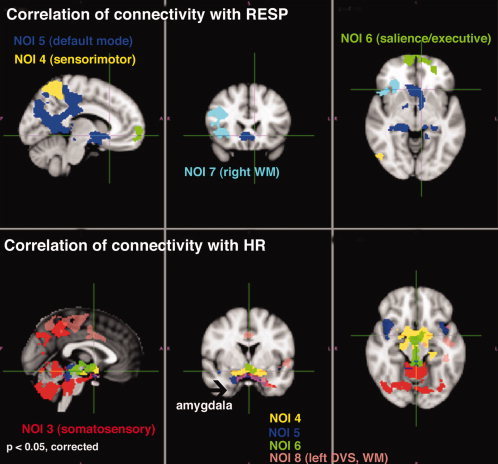
Overlaying maps of voxel‐wise regression of respiration (top) and heart rate (bottom) with connectivity to the 8 template networks (F‐statistics; cluster corrected P < 0.05). See supplemental Figure for effect of including heart rate and respiration in GLM for testing treatment by time interactions.
Interactions of Morphine and Alcohol with RSN Connectivity
Considering the location of regions where including physiological effects in the statistical model resulted in significant differences (white matter and a zone peripheral to the default mode network in relation to respiration), we expect these effect are non‐neuronal and related to global cerebrovascular responses, potentially resulting from a hypercapnic condition due to respiratory depression [Pattinson et al.,2007], or physiological noise [Birn et al.,2008]. Therefore, for the purpose of this study, we have reported drug by time interactions while including these variables in the GLM, to avoid such confounds.
Figure 5 illustrates statistical maps (F‐test; cluster P‐values < 0.05, corrected) of areas where connectivity in relation to a given NOI (in red) was significantly different due to morphine (green) and due to alcohol (blue) over time. Details in terms of cluster size, cluster P‐value and the t‐value of the peak of the cluster are provided in Table II.
Figure 5.
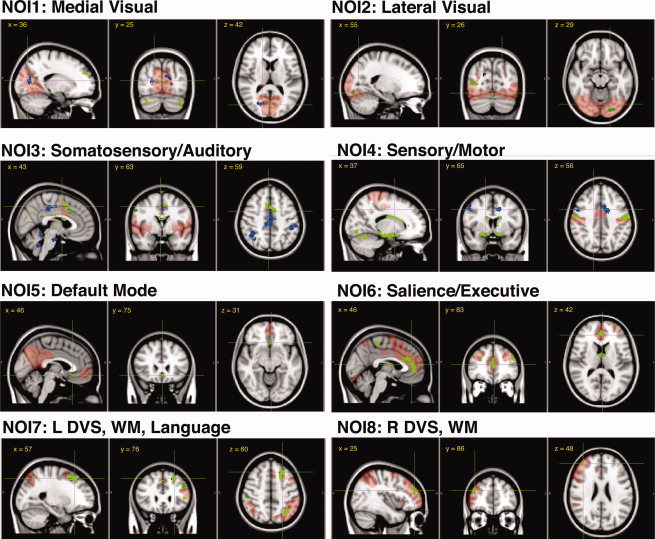
Overlaying maps of drug by time interactions with connectivity to the eight template networks (F‐statistics voxel‐wise P < 0.0001; cluster corrected P < 0.05; average physiological rates included as covariates). The NOIs are represented in pale red, morphine effects in green and alcohol effects in blue. Table II for the effects details. WM, working memory; DVS, dorsal visual stream.
Table II.
Details of drug effects on functional connectivity (F‐tests; at cluster corrected threshold P < 0.05)
| Morphine vs. Placebo | Ethanol vs. Placebo | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Cluster size | Cluster peak | Location | Cluster size | Cluster peak | |||||||
| voxels | t | x | y | z | voxels | t | x | y | z | |||
| NOI1: Medial Visual Network | ||||||||||||
| Includes, calcarine, inferior precuneus, and primary visual cortex and relays visual input through thalamus to primary visual area. | R cerebellum | 149*** | 4.77 | 27 | 28 | 16 | R visual | 140*** | 4.78 | 35 | 27 | 41 |
| L cerebellum | 101** | 4.8 | 64 | 30 | 15 | L visual | 100** | 5.57 | 52 | 26 | 46 | |
| R hippocampus | 65** | 5.05 | 33 | 50 | 29 | L parietal lobule | 67** | −4.91 | 59 | 31 | 56 | |
| R S frontal | 87** | 5.87 | 37 | 88 | 50 | |||||||
| Precentral gyrus | 26* | 4.88 | 44 | 53 | 71 | |||||||
| NOI2: Lateral Visual Network | ||||||||||||
| Includes lateral occipital cortex (superior and fusiform areas) and is involved in visual spatial attention. | R occipital V5 | 91** | 5.52 | 23 | 26 | 40 | R cuneus | 56** | 4.9 | 22 | 33 | 21 |
| L occipital V4 | 70** | 4.87 | 57 | 20 | 29 | Precuneus | 24* | 4.45 | 46 | 32 | 43 | |
| L posterior insula | 45* | −5.24 | 65 | 51 | 45 | |||||||
| R S frontal | 33* | −4.83 | 38 | 88 | 51 | |||||||
| NOI3: Auditory and Somatosensory Network Includes superior temporal cortex, insula, operculum, dorsocaudal ACC, somatosensory cortices and bilateral thalamus.†† | ||||||||||||
| L precuneus | 348*** | −5.78 | 45 | 29 | 53 | Bilateral PCC | 728*** | 5.3 | 45 | 50 | 59 | |
| R paracingulate | 322*** | −4.96 | 44 | 67 | 60 | R S parietal lobule | 310*** | 5.86 | 27 | 39 | 58 | |
| R cerebellum | 96** | −4.57 | 39 | 45 | 26 | L cerebellum | 167*** | 4.97 | 49 | 42 | 25 | |
| L cerebellum (tonsil) | 87** | −5.31 | 54 | 42 | 13 | Bilateral culmen | 153*** | 5.73 | 45 | 37 | 15 | |
| L cerebellum (culmen) | 74** | −4.59 | 50 | 44 | 25 | R cerebellum | 144*** | 5.03 | 41 | 42 | 25 | |
| L amygdala | 63** | −4.6 | 54 | 62 | 25 | Brainstem | 144** | 6.29 | 43 | 54 | 23 | |
| R amygdala | 40* | −4.28 | 35 | 64 | 25 | L supramarginal gyrus | 109** | 5.61 | 68 | 45 | 59 | |
