Abstract
Identification and accurate localization of seizure foci is vital in patients with medically‐intractable focal epilepsy, who may be candidates for potentially curative resective epilepsy surgery. We present a patient with difficult‐to‐control seizures associated with an occult focal cortical dysplasia residing within the deeper left parietal operculum and underlying posterior insula, which was not detected by conventional MRI analysis. Propagated activities from this deeper generator produced misleading EEG patterns both on surface and subdural electrode recordings suggesting initial activation of the perirolandic and mesial frontal regions. However, careful spatio‐temporal analysis of stereotyped interictal activities recorded during MEG, using sequential dipole modeling, revealed a consistent pattern of epileptic propagation originating from the deeper source and propagating within few milliseconds to the dorsal convexity. In this instance, careful dissection of noninvasive investigations (interictal MEG along with ictal SPECT findings) allowed clinicians to dismiss the inaccurate and misleading findings of the traditional “gold‐standard” intracranial EEG. In fact, this multimodal noninvasive approach uncovered a subtle dysplastic lesion, resection of which rendered the patient seizure‐free. This case highlights the potential benefits of dynamic analysis of interictal MEG in the appropriate clinical context. Pathways of interictal spike propagation may help elucidate essential neural networks underlying focal epilepsy. Hum Brain Mapp, 2012. © 2012 Wiley Periodicals, Inc
Keywords: epilepsy, magnetoencephalography, MEG, ICEEG, interictal spike, propagation, nonlesional
INTRODUCTION
Propagated epileptic activities may lead to erroneous interpretation of source localization analysis. Presurgical work‐up is usually difficult in cases with rapidly propagating sources, especially in the absence of an identifiable anatomical MRI abnormality (nonlesional cases). Caution is needed when interpreting interictal spikes to judge if they are stable over time or represent propagated sources. Magnetoencephalography (MEG), with its high spatial and temporal resolution, is inherently suited to analyzing and visualizing the propagation of epileptic activities. Surface EEG studies [Ebersole,1991,1994,1997] and invasive recordings [Ebersole et al.,1995; Merlet and Gotman,1999; Pacia and Ebersole,1997] have both indicated that interictal spikes may share similar propagation pathways as seizure activities. Previous studies have also reported a correlation between the propagation of interictal spikes and surgical outcome. Alarcon et al. has revealed a significant association between poor outcome and non‐removal of leading regions on Electrocorticography (ECoG) in a cohort of 42 patients following temporal lobe surgery [Alarcon et al.1997]. Schulz et al. examined surgical outcome in 58 patients with hippocampal sclerosis and found bitemporal interictal discharges on scalp EEG to be associated with a worse outcome [Schulz et al.,2000]. In this case study, we discuss the value of MEG‐guided interpretation of epileptic activity coming from a deep source.
Case History
An 11‐year‐old boy presented with medically refractory epilepsy. Seizures were three to five times/week. He had normal developmental milestones until seizure onset at two years of age.
Symptomatology
Seizures were predominantly nocturnal, characterized by bilateral asymmetric tonic stiffening involving both limbs, at times more preponderant on the left, followed by thrashing movements, and less frequently secondarily generalized tonic‐clonic seizures. On occasion when awake, the child mentioned episodic sensation of buzzing in his ears. It was unclear if this represented an aura, as no temporal relationship to his habitual seizures was established.
Diagnosis
Scalp video‐EEG (Nihon‐Kohden, Tokyo, Japan) monitoring showed interictal sharp waves in the vertex and left paracentral region (maximum at Cz and C3). Ictal patterns also localized to the same region (maximum at Cz). Three MRI studies, performed separately in 2000, 2004, and 2006, were interpreted as normal. The later two scans were performed using a dedicated high‐resolution epilepsy protocol. A subtraction ictal SPECT coregistered with MRI (SISCOM) study showed nonadjacent areas of hyperperfusion in both hemispheres, with the most prominent and clinically relevant region of hyperperfusion involving the left posterior temporal/temporo‐parietal area, along with the medial frontal region in an interconnected “hourglass” appearance (Fig. 1a). In the multidisciplinary patient management conference (PMC), a decision was made to proceed to intracranial EEG (ICEEG) monitoring. Target areas included the perirolandic cortex along with the mesial fronto‐parietal region, the supplementary motor area, the perisylvian cortex, and the opercular and posterior temporo‐parietal regions. A combination of intracranial subdural grid electrodes along with depth electrodes were implanted as illustrated in Figure 1b. Interictally, a stable spike focus was seen involving four electrodes in the perirolandic region. The ictal map also showed the bulk of ictal activities in the convexity involving grid electrodes mainly in proximity to the perirolandic region.
