Abstract
Dysfunctional default mode network (DMN) has been observed in various mental disorders, including epilepsy (see review Broyd et al. [2009]: Neurosci Biobehav Rev 33:279–296). Because interictal epileptic discharges may affect DMN, resting‐state fMRI was used in this study to determine DMN functional connectivity in 14 healthy controls and 12 absence epilepsy patients. To avoid interictal epileptic discharge effects, testing was performed within interictal durations when there were no interictal epileptic discharges. Cross‐correlation functional connectivity analysis with seed at posterior cingulate cortex, as well as region‐wise calculation in DMN, revealed decreased integration within DMN in the absence epilepsy patients. Region‐wise functional connectivity among the frontal, parietal, and temporal lobe was significantly decreased in the patient group. Moreover, functional connectivity between the frontal and parietal lobe revealed a significant negative correlation with epilepsy duration. These findings indicated DMN abnormalities in patients with absence epilepsy, even during resting interictal durations without interictal epileptic discharges. Abnormal functional connectivity in absence epilepsy may reflect abnormal anatomo‐functional architectural integration in DMN, as a result of cognitive mental impairment and unconsciousness during absence seizure. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, resting‐state, functional connectivity, absence epilepsy, DMN
INTRODUCTION
The default mode network (DMN) has been identified as a resting state of brain function [Andreasen et al., 1995; Raichle et al., 2001], which encompasses the posterior cingulate cortex (PCC)/precuneus, medial prefrontal cortex, bilateral inferior temporal cortex, and bilateral inferior parietal cortex. These brain areas often exhibit both decreased activity resulting from attention‐demanding tasks and increased brain activity when individuals are not focused on the external environment or targeted self‐related mental tasks, such as autobiographical memory retrieval, envisioning the future. DMN has also been referred to as a non‐goal‐directed process, or a self‐referential and reflective activity [Andreasen et al., 1995; Broyd et al., 2009; Gusnard et al., 2001]. Default mode abnormalities have been reported in mental disorders, such as Alzheimer's disease, schizophrenia, depression, epilepsy, and attention‐deficit/hyperactivity disorder [Broyd et al., 2009 for a review]. Altered functional connectivity within the DMN is characteristic of altered network integrity, and is affiliated with mental disorder functions and aging in the resting state [Damoiseaux et al., 2008].
Absence epilepsy is nonconvulsive, generalized epilepsy, characterized by clinically impaired consciousness and bilateral, synchronous, 2.5–4 Hz generalized spike and slow‐wave discharges (SWD) in electroencephalogram (EEG) [Crunelli and Leresche, 2002]. In general, SWD is postulated as a consequence of abnormally synchronized epileptiform activity, which is propagated through thalamocortical circuits [Crunelli and Leresche, 2002; Gloor et al., 1990]. Interictal SWD is an unpredicted and paroxysmal event in epilepsy. In recent studies, simultaneous EEG and functional MRI allowed for analysis of ongoing brain functions related to interictal epileptic discharges (IED). Brain regions exhibiting functional changes presumably involve epileptogenic zones or irritative zones, as well as regions influenced by IED. Specifically, the posterior cingulate may be influenced by electroclinical phenomenon of SWD, and the reduced activity in this region may facilitate the onset of SWD [Archer et al., 2003]. In parallel, decreased blood oxygenation level‐dependent (BOLD) signals associated with interictal SWD have been demonstrated in DMN [Gotman et al., 2005; Laufs et al., 2006a; Salek‐Haddadi et al., 2003]. It was hypothesized that the default brain state was partially suspended by SWD [Gotman et al., 2005]. Kobayashi et al. [2006] also suggested that the slow‐wave component of SWD was more likely to suspend normal brain function during the resting state, resulting in deactivation of default mode regions. Moreover, similar deactivation has been described in medial temporal lobe epilepsy [Laufs et al., 2006b].
In contrast, loss of consciousness has been observed during absence seizure. Impaired cognition, memory dysfunction, and linguistic deficits have been determined as long‐term consequences of absence epilepsy [Caplan et al., 2008; Motamedi and Meadoe, 2003]. In addition, transient cognitive impairment has been shown to be associated with interictal discharge [Hommet et al., 2006]. These disturbances may affect DMN function in epilepsy similar to the aforementioned mental disorders. Recently, a resting state fMRI study in epilepsy patients described a lack of resting‐state, low‐frequency activation in the PCC/precuneus in patients with generalized seizure, but no differences were observed in patients with partial seizure. This dysfunction was perhaps the result of interictal SWD or mental impairment in these patients [Lui et al., 2008]. This study hypothesized that functional connectivity in DMN may be altered even during the resting‐state without IED in absence epilepsy patients, and it might reflect abnormal anatomo‐functional architecture integration in DMN.
A growing number of resting‐state fMRI studies have been performed in healthy controls and patients with mental disorders. The temporal correlations of BOLD signals in distant brain regions are presumed to reflect spontaneous, intrinsic, functional connectivity, and have been demonstrated in several brain function networks, such as vision, language, attention, and DMN [Fox and Raichle, 2007]. In this study, to test the above hypothesis, EEG and fMRI were simultaneously utilized to acquire resting state fMRI data without IED. Cross‐correlation functional connectivity analysis, with seed at the PCC, was adopted to identify DMN spatial patterns, and the DMN connectivity in absence epilepsy patients was further analyzed based on region‐wise analysis in DMN.
SUBJECTS AND METHODS
Participants
A total of 15 patients with absence epilepsy were recruited from epilepsy clinics for the EEG‐fMRI study at Neurology Department in the West China Hospital, Sichuan University. All patients underwent clinical brain structural MRI and 24‐h video EEG. No patient exhibited any radiological abnormalities. Diagnosis was established according to the diagnostic scheme published by the International League Against Epilepsy in 2001 [Engel, 2001]. Absence seizures occurred between 2 and 3 times weekly to 40 and 50 times daily. Of the 15 patients, 10 were newly diagnosed and had not received epilepsy medications. The remaining five patients were mainly prescribed valproate, and continued to suffer from some residual seizures. A total of 14 right‐handed, healthy subjects, who were age and sex matched, were selected for the control group. Informed consent for the study was obtained from each subject.