| L frontal pole | 46* | −4.75 | 57 | 86 | 52 | L precuneus | 45* | 4.92 | 47 | 35 | 41 | |
| L S occipital | 38* | −4.77 | 56 | 26 | 62 | R supramarginal gyrus | 38* | 5.44 | 18 | 45 | 59 | |
| R S occipital | 44* | −4.37 | 37 | 26 | 58 | L OFC | 34* | 5.16 | 70 | 76 | 32 | |
| R postcentral | 53* | −4.58 | 18 | 59 | 57 | R ACC | 22* | 4.8 | 39 | 86 | 36 | |
| NOI4: Sensorimotor Network Includes pre− and postcentral somatosensory somatomotor areas. | ||||||||||||
| L primary somatosensory | 1774*** | 7.52 | 65 | 51 | 65 | R cerebellum/fusiform | 223*** | −5.74 | 27 | 40 | 23 | |
| L hippocampus | 1679 ***† | −7.61 | 55 | 54 | 29 | L dorsocaudal ACC | 132** | −4.02 | 39 | 61 | 59 | |
| R hippocampus | −6.06 | 32 | 56 | 29 | R I parietal lobule | 112** | −4.79 | 19 | 47 | 49 | ||
| L putamen | −4.3 | 58 | 58 | 41 | R precentral R | 98** | −5.3 | 49 | 65 | 56 | ||
| L amygdala | −6.27 | 37 | 61 | 27 | ||||||||
| R amygdala | −6.02 | 52 | 61 | 28 | ||||||||
| L caudate | −4.9 | 52 | 64 | 47 | ||||||||
| Bi primary motor | 1001 *** | 7.18 | 45 | 52 | 66 | |||||||
| L cerebellum VI | 851 ***† | −7.95 | 52 | 30 | 27 | |||||||
| Vermis | −5.37 | 45 | 29 | 28 | ||||||||
| L cerebellum VI | −5.3 | 39 | 30 | 28 | ||||||||
| R primary somatosensory | 722 *** | 5.7 | 25 | 51 | 62 | |||||||
| R putamen | 668 ***† | −6.28 | 32 | 58 | 41 | |||||||
| R caudate | −5.53 | 39 | 64 | 46 | ||||||||
| L occipital fusiform | 494 *** | 6.11 | 54 | 19 | 31 | |||||||
| R occipital fusiform | 314 *** | 6.01 | 33 | 23 | 30 | |||||||
| L posterior insula | 122 ** | 5.39 | 63 | 52 | 45 | |||||||
| R posterior insula | 84 ** | 5.12 | 23 | 56 | 44 | |||||||
| Thalamus | 87 ** | −4.64 | 45 | 52 | 41 | |||||||
| Brainstem | 95 ** | 5.25 | 49 | 47 | 16 | |||||||
| Paracingulate gyrus | 61 * | 5.53 | 47 | 80 | 50 | |||||||
| NOI5: Default Mode Network Includes rostral medial PFC and precuneal and PCC areas. This network is linked to readiness for adaptive response to cognitive and emotional tasks. | ||||||||||||
| L subgenual ACC | 40* | −5.27 | 47 | 76 | 31 | |||||||
| L middle frontal | 40* | −4.81 | 61 | 76 | 59 | |||||||
| PCC | 37* | −4.53 | 45 | 40 | 48 | |||||||
| NOI6: Executive Salience Network Includes medial and inferior PFC. Regions in this network are implicated in executive control, attention and emotional response to pain. | ||||||||||||
| ACC | 490*** | −6.47 | 45 | 85 | 41 | |||||||
| L frontal pole | 392*** | −6.31 | 61 | 94 | 36 | |||||||
| R frontal pole | 350*** | −6.65 | 30 | 89 | 50 | |||||||
| R primary motor | 212*** | −5.7 | 39 | 49 | 69 | |||||||
| L insula | 115** | −6.13 | 63 | 71 | 33 | |||||||
| Bilateral thalamus | 101** | −5.42 | 45 | 62 | 42 | |||||||
| R lingual gyrus | 63** | −5.32 | 46 | 18 | 32 | |||||||
| R I parietal lobule | 50* | −4.82 | 21 | 39 | 51 | |||||||
| L OFC | 42* | −5.43 | 54 | 71 | 27 | |||||||
| L primary motor | 40* | −5.49 | 50 | 49 | 70 | |||||||
| L lingual | 36* | −4.99 | 52 | 30 | 36 | |||||||
| NOI7: Left Dorsal Visual Stream Network: Includes Broca area 44, frontal pole, dorsolateral PFC and parietal lobule. Involved in working memory, visual spatial processing and language. | ||||||||||||
| L S frontal | 31* | 4.83 | 46 | 91 | 45 | |||||||
| R frontal pole | 81** | −5.56 | 25 | 86 | 48 | |||||||
| R middle frontal | 55** | −4.8 | 32 | 73 | 63 | |||||||
| NOI8: Right Dorsal Visual Stream Network Includes homologous regions as NOI7, plus right paracingulate gyrus, left PCC and left medial PFC. | ||||||||||||
| L S frontal | 270*** | −5.16 | 56 | 75 | 58 | L S frontal | 79** | −5.64 | 55 | 74 | 59 | |
| L basolateral PFC | 243*** | −5.22 | 67 | 84 | 34 | |||||||
| L S parietal lobule | 160*** | −5.36 | 62 | 35 | 60 | |||||||
| Paracingulate, medial PFC | 144*** | −5.29 | 47 | 80 | 56 | |||||||
| R primary somatosensory | 63** | −4.83 | 21 | 46 | 63 | |||||||
| L S middle frontal | 52** | −5.5 | 28 | 67 | 63 | |||||||
| R thalamus | 35* | −4.71 | 40 | 53 | 42 | |||||||