Figure 1.
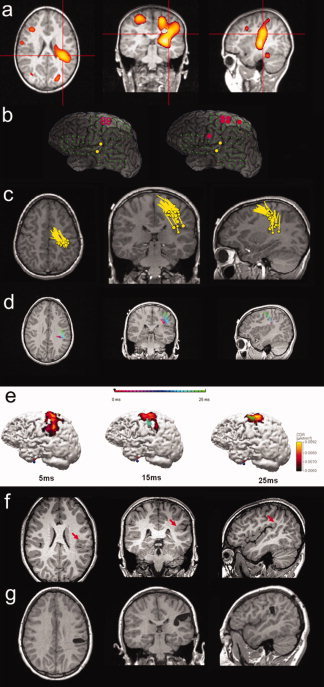
(a) Subtraction ictal SPECT coregistered with MRI (SISCOM) showing areas of hyperperfusion in both hemispheres, with the hyperperfused area involving the left posterior temporal and medial frontal regions showing an “hourglass” morphology. (b) Interictal (left) and ictal (right) maps of Intracranial EEG (ICEEG) reconstructed on top of a coregistered maximum intensity projection (MIP) image of the cortex. MIP was performed using the patient's MRI. Green filled circles: grid ICEEG electrodes; yellow filled circles: depth ICEEG electrodes targeting deeper structures; red circle: interictally involved electrodes; red filled circle: ictally involved electrodes (involved depth electrodes not shown). (c) Result of MEG single dipole analysis shown in a composite image. Dipole positions are superimposed on the patient's MRI. In panels c, d and e, head of the pole indicates estimated position and tail of the pole indicates direction of the dipoles. (d) Result of MEG sequential dipole analysis of one interictal spike showing the trajectory of its propagation. Dipoles are sequentially fitted every 1 ms over 25 ms, and are shown in a color‐coded time trajectory. Dipole positions are superimposed with the patient's MRI. (e) Analysis of one interictal spike (the same as in panel d) using sequential dipole modeling and current density reconstruction (CDR). The CDR is shown on a 3D cortical surface (left view), which is segmented and reconstructed from the patient's MRI. The surface is rendered 60% transparent, so that the dipole underneath the cortical surface can be visualized. Three time snaps are selected here to represent three phases of the propagation: 5, 15, and 25 ms. (f) A 3‐T pre‐op MRI performed after the pre‐surgical evaluations to specifically search for the subtle lesion, indicated by the red arrows. (g) Post‐op MRI showing a limited resection of the parietal operculum and posterior insula which rendered the patient seizure‐free.
Given the complexity of this case and apparent absence of an MRI abnormality, the PMC also recommended evaluation with magnetoencephalography (MEG) prior to implantation of the ICEEG electrodes. A 45‐min resting‐state recording of spontaneous MEG activity using a 306‐channel Neuromag system (Elekta, Helsinki, Finland) was performed. Analyses were done using the vendor software and a commercial program CURRY 6 (Compumedics, Charlotte, NC). We first utilized the single equivalent current dipole (ECD) model (with a single‐shell sphere head model) to localize the source of interictal spikes, using the peak of global field power as the time point to perform the dipole fit. Only spike dipoles with a good dipolar pattern and goodness of fit >85% were accepted. Positions of all (>20) interictal spike occurrences during the recording (as shown in a composite image in Fig. 1c) suggested the presence of two nonadjacent areas of dipole clustering. We then performed sequential dipole fit which contains 80% of the rising slope of each interictal spike [Lantz et al.2003], one example of which is shown in Figure 1d. Sequential dipole fit was performed once every 1 ms; only those with a >80% normalized variance (i.e., >80% data explained by the modeling) were displayed. We found a consistent pattern of propagated interictal activity starting from the left parietal operculum and underlying posterior insula, quickly propagating to the primary and supplementary sensorimotor areas. We also performed a current density reconstruction (CDR) of interictal spikes, using the Minimum Norm Least Squares version with a cutoff threshold of 65%. The reconstruction was constrained by the cortical surface generated with the patient's MRI. Figure 1e represents the current density reconstruction of the same interictal spike as seen in Figure 1d, with a typical pattern of propagation reflected on the cortical surface, as the epileptiform event marches anteriorly and rostrally within 25 ms time (Fig. 1b). We also investigated the scalp EEG simultaneously recorded with the MEG. We noted that in this patient the peak of the EEG spike came approximately 20 ms after that of the MEG spike peak, probably reflecting propagation of the later phase of the MEG spike [Ebersole and Ebersole2010].