Data Acquisition
BOLD‐sensitive MRI data was acquired using gradient‐echo echo‐planar imaging (EPI) sequences in a 3T MRI scanner (EXCITE, GE Milwaukee, WI) with an eight‐channel‐phased array head coil. The imaging parameters were as follows: thickness: 5 mm (no gap), TR = 2,000 ms, TE = 30 ms, FOV = 24 cm × 24 cm, flip angle = 90°, matrix = 64 × 64. Two hundred volumes (30 slices per volume) were acquired during 410 s of an fMRI run. To ensure steady‐state longitudinal magnetization, the first five volumes were discarded. According to patient endurance, two to five fMRI runs were performed. During data acquisition, subjects were required to relax with eyes closed, without falling asleep. Anatomical T1‐weighted images were acquired using a three‐dimensional (3D)‐spoiled gradient recalled (SPGR) sequence, generating 156 axial slices (thickness: 1 mm (no gap), TR = 8.5 ms, TE = 3.4 ms, FOV = 24 cm × 24 cm, flip angle = 12°, matrix = 512 × 512).
During fMRI acquisition, EEG data was continuously recorded through a 10/20 system with 32 Ag/AgCl electrodes attached to the scalp with conductive cream. Two ECG channels were simultaneously recorded. The amplifier was a Mizar 40 (EBNeuro, Florence, Italy), with 32 channels applied for MR. Data was sampled at 4096 Hz. The EEG dynamic range was ±65.5 mV to prevent MRI artifact waveforms that could saturate the EEG/ECG. The MR artifact was filtered online [Garreffa et al., 2003] through the software BE‐MRI Toolbox (Galileo New Technology, Florence, Italy). If IED or seizure was detected in a particular run, fMRI data run would be excluded from the subsequent analysis. Simultaneous EEG was not recorded in healthy subjects.
Data Preprocess Analysis
Preprocessing of fMRI data was conducted through the SPM2 software package (statistical parametric mapping, http://www.fil.ion.ucl.ac.uk/spm). The slice time correction, 3D motion detection and correction, spatial normalization to the MNI template supplied by SPM, and spatial smoothing using an isotropic Gaussian kernel (8 mm full width at half maximum) were included. If subjects with head motion were more than 1 mm or 1° during EEG‐fMRI acquisition, they would be excluded in the following analysis. To extract the time series for cerebrospinal fluid (CSF) and white matter (WM), subject‐specific CSF and WM templates were created. First, all subject high‐resolution structural images were normalized to the MNI template and resampled to 3 × 3 × 3 mm. The images were then segmented through SPM2, and the resulting images were thresholded at a probability of 80%. Finally, we coregistered the thresholded images to functional images and then subject‐specific CSF and WM masks were respectively generated.
DMN Identification With Seed at PCC
A single spherical region (radius 10 mm) was selected as the seed, and it was positioned in the PCC/precuneus area (0, −52, 30) [Fransson, 2005, 2006]. The mean BOLD signal intensity time course was extracted from the seed. Two procedures were used to remove possible variances from the seed averaging time series. (i) Temporal band‐pass filtering (pass band 0.01–0.08 Hz) was conducted through a phase‐insensitive filter, which reduced the effects of low‐frequency drift and high‐frequency noise [Fox et al., 2005; Fransson, 2005]. (ii) Through linear regression, the time series was further corrected to eliminate the effect of six head motion parameters obtained in the realigning step and the effect of the signals from a CSF region, a WM region and global brain signal [Fox et al., 2005, 2009]. After the same procedures were applied to the time series extracted from each brain voxel, cross‐correlation functional connectivity analysis was performed by computing temporal correlation, between the seed and all brain voxels. Correlation coefficients of each voxel were normalized to Z‐scores with Fisher's r‐to‐z transformation. Therefore, an entire brain Z‐score map was created for each subject.
To assess voxel‐wise statistical significance of functional correlations at the group level, as well as differences between controls and patients, SPM2 was used. First, an individual Z‐score map was used in the random effect one‐sample t‐test. A statistical map of significant functional connectivity with PCC was created for each group. The significance level was set at P < 0.05, and was corrected for multiple comparisons using FDR‐criterion [Genovese et al., 2002], with cluster size >540 mm3 (20 adjacent voxels). Two sets of regions were identified for each group: one significantly correlated with PCC (DMN) and the other had a significant negative correlation with PCC. Next, to determine the difference between controls and patients in DMN, the Z‐scores maps were also processed in a random effect two‐sample t‐test in SPM2. To ensure that results could not be accounted for by the potential bias of using a DMN map derived from the control or patient alone, a combined group DMN map was defined from the total group of combined patients and control subjects (see Fig. 1) and was used as a mask for SPM2. The significance level was set to P < 0.05 (FDR‐corrected) and cluster size >540 mm3 (20 adjacent voxels).
Figure 1.