Cluster significance:
P < 0.05;
P < 0.01;
P < 0.001.
Voxel dimension is 2 mm × 2 mm × 2 mm (voxel volume 0.008 mL). S, superior; I, inferior; L, left; R, Right. If no lateralization specified, the effect is in the midline region. ACC, anterior cingulated cortex; OFC, orbitofrontal cortex; PCC, posterior cingulated cortex; PFC, prefrontal cortex. _These clusters included high peaks in several nuclei.
__ Evidence for converging circuitry of auditory and somatosensory function is available (e.g. Rodgers et al, Cerebral Cortex, 2008; Bizley et al, Cerebral Cortex, 2007; Fu et al., Journal of Neurosci, 2003; Foxe et al., Journal of Neurophysiology, 2002.). Considering the prevalence of drug effects on somatosensation, we refer to NOI3 as somatosensory network.
Morphine
Effects of morphine on RSN connectivity over time (compared to placebo) were significant and dissociable in different NOIs. The affected areas include prefrontal regions (subgenual ACC, medial prefrontal and basolateral prefrontal regions), posterior parietal areas (precuneus, posterior cingulate), medial temporal regions (amygdala and the hippocampal), primary sensory, primary motor, basal ganglia, and cerebellum. The most extensive effects of morphine were observed in NOI4 (primary sensory motor network). Within the total areas affected (52.6 ml of brain volume) 39% of the effect was in the bilateral somatosensory areas (inside NOI4), but the rest of the effects were distal to NOI4 (26% in the limbic system and basal ganglia, 16% in the primary motor, 13% in the cerebellum, and 10% in the visual area). The next most extensive effects were in the executive salience network, NOI6 (15.13 mL, 73% of which was prefrontal area that lies inside NOI6).
Alcohol
Compared with morphine, effects of alcohol on RSN connectivity were more limited. The most extensive effects of alcohol were observed in NOI3 (15.15 mL of brain volume), where 62% of the change was in the posterior parietal cortex and the rest in the cerebellum and brainstem (which are outside NOI3). Small changes in connectivity of dorsocaudal ACC and precentral gyrus to NOI4 were present as well (4.52 mL) and in a small area (2.46 mL) inside NOI1.
Overlap
In NOIs 1, 2, 3, 5, 6, and 7, the intersection of the significant clusters of each drug's effects did not reveal overlap, suggesting topographic differences in each drug effect on these functional networks. Only a small (42 voxels = 0.34 mL) overlap was observed in connectivity of the cerebellum to NOI4 and in connectivity of the superior frontal gyrus to NOI8 (52 voxels = 0.416 mL).
Post hoc Examination of Selected ROI Responses
The purpose of the post‐hoc analysis is to demonstrate the range and profile of changes in connectivity in some exemplar ROIs in relation to PK and PD profiles. These ROIs were chosen from NOIs that were most prominently affected by alcohol (NOI3) or morphine (NOI4).
In the sensorimotor network (NOI4), where the negative hippocampal connectivity emerged (Fig. 6a), the hippocampal connectivity in relation to NOI4 was small in alcohol and placebo conditions (95% CI: −1.176 to −0.1104 and −1.230 to 0.09146; respectively). By contrast, morphine infusion increased the absolute value of hippocampal connectivity to NOI4 (95% CI: −4.309 to −3.043). We note that connectivity is defined in terms of linear fitting of spontaneous fluctuations at any given region to the “specific” fluctuations within the entire network (i.e., the weighted average time course of all voxels). Therefore, the negative z‐score suggests a distinct inverse relationship in terms of simple oscillations also a phase shift of the fluctuations of the hippocampus with respect to the sensorimotor network. By contrast, the connection of the central gyrus to NOI4, which was similar prior to both infusions, remained stable for alcohol and placebo (95% CI: 8.08–10.45; and 8.34–10.33, respectively) but became stronger after morphine infusion (95% CI: 11.79–13.20) (Fig. 6b).