Moreover, based on the MEG findings a fourth high‐resolution MRI was specifically requested using a 3‐Tesla magnet (Siemens, Erlangen, Germany) with a 32‐channel surface coil targeting the left posterior perisylvian region. MEG‐directed review of this MRI revealed a previously undetected subtle blurring at the gray white junction of the left supramarginal gyrus, suggestive of an underlying focal cortical dysplasia, as pointed out in Figure 1f.
Treatment
On the basis of the consistent MEG pattern of propagation, the SPECT hyperperfusion findings, the history of an auditory aura and the MRI lesion, we hypothesized that the ictal onset zone would be located in proximity to the area of MRI abnormality, and not within the perirolandic region as suggested by ICEEG.
Following electrical cortical stimulation mapping to identify the posterior language area, the patient underwent a limited resection of the parietal operculum and posterior insula (Fig. 1g—post‐op MRI). Intraoperatively, the surgeon was able to identify an abnormal, firm, and rubbery region, which was subsequently removed. The suctioned tissue was not sent to pathology; therefore, the subtype of dysplasia is unknown. The patient has been seizure and aura free for more than a year.
Discussion
This case of focal cortical dysplasia involving the posterior insula and parietal operculum illustrates the potential value of MEG in the presurgical evaluation of some patients with medically intractable epilepsy with discordant electro‐clinical data and functional imaging. Specifically, this case illustrates the following points.
-
1
Our case underscores the importance of MEG‐directed review of MRI studies in some epilepsy surgery candidates, as this may enable detection of subtle abnormalities not apparent otherwise [Moore et al.,2002].
-
2
MEG results can help the interpretation of the findings from subdural grid monitoring. Interpretation of invasive recordings is dependent on precise targeting. Stereotactic EEG and depth electrodes have the inherent shortcoming of inadequate sampling, unless they completely cover the ictal onset zone. Subdural grid electrodes usually have extensive coverage, but are often difficult to resolve deep sources far away from the cortical surface [Badier and Chauvel1995]. Therefore, when sampling from ICEEG electrodes is inadequate, nonlocalizing and at times misleading results may occur. MEG, when combined with simultaneously recorded scalp EEG, provides for a more complete, global view of whole‐brain activities. Because radially oriented sources are magnetically silent in a sphere volume conductor, one may assume that MEG would be insensitive to deep and gyral sources. Notably, a quantitative study to assess the sensitivity of whole‐head MEG in the human brain showed that only a relatively small proportion of the cortical area (strips of ∼ 2 mm wide) have poor resolvability [Hillebrand and Barnes,2002]. Nonetheless, scalp EEG source localization is a further step to illustrate both the tangential and the radial components of interictal activities [Ahlfors et al.,2010; Ebersole and Ebersole,2010; Goldenholz et al.,2009]. When combined with the information obtained from the simultaneously recorded EEG, MEG can provide a more realistic characterization of cortical sources, and may serve as a powerful tool for: (1) pre‐implantation planning; (2) localizing propagation activities on a ms by ms time resolution to “fill in the gaps” of invasive electrodes. In this patient, the ICEEG results would have directed the surgical resection towards the perirolandic cortex. The MEG information, however, allowed the possibility that the ICEEG results provided only a reflection of propagated epileptic activities. The grid electrodes, in fact, may have detected similar surface patterns to those seen with the later phase of CDR (Fig. 1e), but eluding detection of epileptic activity initiation from a deeper structure.