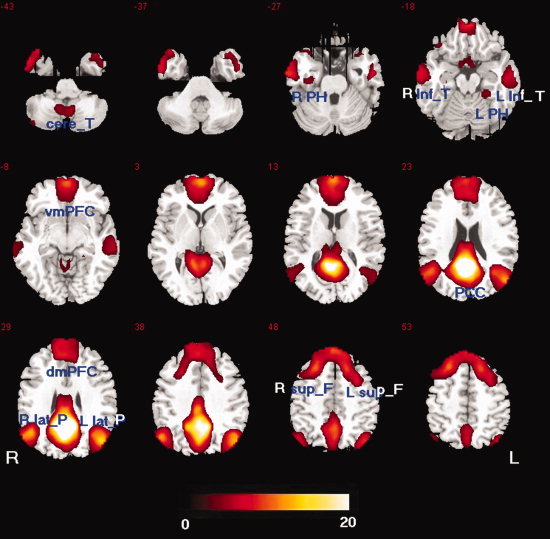
Map of resting state functional connectivity for PCC in group combined patients and control subjects (P < 0.05, FDR‐corrected, 20 adjacent voxels). Total 12 nodes were identified. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Region‐wise Functional Connectivity in DMN
To evaluate functional connectivity within DMN, the major brain regions shown in Figure 1 were selected as graph network nodes, and then a correlation between each pair was analyzed. In combined group DMN map (see Fig. 1), the voxel with maximal activation in a cluster served as the center, or a node of the network. For the frontal lobe, however, a very large cluster obviously included four different DMN regions, which were defined in a previous study (dorsal medial prefrontal cortex, ventral medial prefrontal cortex, left and right superior frontal gyri) [Fair et al., 2008]. Therefore, it was separated into four nodes. A total of 12 DMN nodes were defined (Table I, Fig. 1). These nodes were consistent with a previous DMN anatomical review [Buckner et al., 2008]. Mean time series were extracted by averaging the time series of each peak voxel and its nearest 26 neighbors. The aforementioned procedures were used to remove possible variances from the mean time series of nodes. The resulting time series were correlated between nodes of each subject, and then an N × N (N = 12 in this study) correlation matrix for each subject was obtained. A Fisher's r‐to‐z transformation was applied to normalize correlation coefficients (r). For each group, a Z‐score matrix was averaged across all subjects from each group.
Table I.
Talairach locations of 12 nodes in DMN
| Regions | Abbreviation | Talairach coordinates (x, y, z) |
|---|---|---|
| Dorsal medial prefrontal cortex | dmPFC | −7, 46, 35 |
| Ventral medial prefrontal cortex | vmPFC | 7, 57, 5 |
| Right superior frontal cortex | R sup_F | 19, 36, 50 |
| Left superior frontal cortex | L sup_F | −22, 26, 50 |
| Right parahippocampal gyrus | R PH | 33, −17, −22 |
| Left parahippocampal gyrus | L PH | −28, −33, −13 |
| Right inferior temporal cortex | R inf_T | 60, −13, −15 |
| Left inferior temporal cortex | L inf_T | −60, −15, −15 |
| Right lateral parietal cortex | R lat_P | 53, −60, 35 |
| Left lateral parietal cortex | L lat_P | −49, −63, 35 |
| Posterior cingulate cortex | PCC | 0, −52, 30 |
| Cerebellar tonsils | Cere_T | 10, −52, −36 |
Two‐sample two‐tailed t‐tests were performed on all 66 (C ) possible connections represented in the 12 × 12 correlation matrices between patients and controls [Fair et al., 2008; Liu et al., 2008]. The statistical significance level was set to P < 0.05 (FDR‐corrected).
Graph Definition and Visualization
The regional center of each node was laid out through Pajek software [Batagelj and Mrvar, 1998] (http://pajek.imfm.si/doku.php). The edges (functional connection) between the nodes were constructed by applying a correlation threshold Z (Fisher's r‐to‐z). In terms of the probability of the observed z ij > Z under the null hypothesis that z ij is less than an arbitrary value Z, the threshold Z was defined. Because the multiple, nonindependent tests were for 66 possible connections among the 12 regions, the threshold P < 0.05 (FDR‐corrected) was applied for multiple comparison tests. In other words, a layout of undirected edges was shown only under z ij > Z for each subject and group.
Correlation Between Functional Connectivity and Clinical Features
To investigate the underlying relationship between altered functional connectivity in DMN and clinical variables (initial age of seizure and epilepsy duration), the Pearson's correlation was calculated, with threshold P < 0.05 (uncorrected).
RESULTS
Three of the recruited 15 patients were excluded from further data processing; two of these were excluded due to excessive head motion, and one patient exhibited IEDs during each of the four runs. Twelve patient data sets fulfilled the criteria for this study, and these were included in the patient group. The clinical patient details have been shown in Table II. The control group consisted of 14 healthy subjects.
Table II.
Demographic data of 12 absence epilepsy patients
| Patient no. | Sex | Age | Age at seizure onset | Seizure type frequency | Antiepileptic drugs | Frequency of SWD (Hz) |
|---|---|---|---|---|---|---|
| 1 | M | 7 | 4 | Abs 20/day | VPA | 2.5–3.5 |
| 2 | M | 24 | 10 | Abs several/day | VPA | 3 |
| 3 | F | 16 | 3 | Abs 10/day | None | 3–3.5 |
| 4 | M | 17 | 4 | Abs daily, GTCS 2/year | None | 3–3.5 |
| 5 | M | 21 | 13 | Abs several/day | VPA | 2–4 |
| 6 | M | 5 | 4 | Abs 40/day | None | 3 |
| 7 | M | 18 | 9 | Abs 2–3/week, GTCS 1/month | None | 2.5–3 |
| 8 | M | 8 | 7 | Abs several/day | VPA CZP LTG | 3–4 |
| 9 | M | 14 | 9 | Abs 7–8/day | None | 3 |
| 10 | M | 18 | 5 | Abs 2–3/day | None | 3–4 |
| 11 | F | 9 | 7 | Abs several/day | None | 3–4 |
| 12 | F | 7 | 6 | Abs several/day | None | 3–3.5 |
Abs, absence seizure; GTCS, generalized tonic clonic seizure; VPA, valproic acid; LTG, lamotrigine; CZP, clonazepam.
Connectivity in DMN Identified With Seed at PCC
A connectivity map for each group was generated, and the connectivity patterns of positive and negative correlations appeared to be similar during visual inspection of the two groups (Figs. 2 and 3). The positive correlation maps were primarily found in the medial prefrontal cortex (BA 9, 10, 11), superior frontal gyrus (BA 8), PCC/precuneus (BA 30, 31), inferior parietal cortex/angular gyrus (BA 39), lateral temporal cortex (BA 21), parahippocampal gyrus (BA 36), and cerebellar tonsils for both groups. The map of negative correlation in the control group was observed in the insula (BA 13), precentral gyrus/postcentral gyrus (BA 6, 40/44), supplementary motor area (SMA)/pre_SMA (BA 6, 32), inferior frontal gyrus (BA 45), inferior parietal lobule (BA 40), and superior temporal gyrus (BA 22) (Figs. 2 and 3, Table III). The DMN and anticorrelated network were consistent with previous studies [Buckner et al., 2008; Fox et al., 2005; Fransson, 2005, 2006].