Figure 6.
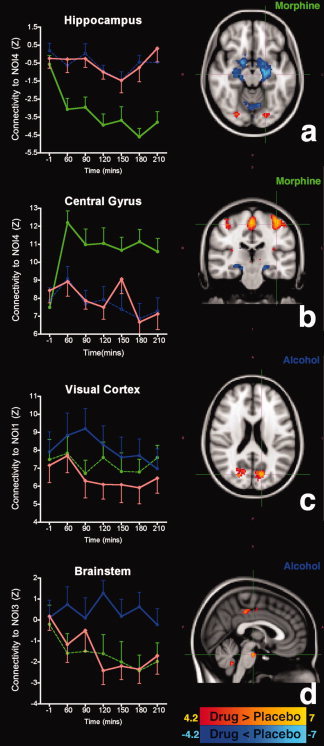
Profile of change in resting‐state connectivity in select ROIs. Statistical parametric maps (t‐statistics, cluster corrected P < 0.05) reflect the amplitude of drug‐induced changes in functional connectivity (drug versus placebo over time). Morphine leads to increased negative connectivity of the hippocampus (a), and positive connectivity of the central gyri (b) in relation to the sensory‐motor network (NOI4). Alcohol enhances the intraconnectivity of the visual network (c) and disrupts adaptive connectivity of brainstem (d). In the ROI diagrams, red corresponds to placebo, green to morphine, and blue to alcohol.
Figure 6c illustrates that alcohol significantly increased the connectivity within NOI1 within the first 90 min of infusion (95% CI: 0.36–2.22). By contrast, in the brainstem (Fig. 6d) morphine and placebo showed an increase in negative connectivity to NOI3 (95% CI: −0.97 to −2.26 and −0.53 to −2.37; respectively), whereas this probably adaptive effect was not present with alcohol (95% CI: −0.07 to 0.87).
DISCUSSION
Effects of morphine and alcohol on changes in the RSN connectivity were measured at several time points (together with the PD and PK profiles of each drug). We detected effects where the within‐subject variation in preinfusion connectivity in relation to a given NOI was minimal, whereas the difference in RSN connectivity (in relation to placebo) increased over the next six time‐points–after plasma concentrations became stable and during the recovery phase. Drugs produced distinct CNS and clinical responses in terms of PD effects, physiological responses, and resting‐state connectivity.
The question of the neurological substrates of resting‐state BOLD fluctuations is still an open one. The BOLD signal originates from changes in cerebral blood flow, cerebral metabolism and oxygen extraction. At the present state of knowledge, interpretations of BOLD resting‐state connectivity are tentative; although evidence for electrical [Britz et al.,2010; Mantini et al.,2007] and arterial perfusion [Chuang et al.,2008] bases of functional connectivity in some of the putative resting‐state networks is already available. Notwithstanding a definite biological interpretation of connectivity, we detected effects in most brain regions where changes in receptor binding, cerebral blood flow, or function in response to these specific substances are previously reported. For instance, PET studies with opiodergic radiotracers have shown that the ACC, opercular/insular cortex, thalamus, amygdala and putamen (the medial parts of the pain system) have the highest, and the primary somatosensory, sensorimotor areas (the lateral parts of the pain system) [Baumgartner et al.,2006; Jones et al.,1991; Zubieta et al.,2001] and occipital areas [Sadzot et al.,1991] to have the lowest binding potentials. As Table II shows, we have detected predominant morphine effects both in areas that, according to PET studies, have low opioid binding potentials (such as primary sensorimotor connectivity to NOI4), and areas that have high opiodergic binding potentials (such as the ACC connectivity to NOI6). This observation precludes interpretation of the effects simply as an outcome of metabolic modulation at the site of receptor action. However, it is possible that the detected effects reflect interactions between these different regions in terms of functional adaptation. Future studies of combined RSfMRI and opiodergic PET studies can shed a light on this central question.
Conversely, we have been able to demonstrate different connectivity effects resulting from morphine and alcohol, which have different sites of action. Unlike morphine, CNS effects of alcohol are primarily mediated via GABAA receptors, which are expressed across the brain, although different parts of the brain have higher affinity for different subunits [Kumar et al.,2009]. Presently, there are no radiotracer studies to have illustrated alcohol‐induced variances in regional GABAA binding potentials in humans. The most direct in vivo evidence for the effect of alcohol on cerebral activation is observed in terms of reduced global metabolism [de Wit et al.,1990; Volkow et al.,2008; Volkow et al.,2006; Wang et al.,2000], with more relative decreases in the occipital cortex [Schreckenberger et al.,2004; Volkow et al.,2008; Wang et al.,2000] and the cerebellum [Volkow et al.,2008; Wang et al.,2000], and relative increases in the brainstem, striatum and the ACC [Schreckenberger et al.,2004]. Also, a radiotracer study of a GABAA ligand ([11C],Ro 15‐4513, which has preferred binding potential for ethanol [Hanchar et al.,2006]) in monkeys indicates that the ACC, insula and the limbic system have the highest, and the occipital cortex and the cerebellum have the lowest binding potential for [11C],Ro 15‐4513 [Maeda et al.,2003]. Here, we observed that alcohol affected the connectivity of the visual cortex (in relation to NOI1), the cerebellum (in relation to NOI3 and NOI4), the ACC (in relation to NOI3 and NOI4) and the brainstem (in relation to NOI3). Surprisingly, the most extensive effect of alcohol was in changing the connectivity of PCC (in relation to NOI3). It is known that alcohol effects can vary with dosage [Blaha et al.,2003; de Wit et al.,1990; Gordon et al.,1995; Luksch et al.,2009; Sano et al.,1993], and they may depend on interindividual differences in regular alcohol consumption. The lesser extent of detected alcohol effects in our study can be hypothetically attributed to these sources of variance and future studies need to address the issue more closely.