-
3
This case highlights the potential benefits of the dynamic analysis of interictal MEG along with ictal SPECT. The “hourglass‐type” hyperperfusion is thought to reflect ictal propagation through a “trail pathway” instead of through contiguous cortical regions [McNally et al.,2005; Varghese et al.,2009]. This interconnected hyperperfusion pattern (Fig. 1a) has been previously shown to reflect both the area of generation along with area(s) of seizure propagation [Dupont et al.,2006]. However, it is usually difficult to distinguish, which of these two interconnected areas is the starting point. The consistent MEG pattern of propagation in this case allowed us to identify the instigator. As has been reported in previous studies, pathways of interictal spike propagation may elucidate neural networks associated with epilepsy [Spencer,2002]. The time scale of the propagation exemplified in Figures 1(d,e), is ∼25 ms, and probably represents subcortical connectivity. Despite their completely different temporal resolutions, interictal MEG and ictal SPECT showed similar propagation patterns, suggesting that the spread of the seizure employed a network similar to the one responsible for the consistent, interictal propagation pattern.
-
4
Our case highlights the importance of data interpretation in the context of all the presurgical noninvasive modalities [Knowlton et al.,2008]. In this patient, the MEG propagation pattern is concordant with the seizure semiology (history of auditory aura preceding the asymmetric tonic seizure suggesting early activation/involvement of the auditory cortex), ictal SPECT findings, and the MRI abnormality.
-
5
This case demonstrates the dynamic relationship between parietal and frontal networks and exemplifies the difficulty to localize seizures arising from the parietal lobe in cases with propagated seizure activity. As illustrated in this case, ictal spread to the frontal lobe and mesial frontal region has previously been observed from the parietal operculum and parieto‐temporal region [Swartz et al.,1990; Williamson et al.,1992]. The findings of scalp interictal/ictal EEG and the variable seizure manifestations usually reflect multiple spread patterns and rich connectivity of the parietal lobe [Williamson et al.,1992]. In a recent study, Eickhoff et al. utilized probabilistic tractography of human diffusion tensor imaging data to elucidate white matter fiber pathway connectivity of the two most widely studied areas of the parietal operculum [operculum parietale (OP) 1 and 4] and their respective connections with either the perceptive parietal network or the frontal motor areas [Eickhoff et al.,2010]. The epileptic activity propagation illustrated in our case may be explained by the closely integrated functional connections between area operculum parietale 4 and the frontal motor region.
REFERENCES
- Ahlfors SP, Han J, Belliveau JW, Hamalainen MS ( 2010): Sensitivity of MEG and EEG to source orientation. Brain Topogr 23: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon G, Garcia Seoane JJ, Binnie CD, Martin Miguel MC, Juler J, Polkey CE, Elwes RD, Ortiz Blasco JM ( 1997): Origin and propagation of interictal discharges in the acute electrocorticogram. Implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain 120( Part 12): 2259–2282. [DOI] [PubMed] [Google Scholar]
- Badier JM, Chauvel P ( 1995): Spatio‐temporal characteristics of paroxysmal interictal events in human temporal lobe epilepsy. J Physiol Paris 89( 4‐6): 255–264. [DOI] [PubMed] [Google Scholar]
- Dupont P, Van Paesschen W, Palmini A, Ambayi R, Van Loon J, Goffin J, Weckhuysen S, Sunaert S, Thomas B, Demaerel P, et al. ( 2006): Ictal perfusion patterns associated with single MRI‐visible focal dysplastic lesions: implications for the noninvasive delineation of the epileptogenic zone. Epilepsia 47: 1550–1557. [DOI] [PubMed] [Google Scholar]