Figure 2.
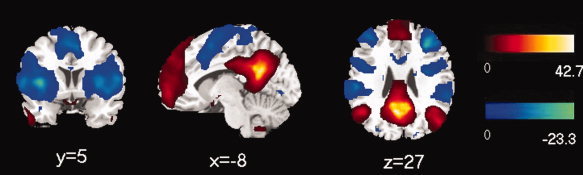
Map of resting state functional connectivity for PCC in control group. The positive (hot) and negative (cool) relationships were showed. The statistical threshold was P < 0.05 (FDR‐corrected, 20 adjacent voxels). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
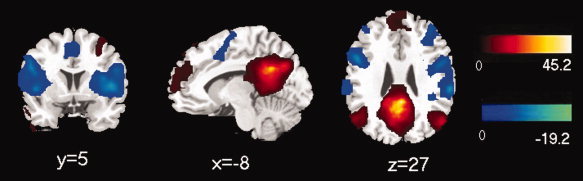
Map of resting state functional connectivity for PCC in absence epilepsy group. The positive (hot) and negative (cool) relationships were showed. The statistical threshold was P < 0.05 (FDR‐corrected, 20 adjacent voxels). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table III.
Significant difference of functional connection to PCC between two groups
| Regions | Talairach coordinates | Peak T‐score | Cluster voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Patents < control | |||||
| Left posterior cingulate | −6 | −48 | 21 | 6.31 | 176 |
| Right precuneus | 6 | −53 | 30 | 4.35 | |
| Left angular gyrus | −50 | −71 | 33 | 6.03 | 126 |
| Right medial frontal gyrus | 9 | 54 | 36 | 4.67 | 239 |
| Left superior frontal gyrus | −9 | 48 | 28 | 4.13 | |
| Right superior frontal gyrus | 9 | 46 | 47 | 3.78 | |
| Right inferior parietal lobule | 50 | −59 | 38 | 3.85 | 74 |
Between‐Group Analysis of DMN Connectivity Identified With Seed at PCC
Between‐group analysis was performed using the SPM2 two‐sample t‐test in the DMN mask, with a statistical threshold of P < 0.05 (FDR‐corrected), and the cluster corrected to >20 adjacent voxels. The between‐group difference in DMN was obtained, which was defined by the cross‐correlation functional connectivity analysis with seed at PCC (Fig. 4, Table III). Compared with the control group, no increase in connectivity was found in the patient group. However, decreased connectivity was detected at the bilateral medial prefrontal cortex (BA 8, 9), bilateral angular gyri and inferior parietal lobule (BA 39), and posterior cingulated cortex (BA 30). Results revealed that DMN functional connectivity was reduced in the absence epilepsy patients.
Figure 4.
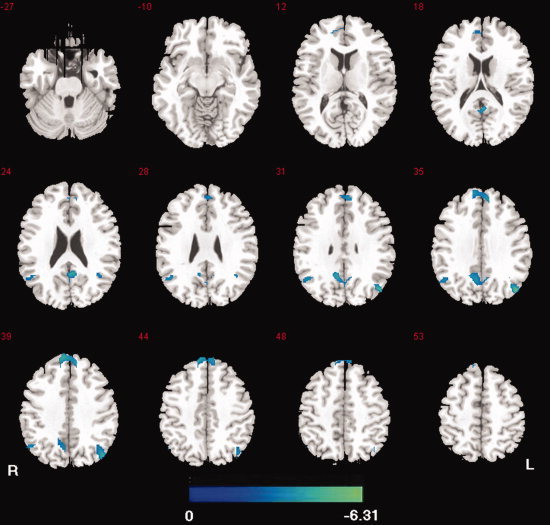
Map of functional connectivity differences for PCC between the two groups. Compared with controls, no increased connection was found, and decreased connectivity was showed (cool) in absence epilepsy patients. The statistical threshold was P < 0.05 (FDR‐corrected, 20 adjacent voxels). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Region‐wise DMN Functional Connectivity in Patients and Controls
The mean correlation matrix was calculated by averaging the correlation matrix across all subjects within both groups. For better visualization of structural patterns within those connection matrices, a layout of nodes (individual ROIs) and undirected edges were represented as networks (Fig. 5A,B). The edges between nodes were constructed by setting the significance level of P < 0.05 (FDR‐corrected). The correlation coefficient threshold determined by P < 0.05 (FDR‐corrected) was variable for each subject, with a value range of 0.16–0.18. According to Figure 5, functional connectivity in the patient group appeared to be less. To quantitatively evaluate region‐wise functional connectivity in DMN, the sparsity of network from each subject was compared between the two groups. Here, the sparsity of a network is the ratio of the number of existing edges and the possible maximum edges in the network (C ) [He et al., 2009]. Results showed that connectivity was significantly sparse (t = 3.39, P = 0.0024, two‐sample two‐tailed t‐tests) in the patient group.
Figure 5.