With the exception of a small overlap in the cerebellum (in relation to NOI4) and the superior frontal gyrus (in relation to NOI8), drug effects on connectivity were distinct. Similarly, overlap in pharmacodynamic effects was limited. Alcohol significantly increased a feeling of drunkenness and morphine significantly increased nauseous sensation. The CNS effects expected to be common in both drugs such as calmness, alertness, and mood were not significant for alcohol (although calmness was significantly increased by morphine). We cannot conclude that alcohol and morphine do not interact on common brain circuitry. In this experiment, the VAS scores indicate little overlap in terms of subjective changes in mood and feelings induced by alcohol and morphine. However, alcohol (ethanol) was administered at relatively low levels (0.6 g/L), whereas morphine was given in a therapeutically relevant dose (14.5 mg) with considerable functional impact. It is likely that higher doses of alcohol intoxication that result in stronger cognitive and affective responses, will elicit more pronounced changes in local functional connectivity. Presently, without extensive psychometric tests the behavioral correlates of the observed CNS effects cannot be discussed. More precise recordings of certain aspects of emotion and cognition would have enabled us to study the functional significance of RSN connectivity changes. In this experiment, we omitted cognitive testing between different resting‐state measurements to avoid introducing performance‐related confounds. In fact, our study underlines an important advantage of the resting‐state pharma‐fMRI (RS‐PhfMRI). Significant changes were detected in regions that are expected to be associated with the CNS effects of these drugs; without a priori models and performance‐ or task‐related confounds.
It is crucial to note the effects of global physiological variations due to morphine and alcohol. Previous studies have shown that respiratory depression due to opiodergic drugs generates hypercapnic conditions that increases cerebral blood flow [MacIntosh et al.,2008], and causes focal BOLD signal reduction in response to hypercapnic condition in the sensorimotor brain regions [Pattinson et al.,2007]. On the other hand, it has been shown that BOLD fluctuation correlating with respiration and heart rate can compromise delineation of the default mode network [Chang et al.,2009; Chang and Glover2009b]. Nevertheless, the impact of physiological noise on BOLD is nearly global and including the timecourse of physiological fluctuations do not drastically change the results of connectivity estimation [Birn et al.,2006; Chang and Glover2009a]. We observed a significant respiratory depression after morphine injection and an increase in heart rate due to alcohol. Our study does not address effect of physiological variables in dual regression outcome, but we have examined the effects of overall physiological variations (in terms of average respiration and average heart rates per each subjects per each session) on resting‐state connectivity, and have shown that inclusion of physiological covariates in a repeated measures model does not change the main effects of the drug by time interaction. Interestingly, including respiration and heart rate in the model removes emerging connections from white matter to NOI4, and reduces the extent of morphine effect on connections to NOI5, the default mode network, whose fluctuations seem to overlap in frequency with spontaneous BOLD changes in this network [Birn et al.,2006; Wise et al.,2004]. It should be noted that our framework is based on examining connectivity to eight NOI templates corresponding to the most consistently determined RSNs. These template networks are initially obtained from independent component analyses (ICA). It has been suggested that the default mode network obtained from ICA might not be fully respiration‐proof [Birn et al.,2008]. It is therefore striking that modeling a global measure of respiration change at the group level analysis would demonstrate an effect expected from more careful analysis of the respiration spectra [Birn et al.,2006; Wise et al.,2004]. We cannot preclude the possibility that variation in the temporal physiological profiles have a stronger explanatory power on effects of some of these networks, such as NOI4. Our aim in this study was to show that RS‐fMRI is a sensitive and applicable method for detecting drug effects in the brain. Future studies that aim to validate RS‐fMRI in relation to specific metabolic effects of especially opiodergic drugs should make precise recordings of variables such as end‐tidal CO2 or arterial CO2 tension during the RS‐fMRI session and incorporate them in such analyses.
It is worth mentioning that, without considering the time and the session effects in the model, we have detected significant associations between the heart rate and connectivity of several regions outside of NOI3 in relation to this network, in addition to changes in connectivity of a region near major arteries (circle of Willis) that also extends to the amygdala, in relation to NOI3, NOI4, NOI5, NOI6, and NOI8. By contrast, respiration changes affected connectivity to NOI4, NOI5, NOI6, and NOI7. An understanding of the neural or vascular substrates of physiological modulation is potentially crucial to interpretation of the neurological substrate of resting‐state BOLD connectivity. Especially in pharmacological studies, the autonomic responses to the CNS drug are central to the research and these questions should be carefully considered in the study design. For instance, heart rate modulated the connectivity of amygdala to NOI4 (sensorimotor), NOI5 (default mode network), NOI6 (salience executive), and NOI8 (right dorsomedial visual stream). This observation is striking, since amygdalar association with visual‐emotional [Critchley et al.,2005; van Marle et al.,2009] or executive‐motor [Napadow et al.,2008] modulation of the heart rate is previously reported. Regardless of neurobiological interpretations, our findings illustrate that different NOIs vary in degree of susceptibility to physiological modulation; and highlight a need for considering different statistical models to ensure important results are not obscured.