- Ebersole JS ( 1991): EEG dipole modeling in complex partial epilepsy. Brain Topogr 4: 113–123. [DOI] [PubMed] [Google Scholar]
- Ebersole JS ( 1994): Non‐invasive localization of the epileptogenic focus by EEG dipole modeling. Acta Neurol Scand Suppl 152: 20–28. [DOI] [PubMed] [Google Scholar]
- Ebersole JS ( 1997): Defining epileptogenic foci: Past, present, future. J Clin Neurophysiol 14: 470–483. [DOI] [PubMed] [Google Scholar]
- Ebersole JS, Ebersole SM ( 2010): Combining MEG and EEG source modeling in epilepsy evaluations. J Clin Neurophysiol 27: 360–371. [DOI] [PubMed] [Google Scholar]
- Ebersole JS, S.Hawes S, Scherg M ( 1995): Intracranial EEG validation of spike propagation predicted by dipole models. Elec‐Troencephalogr Clin Neurophysiol 95: 18–19. [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TE ( 2010): Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci 30: 6409–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenholz DM, Ahlfors SP, Hamalainen MS, Sharon D, Ishitobi M, Vaina LM, Stufflebeam SM ( 2009): Mapping the signal‐to‐noise‐ratios of cortical sources in magnetoencephalography and electroencephalography. Hum Brain Mapp 30: 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Barnes GR ( 2002): A quantitative assessment of the sensitivity of whole‐head MEG to activity in the adult human cortex. Neuroimage 16( Part 1): 638–650. [DOI] [PubMed] [Google Scholar]
- Knowlton RC, Elgavish RA, Limdi N, Bartolucci A, Ojha B, Blount J, Burneo JG, Ver Hoef L, Paige L, Faught E, et al. ( 2008): Functional imaging: I. Relative predictive value of intracranial electroencephalography. Ann Neurol 64: 25–34. [DOI] [PubMed] [Google Scholar]
- Lantz G, Spinelli L, Seeck M, de Peralta Menendez RG, Sottas CC, Michel CM ( 2003): Propagation of interictal epileptiform activity can lead to erroneous source localizations: A 128‐channel EEG mapping study. J Clin Neurophysiol 20: 311–319. [DOI] [PubMed] [Google Scholar]
- McNally KA, Paige AL, Varghese G, Zhang H, Novotny EJ Jr, Spencer SS, Zubal IG, Blumenfeld H ( 2005): Localizing value of ictal‐interictal SPECT analyzed by SPM (ISAS). Epilepsia 46: 1450–1464. [DOI] [PubMed] [Google Scholar]
- Merlet I, Gotman J ( 1999): Reliability of dipole models of epileptic spikes. Clin Neurophysiol 110: 1013–1028. [DOI] [PubMed] [Google Scholar]
- Moore KR, Funke ME, Constantino T, Katzman GL, Lewine JD ( 2002): Magnetoencephalographically directed review of high‐spatial‐resolution surface‐coil MR images improves lesion detection in patients with extratemporal epilepsy. Radiology 225: 880–887. [DOI] [PubMed] [Google Scholar]
- Pacia SV, Ebersole JS ( 1997): Intracranial EEG substrates of scalp ictal patterns from temporal lobe foci. Epilepsia 38: 642–654. [DOI] [PubMed] [Google Scholar]
- Schulz R, Luders HO, Hoppe M, Tuxhorn I, May T, Ebner A ( 2000): Interictal EEG and ictal scalp EEG propagation are highly predictive of surgical outcome in mesial temporal lobe epilepsy. Epilepsia 41: 564–570. [DOI] [PubMed] [Google Scholar]
- Spencer SS ( 2002): Neural networks in human epilepsy: Evidence of and implications for treatment. Epilepsia 43: 219–227. [DOI] [PubMed] [Google Scholar]
- Swartz BE, Halgren E, Delgado‐Escueta AV, Feldstein P, Maldonado H, Walsh GO ( 1990): Multidisciplinary analysis of patients with extratemporal complex partial seizures. II. Predictive value of semiology. Epilepsy Res 5: 146–154. [DOI] [PubMed] [Google Scholar]
- Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Paige AL, Zubal IG, et al. ( 2009): Clinical use of ictal SPECT in secondarily generalized tonic‐clonic seizures. Brain 132( Part 8): 2102–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson PD, Boon PA, Thadani VM, Darcey TM, Spencer DD, Spencer SS, Novelly RA, Mattson RH ( 1992): Parietal lobe epilepsy: Diagnostic considerations and results of surgery. Ann Neurol 31: 193–201. [DOI] [PubMed] [Google Scholar]