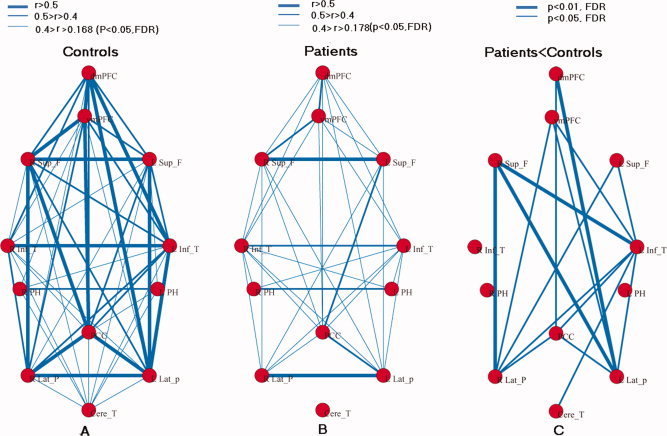
Graph visualization of the mean connectivity network in controls (A) and absence epilepsy patients (B). Undirected edges (functional connectivity) are demonstrated with three different width lines according to three different connection strengths. The connective threshold was P < 0.05 (FDR‐corrected), e.g., r > 0.168 for control group (A) and r > 0.178 for absence epilepsy group (B). Right pattern (C) represents the differences in region‐wise functional connectivity between the two groups. The undirected edges are illustrated by lines of two width according to the two significant levels (P < 0.05 and P < 0.01, FDR‐corrected). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To directly compare connectivity (r) of each pair node between the two groups, two‐sample two‐tailed t‐tests were performed on all 66 potential connections included in the correlation matrices. Compared with the controls, 16 pairs of cross‐correlations were significantly decreased (P < 0.05, FDR‐corrected) in the patient group. No significant increase was observed in the patient group. Decreased connectivity was primarily detected among the frontal and parietal lobes, as well as the left temporal neocortex nodes (Fig. 5C).
Relationship Between Functional Connectivity and Clinical Features
Functional connectivity between R_SFG and L_LP (R = −0.586, P = 0.045), which was significantly decreased in the patients, had significant negative correlation with epilepsy duration (see Fig. 6). The correlation between functional connectivity and initial age of seizure was not significant.
Figure 6.
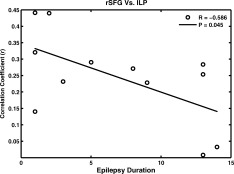
Functional connectivity and epilepsy duration.
DISCUSSION
DMN Aberration in Patients With Absence Epilepsy
To date, very few studies have focused on resting‐state fMRI in absence epilepsy patients, particularly analysis of interictal duration without IED. In this study, to avoid IED effects, resting‐state fMRI data sets of patients with absence epilepsy were acquired during interictal duration without IED. Functional connectivity analysis, based on regions of interest, is considered to be an efficient method and has been widely adopted to identify spatial patterns of spontaneous coherent BOLD activity [Fox and Raichle, 2007]. PCC plays a crucial role in DMN, and it is typically used as a seed to obtain DMN spatial patterns [Fox et al., 2005; Fransson, 2005, 2006]. In previous studies, this method has been used to assess DMN functions in Alzheimer's disease, schizophrenia, and attention‐deficit/hyperactivity disorder [Broyd et al., 2009]. In this study, the same method has been utilized to identify functional connectivity maps in DMN. Combined with region‐wise functional connectivity analysis in DMN, the differences between absence epilepsy patients and controls were subsequently estimated, and the following principal observations were made: (1) DMN spatial distribution in the control group was consistent with previous studies, and the patient group map was also similar (Figs. 1, 2, 3, 1, 2, 3). This implied that the cross‐correlation functional connectivity analysis seed on PCC acquired spontaneous low‐frequency fluctuations and represented a default mode state in absence epilepsy patients. (2) Compared with the control group, spontaneous coherent BOLD activity decreased in DMN, which reflected reduced DMN integration in the patient group. (3) Region‐wise functional connectivity among the frontal, parietal, and temporal lobe was significantly decreased in the patient group. Moreover, functional connectivity between the frontal and parietal lobe appeared to show a negative correlation with epilepsy duration, although this particular finding did not survive correction for multiple comparisons so further study is required to confirm this finding. Our findings indicated aberrant default mode functional connectivity in patients with absence epilepsy during a resting state without IED. In addition, decreased functional connectivity may be aggravated by long epilepsy duration.
Possible Reasons for Decreased DMN Functional Connectivity in Absence Epilepsy
Abnormal myelination could have been responsible for the decreased integration in patients. Increased signal propagation efficiency, with elaboration of the myelin sheath, may support functional integration of distant regions [Fair et al., 2008; Luna and Sweeney, 2004]. Although epilepsy is not considered to be a typical white matter or myelin sheath disease, several studies have suggested that abnormal white matter and demyelination occurred in conjunction with epilepsy [Chahboune et al., 2009; Grant, 2005; Hoffmann et al., 2008; Luat and Chugani, 2008]. Recently, a DTI study of absence epilepsy in a rat model has demonstrated that chronic abnormal epileptic activity in the cortex is along with microstructural changes in white matter pathways interconnecting seizure discharge regions [Chahboune et al., 2009].
Besides, the posterior cingulate might be influenced by SWD, and reduced activity in this region may facilitate onset of SWD [Archer et al., 2003]. Recently, Vaudano et al. [2009] have suggested that the precuneus plays a permissive role in absence seizures and that functional change of PCC and SWD may influence each other. Therefore, anything that induces SWD could indirectly result in connectivity changes in the PCC and ultimately increase the possibility of seizure.
Decreased DMN Functional Connectivity and Brain Function
Although neuropsychological tests were not performed in this study, some previous studies have demonstrated that cognitive and behavioral deficits, such as memory, attention, and linguistic function, are exhibited in absence epilepsy patients [Henkin et al., 2005; Pavone et al., 2001]. In addition, subclinical SWD has been shown to be associated with transient cognitive impairment and behavioral deficits [Aldenkamp and Arends, 2004]. Therefore, decreased DMN connectivity in absence epilepsy may be related to attention lapses and memory deficits, just like the cases in Alzheimer's disease [Greicius et al., 2004; He et al., 2007], attention‐deficit/hyperactivity disorder [Castellanos et al., 2008], and schizophrenia [Pomarol‐Clotet et al., 2008]. For example, autobiographic memory retrieval activates the major extent of the default network [Buckner et al., 2008; Cabeza and St Jacques, 2007], and abnormal DMN activity has been observed in an fMRI study using autobiographic memory tasks in temporal lobe epilepsy patients [Addis et al., 2007]. Moreover, reduced connectivity strength within the left temporal lobe and DMN was reported in resting‐state fMRI studies in temporal lobe epilepsy patients [Bettus et al., 2009; Frings et al., 2009].