Our study overcomes some limitations in earlier pharmacological fMRI studies, and poses challenging questions. We have successfully detected effects reported in previous studies of morphine and alcohol‐without subjectively delineating an ROI necessary for commonly used seed‐based RS‐fMRI analysis [Anand et al.,2007; Hong et al.,2009; Kelly et al.,2009; Rack‐Gomer et al.,2009]. It may be argued that predefined NOIs limit the scope of observations by restricting the criterion of connectivity to temporal similarity with a given NOI. However, these networks were chosen on the basis of previous studies that robustly reproduced them in entirely different population [Beckmann et al.,2005; Damoiseaux et al.,2006]. By choosing these networks we avoided typical ambiguities associated with ICA approach that depends on choosing the model order, or on subjective selection of similar or relevant components for further analysis. Nevertheless, we have detected significant changes in connectivity of regions such as the cerebellum and the hippocampus that are outside the reference NOIs. Therefore this method seems to be robust and less limiting than alternative ICA or ROI methods in detecting regions where the drug effects may not be expected. New approaches such as eigenvector centrality mapping (ECM) (Lohmann et al., 2010) might bring us closer to the goal of total model‐free analyses, but such methods are computationally more costly than dual‐regression using predefined NOI templates, as we did. It would be interesting to compare results from ECM analysis and our proposed methodology.
An important advantage of resting‐state connectivity, compared to other (more quantitative) neuroimaging methods is that it reveals the complexity of the temporal profile of drug effects in each region or interest. For instance, no significant connection is present between the hippocampus and the sensorimotor network (NOI4) under placebo or alcohol conditions, but during morphine infusion, a significant negative connectivity emerges. At the same time, morphine strengthens connectivity within some regions of the NOI4. To date, the issue of negative connectivity remains a topic of debate [Murphy et al.,2009]. Several hypothetical explanations are possible: It may be argued that global vascular effects of morphine (especially due to respiratory depression) would give rise to the observed negative connectivity demonstrated in the hippocampus. However, these particular emerging negative correlations are anatomically confined to bilateral hippocampus, and although we cannot comment on the neurophysiological basis of the effect, they are unlikely to be non‐specific artifacts. Another possibility is that reduction of physiological noise (e.g., due to respiratory depression due to morphine) would enhance negative correlations [Fox et al.,2009]. Alternatively, the negative connectivity in the hippocampus may correspond to a delayed, and perhaps adaptive function of this structure [Bast2007; Khalili‐Mahani et al.,2010]. We remind that these particular effects were not affected by inclusion of respiration and heart rate averages in the group‐level statistical model. Such interregional and internetwork heterogeneity of effects raises questions about compensatory and adaptive mechanisms that interact with the drug (which reflects interactions of different brain regions with each other); and underlines the added advantage of resting‐state connectivity analysis compared to other neuroimaging modalities.
In conclusion, we have shown that drug‐class specific effects on dynamics of brain function at resting‐state can be detected even without a priori models. An important advantage of with RS‐PhfMRi is that it is not confounded by differences in alertness or test strategies and other factors that influence the performance and outcomes of task‐induced fMRI, and demonstrates effects even if no appropriate task is available. This study was designed to exhibit limited pharmacokinetic variability, and was hence unsuitable to investigate concentration‐effect relationships, although the time profiles of the MRI‐effects roughly followed those of the clinical responses. This study demonstrates that RS‐fMRI could be useful for “finger‐printing” different drug actions within the same individual's brain. Notwithstanding the exploratory nature of this study, and the inability to characterize the neurophysiological substrates of resting‐state BOLD fluctuations, many of our results correspond to findings from previous quantitative PET studies. Because RS‐fMRI is noninvasive, widely available and frequently repeatable, it may become an important biomarker for CNS‐drug development, able to distinguish detailed drug‐induced response patterns in healthy subjects and patients.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors acknowledge support from the UK NIHR Biomedical Research Center funding scheme. They thank Jordan Gross and Rene Post (CHDR) for assisting with the data acquisition, and Ilya Veer, Luca Ferrarini and Dr Julien Milles (LUMC) for helpful discussions on the data analysis. Technical support from the fMRIB Center of Oxford University is appreciated. Comments and suggestions of Dr Paul de Bruin (LUMC) and Dr Sander Nieuwenhuis (LIBC) on the manuscript, and consultations with Dr Pedro Rosa‐Neto and Dr Thijs Van Osch are gratefully acknowledged.
REFERENCES
- Anand A, Li Y, Wang Y, Gardner K, Lowe MJ ( 2007): Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsychiatry Clin Neurosci 19: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T ( 2007): Toward an integrative perspective on hippocampal function: From the rapid encoding of experience to adaptive behavior. Rev Neurosci 18( 3‐4): 253–281. [DOI] [PubMed] [Google Scholar]
- Baumgartner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeier T, Rolke R, Hohnemann S, Piel M, Rosch F, Wester HJ, Henriksen G, Stoeter P, Bartenstein P, Treede RD, Schreckenberger M ( 2006): High opiate receptor binding potential in the human lateral pain system. Neuroimage 30: 692–699. [DOI] [PubMed] [Google Scholar]
- Beckmann C, Mackay C, Filippini N, Smith S ( 2009): Group comparison of resting‐state FMRI data using multi‐subject ICA and dual regression. NeuroImage 47( Suppl 1): 1.19376248 [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM ( 2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA ( 2006): Separating respiratory‐variation‐related fluctuations from neuronal‐activity‐related fluctuations in fMRI. Neuroimage 31: 1536–1548. [DOI] [PubMed] [Google Scholar]
- Birn RM, Murphy K, Bandettini PA ( 2008): The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum Brain Mapp 29: 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP ( 2010): Toward discovery science of human brain function. Proc Natl Acad Sci USA 107: 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha M, Aaslid R, Douville CM, Correra R, Newell DW ( 2003): Cerebral blood flow and dynamic cerebral autoregulation during ethanol intoxication and hypercapnia. J Clin Neurosci 10: 195–198. [DOI] [PubMed] [Google Scholar]
- Bond A, Lader M. ( 1974): Use of analog scales in rating subjective feelings. Br J Med Psychol 47( Sep): 211–218. [Google Scholar]
- Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy‐Byrne PP ( 1998): Psychedelic effects of ketamine in healthy volunteers—Relationship to steady‐state plasma concentrations. Anesthesiology 88: 82–88. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE ( 1997): Acute effects of cocaine on human brain activity and emotion. Neuron 19: 591–611. [DOI] [PubMed] [Google Scholar]
- Britz J, Van De Ville D, Michel CM ( 2010): BOLD correlates of EEG topography reveal rapid resting‐state network dynamics. Neuroimage 52: 1162–1170. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O ( 2009): Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10: 186–198. [DOI] [PubMed] [Google Scholar]
- Chang C, Cunningham JP, Glover GH ( 2009): Influence of heart rate on the BOLD signal: The cardiac response function. Neuroimage 44: 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH ( 2009a): Effects of model‐based physiological noise correction on default mode network anti‐correlations and correlations. Neuroimage 47: 1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH ( 2009b): Relationship between respiration, end‐tidal CO2, and BOLD signals in resting‐state fMRI. Neuroimage 47: 1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KH, van Gelderen P, Merkle H, Bodurka J, Ikonomidou VN, Koretsky AP, Duyn JH, Talagala SL ( 2008): Mapping resting‐state functional connectivity using perfusion MRI. Neuroimage 40: 1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K ( 2004): Mu opioid receptor: A gateway to drug addiction. Curr Opin Neurobiol 14: 370–378. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ ( 2005): Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage 24: 751–762. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF ( 2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Metz J, Wagner N, Cooper M ( 1990): Behavioral and subjective effects of ethanol: Relationship to cerebral metabolism using PET. Alcohol Clin Exp Res 14: 482–489. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE ( 2009): Distinct patterns of brain activity in young carriers of the APOE‐epsilon4 allele. Proc Natl Acad Sci USA 106: 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME ( 2007): Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56: 171–184. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME ( 2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101: 3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EL, Nguyen TS, Ngai AC, Winn HR ( 1995): Differential effects of alcohols on intracerebral arterioles. Ethanol alone causes vasoconstriction. J Cereb Blood Flow Metab 15: 532–538. [DOI] [PubMed] [Google Scholar]
- Greicius M ( 2008): Resting‐state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 21: 424–430. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW ( 2006): Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15‐4513 to alpha4/6beta3delta GABAA receptors. Proc Natl Acad Sci USA 103: 8546–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey G, Bullmore E ( 2004): Human pharmacological MRI. Trends Pharmacol Sci 25: 366–374. [DOI] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA ( 2009): Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry 66: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Qi LY, Fujirawa T, Luthra SK, Ashburner J, Bloomfield P, Cunningham VJ, Itoh M, Fukuda H, Jones T ( 1991): In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neurosci Lett 126: 25–28. [DOI] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K ( 2009): L‐dopa modulates functional connectivity in striatal cognitive and motor networks: A double‐blind placebo‐controlled study. J Neurosci 29: 7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili‐Mahani N, Dedovic K, Engert V, Pruessner M, Pruessner JC ( 2010): Hippocampal activation during a cognitive task is associated with subsequent neuroendocrine and cognitive responses to psychological stress. Hippocampus 20: 323–334. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo‐Pich E, Weiss F ( 1998): Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res 22: 3–9. [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz‐Granados JL, Helfand RS, Morrow AL ( 2009): The role of GABA(A) receptors in the acute and chronic effects of ethanol: A decade of progress. Psychopharmacology (Berl) 205: 529–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G, Margulies DS, Horstmann A, Pleger B, Lepsien J, Goldhahn D, Schloegl H, Stumvoll M, Villringer A, Turner R ( 2010): Eigenvector centrality mapping for analyzing connectivity patterns in FMRI data of the human brain. PLoS ONE 5(4):e10232. doi:10.1371/journal.pone.0010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luksch A, Resch H, Weigert G, Sacu S, Schmetterer L, Garhofer G ( 2009): Acute effects of intravenously administered ethanol on retinal vessel diameters and flicker induced vasodilatation in healthy volunteers. Microvasc Res 78: 224–229. [DOI] [PubMed] [Google Scholar]