Decreased Frontoparietal Connectivity and Consciousness Impairment
Behavioral manifestations in absence seizure are thought to be a result of brief impaired consciousness [Crunelli and Leresche, 2002]. A transitory suspension of the “default state” of the brain may be associated with an altered level of consciousness observed during absence seizure [Gotman et al., 2005; Laufs et al., 2006a; Salek‐Haddadi et al., 2003]. Previous functional neuroimaging studies have shown that frontoparietal association areas play an important role in consciousness and the genesis of consciousness. Moreover, states of extremely low‐ or high‐ activation in frontoparietal regions were associated with unconsciousness [Boly et al., 2008]. Low‐metabolic rates in the frontal‐parietal cortex have been demonstrated in some unconscious states, such as deep sleep [Steriade, 2001], general anesthesia [John et al., 2001], and coma/vegetative states [Laureys et al., 1999]. In several studies of epilepsy, generalized seizure resulted in abnormal activity in the frontoparietal association cortex and midline “default mode” networks, and complex partial seizures resulted in abnormal decreased activity. Both were associated with loss of consciousness [see review by Blumenfeld and Taylor, 2003 and Cavanna and Monaco, 2009]. Results from this study revealed significantly decreased functional connectivity between the frontal and parietal nodes during resting nonseizure states in absence epilepsy patients, compared with controls. This decrease likely reflects underlying neuronal functional impairment or altered integration in specific brain regions of absence epilepsy patients. It has been assumed that potential abnormal frontoparietal functional connectivity in a conscious state is likely to reflect pathological mechanisms that results in impaired consciousness during absence seizures. These results were in accordance with a previous proposal of network inhibition or network disruption as a plausible mechanism for impaired consciousness during epilepsy seizures [Yu and Blumenfeld, 2009]. Moreover, an interesting link between decreased functional connectivity and disease state has been provided by significant negative correlations with epilepsy duration (see Fig. 6). In addition, decreased functional connectivity related to epilepsy duration was observed between the lateral frontal node and lateral parietal node. This further supported that decreased functional connection reflected the pathological state of functional brain organization in patients with absence epilepsy. These alterations were gradually aggravated with increased epilepsy duration.
Decreased Temporal, Frontal, and Parietal Connectivity and Language Impairment
Functional connectivity between the left temporal neocortex node and frontoparietal nodes was significantly decreased in this study. These results might be associated with language impairment in absence epilepsy. The control subjects exhibited typical, left‐hemispheric dominance for language. However, absence epilepsy patients demonstrated decreased functional connectivity between the left temporal neocortex node and frontoparietal nodes. Because these regions play crucial roles in language, altered connection might indicate a reorganization of the language system, which could result in the language impairment. In accordance with this, connectivity decreases in language networks such as between frontal and parietal areas, have been found in a previous study with language task in temporal lobe epilepsy [Waites et al., 2006]. In addition, similar connectivity decreases were observed in a resting‐state study of temporal lobe epilepsy [Bettus et al., 2009], which suggested that the decrease might be related to the language impairment.
Methodological Considerations
Several considerations regarding methodology used in this study, however, should be addressed. Nonneuronal physiological processes could cause widely shared variances in fMRI data [Birn et al., 2006; Desjardins et al., 2001; Wise et al., 2004], because global signals that were ubiquitously presented across gray matter and could obscure underlying neuroanatomical relationships [Fox et al., 2009]. These signals should be removed by global (whole brain) signal correction to prevent an increased correlation [Aguirre et al., 1998; Desjardins et al., 2001; Macey et al., 2004]. In this study, CSF, WM and global brain signals were used to remove confounding variance [Fox et al., 2005, 2009]. However, global signal correction through global regression as a preprocessing step could result in false resting‐state correlations [Murphy et al., 2009]. For example, in a resting fMRI study, global correction could introduce negative correlations and reduce positive correlation [Murphy et al., 2009]. Still now the removal of global effects by regression remains a problem. On the other hand, we performed preprocess without global signal regression, too. We found that the global effect might enhance the negative correlation whereas change in “positive correlation regions” was almost invisible. The differences observed with this approach did not alter the major results in our study.
To avoid the possibility of various complications, respiratory and cardiac fluctuations should be considered, which might reduce the specificity of low‐frequency fluctuations to functional connected regions [Lowe et al., 1998]. However, temporal band‐pass filter would not completely eliminate these problems, because the fMRI sampling rates (1/TR) were often too low, and respiratory and cardiac fluctuation information became distorted by subsampling [Rombouts et al., 2003]. In addition, respiratory and cardiac fluctuations should be simultaneously collected with fMRI for controls and patients to estimate the cardiac and respiratory‐linked changes [Birn et al., 2006; Chang et al., 2008]. These fluctuations should also be considered for artifact removal by regression [Smyser et al., 2001].
There were several limitations in this study. Antiepileptic drugs have been shown to impair cognitive function [Brunbech and Sabers, 2002]. In this study, partial patients (4/12, Table II) were treated with valproate monotherapy. However, valproate is generally thought to induce very few cognitive effects [Kang et al., 2006; Legarda et al., 1996]. Therefore, antiepileptic drugs were unlikely to result in altered DMN functional connectivity. Further studies are needed to effectively determine mechanisms of antiepileptic drugs with regard to DMN functional connectivity. Besides, neuropsychological testing was not performed in this study. As mentioned above, cognitive and behavioral deficits have been previously reported in absence epilepsy patients in many previous studies [Henkin et al., 2005; Pavone et al., 2001]. Because these cognitive functional studies consisted of larger sample sizes, these results should reflect the common neuropsychological abnormalities. Furthermore, altered connectivity may correlate with epilepsy duration, initial age and seizure frequency. However, due to the limited sample size, only the correlation with epilepsy duration was reported in this study and it was only significant without correction for multiple comparisons. Future studies should include larger sample sizes to determine these mechanisms.
CONCLUSION
Results from the present resting‐state fMRI study provided compelling evidence for DMN abnormalities in patients with absence epilepsy, even during resting interictal durations without IED. Compared with previous studies, which stated DMN functional effects were caused by IED propagation and momentary suspension [Gotman et al., 2005; Laufs et al., 2006a], the current results suggested that abnormal functional connectivity might also reflect chronic, abnormal, anatomo‐functional integration in DMN, which was derived from anatomical abnormalities or functional reorganization. These alterations could be the specific characteristics of absence epilepsy, which involve the position of irritative zones, dissemination of slow‐wave discharges, seizures, etc., and which might also lead to partial cognitive mental impairment and unconsciousness during absence seizure.
REFERENCES
- Aguirre GK, Zarahn E, D'Esposito M ( 1998): The inferential impact of global signal covariates in functional neuroimaging analyses. NeuroImage 8: 302–306. [DOI] [PubMed] [Google Scholar]
- Addis DR, Moscovitch M, McAndrews MP ( 2007): Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain 130: 2327–2342. [DOI] [PubMed] [Google Scholar]
- Aldenkamp AP, Arends J ( 2004): Effects of epileptiform EEG discharges on cognitive function: Is the concept of “transient cognitive impairment” still valid? Epilepsy Behav 5: S25–S34. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD ( 1995): Remembering the past: Two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 152: 1576–1585. [DOI] [PubMed] [Google Scholar]
- Archer JS, Abbott DF, Waites AB, Jackson GD ( 2003): fMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage 20: 1915–1922. [DOI] [PubMed] [Google Scholar]
- Batagelj V, Mrvar A ( 1998): Pajek‐Program for large network analysis. Connections 21: 47–57. [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort‐Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F, Guye M ( 2009): Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp 30: 1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA ( 2006): Separating respiratory‐variation‐related fluctuations from neuronal‐activity‐related fluctuations in fMRI. Neuroimage 31: 1536–1548. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Taylor J ( 2003): Why do seizures cause loss of consciousness? Neuroscientist 9: 1–10. [DOI] [PubMed] [Google Scholar]
- Boly M, Philips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang‐Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S ( 2008): Intrinsic brain activity in alteres states of consciousness how conscious is the default mode of brain function? Ann NY Acad Sci 1129: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga‐Barke EJS ( 2009): Default‐mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev 33: 279–296. [DOI] [PubMed] [Google Scholar]
- Brunbech L, Sabers A ( 2002): Effect of antiepileptic drugs on cognitive function in individuals with epilepsy: A comparative review of newer versus older agents. Drugs 62: 593–604. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P ( 2007): Functional neuroimaging of autobiographical memory. Trends Cogn Sci 11: 219–227. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, Koh S, Sanker R, Shilds WD ( 2008): Childhood absence epilepsy: Behavioral, cognitive, and linguistic comorbidities. Epilepsia 49: 1838–1846. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly AMC, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal BB, Sonuga‐Barke EJS, Rotrosen J, Adler LA, Milham MP ( 2008): Cingulate‐precuneus interactions: A new locus of dysfunction in adult attention‐deficit/hyperactivity disorder. Biol Psychiatry 63: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Monaco F ( 2009): Brain mechanisms of altered conscious states during epileptic seizures. Nat Rev Neurol 5: 267–276. [DOI] [PubMed] [Google Scholar]
- Chahboune H, Mishra AM, DeSalvo MN, Staib LH, Purcaro M, Scheinost D, Papademetris X, Fyson SJ, Lorincz ML, Crunelli V, Hyder F, Blumenfeld H ( 2009): DTI abnormalities in anterior corpus callosum of rats with spike‐wave epilepsy. NeuroImage. 47: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Cunningham JP, Glover GH ( 2008): Influence of heart rate on the BOLD signal: The cardiacresponse function. Neuroimage 44: 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Leresche N ( 2002): Childhood absence epilepsy: Genes, channels, neurons and networks. Nat Rev Neurosci 3: 371–382. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Sanz Arigita EJ, Barkhof F, Scheltens P, Smith SM, Rombouts SARB ( 2008): Reduced resting‐state brain activity in the ‘default network’ in normal aging. Cereb Cortex 18: 1856–1864. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF ( 2001): Removal of confounding effects of global signal in functional MRI analyses. Neuroimage 13: 751–758. [DOI] [PubMed] [Google Scholar]
- Engel J Jr ( 2001): A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE task force on classification and terminology. Epilepsia 42: 796–803. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL ( 2008): The maturing architecture of the brain's default network. Proc Natl Acad Sci USA 105: 4028–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME ( 2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, van Essen DC, Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME ( 2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101: 3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P ( 2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P ( 2006): How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44: 2836–2845. [DOI] [PubMed] [Google Scholar]
- Frings L, Schulze‐Bonhage A, Spreer J, Wagner K ( 2009): Remote effects of hippocampal damage on default network connectivity in the human brain. J Neurol 256: 2021–2029. [DOI] [PubMed] [Google Scholar]