- MacIntosh BJ, Pattinson KT, Gallichan D, Ahmad I, Miller KL, Feinberg DA, Wise RG, Jezzard P ( 2008): Measuring the effects of remifentanil on cerebral blood flow and arterial arrival time using 3D GRASE MRI with pulsed arterial spin labelling. J Cereb Blood Flow Metab 28: 1514–1522. [DOI] [PubMed] [Google Scholar]
- Maeda J, Suhara T, Kawabe K, Okauchi T, Obayashi S, Hojo J, Suzuki K ( 2003): Visualization of alpha5 subunit of GABAA/benzodiazepine receptor by 11C Ro15‐4513 using positron emission tomography. Synapse 47: 200–208. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M ( 2007): Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA 104: 13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mearadji B, Straathof JWA, Biemond I, Lamers CBHW, Masclee AAM ( 1998): Effects of somatostatin on proximal gastric motor function and visceral perception. Alimentary Pharmacol Therapeutics 12: 1163–1169. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA ( 2009): The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? Neuroimage 44: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R ( 2008): Brain correlates of autonomic modulation: Combining heart rate variability with fMRI. Neuroimage 42: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP ( 2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S, Morzorati S, Christian J, Li TK ( 1998): Clamping breath alcohol concentration reduces experimental variance: Application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res 22: 202–210. [PubMed] [Google Scholar]
- Pattinson KT, Rogers R, Mayhew SD, Tracey I, Wise RG ( 2007): Pharmacological FMRI: Measuring opioid effects on the BOLD response to hypercapnia. J Cereb Blood Flow Metab 27: 414–423. [DOI] [PubMed] [Google Scholar]
- Rack‐Gomer AL, Liau J, Liu TT ( 2009): Caffeine reduces resting‐state BOLD functional connectivity in the motor cortex. Neuroimage 46: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadzot B, Price JC, Mayberg HS, Douglass KH, Dannals RF, Lever JR, Ravert HT, Wilson AA, Wagner HN Jr, Feldman MA, et al. ( 1991): Quantification of human opiate receptor concentration and affinity using high and low specific activity [11C]diprenorphine and positron emission tomography. J Cereb Blood Flow Metab 11: 204–219. [DOI] [PubMed] [Google Scholar]
- Sano M, Wendt PE, Wirsen A, Stenberg G, Risberg J, Ingvar DH ( 1993): Acute effects of alcohol on regional cerebral blood flow in man. J Stud Alcohol 54: 369–376. [DOI] [PubMed] [Google Scholar]
- Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L, Dahan A ( 2000): Sex differences in morphine analgesia: An experimental study in healthy volunteers. Anesthesiology 93: 1245–1254; discussion 6A. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Amberg R, Scheurich A, Lochmann M, Tichy W, Klega A, Siessmeier T, Grunder G, Buchholz HG, Landvogt C, Stauss J, Mann K, Bartenstein P, Urban R ( 2004): Acute alcohol effects on neuronal and attentional processing: Striatal reward system and inhibitory sensory interactions under acute ethanol challenge. Neuropsychopharmacology 29: 1527–1537. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD ( 2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH ( 2007): Low‐frequency fluctuations in the cardiac rate as a source of variance in the resting‐state fMRI BOLD signal. Neuroimage 38: 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF ( 2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106: 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS ( 1998): Nicotine‐induced limbic cortical activation in the human brain: A functional MRI study. Am J Psychiatry 155: 1009–1015. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G ( 2009): From specificity to sensitivity: How acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry 66: 649–655. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ma Y, Zhu W, Fowler JS, Li J, Rao M, Mueller K, Pradhan K, Wong C, Wang GJ ( 2008): Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res 162: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D, Fowler JS, Thanos PP, Maynard L, Gatley SJ, Wong C, Veech RL, Kunos G, Kai Li T ( 2006): Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage 29: 295–301. [DOI] [PubMed] [Google Scholar]
- Wagner KJ, Willoch F, Kochs EF, Siessmeier T, Tolle TR, Schwaiger M, Bartenstein P ( 2001): Dose‐dependent regional cerebral blood flow changes during remifentanil infusion in humans: A positron emission tomography study. Anesthesiology 94: 732–739. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N, Pappas N, Wong CT, Hitzemann RJ, Felder CA ( 2000): Regional brain metabolism during alcohol intoxication. Alcohol Clin Exp Res 24: 822–829. [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I ( 2004): Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage 21: 1652–1664. [DOI] [PubMed] [Google Scholar]
- Zoethout RW, Schoemaker RC, Zuurman L, van Pelt H, Dahan A, Cohen AF, van Gerven JM ( 2009): Central nervous system effects of alcohol at a pseudo‐steady‐state concentration using alcohol clamping in healthy volunteers. Br J Clin Pharmacol 68: 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoethout RW, van Gerven JM, Dumont GJ, Paltansing S, van Burgel ND, van der Linden M, Dahan A, Cohen AF, Schoemaker RC ( 2008): A comparative study of two methods for attaining constant alcohol levels. Br J Clin Pharmacol 66: 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS ( 2001): Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293: 311–315. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP ( 2010): Reliable intrinsic connectivity networks: Test‐retest evaluation using ICA and dual regression approach. Neuroimage 49: 2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