- Garreffa G, Carnì M, Gualniera G, Ricci GB, Bozzao L, De Carli D, Morasso P, Pantano P, Colonnese C, Roma V, Maraviglia B ( 2003): Real‐time MR artifacts filtering during continuous EEG/fMRI acquisition. Magn Reson Imaging 21: 1175–1189. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Gloor P, Avoli M, Kostopoulos G ( 1990): Thalamo‐cortical relationships in generalized epilepsy with bilaterally synchronous spike‐andwave discharge In: Avoli M, Gloor P, Kostopoulos G, Naquet R, editors. Generalized Epilepsy: Neurobiological Approaches. Boston: Birkhauser; pp 190–212. [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F ( 2005): Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA 102: 15236–15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PE ( 2005): Imaging the developing epileptic brain. Epilepsia 46 ( Suppl 7): 7–14. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V ( 2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin Y, Sadeh M, Kivity S, Shabtai E, Kishon‐Rabin L, Gadoth N ( 2005): Cognitive function in idiopathic generalized epilepsy of childhood. Dev Med Child Neurol 47: 126–132. [DOI] [PubMed] [Google Scholar]
- He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T ( 2007): Regional coherence changes in early Alzheimer's disease: A combined structural and resting‐state functional MRI study. Neuroimage 35: 488–500. [DOI] [PubMed] [Google Scholar]
- He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, Tang H, Zhu C, Gong Q, Zang Y, Evans AC ( 2009): Uncovering intrinsic modular organization of spontaneous brain activity in human. PLoS ONE 4: e5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K, Lindner M, Groticke I, Stangel M, Loscher W ( 2008): Epileptic seizures and hippocampal damage after cuprizone‐induced demyelination in C57BL/6 mice. Exp Neurol 210: 308–321. [DOI] [PubMed] [Google Scholar]
- Hommet C, Sauerwein HC, De Toffol B, Lassonde M ( 2006): Idiopathic epileptic syndromes and cognition. Neurosci Biobehav Rev 30: 85–96. [DOI] [PubMed] [Google Scholar]
- John ER, Prichep LS, Kox W, Valdes‐Sosa P, Bosch‐Bayard J, Aubert E, diMichele F, Gugino LD ( 2001): Invariant reversible QEEG effects of anesthetics. Conscious Cogn 10: 165–183. [DOI] [PubMed] [Google Scholar]
- Kang KH, Lee JM, Lee HW, Jung DK, Suh CK, Kwon SH, Park SP ( 2006): Cognitive profiles of lamotrigine in epilepsy patients: A comparative study with valproate. J Korean Epilepsy Soc 10: 146–152. [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J ( 2006): Negative BOLD responses to epileptic spikes. Hum Brain Mapp 27: 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Lengler U, Hamandi K, Kleinschmidt A, Krakow K ( 2006a): Linking generalized spike‐and‐wave discharges and resting state brain activity by using EEG/fMRI in a patient with absence seizures. Epilepsia 47: 444–448. [DOI] [PubMed] [Google Scholar]
- Laufs H, Hamandi K, Salek‐Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L ( 2006b): Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp 28: 1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Lemaire C, Maquet P, Phillips C, Franck G ( 1999): Cerebral metabolism during vegetative state and after recovery to consciousness. J Neurol Neurosurg Psychiatry 67: 121–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legarda SB, Booth MP, Fennell EB, Maria BL ( 1996): Altered cognitive functioning in children with idiopathic epilepsy receiving valproate monotherapy. J Child Neurol 11: 321–330. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T ( 2008): Disrupted small‐world networks in schizophrenia. Brain 131: 945–961. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA ( 1998): Functional connectivity in single and multislice echoplanar imaging using resting‐state fluctuations. NeuroImage 7: 119–132. [DOI] [PubMed] [Google Scholar]
- Luat AF, Chugani HT ( 2008): Molecular and diffusion tensor imaging of epileptic networks. Epilepsia 49 ( Suppl 3): 15–22. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA ( 2004): The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann NY Acad Sci 1021: 296–309. [DOI] [PubMed] [Google Scholar]
- Lui S, Ouyang L, Chen Q, Huang X, Tang H, Chen H, Zhou D, Gramham JK, Gong Q ( 2008): Differential interictal activity of the precuneus/posterior cingulate cortex revealed by resting state functional MRI at 3T in generalized vs. partial seizure. J Magn Reson Imaging 27: 1214–1220. [DOI] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM ( 2004): A method for removal of global effects from fMRI time series. Neuroimage 22: 360–366. [DOI] [PubMed] [Google Scholar]
- Motamedi G, Meadoe K ( 2003): Epilepsy and cognition. Epilepsy Behav 4: S25–S38. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA ( 2009): The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? Neuroimage 44: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone P, Bianchini R, Trifiletti RR, Incorpora G, Pavone A, Parano E ( 2001): Neuropsychological assessment in children with absence epilepsy. Neurology 56: 1047–1051. [DOI] [PubMed] [Google Scholar]
- Pomarol‐Clotet E, Salvador R, Sarró S, Gomar J, Vila F, Martínez A, Guerrero A, Ortiz‐Gil J, Sans‐Sansa B, Capdevila A, Cebemanos JM, McKenna PJ ( 2008): Failure to deactivate in the prefrontal cortex in schizophrenia: Dysfunction of the default‐mode network? Psychol Med 38: 1185–1193. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SARB, Stam CJ, Kuijer JPA, Scheltens Ph, Barkhof F ( 2003): Identifying confounds to increase specificity during a “no task condition”: Evidence for hippocampal connectivity using fMRI. Neuroimage 20: 1236–1245. [DOI] [PubMed] [Google Scholar]
- Salek‐Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR ( 2003): Functional magnetic resonance imaging of human absence seizures. Ann Neurol 53: 663–667. [DOI] [PubMed] [Google Scholar]
- Smyser C, Grabowski TJ, Frank RJ, Haller JW, Bolinger L ( 2001): Real‐time multiple linear regression for fMRI supported bytime‐aware acquisition and processing. Magn Reson Med 45: 289–298. [DOI] [PubMed] [Google Scholar]
- Steriade M ( 2001): Active neocortical processes during quiescent sleep. Arch Ital Biol 139: 37–51. [PubMed] [Google Scholar]
- Vaudano AE, Laufs H, Kiebel SJ, Carmichael DW, Hamandi K, Guye M, Thornton R, Rodionov R, Friston KJ, Duncan JS, Lemieux L ( 2009): Causal hierarchy within the thalamo‐cortical network in spike and wave discharges. PLoS ONE 4: e6475; doi:10.1371/journal.pone. 0006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD ( 2006): Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol 59: 335–343. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Ide K, Poulin MJ, Tracey I ( 2004): Resting state fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage 21: 1652–1664. [DOI] [PubMed] [Google Scholar]
- Yu L, Blumenfeld H ( 2009): Theories of impaired consciousness in epilepsy. Ann NY Acad Sci 1157: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


